Abstract
Addiction is a chronic brain disease that has dramatic health and socioeconomic consequences worldwide. Multiple approaches have been used for decades to clarify the neurobiological basis of this disease and to identify novel potential treatments. This review summarizes the main brain networks involved in the vulnerability to addiction and specific innovative technological approaches to investigate these neural circuits. First, the evolution of the definition of addiction across the Diagnostic and Statistical Manual of Mental Disorders (DSM) is revised. We next discuss several innovative experimental techniques that, combined with behavioral approaches, have allowed recent critical advances in understanding the neural circuits involved in addiction, including DREADDs, calcium imaging, and electrophysiology. All these techniques have been used to investigate specific neural circuits involved in vulnerability to addiction and have been extremely useful to clarify the neurobiological basis of each specific component of the addictive process. These novel tools targeting specific brain regions are of great interest to further understand the different aspects of this complex disease.
1. Introduction
Addiction is a chronic relapsing disorder characterized by the loss of inhibitory control over drug-seeking and taking, and maintenance of drug use despite negative consequences (Koob and Volkow, 2016). This brain disease has a multifactorial origin with several environmental factors and gene networks interacting among them, leading to a vulnerable phenotype to develop the addictive process (Hamer, 2002). The Diagnostic and Statistical Manual of Mental Disorders (DSM), the tool for diagnosing mental illness used by the American Psychiatric Association, has highly evolved from the eighties to the nineties in the criteria used to diagnose addiction (Fig. 1). In 1980, the third edition of the DSM (DSM-III) defined addiction mainly based on the two significant physical effects produced by the long-term exposure to drugs of abuse, tolerance to the drug, and withdrawal symptoms when stopping drug use (American Psychiatric Association, 1980). In 1994, this drug-centered definition changed with the DSM-IV, and these two criteria were no longer necessary for the diagnosis of addiction. The diagnosis was given in the DSM-IV only when the patient met a minimum of three out of seven criteria, and five of the seven diagnosis criteria were focused on behavioral markers (American Psychiatric Association, 1994).
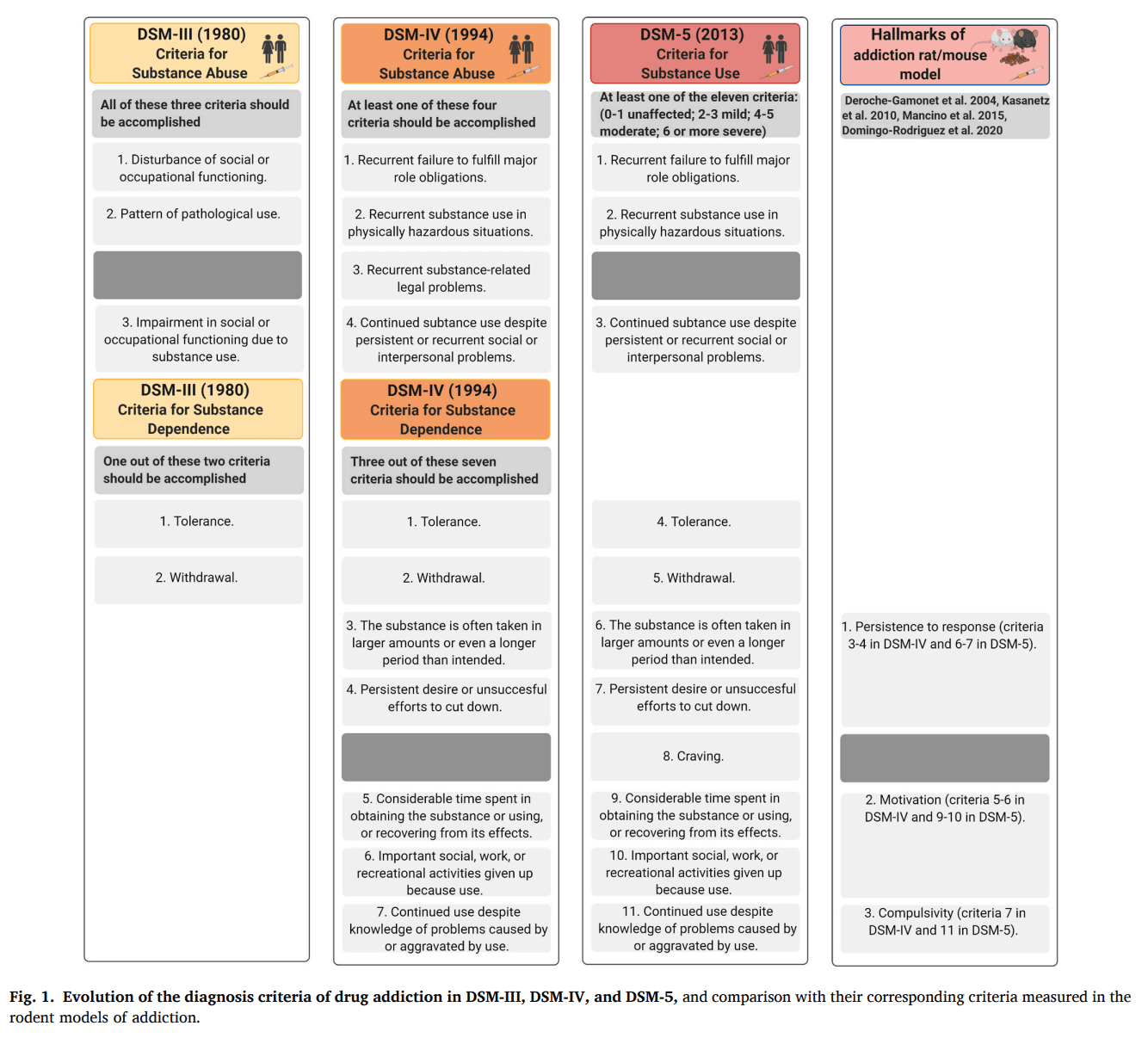
The current DSM fifth edition (American Psychiatric Association, 2013) combines the DSM-IV categories of substance abuse and substance dependence into a single substance use disorder, measured with eleven criteria on a continuum from mild to severe. Thus, the categorical approach of DSM-IV has evolved into a dimensional approach in the DSM-5 that consists of condensing abuse and dependence as dimensions into a single manifestation of a disorder with varying levels of severity (Kopak et al., 2012). Each specific substance is a new separate use disorder, but nearly all substances are diagnosed based on the same overarching criteria (American Psychiatric Association, 2013). A diagnosis of substance dependence previously required three criteria in the DSM-IV, but now mild substance-use disorder in the DSM-5 requires only two symptoms, while severe requires a minimum of six. In the DSM-5, the term addiction is now synonymous with the concept of severe substance-use disorder (Volkow et al., 2016). Problems with law enforcement are not present anymore because cultural considerations make the criteria challenging to apply internationally. Hence, the concept of addiction in the DSM-IV and DSM-5 remains behavioral-centered, and the alterations of loss of inhibitory control emerge as relevant for the transition to addiction. In agreement, the Tenth Revision of the International Classification of Diseases and Health Problems (ICD-10) defines the dependence syndrome as being a cluster of behavioral, physiological, and cognitive phenomena in which the habit of substance use occurs on a much higher urgency for an individual than other activities that once had more salient value. However, the crucial feature of dependence in the ICD-10 is the desire to take the drug and the high probability of relapse after a period of abstinence (WHO, 2020). The DSM-5 also includes gambling disorder as the only diagnosable condition in a new category of behavioral addictions. The controversial concept of food addiction is not included in the DSM-5, although this concept is evolving, and the Yale Food Addiction Scale (YFAS) is the current tool used for the diagnosis of food addiction (Gearhardt et al., 2009) in its updated version YFAS v2.0 (Gearhardt et al., 2016).
The individual vulnerability to addiction is a crucial component in the development of the pathology. Thus, drug-taking initiation does not necessarily lead to addiction, and not all drug users become addicted. Out of 100 people initiating drug use, around 15 to 17 develop an addiction (Anthony et al., 1994), and the range of variation depends on the pharmacological drug properties, the environment, and the genetic susceptibility (Nestler et al., 2016). After the initiation of drug use, resilient individuals can stop and control consumption. In contrast, occasional use in vulnerable individuals is followed by regular use, and finally, addiction develops with a high risk of relapse even after prolonged periods of abstinence. Currently, relapse is a cause of maximum preoccupation at the clinical level with difficult therapeutic approaches.
The diagnostic criteria used in the manuals of psychiatry must be considered when animal models are used to study addiction's neurobiology (Piazza and Deroche-Gamonet, 2013). An animal model of drug addiction has been generated based on five behavioral criteria of DSM-IV/5 clustered in three particular behavioral hallmarks of addiction with the following correspondence (Fig. 1): 1) persistence to response (criteria 3 and 4 of DSM-IV, and 6 and 7 of DSM-5), 2) motivation for the drug (criteria 5 and 6 of DSM-IV, and 9 and 10 of DSM-5), 3) compulsivity defined as an alteration of inhibitory control despite negative consequences (criterion 7 of DSM-IV, and 11 of DSM-5) (Deroche-Gamonet et al., 2004). As the DSM-IV addiction criteria were maintained in the DSM-5, the three hallmarks that englobe them have been continuously used as a reference to develop animal models of drug and food addiction and to elucidate the neurobiological mechanisms underlying these processes (Augier et al., 2018; Deroche-Gamonet et al., 2004; Domingo-Rodriguez et al., 2020; Kasanetz et al., 2010; Mancino et al., 2015).
Here we review the correspondence between the diagnostic criteria in the DSM and the behavioral hallmarks used in translational research with animal models to link these behavioral responses with recent advances in the neurobiology of addiction. Operant behavioral approaches are usually required to evaluate in animals these complex responses, and novel techniques are now available to be combined with these operant approaches in order to dissect the brain interconnected networks that underline these responses. These techniques include optogenetics, DREADDs, calcium imaging, electrophysiology, and whole-brain 3-D imaging. Whole-brain 3-D imaging approaches comprise optical clearing methods that allow a three-dimensional reconstruction of brain tissue structures. Among different clearing methods, PACT (passive CLARITY technique) and uDISCO (ultimate DISCO) possess excellent clearing capability on mouse brain samples. PACT removes cell membranes and protein by sodium dodecyl sulfate to decrease the sample's scattering while preserving native epitope with acrylamide hydrogel. uDISCO employs essential organic compounds that render tissue transparent by protein denaturation and lipid removal. Among others, these clearing methods have provided essential tools for mapping brain wiring diagrams and promoted advances in circuit-based approaches (Kimbrough et al., 2020; Parra-Damas and Saura, 2020; Ueda et al., 2020; Vigouroux et al., 2017). Moreover, optogenetic manipulation of specific neural subpopulations has become a powerful strategy to understand behavioral paradigms, already discussed in excellent recent review articles (Chen et al., 2018; Deubner et al., 2019; Rodriguez-Romaguera et al., 2020). The recent advances obtained by combining DREADDs, calcium imaging, and electrophysiology with operant behavioral approaches will be discussed in this review. All together, these techniques have identified particular brain networks that could be targeted as potential therapies for future prevention and treatment of addiction.
2. Neurocircuitry of addiction
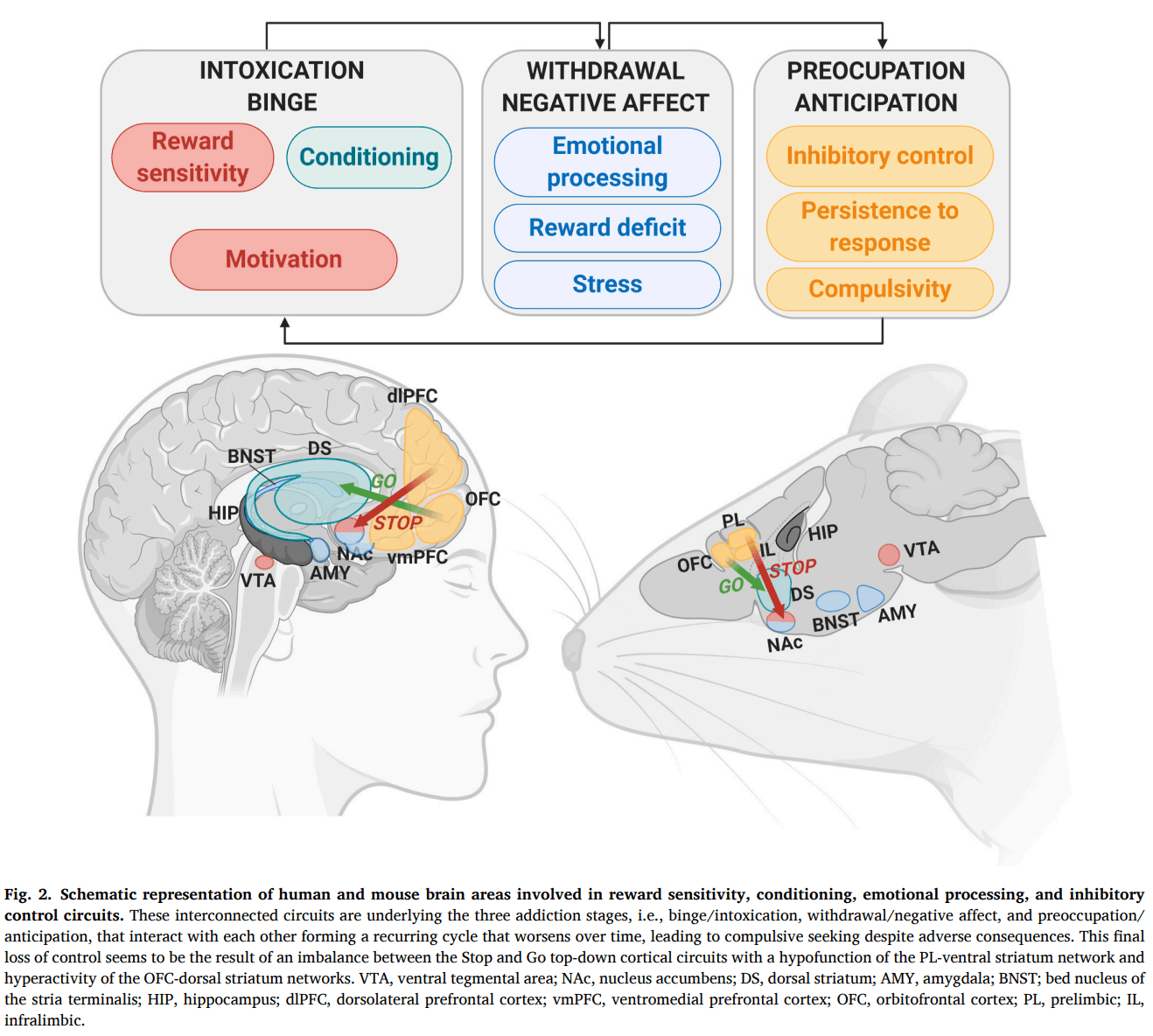
The neurocircuitry underlying addiction lies initially in the brain reward system. Within this system, several interconnected networks involved in reward sensitivity, conditioning, emotional processing, and inhibitory control are first recruited by drug exposure and later modified by long-lasting neuroadaptations (Volkow et al., 2019). These networks can be clustered in three major neurobiological circuits: the basal ganglia, the extended amygdala, and the prefrontal cortex (Koob and Volkow, 2016). These three major neurobiological circuits have its correspondence to three stages of the transition to addiction, i.e., binge/intoxication, the withdrawal/negative affect, and the preoccupation/anticipation stage, that interact with each other forming a recurring cycle in which addiction can be conceptualized (Koob and Volkow, 2010). The three behavioral hallmarks used in the addiction animal model, motivation, persistence to response, and compulsivity, can be integrated into these three stages of the addictive process. Thus, the motivation for the drug has its correspondence with the intoxication stage; whereas, the persistence to response and the compulsivity-like behavior have their phenotypic similarity in the preoccupation/anticipation stage (Fig. 2). Motivation is connected to reward processing and is related to the mesolimbic dopamine neurons projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) (Lindgren et al., 2017). Persistence to response reveals the difficulty of animals to stop reward-seeking. This behavioral hallmark is related to a persistent desire and an ineffective effort to cut down the response due to habit formation or disruption of extinction learning and mainly involves the participation of the prefrontal cortex (PFC), the dorsal striatum, and the hippocampus (Schmitzer-Torbert et al., 2015). The compulsivity-like behavior is related to the connectivity strength of the medial PFC (mPFC) glutamatergic projections to the NAc (Domingo-Rodriguez et al., 2020). This establishment of addiction behavioral hallmarks in animal research based on human diagnostic criteria has helped to study the neurobiological substrates underlying addiction, taking into account each specific endophenotype that integrates this complex behavioral disease.
During the intoxication stage, the rewarding effects of drugs of abuse are related to a massive burst of dopamine in the NAc (Di Chiara and Imperato, 1988; Koob and Bloom, 1988). The dopaminergic neurons that project from the VTA to the NAc involved in these rewarding effects integrate the mesolimbic pathway critically involved in goal-directed behavior (Lammel et al., 2012; Yang et al., 2018). This reward pathway drives the motivation for natural reinforcers, such as food, indicating that drugs are hijacking the circuits underlying natural rewards (Kelley and Berridge, 2002). The physiological role of this circuit provides a neurobiological explanation for the capability of foods, mostly those rich with sugars, to trigger addictive behaviors similar to drugs of abuse (Volkow et al., 2013; Wise, 2002).
Phasic dopamine firing in the NAc produced by addictive substance intake promotes a strong association between environmental neutral stimuli and the reward through the process of conditioning (Koob and Volkow, 2010). With the repeated pairing stimulus-reward over time, the learned cue becomes salient and acquires the ability to increase dopamine in the NAc by itself in anticipation of the reward (Peciña and Berridge, 2013). Thus, the motivational drive towards the reinforcer now may occur with the addictive substance-predictive cue's exposure, in the absence of drug or food presentation and the absence of physiological needs, facilitating the transition to habit-like compulsive seeking (Everitt and Robbins, 2005). This transition from controlled actions to more habit-based behaviors involves a shift from the ventral striatum in favor of progressive recruitment of the dorsal striatum (Balleine and O’Doherty, 2010; Everitt and Robbins, 2013). Within the dorsal striatum, the dorsolateral portion of this area (DLS) has been proposed to play a particular role in the formation and expression of stimulus-response habits in contrast to the dorsomedial portion (DMS), involved in the acquisition of drug-seeking and goal-directed behavior (Everitt and Robbins, 2016; Lipton et al., 2019). Thus, dopamine receptor blockade in the DLS decreased cocaine-seeking after prolonged training in mice but was ineffective during the acquisition phase. In contrast, dopamine receptor blockade in the DMS modified cocaine-seeking acquisition but did not affect prolonged training when habit formation was established (Murray et al., 2012). In agreement, electrophysiological studies revealed a DMS neuronal activation during the acquisition of a specific learning task, which was progressively decreased over training corresponding to the time frame when DSL activity emerged to drive habitual performance (Gremel and Costa, 2013; Smith and Graybiel, 2013; Thorn et al., 2010). Therefore, the repetitive consummatory behavior leads to a reduction of the glutamate inputs from the PFC to the NAc and the DMS governing the goal-directed behavior in favor of glutamate projections from sensory-motor cortical areas and dopaminergic projections from the substantia nigra to the lateral portion of the dorsal striatum leading to the habitual drug-seeking (Everitt and Robbins, 2013, 2016). However, recent data have raised questions about the relevance of ‘habit-formation’ in addiction development since drug-taking behaviors were reported to only require dopamine inputs from the ventral striatum but not from the dorsal striatum (Singer et al., 2018).
As addiction progresses, abstinence of the addictive substance results in a strong negative emotional state (anhedonia, anxiety, depression, among other symptoms) that leads to an increased urge to consume the addictive substance to ameliorate these negative symptoms rather than for its primary reinforcing value (Parylak et al., 2011). The neurobiological substrate underlying this stage is the recruitment of the brain's stress systems in the extended amygdala and the activation of the hypothalamic-pituitary-adrenal axis triggered by stress mediators, such as the corticotropin-releasing factor (Koob and Volkow, 2016). In parallel, the chronic activation of the reward system leads to neuroadaptations producing a decreased reward function characterized by loss of motivation for natural rewards and the lack of interest in activities not associated with the reinforcer. Indeed, neuroimaging studies revealed a lower availability of dopamine type 2 receptors (D2Rs) and decreased dopamine release in the striatum in drug-addicted individuals, similar to the downregulation of these receptors reported in obese individuals (Volkow and Wise, 2005; Wang et al., 2001). Striatal D2Rs availability correlates with lower metabolic activity in PFC regions in these subjects (Goldstein and Volkow, 2011; Volkow et al., 2008). These neuroadaptations contribute to the emergence of the negative state associated with the withdrawal, driving to a craving that increases the vulnerability to relapse.
During the preoccupation stage, the exposure to conditioned cues leads to an augmentation of the craving associated with a hypofunction of frontal areas involved in the top-down inhibitory control (Goldstein and Volkow, 2011). This loss of inhibitory control over drug or food-seeking leads to continue use despite negative consequences. Only a small percentage of people who consume addictive substances reach this stage, pointing out the individual differences between vulnerable and resilient phenotypes (Deroche-Gamonet et al., 2004; Piazza and Deroche-Gamonet, 2013). One frontal area involved in behavioral control that has received considerable attention is the PFC (Miller and Cohen, 2001). The PFC is a collection of several brain areas involved in cognitive processes that are closely implicated in addiction (Moorman et al., 2015). The PFC receives dopamine inputs from the VTA and sends glutamatergic projections to the dorsal and ventral striatum, modulating the striatal-pallidal-thalamocortical system to the VTA, exercising feedback control over the dopaminergic system (Koob and Volkow, 2016). Drug-induced PFC alterations contribute to the dysregulation of reward circuits and higher-order executive functions. The PFC is crucial in the transition to addiction, as the disorder emerges from an imbalance between execution and inhibition of behavior, with excessive motivation uncontrolled by impaired self-regulation, through altered PFC performances. The PFC includes the orbitofrontal cortex (OFC) and the medial PFC (mPFC) (Koob and Volkow, 2010). The mPFC contains several sub-regions that seem to play distinct functional roles: the medial precentral, anterior cingulate, prelimbic (PL), and infralimbic (IL) cortex (Heidbreder and Groenewegen, 2003). These three last sub-regions of the mPFC have been widely related to drug addictive processes. The PL cortex has been reported to promote cocaine-seeking, whereas the IL cortex has been proposed to suppress cocaine-seeking after extinction (Moorman et al., 2015). However, both PL and IL cortices drive and inhibit drug-seeking depending on the behavioral context, the type of drug, and the previous history of drug consumption, suggesting that multiple subcircuits within each of these mPFC areas may play unique behavioral functions (Moorman et al., 2015; Riga et al., 2014). The anterior cingulate cortex has been mainly involved in attentional selectivity and discrimination learning participating in the generation of impulsive actions (Perry et al., 2011) and cues-induced cocaine reinstatement (Kalivas and Volkow, 2005). Another area recently related to cue-induced cocaine reinstatement is the lateral habenula, which regulates dopaminergic signaling from the limbic forebrain to the brainstem nuclei. The main role attributed to the lateral habenula is the processing of aversive information and behavioral flexibility (Nair et al., 2020).
The orbitofrontal cortex is involved in appropriate flexible behavior and goal-directed decision making (Wikenheiser and Schoenbaum, 2016). Early studies support a crucial role of this area in the inhibitory control of inappropriate responses in a current context (Perry et al., 2011). In contrast, more recent studies argued against response inhibition as the primary role of the orbitofrontal cortex, suggesting a more relevant role in associative learning and decision-making (Stalnaker et al., 2015). The orbitofrontal cortex in rodents is part of the olfactory system, and the possible differential role of the sub-regions of this cortical area has not yet been well defined (den Hartog et al., 2016; Lucantonio et al., 2012; Wikenheiser and Schoenbaum, 2016).
Within the PFC, the anterior insular cortex that corresponds to the agranular insula in rodents (Naqvi and Bechara, 2009) appears to have an interoceptive function integrating autonomic and visceral information with emotion and motivation and has been shown to be involved in the maintenance of drug addiction (Naqvi and Bechara, 2009), and food anticipatory activity (Gavrila et al., 2017). The role of the insula in drug addiction seems related to maladaptive interoceptive control over behavior (Naqvi and Bechara, 2009; Stewart et al., 2014; Verdejo-Garcia et al., 2012; Verdejo-García and Bechara, 2009). Accordingly, this brain structure is activated during craving (Bonson et al., 2002; Brody et al., 2002; Naqvi et al., 2007), and tobacco smokers with insula damage stopped smoking easily without experiencing craving or relapse (Naqvi et al., 2007). Furthermore, anterior insular cortex lesions modulate the loss of control over cocaine intake in rats (Rotge et al., 2017). Other frontal cortical areas, such as the granular insula (Forget et al., 2010), have also been involved in drug addiction. Interestingly, the granular insula's integrity is necessary for exhibiting motivation to take nicotine and relapse to nicotine seeking, but not similar behavioral responses mediated by food (Forget et al., 2010).
The frontal areas send glutamatergic projections in a top-down manner to different brain areas such as VTA, NAc, dorsal striatum, hippocampus, and amygdala, controlling the basal ganglia and the extended amygdala microcircuits (Parsons and Hurd, 2015). Specific ‘Go circuits' and ‘Stop circuits' have been identified depending on if their recruitment promotes or difficult the transition to addiction-like behaviors (Bock et al., 2013; Picciotto, 2013). The Go circuit produces an activation of the OFC neurons that directly connect to the medial striatum cluster, which includes the NAc core and the medial wall of the dorsal striatum (Hu et al., 2019; Pascoli et al., 2018; Wall et al., 2019). In turn, the ‘Stop circuit’ activates the excitatory synaptic transmission in PL mPFC neurons projecting to the NAc core inhibiting compulsive-like behavior (Fig. 2) (Domingo-Rodriguez et al., 2020; Hu et al., 2019). An imbalance between both circuits is crucial in the transition to addiction. Identifying the individual strength of both networks may help for restoring the physiological balance between them.
The dorsolateral PFC (dlPFC) in humans is equivalent to the PL area in rodents, and the ventromedial PFC (vmPFC) in humans corresponds to the IL area in rodents (Heidbreder and Groenewegen, 2003). Human neuroimaging studies showed that dlPFC activity increased in response to cocaine-related cues, and this activation was associated with craving and a high risk of relapse (Wexler et al., 2001). Similarly, rodent studies using drug self-administration reinstatement paradigms reported decreased cocaine- and food-reinstatement behavior after pharmacological PL inactivation (McFarland and Kalivas, 2001). However, compelling evidence suggests that dlPFC and PL circuits also have an essential inhibitory role in regulating reward-seeking behavior. Thus, brain imaging studies revealed a reduced dlPFC activation in cocaine abusers performing response inhibition tasks (Crunelle et al., 2012). In parallel, the PL area's pharmacological inactivation reduced a footshock-associated stimulus's ability to decrease cocaine responding and increased punishment resistance in animals responding for sucrose (Limpens et al., 2015). A reduction of PL neurons excitability was revealed after prolonged cocaine self-administration exposure, with the most substantial effect shown in shock-resistant rats (Chen et al., 2013). In some studies, the dlPFC appears to stimulate cocaine-seeking in the reinstatement model while, in others, it suppresses cocaine-seeking when a shock is associated. This contradiction may be explained considering the associative learning process where the activity in the dlPFC promotes the performance of the most potent behavioral response at the expense of a minus well-learned response (Jasinska et al., 2015; Smith and Laiks, 2018). Therefore, the dorsal dlPFC, equivalent to the PL area, is recruited in appetitive associative learning promoting drug-seeking responses in reinstatement and also in aversive learning producing avoidance in the presence of harmful consequences of compulsive taking. It is important to underline that the specific circuits driving or suppressing behavior may depend on multiple factors, including the animal model, the behavioral context, and the nature of the drug investigated (Moorman et al., 2015). Indeed, the role of mPFC neurons in reward processing is more complicated than a simple PL vs. IL dichotomy, and both subregions have overlapping functions depending on these factors. The heterogeneous functions within the mPFC may reflect the importance of multiple interconnected subcircuits within each of these areas of mPFC necessary to support unique functions (Moorman et al., 2015). Therefore the mPFC must be considered a structurally subdivided region with multiple specific neural networks that connect different sets of neurons to achieve its complex function in the rational control of the behavioral responses.
3. DREADD approaches on behavioral studies
Innovative techniques have been combined in the last years with behavioral approaches to study the neurobiological substrate of addiction. A chemogenetic technique that has been recently used to study the implication of specific cell populations in addiction is Designer Receptors Exclusively Activated by Designer Drugs (DREADDs). DREADD is based on G protein-coupled receptors (GPCRs) that allow the modification of a specific cell population to modulate the neuronal activity of a brain region or, even more precisely, the activity of a neuronal pathway. This modulation is done through the depolarization or hyperpolarization of the neurons infected by a viral vector that contains the genetic information for excitatory (hM3Dq or hM3Ds) or inhibitory (hM4Di) GPCRs, respectively (Roth, 2016). The first and prototypical DREADD agonist, clozapine-N-oxide (CNO), has low brain penetrance and, via metabolic degradation, produces the antipsychotic drug clozapine in low concentrations that represents an additional active DREADD ligand (Gomez et al., 2017). In spite of this metabolic conversion, multiple studies support the use of CNO, taken into account several mandatory requirements. Indeed, CNO use requires appropriate controls by the administration of CNO to non-DREADD-expressing animals in order to exclude potential off-target effects of CNO and/or clozapine (Mahler and Aston-Jones, 2018).
Alternative DREADD agonists have been used instead of CNO, such as perlapine and compound 21, although substantial systemic doses are required to activate DREADDs in vivo, and the use of appropriate controls are also necessary for potential off-target actions (Chen et al., 2015; Thompson et al., 2018). Recently, a potent and selective agonist for muscarinic-based DREADDs named deschloroclozapine has been reported to show 100-fold better affinity and potency for hM3Dq and hM4Di than CNO or compound 21 (Nagai et al., 2020). Deschloroclozapine quickly penetrated the brain and activated hM3Dq and hM4Di in less than 10 min post-injection in rodents and decreased off-target binding compared to CNO in vitro (Nagai et al., 2020).
Although cholinergic DREADDs are the most widely chemogentic tool used in the neuroscience field, other non-cholinergic DREADDs have also been developed. Based on the k-opioid receptor, KORD (k-opioid DREADD) has been developed to be activated by the selective inert ligand salvinorin B (SALB). KORD can be expressed in mice together with CNO-responsive DREADDs, a promising bidirectional chemogenetic manipulation of neural circuits (Vardy et al., 2015). Ion channel approaches have also been developed to obtained DREADDs. Thus, modified glutamate-gated chloride channels have been developed to inhibit neuronal transmission using the drug ivermectin as a selective ligand (Frazier et al., 2013). In addition, a promising set of modular ion channels and ultrapotent ligands have been developed, such as novel chemogenetic agonists derived from the clinically approved drug varenicline and novel sub-nanomolar potency agonists with high selectivity for the chemogenetic receptors (Magnus et al., 2019).
In the last years, DREADDs have been widely combined with behavioral studies to elucidate the neurobiological correlates of addiction to different drugs in rodents (Urban and Roth, 2015). Here, we briefly summarized only the main studies that have used DREADDs to dissect the role of specific neuronal pathways in the binge intoxication, withdrawal/negative affect, and preoccupation/anticipation stages of addiction. The use of DREADDs in other responses related to addictive processes has been revised in recent articles (Dobrzanski and Kossut, 2017; Runegaard et al., 2019; Yager et al., 2015).
Most DREADD studies focus on the reinforcing effects of drugs of abuse responsible for the binge intoxication stage of the addictive process. The reinforcing effects are regulated by the release of dopamine from the VTA to the NAc, and VTA neurons are regulated at the same time by the GABAergic medial spiny neurons of the NAc (Koob and Volkow, 2016). In agreement, the activation of the excitatory DREADD, hM3Dq, by CNO chronically in the NAc reduces binge alcohol consumption (Pozhidayeva et al., 2020; Purohit et al., 2018). Cell-type-specific in vivo chemogenetic manipulation in a model of repeated cycles of alcohol consumption and withdrawal in mice demonstrated that chemogenetic excitation of D1-medium spiny neurons in the NAc mimicked glutamatergic strengthening, promoting alcohol consumption. Inhibition of D2-medium spiny neurons produced the same effect by mimicking strengthening of GABAergic inputs (Cheng et al., 2017). In cocaine self-administration, DREADD inhibition of D2-medium spiny neurons using a chemogenetic approach, enhanced the motivation to obtain cocaine (Bock et al., 2013). In contrast, DREADD activation of the mesolimbic dopaminergic pathway using Cre-recombinase expressing CAV2 vector increased cocaine motivation in rats (Boender et al., 2014). The enhancement of VTA dopamine activity via excitatory DREADDs increased both the affinity of cocaine for DAT and the development of cocaine conditioned place preference in mice with a clear sexual dimorphism (Calipari et al., 2017).
DREADDs have also been used to investigate the role of limbic regions in the negative affect rise during drug abstinence and relapse. Interestingly, inhibition of the insula with DREADDs reduces the hyperactivity of the BNST in abstinence from alcohol, and the excitation of the BNST with DREADDs enhances the negative affect that leads to relapse (Centanni et al., 2019). Inhibition of the lateral habenula also reduces cocaine self-administration and diminishes cue-induced cocaine reinstatement (Nair et al., 2020). In contrast, DREADD BNST inhibition enhances cue-induced reinstatement (Perez et al., 2020). In the NAc, DREADD-mediated glutamate release by glial cells inhibited cue-induced reinstatement of cocaine-seeking by stimulating release-regulating mGluR2/3 autoreceptors (Scofield et al., 2015). Methamphetamine reinstatement was also modulated by chemogenetic manipulations of glial glutamate release within the NAc core, and astrocyte-specific Gq-coupled expression DREADDs inhibited methamphetamine seeking promoted by cues (Siemsen et al., 2019).
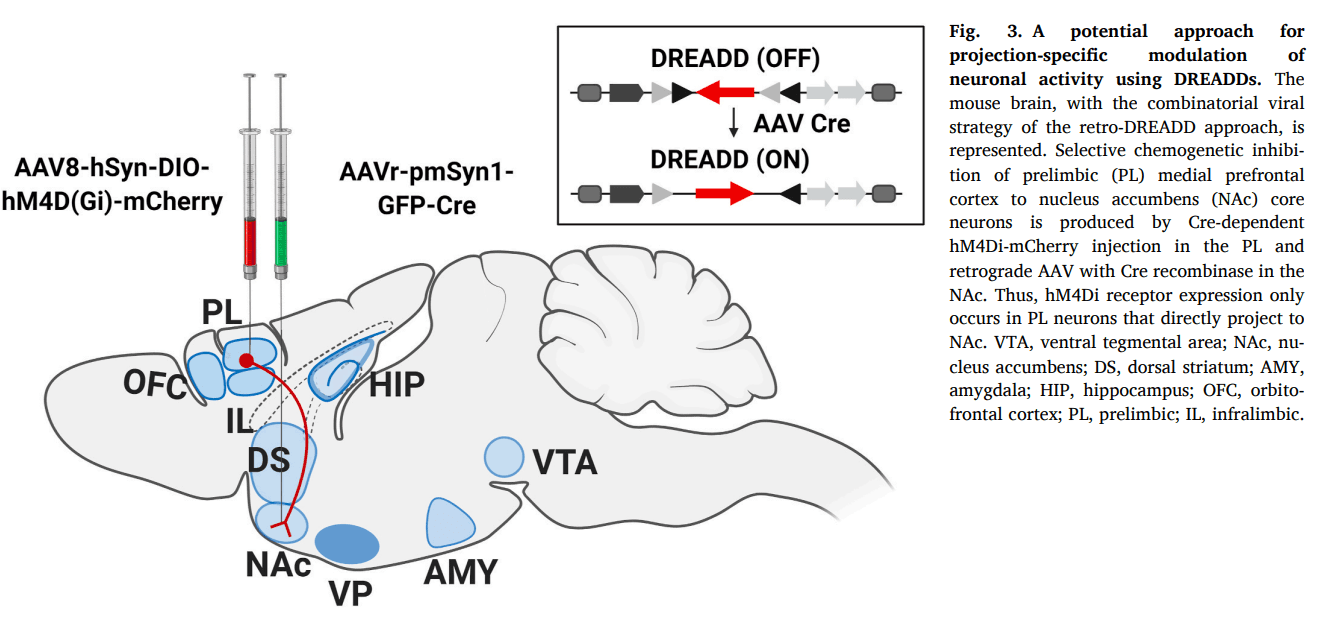
In the phase of preoccupation and anticipation of the addiction cycle, the neurobiology of compulsivity has been studied using DREADDs. Cocaine-induced perseverative behaviors in mice were associated with altered synaptic plasticity in accumbal D2-medium spiny neurons integrated into the ‘Stop circuit’. A potentiation of glutamatergic input to this ‘Stop circuit’ produced resilience toward compulsive cocaine-seeking (Bock et al., 2013). These data provide a possible input-output link of a protective mechanism against compulsive behavior due to the enhanced activity induced by cocaine of glutamatergic circuits synapsing onto D2-medium spiny neurons within the NAc. Strengthening the accumbal D2-medium spiny neurons promoted resilience to compulsive cocaine use (Bock et al., 2013). The loss of inhibitory control over palatable food has been studied using the retro-DREADD technique. The retro-DREADD technique consists of the inhibition or excitation of a neuronal population in a specific pathway within a brain region, taking advantage of retrograde viral microinjection, which contains an enzyme that recombines the DREADD. A study using the retro-DREADD technique from the mPFC to the NAc administrating CNO chronically in a constant release for four weeks through minipumps evaluated the role of this pathway in an addictive-like behavior promoted by palatable food. The inhibition of these mPFC projections to the NAc core resulted in compulsive consumption of palatable food and enhancement of the addictive-like behavior (Fig. 3) (Domingo-Rodriguez et al., 2020).
4. Calcium approaches in combination with behavioral studies
Another pioneering technique easy to combine with behavioral approaches to determine neuronal activity in vivo in animals is calcium imaging. Calcium imaging is based on the recordings of intracellular calcium influxes caused by depolarization of the neuronal cells. Several optical techniques capture the intracellular calcium influxes based on genetically encoded calcium indicators (Yang and Yuste, 2017). In this review, we focus on the head-mounted miniscopes technology as the most promising technique to combine with behavioral studies that may provide crucial advances in the neuroscience field in the following years. Indeed, miniscopes allow for the first time the optical recordings of neuronal activity in awake, freely moving rodents that can be used in different behavioral models (Ghosh et al., 2011).
Few studies have used head-mounted miniscopes technology in behavioral studies to investigate the neuronal activity patterns during addiction. A recent study has combined calcium imaging and DREADDs to modulate neuronal activity and observe the resulting neuronal activity pattern during cocaine-seeking behavior through calcium imaging (Heinsbroek et al., 2020). Activation of glutamate or GABA neurons in the ventral pallidum regulated differently cue-induced cocaine-seeking. Indeed, activation of glutamate neurons inhibited cocaine-seeking, while activating GABA or enkephalin neurons induced cocaine-seeking (Heinsbroek et al., 2020). Differential neuronal activity patterns were also recently reported combining electrophysiological and calcium recordings. Thus, increased activity of neurons projecting from the IL cortex to the NAc was revealed during cocaine-seeking, whereas the decreased activity of the same neurons was observed in a cocaine-free period (Cameron et al., 2019). Specifically, selective recordings were performed from IL-NAc neurons during drug-seeking, and the activation of this network was associated with relapse prevention. This decreased activity with susceptibility to relapse suggests that strengthening the activity of IL to NAc neurons could be a promising strategy for relapse prevention. Therefore, the calcium imaging technique is a promising optical tool that may provide a considerable contribution in the near future to understand better the neuronal activity patterns of brain regions of the brain currently involved in addiction.
Miniscopes can determine in a quantitative and qualitative strategy within cell resolution the particular neural activity in contrast to photometry that only captures the overall amount of fluorescence emitted in a quantitative strategy. Although miniscopes are currently the most contrasted in vivo calcium imaging system, new promising approaches are emerging, such as the optical fiberscope that also allows high optical resolution in quantitative and qualitative strategies (Dussaux et al., 2018). It should also be mentioned that calcium ions are the most used target due to their critical role in neuronal depolarization. However, other ion indicators, including potassium or sodium, and other potential markers like pH and membrane, are also emerging (Bischof et al., 2019).
5. Electrophysiological approaches combined with behavioral studies
In vivo electrophysiology is employed to record the electrical activity of neurons in combination with behavioral approaches. This technique enables a real-time readout of neural functions and network capability in different brain states across a wide range of temporal and spatial scales. Electroencephalography (EEG) is a non-invasive technique that has been widely used in the last four decades to record on the scalp of patients suffering from addiction. EEG data have been used to get an insight into the pharmacological modulation by drugs of abuse of ongoing neural oscillations, which are electric waves that travel across the brain. The acute effect of different addictive drugs has been extensively studied. Acute administration of drugs of abuse, such as cocaine (Herning et al., 1985), alcohol (Little, 1999; Lukas et al., 1986; Stenberg et al., 1994), THC (Lukas et al., 1995), and benzodiazepines (Benowitz et al., 1980; Manmaru and Matsuura, 1989) induced an increase in alpha and beta activity in healthy volunteers. By contrast, MDMA administration reveals a decrease in alpha and theta power in MDMA naïve volunteers (Frei et al., 2001). Theta activity is also decreased after acute THC administration in healthy volunteers (Aaron B et al., 2004; Böcker et al., 2010; Zuurman et al., 2008). Thus, it is well known that acute administration of drugs of abuse tunes the rhythmic activity of neurons' in the human brain. Other authors have focused on studying the acute effect of drugs on consumers. During resting states, cannabis users exhibited decreased delta and increased theta, beta, and gamma power compared to controls (Shikha Prashad and Dedrick, 2018). Alcohol users showed a decrease in delta and theta bands (Coutin-Churchman et al., 2006). Cocaine experienced users did not evidence persistent beta increase, which is commonly observed in inexperienced intranasal cocaine users (Heming et al., 1994). Furthermore, abnormal brain activities, predominantly in the beta band, were detected in heroin addicts compared to healthy controls (Motlagh et al., 2018).
EEG has also been used to study reward valuation processes and cognitive impairments using drug-related cues in patients with previous drug intake stories. These studies have focused on event-related potentials (ERPs), which are the means of EEG recordings that are time-locked to a stimulus or a response. Among different ERPs types, P300 is the most frequently employed index of neural resources assigned to reward studies. It is a positive ERP that appears 300–600 ms following stimulus presentation. P300s are implicated in memory, motivation, attention, and response inhibition using cognitive and emotional paradigms (Stewart and May 2016). Using oddball visual tasks, opioid (Lubman et al, 2007, 2008, 2009), nicotine (Littel and Franken, 2011), alcohol (Bartholow et al., 2010; Petit et al., 2012), and cannabis (Henry et al., 2014) users displayed greater P300s for substance-related cues than for non-substance-related cues. Furthermore, drug users exhibited generally higher P300s compared to controls. These studies indicate that consumers increased neural activity to process stimuli related to the selected drug of choice at the expense of other stimuli.
Despite the advances in understanding the consequences of exposure to drugs of abuse by recording neural activity on patients' scalp, EEG research in addiction and reward processes has significant limitations. Indeed, the electrical activity recorded by EEG electrodes is distant from the input source, coming from neurons in deep brain areas, which prevents the accurate study of brain regions altered during addiction. For that reason, direct extracellular electrophysiological recordings in specific brain areas of animals have emerged to acquire a better understanding of the neurocircuitry basis underlying addiction.
In vivo extracellular recordings may detect neural activity in awake animals during different behavioral performances. Animals undergo stereotaxic surgery to implant electrodes to permit real-time monitoring of neural network activity. Neural activity is detected as local field potentials (LFP) and multi-unit (MUA) or single-unit activity (SUA) (Hong and Lieber, 2019). LFPs are voltage waves generated by the synchronization of the activity of neuron populations interconnected in neural networks. MUA and SUA provide spike firing rate information of a group of neurons and single neurons, respectively. Therefore, crucial information on brain circuitry and connectivity related to addictive-like behaviors can be obtained using in vivo extracellular recordings.
The effect of drugs of abuse in neurons' firing rate has been extensively studied in anesthetized and awake animals. In anesthetized rats, cocaine (Koulchitsky et al., 2012) and opioids (Khodayari et al., 2019) produced a general decrease in the firing rate and bursting of VTA neurons after injection. Moreover, THC (Norris et al., 2019; Renard et al., 2017) and ethanol (Tu et al., 2007) decreased medium spiny neurons activity in the anterior NAc and/or PFC. By contrast, nicotine (Morel et al., 2018) and amphetamines (Shi et al., 2000) enhanced VTA DA cells' firing and bursting activity. Many studies have evaluated neuron synaptic plasticity in anesthetized animals with previous chronic drug-intake (Moussawi et al., 2009; Shen and Kalivas, 2013). Nevertheless, our review focuses on electrophysiological studies combined with operant behavioral approaches, and these studies in anesthetized animals are out of scope.
Many electrophysiological studies in addiction have focused on neuronal firing responses related to brain reactivity to contingent drug-self administration. These studies record chronic activity in awake, freely moving animals trained for operant self-administration and examine correlations between neural activity (LFPs and single-unit) and lever-pressing for intravenous administration of drugs or drug-related cues (Fig. 4). Through an entire self-administration session, amphetamine (Haracz et al., 1998) and cocaine (Kiyatkin, 2002; Peoples et al, 1997, 2004) infusions increased dopamine levels that tonically inhibited the majority of neurons in the striatum and the NAc. Nicotine self-administration sessions produced higher activity in VTA DA neurons compared to saline self-administration sessions (Caillé et al., 2009). Moreover, lever-pressing for drugs of abuse correlates with increases or decreases of glutamate-mediated firing in response to drug reward or drug-related cues. During cocaine and heroin self-administration sessions, distinct firing rates patterns were associated to the reinforced lever-presses (Carelli et al., 1993; Carelli and Deadwyler, 1996; Chang et al, 1994, 1997; Deadwyler et al., 2004). In the NAc of rats trained to self-administer cocaine, a small percentage of neurons exhibited either increased or decreased firing rates seconds before lever-pressing (anticipatory responses). Half of the neuron population predominantly decreased firing rates minutes after lever-pressing (post-cocaine responses) (Chang et al., 1994). In heroin self-administration sessions, NAc neurons displayed more post-heroin responses than PFC neurons, while the percentage of neurons firing before the lever-press was similar in the PFC and the NAc (Chang et al., 1997). In ethanol self-administration, the recording from ensembles of single-units primarily located within the shell of the NAc during operant responding for oral ethanol in trained rats exhibits significant phasic changes, with alterations in firing rate related to operant response, tone stimulus, and ethanol delivery (Janak et al., 1999). These results reveal the crucial role of the NAc in linking together conditioned and unconditioned internal and external stimuli with motor plans to allow for ethanol-seeking behavior to occur.
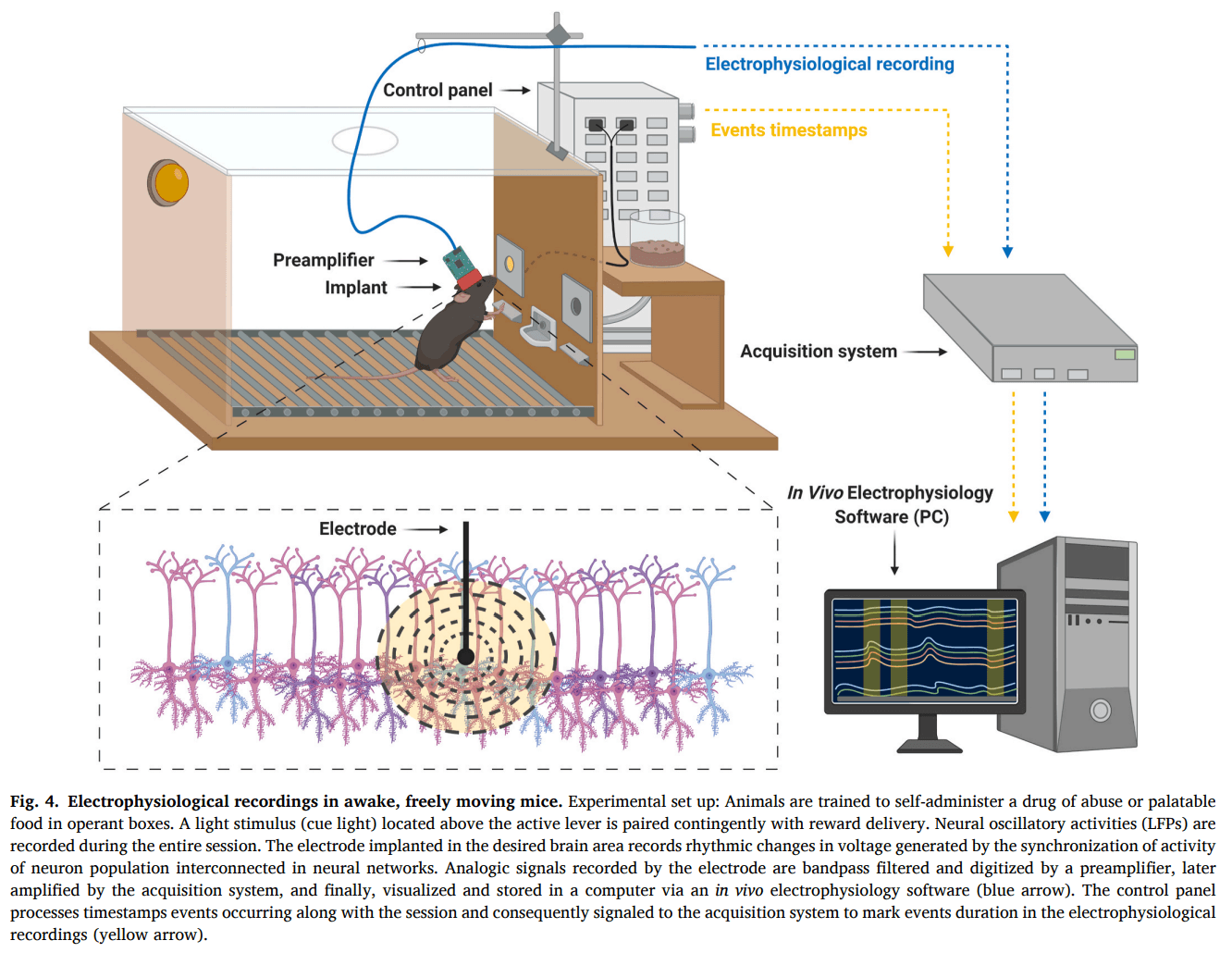
Several studies have also focused on neuronal responses related to particular behavioral responses involved in the addictive process. Evidence suggests that oscillatory activities of the mPFC and NAc correlate with impulsivity and reward outcomes. According to neuron firing rates, more impulsive rats showed a more significant shift in the proportion of small/immediate PL neurons than less impulsive rats (Sackett et al., 2019). Power in gamma frequency LFP oscillations transiently increased in the PFC and the NAc during signaling cue anticipation (Donnelly et al., 2014). Moreover, theta LFP power increased during the waiting period in these brain areas (Donnelly et al., 2014). These neurophysiological findings further implicate the PFC and the NAc in reward outcome abnormalities (Koob and Volkow, 2010). However, connectivity measures between brain areas involved in addictive behaviors during reward processes remain mostly unknown. The elucidation of these interconnectivities may be crucial to clearly understand the neurophysiological mechanisms responsible for addictive behaviors.
6. Possible novel therapeutic approaches for addiction
Despite the amount of work devoted to addiction research, few treatments are currently available for substance use disorders. In most cases, these treatments combine psychosocial approaches with pharmacotherapy. Up to date, the most effective pharmacological treatments approved by the U.S. Food and Drug Administration (FDA) are agonist treatments, also called ‘substitution therapies' (Randesi et al., 2020). They have proven to be the most robust approach to treat opioid and nicotine use disorders. They consist of a molecule that mimics the drug's molecular mechanism by binding to the same receptor. Their efficacy relies on their specific pharmacological properties, which make them less abusable than the original drug. Thus, methadone is an opioid agonist used to treat opioid use disorder due to its slower onset of action on the μ-opioid receptor (Schuckit, 2016), the receptor responsible for the addictive properties of all the prototypical opioid drugs (Lofwall et al., 2018). Buprenorphine presents a partial agonist activity on μ-opioid receptor to treat opioid use disorder (Schuckit, 2016). Varenicline is a partial agonist that activates α4β2 nAChR, only to 50% of the maximal nicotine effect, and is used to treat nicotine use disorder (Prochaska and Benowitz, 2019). Another partial α4β2 nAChR agonist of interest for nicotine abuse treatment termed cytisine has not yet been approved by the FDA (Prochaska and Benowitz, 2019).On the other hand, opioid antagonists, such as naloxone and naltrexone, reverse and prevent opioid effects by blocking the opioid receptors. Naloxone is a μ-opioid antagonist used to reverse an opioid overdose, and take-home naloxone programs aim to prevent fatal overdose (Bell and Strang, 2020). Naltrexone mainly antagonizes μ-opioid receptors and has been approved by the FDA to treat alcohol (Kranzler and Soyka, 2018) and opioid (Strang et al., 2020) use disorder. Nalmefene, a μ and δ opioid receptor antagonist and κ opioid receptor partial agonist, appeared to act similarly as naltrexone and effectively reduced alcohol consumption in heavy drinkers when combined with psychological support (Wang et al., 2020). A recent approach to improve adherence and safety in opioid use disorder is based on a new depot and implant formulations of buprenorphine and naltrexone (Bell and Strang, 2020). Depot buprenorphine compared with sublingual buprenorphine plus naltrexone has revealed not inferior effect (Lofwall et al., 2018), whereas another depot buprenorphine preparation revealed a superior effect to a placebo (Haight et al., 2019).In addition to agonist and antagonist treatments, of particular importance are other approaches such as topiramate, bupropion, and disulfiram. The FDA first approved topiramate, a GABA/glutamatergic modulator, for the treatment of seizures, epilepsy, and migraines. Topiramate eventually decreases the activity of the mesocorticolimbic dopaminergic system and is currently used with limited efficacy in the treatment of alcohol (Kranzler and Soyka, 2018), cocaine (Kampman, 2019), and methamphetamine/amphetamine (Siefried et al., 2020) use disorder. Bupropion, a non-tricyclic antidepressant that inhibits mainly dopamine reuptake, reduces cocaine craving (Frishman, 2007). Bupropion has also been approved to treat nicotine use disorder (Prochaska and Benowitz, 2019). Interestingly, naltrexone in combination with bupropion is rising as a possible treatment for methamphetamine/amphetamine use disorder (Siefried et al., 2020) and binge eating disorder (Valbrun and Zvonarev, 2020). In 2014, the FDA approved this combination of naltrexone and bupropion for obesity management. The fact that a drug used to treat drug addiction provides such an outcome when administered to patients suffering from binge eating disorder and abnormal eating patterns underlines the similarities in neuronal circuits shared with drug use disorders (Lindgren et al., 2017). The Disulfiram was the first medication approved for the treatment of alcohol use disorder. Disulfiram mechanism of action consists of inhibiting aldehyde dehydrogenase and increasing toxic metabolites of alcohol if ingested. Instead of having a direct pharmacological effect, its effectiveness lies in the patient's will to avoid the subsequent unpleasant effects (Kranzler and Soyka, 2018). However, disulfiram also inhibits dopamine β-hydroxylase, which converts dopamine to norepinephrine (Kampangkaew et al., 2019) and has also been used for the treatment of cocaine use disorder (Kosten et al., 2013). Notably, a genetic variation in the SLC6A3 gene, encoding DAT, has been associated with disulfiram effectiveness for cocaine addiction, with patients with higher DAT levels having better treatment outcomes than those with lower DAT levels (Kampangkaew et al., 2019). As pharmacologic treatments have proven to be insufficient in fighting addiction, new techniques are rising as promising options to treat substance use disorders. One of these techniques is transcranial magnetic stimulation (TMS), which consists of the induction of an electromagnetic field to depolarize neurons and eventually modulate brain excitability (Terraneo et al., 2016; Volkow et al., 2019). Applying repetitive TMS in the mPFC of patients suffering from cocaine use disorder showed promising results (Terraneo et al., 2016). The possible efficacy of this treatment relies on the role of top-down inhibition exerted by the mPFC on the reward system, a function diminished in cocaine use disorder (Volkow et al., 2019). A variation of TMS is termed deep TMS (dTMS). The H-coil use in this technique allows a more in-depth, bilateral brain structure stimulation compared to the traditional repeated TMS 8-coil, without increasing the stimulation intensity in the cortical regions and possible consequent side effects (Addolorato et al., 2017). Promising results with dTMS were obtained when applied to the PFC of patients suffering from nicotine (Dinur-Klein et al., 2014) and alcohol (Addolorato et al., 2017) use disorders. This technique has also been reported to reduce food cravings in obese individuals (Ferrulli et al., 2019). Deep brain stimulation is another promising technique for treating addiction in humans that has been revert compulsivity by targeting the NAc (Xu et al., 2020). It is based on a surgical intervention that consists of placing bipolar electrodes over specific brain regions and stimulating them with an implanted pulse generator (Salling and Martinez, 2016). Finally, the current development of innovative neuroimaging techniques, such as task-based functional magnetic resonance imaging, positron emission tomography, and diffusion tension imaging, among others, is opening the possibility to study individual predisposition to addiction to tailor treatments and predict outcomes (Voon et al., 2020).
7. Concluding remarks
The knowledge of the neurobiological substrate underlying addictive processes has been improved largely in recent years due, in part, to important technical innovations in neurosciences and the availability of novel behavioral models. The previous behavioral models in animals to evaluate addiction were focused on particular effects of drugs of abuse or specific behavioral responses related to drug exposure. In contrast, we have now ground-breaking behavioral models validated in rodents that incorporate the main hallmarks that characterized this disease in human patients. These models are based on complex operant behavioral techniques that mimic the development of the addictive processes and allow to identify individuals vulnerable and resilient to develop the disease imitating the human conditions, where only a minority of individuals that consumed the drug of abuse become addict. The current use of these novel behavioral models with high translational value is offering valuable information more easier to translate to the human clinical conditions than that obtained with the early behavioral models.New techniques have also been recently developed to be combined with these sophisticated behavioral approaches in order to identify the specific neuronal pathways involved in these complex behaviors. Early optogenetic studies combined with behavioral approaches have first provided significant advances to understand the specific involvement in the addiction of relevant neuronal pathways. The current availability of novel chemogenetic techniques that can also be combined with these behavioral approaches has allowed to advance an additional step to dissect the precise role of these specific brain networks. The possibility to maintain the modifications in the activity of these neuronal networks during long-term periods represents an additional advantage of these chemogenetic techniques.The use of head-mounted miniscopes and in vivo electrophysiological techniques in awake, freely moving rodents in combination with the behavioral approaches are other pioneering techniques that have recently allowed to better understand the neuronal activity patterns during addiction. These technologies now allow a real-time readout of neural functions and network capability in specific brain areas and pathways and have recently provided important results to clarify the substrate of addiction. At the present moment, few studies have combined these innovative technological and behavioral approaches together in the field of addiction. The results obtained have already been of great interest, but they represent only the first step for future studies that will certainly provide definitive advances in understanding the neurobiological bases of this complex disease. The identification of the precise involvement of the crucial neuronal pathways underlying each stage of the addiction process will allow to identify potential targets to develop more efficient therapeutic approaches. The possibility to apply in a near-future novel technologies to selectively modulate the excitability of these specific neuronal pathways may open new avenues for the treatment of addictive processes, which have enormous health and socio-economic burden worldwide at the present moment.