Abstract
Objective: One route to improve adolescent addiction treatment outcomes is to use translational approaches to help identify developmental neuroscience mechanisms that undergird active treatment ingredients and advance adolescent behavior change.
Methods: This sample included 163 adolescents (ages 15-19) randomized to motivational interviewing (MI) vs. brief adolescent mindfulness (BAM). Youth completed an fMRI paradigm assessing adolescent brain response to therapist language (complex reflection vs. mindful; complex reflection vs. confront; mindful vs. confront) at pre- (prior to the completion of the full intervention) and post-treatment (at 3-month follow-up) and behavioral measures at 3, 6 and 12 months.
Results: Youth in both treatment groups showed significant problem drinking reductions at 3 and 6 months, but MI youth demonstrated significantly better treatment outcomes than BAM youth at 12 months. We observed several significant treatment group differences (MI > BAM) in neural response to therapist language, including at pre-treatment when examining complex reflection vs. mindful, and complex reflection vs. confront (e.g., superior temporal gyrus, lingual gyrus); and at post-treatment when examining mindful vs. confront (e.g., supplementary motor area; middle frontal gyrus). When collapsed across treatment groups (MI + BAM), we observed significant differences by time, with youth showing a pattern of brain change in response to complex reflection vs. mindful, and complex reflection vs. confront (e.g., precuneus; postcentral gyrus). There was no evidence of a significant group × time interaction. However, brain change in response to therapist language (complex reflection vs. confront) in regions such as middle frontal gyrus, was associated with reductions in problem drinking at 12 months. Yet, few treatment group differences were observed.
Conclusions: These data underscore the need to better understand therapist language and it's impact on the developing brain, in order to inform and aggregate the most impactful elements of addiction treatment for future treatment development for adolescents.
1. Introduction
Adolescence is a unique developmental period. During this neurodevelopmental phase, youth in many regions of the globe are encouraged to begin taking responsibility for more “adult” decisions (e.g., driving, dating, unsupervised social events), decisions that often include drinking. Yet, the brain regions responsible for weighing consequences, judging costs and benefits, and self-regulating are still very much in development during this period (Luna et al., 2010). This matters because data suggest that alcohol use during adolescence may be neurotoxic (Feldstein Ewing et al., 2014a). In turn, drinking, particularly in high-risk patterns like binge drinking, may negatively influence adolescents’ neurodevelopmental trajectory, subsequently placing adolescents at greater risk for sustained patterns of alcohol use and related problems as they transition into adulthood (Nguyen-Louie et al., 2018).
Definitions of addiction are being increasingly revised to reflect the nature of use in this age group. Historically, categorizations of alcohol abuse/dependence as defined through standard mental health diagnostic criteria (e.g., DSM; APA, 2013) were observed to clinically be poor fits with the nature of alcohol addiction observed in this age group (Clark, 2004). More recent calls have encouraged the use of “problem drinking” as the best representation of alcohol addiction (and its resolution) in this age group (Silvers et al., 2019). This is because problem drinking represents one of the most robust metrics of “interference in functioning”, which more accurately captures the manifestation of addiction in this age group, largely revolving around the adverse impact that drinking has upon social/peer, school/academic, work, and family spheres (Silvers et al., 2019).
Not only do youth exhibit high levels of problem drinking (Johnston et al., 2018), but unlike their adult counterparts, adolescents engaged in problem drinking are even more unlikely to seek, receive, or complete indicated alcohol treatment (Lipari et al., 2016). Thus, improving the effectiveness of brief behavioral alcohol treatments is integral to decreasing problem drinking and related harms for this age group. While existing addiction treatments show promise, they are not universally effective (Silvers et al., 2019). Even among the strongest evidence-based addiction treatments for this age group, including motivational interviewing (MI) (Miller and Rollnick, 2013), effect sizes for adolescents suggest that there is still room for improvement (Feldstein Ewing et al., 2016a).
One reason for the lower effect sizes observed for treatment outcomes in this age group is likely due to differences in the developing brain (Silvers et al., 2019). To that end, the adolescent brain is gaining increased recognition for its unique structure and function throughout this developmental stretch (Giedd, 2015). And, data indicate different patterns of neural response between adults and adolescents within some types of behavioral addictions treatment, including MI (Feldstein Ewing et al., 2011, Feldstein Ewing et al., 2013). However, this line of research has only begun.
Early translational data support the empirical relationship between neural response to task conditions of adolescent within-session client language [youth statements in favor of changing behavior (change talk) vs. youth statements in favor of not making behavioral changes (sustain talk)] and post-treatment behavior change (Feldstein Ewing et al., 2011, Feldstein Ewing et al., 2013). Studies have showed the capacity of brief (2 session) behavioral addiction treatments to reduce adolescent substance use. These studies reflect that, in contrast to adults, adolescents show a distinctly different pattern of neural activation, with greater BOLD response to task conditions of adolescent within-session client language (change talk > sustain talk) in areas important to self-awareness (e.g., precuneus and posterior cingulate cortex; PCC), rather than the pattern of mesocortiocolimbic reward response during task conditions of within-session client language (sustain talk > change talk) observed among adults.
Moreover, youth engaged in problem drinking who naturally generated their own client language with a therapist in the context of a real-world session with a behavioral addiction therapist show significantly greater BOLD response in task conditions of their own naturally-generated change talk and naturally-generated sustain talk as compared with youth who read and recorded an ecologically-valid, pre-provided script of change talk and sustain talk, but did not meet with a behavioral addiction therapist prior to the scan (Feldstein Ewing et al., 2014b). These data indicate two important patterns: first, the importance of engaging in a true therapeutic exchange with a skilled therapist, as parallel levels of neural activation were not observed in the absence of this therapeutic exchange. Second, overlaid with other findings in this area (Feldstein Ewing et al., 2017), these data point to a unique pattern of neural response, likely to subserve a different developmentally-specific pattern of neurocognitive response to addiction treatment within the period of adolescence (Silvers et al., 2019).
In terms of potential neural substrates of brief adolescent mindfulness (BAM), nascent work in this area has suggested that reward, learning/memory, interoception, executive control, and stress response may serve as mechanisms of neural response for adults engaged in mindfulness as a treatment for alcohol addiction (e.g., Becker et al., 2017, Witkiewitz et al., 2013). Yet, the adolescent literature in this area is quite limited. We could find no peer-reviewed, empirical studies examining neural mechanisms of mindfulness as a treatment for adolescent alcohol addiction. In turn, our hypotheses for these brain mechanisms were informed by the extant adult literature. Critically, following the translational neurodevelopmental literature in MI, we anticipated that neural networks of adolescent brain response to BAM would likely be disparate from the networks observed among adults (Silvers et al., 2019).
Emerging adolescent translational data in the sphere of mood and anxiety disorders has indicated that mindfulness (mindfulness-based cognitive therapy for children) may catalyze response within the fronto-parietal and cingulo-opercular networks via modulation of global efficiency and characteristic path length, along with enhanced functional connectivity of frontal and limbic areas within the default mode and cingulo-opercular networks (Qin et al., 2021). Similarly, two separate studies of children and adolescents who received a mindfulness-based intervention [mindfulness training; Training for Awareness, Resilience, and Action (TARA), respectively] also showed significant improvements in terms of stress, anxiety symptoms, anhedonia, and negative affect subserved by right amygdala (Bauer et al., 2019, Tymofiyeva et al., 2021). Among youth without behavioral disorders, mindfulness interventions (self-compassion) correlated with activation in right posterior cingulate cortex/precuneus during an emotional face recognition task, which notably comprise a similar set of behavioral and neurocognitive activities relevant to participation and success within behavioral therapy (Liu et al., 2020).
Overall, given that default mode network (DMN) is composed of subnetworks highly interconnected to the inherent cognitive processes involved in behavioral therapy (e.g., memory, understanding others’ minds) (Bathelt and Geurts, 2021), and the early data showing the involvement of DMN-related regions (e.g. precuneus, posterior cingulate cortex) among early studies of both MI and other mindfulness approaches, we posited response within DMN for both the MI and BAM interventions. However, due to the early stage of this field of research in the overall translational sphere (Ray et al., 2021), and among adolescents specifically (Silvers et al., 2019), we were not able to anticipate or propose potential differences in DMN response between these two adolescent interventions.
Despite early work suggesting a relationship between therapist language and adolescents’ unique pattern of processing of addiction treatment within self-reflection networks (Feldstein Ewing et al., 2016b), the field continues to need a randomized controlled trial (RCT) where youth are randomized to empirically-supported behavioral addictions treatments, and followed at more distal post-treatment timepoints (e.g., 3, 6, and 12 months), to illuminate how adolescent brain response may operate as a mechanism connecting salient within-session active ingredients (e.g., task conditions of therapist language) with treatment response (e.g., problem drinking reductions). This is important, given adolescents’ likely neurodevelopmentally-specific response to indicated behavioral addiction treatments (Silvers et al., 2019).
In this study, we thus employed an innovative translational design, integrating a neuroimaging (fMRI) paradigm within an RCT. We randomized adolescents engaged in problem drinking to two empirically-supported behavioral treatments for adolescent addiction (MI and BAM). Directly in line with our prior adolescent translational research designs (e.g., Feldstein Ewing et al., 2016c, Feldstein Ewing et al., 2013, Feldstein Ewing et al., 2014b), we conducted an fMRI task prior to the completion of the full intervention (henceforth referred to as “pre-treatment”) and at post-treatment in order to examine youths’ change in brain response to our target mechanism (therapist language), and its impact on their behavioral treatment response post-treatment (problem drinking). We proposed that youth would show greater behavior change in the MI vs. BAM intervention. In tandem, we expected that youth would show greater neural change in the precuneus/PCC to task conditions of specific therapist language (complex reflection > mindful; mindful > confront), and that this would be associated with problem drinking reductions post-treatment at 3, 6, and 12 months. Via this design, we hoped to disaggregate how adolescent brains respond to task conditions of therapist language in the context of behavioral addiction treatment. Further, we aimed to determine how that neural response may serve as a mechanism between salient active ingredients in addiction treatment and real-world adolescent behavior change in youth once they leave the therapist’s office. Ultimately, the goal of this study was to begin to pave the way for new translational studies in the field of adolescent addiction.
2. Materials and methods
2.1. Experimental design
This was a translational design, integrating a neuroimaging (fMRI) paradigm within an RCT for adolescent addiction with two behavioral treatment arms, motivational interviewing (MI) and mindfulness (BAM). All study procedures were conducted with University Institutional Committee on Human Subjects approval, and a federal Certificate of Confidentiality.
Youth were randomized to receive time-matched individual sessions of MI or BAM focused on reducing drinking, and an fMRI paradigm to assess the impact of neural processing of active ingredients of adolescent addiction treatment (here, therapist language). Youth completed the pre-treatment behavioral assessment and the first treatment session during their first appointment. Approximately one week later, youth completed the first scan, immediately followed by their second treatment session. The post-treatment scan occurred 3 months after the first scan. All youth also completed behavioral follow ups at 3-, 6-, and 12-months post-treatment. All youth received two one-hour intervention sessions, spaced to provide youth an opportunity to practice newly acquired skills in the intervening weekend. All analyzed participants (100%) attended both treatment sessions.
2.2. Participants
This sample included N = 163 adolescents (age: M = 18.71, range: 15.07–19.98) recruited through community outreach in a northwestern metropolitan area (Table 1). Eligible youth were 14–19 years of age and were currently engaged in problem drinking (following previous adolescent addiction studies, defined as ≥ 1 binge drinking episode during prior 2 months) (Feldstein Ewing et al., 2015). Exclusion criteria included left-handedness, >3 past-month non-tobacco- or cannabis-substance use events (e.g., methamphetamine), evidence of brain injury/illness or neurological disorder including psychosis, loss of consciousness ≥ 2 min, and/or MRI contraindications. Participants were breathalyzed to ensure BrAC = 0 and provided a urine drug screen before all study visits to corroborate self-report. Youth age 18 provided informed independent consent, while informed parent consent/adolescent assent was obtained for youth under age 18. Project staff randomized participants to treatment condition (MI or BAM) via coin toss. Post-hoc examination following randomization reflected that treatment groups (MI vs. BAM) did not differ based on cannabis or nicotine use (p’s = 0.77 and 0.64, respectively). Youth received up to $250 for participation in the study.
Table 1.
2.3. Adolescent alcohol treatments
Building upon prior work, which had largely used single-treatment arm within-subjects designs (Feldstein Ewing et al., 2016c, Feldstein Ewing et al., 2013), adolescents were randomized to one of two empirically supported behavioral treatments for addiction, MI and BAM . Participants across both conditions discussed factors relevant to problem drinking and received two individual 60-minute sessions of 1:1 treatment contact. Both conditions were time-matched to ensure equivalent duration of therapist contact. All sessions took place in a confidential room at the university dedicated to this purpose.
2.3.1. Therapist training and monitoring
In line with prior RCT approaches by this team (e.g., Feldstein Ewing et al., 2015), we ensured distinction of therapeutic content via a six-step approach. First, all interventions were manualized (manuals are available upon request to senior author). Second, therapists were distinct across conditions. Specifically, they were assigned to, trained in, and conducted only one of the two intervention approaches. Third, all therapists were carefully trained in their own intervention-specific training group prior to conducting their assigned intervention. Fourth, all intervention sessions were audiorecorded with participant permission. Fifth, random segments from the audiorecorded interventions were reviewed with the senior author during weekly therapist supervision (also separate by condition) to ensure treatment fidelity, prevent therapist drift, and maintain integrity of the interventions. Sixth, we utilized a final fidelity metric to ensure the distinction of each intervention.
2.3.2. Intervention fidelity and distinction
In line with previous adolescent behavioral RCTs including our own (e.g., Bryan et al., 2018, Feldstein Ewing et al., 2015), immediately following each intervention, all study therapists completed a fidelity measure (Chawla et al., 2010, Feldstein Ewing et al., 2012) to assess the presence of key elements of each intervention (range: 6–30, with 6 as “strongly agree” and 30 as “strongly disagree”). As expected, therapists showed distinction across interventions; MI therapists reported significantly more use of MI elements as compared to BAM elements (t(74) = -48.18, p < .001), and BAM therapists reported significantly more use of BAM elements as compared to MI elements (t(85) = 3.69, p < .001). This aligns with our prior adolescent RCTs, reflecting our team’s capacity to ensure fidelity and distinction across adolescent intervention approaches (e.g., Bryan et al., 2018, Feldstein Ewing et al., 2015).
2.3.3. Motivational interviewing (MI)
The goal of the MI treatment was to introduce, for the first time for many youth, a conversation about alcohol use, and the personally-experienced consequences of problem drinking. Following the empirically-supported approach for MI with non-treatment-seeking adolescents, this manualized treatment (Feldstein Ewing et al., 2009) explored youths’ stories around their substance use, the factors in youths’ lives that support problem drinking (e.g., what they like about drinking, aspects of their immediate community that facilitate drinking), and the consequences of their recent or previous problem drinking (e.g., getting in trouble at school; getting in trouble with parents). Youth were provided personalized feedback about how their problem drinking compared to age-matched norms in the U.S. The ultimate goal of the MI sessions was to engage youth in a thoughtful conversation about their problem drinking and the implications that their problem drinking may have on their lives, with an eye to bolstering and supporting youths’ own inherent drive for behavior change.
2.3.4. Brief adolescent mindfulness (BAM)
The goal of the BAM treatment was to introduce, also novel for many youth, a conversation about what BAM is, and ways that it might be personally-relevant to adolescents’ current experiences. Following empirically supported approaches for BAM (Crane et al., 2017), this manualized treatment (Feldstein Ewing and Somohano, 2015) introduced concepts of eastern thought in a manner articulated to adolescents specifically, and aligned to their phase of socio-cognitive development. This included a discussion of factors in the youth's life that could be positively impacted by using or engaging mindful approaches (e.g., current experiences of stress or adversity) and a link to how mindful approaches might be applicable or relevant to the adolescent's problem drinking. The ultimate goal was to engage youth in a thoughtful conversation about BAM and how eastern thought and mindful approaches could unburden some aspects of their current lived experiences; this treatment aimed to demystify basic BAM practice and introduce it as a tool to help adolescents navigate current experienced stresses and difficulties, including their problem drinking.
2.4. Measurements
2.4.1. Target treatment outcome: Problem drinking
Participants completed several questionnaires, including an evaluation of demographic factors. In line with recent calls (Silvers et al., 2019), the target treatment outcome for this study was problem drinking, measured by the Rutgers Alcohol Problems Index (RAPI; White and Labouvie, 1989). The RAPI is a well-validated 23-item self-report measure for adolescents (e.g., “Missed out on things because you spend too much money on alcohol”). Response options for each item (Never, 1–2 times, 3–5 times, 6–10 times, More than 10 times) were coded and summed for a total score at each timepoint (pre-treatment; 3, 6, and 12 months post-treatment).
Objective biological measures were collected at each study visit; a breathalyzer estimated blood alcohol content (BrAC) and a urine sample (Alere iCup, 10 panel; Abbot) provided an immediate screen for cannabis, amphetamine, barbiturates, benzodiazepines, methamphetamine, opiates, oxycodone, and MDMA. The use of biometrics in this study served as a safety screen to ensure that participations were not intoxicated at the time of MRI data collection, which could interfere with participant safety and the integrity of the MRI data. Biometric data were not, on their own, utilized as a data collection tool in this study.
2.5. fMRI data acquisition and processing
2.5.1. fMRI task
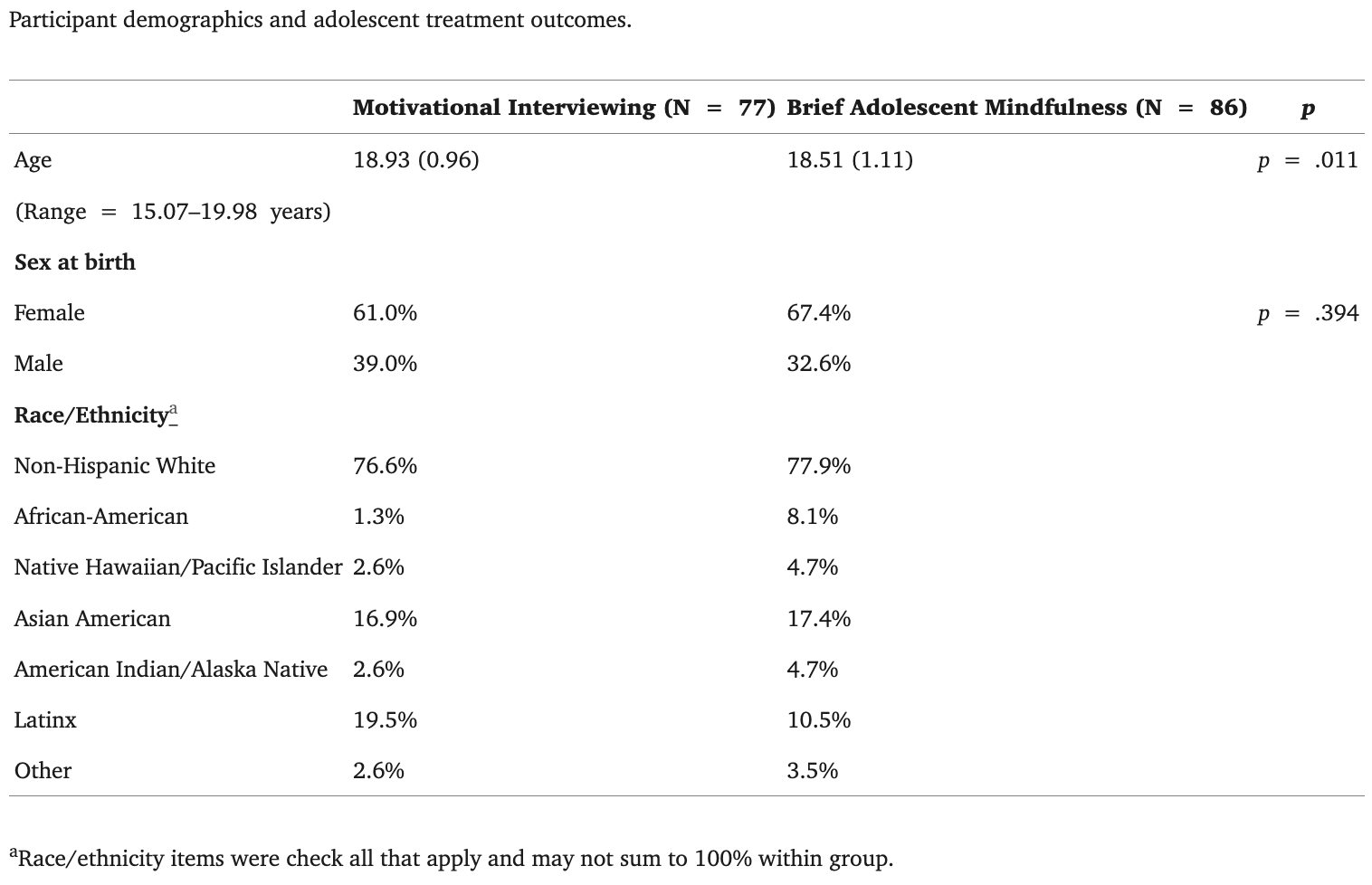
Fig. 1 represents the schematic of the fMRI task utilized to examine brain activation in response to task conditions. In the Neural In-Session Language Task (NILE) task, youth were re-presented their therapist’s voice and words in the imaging environment. Specifically, youth saw/heard n = 60 therapist statements. One third (n = 20) aligned with the approach/content of their assigned behavioral treatment condition. For example, youth in the MI condition heard n = 20 complex reflections capturing therapist language intended to bolster and support youths’ internal motivation to change (e.g., “You are worried that you might lose your friends because of your drinking”). Similarly, youth in the BAM condition heard n = 20 mindful statements capturing therapist language intended to foster non-judgmental acceptance (e.g., “You are noticing that your drinking is starting to change”). The final comparison condition was designed to capture the nature of provider interactions that youth often receive in traditional adolescent addiction counseling, emergency and/or urgent care treatment settings, assessed here in the confront condition (e.g. “Your drinking is starting to get out of control!”) Practically, in addition to the aligned content (complex reflections for MI youth; mindful statements for BAM youth), each youth was presented with a “matched” set of comparison statements. As an example, youth in the MI condition also received a parallel set of n = 20 mindful statements stated in the voice of their study therapist. Further, youth in the BAM condition also received a parallel set of n = 20 complex reflections stated in the voice of their study therapist. All youth received n = 20 confront statements stated in the voice of their study therapist. All statements presented to youth were recorded by their treating study therapist, and every effort was made to match statements in content, length, and format.
Fig. 1.
Immediately following the first session, study therapists made an explicit effort to match the semantic correspondence between the client and statement therapists for each trial; with the therapist statement directly responding to the overall content and nature of their initial meeting. MI therapists began by selecting “complex reflections” for the individualized NILE task, which were then matched with mindful and confront statements that had parallel content. Similarly, BAM therapists began by selecting mindful statements for the individualized NILE task, which were then matched with complex reflections and mindful statements that had parallel content. To be consistent with the youths’ clinical experience with the study therapist, all therapist statements were then audio-recorded by the study therapist in preparation for the fMRI paradigm. In other words, therapists selected their statements for the NILE task to be responsive to the content and nature of their therapy session, which were then recorded specifically for the experiment.
In line with prior approaches by this study team (e.g., Feldstein Ewing et al., 2016c, Feldstein Ewing et al., 2013, Feldstein Ewing et al., 2014b), all participants did not receive the same set of statements. Rather, they received unique statements specifically articulated to their therapeutic exchange, recorded by their treating therapist. However, all participants received the same set of individually-articulated statements at their pre- and post-treatment scans, in order to facilitate our evaluation of their change in neural response to the proposed therapeutic active ingredients (here, therapist language).
Participants received one 13-minute run of the task, presented using Presentation (https://www.neurobs.com/), which consisted of 60 trials, each beginning with a 500 ms “listen” cue, followed by the individualized text and audio of the therapist statements for each youth (for 5,000 ms). Trials were separated with jittered fixations 4,000–10,000 ms in length. Condition and fixation orders (during the ITI) were optimized using Optimize X (http://www.bobspunt.com/easy-optimize-x/), with no condition presented for more than two consecutive trials, and all therapist language randomized within each condition before each run.
2.5.2. MRI scan procedure
During the MRI, participants had a high-resolution anatomical scan prior to completing the fMRI task. Stimuli were back-projected and viewed with a mirror mounted on a head coil. Participants wore MRI-compatible electrostatic ear bud headphones to hear auditory stimuli. Foam padding was used to constrain head motion. Youth completed a series of validation checks to ensure task engagement, including a sound check to verify that they were able to hear the audio statements and a visual check to ensure that they could see the presented statements. Youth were also asked to report the percent of fMRI task statements that they could hear clearly to ensure effective audio delivery and participant engagement. Only youth who heard (and were able to engage in) ≥ 80% of the task were included in imaging analyses.
2.5.3. MRI data acquisition
Data were acquired on a Siemens 3 Tesla Prisma scanner (Siemens Medical Solutions, Erlangen, Germany) at the Advanced Imaging Research Center (AIRC) with a Siemens 32-channel head coil. A high-resolution, T1-weighted anatomical scan was collected for alignment and normalization of functional images for each participant (176 slices 1 mm isotropic, matrix = 256×256, TR/TE/TI = 2500/2.88/1060 ms, flip angle = 8°, pixel bandwidth = 240 Hz); volumes were acquired with Volumetric Navigators (vNAVS) to correct for intra-scan motion. The whole-brain fMRI task scan was acquired using a T2*-weighted echoplanar (EPI) sequence (2.4 mm isotropic, matrix = 90x90, TR/TE = 800/30 ms, flip angle = 52°, field of view = 216 mm, slices = 60) so that middle volume aligned with the subject’s inter-commissural line (AC-PC). A pair of field maps were acquired, with phase encoding direction reversed, to correct for distortions. Scanning parameters optimized BOLD signal quality while maximizing whole brain coverage.
2.5.4. fMRI Pre-processing
fMRI and anatomical images were analyzed using Analysis of Functional NeuroImages (AFNI) software package (https://afni.nimh.nih.gov/afni/version AFNI_18.2.04). The T1-weighted anatomical images were manually segmented, producing a skull-stripped image. A secondary segmentation and warping was applied using @SSwarper to skull-strip images to refine segmentation and conform to Montreal Neurological Institute stereotactic space (MNI152 2009). Using AFNI’s afni_proc.py script, a processing pipeline was generated to discard the first 6 EPI volumes, implement slice time correction, correct for distortions using the field map pairs, register fMRI volumes to the minimum outlier, align and warp volumes to template space provided by anatomical transformation, apply spatial smoothing using a Gaussian filter (5 mm full-width-at-half-maximum), and scale each of the voxel time series to a mean of 100. Frame-to-frame displacement was calculated for each volume. A generalized additive model (GAM) was run for each participant modeling each of the 3 task conditions (complex reflection, mindful, and confront) and 6 motion parameters, separately pre-and post-treatment.
2.6. Statistical analysis
2.6.1. Behavioral data
Target treatment response (problem drinking; derived from the RAPI total) was analyzed using a repeated measures ANCOVA with the repeated factor of Time (Post-Treatment: 3, 6, and 12 months) and the fixed factor of Treatment (MI; BAM) with pre-treatment problem drinking included as a covariate. An alpha of 0.05 was used for all behavioral analyses. Youth with full problem drinking data at all follow up time points were included in treatment outcome analyses. Of note, the sample with behavioral data (only) was slightly larger than the sample with both brain and behavioral data, due to more stringent criteria required for the inclusion of imaging data in analyses (see Supplemental Fig. 1 for study CONSORT).
2.7. fMRI Data
2.7.1. Pre-treatment analyses
Pre-treatment analyses were conducted to validate activation during each proposed mechanisms (each type of task condition - complex reflection; mindful; confront) and to ensure absence of pre-treatment group differences. Paired and independent group t-tests were conducted using AFNI’s 3dttest++ for differences in activation between task condition at pre-treatment (complex reflection vs. mindful, complex reflection vs. confront, mindful vs. confront). The –Clustsim option is the non-parametric correction tool utilized to determine minimum cluster size threshold for a voxel-wise threshold of p < .01 to obtain a corrected p < .05.
2.7.2. Multivariate modeling
Two series of multivariate statistical models using AFNI’s 3dMVM program were constructed (G. Chen et al., 2014; G. Chen et al., 2015). For the first series, a separate model was completed for each of the following contrasts: complex reflection vs. mindful, complex reflection vs. confront, and mindful vs. confront. Factors in the analyses were Time (pre-treatment; post-treatment) and Treatment (MI; BAM). AFNI’s –Clustsim option was used to determine minimum cluster size threshold for a voxel-wise threshold of p < .01 to obtain a corrected p < .05 for multiple comparisons. Results were thresholded at a voxel-wise p < .01 and whole-brain corrected at a p < .05. We selected a threshold that balances careful negotiation of Type 1 and Type 2 errors, which are the two central areas of attention in neuroimaging analyses. This selection represents the most contemporary recommended approach for nonparametric correction for multiple comparisons and utilizes the latest version of 3dClustsim to protect against Type 1 errors (Cox et al., 2017, Eklund et al., 2019). Our threshold was selected to also mitigate Type 2 errors, which have been identified as highly relevant in innovative fMRI data analyses (Hopfinger, 2017). Post-hoc t-tests and simple main effect analyses (3dMVM) were utilized to interpret all significant findings and their directionality.
The second series of analyses added models of each contrast (complex reflection vs. mindful, complex reflection vs. confront, and mindful vs. confront) with problem drinking at 12 months, allowing us to examine problem drinking regressed on the change in whole brain activation (for each of the identified contrasts) from pre- to post-treatment. Results were thresholded at a voxel-wise p < .05, uncorrected and clustered at 300 voxels. Significance for associations was determined through the 3dMVM platform in AFNI. Percent signal change for a 5-mm sphere around the peak coordinates of all significant clusters was extracted for pre- and post-treatment for the three task conditions to better understand the nature of the effects. For significant findings from models (examining problem drinking treatment outcomes), average percent signal change representing the change (post-treatment – pre-treatment) in activation between conditions was extracted and correlated with problem drinking at 12 months to quantify the relationship and determine directionality. Brain-behavior correlations for main effects of task conditions were not corrected for multiple comparisons, as they were proposed a priori .
3. Results
3.1. Adolescent treatment outcomes
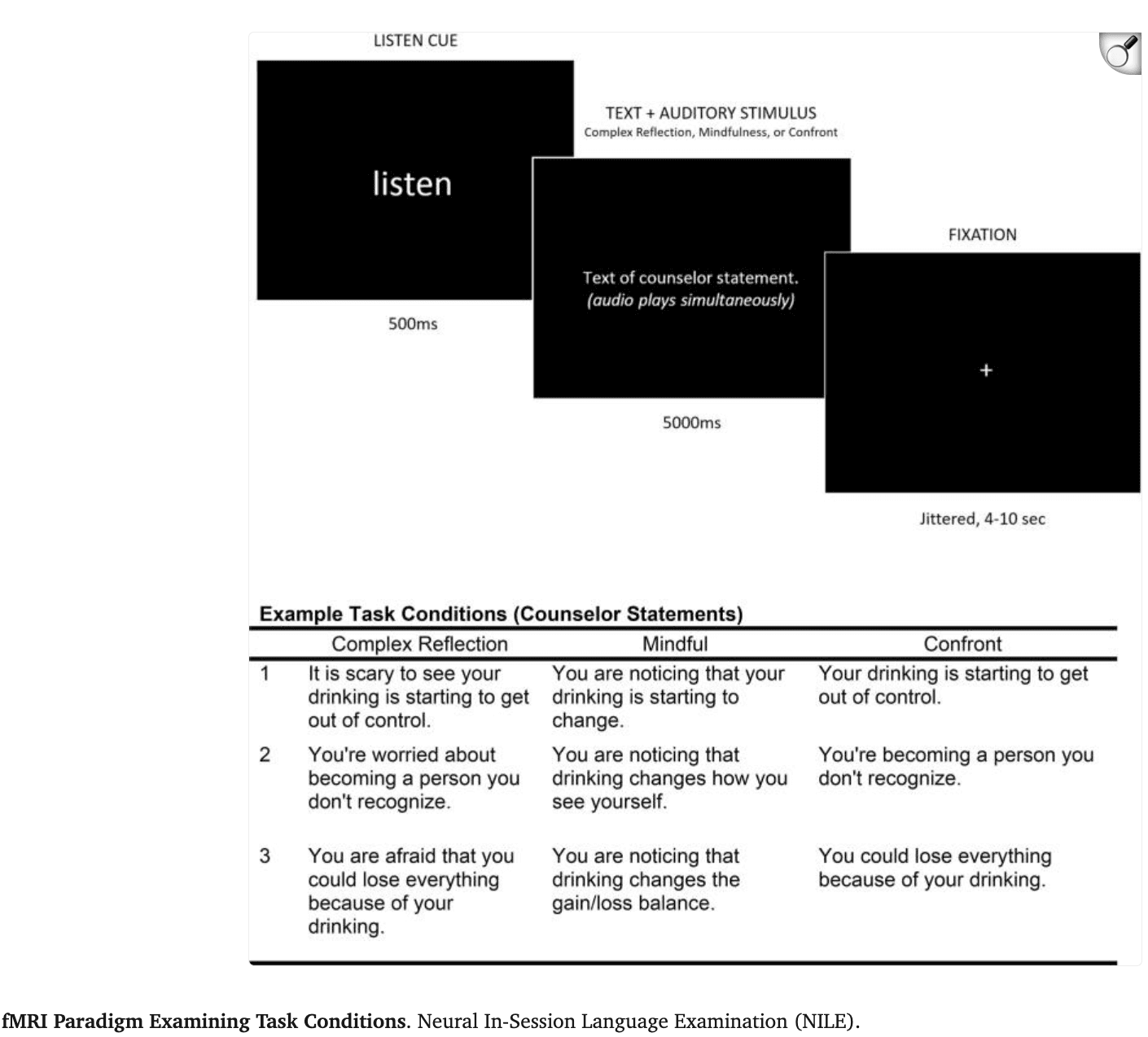
The repeated measures ANCOVA of Time (Post-Treatment: 3, 6, 12 months) × Treatment (MI, BAM) with the covariate of pre-treatment problem drinking revealed no main effect for Time (F(2,288) = 2.55, p = .080) or Treatment condition (F(2,288) = 3.96, p = .069). Yet, there was a significant Time × Group interaction (F(2,288) = 3.96, p = .020), wherein MI and BAM youth demonstrated similar problem drinking at 3 (t (145) = 0.82, p = .413) and 6 months (t (145) = −0.26, p = .793), but MI youth demonstrated significantly better treatment outcomes than BAM youth at 12 months (t (124.44) = −2.42, p = .0173) (Table 1; Fig. 2). Of note, all data collected for this study was conducted/completed pre-pandemic (final follow-up on January 2020), so observed drinking reductions within the MI cohort would not stem from pandemic-related absence of drinking opportunities.
Fig. 2.
3.2. Pre-treatment data per group
3.2.1. Pre-treatment response within the MI group
For pre-treatment brain response to complex reflections as contrasted with mindful, MI youth showed robust response with large loci of activation around the lingual gyrus (15,992 voxels), inferior frontal gyrus (IFG) (7,845 voxels) and supplementary motor area (SMA;6,360 voxels). Similarly, for pre-treatment brain response to complex reflections as contrasted with confront, MI youth also showed response around lingual gyrus (24,890 voxels) and bilateral superior temporal gyrus (6,627 voxels; 2,638 voxels, respectively). For pre-treatment brain response to mindful as contrasted with confront, MI youth showed large areas of response including cuneus (3,859 voxels), superior temporal gyrus/supramarginal gyrus (3,522 voxels; Table 2).
Table 2.
Task conditions and adolescent brain response across each treatment group (pre-treatment and change data). Activation for paired t-tests is presented by treatment type (MI, BAM) at pre- and post-treatment. Voxel level significance of p < .01, with whole-brain correction of p < .05. Peak z score is presented, extracted for each treatment type (MI, BAM) and the independent t-test of MI > BAM. Talairach coordinates. R = right. L = left. MI = motivational interviewing. BAM = brief adolescent mindfulness. B = bilateral. SMA = supplementary motor area.† = p < .05, uncorrected. * = p < .01, uncorrected. ** = p < .001, uncorrected.
Region | BA | Voxels | x | y | z | Z | ||
MI | BAM | MI > BAM | ||||||
Complex Reflection > Mindful | ||||||||
Pre-Treatment | ||||||||
MI | ||||||||
B Lingual Gyrus | 18 | 15,992 | 1 | −91 | −1 | 4.85 | 6.47 | −0.55 |
L Inferior Frontal Gyrus | 47 | 7,845 | −53 | 25 | −7 | 4.49 | 3.48 | 0.57 |
L SMA | 6 | 6,360 | −7 | 11 | 73 | 4.80 | 3.28 | 0.67 |
R Medial Temporal Pole | 38 | 3,336 | 55 | 15 | −25 | 5.84 | 5.45 | 0.052 |
L Precentral Gyrus | 6 | 1,205 | −49 | 5 | 55 | 4.72 | 2.43 | 1.70 |
BAM | ||||||||
B Lingual Gyrus | 18 | 20,021 | 1 | −89 | −9 | 4.40 | 6.61 | −3.26* |
L Medial Temporal Pole | 38 | 7,415 | −35 | 25 | −31 | 4.51 | 3.97 | −0.30 |
L Superior Medial Gyrus | 10 | 7,278 | 1 | 65 | 23 | −1.35 | 4.19 | −4.10** |
R Superior Temporal Gyrus | 22 | 6,356 | 65 | −1 | −7 | 4.20 | 5.60 | −2.65* |
Post-Treatment | ||||||||
MI | ||||||||
B Lingual Gyrus | 18 | 5,163 | 3 | −95 | 1 | 6.33 | 3.75 | 1.90 |
L Temporal Pole | 38 | 1,946 | −61 | 9 | −11 | 5.29 | 4.81 | 0.12 |
R Superior Temporal Gyrus | 22 | 1,678 | 67 | −1 | 5 | 3.69 | 3.60 | −0.15 |
BAM | ||||||||
B Lingual Gyrus | 18 | 5,474 | 1 | −91 | −5 | 4.68 | 5.82 | −1.54 |
R Superior Temporal Gyrus | 22 | 2,686 | 65 | −1 | −7 | 2.74 | 4.71 | −1.55 |
L Middle Temporal Gyrus | 22 | 2,583 | −61 | 7 | −15 | 4.06 | 5.04 | −1.63 |
Complex Reflection > Confront | ||||||||
Pre-Treatment | ||||||||
MI | ||||||||
B Lingual Gyrus | 18 | 24,890 | 1 | −89 | −5 | 13.00 | 13.00 | −2.43 † |
L Superior Temporal Gyrus | 41 | 6,627 | −69 | −17 | 11 | 5.11 | 4.87 | −1.35 |
R Superior Temporal Gyrus | 41 | 2,638 | 59 | −9 | 5 | 6.74 | 6.95 | −1.13 |
L Superior Frontal Gyrus | 6 | 1,390 | −7 | 1 | 77 | 2.84 | 2.99 | 0.33 |
BAM | ||||||||
B Lingual Gyrus | 18 | 35,553 | 1 | −91 | −7 | 7.01 | 13.00 | −3.10* |
R Superior Temporal Gyrus | 41 | 4,255 | 67 | −17 | 13 | 4.94 | 5.67 | −3.40** |
Post-Treatment | ||||||||
MI | ||||||||
B Lingual Gyrus | 18 | 11,956 | 5 | −97 | −9 | 5.25 | 2.03 | 1.80 |
L Superior Temporal Gyrus/Postcentral Gyrus | 41 | 7,052 | −67 | −13 | 9 | 5.59 | 3.48 | 1.76 |
R Superior Temporal Gyrus | 41 | 2,390 | 59 | −11 | 7 | 7.42 | 6.18 | −0.47 |
L Precentral Gyrus | 6 | 1,400 | −51 | −5 | 57 | 5.55 | 4.65 | −0.018 |
BAM | ||||||||
B Lingual Gyrus | 18 | 8,350 | 1 | −91 | −5 | 7.01 | 7.47 | −1.69 |
L Superior Temporal Gyrus/Postcentral Gyrus | 41 | 3,949 | −59 | −17 | 9 | 6.97 | 5.90 | −0.93 |
R Superior Temporal Gyrus | 41 | 2,382 | 61 | −9 | 5 | 7.02 | 6.24 | −1.00 |
L Precentral Gyrus | 6 | 1,412 | −49 | 1 | 57 | 4.65 | 4.02 | −1.40 |
Mindful > Confront | ||||||||
Pre-Treatment | ||||||||
MI | ||||||||
B Cuneus | 18 | 3,859 | 3 | −101 | 11 | 2.99 | 0.71 | 1.98 † |
L Superior Temporal Gyrus/ Supramarginal Gyrus | 40 | 3,522 | −57 | −19 | 11 | 6.41 | 5.33 | 0.29 |
R Superior Temporal Gyrus | 41 | 2,741 | 57 | −11 | 7 | 7.11 | 4.93 | 0.79 |
L Temporal Pole | 38 | 6,886 | −31 | 23 | −31 | −4.76 | −2.91 | −1.64 |
R Inferior Frontal Gyrus | 47 | 6,874 | 53 | 25 | −9 | −4.89 | −3.11 | −1.79 |
R SMA | 6 | 5,845 | 3 | 17 | 69 | −4.16 | −1.11 | −2.25 † |
BAM | ||||||||
B Cuneus | 18 | 4,793 | 1 | −93 | 33 | 1.99 | 2.68 | −0.25 |
L Superior Temporal Gyrus | 40 | 4,683 | −57 | −19 | 9 | 7.40 | 5.63 | −0.41 |
R Superior Temporal Gyrus | 41 | 2,911 | 69 | −17 | 13 | 4.49 | 4.29 | −2.10 † |
R Medial Temporal Pole | 38 | 4,255 | 55 | 15 | −27 | −5.74 | −5.99 | 0.68 |
R Superior Medial Gyrus | 10 | 4,011 | 3 | 65 | 23 | −2.66 | −2.98 | 1.79 |
L Temporal Pole | 38 | 1,742 | −31 | 23 | −29 | −3.90 | −3.42 | −0.54 |
L Precuneus | 31 | 1,368 | −1 | −55 | 29 | −3.63 | −4.26 | 1.28 |
Post-Treatment | ||||||||
MI | ||||||||
L Superior Temporal Gyrus/L Supramarginal Gyrus | 40 | 5,287 | −55 | −19 | 9 | 7.85 | 5.06 | 0.61 |
L Cuneus | 18 | 4,394 | −7 | −97 | 20 | 2.76 | 1.81 | 0.71 |
R Superior Temporal Gyrus | 22 | 3,976 | 61 | −9 | 7 | 5.75 | 4.44 | 0.48 |
L SMA | 6 | 3,669 | −7 | 19 | 71 | −3.30 | −1.19 | −2.06 † |
R Inferior Frontal Gyrus | 47 | 3,253 | 53 | 27 | −9 | −4.79 | −3.41 | −0.32 |
L Middle Frontal Gyrus | 10 | 3,059 | −49 | 51 | −5 | −2.89 | −0.81 | −2.20 † |
BAM | ||||||||
R Inferior Frontal Gyrus | 47 | 3,455 | 55 | 25 | −7 | −3.59 | −3.55 | 0.065 |
L Superior Temporal Gyrus | 22 | 3,371 | −55 | −25 | −7 | 7.85 | 5.06 | 0.61 |
L Medial Temporal Pole | 38 | 2,462 | −35 | 25 | −31 | −1.93 | −4.11 | 0.41 |
R Superior Temporal Gyrus | 41 | 2,343 | 63 | −9 | 7 | 5.51 | 4.21 | 0.39 |
B Cuneus | 18 | 2,140 | 5 | −91 | −9 | 2.94 | 4.15 | −0.92 |
R Superior Medial Gyrus | 9 | 1,326 | 7 | 61 | 39 | −1.23 | −3.28 | 1.64 |
3.2.2. Pre-treatment response within the BAM group
For pre-treatment brain response to complex reflections as contrasted with mindful, BAM youth showed large loci of activation around the lingual gyrus (20,021 voxels), medial temporal pole (7,415 voxels), and superior medial gyrus (7,278 voxels). Similarly, for pre-treatment brain response to complex reflections as contrasted with confront, BAM youth also showed response around lingual gyrus (35,553 voxels) and superior temporal gyrus (4,255 voxels). For pre-treatment brain response to mindful as contrasted with confront, BAM youth showed large areas of response including cuneus (4,793 voxels) and bilateral superior temporal gyrus (3,683 voxels; 2,911 voxels, respectively; Table 2).
3.3. Post-treatment data per group
3.3.1. Post-treatment response within the MI group
For post-treatment brain response to complex reflections as contrasted with mindful, MI youth showed large loci of activation largely around the lingual gyrus (5,163 voxels). Similarly, for post-treatment brain response to complex reflections as contrasted with confront, MI youth brain response centered around lingual gyrus (11,956 voxels) and superior temporal gyrus/postcentral gyrus (7,052 voxels). For post-treatment brain response to mindful as contrasted with confront, MI youth showed large brain response in regions such as superior temporal gyrus/supramarginal gyrus (5,287 voxels), cuneus (4,394 voxels), and superior temporal gyrus (3,976 voxels; Table 2).
3.3.2. Post-treatment response within the BAM group
For post-treatment brain response to complex reflections as contrasted with mindful, BAM youth showed robust brain response around lingual gyrus (5,474 voxels), along with superior and middle temporal gyrus (2,686 voxels; 2,583 voxels, respectively). Similarly, for post-treatment brain response to complex reflections as contrasted with confront, BAM youth also showed response around lingual gyrus (8,350 voxels) and superior temporal gyrus/postcentral gyrus (2,383 voxels). For post-treatment brain response to mindful as contrasted with confront, BAM youth showed large areas of brain response in regions including IFG (3,455 voxels), medial temporal pole (2,462 voxels), and bilateral superior temporal gyrus (3,371 voxels; 2,343 voxels, respectively; Table 2).
3.4. Intervention group effect (MI vs. BAM), time effect (pre vs. post), and group × time interaction
3.4.1. Differences between treatment groups
At pre-treatment, adolescents within the MI and BAM intervention conditions showed significant between-treatment-group differences in response to task conditions. When comparing MI > BAM responses to complex reflection as compared with mindful, we found significant differences in L superior medial gyrus (BA 10; z = −4.10, p < .001), R superior temporal gyrus (BA 22; z = -2.65, p < .01) and lingual gyrus (BA 18, z = -3.26, p < .01). Similarly, when comparing MI > BAM responses to complex reflection as compared with confront, we found significant differences in R superior temporal gyrus (BA 41; z = -3.40, p < .001) and bilateral lingual gyrus (BA 18, z = 2.43, p < 0.05; −3.10, p < .01, respectively). Also, when comparing MI > BAM responses to mindful as compared with confront, we found significant differences across cuneus (BA 18; z = -1.98, p < .05), R SMA (BA 41; z = 2.25, p < .05), and R superior temporal gyrus (BA 41; z = -2.10, p < .05; see Table 2). There were no additional regions where BAM > MI.
At post-treatment, adolescents within the MI and BAM intervention conditions showed significant between-treatment-group differences in response to task conditions. When comparing MI > BAM responses to mindful as compared with confront, we found significant differences across L SMA (BA 6; z = 2.06, p < .05) and L middle frontal gyrus (BA 10; z = 2.20, p < .05). At post-treatment, when comparing MI > BAM responses to complex reflection versus mindful, along with complex reflection versus confront, we no longer observed the significant intervention group differences that we had observed at pre-treatment (see Table 2 and Fig. 4). There were no additional regions where BAM > MI.
Fig. 4.
3.4.2. Differences by time
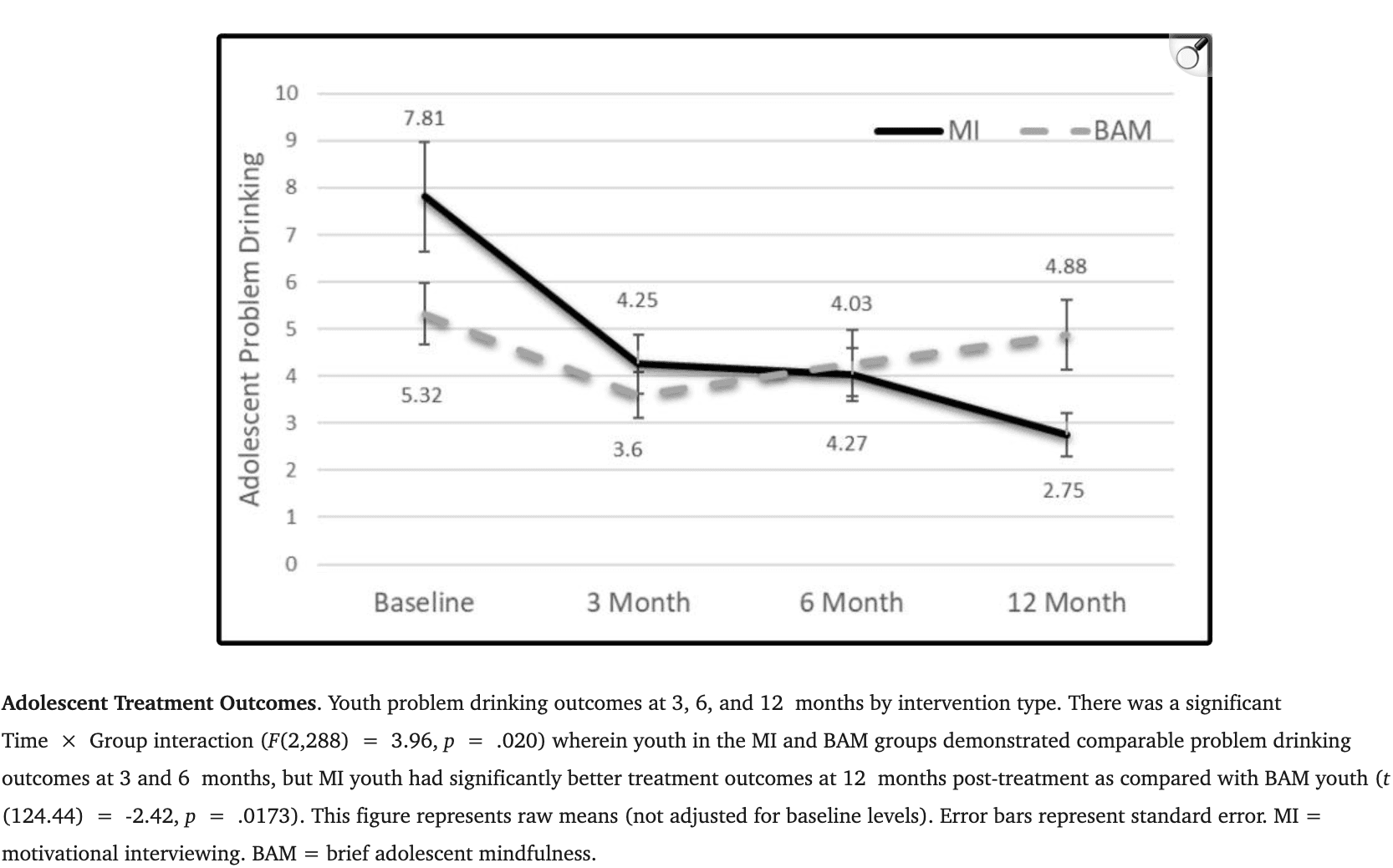
When collapsed across treatment groups, adolescents showed a pattern of brain change in response to complex reflection compared with mindful across multiple regions spanning precuneus (BA 4/19)/postcentral gyrus (BA 7), inferior parietal lobe (BA 40), R superior frontal gyrus, and medial frontal gyrus (BA 10) (2,252 voxels; 1,109 voxels; 867 voxels; 729 voxels respectively). There was also a pattern of adolescent brain change for complex reflection as compared with confront across bilateral precuneus (BA 7) and bilateral cuneus (BA 19) (2,548 voxels; 743 voxels, 517 voxels, 500 voxels, respectively; Table 3; Fig. 3). Across contrasts and regions, significant changes in activation reflected decreases in BOLD response to complex reflection. No significant differences emerged for brain change in response to mindfulness as compared with confront. These relationships at pre- and post-treatment are also represented as columns graphically depicting the areas with differences in Fig. 4.
Table 3.
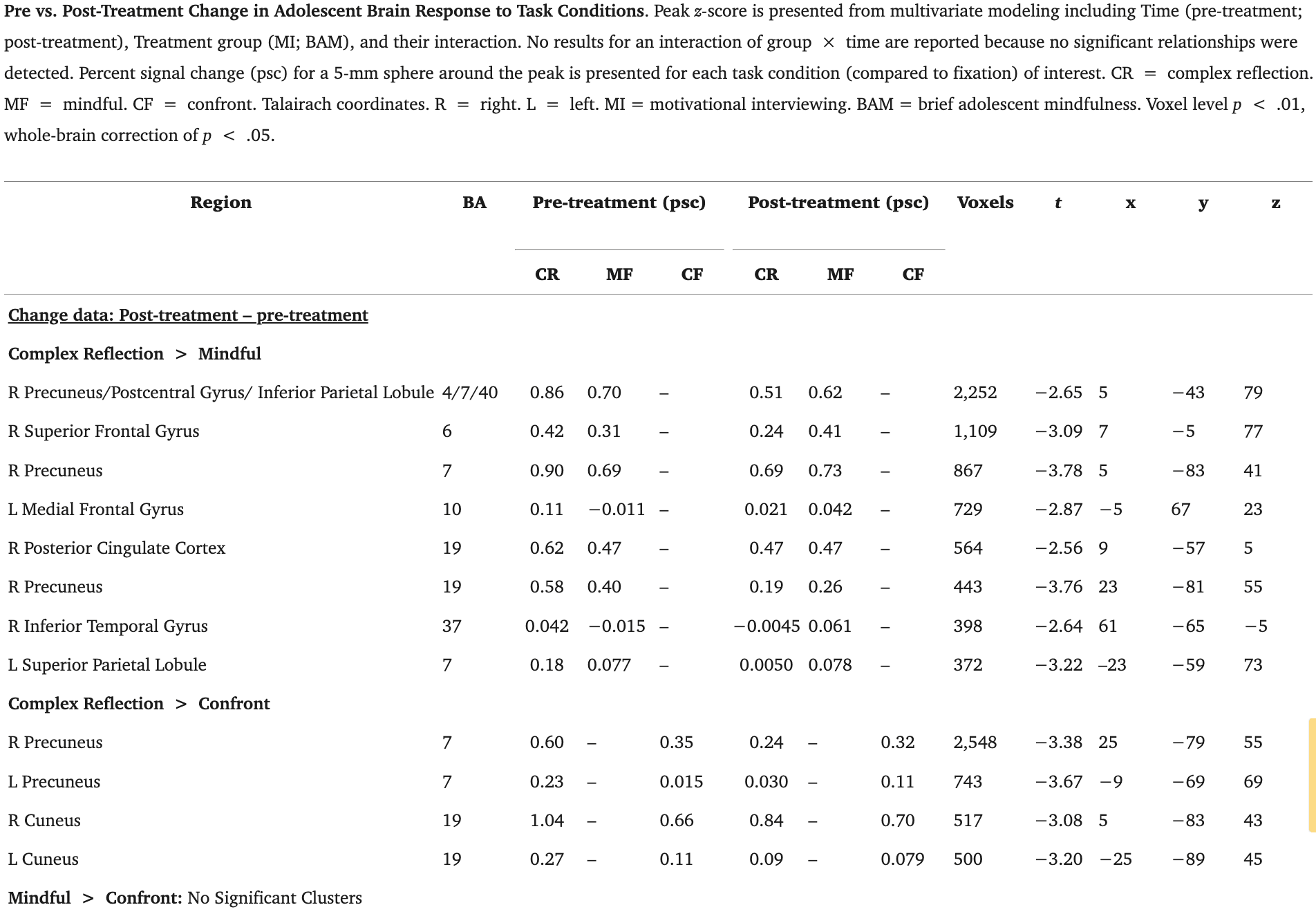
Fig. 3.
Pre-to-Post Treatment Change in Adolescents’ Brain Response to Task Conditions. Percent signal change (post-treatment - pre-treatment) for each task condition included in the contrast (examined across treatment groups); p < .01, corrected at p < .05. R = right. L = left. IPL = inferior parietal lobule. aMedFG = anterior medial frontal gyrus. MedFG = medial frontal gyrus. Talairach coordinates.(A) Adolescents’ response to complex reflection as compared with mindful . (B) Adolescents’ response to complex reflection as compared with confront . (C)* Adolescents’ response to mindful as compared with confront is not included as there were no significant findings for this contrast.
3.4.3. Interaction
There was no evidence of a significant group × time interaction.
3.5. Adolescents’ brain response to task conditions and associated 12 month treatment outcomes
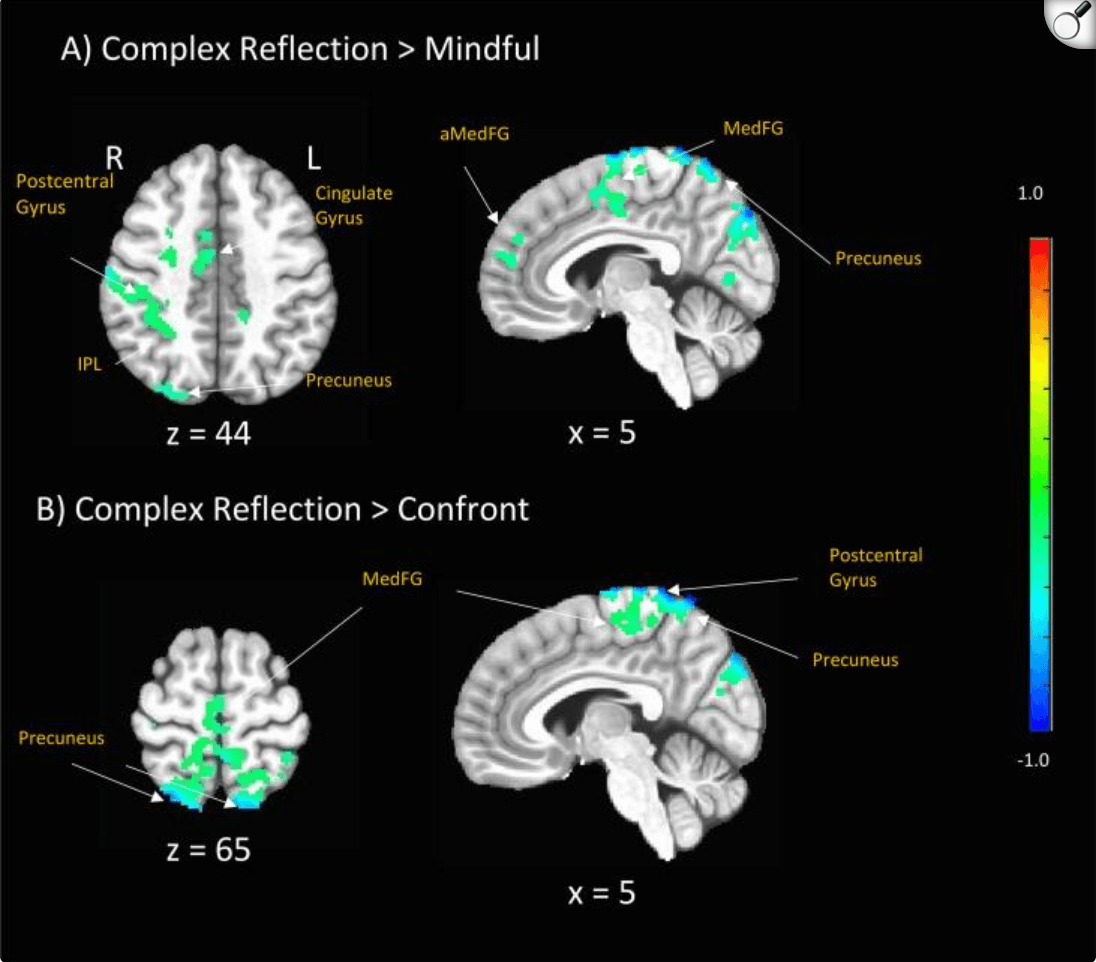
Adolescent brain change in response to complex reflection as compared with mindful was associated with long term (12 month) behavior change, here measured as reductions in problem drinking; however, no significant treatment group differences were observed for this comparison. Adolescent brain change in response to complex reflection as compared with confront was also associated with long term (12 month) behavior change, here also measured as reductions in problem drinking. HWe did observe a few treatment group differences, including. significant correlations for the BAM treatment group across middle frontal gyrus (t(114) = 2.24, p = .025). Finally, adolescent brain change in response to mindful compared with confront was associated with long term (12 month) behavior change, here as well, measured as reductions in problem drinking. We found significant treatment group differences, primarily significant for youth in the BAM treatment group across thalamus (t(114) = -2.04, p = .042) (Table 4; Fig. 5).
Table 4.
Adolescents’ Brain Response to Task Conditions and 12 month Treatment Outcomes. Regression of adolescent problem drinking on whole-brain activation for change in adolescent brain response to therapist language. Peak F score is presented for the regression. r values and corresponding z values are presented for significant whole-brain associations across treatments and separately for each treatment. Talairach coordinates. R = right. L = left. MI = motivational interviewing. BAM = brief adolescent mindfulness. Voxel level significance of p < .05, uncorrected. Only clusters ≥ 300 voxels are presented.
Region | BA | Voxels | F | x | y | z | r |
Complex Reflection > Mindful | |||||||
L Inferior Temporal Gyrus | 21 | 404 | 31.97 | −57 | −7 | −19 | −0.19 |
MI | – | – | – | – | – | – | −0.16 |
BAM | – | – | – | – | – | – | −0.20 |
L Posterior Cingulate Cortex | 30 | 373 | 13.42 | −7 | −53 | 3 | −0.22 |
MI | – | – | – | – | – | – | −0.22 |
BAM | – | – | – | – | – | – | −0.19 |
Complex Reflection > Confront | |||||||
L Superior Frontal Gyrus | 10 | 811 | 18.09 | −29 | 65 | −11 | −0.053 |
MI | – | – | – | – | – | – | −0.15 |
BAM | – | – | – | – | – | – | 0.006 |
R Middle Frontal Gyrus | 11 | 411 | 15.94 | 49 | 51 | −15 | −0.14* |
MI | – | – | – | – | – | – | 0.13 |
BAM | – | – | – | – | – | – | −0.29 |
R Insula | 13 | 352 | 15.62 | 29 | 21 | 1 | −0.18 |
MI | – | – | – | – | – | – | −0.28 |
BAM | – | – | – | – | – | – | −0.14 |
L Thalamus | – | 310 | 16.65 | −25 | 7 | −5 | −0.22 |
MI | – | – | – | – | – | – | −0.29 |
BAM | – | – | – | – | – | – | −0.17 |
R Middle Frontal Gyrus | 9 | 301 | 14.85 | 37 | 33 | 35 | −0.006 |
MI | – | – | – | – | – | – | −0.10 |
BAM | – | – | – | – | – | – | 0.049 |
Mindful > Confront | |||||||
R Superior Temporal Gyrus | 38 | 764 | 19.60 | 27 | 5 | −27 | −0.35 |
MI | – | – | – | – | – | – | −0.26 |
BAM | – | – | – | – | – | – | −0.43 |
R Thalamus | – | 559 | 17.20 | 11 | −31 | 19 | 0.30* |
MI | – | – | – | – | – | – | 0.057 |
BAM | – | – | – | – | – | – | 0.42 |
R Medial Frontal Gyrus | 6 | 401 | 13.09 | 3 | −17 | 65 | −0.12 |
MI | – | – | – | – | – | – | −0.14 |
BAM | – | – | – | – | – | – | −0.14 |
R Middle Frontal Gyrus | 10/46 | 376 | 14.52 | 51 | 49 | 3 | −0.11 |
MI | – | – | – | – | – | – | −0.26 |
BAM | – | – | – | – | – | – | −0.04 |
L Caudate | – | 369 | 18.89 | −29 | −1 | 27 | 0.29 |
MI | – | – | – | – | – | – | 0.28 |
BAM | – | – | – | – | – | – | 0.29 |
L Inferior Frontal Gyrus | 47 | 360 | 11.43 | −45 | 13 | 7 | −0.31 |
MI | – | – | – | – | – | – | −0.20 |
BAM | – | – | – | – | – | – | −0.40 |
Fig. 5.
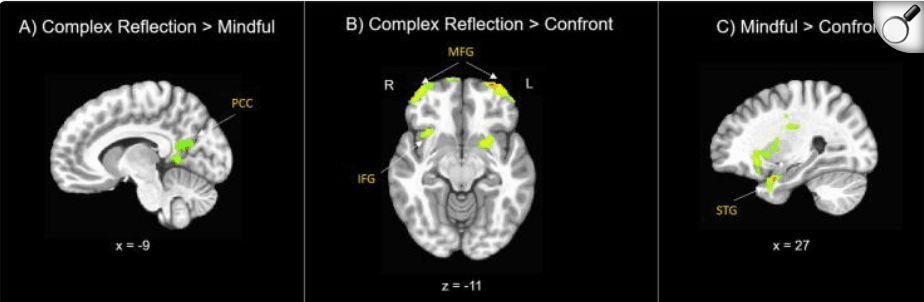
Correlations between Adolescents’ Brain Response to Task Conditions and 12 month Treatment Outcomes. These are the primary areas of brain-behavior correlations for each of the 3 language contrasts. Figure shows associations between neural change in activation to task conditions associated with long term behavior change, measured as adolescent problem drinking outcomes at 12 months post-treatment (examined across treatment groups). p < .05, uncorrected. R = right. L = left. PCC = posterior cingulate cortex. MFG = middle frontal gyrus. IFG = inferior frontal gyrus. STG = superior temporal gyrus. Talairach coordinates.
3.6. Discussion
Despite the critical role of therapists within behavioral addiction treatment (Magill et al., 2018), only a handful of published studies have investigated the role of therapist factors in adolescent treatment response (Feldstein Ewing et al., 2015, Gaume et al., 2016). Fewer have evaluated youths’ within-session brain response in the context of behavioral addiction treatment (Feldstein Ewing et al., 2016c, Feldstein Ewing et al., 2013). This is critical because behavioral-only studies may be unable to detect subtle, but highly important clinical changes and/or mechanisms that underlie successful treatment response (Silvers et al., 2019). Thus, one asset of this study is that it offers a distinctly sensitive method (neuroimaging) to facilitate the detection of salient brain responses likely to escape other methods (Gaume et al., 2019). Further, these data are critical to guide improvements in adolescent addiction treatment ..
Within this study, we employed an innovative translational design, integrating a neuroimaging paradigm within an RCT. We randomized adolescents engaged in problem drinking to two empirically-supported behavioral treatments (MI and BAM), and examined behavioral treatment outcomes (problem drinking) at 3, 6, and 12 months post-treatment. We then conducted an fMRI task pre- and post-treatment to evaluate youths’ change in brain response to our target mechanism (therapist language), and its impact on their long term (12 month) behavior change, here measured as reductions in problem drinking.
In terms of overall treatment outcomes, prior studies including our own, have highlighted the success of MI in catalyzing and sustaining behavior change in this age group (D'Amico et al., 2018, Feldstein Ewing et al., 2020, Feldstein Ewing et al., 2013). Thus, we anticipated that this sample of adolescents engaged in problem drinking would show greater behavior change following the receipt of MI, as compared with BAM. Contrary to expectations, but in line with recent studies in adolescent addiction treatment response (Silvers et al., 2019, Yeager et al., 2017), we did not observe differential treatment outcomes at 3 and 6 months. However, we did observe significant differential reductions in problem drinking at 12 months, wherein MI youth displayed continued treatment gains at 1 year post-treatment. In contrast, parallel reductions were not observed for BAM youth, who began to return to problem drinking at the 12 month follow-up.
These findings are clinically meaningful for three reasons; first, one encouraging aspect of these data is that they support the use of brief behavioral treatments (two 60-minute 1:1 sessions with therapists) to promote and foster adolescent behavior change in the domain of problem drinking – and, equivalently so, for short-term outcomes (at 3 and 6 months). Second, these findings follow the large and impressive body of work reflecting the capacity of MI to sustain long-term treatment gains, a literature that is now increasing in size and breadth for this age group (D'Amico et al., 2018, Steele et al., 2020), and, which hasve historically been less well examined in the youth addiction field (Cronce and Larimer, 2011). Third, while some of the larger RCTs have compared MI against other treatments, this examination represents the only empirical study that we could find that integrates a translational paradigm into an RCT for adolescent addiction, allowing for the comparison of neurodevelopmental mechanisms across two different active behavioral treatments.
The integration of a translational design also allows us to move beyond examination of simple black-box treatment outcomes to dive deeper into the neural mechanisms below the surface, which reveal a more nuanced picture of what is happening in the adolescent brain and how it is connected to youth behavioral treatment response. Further, this generates a compelling window into which mechanisms might be specific to certain treatments, and which might represent global factors undergirding adolescent behavior change across treatments (referred to in the behavioral treatment literature as “common factors”) (Miller and Moyers, 2014). To better disaggregate how adolescent brains respond to therapist language in the context of brief addiction treatments, we examined adolescents’ pre-to-post treatment brain response to therapist language. We proposed that youth would show greater change in the precuneus and PCC pathway in response to task conditions of specific therapist language (complex reflection > mindful; mindful > confront).
In terms of adolescent brain change, we observed an overall pattern of differential neural response from pre-to-post treatment across default mode network (DMN, precuneus, posterior cingulate cortex, medial frontal gyrus) in response to task conditions of therapist language, including those consistent with MI (complex reflection), those consistent with BAM (mindful), and confrontational therapist language (confront). Yet, the nature of this pattern was not precisely as anticipated.
Specifically, when examining task conditions, youth showed robust pre-treatment brain response to all three therapist language comparisons (complex reflection vs. mindful; complex reflection vs. confront; and mindful vs. confront), suggesting that therapist language effectively, and differentially, elicited adolescent brain response. In terms of treatment group differences, we observed significant treatment group differences at pre-treatment. Here, MI youth showed significantly greater BOLD response than BAM youth to task conditions of complex reflection as compared with mindful and confront, respectively, across R superior temporal gyrus and lingual gyrus. Different, but still significant treatment group responses were observed at pre-treatment for MI youth as compared to BAM youth when evaluating pre-treatment task conditions of mindful with confront, across cuneus, R SMA, and R superior temporal guys.
At post-treatment, we still observed significant treatment group differences. Here, MI youth showed significantly greater BOLD response than BAM youth to task conditions of mindful as compared with confront, across L SMA and L middle frontal gyrus. However, at post-treatment, we no longer observed significant treatment group differences in response to task conditions of complex reflection versus mindful or confront.
In sum, youth in both the MI and BAM groups showed robust, but differential, brain response to task conditions of complex reflections as compared with mindful and confront, particularly at pre-treatment. Not only do these findings have synergy with behavioral treatment literature, hese findings have a high degree of overlap with the neurodevelopmental linguistic processing literature. The consistency between the domains of linguistics and behavioral response have a compelling and strong history in the scientific background and later treatment foci for MI (Amrhein et al., 2003), including formative psycho-linguistic studies indicating the role of change language as a key mechanism of behavioral response in MI (Moyers et al., 2007).
To this end, early work in the neurodevelopmental processing of emotional semantics and prosody, including the capacity to derive meaning from things that we see and hear in adolescence indicates bilateral processing, includingin IFG, lingual gyrus, PCC, middle and superior temporal gyri, insula, SMA, and precuneus (Castelluccio et al., 2016, Enge et al., 2021, Vaughn et al., 2021). Additional findings from the large-scale ABCD Study also indicate that verbal processing in adolescents might rely more heavily on the engagement of right hemisphere structures than the left hemisphere structures often observed among adults (Gonzalez et al., 2021). Further, the neurodevelopmental language processing literature also denotes the importance of DMN network organization in terms of increased network homogeneity in not only DMN, but also in salience and language networks as essential precursors to effective early learning (B. Chen et al., 2021). In sum, these regions are relevant within the behavioral response literature, as well as the neural response of language, suggesting critical areas for future exploration. Specifically, future work will disaggregate the implications of these different regions of response (e.g., those associated with substance use and its remediation, those associated with the development of language processing in this age group, and their integration in terms of adolescent behavior change). Here, network based approaches may generate more information about how these regions overlap, and to what degree that synchrony may help inform more impactful therapist language and therapeutic approaches with this age group.
In terms of the relevance of these regions, beyond the linguistic literature, superior temporal gyrus has been identified as salient within neurodevelopmental processing of valence vs. risk (Blankenstein and van Duijvenvoorde, 2019). As well, recent translational research in the domain of adolescent behavioral treatment (in this study, response to trauma-focused therapy in the context of youth PTSD), found that a network centered on bilateral superior temporal gyrus predicted positive youth treatment outcomes (Zhutovsky et al., 2021).
In addition to the adolescent addiction literature (Hammond, Allick et al., 2019), middle temporal gyrus has played a role in both youth social anxiety (Wang et al., 2021), as well as processing of prosocial decisions with preferred peers (Schreuders et al., 2019), and estimates of believability and trust (Jiang et al., 2018). Middle temporal gyrus has also been found to be integral in processing of stress during problem solving (Nair et al., 2019), as well as learning about and categorizing sequential cause/effect events (Leshinskaya and Thompson-Schill, 2020). In sum, middle temporal gyrus clearly has a role in social competence and relationship building during this phase of adolescent development.
As with SMA (Sweitzer et al., 2016), lingual gyrus has consistently shown a role in youth responding to substance use information across numerous substances, including alcohol and cannabis use, and more recently, tobacco and e-cigarettes/vaping (Y. Chen et al., 2018, Filbey et al., 2018, Leiker et al., 2019). Lingual gyrus has been involved in the processing of social experience, salience detection, social cognition and emotional memory among youth (Rudolph et al., 2021). Moreover, early translational studies in the context of the behavioral treatment (here, cognitive behavioral therapy in the treatment of OCD), have shown the role of lingual gyrus among positive behavioral treatment responders (Cao et al., 2021).
When examined across treatment groups, collectively, adolescents showed a pattern of brain change in response to complex reflection as compared with mindful and confront across multiple regions (e.g., precuneus, cuneus, postcentral gyrus, right inferior parietal cortex, medial frontal gyrus). Ultimately, when collapsed across treatment conditions, we observed these patterns within the contrasts across the entire sample. As shown in Fig. 3, Fig. 4, we observed that overall, adolescents tend to show differential response to these three types of therapist language in the following patterns. Here, as the role of DMN is increasingly being found as relevant in systematic reviews of the broader mood/affective adolescent behavioral therapy response literature (La Buissonniere-Ariza et al., 2021), in this initial translational adolescent addiction study, we found that responses to the complex reflection in DFM regions like precuneus and PCC, indicate that therapist language that includes language around emotion generation (e.g., “You are embarrassed about how much you have been drinking”), tends to activate regions relevant in self-reflection. Further, between pre-and post-treatment, we saw a pattern of reduced response to complex reflections at T2 but relatively stable responses in these regions to the mindful and the confront statements.
Adolescent brain response to mindful largely aligned to some degree with the pattern observed within complex reflection; however, it showed two notable differences. For mindful, the overall pattern of activity tended more toward superior temporal gyrus and superior medial gyrus, regions associated with auditory comprehension, and also social cognition (Kral et al., 2017). Further, between pre- and post-treatment, responses in these regions were fairly stable. Similarly, when collapsed across treatment groups, we observed a consistent pattern of limited brain response to confront in observed regions, as well as a fairly stable set of responses between pre- and post-treatment. These data indicate that whereas therapist language that describes an adolescent’s emotions, or highlights an adolescent’s current lived experience, activates relevant self- and social-processing regions, standard condemnatory language often used in adolescent addiction interventions (e.g., “Don’t you see how your drinking is affecting you??”) did not generate comparable responses in those relevant self- and social-change regions. In sum, these data support that these relatively subtle differences in therapist language (as shown in Fig. 1) can lead to notably consistent impacts on neural activation.
We also examined how those neural changes would interact with adolescent treatment response. Specifically, we proposed that pre-to-post adolescent brain change would be associated with long term (12 month) behavior change, here measured as reductions in problem drinking; we examined this at 12 months, the follow-up period for which there was a significant difference in drinking outcomes by treatment group.
Across both treatment groups, adolescent brain change in response to complex reflection as compared with mindful as well as with confront were associated with 12-month problem drinking reductions (e.g., inferior temporal gyrus, PCC; superior frontal gyrus, insula, thalamus, respectively). Treatment groups showed comparable response, with the exception of BAM youth showing significantly stronger relationships in middle frontal gyrus (MFG) as compared with MI youth. Adolescent brain change in response to mindful as compared with confront was also associated with 12-month problem drinking reductions (e.g., MFG, superior temporal gyrus, medial frontal gyrus, caudate, IFG); here, BAM youth showed stronger response in the thalamus, as compared with MI youth.
In sum, these data suggest an overall compelling pattern of adolescent change in brain response across DMN hubs, including, but not limited to: precuneus, PCC, and MFG, that differentially emerged by therapist language, and was associated with long term (12 month) behavior change, here measured as reductions in problem drinking. This aligns with increasing work in the field of translational efforts integrating behavioral treatment with neuroimaging in addiction (Konova, Moeller, and Goldstein, 2013).
The precuneus and the posterior cingulate cortex (PCC) are particularly relevant in the picture of adolescent behavioral treatment response, and represent key loci of DMN (Raichle, 2015). Both are central to self-reflection, past/future thinking, emotional processing, and self-referential processing. These functions are already naturally elevated in the adolescent developmental period, due to inherent increased escalation of self-examination and focus within this neurodevelopmental stretch, requisite to the development of the emerging “self” that occurs within this window (Davey et al., 2019). Recent studies are increasingly reflecting the salient role of DMN response – particularly in these self-referential cognitive skills (e.g., theory-of-mind, episodic memory, prospection), factors integral to social development, mentalizing, and social exchanges; factors highly relevant to the capacity to perform impactfully within a therapeutic exchange (Washington and VanMeter, 2015). The same factors relevant in adolescents’ self-examination and self-scrutiny may undergird parallel neurocognitive processes that adolescents are engaging in behavioral treatments, and that when successfully deployed, lead to successful behavior change in the context of problem drinking.
Further, precuneus continues to emerge as an important region within a central network in adolescent behavioral treatment response, particularly in the context of MI-based interventions (Feldstein Ewing and Chung, 2019, Feldstein Ewing et al., 2013, Grodin et al., 2019). And, also interesting in terms of outcomes observed here, the posterior cingulate cortex (PCC) is being recognized for its probable role in positive response to BAM -based approaches (Brewer and Garrison, 2014, DeWitt et al., 2015).
Potentially due to its role in orienting and attention (Japee et al., 2015), MFG is emerging as a highly relevant region in the treatment context. Outside of addiction, treatment approaches have been observing the salient contribution of MFG response to treatment processing and outcomes in both BAM and cognitive behavioral therapy when utilized to treat distress (Du et al., 2016, Taren et al., 2017). Less well-examined in adolescent addiction treatment, MFG may have a role in adolescent resilience and recovery. Some studies have shown that treatment responders in adolescent depression show greater MFG response over time than youth with more persistent depression (Fischer et al., 2019). With its placement between the superior and inferior frontal sulci, rostral to the precentral gyrus, MFG may truly be a site of “convergence” (Japee et al., 2015). Aligning with its integral role in managing and deploying brain resources to manage attention (e.g., interrupting and reorienting to attentional processing), MFG may be instrumental in the neural connectivity undergirding behavioral treatment participation and success within this age group.
3.7. Clinical implications
These data support the strength of two treatment approaches – MI and BAM - for short-term adolescent behavior change (3 and 6 months), as well as differential improvements of MI over BAM for more long-term (12 month) adolescent addiction treatment outcomes. In terms of adolescent brain mechanisms, this study supports that therapist language congruent with these treatment approaches (complex reflections and mindful) both appear to successfully activate the adolescent brain in the context of behavioral therapy. Further, significant changes in the adolescent brain in response to these therapeutic approaches (particularly across DMN hubs including, but not limited to, precuneus, PCC, MFG), were directly associated with adolescent behavior change. .
While this study is a substantive step in the right direction, we believe that these outcomes generate more questions than answers in terms of next steps, particularly with respect to the configuration of results observed here. To this end, the overall gestalt of these outcomes represents a complicated picture, with some more optimal results for MI elements, overlaid with some more impactful results for BAM elements. Together, this amalgam of brain/behavioral outcomes for this adolescent age group suggest the need for a paradigm shift in adolescent addiction treatment development. Specifically, these data underscore the need to continue hone in on particular types of therapist language and their impact on the developing brain, to inform a step-wise approach to aggregate and organize the most impactful elements of addiction treatment into a novel treatment for adolescents. While the MI and BAM interventions showed treatment gains, stepping back from existing treatments to determine novel avenues to approach adolescent behavior change may be highly valuable to advancing behavior change in this age group (Silvers et al., 2019, Yeager et al., 2017).
3.8. Limitations and future direction
While there are several strengths within this study, our findings should be interpreted with an eye to the following limitations. First, and most importantly, while advances are being made in this direction, it is still not possible to conduct live adolescent addiction behavioral treatments while receiving an MRI; an in vivo, temporal evaluation of these relationships would offer the next step in understanding these data. Second, due to the nature of the design, we were only able to compare two active treatment conditions (MI vs. BAM); thus, at this time, we do not know how these findings might compare with other widely-used interventions for adolescent addiction (e.g., cognitive behavioral therapy). Yet, we believe these data suggest that common elements highlight promise of generalizabilty to other behavioral treatments . Future work will continue to examine novel elements that might represent additioanl important “common factors”. Third, integrative (clinical-neuroimaging) studies, and particularly translational RCTs, are complicated; it is important to note that this is still a highly emergent area where the road has not been well-developed. Fourth, there may be concern that giving youth all three types of statements in the scanner potentially exposes them to both therapeutic strategies, and thus reduces the ability to observe differences between them; in turn, one recommended design would be to only present examples of statements from the therapy assigned with the confrontational statements, as a comparison in both conditions. Fifth, we note that statistical analytic approaches can be quite different across neuroimaging and behavioral (traditional RCT) studies. We offer our approach and methodology as one example for those interested in conducting clinical translational neuroscience in adolescent behavioral treatment. Finally, we acknowledge that it would be impractical to use neuroimaging in real-world treatment contexts; in turn, we do not suggest that all youth should be imaged before receiving outpatient addiction treatment. Rather, we suggest that this approach is useful in that it offers a creative and innovative approach to accelerate discovery; it is precisely the inclusion of the neurocognitive component that makes this study responsive to recent calls to utilize translational approaches to improve adolescent addiction treatment response (Feldstein Ewing et al., 2016c, Gabrieli, 2018).