Abstract
Substance-related disorders are complex psychiatric disorders that are characterized by continued consumption in spite of harmful consequences. Addiction affects various brain networks critically involved in learning, reward, and motivation, as well as inhibitory control. Currently applied therapeutic approaches aim at modification of behavior that ultimately leads to decrease of consumption or abstinence in individuals with substance use disorders. However, traditional treatment methods might benefit from recent neurobiological and cognitive neuroscientific research findings. Novel cognitive-behavioral approaches in the treatment of addictive behavior aim at enhancement of strategies to cope with stressful conditions as well as craving-inducing cues and target erroneous learning mechanisms, including cognitive bias modification, reconsolidation-based interventions, mindfulness-based interventions, virtual-reality-based cue exposure therapy as well as pharmacological augmentation strategies. This review discusses therapeutic strategies that target dysregulated neurocognitive processes associated with the development and maintenance of disordered substance use and may hold promise as effective treatments for substance-related disorders.
Introduction
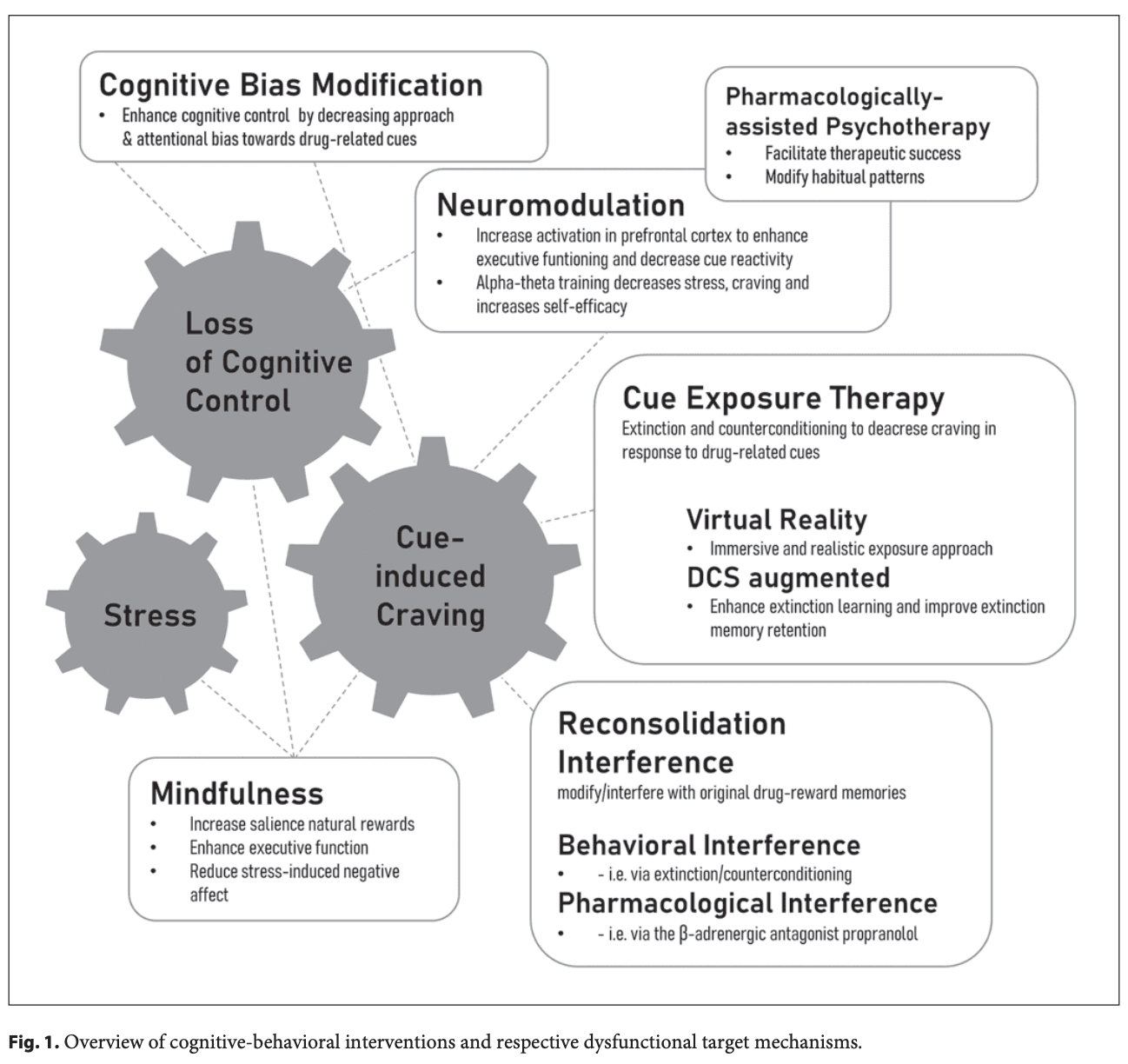
Substance use disorders (SUDs) are characterized by continued harmful consumption of psychoactive substances despite severe adverse consequences for the individual such as clinical and social impairment and persistent relapse over time as well as for society. According to comprehensive research in this field, one hallmark of addictive disorders is an increased salience attribution toward addiction-related stimuli. As stated in this incentive-sensitization theory of addiction, environmental stimuli associated with substances of abuse acquire motivational properties via Pavlovian learning processes and subsequently act as powerful motivators that drive operant alcohol seeking and consumption (i.e., cue reactivity), thereby undermining the goal to stay abstinent. Moreover, these processes are accompanied by aberrant brain responses and functional connectivity within dopaminergic pathways. Although there is a growing body of basic research aiming to inform potential therapeutic approaches, results of interventions are inhomogeneous and complex. The present narrative review aims at giving a broad overview on current results on novel therapeutic approaches for SUD with a focus on cognitive-behavioral content. When reviewing randomized-controlled trial (RCT) studies, we will also report effect sizes where available, in order to guide comparison between treatment approaches. Since stress is known to play a major role in the development and maintenance of SUD, we will introduce mindfulness-based interventions (MBIs) aiming at reducing stress and habitual responding while strengthening goal-directed behavior and salience of natural rewards and thus strengthening self-efficacy. To also reduce habitual responding and to enhance cognitive control in subjects with SUD, so-called cognitive bias modification (CBM) is used to modify automatic cognitive biases. We will moreover introduce virtual-reality-based cue exposure therapy (VR-CET) as well as pharmacologically augmented extinction therapies targeting the aforementioned maladaptive Pavlovian associations via repeated confrontation (exposure) with addiction-related cues without the subsequent reinforcing consumption. Complementary, reconsolidation-based therapies will be introduced; here, the critical reconsolidation window is used to modify cue-drug associations. Besides cue-induced processes, we will subsequently focus on specific neurobiological therapeutic approaches. We will present neuromodulation such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and neurofeedback, the latter mostly in combination with cue exposure. Finally, we will give an overview of new pharmacological approaches, such as psychedelic-assisted psychotherapies (PAPs). For a schematic overview, please see Figure 1. In the field of more intensively researched areas such as CBM and neuromodulation, we will focus on comparative and summarizing work such as meta-analysis and reviews, while we will concentrate on detailed study information for intervention with high novelty such as VR-CET or reconsolidation-based interventions.
Mindfulness-Based Interventions
Mindfulness has been described as a state of consciousness in which one purposefully attends to their experiences, feelings, and perceptions in a noncritical manner. Mindfulness has its conceptual roots in Eastern contemplative tradition and has been shown to be increased with practice of mindfulness meditation. Nowadays, MBIs have been developed and applied in a variety of secular settings, most prominently psychiatric disorders, due to proven positive effects on mood and anxiety. More recently, MBIs tailored to treat a variety of addictive disorders from SUD to behavioral addictions have been introduced. These include, among others, mindfulness-based relapse prevention, mindfulness-oriented recovery enhancement (MORE), and acceptance and commitment therapy. Due to the growing interest in MBIs in the context of addictive disorders, various meta-analyses have validated their efficacy in the context of addiction. Here, effect sizes of the efficacy of craving reduction were OR = 0.72 and d = −0.68, respectively. In this context, the benefit of MBIs in patients with SUDs has been found to be increased in subgroups with higher SUD and affective symptom severity.
Parallel to the growing body of efficacy studies, the underlying cognitive mechanisms, e.g., cue reactivity, cognitive control, negative affect, and stress susceptibility, have been applied in clinical MBI studies. For instance, in tobacco use disorder (TUD) neural cue reactivity was reduced in prefrontal areas as well as in the ventral striatum after 10 weeks of receiving a manualized MBI (MORE). In the former study, a reduction in neural cue reactivity was related to a reduction in cigarettes smoked (d = 0.79). In a sample of opioid users, 8 weeks of treatment with an MBI resulted in decreased self-reported as well as physiological cue reactivity reflected by lower heart rate and increased heart rate variability (HRV) (ηpartial2 = 0.09). This was paralleled by reduced opioid misuse and craving. Neurophysiologically, reductions in late positive potential in response to drug-cues indicate a reduction in cue reactivity in another cohort of opioid users after MBI treatment (ηpartial2 = 0.12). In addition, these groups of opioid misusers displayed enhanced reactivity toward natural rewards after treatment (ηpartial2 = 0.16). As drug cues gain salience over natural rewards during the course of addictive disorders, this indicates that craving and substance use might be reduced through shifting valuation from drug-related rewards back to natural rewards.As addictive disorders are often accompanied by a decrease of executive control that further ameliorates the manifestation of craving and relapse, it was found that MBIs affect clinical outcome through enhancement of top-down control. For instance, a brief MBI led to P3 amplitude decreases in a Go/No-Go task in smokers. The authors link this proposed decrease in effort to inhibit responses to positive clinical outcomes in consumption measures.
In a cohort of subjects with TUD, implementation of an MBI led to increased neural activation in anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) which form part of a cognitive control network. This increase in turn was associated with improved emotional regulation. An increase of reflected decision-making as well as goal-directed control and working memory function were seen in polysubstance users post-MBI intervention. MORE has been further researched in cohorts of patients with chronic pain treated with prescription opioids at risk for misuse. Here, improvement in executive functions such as decreased errors of commission in a pain-related Go/No-Go task when faced with pain-related distractors was seen (ηpartial2 = 0.07). It was furthermore seen that drug-related attentional bias was decreased by MORE (d = 0.75) as well as that HRV during meditation was increased after reception of MORE, which finally correlated with reduced drug intake post-intervention (d = 1.07). Prefrontal theta power increases after MORE treatment which was related to the observation of reduced opioid intake. Withal, MORE led to reductions in attentional bias in patients with alcohol use disorder (AUD).
With negative affect and stress being directly linked to substance use as well as behavioral addictions, MBIs’ effects on these domains have been investigated in addiction populations as well. For instance, in a group of patients with SUD, increased HRV as well as lower anxiety and stress reactivity was found after MBI treatment (ηpartial2 = 0.3–0.4). In a TUD cohort, similarly, changes in subjective stress reactivity but not vagal control indicated by HRV were found after a brief MBI. In opioid-treated chronic pain patients, MORE increased positive affect and reduced stress (OR = 2.75), which was related to reduced craving as well as misuse of opioids after treatment. Reductions in stress (d = −0.77) also mediated substance use (d = −0.58) after undergoing MBI in patients with SUD. In line with this, a brief MBI in AUD subjects, stress-induced negative mood (ηpartial2 = 0.41) as well as alcohol seeking was decreased (ηpartial2 = 0.02), while subjective mood improved (ηpartial2 = 0.07). Moreover, an fMRI study suggested that lower stress reactivity following an MBI was associated with decreases in amygdala and anterior/mid-insula activation.
Conclusion
The research conducted thus far indicates the efficacy of MBIs in the field of addiction, while underlying cognitive mechanisms such as increased cognitive control have been suggested to impinge treatment success. Across the limited body of research that considered adverse events caused by MBIs, the treatment method is considered relatively safe. While acceptance and MBIs have been rated as high, some issues regarding treatment retention have been reported and it has been suggested to assess clients on motivation to sustain engagement in the intervention.
Cognitive Bias Modification
The term “cognitive bias modification” (CBM) summarizes different approaches which aim at enhancing cognitive control in subjects with different mental health issues by modifying so-called cognitive biases. In recent years, evidence from experimental psychology also led to a better understanding of unconscious and automatic responses underlying addictive behavior in subjects with SUD. Such cognitive alterations are referred to as attentional and approach biases. Concretely, attentional bias means the tendency for attention to be allocated toward substance-related cues, while approach bias refers to automatic behavioral tendencies when being confronted with substance-related cues. One assumes that most psychotherapy approaches target mainly more conscious aspects of cognitive control and therefore do not affect more automatic processes like cognitive biases. Thus, in the field of treatment of subjects with SUD, existing evidence on cognitive biases in SUD is translated into interventions and trainings which are intended to decrease approach and/or attentional bias toward drug-related cues. Depending on the specific operationalization, a distinction is made between attentional bias interventions and approach bias modification.As an example, a CBM intervention as used in the context of SUD should be described here in more detail. CBM interventions are intended to modify an approach toward, e.g., alcohol-related cues by pairing such cues with a motor avoidance response, i.e., pushing a joystick, in contrast to other (soft) drinks. Training sessions may vary in frequency and duration. Further, sham training with equal proportions of approach-avoidance responses is used as control condition. Thus, subjects with SUD can retrain approach-avoidance tendencies in a self-administered computerized training. As an example, by using such a training, CBM has been shown to reduce alcohol relapse rates up to 1 year after training measured with moderate effect sizes compared to sham training and no training (ηpartial2 = 0.09). In line with this, a more recent CBM study found that training in comparison with a sham intervention significantly increased abstinence rates in AUD patients.
Recent reviews and meta-analyses focusing on the effectiveness of bias modification training in SUD came to mixed conclusions. In their meta-analysis, Cristea et al. focused on RCTs of CBM interventions in SUD and included 18 RCTs for problems related to alcohol and seven RCTs for smoking behavior. This meta-analysis found no significant effects of CBM interventions on addiction symptoms in general (g = 0.08), nor on craving (g = 0.05) as a specific characteristic. Nevertheless, there was a moderate significant effect of CBM interventions on the extent of cognitive bias itself (g = 0.60). Further, a small significant effect on addiction outcomes during follow-up measures, as reported by a subgroup of seven studies, was observed (g = 0.18). Based on this and also on further methodological analyses, the authors concluded that the clinical utility of CBM interventions in SUD has to be doubted and methodologically improved trials are necessary to conclude on this issue.Consequently, Boffo et al. expanded the work by Cristea et al. They conducted a Bayesian meta-analysis of 14 studies with a total of 2,435 participants on CBM interventions in subjects with AUD and TUD. Analyses of outcomes included alterations in cognitive biases, in substance use as well as relapse rates (short and long term). No effects on reduction on substance use were seen (θ = 0.05) but small significant effects of CBM interventions on cognitive bias (θ = 0.10) and relapse rates (θ = 0.28). When including moderating covariates such as severity of substance use, type of SUD, or number of completed training trials, no effect on the outcomes was seen. These authors also doubted the reliability of the so far available evidence. They concluded that research in this field would benefit from more high-standard RCTs using shared protocols of CBM interventions.Besides the above-reported meta-analyses, systematic reviews focused on attentional bias modification (ABM) as a specific form of CBM. For example, Heitmann et al. included and reported on 18 studies on ABM in SUD (nine studies on alcohol, six on nicotine, and three on opiate use). As already criticized by Boffo et al., the reported studies differed regarding relevant variables, like type and frequency of ABM interventions and outcome measures as with regard to the reported results of ABM interventions on addiction symptoms. Therefore, no clear conclusions regarding the effectiveness of ABM interventions could be drawn thus far.With a similar intention, Zhang et al. provided a systematic review on ABM, but with a focus on subjects with the use of stimulants, cannabis, and opioids. The authors reported on six randomized trials highlighting the relevance of attentional biases in opioid use disorder (OUD) and cocaine use disorder (CUD), but they did not perform further analyses of secondary outcome measures due to methodological limitations. Despite the controversial findings regarding the clinical utility of CBM in SUD, studies have been undertaken to understand its neural correlates and related brain functional alterations after the intervention. This is also related to the fact that previous studies showed that the effect of a CBM intervention, like described above, on alcohol relapse rates is mediated by its effectiveness in building an alcohol avoidance bias, while changes in executive functions (Stroop task training) did not function as a mediator. To further investigate the underlying cause of interindividual differences in training success, various predictors such as addiction severity, psychopathology, or demographics were examined. Results demonstrated that patients profited most from CBM with increased age. To extend these findings, Eberl et al. further investigated the effect of training sessions on treatment success and concluded that optimal intervention effects were seen after six sessions. Since action tendencies and executive functions are known to be associated with different functional systems in the brain, this evidence suggests that distinct mechanisms on neuronal level may play a role here. Therefore, Wiers et al. conducted two fMRI studies in order to measure the neural effects of a 3-week CBM intervention (six sessions) in subjects with AUD. The authors reported a significantly reduced activation in the mPFC post-training related to bias modification. At the same time, the training showed no effects on the nucleus accumbens, as a reward-related region in the brain. Instead, the CBM intervention diminished alcohol-related cue reactivity in the amygdala which was correlated with self-reported craving. Taken together, the neuroimaging evidence suggests alterations in the motivational system of the brain underlying the effects of CBM interventions, but further studies are needed for a better understanding.
Conclusion
As seen above, different studies confirm the relevance of cognitive biases in the development and maintenance of SUD. Further, several studies focused on the effectiveness of bias modification interventions of different kinds in substance-using individuals. Structured reviews as well as meta-analyses came to mixed results, and therefore, authors recommended generating more extensive evidence of higher methodological quality before drawing final conclusions. Notably, CBM interventions and its application to subjects with SUD are not without challenges. For example, it has been observed that the existence and magnitude of cognitive biases show a high interindividual variability and CBM interventions are highly dependent on the selection of the appropriate task for bias assessment and modification.
Most recent studies tried to overcome previous methodological limitations and increasingly aim at applying CBM interventions in more naturalistic settings, e.g., home-delivered and Internet-based. For example, Heitmann et al. used an ABM intervention and investigated its effectiveness in a home-delivered, multi-session, Internet-based form as an add-on to treatment-as-usual (TAU) in subjects with AUD or CUD and found no significant effects on attentional bias nor on substance use, craving, relapse rates, or other complaints. Other studies on the development and validation of app-based CBM interventions are ongoing. In general, CBM has been considered as cost-effective, easy to implement, and relatively safe. However, adverse events due to exposure to alcohol-related cues during withdrawal should be monitored. Thus, while considered a tolerable, low-cost intervention, evidence on CBM interventions in SUD all in all remains controversial.
Virtual-Reality-Based Cue Exposure Therapy
Repeated confrontation (exposure) with addiction-related cues without the subsequent reinforcing consumption has been applied to reduce the association between the drug and the cue and thus to reduce craving. This so-called CET is believed to utilize the well-established principle of habituation, i.e., the decrease of psychobiological responses to substance-related stimuli via repeated presentation as well as the development of alternative behavioral strategies in high-risk situations potentially leading to relapse. Although there have been some promising results, CET is not a routine part of clinical care and its efficacy has been called into question. One reason could be lacking feasibility within the context of psychotherapeutic practice (i.e., immense time and organizational effort; high costs) as well as lacking transferability of confrontation with drugs of abuse within laboratory settings to daily life encounters in natural settings. One promising approach to successfully deal with these pitfalls is the so-called VR-CET, i.e., the confrontation with addiction-related stimuli within a realistic VR environment. Originally, VR-based interventions have been developed for specific phobia in the 1990s, but since then have continuously been adapted to other psychiatric conditions such as other anxiety disorders, posttraumatic stress as well as SUD.
VR Techniques
There are four key elements defining the operating principles of VR: (1) the Virtual World as an imaginary space with objects and rules governing these objects; (2) (Mental) Immersion as a state of being deeply engaged into this alternative reality/environment; (3) Sensory Feedback based on the physical positions of the participants within the VR environment; and (4) Interactivity as the ability of the participant to interact with the computer-based world. Here, “cybersickness” (i.e., nausea, headaches, disorientation, and tiredness, typically experienced by stationary users that perceive that they are moving in a virtual scene) is the most common side effect of VR simulation. Besides this factor, VR-CET is safe and is accompanied by high patients’ compliance: in a study with a focus on specific phobias, 76% of all patients preferred a VR-based exposure compared to in vivo exposure. An online survey on the public opinion on VR technique application via a social media platform showed mostly positive perceptions (ca. 75% of all given comments) about the use of VR in a wide range of health care settings. To date, the application of head-mounted displays with motion tracking is the most common medium of VR with the advantage of isolating the user from all external visual stimuli while reducing costs for other expensive equipment such as 3D walls, etc.. The majority of VR environments focus on visual simulations, but there are also multisensory VR environments available with auditory and haptic or even olfactory and gustatory stimuli.
VR-Based Approaches Focusing on Craving
Up to now, there is a wide range of studies probing VR to elicit and assess craving as one of the core symptoms of addictive disorders. Here, as a first step, the evaluation of potentially relevant cues via interviews or questionnaires is of importance: early studies in AUD incorporated visual, auditory, and olfactory stimuli in a VR environment to evoke the urge to drink: Bordnick et al.; Ryan et al.; and Ghita et al. determined relevant contextual triggers of alcohol craving, including situations (e.g., being in a restaurant, bar, or pub; being at a party or at home) as well as emotional states (e.g., sadness, stress, frustration, anxiety, tension, or irritability). Here, the role of social pressure as a possible moderator might be of relevance: Cho et al. observed significantly higher alcohol craving in situations with an avatar (social pressure) than in situations without an avatar (no social pressure), while Lee et al. showed that patients with AUD reported extremely high levels of craving immediately upon exposure to a virtual environment with alcohol cues, regardless of social pressure. Similar results have been shown in other substances such as nicotine, cannabis, cocaine, and methamphetamine: virtual environments significantly increased the urge to consume the drug in individuals with SUD with and without avatar interactions.
VR-Based Approaches Focusing on Therapy
Up to now, there are only a few studies with rather limited sample sizes focusing on VR implementation in therapeutic approaches, most of them applying VR-CET. In AUD, different studies observed a significant reduction in cue-elicited craving after VR-CET. Lee et al. showed that a series of ten VR-CET sessions compared to the same amount of cognitive-behavioral therapy sessions led to a greater decrease in craving. An interesting cohort study by Hernández-Serrano et al. explored potential predictors of changes within craving for alcohol in patients with AUD: they showed that TAU supplemented with VR-CET compared to TAU only was especially beneficial among patients with intense alcohol craving and individuals having used illicit substances prior to treatment. In different studies using VR-CET in nicotine dependence, a reduction in (cue-induced) craving was shown as well as a reduction in consumption.
Regarding potential underlying neurobiological mechanisms, Hyun et al. observed that VR-CET led to a decrease in craving as well as a decrease in brain metabolism (measured with 18F-fluorodeoxyglucose positron emission tomography) after ten VR-CET sessions compared to baseline in a small sample of alcohol-dependent subjects. This finding is in line with a glucose positron emission tomography study by Son et al.: after VR-CET, alcohol-dependent individuals showed decreased brain metabolism in lentiform nucleus and temporal lobe relative to that at baseline (the latter being heightened compared to healthy individuals).Besides VR-CET, VR is increasingly used to enhance other therapeutic approaches, such as serious games. In a study by Metcalf et al., users were instructed to hit or kick away drug-related cue images (alcohol- and nicotine-related) as they fly toward the user in a four-session intervention. If a user successfully hits the image, it explodes and the user gains points. The authors observed that craving and substance use significantly decreased while self-efficacy increased. Similarly, the group of Girard et al. used a VR game where participants crushed virtual cigarettes via arm motions: although there were no effects after 4 weeks of treatment, they observed significant higher abstinence rates for the cigarettes crushing group at the end of the 12-week program and at a 6-month follow-up.Moreover, VR was used in studies pairing drug-related cues with unpleasant stimuli. Choi and Lee enhanced an imagery-based aversive treatment (i.e., repeatedly pairing heavy drinking with unpleasant aversive stimuli) with VR. Although they used a single treatment session, AUD participants changed explicit, self-reported craving as well as implicit craving (as assessed via alcohol implicit association test, eye-tracking test, and alcohol Stroop test). Regarding methamphetamine dependence, a study by Wang et al. combined VR videos depicting methamphetamine-related cues with VR videos showing adverse consequence caused by methamphetamine use (arrestment; severe bodily conditions; sudden death): they observed a significant decrease in subjective craving and a more negative attitude toward methamphetamine use compared to TAU.Most recently, Mellentin et al. published a study protocol of a RCT to examine the effectiveness of an alcohol-associated approach-avoidance training program (AATP) within a VR environment compared to AATP without VR as well as to TAU in a large sample of AUD individuals. The authors hypothesize that AATP is more effective than TAU and VR AATP more effective than standard AATP. Regarding cannabis and cocaine use, there are currently no studies on VR-based treatment approaches indicating the urgent need for future research.
Conclusion
Taken together, studies up to now suggest that VR is a promising tool for the assessment and treatment of craving among individuals with diverse SUDs. Especially since SUD is associated with different social aspects (i.e., increased consumption and craving under social pressure, VR might be able to mimic socially difficult situations better than in vivo laboratory exposure. VR allows for a number of advantages over in vivo exposure therapy by (1) significantly increasing practicability of exposure therapy, (2) high comparability between different treatment providers, e.g., therapists via structured and standardized stimulation protocols plus adaptation toward individual needs (setting, drug, etc.), (3) safer exposure to cues of illicit substances, and (4) high ecological validity combining the benefits of a laboratory setting with the advantages of a realistic environment.
D-Cycloserine-Augmented Cue Exposure Therapy
As the efficacy of CET for substance use is limited the partial N-methyl-D-aspartate (NMDA) receptor agonist D-cycloserine (DCS) has been proposed as a cognitive enhancer to improve CET outcomes. CET is thought to rely at least partly on the principle of extinction learning, an NMDA receptor-dependent form of learning. DCS binds at the glycine site of the NMDA glutamate receptor, thereby increasing its activation probability. Research has documented NMDA receptor involvement in synaptic plasticity, learning, and memory. In line with that, preclinical models of drug addiction demonstrated that systemic administration or local infusion of DCS in relevant brain structures (i.e., amygdala, hippocampus, ventromedial prefrontal cortex) before or immediately after extinction training enhanced extinction learning of drug-associated cues and improved extinction memory retention. Corroborating preclinical studies showing robust and strong improvement of fear memory extinction under DCS (d = 1.19), meta-analytic investigations from clinical trials of DCS-augmented exposure therapy in various anxiety disorders revealed an overall beneficial effect of DCS, although effect sizes might be rather small (d = 1.19). While open questions about potential moderating factors still remain, the benefit of DCS-augmented exposure therapy might primarily lie in a speeding-up of treatment responses rather than a long-term superiority compared to standard exposure therapy. In contrast to these encouraging findings, clinical trials investigating DCS-augmented CET for SUD yielded quite heterogeneous results.
Santa Ana et al. were the first to investigate DCS-augmented CET for smoking cessation. They found two sessions of DCS-augmented CET to reduce subjective and physiological cue reactivity relative to placebo. Although groups did not differ in reported smoking behavior, expired carbon monoxide in the DCS group was significantly lower at the first of two follow-up sessions (d = 1.1). In line with this, Otto et al. investigated DCS-augmented CET after treatment-seeking TUD patients completed a smoking cessation treatment, showing reduced subjective craving and physiological reactivity to smoking cues (d = 0.8–1.21), but only a tendency of higher abstinence rates at 6-week follow-up in DCS-treated participants relative to placebo. However, a third study found no effect of DCS + CET on cue-induced craving or attentional bias, but a statistical trend indicating DCS might have reduced tonic craving at 2-week follow-up, as assessed in the emotional subscale of the Tobacco Craving Questionnaire.
Watson et al. investigated DCS-augmented CET in recently detoxified AUD patients and found no benefit of DCS relative to placebo on subjective or physiological cue reactivity. However, a substantial portion of patients exhibited no or only very low levels of cue reactivity throughout the sessions, making it difficult to detect a pharmacological augmenting effect. Contrary to this, defining alcohol cue-induced craving as inclusion criterion, DCS has been shown to attenuate subjective craving in the first of four CET sessions (η2partial = 0.13). This reduction was sustained up to a 3-week follow-up period. Moreover, patients receiving DCS drank significantly less and reported viewer drinking days by the end of treatment, although these effects did not sustain until the follow-up period. Corroborating these results, in recently detoxified AUD patients showing neural alcohol cue reactivity before treatment, DCS-augmented CET significantly decreased ventral and dorsal striatal cue reactivity compared to placebo. Two additional studies investigated the effect of DCS in a high-risk population of heavy social drinkers (η2partial = 0.25), suggesting that DCS had no effect on subjective, physiological, or attentional measures of cue reactivity in this population or might even increase short-term craving.
The line of research investigating DCS-augmented cue exposure for CUD reveals a comparably homogenous picture, as DCS failed to attenuate cue-induced craving responses across studies. Intriguingly, some of these studies rather suggest detrimental effects of DCS. Specifically, DCS has been found to impede within-session extinction and enhance cue-induced craving compared to placebo. One neuroimaging study assessed the effect of DCS-augmented CET using a cue reactivity paradigm before and after treatment. While both groups showed significant decreases in brain activation in a variety of frontostriatal regions (i.e., nucleus accumbens, caudate, frontal pole), placebo but not DCS participants also showed reduced blood oxygen level-dependent signal in left angular gyrus, middle temporal gyri, and lateral occipital cortex, indicating more widespread reductions in cue-induced neural signals in the placebo group, although subjective craving did not differ between groups.
Conclusion
Although DCS has been considered a well-tolerated and safe pharmacological adjunct with minimum side effects at low doses, the literature on DCS-augmented CET for SUDs appears rather disillusioning. Preliminary evidence for the usefulness of DCS as a cognitive enhancer for CET stems from trials investigating patients with TUD and AUD. Intriguingly, some studies even suggest detrimental effects of DCS, especially for CUD. As the efficacy of DCS might depend on extinction success, several methodological challenges have been discussed that might explain some of these negative findings. These moderators include insufficient craving reduction by the end of CET sessions or concerns about reconditioning experiences under the influence of DCS between CET sessions in studies that did not control for between-session drug use.
Indeed, studies controlling for between-session sensitization experiences reported positive effects for DCS-augmented CET in patients with AUD and TUD. Moreover, all reported studies suffer from small sample sizes, making it difficult to detect the presumably small effect suggested from clinical trials in anxiety disorders. An individual patient data meta-analysis on DCS-augmented exposure therapy for anxiety disorders related fewer sessions of DCS-augmented exposure (<9) and a short time interval between DCS administration and exposure (60 min or less) to diminished efficacy of this treatment approach, which might potentially also translate to DCS-augmented CET for SUD. Finally, although DCS facilitates appetitive extinction learning in preclinical models and reduced cue-evoked BOLD activation after extinction in a human laboratory study, the mechanism of action of CET is not limited to extinction learning; e.g., higher order cognitive processes like self-control might be even more important for treatment success.
Reconsolidation-Based Interventions
Another strategy aims to interfere with the reconsolidation process of maladaptive learned cue-drug associations, using either pharmacological or behavioral interventions. According to reconsolidation theory, the reactivation of an already consolidated memory renders it into a labile state, in which it is susceptible to change. The process of reconsolidation restabilizes this activated memory, serving as an update mechanism in order to add new information to an already consolidated memory or to strengthen the memory with repeated retrieval. A timed intervention during the critical “reconsolidation window” spanning several hours post-reactivation should be able to directly modify the original cue-drug associations, leading to long-lasting reductions in cue-induced craving. Indeed, preclinical and experimental clinical research on reconsolidation-based interventions for SUD flourished over the last two decades, with quite encouraging results.
Behavioral Reconsolidation Interference
Extinction and counterconditioning are the most often used behavioral interventions that have been provided after a reactivation procedure in order to incorporate new learning into drug-related memories during reconsolidation. In a seminal study, Xue et al. investigated the effectiveness of a retrieval extinction procedure to reduce cue reactivity in inpatient detoxified heroin addicts. A short video sequence with heroin-related content served as the retrieval cue to activate drug-cue memories. Three experimental groups were examined, receiving a 45-min cue exposure session either (a) 10 min after the reactivation (i.e., within the reconsolidation window, (b) 6 h after reactivation (i.e., outside the reconsolidation window), or (c) only cue exposure without former reactivation. Consistent with a reconsolidation update effect, only the retrieval extinction manipulation with a 10-min delay caused a long-lasting attenuation of cue-induced craving and blood pressure, but not heart rate for at least 6 months. This effect has been replicated in nicotine-dependent patients, where a retrieval extinction procedure compared to extinction alone reduced cue-induced craving (d = 0.44) as well as smoking behavior (d = 0.50) and corresponding CO breath levels (d = 0.47) at 1-month follow-up. Importantly, reductions in subjective craving also generalized to novel cues and group differences in cue-induced craving increased over the follow-up period. These results are in line with the idea that, in contrast to standard cue exposure, the retrieval extinction procedure produces long-lasting effects that are less susceptible to Pavlovian relapse phenomena like spontaneous recovery or renewal. Recently, Zandonai et al. showed that a brief VR retrieval extinction manipulation was successful in reducing cue-induced craving in smokers, tested 24 h later.Two studies combined memory reactivation via retrieval cues with counterconditioning in hazardous drinkers. During counterconditioning, drug-cues were reassociated with disgust-inducing outcomes (i.e., disgusting images and bitter tasting drink). Following evidence that a prediction error (PE) is critical for memory destabilization, both studies explicitly manipulated expectancy violations to drink alcohol during retrieval (i.e., the generation of a PE). Here, retrieval with PE in comparison with retrieval without PE led to significant reductions in subjective value (η2partial = 0.09) and attentional bias (η2partial = 0.11) toward alcohol cues at 1-week follow-up. Independent of the PE manipulation, retrieval + counterconditioning has been further associated with long-term reductions in alcohol consumption at 9-month but not 1-week follow-up. However, only in the retrieval with PE group, effectiveness of counterconditioning was associated with reduced reactivity to alcohol cues and actual alcohol consumption.
Besides extinction and counterconditioning, other behavioral interventions have been applied during the proposed reconsolidation window in order to interfere with maladaptive drug-cue memories. In abstinent patients with heroin use disorder (HUD), acute social stress following memory reactivation compared to a control condition significantly impaired free recall of previously learned heroin-related but not neutral words. Likewise, cognitive reappraisal following alcohol-related memory reactivation with PE resulted in reduced verbal fluency for positively valenced alcohol-related words in at-risk drinkers, suggesting that this procedure could alter drug-related semantic networks. Kaag et al. used a working memory task in order to interfere with drug memory reconsolidation in heavy drinkers. Here, unexpectedly, high working memory load before but not after memory reactivation reduced subjective craving at 1-month follow-up, an effect that warrants further investigation. Finally, preliminary evidence suggests that CBM training following drug memory reactivation compared to a control reactivation procedure differentially influenced brain oscillation during resting state EEG in abstinent AUD patients.
Pharmacological Reconsolidation Interference
Considerable evidence from animal addiction models showed that pharmacological reconsolidation blockade by protein synthesis inhibitors, β-adrenergic or NMDA receptor antagonists is efficacious in preventing drug relapse. However, most of the tested substances are not safe for humans. One exception is the β-adrenergic antagonist propranolol. A single session of oral propranolol administration 1 h before a reactivation procedure has been shown to reduce subjective liking of smoking cues (d = 0.57–0.69) as well as cue (d = 0.64) or priming dose (d = 1.15)-induced craving in smokers when tested up to 48-h later, in contrast to placebo + reactivation or propranolol administration 6 h before memory reactivation. In abstinent patients with HUD, propranolol administration before memory reactivation significantly impaired free recall of positively and negatively valenced heroin-related but not neutral words tested 24 h later. Critically, the effect of propranolol was dependent on memory reactivation, as patients receiving propranolol without memory reactivation did not perform differently from the placebo groups. While Saladin et al. support the aforementioned short-term effects of propranolol by showing that one session of propranolol versus placebo administration immediately after memory retrieval reduced subjective as well as physiological cue-induced craving in patients with CUD 24 h later, these effects could not be maintained up to 1-week follow-up, as would be expected under the assumption of reconsolidation blockade. Likewise, two other studies found no beneficial effect of propranolol + memory reactivation on cue reactivity measures in smokers or patients with HUD when tested 1 week later. The latter study further suggested detrimental acute effects of propranolol with increased craving during the intervention session.
So far, only isolated studies investigated alternative reconsolidation blocker other than propranolol in subjects with SUD. However, first promising evidence suggests that post-retrieval intravenous ketamine – an NMDA receptor antagonist – can reduce alcohol craving and consumption in at-risk drinkers up to 9-month follow-up, compared to ketamine alone or placebo infusion post-retrieval. Apart from that, combining memory retrieval with memantine, nitrous oxide gas, or lidocaine revealed null results.
Conclusion
To conclude, targeting drug memory reconsolidation by means of behavioral or pharmacological interventions constitutes a promising avenue for future translational and clinical work. Providing new Pavlovian learning associations (i.e., extinction or counterconditioning) during the reconsolidation window has been shown to result in marked and long-lasting reductions in drug craving and consumption, thereby outperforming the very limited efficacy of stand-alone extinction learning provided by cue exposure or its pharmacological augmentation using DCS. In contrast, studies that used pharmacological interventions like propranolol to interfere with reconsolidation yielded more heterogeneous results, and it remains to be further investigated whether the reported acute beneficial effects are longer lasting. Although based on a small number of studies published thus far (n = 8), a meta-analysis across retrieval interference strategies in (sub)clinical SUD identified interference type (behavioral vs. pharmacological) as a significant moderator, as only behavioral post-retrieval interventions were associated with a significant moderate effect size (g = 0.6), in contrast to pharmacological interference strategies (g = −0.03). However, post-retrieval ketamine infusion has been associated with long-lasting reductions in alcohol consumption and is therefore a promising new candidate worth investigating further. This also matches preclinical evidence showing that NMDA receptor antagonists are more effective reconsolidation blocker than β-adrenergic antagonists. While reconsolidation-based strategies represent a relatively short intervention that could be easily incorporated into a larger treatment protocol, several “boundary conditions” on memory destabilization have been discussed that might limit the clinical applicability of this treatment approach, including the age and strength of drug memories, or the specificity of the applied retrieval cues.
Neuromodulation
As the underlying neural basis of addictive disorders has been vastly studied and altered structures within the brain of patients with SUD have been identified, the field of neuromodulation aims to translate this knowledge into treatment by targeting and modulating impaired brain circuits.
tDCS and TMS
In the field, noninvasive brain stimulation techniques such as tDCS and TMS are safe and accessible methods of modulating brain activity. With tDCS, a continuous weak electrical current (ranging from 0.5 mA to 2 mA) is applied directly to the scalp. Initially, the current modifies resting membrane potentials by depolarization or hyperpolarization and respective increase or decrease of neural activity (depending on an anodal or cathodal stimulation protocol). Eventually, prolonged stimulation enables effects of long-term potentiation/depression. On the other hand, with TMS, distinct electromagnetic induction generates electrical activity in underlying cortical tissue. While single stimulation protocols have been unsuccessful in targeting populations with SUD, repetitive TMS (rTMS) has been more promising. With a stimulating coil, a magnetic field is applied to the skull that induces action potentials in cortical regions. Hereby, stimulation protocols differ between excitatory high-frequency and inhibitory low-frequency rTMS. tDCS and TMS studies in populations with addictions have mostly focused on the dorsolateral prefrontal cortex (DLPFC) for its role in craving and cue reactivity as well as executive functioning such as attention, inhibition, or awareness.
Clinical trials in addiction populations found active stimulation protocols to be related to reduced craving, posttreatment consumption, and relapse rates in populations with various SUDs. A meta-analysis by Song et al. evaluated the effects of DLPFC neuromodulation in SUD and food addiction populations and found that craving was reduced (g = 0.46). The effect was largest in TUD (g = 0.63). When investigating consumption, which was only assessed on TUD populations at this point, a large effect on smoking reduction was revealed (g = 1.14). For both consumption and craving reduction, multiple sessions turned out to be more effective than single-session protocols. A more recent meta-analysis by the same authors aimed at investigating follow-up effects of neuromodulation. In line with the previous analysis, craving (g = 0.73) as well as consumption (g = 0.53) was effectively reduced right after the interventions. Follow-up indicated the sustained efficacy in regard to abstinence levels in all SUD and eating disorder populations (g = 0.70).
When investigating potential moderating factors on the efficacy of neuromodulation interventions, it was found that stimulation of the left DLPFC compared to the right hemisphere pendent was more effective, while an earlier analysis found no difference. This is in line with another meta-analysis that found no difference in efficacy comparing substance type, stimulation sites, current intensities, duration of stimulation, and study design.
To exemplify, for instance, in comparison with TAU, short bilateral DLPFC tDCS as well as sham tDCS in five sessions was associated with reduced craving and enhanced cognitive functioning in AUD patients. However, improved long-term relapse rate was only seen after the active tDCS sessions. In another recent study by Klauss et al., craving was significantly reduced in the real tDCS group after ten sessions (η2partial = 0.23).In a study combining tDCS with ABM, AUD patients received a stimulation or sham protocol during four training sessions. Results indicated no training effect nor an enhancing effect of tDCS on the training. Post hoc logistic-regression indicated a positive trend effect on relapse rates. Some studies did not conclude tDCS treatment to be superior to sham interventions, while generally sample sizes are small and studies lack assessment of long-term effects in addiction.As indicated by various meta-analyses, studies that employ rTMS show some preliminary positive results in the treatment of SUDs as well. In populations with AUD, high-frequency DLPFC stimulation led to reductions in craving after ten sessions (η2 = 0.40) and 15 sessions. Ceccanti et al. found decreased consumption in addition to reduction in craving after ten rTMS sessions in patients with AUD. Intake but not craving was reduced in a small sample after rTMS as well as that dopamine transporter availability was decreased in the striatum as indicated by single-photon emission computerized tomography. Incongruent to these findings, several studies failed to find effects of rTMS on craving and consumption.
Neurofeedback
An additional appealing neuromodulatory approach is neurofeedback, a biofeedback technique that converts recorded neural activity into visual and auditory cues. Consequently, patients can modulate their own brain activity and cognitive processes and behaviors can be altered through training. In light of the deleterious neuropsychological effects related to onset and course of SUDs, neurofeedback has been applied in the context of treatment.
While to date no meta-analytic reviews on this topic exist yet, various studies in populations with SUD have been applying alpha/theta training in combination with variants of a beta-sensory motor rhythm protocol in order to reduce craving, stress, or depression. Most interventions are based on a study by Scott et al. that included patients with mixed SUD that showed better compliance and abstinence rates after training compared to a control group at 12-month follow-up.In one study, patients with AUD received alpha-theta training, a neurofeedback paradigm that aims at amplification of slow-wave bands to ultimately increase well-being and cognitive performance. After twelve sessions of biweekly neurofeedback, the experimental group improved in terms of severity of clinical scales such as the Beck Depression Inventory (BDI) or the Brief Symptom Inventory (BSI). In the same cohort, an improvement of a subscale of the Inventory of Clinical Personality Accentuations (ICP), namely, Avoidant Personality Accentuation, was observed. Furthermore, participants with AUD were subjected to alpha-beta neurofeedback aiming at normalizing a low alpha to high beta wave ratio. Improvement in for example self-efficacy and self-regulation was seen.
Three studies assessed alpha-theta in combination with SMR training in OUD. In 20 patients with OUD, it was found that substance use-related measures such as craving or general psychiatric symptomatology measured by the Symptom Checklist-90-Revised (SCL-90-R) were mitigated along with abnormal EEG functions (g = 0.37). In the same group, restitution of, e.g., depressive, somatic, and general mental health symptoms along with reductions in desire to use opioids, craving, and withdrawal was seen (η2 = 0.26). In addition, a large group (n = 100) of crystal meth-dependent patients undergoing the alpha-theta SMR protocol showed decreased addiction severity and improvements in psychological well-being.
Next to EEG, real-time fMRI as a neurofeedback modality has the advantage of higher spatial resolution, thus enabling training of activity or functional connectivity between regions. A vast amount of interventional studies has been conducted thus far – mostly in populations with TUD. All of them aimed at decreasing craving-related ACC activity in response to drug-cues. For instance, studies showed that training sessions decreased ACC activity, which was correlated to decreased nicotine craving. In contrast, increasing mPFC activity to enhance urge resistance did not yield positive results. In terms of treatment success, it was found that the reduction of craving-related activation was correlated with treatment success in terms of abstinence rates. In extension of previous studies, Kim et al. contrasted ACC activity reduction with additional modulation of connectivity between craving-related regions of interest and found functional connectivity neurofeedback to be superior in efficacy. A recent study also investigated the effect of neurofeedback on reward sensitivity to natural rewards in cocaine users. Here, neurofeedback training improved the ability to activate reward-related regions in response to previously trained rewarding imaginary scenarios.Two studies have been conducted in the context of AUD so far. It was shown that neurofeedback also had an effect on reducing craving-related neural activation in prefrontal areas as well as in ventral striatum.
Conclusion
Considering the relationship between SUD and alterations in cortical functioning, targeting specific prefrontal areas by neuromodulation techniques has shown efficacy in reducing craving, consumption as well as enhancing cognitive functioning. Some results of neuromodulation studies seem promising; however, the sample sizes were very small as well as duration of treatment was short over all reviewed studies. At this point, the lack of studies with adequate sample sizes and methodology produce conflicting results. Concerning the application of tDCS and TMS, the occurrence of side effects, such as seizures, has been extremely rare, available literature is still relatively sparse, and the long-term safety needs to be confirmed. Regarding the well-established safety and tolerability of EEG and fMRI, no concerns regarding long-term consequences have been raised; however, the use of fMRI in particular is very costly.
Pharmacologically Assisted Psychotherapy in SUD
Pharmacological Therapy and CBT
There is approved medication for the relapse prevention of SUD as well as promising off-label medication. Relating to the most frequent use disorder TUD, approved medication includes for example the antidepressant bupropion and the partial nicotine receptor agonist varenicline. With regard to AUD, relapse prevention substances such as the NMDA receptor antagonist acamprosate and the opioid-receptor antagonist naltrexone can be prescribed. In the treatment of SUD, a recent review analyzing more than 30 RCTs recommends adding CBT to a regular pharmacological therapy especially in the treatment of AUD. The authors suggest that while waiting for the medication effects to become apparent, CBT can provide support and skills, enhance treatment adherence, and improve treatment and study retention Combining behavioral therapy and medication does indicate advantage not only in the treatment of AUD but also in OUD and TUD. However, the efficacy of adding psychosocial interventions varied across studies and within types of interventions. Especially looking at CBT, results of these rather small sample-sized studies are heterogeneous. No study could show a clear benefit of combining pharmacotherapy with CBT concerning different outcomes such as treatment retention.
Psychedelic-Assisted Psychotherapies
Research on the therapeutic use of psychedelics started in the 1940s after synthetization of the first hallucinogenic substance, lysergic acid dimethylamine. Already back then, it was used as an adjunctive psychotherapy medication and served for studies on the nature of psychoses.Research was halted by regulatory restrictions in the 1970s and has been resumed in the last 20 years in the context of PAPs. PAPs combine psychotherapy and new pharmacological treatment approaches and have been given rising attention especially in the treatment of affective disorders in the past decade. By now, some studies on their benefit in the treatment of SUD exist. Psychedelic substances include the glutamate receptor agonist ketamine, the entactogen MDMA as well as serotonergic substances known as “classical psychedelics,” such as LSD, psilocybin, and dimethyltryptamine (DMT). Nowadays, the most studied psychedelic substance is ketamine, followed by MDMA, and psilocybin. PAP usually includes a preparatory session, a medication session in a comfortable room with music and an integration session. Frequency and type of therapy vary across studies, and studies often integrate elements from different psychotherapeutic approaches. All elements in combination – the psychedelic drug itself, the PAP experience, and in this the drug-facilitated enhancements in the therapeutic alliance – are suggested to promote the therapeutic effect.
Although their exact neurochemical modes of action remain unknown, evidence suggests that psychedelics increase the expression of brain-derived neurotrophic factor, facilitating neuromodulation. Ketamine exerts a neuromodulatory effect by induction of synaptogenesis and could potentially improve adaptation of learned behavioral patterns. Moreover, psychedelics’ effect on neural network communication seems to play an important role. It has been reported that “classical psychedelics” decrease communication within neural networks and increase communication between them, leading to enhanced global brain connectivity. Also, they are proposed to relax the precision of pathological overweight priors and beliefs. Psychedelic substances are described to cause powerful subjective experiences and altered states of consciousness. These possible mechanisms may allow psychedelics to change narrow mental states with inflexible habits of thought and behavior. Doing so, they are presumed to facilitate psychotherapeutic interventions according to their catalyzation and augmentation, attaining positive long-term mental health consequences. Indeed, not only studies on mood disorders, but also a few studies on SUD describe that even a few treatment sessions may allow for long-lasting therapeutic effects of weeks to months, but further research is clearly necessary.
Ketamine
While ketamine works as anesthetics in high dose, whereas lower doses are used as psychiatric pharmacotherapy. Studies suggest that ketamine has a significant overall antidepressant effect on treatment-resistant depressions in disparate formulations, at least in short-time administration. Evidence from preclinical studies shows that acute administration also reduces alcohol intake in rodents. In humans with cocaine use, ketamine leads to a reduction of craving, a rising motivation to quit cocaine, and decreased rates in cocaine use. Furthermore, ketamine-assisted psychotherapy indicates to increase abstinence rates as well as suppress physiologic response to withdrawal in OUD, while reducing withdrawal-related benzodiazepine requirements in AUD. Of note, these studies are limited either because of limited sample sizes, homogeneous populations, short follow-up periods or due to lack of placebo control. Nevertheless, promising studies are ongoing. In a recent randomized-controlled pilot study, single ketamine infusion was found to improve measures of drinking in persons with alcohol dependence engaged in motivational enhancement therapy. Finally, ketamine’s addictive potential should be considered in SUD populations especially; however, lower dosages are usually not seen as producing stronger psychomimetic effects that might be responsible for the rewarding and addictive effect.
“Classical Psychedelics” and MDMA
Data from studies on the effect of “classical psychedelics” on SUD are still very limited. A meta-analysis of six relevant RCTs evaluated the clinical efficacy of LSD in AUD and found small to moderate effects (OR = 1.96). The authors found evidence that a single dose of LSD has a significant beneficial effect on alcohol misuse in the short term (2–3 months posttreatment) and medium term (6 months posttreatment), which was not statistically significant in the long term (12 months posttreatment). Among the three trials that reported maintained abstinence from alcohol use, the authors describe a beneficial effect of LSD at the first and second follow-up (1–3 months posttreatment), but not at medium-term follow-up (6 months posttreatment). In five of the six trials, a total of eight participants reported acute adverse reactions without lasting harmful effects. In more recent literature, psilocybin is promoted as a safe, feasible, and potentially efficient drug in the treatment of TUD. In an open-label trial, 10 patients with AUD received moderate to high dosages of psilocybin during a 12-week manualized psychotherapeutic intervention. After 4 weeks, patients showed significant reductions in drinking days and heavy drinking days and these reductions were largely maintained throughout the study until the last follow-up, 32 weeks after their first psilocybin treatment. Although to date no well-designed studies have yet been published, some promising preliminary findings on the therapeutic use of DMT in SUD exist as well. Thomas et al. evaluated the impact of the administration of DMT-containing ayahuasca broth in group therapy for problematic substance use and stress. Statistically significant increases were seen in hopefulness, empowerment, mindfulness, quality of life measures, while self-reported alcohol, tobacco, and cocaine use decreased.
Concerning the use of MDMA in the treatment of AUD, evidence (so far) is limited to the support of its safety and tolerability. However, MDMA has risen prominence in the past years in the treatment for social phobia in autistic adults and posttraumatic stress disorder: only recently, it has been shown to be highly efficacious in individuals with severe posttraumatic stress disorder.
Conclusion
Adding psychotherapy to a regular pharmacological therapy in SUD may provide support and skills and is recommended to improve treatment outcomes and treatment retention. In recent years, interest in PAP has resumed, representing a treatment approach which is unique as pharmacological and psychotherapeutic dimensions are combined and closely interrelated to each other. While there is some evidence on a beneficial use of PAP in the therapy of affective disorders, a wider transfer to its use to treat SUD is suggested. In this field, most evidence exists on the use of LSD and psilocybin. Evidence on other psychedelics’ use in the treatment of SUD is still very limited. Furthermore, established as physiologically safe, there are psychological and psychiatric side effects of psychedelics. Most commonly, they include challenging experiences during the session, while the risk of prolonged psychoses is very low. Although their use could already be evaluated as safe and tolerable, randomized-controlled studies with greater samples on the efficacy of PAP in SUD are still needed.
Overall Conclusion
As demonstrated in this review of the current state of novel cognitive-behavioral treatments in SUD, there are several different emerging approaches in this field including MBIs, CBM interventions, neuromodulation techniques, virtual-reality-based and pharmacologically enhanced cue exposure as well as reconsolidation-based interventions. With regard to MBIs, it is possible to conclude so far that a good efficacy of MBIs in the field of addiction can be assumed. Nevertheless, the underlying mechanisms are not yet clear. An increase in cognitive control might be one relevant factor here, an aspect that is also targeted by CBM interventions. To date, several studies assessed the effectiveness of different bias modification interventions in SUD, but also meta-analyses were not able to draw clear conclusions so far, especially due to methodological limitations of the available studies. Main future perspectives in this field are the improvement of available evidence as well as the development of Internet/app-based applications. Also different neuromodulatory interventions are discussed including tDCS, rTMS, and neurofeedback. Based on the neurobiological knowledge on alterations in cortical functioning of specific prefrontal and limbic areas in SUD, such techniques showed some efficacy in reducing craving, consumption as well as enhancing cognitive functioning. Although promising, the results do not yet justify neuromodulation as a standard treatment option for patients with SUD given the methodological constraints of the available literature. One of the most promising tools with high clinical relevance is the assessment and treatment of craving among individuals with different SUD via VR settings. Given a number of advantages over in vivo exposure therapy and some positive preliminary findings, further research is needed to determine the specific relevant variables in VR-based interventions in SUD and to foster translation into patient care. Finally, various pharmacologically augmented interventions are discussed for SUD with mixed results. While there is little clear evidence on the usability of DCS-augmented CET for SUD, targeting drug-related memory reconsolidation by means of behavioral or pharmacological interventions seems to be an encouraging approach for future translational and clinical studies. Further, studies on PAP are ongoing with unclear results for the application in subjects with SUD.Notably, digital technology is gaining momentum as a powerful tool to deliver interventions in a personalized, accessible, and engaging format. On the one hand, digital interventions have been designed to deliver evidence-based therapeutic interventions as stand-alone or supplementary treatment to effectively reduce substance use outcomes. On the other hand, digital phenotyping technology has been fundamental in advancing personalized care in a mental health setting: dense data collection in real-life settings is used to predict relapse risk and monitor clinical symptoms to deliver specific interventions. In light of the suspension of in-person treatment due to the COVID-19 pandemic, it could be vital to expand clinical support to accessible digital platforms.One important and open point of discussion is the differentiation of the described treatment approaches regarding the present type of substance. Although the discussed treatment approaches aim at providing clinically evident improvement across addictions, research about drug-specific mechanisms of action would be recommendable. Up to date, there is no detailed information given that could have been taken account of in this review. Taken together, the literature on novel cognitive-behavioral treatments in subjects with SUD so far appears rather disillusioning, although there is a rocky road ahead for more high-quality research projects on the most promising candidates.