Abstract
Objective: The purpose is to determine whether a facilitated local change team (LCT) intervention improves linkage to medication for opioid use disorder (MOUD) and implementation outcomes, and whether participant-level outcomes are further enhanced by use of peer support specialists (PSS). Methods: This Type 1 hybrid implementation-effectiveness study involves a pre-post design (implementation study) followed by a randomized trial of PSS (effectiveness study). Participants are at least 114 justice and service staff from 7 sites in three states: probation officers, community treatment providers, a supervisor from each agency, and key stakeholders. The study will recruit up to 680 individuals on probation from seven adult community probation offices; eligible individuals will be recently committed, English speakers, with opioid use disorder (OUD). Core Implementation Study: The study will use the exploration, preparation, implementation, sustainability (EPIS) framework to guide system-change through facilitated LCTs of probation and community treatment staff given a core set of implementation strategies to set goals. The study will collect program-level and staff survey data at the end of each EPIS stage. Implementation Outcomes: Organizational engagement in MOUD (primary), plus changes in staff knowledge/attitudes and organizational outcomes (secondary). Effectiveness Study of PSS: After completing implementation, the study will randomize adults on probation to receive PSS vs. treatment as usual, with assessments at baseline, 3, 6 and 12 months. Effectiveness outcomes include participant engagement in MOUD (primary), probation revocation, illicit opioid use, and overdoses. Other aims include identifying barriers and facilitators, and cost-benefit analysis of PSS. Adaptations in response to COVID-19 included moving many procedures to remote methods.
1. Rationale and overview
Individuals released from incarceration have increased risk of overdose death in the first two weeks postrelease (Binswanger et al., 2010; Ranapurwala et al., 2018). Although rates of substance use disorder (SUD) are nearly 50%, there is limited access to SUD treatment while on community supervision. However, providing medication for opioid use disorder (MOUD) significantly decreases relapse and recidivism, and increases retention in SUD treatment (Gordon et al., 2015; Clark et al, 2014). While a department of corrections (DOC) system-wide program offering MOUDs in all prison/jail settings resulted in a significant decrease in state-wide overdose deaths (Green et al., 2018), only 33% of individuals newly on MOUD continued with treatment postrelease (Martin et al., 2018).
Community supervision provides a unique opportunity to engage individuals in treatment. Probation agencies are not structured for service delivery (Taxman 2012; Taxman & Belenko, 2012). Probation officers (POs) often lack the training and motivation to refer individuals to OUD treatment, indicating a need for informed partnerships to supplement current practices for those with OUD. Improved contact, communication, and role expectations between community corrections and community service providers stands to improve the continuum of care (i.e., screening, referral and linkage) for MOUD (Monico et al., 2016; Taxman & Belenko, 2012; Welsh et al., 2016). Additionally, peer support specialists (PSSs) may assist in linking individuals on probation to MOUD and other services; research has shown that PSSs are increasingly used, with limited but strong empirical evidence among criminal justice (CJ) populations (Bauldry et al., 2009; Cos et al., 2019; Marlow et al., 2015; Reingle Gonzalez et al., 2019).
Study Overview:
We evaluate a systems-change approach for increasing MOUD engagement in seven probation sites across Rhode Island, North Carolina, and Pennsylvania, using a hybrid Type 1 implementation-effectiveness design (Figure 1). Stage 1 — Core implementation study: The exploration, preparation, implementation, sustainability (EPIS) framework guides system-change through facilitated local change teams (LCTs) of CJ and community treatment providers using a core set of implementation strategies at the agency level, with assessments after each stage. The study’s objective is to improve linkage to the continuum of evidence-based care for probation-involved adults with OUD. Stage 2 — PSS effectiveness study: After implementation, the study will randomize 680 individuals on probation to receive PSS vs. treatment as usual (TAU), with 3-, 6- and 12-month follow-up assessments. This trial tests whether having a trained peer improves clinical outcomes beyond effects of core implementation.
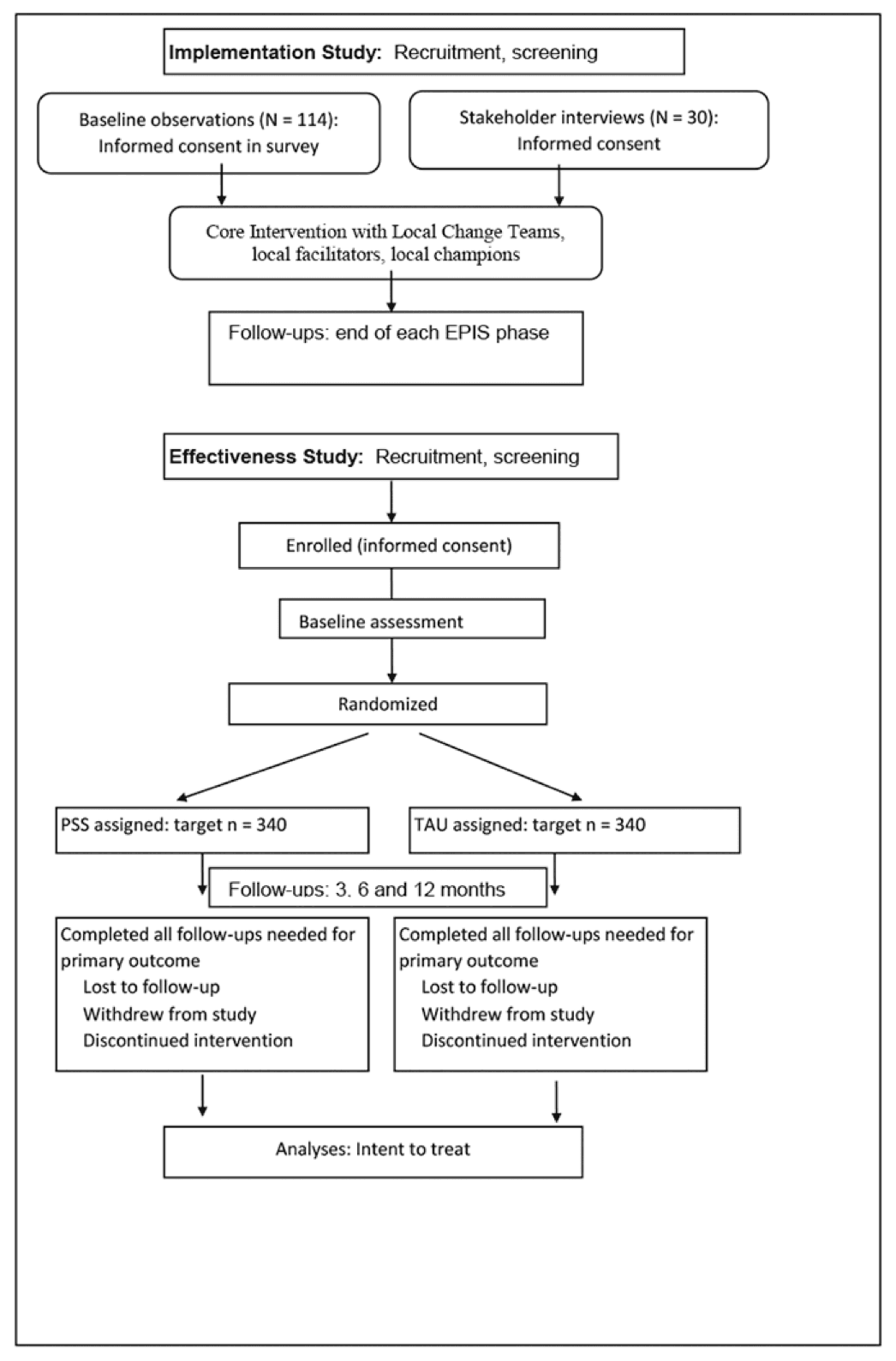
2. Study protocol
2.1. Aims and hypotheses
The study aims are: 1) To compare PSS to TAU (agency approach after implementation) on outcomes of individuals on probation: Engagement in MOUD (primary effectiveness outcome), probation revocation (secondary), illicit opioid use (secondary), and overdose (tertiary). 2) To test the effectiveness of the EPIS core implementation intervention relative to baseline on engagement in MOUD (primary implementation outcome). 3) To test the effectiveness of the EPIS-based core implementation intervention relative to baseline on organizational and staff-level outcomes: staff MOUD knowledge and attitudes, commitment and efficacy, readiness for change; organizational readiness for change, commitment and efficacy; penetration, adoption, sustainability. 4) To conduct a cost-benefit analysis of the societal cost implications of implementing a PSS model compared to TAU. 5) To identify organizational and staff barriers and facilitators to intervention implementation by conducting qualitative interviews with key probation and community treatment stakeholders who are managing and delivering the MOUD program. We hypothesize that the core implementation intervention will improve MOUD engagement compared to baseline (implementation outcome), that PSS will improve MOUD engagement relative to TAU after implementation (participant-level outcome), and that staff attitudes and perceptions regarding MOUD will improve over time (implementation outcomes).
2.2. Design
A Type 1 hybrid implementation-effectiveness study.11 The core implementation study involves a pre-post design. The effectiveness study uses randomized parallel assignment with two arms.
2.3. Participants and sites
Program-level core implementation study (Stage 1):At least 114 POs and service staff from seven adult probation offices in three states: 8–10 POs and 1 supervisor from each office, 2–3 treatment providers and 1 supervisor from each community treatment agency, and up to 30 key stakeholders.
Probation participant-level effectiveness study (Stage 2): Up to 680 individuals on probation recruited from the same probation offices.
Inclusion criteria. Participants will be at least 18 years old, English speakers able to provide written and/or verbal informed consent.
A. Treatment provider staff. Treatment provider at a participating agency who provides support to MOUD clients, has an active caseload including some individuals on probation, and has been employed at least 3 months. One supervisor of such staff per agency.
B. Probation staff. PO at a participating office who has an active caseload and has been employed at least 6 months. One supervisor from each probation office. A local champion will be recruited to lead the LCT.
C. Key stakeholders. Key stakeholders include POs, medical staff, administrative staff, community MOUD staff, case managers, and leadership currently employed by the probation offices or community treatment agency; or involved in the implementation, oversight, or provision of MOUD at each site.
D. Probation participants. Adults committed to probation within 90 days prior to study enrollment; diagnosed with OUD; able to provide locator information (see section 2.7.2.1).
2.4. Recruitment
Staff surveys and stakeholder interviews. The study will hold an orientation meeting at each site. Research staff will verify the few inclusionary criteria then discuss the consent form. Participants will give consent on-line at the start of the survey. The study will obtain informed consent for key stakeholders in person or via videoconferencing.
Probation participants. Research staff will inform potential participants that study participation is completely voluntary and will not affect their terms of probation or status, and will review the informed consent form before obtaining consent. Research staff screen for inclusionary criteria in a private space, and confirm OUD diagnosis and capability to consent with agency staff. POs will not be involved in recruitment or informed as to the participation of any individual.
2.5. Incentives
In sites that allow incentives, POs and treatment staff will receive a $10 gift card for each completed assessment ($40 total), with a $100 gift card drawing (per site) every 3 months for staff who complete that cycle’s assessment. Stakeholders will receive a $5 gift card for each completed interview ($20 total). Probation participants receive $30 for the baseline survey and $35 for each completed follow-up ($135 total).
2.6. Stage 1: Core implementation study
2.6.1. Baseline observation period
Rates of OUD referrals and MOUD treatment engagement are collected at each site for the 6-month period before the core intervention. The study also assesses PO and supervisor MOUD knowledge and attitudes toward innovation.
2.6.2. Core intervention
An orientation with PO and community treatment stakeholders discusses project goals and procedures, and agency leadership demonstrate study endorsement.
Needs assessment and systems mapping. This exercise identifies processes that POs use to screen and assess OUD, and link individuals to treatment. A focus group with probation and treatment staff will construct a systems map showing linkage points for screening, assessment, and referral, and the agencies/staff involved in these activities.
Local change teams (LCT). Each state forms a LCT that an implementation facilitator leads. Core interventions include the needs assessment/systems mapping, and development of community-specific best practices for POs and community treatment providers, and trainings. The LCT will be led through a goal selection exercise to identify part(s) of the OUD screening/referral process in need of improvement. Trainings, pending goal selection, include SMART (specific, measurable, achievable, relevant, timely) goals; Plan-Do-Study-Act; evidence for MOUD; stigma (effects, causes, how to address), and clarification of needs/expectations/roles of POs and community treatment providers. Such clarification involves a walk-through of justice and service agencies, and working knowledge of recovery supports and MOUD. The facilitator assists the LCT in developing commitment to MOUD, identifying facilitators/barriers to MOUD, and problem-solving to reduce barriers and increase PO and treatment provider efficacy for linkage to MOUD. The facilitator will explicitly address the need for communication between justice- and community treatment partners to enhance MOUD linkage. Strategies used for systems change emphasize knowledge about OUD and MOUD, commitment to change (implementation benefits outweigh challenges), and efficacy for change (e.g., probation has means of facilitating MOUD). LCTs will be asked to choose goals and strategies for long-term sustainment. Facilitators will work with LCTs for 12 months following a written action plan based on goal selection. Each LCT (N = 8–10 per LCT) will comprise a PO leader to assist the facilitator, POs, community treatment providers, and at least one PO supervisor.
Implementation facilitators (study staff, one per state) will guide each LCT through the process of organizational improvement. The facilitator will train a local champion (LCT leader) who will become a local facilitator to move toward sustainability using a transition protocol. LCT and facilitator will be guided by detailed manuals with extensive guidance on strategies, activities, and practical resources to foster rapid-cycle changes through the use of Plan-Do-Study-Act. Facilitators will be centrally trained. The study will monitor fidelity of implementation in terms of adherence, dose, delivery quality and participant responsiveness (Dane & Schneider, 1998).
2.6.3 Measures for core intervention
2.6.3.1. Timing of assessments
Assessments are timed with EPIS phases. Baseline assessment occurs before the core intervention (exploration phase); preparation ends with choosing goals from needs assessment; implementation ends with LCT accomplishment of goal(s); sustainability will be assessed over 12 months.
2.6.3.2. Primary implementation outcomes
The study defines MOUD engagement as enrollment in MOUD treatment program, or filling a prescription for buprenorphine from a provider (if not on MOUD at time of recruitment), or remaining in MOUD treatment (if on MOUD when recruited or at previous follow-up), coded dichotomously. The study will track rate of MOUD engagement via medical and probation records.
2.6.3.3. Staff surveys: Experiences, attitudes, and perceptions
Staff will complete a web-based survey (mailed paper versions optional) across two weeks (reminder after one week) at each time point. The survey includes: The Inter-Organizational Relationships (IOR) survey (Welsh et al., 2016), which focuses on organizational linkages for collaboration and coordination between correctional and treatment agency dyads. Measures of organizational climate, functioning, innovation support, leadership, and staff attributes were adapted from the Evidence-Based Practices Attitudes Scale (Aarons, 2004), Survey of Organizational Functioning (IBR, 2005), and Organizational Readiness for Change (IBR, 2009). The Medications Opinion Survey(Friedmann et al, 2012) assesses PO and supervisor knowledge/attitudes toward MOUD.
2.6.3.3. Qualitative interviews with key stakeholders: Barriers and facilitators
Study staff will conduct semistructured qualitative interviews (60–90 min) with key stakeholders three times: pre-implementation, early implementation, and late implementation, to assess sustainability and learning. Interviews will identify organizational and staff inner context factors: 1) organizational characteristics (i.e., readiness for change); 2) individual adopter characteristics; 3) fidelity (e.g., integrity and quality of the program); and 4) penetration of the program (i.e., has the program become standard). Outer context issues include the sociopolitical environment (e.g. funding, legislative landscape, policy, community access to MOUD). The study will transcribe audio-recorded interviews for analysis.
2.7. Stage 2: Randomized trial of PSS
2.7.1. PSS and control conditions
At the conclusion of the core implementation intervention, the study will recruit and randomize individuals on probation with OUD to PSS vs. TAU.
Participants in the experimental arm will meet with a PSS for 12 months. The schedule and content of meetings will be based on the participant’s needs and wants, plus written guidance for PSS-participant interactions. PSSs have lived experience with addiction, recovery, and CJ involvement. PSSs establish linkages to community treatment providers; educate about recovery support services, transportation assistance, and MOUD; provide experiential, nonclinical support to individuals with SUD; share skills, offer support for setting goals and navigating the recovery process (Bassuk et al., 2016; Reif et al., 2014; Kelly & Hoeppner, 2015); and provide referrals and support for treatment, housing, employment, drug court, and probation (SAMHSA, 2009).
PSS training will cover both state requirements for certification (46–75 hr specialist training) and the skills and knowledge needed for peer work with this population. PSSs will be certified according to each state’s specifications and be supervised by a peer supervisor. The study will assess delivery via PSS logs of participant contacts (date, type, duration). The study will track fidelity via participant and PSS ratings of 12 core competencies, each including 4–7 indicators (Bassett et al., 2016; SAMHSA, 2015).
Participants randomized to TAU will receive the usual services offered at the sites.
2.7.2. Measures for effectiveness trial
2.7.2.1. Assessment procedures and timing
The study will conduct assessments (about 60 min each) at baseline, 3, 6, and 12 months. Locator information (consent to contact a family member, close friend, employer; numerous methods of contact) will facilitate tracking participants. Blinded research staff will conduct interviews. The study will conduct urine drug screens at each time point. Research staff will access medical records data on opioid use and overdoses from participating treatment agencies and public health records, and records from participating CJ agencies.
2.7.2.2. Participant-level baseline
Obtained from survey: Age, sex, gender identity/sexual orientation, race, ethnicity, marital status, education, employment, criminal justice history, substance use history, social supports, other health conditions, health services utilization, HIV risk behaviors, perceived discrimination/stigma, and history of MOUD. Obtained from agency record: OUD diagnosis.
2.7.2.3. Participant-level outcomes
Primary outcome: Engagement in MOUD across 12 months is assessed with The Treatment Services Review (TSR-6) and medical record review from participating treatment agencies (dichotomous at each time point).
Secondary outcomes: (1) Probation revocation obtained from DOC data. (2) Opioid use per self-report and urine toxicology screen. (3) Overdoses (incidence and death), per medical records and surveys.
Adverse events: Research staff will report unexpected adverse events or unexpected ill health to the PI for evaluation, with serious adverse events or loss of confidentiality reported per NIDA’s reporting requirements.
2.7.2.5. Cost-benefit data for PSS
Input costs: The study will record the time PSSs spend with participants in logs at the person-level and valued using the human capital approach based on appropriate wage rates from the Bureau of Labor Statistics.2 The study will track for each participant visits to medical providers from participant assessments (outpatient, emergency, hospitalizations) and use of MOUD. These will be valued at Medicare fee-for-service payment rates.3
Outcome costs: The study will track criminal offenses and re-incarceration time and value it for costs associated with criminal activity and jail time.
2.8. Power analysis
Power for Aim 1: For a 35% increase in MOUD engagement (Hazard ratio=1.30) in PSS versus TAU (modest effect size), N=680 will give ≥80% power at 3 months after adjustments for clustering effects and up to 25% attrition, with greater power at later follow-ups due to greater disengagement with MOUD over time in the TAU arm.
Power for Aims 2–3: The EPIS core intervention should have a small to medium effect size on staff and organizational outcomes (Knight et al., 2015). Assuming d=.3 and N=114, power is ≥80% after adjustments for clustering effects.
2.9. Statistical data analysis methods
Aim 1: Effectiveness study. First we will investigate differences in relevant characteristics between arms to enter as covariates (e.g., years of OUD, past MOUD). Log binomial regression will model condition differences in MOUD engagement at each follow-up. Generalized estimating equations (GEE) will analyze group differences over time while accounting for state-level clustering. Using intent-to-treat analysis, the study will code participants lost to follow-up as not engaging in MOUD; incarceration or death will be censored at the time of event. To examine efficacy (the true impact had everyone adhered to the intervention), we will address loss to follow-up as missingness (attrition) and use inverse probability of attrition weighting to examine what would happen if everyone had adhered to their assigned intervention. The study will analyze secondary outcomes the same way.
Aim 2 and 3: Implementation study. The study will analyze engagement in MOUD, knowledge, attitudes, organizational readiness for change, commitment and efficacy among agency staff pre-to post-EPIS implementation with Poisson regression with GEE to control for facility-level clustering.
Aim 4: Cost analysis. For each condition we will evaluate costs using the Second Panel on Cost-Effectiveness in Health and Medicine cost-benefit analysis framework.4 We estimate the differences in input and outcome costs by condition, allowing for clustering within states or PSS, adjusting for person-level characteristics, using two-part models with boot-strapped standard errors, if there are large numbers of zero costs for services. Finally, the study will compare adjusted input and outcome costs for each intervention using net savings, benefit-to-cost ratios, and return on investment calculations.
Aim 5: Qualitative data. The study will analyze qualitative data iteratively and rapidly to optimize intervention implementation. Interviewers will complete debriefing forms and discuss them at weekly meetings, with additional probes as needed to follow up on unanticipated themes. The study will develop meaningful analytical units by using a coding scheme based on the dominant themes in the data, with each theme and subtheme assigned a code compiled in a codebook. We will use open and axial coding, with analysis guided by a thematic approach due to its emphasis in pin-pointing, examining, and recording patterns in the data. After initial coding, researchers will summarize and organize the resulting data in Nvivo 11 (QSR International, Burlington, Massachusetts, USA). Two coders will work in tandem to code data after attaining inter-rater reliability of 90%.
3. Potential adaptations in response to COVID-19
The study may conduct orientation meetings via videoconferencing, with eligibility screening and informed consent discussion done individually by telephone or videoconferencing. Informed consent forms for staff are embedded in electronic surveys. The study may conduct informed consent for key stakeholder interviews by telephone or videoconferencing, with electronic copies of the informed consent form shared, and verbal consent obtained. Informed consent for participants may change to these methods. All surveys are electronic. The study may conduct qualitative interviews via videoconferencing. LCTs (agency employees) will use whatever interaction methods their sites have approved. PSSs will meet with participants either in person or via telehealth methods, based on participant and agency preferences.
4. Conclusions
This study is uniquely poised to evaluate the implementation and impact of MOUD among CJ-involved individuals, and changes in staff attitudes and behaviors related to MOUD; answer questions of urgent public health significance; improve service delivery methods via implementation science; and assess the efficacy of adding a PSS to MOUD treatment among individuals on probation.