Abstract
Marijuana is perceived as a harmless drug, and its recreational use has gained popularity among young individuals. The concentration of active ingredients in recreational formulations has gradually increased over time, and high-potency illicit cannabinomimetics have become available. Thus, the consumption of cannabis in the general population is rising. Data from preclinical models demonstrate that cannabinoid receptors are expressed in high density in areas involved in cognition and behavior, particularly during periods of active neurodevelopment and maturation. In addition, growing evidence highlights the role of endogenous cannabinoid pathways in the regulation of neurotransmitter release, synaptic plasticity, and neurodevelopment. In animal models, exogenous cannabinoids disrupt these important processes and lead to cognitive and behavioral abnormalities. These data correlate with the higher risk of cognitive impairment reported in some observational studies done in humans. It is unclear whether the effect of cannabis on cognition reverts after abstinence. However, this evidence, along with the increased risk of stroke reported in marijuana users, raises concerns about its potential long-term effects on cognitive function. This scientific statement reviews the safety of cannabis use from the perspective of brain health, describes mechanistically how cannabis may cause cognitive dysfunction, and advocates for a more informed health care worker and consumer about the potential for cannabis to adversely affect the brain.
Marijuana, or cannabis, was considered an illicit drug for decades. However, in many parts of the world, cannabis has been legalized for medical use or decriminalized for recreational or medicinal applications. This shift in attitude has resulted in a rapid increase in its use. It has been estimated that ≈183 million people in the world used marijuana in 20141 and that 22 million met criteria for cannabis use disorder in 2016.2 In addition, according to the 2002 to 2019 National Survey on Drug Use and Health, the proportion of the US population >12 years of age who used marijuana in the past year increased gradually from 11% in 2002 to 18% in 2019.3 The use of marijuana has gained popularity, particularly among adolescents and young adults, with ≈36% of 12th graders and 43% of college students reporting having used it in the past year.4 In parallel, evidence suggests that the potency of cannabis products in the United States, measured by the concentration of the primary psychoactive constituent of marijuana, Δ9-tetrahydrocannabinol (THC), has gradually increased from ≈4% in 1995 to 15% in 2018.5
Cannabinoid receptors are expressed in high density in areas of the brain involved in executive function and memory such as the hippocampus, amygdala, and prefrontal cortex (PFC), particularly during periods of active brain development.6 Acute intoxication with cannabinoids can impair memory and behavioral inhibition.7 Cannabinoids also regulate anxiety and can produce psychosis-like effects.6 Evidence shows that age at exposure may influence the effect of cannabinoids on cognitive function. For example, the prenatal, perinatal, and adolescent periods may be particularly sensitive to these compounds.8 Data obtained in preclinical models have shown that cannabis and its associated signaling pathways regulate neurotransmission and play an active role in key cerebral processes, including neuroinflammation, neurogenesis, neural migration, synaptic pruning, and white matter development.6,9 Furthermore, experimental data show that cannabinoids can regulate the functioning of different cytochrome-P450 isoforms and uridine 5′-diphospho-glucuronosyltransferases. Thus, there is a potential risk for drug-to-drug interactions with medications commonly used by the elderly such as warfarin, antiarrhythmic agents, sedatives, and anticonvulsants.10
These factors have raised concerns about the potential effect of cannabis on cognitive vitality. The goal of this scientific statement is to critically appraise the safety of cannabis use from the perspective of brain health.
Cannabis and Endocannabinoids
Anandamide and 2-arachidonoyl-glycerol are endogenous bioactive lipids that activate 2 G-protein–coupled receptors designated as cannabinoid receptor type 1 (CB1) and 2 (CB2). These lipids, called endocannabinoids, are not stored in vesicles but are synthesized on demand. The system formed by the cannabinoid receptors CB1 and CB2, endogenous ligands, and enzymes involved in their production and degradation is known as the endocannabinoid system (ECS). A detailed description of the composition and regulation of the ECS is beyond the scope of this publication; this topic has been reviewed extensively elsewhere.9,11,12
Phytocannabinoids are exogenous cannabinoids extracted from flowering plants from the cannabis genus, including Cannabis sativa, Cannabis indica, and Cannabis ruderalis. Whether these are species or subspecies is a matter of debate. More than 100 phytocannabinoids have been extracted from these plants, with THC and cannabidiol (CBD) being the most abundant. The relative concentration of THC and CBD in these strains is variable. In general, cannabis cultivars can be classified according to the cannabinoid produced as chemotype I (THC rich), II (THC/CBD balanced), III (CBD rich), IV (cannabigerol rich), or V (cannabinoid free).13
THC is a psychoactive alkaloid that signals through CB1 and CB2 receptors. Cannabinoid receptor type 1 is expressed abundantly in peripheral and central neural cells. In the periphery, CB1 localizes to sympathetic nerve terminals and sensory neurons. In the central nervous system, it is expressed mainly in presynaptic membranes of excitatory and inhibitory neurons, where it regulates the vesicular release of dopamine, GABA, and glutamate. In comparison, CB2 is expressed mainly in immune cells, including microglia.9
CBD is a nonpsychoactive cannabinoid that has antioxidant and anti-inflammatory properties. It is thought that CBD exerts some of the beneficial effects that phytocannabinoids have in Dravet syndrome and Lennox-Gastaut syndrome. Furthermore, studies done in preclinical models suggest that CBD is beneficial in Alzheimer disease, cerebral ischemia, multiple sclerosis, and other neurologic disorders.9,14 The therapeutic potential of CBD is being investigated in different clinical trials. Compared with THC, CBD signals through different pathways but does not activate CB1 and CB2. At low concentration, CBD blocks the orphan G-protein–coupled receptor-55, the equilibrative nucleoside transporter 1, and the transient receptor potential of melastatin type 8 channel. It also activates the serotonin (5-hydroxytryptamine) 1A receptor, the transient receptor potential of ankyrin type 1 channel, and α3 and α1 glycine receptors. At high concentration, CBD activates the nuclear peroxisome proliferator-activated receptor γ and the transient receptor potential of vanilloid types 1 and 2.12,14
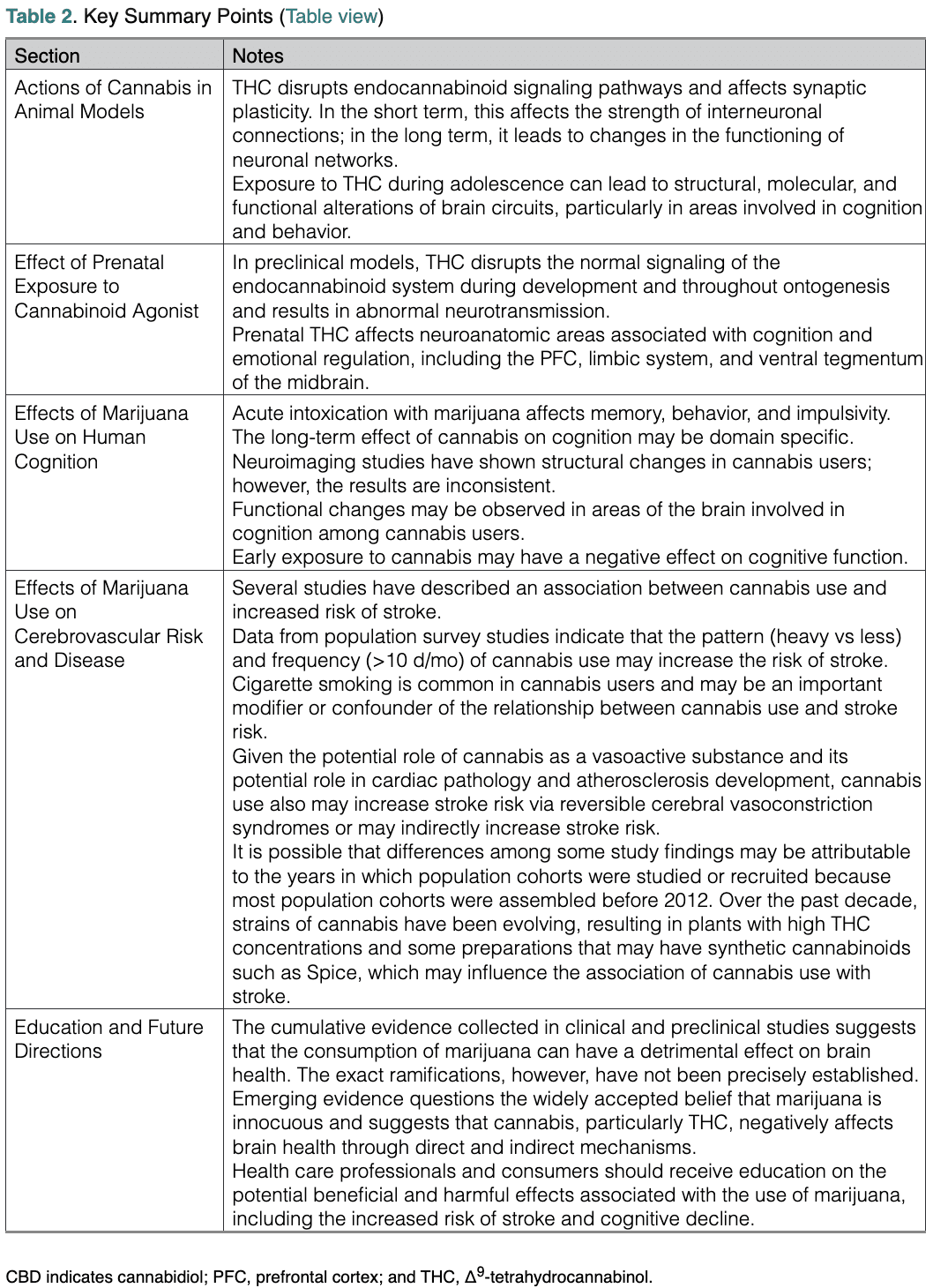
Several cannabinoids have received approval in different countries for the treatment of specific medical conditions. In addition, high-potency synthetic cannabimimetics such as Spice are available in the illegal market (Table 1).15–17
Table 1. Synthetic and Semisynthetic Cannabinoids
CBD indicates cannabidiol; and THC, Δ9-tetrahydrocannabinol. *Approved by the US Food and Drug Administration. †Approved by the Health Products and Food Branch of Health Canada.
Neurobiological Actions of Cannabis in Animal Models
Molecular and cellular mechanisms underlying the effects of cannabis on the developing brain are inferred mainly from preclinical studies that permit controlling for social and environmental factors that could influence outcomes of interest. In addition, animal models allow the investigation of a range of human age-related behavioral factors (eg, novelty and sensation seeking, impulsivity, risk-taking behaviors) and key stages of neurodevelopment that are conserved across many mammalian species. However, many individual (eg, species, strain, age) and experimental (eg, design, drug, dose, delivery, regimen) variables, along with objective end points (eg, behavioral paradigm, experimental technique), have contributed to equivocal findings across studies. Nonetheless, experimental animal models of prenatal and adolescent cannabis exposure have proved fundamental in disclosing the underlying neurobiological mechanisms that might explain several clinical neuropsychiatric outcomes outlined here.
Animal models have been used to examine the role of the ECS in the modulation of synaptic plasticity, a process that allows the brain to change and adapt to new information.18 The ECS modulates synaptic plasticity by affecting the strength of interneuronal connections and, ultimately, the functioning of neuronal networks. From the mechanistic standpoint, THC activates cannabinoid receptors in the brain, thus interfering with physiological actions of endocannabinoids. Spatial and time resolution of endocannabinoid production is pivotal for correct processing of different brain functions such as higher-order cognition, memory, reward, mood, and stress sensitivity.8,19,20 Consequently, THC, activating nonspecifically CB1 receptors in the brain, disrupts the fine-tuning of synaptic activity exerted by endocannabinoids, eventually impairing connectivity of neuronal networks and brain functionality.
Although incompletely understood, the way in which THC disrupts memory and learning may be through its differential effect on neurotransmitter release and binding to CB1 receptors.19 For example, THC activates CB1 receptors located on GABAergic interneurons, which represent nearly three-quarters of the brain CB1 receptors, and astrocytes, resulting in the release of hippocampal glutamate. Concomitantly, THC affects the transmission of other neurotransmitters involved in the modulation of memory such as acetylcholine, adenosine, and serotonin.19,20 Furthermore, THC activation of CB1 receptors present on mitochondria leads to decreased cellular respiration and ATP supply.19 ATP is fundamental in maintaining and regulating neurotransmission, and its reduction might contribute to THC-induced cognitive deficits.
Repeated exposure to cannabis, especially during the adolescent developmental period, may be especially harmful to brain health and cause structural, molecular, and functional alterations of brain circuits, particularly in the PFC and hippocampus.8,21,22 Long-term THC exposure induces CB1 receptor downregulation and desensitization that appear more intense and widespread after adolescent exposure as opposed to adulthood exposure.22 Data obtained in experimental models showed that these effects could have implications for neurodevelopmental processes in which the ECS plays a role. Accordingly, long-term THC exposure during adolescence may disrupt dynamic changes occurring in glutamatergic and GABAergic systems, leading to excessive synaptic pruning (ie, loss of synaptic contacts), long-term dysfunction in prefrontal excitatory/inhibitory balance, and desynchronization of PFC neuronal networks, which also dysregulate the mesolimbic dopaminergic pathway (Figure).23 These changes may represent the molecular underpinnings of cognitive deficits and altered emotional reactivity and social behavior observed long after adolescent long-term THC exposure.22 Long-term changes in brain functionality induced by THC exposure during adolescence might also arise from epigenetic modifications with a marked reprogramming of the transcriptome, affecting mainly genes related to synaptic plasticity processes.8,19 These effects have not been reported after adult THC exposure.19
In addition to the effects on neuron cellular and subcellular components, recent evidence suggests that alterations in glial cells have a key role in the actions of THC.24 Long-term THC exposure activates microglia and astrocytes to produce inflammatory cytokines. For example, long-term administration of THC during adolescence increased the microglial expression of the proinflammatory mediators tumor necrosis factor-α, inducible nitric oxide synthase, and cyclooxygenase-2 by 60%, 130%, and 80%, respectively, and reduced the expression of the anti-inflammatory cytokine interleukin-10 by 30% in the PFC. The resulting neuroinflammatory response was associated with memory impairment during adulthood.25
Dose constitutes an additional important variable to consider. Most studies describe detrimental effects of THC in models of heavy cannabis use in middle adolescence. However, even lower doses may produce these same effects when administered earlier in adolescence.22
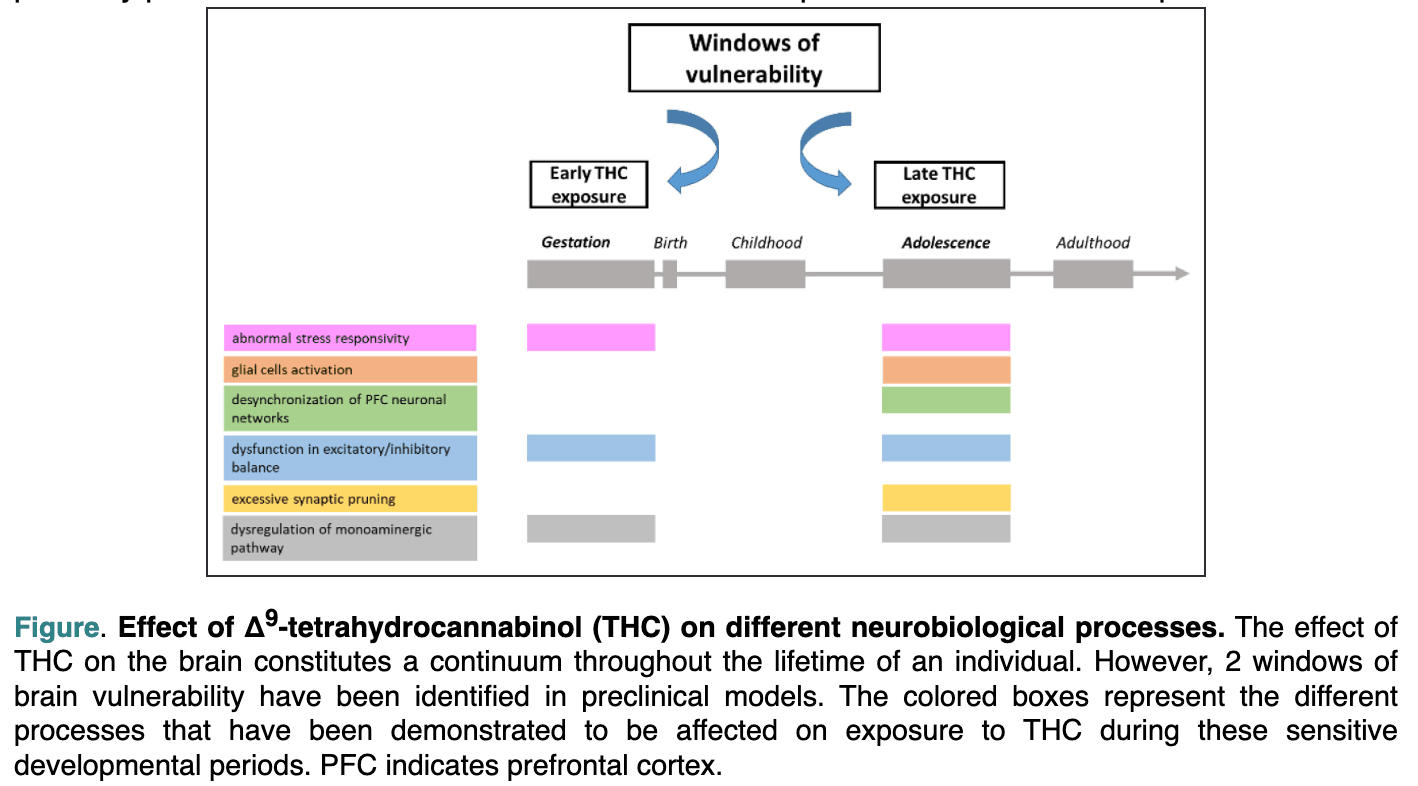
Figure. Effect of Δ9 -tetrahydrocannabinol (THC) on different neurobiological processes. The effect of THC on the brain constitutes a continuum throughout the lifetime of an individual. However, 2 windows of brain vulnerability have been identified in preclinical models. The colored boxes represent the different processes that have been demonstrated to be affected on exposure to THC during these sensitive developmental periods. PFC indicates prefrontal cortex.
Effect of Prenatal Exposure to Cannabinoid Agonists
A recent study examined associations between prenatal cannabis exposure (PCE) and various indicators of mental and neurocognitive health in a sample of 11,489 youth.26 Self-report of maternal cannabis use during pregnancy was associated with various adverse outcomes among youth at 9 to 10 years of age, including poorer performance on tests of neurocognitive functioning and total intracranial volumes, even after controlling for potential confounders. Several reviews describe PCE sequelae in preclinical models.8,24,27–30 Here, we focus on mechanistic insights inferred from animal studies recapitulating the neuropsychiatric features of clinical outcomes.31 The detrimental effect of PCE on cognitive processing and emotional regulation of the progeny has been ascribed to changes in intrinsic and synaptic properties and plasticity of cortical (eg, PFC), limbic (eg, amygdala, hippocampus), and midbrain (eg, ventral tegmentum) regions. Changes in the balance of excitatory and inhibitory input strength, along with alterations in how principal neurons and interneurons receive, integrate, and convey information, have been observed in these neuroanatomic areas (Figure).8,24,27–30 Aberrant glutamatergic function is a common hallmark, as indexed by changes in the expression and function of ionotropic and metabotropic receptors and in dynamic regulation of glutamate levels by glutamate transporters at both synaptic cleft and extrasynaptic spaces. These changes depend largely on the alterations of endocannabinoid signaling pathways caused by exogenous cannabinoids during development and throughout ontogenesis (eg, neural proliferation, survival, directional axonal growth).8,24,27–30 Defects in ECS function also may account for the interneuronopathy observed in many brain regions of PCE offspring, a phenomenon often more prominent in female than in male animals.8,27–29 In the PFC, this persistent inhibitory circuit deficit also is associated with a delayed switch of GABA from its excitatory role early in development to a classic inhibitory function exerted throughout the central nervous system later in life.8,29 This is particularly relevant because the GABA switch represents a critical milestone during neurodevelopment. Any alteration in the normal and predictable temporal sequence of these periods such as delays, stalls, or accelerations imposed by PCE may lead to perturbations of offspring cognitive processing and emotional behavior.8,29
It was observed that marijuana use leads to dysregulation of monoaminergic pathways and stress response systems.8,27–29 PCE hampers the maturation of monoamines, which also exert trophic actions on target neurons and afferent terminals. This phenomenon may depend on epigenetic modifications and may be implicated in aberrant reward signaling. Furthermore, PCE is associated with an endophenotype in the offspring, which displays protracted dysregulation of stress responsivity that is not explained by glucocorticoid levels. A susceptibility to acute and chronic stress is tied to many psychiatric disorders, ranging from depressed mood and psychosis to substance use disorders and anxiety. A deeper understanding of how PCE interferes with endocannabinoid signaling during neurodevelopment would allow us to explore potential interventions aimed at restoring or reprogramming the hierarchical progression of developmental milestones.
Effects of Marijuana Use on Human Cognition
Acute intoxication from marijuana is associated with impairment of working and episodic memory, behavioral disinhibition, and impulsivity, which can affect performance in real-world activities.6 For example, a meta-analysis from 2016 showed that the odds of being involved in a motor vehicle accident was increased 36% in cannabis users relative to nonusers.32 In addition, a crossover clinical trial published in 2020 investigated the effect of different cannabis products in relation to on-road driving tests. The SD of lateral position, a measure of lane weaving, swerving, and overcorrection, was 20.29 cm at 40 to 100 minutes after inhalation of THC-dominant cannabis and 21.09 cm after inhalation of a mixture of THC and CBD. It is interesting to note that the SD of lateral position after inhalation of CBD-dominant cannabis was similar to that in the placebo group (18.21 cm versus 18.26 cm).33 These observations illustrate the differential short-term effect of THC and CBD on cognition. Evidence also suggests that the short-term effects of cannabinoids are transient and can be influenced by the development of tolerance and the use of other drugs.
The long-term effect of cannabis on cognition, however, is less well established. Recent meta-analyses report residual effects of cannabis use on neurocognition, consistent with prior research.34 A meta-analysis by Lovell et al35 in 2020 focused on adult near-daily cannabis use for >2 years and found global neurocognition among users (n=849) to be about one-quarter of an SD worse than that of nonusers (n=764). Four of the 7 domains investigated (decision-making, verbal learning, retention, executive function) showed significant effect sizes ranging from Hedges g=−0.52 to −0.18. A meta-analysis of cannabis users <26 years of age (n=2152) and nonusers (n=6575) also showed a one-quarter of an SD difference in global neurocognitive performance but with more specific domains affected,36 albeit with smaller effect size compared with that found by Lovell et al.35 Both lacked support for worse neurocognition in early adolescence in that neither found that age at onset of cannabis use influenced the association between exposure and cognitive performance.
In contrast to these meta-analyses, large longitudinal studies provide stronger causal inferences by examining change over time. In the CARDIA study (Coronary Artery Risk Development in Young Adults), 3385 participants 18 to 30 years of age were followed up longitudinally. Marijuana use was assessed periodically in the 25-year follow-up. In addition, cognitive assessment was completed 25 years after inception. In this study, cumulative years of exposure to marijuana was associated with worse verbal memory (0.13 lower SD in the verbal memory test for each additional 5 years of exposure to marijuana).37 Longitudinal co-twin studies use a research design that additionally controls for shared variance from genetic and environmental factors. Two large longitudinal twin studies (n=3066) with neurocognitive measures collected before (at 9–12 years of age) and after (17–20 years of age) cannabis exposure reported that declines in vocabulary and general knowledge were associated with being a cannabis user but not with amount of cannabis consumed.38 Twins discordant for cannabis use showed no differences in IQ declines. Thus, differences were likely caused by shared risk factors. Using a similar design, Meier et al39 reported that lower IQ predated cannabis use with no evidence of actual IQ declines among 1989 twins assessed at 5, 12, and 18 years of age. Ross et al40 evaluated other aspects of neurocognition among 856 individual twins and reported only 1 within-family effect of 70 tested. Specifically, frequency of cannabis use at 17 years of age was associated with poorer executive functioning at 23 years of age, but executive functioning problems predating cannabis use could not be ruled out.
Magnetic resonance imaging (MRI) techniques demonstrate differential associations of cannabis use with brain structure and function. In terms of brain structure, alterations related to cannabis use have been mixed. In a longitudinal study including 1598 MRIs done in adolescents at baseline and the 5-year follow-up, a dose-dependent association was observed between cannabis use and PFC thinning.41 On the other hand, although a meta-analysis found that regular cannabis consumption was associated with smaller hippocampal (standardized mean difference, 0.14 [95% CI, 0.02–0.27]), medial orbitofrontal cortex (standardized mean difference, 0.30 [95% CI, 0.15–0.45]), and lateral orbitofrontal cortex (standardized mean difference, 0.19 [95% CI, 0.07–0.32]) volumes relative to nonuse, brain volumes were not associated with cannabis use duration and dosage.42 Other large studies have reported null effects. In 2 large twin samples from the United States (n=474) and Australia (n=622), cannabis use was unrelated to volumes of the thalamus, caudate nucleus, putamen, pallidum, hippocampus, amygdala, and nucleus accumbens.43 A multisite study of cortical surface measures (n=262) reported no difference in cortical thickness, surface area, and gyrification index in cannabis users versus nonusers, in cannabis dependence versus nondependence versus nonusers, and in early adolescent versus late adolescent onset of cannabis use versus nonuse.44 Thus, brain structural abnormalities related to cannabis use are inconsistent.
Functional MRI studies report more robust effects, particularly after prolonged cannabis use. A meta-analysis of task-based functional MRI studies in current adult and adolescent users found abnormalities in activation in both age groups. Relative to nonusing control subjects, adult cannabis users had greater brain activation in the superior (seed-based d mapping [SDM-Z], 1.561; P<0.002) and posterior (SDM-Z, 1.479; P<0.003) transverse temporal and inferior frontal gyri (SDM-Z, 1.568; P<0.002) and less activation in the striatum (SDM-Z, −1.843; P<0.001), insula (SDM-Z, −1.637; P<0.001), and middle frontal gyrus across different tasks. Adolescent cannabis users also had greater activation in the inferior parietal gyrus (SDM-Z, 1.06; P<0.001) and putamen (SDM-Z, 1.008; P<0.001) compared with nonusers across various tasks, suggesting compensatory neuroadaptive mechanisms.45 These functional abnormalities persist despite cessation of cannabis use and beyond the period when THC metabolites are detectable. A meta-analysis of the same adolescent studies found that >25-day abstinent adolescent cannabis users exhibited greater activation in the right inferior frontal gyrus in addition to other areas relevant for executive functioning and self-regulatory mechanisms.46
Several recent studies examined cannabis effects in populations with premorbid clinical risk factors and those using medical marijuana. A meta-analysis focused only on cannabis users with psychosis <25 years of age (n=529) and nonusing control subjects with psychosis (n=901). In this study, there were significant differences in 3 of 11 domains assessed (premorbid IQ, Hedges g=0.40 [standardized effect size]; current IQ, Hedges g=−0.17; working memory, Hedges g=−0.76).47 Among a sample of 215 adult patients with chronic pain provided daily herbal cannabis containing 12.5% THC for 1 year, no significant neurocognitive differences were found compared with 216 control subjects.48 This is in line with a study of patients with multiple sclerosis in response to oral dronabinol that found no significant differences in MRI-derived measures, including annual percentage of brain volume change and occurrence of new lesions, after 12 months of use.49 These clinical trials suggest no significant adverse effect of THC on neurocognitive symptoms in specific clinical populations.
Cerebrovascular Risk and Disease
Cerebrovascular Risk Factors
Similar to the literature linking marijuana use with cardiovascular outcomes,10 evidence that marijuana consumption increases the prevalence of specific cerebrovascular risk factors and disease is limited by a preponderance of observational studies, cross-sectional studies, case reports, and case series prone to potential publication and other biases. Postulated adverse effects of marijuana use may include sympathetic nervous system activation, blood pressure changes, platelet activation, and electrophysiological effects.50–52 Concomitant tobacco smoking and other substance use and abuse possibly contribute to these effects, which may be short term and have been studied mostly in low-risk populations such as younger adults. These factors may explain why many longitudinal studies linking marijuana use and cardiovascular or metabolic risk factors have been negative after multivariable adjustment for unhealthy behaviors such as diet and tobacco smoking.53–55
Hypertension, in particular, is an important risk factor for ischemic stroke, hemorrhagic stroke, and subarachnoid hemorrhage. With marijuana use, the most common acute reaction in humans is a decrease in blood pressure resulting from cannabinoid effects on the vasculature and autonomic nervous system.52 Despite this physiological reaction, limited studies using the National Health and Nutrition Examination Survey showed a modest association of recent cannabis use with higher systolic blood pressure and higher prevalence of hypertension among current users 30 to 59 years of age.56 Heavy users, defined as use of marijuana or hashish in >20 of the past 30 days, had higher odds of abnormal blood pressure compared with never-users. Although this difference remained statistically significant after adjustment for age, sex, race, ethnicity, body mass index, education, and survey year, it was no longer statistically significant after additional adjustment for current tobacco and binge alcohol use (adjusted odds ratio, 1.47 [95% CI, 0.99–2.16]).57 The relationship between marijuana use and elevated blood pressure, especially among heavy users, may drive longer-term associations with cerebrovascular outcomes, although this mechanism remains to be studied.
Prior cardiovascular disease such as myocardial infarction (MI) or atrial fibrillation (AF) is also an important risk factor for stroke.58 Case reports of MI after marijuana use are mainly among young adults who lack vascular risk factors, with onset of MI shortly after use.59 Risk of MI was elevated 4.8-fold within an hour after smoking marijuana compared with periods of nonuse. This association demonstrates the potential role of marijuana as an acute trigger for cardiovascular disease.60 Over 25 years of follow-up, among 5113 adult participants in the Coronary Artery Risk Development in Young Adults study, cumulative or recent marijuana use was not associated with coronary heart disease, stroke, or cardiovascular disease mortality.61 This finding contrasts with a population-based, multi-institutional database study that observed an increased risk of 3-year cumulative incidence of MI among marijuana users compared with control subjects (1.37% vs 0.54%; relative risk, 2.54 [95% CI, 2.45–2.61]).62
Similarly, marijuana use appears to be a trigger for AF. Data from the Nationwide Inpatient Sample show that the percentage of individuals with cannabis use disorder discharged in the postlegalization period (2010–2014) with the diagnosis of arrhythmia increased 31%.63 However, in a study of patients hospitalized for heart failure, marijuana users had a reduced odds of AF compared with nonusers (adjusted odds ratio, 0.87 [95% CI, 0.77–0.98]).50 Simultaneous use of cocaine, stimulants, and other drugs may be responsible for observations of AF among marijuana users, although this remains to be fully studied outside of observational and cross-sectional reports.
Risk of Stroke and Transient Ischemic Attack
Several case reports and case series mostly in young individuals suggest a relationship between recent and heavy cannabis use and risk of stroke.64–66 In contrast, and as reviewed below, findings among case-control studies,67 population-based studies,68 and studies conducted using outpatient69,70 or inpatient71,72 national databases or hospital electronic health records73 have been equivocal, depending on the study design, covariates considered in the analysis, and source of the population being studied. Inconsistent associations also can be attributable to the presence of comparison groups and whether adjustment of other important risk factors was considered, along with attention to potential confounding by other risk factor and lifestyle features between cannabis users and nonusers.
In 1 case-control study using cannabis urine screens to identify cannabis users, the authors found an association between cannabis use and the risk of ischemic stroke and transient ischemic attack, but the association was not significant when tobacco use was included as a covariate (adjusted odds ratio, 1.59 [95% CI, 0.71–3.70]) among subjects 18 to 55 years of age with and without stroke.67 Similarly, after adjustment for cigarette smoking and alcohol use, another study found no association between cannabis use in young adulthood and the occurrence of fatal and nonfatal stroke later in life among Swedish men in up to 38 years of follow-up.68
Data from studies that have examined more specifically the dose or amount of cannabis consumed within a designated time frame suggest that regular cannabis use may increase the risk of stroke. Using data from population-based surveys, investigators have reported that when no cannabis use was compared with heavy cannabis use in the past year, cannabis use was associated with an increased risk for the occurrence of nonfatal stroke and transient ischemic attack.70 Similarly, another study found that recent (within the past 30 days) and frequent (>10 d/mo) cannabis use was associated with increased risk for the occurrence of stroke compared with nonuse, whereas less frequent cannabis use (≤10 d/mo or less than weekly in the past year) was not associated with increased risk.69,70
Using several International Classification of Diseases, Ninth Revision, Clinical Modification codes for marijuana use, a Nationwide Inpatient Sample study found that cannabis use among men and women hospitalized between 2004 and 2011 was associated with a 17% increased relative risk for acute ischemic stroke in a multivariable-adjusted analysis. Concomitant use of tobacco with cannabis increased the risk to 31%.71 Similarly, a separate study using the Nationwide Inpatient Sample but between 2009 and 2010 observed a higher odds of stroke among cannabis users (odds ratio, 1.24 [95% CI, 1.14–1.34]).72 In contrast, investigators using electronic health record data from patients admitted to a single center between 2015 and 2017 found that testing positive for cannabis use was not associated with the risk of ischemic stroke compared with testing negative, even after adjustment for numerous confounders, including age, cigarette smoking, and comorbidities.73
There may be certain populations or scenarios in which cannabis use can be meaningfully linked to stroke. A study of a large longitudinal cohort of Canadian pregnant women that included >1 million participants between 1989 and 2019 with follow-up at 30 years observed that cannabis use disorder was associated with a doubling of risk for hemorrhagic stroke (hazard ratio, 2.08 [95% CI, 1.07–4.05]) but no increased risk for ischemic or other cerebrovascular disease.74 Because of the theoretical vasoactive effect of cannabis, its use has been implicated in some cases of reversible cerebral vasoconstriction syndrome, with 6 of 24 nonidiopathic reversible cerebral vasoconstriction syndrome cases at a Colorado stroke center attributed to marijuana use.75 In addition, an elevated risk of stroke from intracranial arterial stenosis has been described among young cannabis users 18 to 45 years of age wherein vasospasm or reversible cerebral vasoconstriction syndrome may be a potential mechanism.76 Studies done in experimental models have shown that cannabinoids exert complex effects on cardiac contractility, vascular tone, and atherogenesis. Both vasodilatation and vasoconstriction responses were observed, depending on the experimental model and cannabinoid used. CB1 activation promotes inflammation, upregulates the production of reactive oxygen species, and activates proapoptotic pathways in endothelial cells and cardiomyocytes. In addition, it induces endothelial dysfunction and vascular smooth muscle cell proliferation and migration. These processes have been linked to cardiac dysfunction and the development of atherosclerosis.52 This is in contrast to the atheroprotective role associated with CB2.
Acute cardiovascular events and stroke also have been reported in patients using synthetic cannabinoids.77 Spice is associated with idiopathic thrombocytopenic purpura, which increases the risk of major hemorrhage.78 In addition, intracranial hemorrhage in Spice users has been linked to the presence of brodifacoum, an adulterant considered a superwarfarin.79
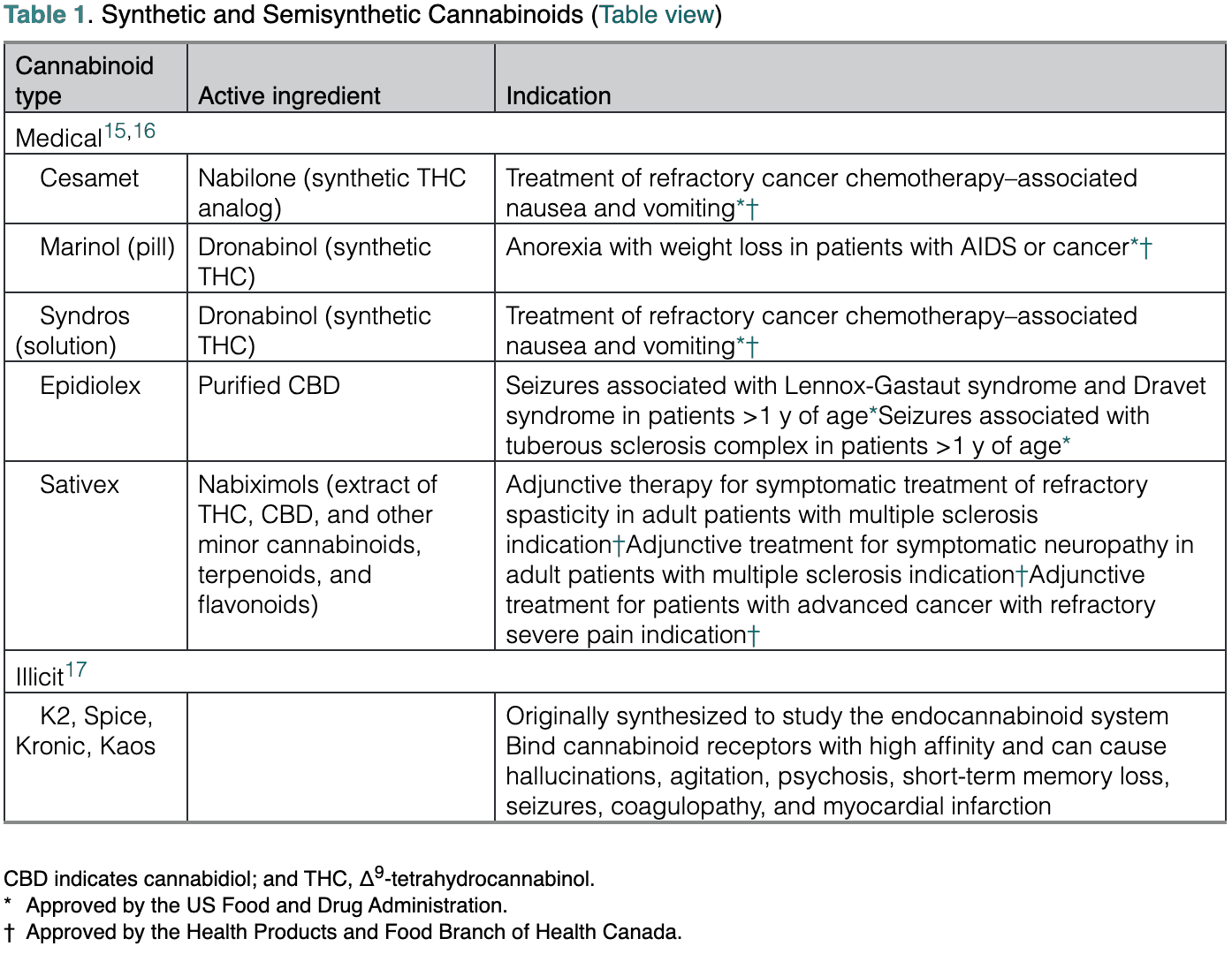
Education and Future Directions
Our understanding of the ramifications of cannabis consumption on brain health is limited but rapidly evolving. Observational studies have produced conflicting results in relation to the effect of marijuana on different outcomes of interest, including hypertension, AF, MI, and cognition. Several methodological factors may explain these apparent contradictions. First, given its historical classification as an illicit drug, the use of marijuana has been underreported for generations. The inclusion of marijuana users in the control group of observational studies that rely on self-reported use could underestimate its effect on brain health. Second, several behaviors such as smoking and alcohol use are associated with marijuana consumption and can influence stroke risk and brain connectivity.80,81 The often missing information on frequency of exposure to these factors limits our ability to determine with accuracy the independent effect of marijuana. Third, the time of exposure, frequency of use, and bioavailability of marijuana, which is affected by the route of administration, diet, and concomitant use of medications that may affect its metabolism, are reported inconsistently.10 Fourth, THC and CBD have different pharmacological effects. Although the use of THC has been associated with detrimental effects, CBD appears to have therapeutic potential in some neurologic disorders.9 The absolute and relative concentrations of these compounds differ according to the strain of cannabis plant and the methodology used to extract the active ingredients.82 Fifth, the gradual increase in the potency of marijuana used recreationally limits the relevance of older studies.5 Sixth, different factors impede the development of long-term placebo-controlled studies, including ethical reasons and the psychotropic effect of THC, which cannot be blinded.
Social media may emphasize a beneficial role for marijuana, and the general population may perceive it as a harmless drug. However, the emerging evidence linking marijuana use to cardiovascular events and stroke, as well as the potential and demonstrated drug-to-drug interactions between marijuana and medications commonly used in the general population, calls for caution and highlights the potential importance of active surveillance programs.10,83 In addition, the high density of cannabinoid receptors in areas involved in executive function and memory, the dose-dependent detrimental effect of THC on working and episodic memory, and the role of cannabinoid-associated biochemical pathways on synaptic plasticity and neuronal development raise concern that long-term exposure to marijuana may affect brain health. There is lack of agreement on whether the effects of marijuana resolve completely after months of abstinence. However, the disruption of endocannabinoid signaling pathways during the prenatal and perinatal periods and in adolescence may be detrimental to neurodevelopment.6,8,9 Key points discussed in this scientific statement are summarized in Table 2. It should be noted that the overarching goal of this scientific statement was to discuss mechanisms by which marijuana use could influence brain health. However, as the field is developing, several important aspects require additional research. As an example, there is limited information comparing the differential effect of recreational, illicit, and medicinal uses of marijuana, as well as the type of cannabis product consumed. Similarly, the modulatory effects of social determinants of health and race and ethnicity on the interaction of brain health and marijuana use are largely unexplored. The latter area of research may be particularly important because communities of color in the United States may be disproportionately affected by natural and synthetic cannabinoids in relation to use and exposure and the legal implications of criminalization of marijuana.84
Public health efforts should be considered to raise awareness about the potential negative effects associated with the use of marijuana in the general population. Possible strategies include the use of standardized concentrations of biologically active components and health warning labels on available formulations. In addition, the use of marijuana should be individualized and closely monitored. Health care professionals and patients should receive unbiased education about the potential consequences of medicinal, recreational, and illicit marijuana use on brain health, particularly when the exposure occurs during vulnerable vital periods. It also may be important for professionals to monitor cognitive performance of marijuana users and to review their medications to identify potential drug-to-drug interactions. Knowledgeable health care professionals will be able to properly educate potential or active marijuana users about its possible adverse effects, empowering them to make an informed decision that is based on unbiased data.
Table 2. Key Summary Points