Abstract
Substance use disorder (SUD) is common in psychiatric patients and has a negative impact on health and well-being. However, SUD often goes untreated, and there is a need for psychiatrists, of all specialties, to address this pervasive clinical problem. In this review, the authors’ goal is to provide a resource that describes treatments for SUD, using neuroscience as a framework. They discuss the effect of pharmacotherapy on craving, intoxication, and withdrawal and its ability to interrupt the cycle of substance use in SUD. The neuroscience of stress is reviewed, including medications targeting neurotransmitter systems activated by alarm and fear. Neuroplasticity and promising treatments that use this mechanism, including ketamine, psilocybin, and transcranial magnetic stimulation (TMS), are discussed. The authors conclude by listing resources and practice guidelines for physicians interested in learning more about treatments for SUD.
Habits define many of our actions, whether good, bad, or neutral. Habitual behavior frees up cognitive resources, allowing novel or complex situations to be evaluated while the networks in charge of our routines function in the background. However, habits can also impede the ability to change patterns of behavior when there is a need to adapt.
A key feature of substance use disorder (SUD) is the difficulty patients face in modifying their habitual substance use, even as circumstances change, and their use leads to harm or worsening health. Symptoms that elicit habitual substance use—and impede recovery—include craving, withdrawal, and stress. Research shows that these factors involve a range of brain regions and neurotransmitter systems. Thus, understanding the neurocircuitry of SUD has the potential to improve patient outcomes.
In this review, we focus on the neuroscience of SUD and the impact of treatment on patients’ ability to change, including medication, psychotherapy, and targeted approaches to stress. We also review the neuroscience of plasticity and the ability of neural networks to modify their structure and connections as they adapt to a changing environment. This discussion includes interventions using ketamine, psilocybin, and transcranial magnetic stimulation (TMS), which activate neuroplastic mechanisms.
Studies show that SUD is common among patients with psychiatric disorders, including major depression, attention deficit hyperactivity disorder, posttraumatic stress disorder, anxiety disorders, and more. Thus, our goal is to provide a resource for psychiatrists who wish to integrate SUD treatment into their practice, since combining neuroscience with clinical expertise increases access to evidence-based treatments for patients in need.
THE IMPACT OF TREATMENT ON SUBSTANCE USE AND CHANGE
In animal studies, habitual behavior can be defined as actions that result from extensive training and become autonomous (independent of outcome value). However, we use “habit” to denote a repeated pattern of behavior with an expected result, for better or worse, such as eating healthily or excessive cell phone use. The desire to adopt healthy habits, and to shed harmful ones, is a common human condition—including among people grappling with SUD. In this section, we review the neurocircuitry of SUD and explain how treatment can help patients reach their goals.
Habitual Behavior, Cognitive Control, and SUD
The psychoactive effects of substances, such as euphoria, intoxication, and stress relief, increase the likelihood of developing a habit. In the setting of rewards, habitual behavior tends to form quickly, since an individual is likely to repeat the actions that lead to pleasure. Similarly, behaviors that reduce negative experiences (such as stress) can develop into a habit. In SUD, both the positive effects of substance use and the avoidance of negative effects contribute to the development of habitual use.
The crux of the habit network is the striatum, which underlies the shift from experience-based learning to habitual behavior. In the setting of repeated substance use, increased involvement of the habit network contributes to the emergence of drug-seeking behavior and problematic use. In SUD, imaging studies show that altered signaling in the striatum is associated with the severity of illness and difficulty responding to treatment.
Cognitive control refers to the mental processes that represent goals and the actions needed to reach these goals, such as the ability to learn and adapt. To enact change, the networks regulating cognitive control must modify patterns of brain activity and select the behavior that best meets the demands of the environment. Substance use that persists, despite the need to adapt, can be viewed as an imbalance between cognitive control and habitual behavior.
The prefrontal cortex (PFC) serves as a hub for cognitive control, and imaging studies show that SUD is associated with altered signaling in this brain region. Zilverstand et al. showed that the PFC engages with greater activation in response to drug-related cues compared with non-drug stimuli in SUD. Additionally, blunted activity in the PFC is associated with less impulse control and relapse in participants with SUD. Recent imaging studies have also shown that the PFC plays a role in modulating the experience of craving in SUD.
Encouragingly, imaging studies show that recovery in SUD is associated with improvement in the function and connectivity of the PFC. The duration of remission has an effect: individuals with longer abstinence show increased synchrony (patterns of brain activity that change together) in the PFC and decreased synchrony in subcortical networks (compared to individuals with short-term abstinence). In other words, as patients experience more sustained recovery, the brain regions regulating cognitive control increase their ability to coordinate brain activity compared to regions in charge of habitual behavior.
The Impact of Treatment
SUD is often driven by a cycle of craving, intoxication, and withdrawal. As this pattern continues, a patient’s SUD becomes more severe and ingrained. However, treatment can interrupt this cycle. As shown in Figure 1, pharmacotherapy can shift the balance away from substance use by reducing the impact of craving, inhibiting the positive and reinforcing effects of substances (including intoxication), and alleviating withdrawal (negative reinforcement). In the following section, we review treatments for SUD and their impact on craving, intoxication, and withdrawal.
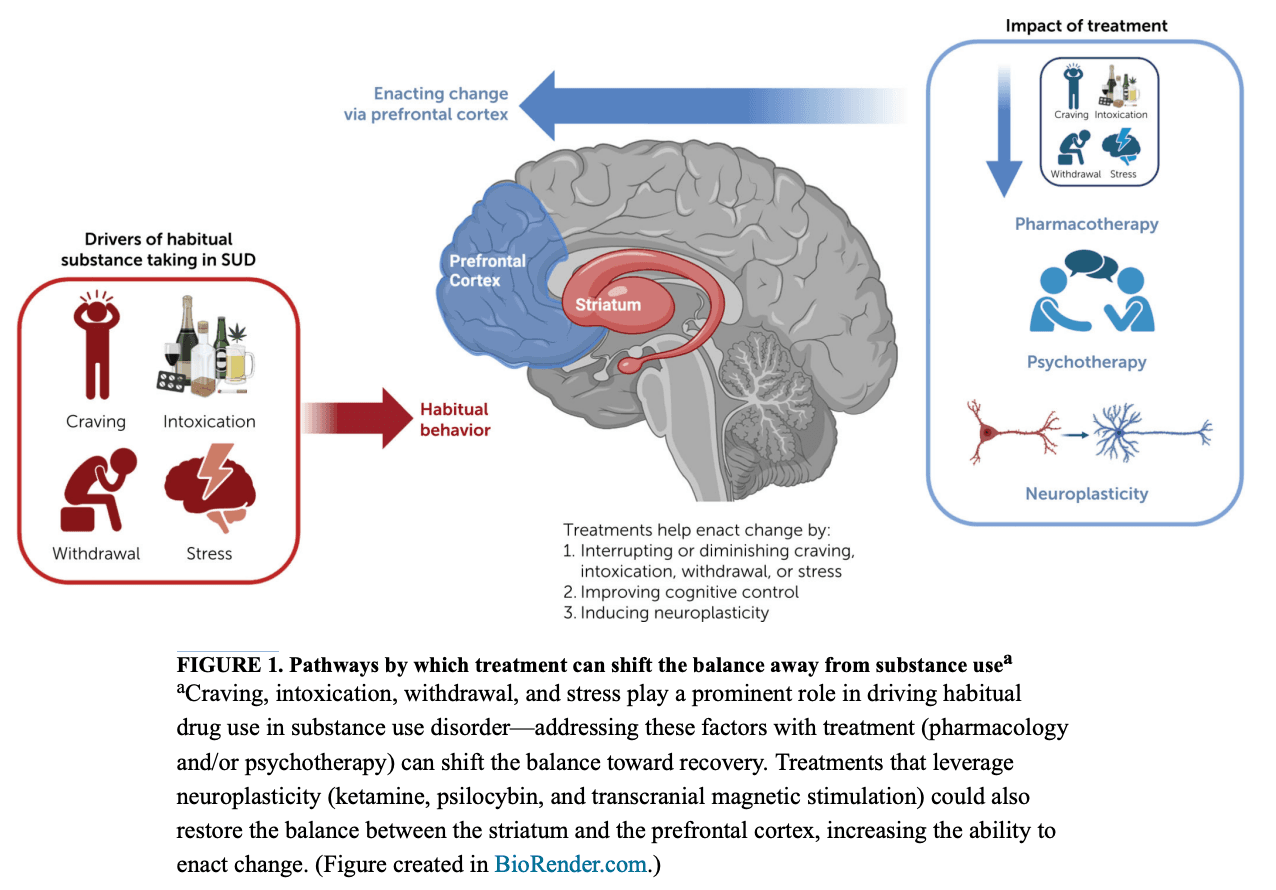
Alcohol use disorder (AUD).
Treatments for AUD include medications approved by the U.S. Food and Drug Administration (FDA) and off-label use of topiramate and gabapentin (Table 1). The FDA-approved medications include naltrexone, an opioid receptor antagonist that reduces craving and inhibits the positive effects of alcohol, which decreases the frequency of heavy drinking; acamprosate, an NMDA receptor modulator that inhibits alcohol-induced craving, reduces the sleep disturbance associated with alcohol withdrawal, and enhances recovery; and disulfiram, which increases acetaldehyde (by inhibiting aldehyde dehydrogenase) and causes diaphoresis, flushing, nausea, vertigo, hypotension, palpitations, and tachycardia when alcohol is consumed. Disulfiram interferes with the intoxicating effects and relies on patients’ ability to control drinking.
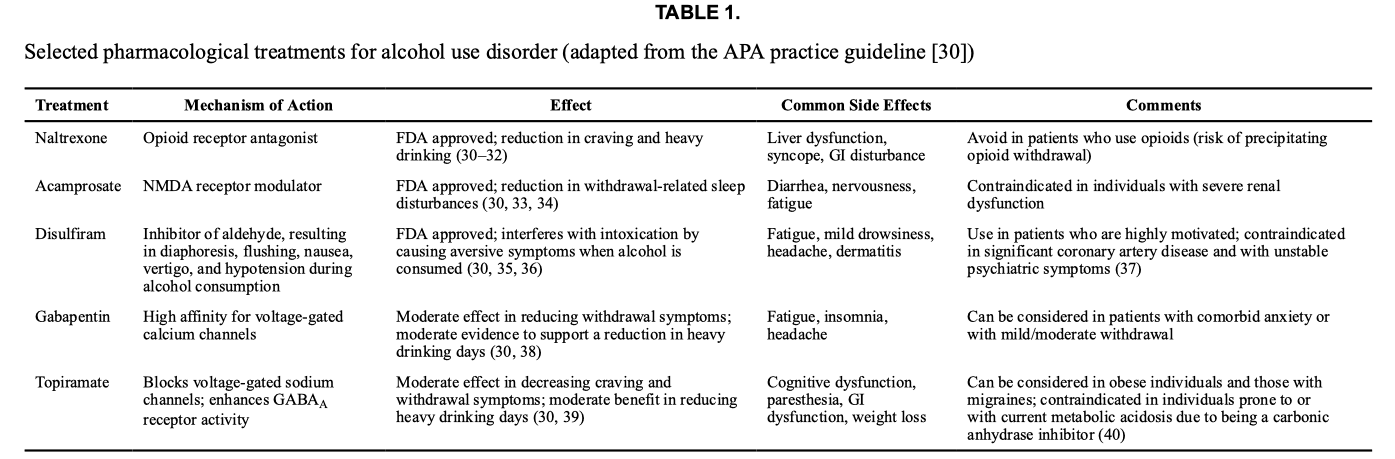
Clinical trials also support the use of topiramate and gabapentin, although these are not FDA approved for AUD. Topiramate decreases craving, frequency of heavy drinking, and symptoms of anxiety and depression during the initial phase of alcohol cessation. Gabapentin also has efficacy for AUD, and research shows that it reduces heavy drinking compared to placebo. A recent randomized controlled trial of gabapentin reported that it has efficacy in patients who experience withdrawal symptoms (even when mild) when they cut down or stop drinking. Additionally, varenicline, a medication typically used for smoking cessation, has shown promise in promoting reductions in heavy drinking days and increases in percentage of days abstinent from alcohol.
Cannabis use disorder.
Although several clinical trials have investigated medications for cannabis use disorder, there remains no FDA-approved pharmacotherapy. Nonetheless, there are some promising options that attenuate craving and withdrawal (Table 2). Medications that reduce craving include naltrexone, bupropion, and modulators of the type 1 cannabinoid receptor (dronabinol and nabilone). In patients with cannabis use disorder, withdrawal is a significant deterrent to stopping or reducing their use, and it can persist for weeks. However, pharmacotherapy can help alleviate withdrawal symptoms (see Table 2).
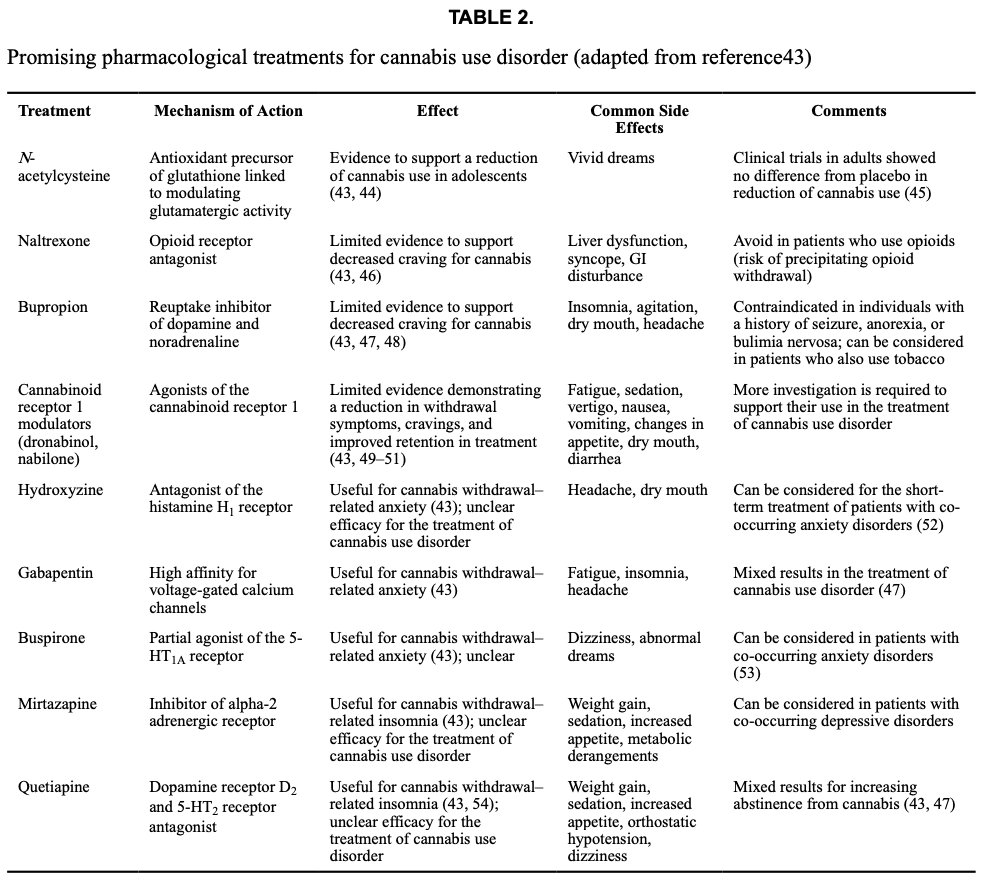
In clinical trials of cannabis use disorder, abstinence from cannabis is the standard outcome measure. However, medications that meet this criterion are lacking. One exception to this is N-acetylcysteine, which has been found to increase abstinence in adolescents, although it is less effective in adults. More recently, there has been a movement toward using a decrease in cannabis use, rather than abstinence, as an endpoint in clinical trials. A secondary analysis showed that three forms of pharmacotherapy (quetiapine, dronabinol, and dronabinol combined with lofexidine) can reduce cannabis use in participants with heavy use (where moderate use replaced heavy use) (see Table 2). Additionally, early studies indicate that cannabidiol (CBD) and nabiximols (a 1:1 concentration of CBD: THC) might produce moderate reductions in use. Taken together, these studies show promise for developing treatments for cannabis use disorder, although definitive clinical trials are warranted.
Opioid use disorder (OUD).
Methadone, buprenorphine, and naltrexone are the first-line FDA-approved treatments for OUD (Table 3). Methadone is a long-lasting opioid receptor agonist that reduces craving and mitigates opioid withdrawal. Buprenorphine, a partial opioid receptor agonist, eases craving and produces less intoxication compared to opioids (like heroin). Naltrexone is an antagonist at opioid receptors that blocks the intoxicating effects of heroin and other opioids, which increases the likelihood of sustained recovery.
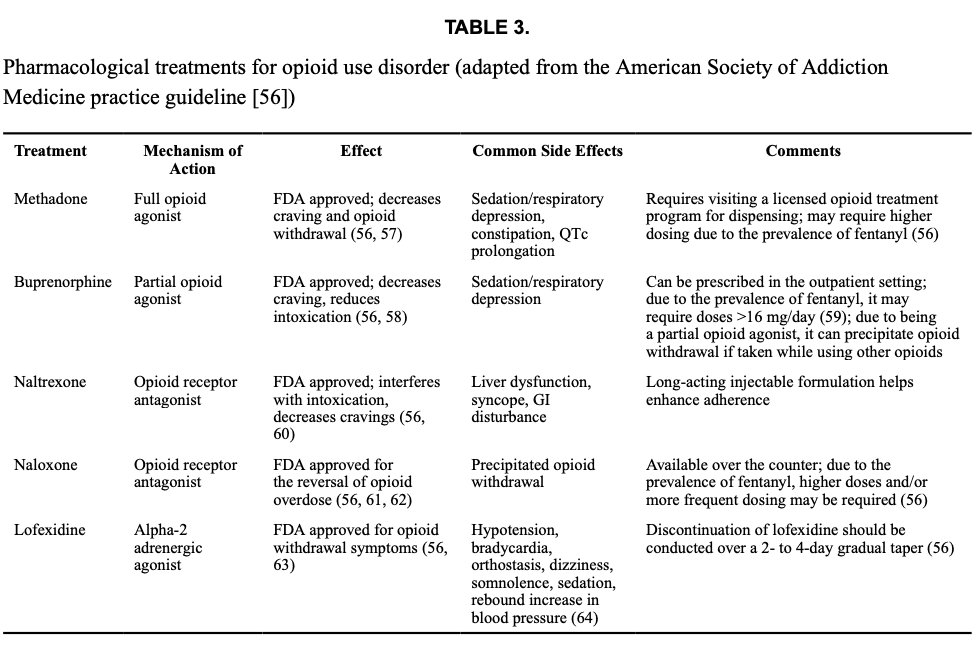
Despite the availability of FDA-approved medications, retention in treatment remains an issue in OUD, often due to withdrawal symptoms that occur as patients begin pharmacotherapy. However, research shows that induction onto medication can be facilitated with clonidine or lofexidine, alpha-2 agonists that reduce opioid withdrawal. Recently, sublingual dexmedetomidine, a selective alpha-2 agonist, has also been shown to effectively reduce opioid withdrawal.
Lastly, given the risk of opioid overdose, current recommendations are for any individuals with OUD (and their close contacts) to be trained in the use of naloxone, which is approved for the reversal of opioid overdose.
Tobacco use disorder.
Approved medications for this disorder include nicotine replacement therapy, varenicline, and bupropion (Table 4). Nicotine replacement therapy can reduce craving, ameliorate withdrawal, and extend periods of recovery. Nicotine products are available over the counter (gum, lozenge, patch) as well as in prescription forms (inhaler, nasal spray). Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, also reduces craving, decreases the reward value of smoking, and lessens withdrawal symptoms. Bupropion is a reuptake inhibitor of dopamine and noradrenaline that also blocks several neuronal nicotinic acetylcholine receptors. Bupropion can attenuate craving and reduce symptoms of anxiety and depression.
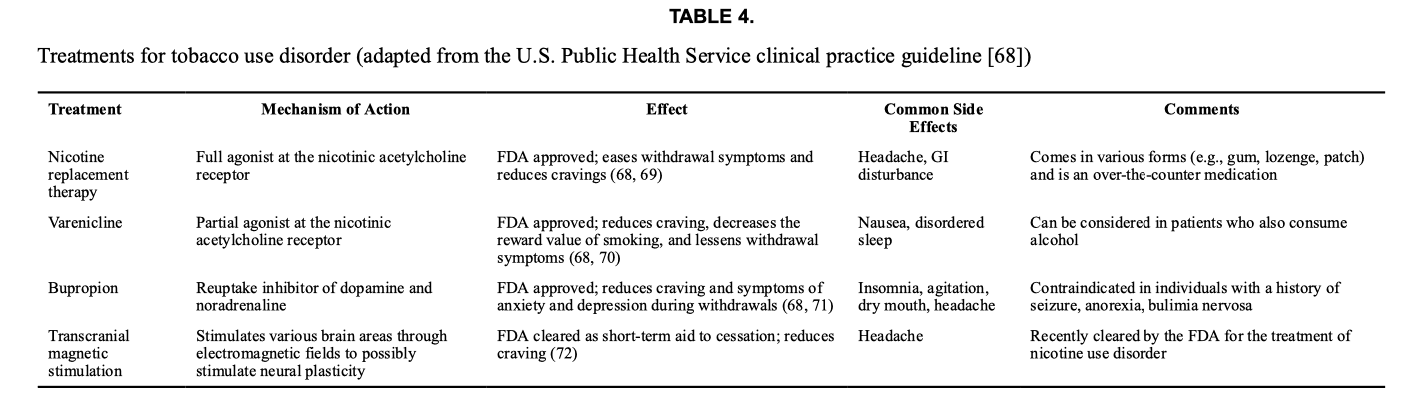
TMS (delivered to the PFC and insula) was recently cleared by the FDA as an aid for short-term smoking cessation. This determination was based on a study showing that TMS (daily for 3 weeks followed by once weekly for 3 weeks) reduced craving and increased quit rates.
In treating tobacco use disorder, combining therapies can be beneficial. For example, the use of medication with individual, group, or telephone counseling (such as 1–800-QUIT-NOW, a free service) increases the chances of success. Combining different types of nicotine replacement (such as a patch and a fast-acting form, like gum or spray) also improves the ability to stop smoking. Additionally, a recent study showed that combining varenicline with TMS (directed to the insula) is more effective than varenicline alone in achieving abstinence from tobacco.
Stimulant use disorder.
Although there are no FDA-approved medications for stimulant use disorder, research shows that pharmacotherapy can be helpful (Table 5). A recent meta-analysis showed that bupropion (a dopamine reuptake inhibitor) and topiramate (a GABAergic medication) moderately increased the rates of abstinence in cocaine use disorder. For stimulant use disorder (involving both amphetamine and methamphetamine) a similar pattern was seen: topiramate and naltrexone had moderate efficacy in decreasing stimulant use. Other medications, such as bupropion, mirtazapine, and riluzole, have mixed results in their ability to treat amphetamine and methamphetamine use disorders.
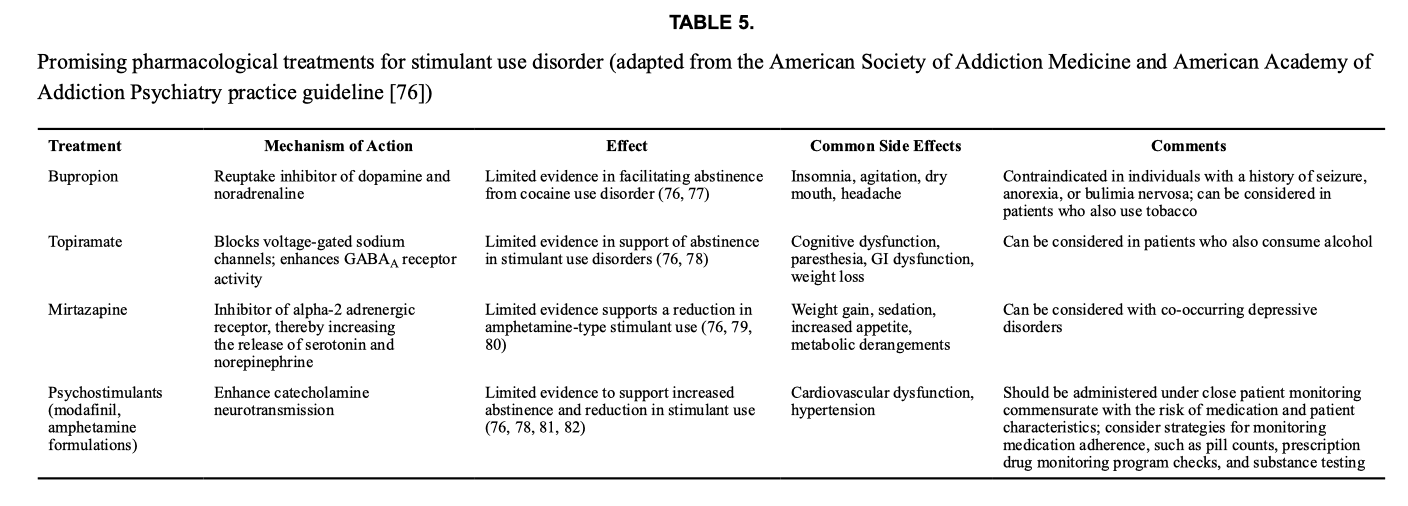
Clinical trials also show that prescription psychostimulants can reduce use in stimulant use disorder, including dexamphetamine, mazindol, methamphetamine, methylphenidate, mixed amphetamine salts, modafinil, and lisdexamfetamine. These medications enhance catecholamine neurotransmission and have yielded increased rates of abstinence and reduced stimulant use. However, it is crucial to carefully weigh the risks and benefits before prescribing psychostimulants due to the potential for misuse.
More recently, combinations of medications have also shown promise in the treatment of stimulant use disorders. In a study of frequent cocaine users, administration of topiramate combined with extended-release mixed amphetamine salts showed increased abstinence rates. In methamphetamine use disorder, the combination of extended-release injectable naltrexone and bupropion was associated with a small but significant increase in abstinence compared to placebo after 12 weeks of treatment.
Behavioral Therapies
Behavioral therapies can be used to treat SUD and can serve to increase motivation, shift behavior, and reduce stress reactivity, as we briefly describe here.
Contingency management/community reinforcement approach.
Contingency management uses positive rewards (like vouchers or gift cards) for abstaining from substance use. It is often combined with the community reinforcement approach, which focuses on positive influences in patients’ lives, like support from family and friends or progress at work or school. This therapy promotes recovery by shifting decision making away from substance use and toward other reinforcers (including money, consumer goods, and/or social connection). Contingency management can also promote goals beyond substance use, such as adhering to treatment and medication for co-occurring disorders. Although contingency management is effective in SUD, it is not often implemented, given issues associated with providing monetary reward for abstinence, including regulations regarding payment to patients.
Motivational enhancement treatment.
This therapy helps patients identify and resolve ambivalence regarding their substance use and the decision to pursue treatment. The components include empathy and understanding that change is difficult, setting goals, identifying sources of ambivalence, and strategizing to enact change. A manual that can be used to deliver motivational enhancement treatment is freely available from the National Institute on Alcohol Abuse and Alcoholism.
Cognitive-behavioral therapy (CBT).
CBT enhances patients’ ability to identify triggers for substance use and to mobilize resources that can counteract these factors. Imaging studies show that CBT reduces the recruitment of the brain’s reward circuitry and improves impulse control in SUD. Cognitive reappraisal is a similar technique, where the meaning of a stressful or adverse event is reinterpreted to reduce its negative emotional valence. For example, research shows that individuals with cocaine use disorder undergoing cognitive reappraisal show decreased attention bias to substance-related cues.
Mindfulness.
This approach uses awareness of the moment without judgment or interpretation. Studies have shown that mindfulness can be helpful for SUD, including a meta-analysis of controlled trials that compared different forms of mindfulness (mindfulness-based stress reduction, mindfulness-based cognitive therapy, and mindfulness-based relapse prevention). Additionally, imaging studies show that mindfulness can change connectivity across brain regions, including the PFC and striatum. For example, individuals with OUD and chronic pain can benefit from mindfulness-oriented treatment (combined with methadone) to decrease their substance use and pain while improving response to treatment.
Taken together, the research shows that treatment can improve outcomes in SUD. Resources for clinicians wishing to learn more about these modalities (including medical education and guidelines) are provided below, in the Discussion section.
In the next section, we review the impact of stress in SUD and treatments under development that target stress (none are currently FDA approved for this indication).
STRESS AND SUD
Stress is the emotional and cognitive response to an event that threatens well-being. The experience of stress can overwhelm other thought processes, making it difficult to concentrate on anything other than worry and fear. Under stress, the brain relies on habitual behavior, including the use of substances, by engaging the striatum while neuronal activity in the PFC is diminished. As a result, stress can make SUD difficult to treat and contributes to relapse. In this section, we review the neurocircuitry of stress in SUD and describe treatments being developed to address this symptom.
Neurobiology of Stress and SUD
Stress is triggered by adverse or demanding circumstances. It mobilizes the individual and elicits a reaction to the threat by activating a range of neurotransmitter systems. These include corticotropin-releasing factor, which modulates the brain’s response to stress; norepinephrine, which mediates the fight-or-flight response within the autonomic nervous system; orexin, which plays a role in wakefulness, fear, and the formation of aversive memories; and dynorphin, which increases motivation to escape threat and contributes to negative emotional states.
The stress-induced activation of networks regulating apprehension, fear, vigilance, and negative emotion can eclipse a patient’s ability to change behavior. This is especially the case in chronic stress, which impairs the brain networks required for cognitive flexibility. Studies in animal models and human volunteers show that chronic stress impedes flexible choice behavior and reversal learning, both of which reduce the ability to adapt to a changing environment. In SUD, imaging studies show that stress disrupts the functional activity of the PFC and increases the likelihood of choosing an immediate reward (such as substance use) over more adaptive behaviors that promote health.
Stress has been implicated in several aspects of SUD. Early-life stress predisposes an individual to the rewarding properties of psychoactive substances, which can potentiate the development of SUD later in life. Furthermore, a history of early adverse childhood experiences is associated with lower responsiveness to SUD treatment. Chronic, heavy substance use alters stress biology such that withdrawal symptoms (even if mild) are associated with increases in cortisol and cognitive impairment. Thus, stress plays a prominent role in relapse and can provoke a return to substance use even in patients who have experienced a prolonged period of recovery.
Alleviating Stress and Inducing Change
Chronic stress in patients with SUD comes from many sources. These can include problems with relationships, social support, housing, comorbidity, and employment. Black patients with SUD face the additional threats of bias and racism. For example, the experience of discrimination (based on race and ethnicity) in minority groups has been linked to an increased risk and severity of AUD.
While addressing the environmental sources of stress can improve recovery, research shows that SUD itself is associated with an increased sensitivity to stress Thus, targeting the neurobiology of stress could potentially improve recovery. In the following paragraphs, we briefly review promising therapeutic approaches (please note that none of these are FDA approved or included in practice guidelines at this time).
Alpha-2 adrenergic receptors can dampen sympathetic activity and reduce the physiological effects of norepinephrine. The alpha-2 agonists clonidine and lofexidine can improve response to treatment in opioid and alcohol use disorder, especially in the setting of withdrawal, which is stressful in itself. Additionally, guanfacine and clonidine have been shown to inhibit stress-induced craving in cocaine use disorder, and a clinical trial is being conducted to investigate the impact of lofexidine on stress reactivity in opioid use disorder. These findings show the importance of research that specifically addresses the stress response in SUD.
The kappa opioid receptor is a promising target, given its role in stress and negative affect. An imaging study showed that kappa receptor availability correlated with stress-induced substance use in cocaine use disorder, which is consistent with animal studies showing that kappa receptor activation reinstates stress-induced drug-seeking behavior. In healthy volunteers, greater kappa receptor availability in the brain correlates with low social support, and buprenorphine (which blocks the kappa opioid receptor) can blunt stress caused by a taxing social situation. However, there remains a lack of research investigating the effect of kappa receptor antagonists on stress in SUD. Studies are being conducted with aticaprant, a kappa receptor antagonist, for major depression that could be used for SUD in the future.
Mifepristone is a glucocorticoid receptor antagonist that is FDA approved for Cushing’s syndrome and to medically terminate pregnancy. However, it also attenuates stress-induced hypothalamic-pituitary-adrenal axis activity. Mifepristone has been shown to inhibit stress-induced craving for alcohol and to reduce intake in individuals with AUD. A clinical trial is under way to investigate the effect of mifepristone on stress and drinking (Clinical-Trials.gov identifier, NCT02989662). Similar findings have been reported with pregnenolone, which inhibits cortisol production and has been shown to reduce stress-induced cravings in alcohol and cocaine use disorders.
The orexins are neuropeptides that are involved in arousal, energy metabolism, and stress response. Research in animal models of SUD, including cocaine, alcohol, and opioids, shows that orexin receptor antagonists reduce stress-induced drug-seeking behavior. A preliminary study in cocaine use disorder showed that suvorexant improved sleep and reduced stress response, and a clinical trial is currently investigating suvorexant as a potential treatment. Furthermore, the administration of suvorexant during a buprenorphine/naloxone taper increased the total amount of sleep, decreased opioid withdrawal, and lowered opioid craving. Thus, the orexin antagonists (suvorexant, lemborexant, and daridorexant), which are FDA approved for insomnia, might have a role in the treatment of SUD.
These studies, in animal models and human volunteers, suggest that addressing stress has the potential to improve the treatment of SUD. These approaches are currently being investigated with a focus on patients at risk for stress-induced relapse.
An additional approach in developing new treatments for SUD is to leverage mechanisms in the brain that induce neuroplasticity, which is discussed in the next section.
PLASTICITY AND PROMISING TREATMENTS
Plasticity refers to the ability of brain networks to adapt to a changing environment by modifying their structure, function, or connections. On the level of neurons, plasticity involves mechanisms like long-term potentiation and long-term depression, which describe the strengthening and weakening of synaptic communication between neurons. On the level of behavior, modifying the synaptic strength between networks improves the ability to enact change. In SUD, this could include adopting new patterns of behavior, like replacing a smoke break with a phone call to a quit-line counselor.
In SUD, plasticity plays a role in both the development of addiction and the ability to recover. Repeated substance use leads to changes in synaptic density, which strengthens this pattern of behavior and contributes to habitual substance use. However, plasticity can also reverse this process and allow the adaptation of more healthful behavior over problematic substance use.
In the development of SUD, neuroplastic changes in the striatum strengthen the networks associated with habitual substance use. Dopamine signaling is activated in response to cues associated with use (such as people or places) and mental states (loneliness, stress, expectation). Neuroplasticity contributes to this conditioning of the dopamine system, through the expression of genes and trophic factors that alter synaptic connections. These changes lead to long-term potentiation and long-term depression, which make neurons more likely to react (or not react) to the input received. In terms of behavior, events in the person’s life that are associated with substances become triggers for drug use, while other events are less likely to spark interest or motivation.
However, it is possible to treat SUD—and to use the brain’s ability to adapt—through therapies that leverage neuroplasticity. Research with ketamine, psilocybin, or TMS is being conducted to investigate this possibility, as reviewed here.
Ketamine is a noncompetitive NMDA glutamate receptor antagonist that produces a rapid antidepressant effect. Ketamine induces a cascade of cellular events that include glutamate and GABA signaling, transcriptional regulators, and neurotrophic factors. Animal models show that ketamine induces homeostatic plasticity, which refers to the resetting of synaptic strengths in order to restabilize networks that had become unbalanced. Thus, in SUD, ketamine may disrupt maladaptive reward memory and readjust the imbalance between the networks regulating excessive drug use and change.
In AUD, three randomized controlled studies have investigated ketamine as a treatment, with promising results. Dakwar et al. investigated ketamine, combined with motivational enhancement therapy, and showed a reduction in alcohol use. Das et al. combined ketamine with a memory retrieval protocol and showed a decrease in harmful drinking. Recently, Grabski et al. compared ketamine (three sessions) to placebo and showed that ketamine, along with mindfulness-based relapse prevention therapy, increased abstinence from alcohol.
Ketamine has been studied in other forms of SUD. Three studies have been conducted on cocaine use disorder, including two that used relaxation exercises and one that combined ketamine with mindfulness-based relapse prevention. The results of these trials showed that ketamine reduces cocaine-seeking behavior and improves rates of abstinence. A recent proof-of-concept study in cannabis use disorder showed that ketamine (combined with motivational enhancement therapy and mindfulness-based relapse prevention) reduced cannabis use.
Psilocybin, a 5-HT2A receptor agonist, has gained attention for its therapeutic effects in psychiatric disorders. Animal studies show that psilocybin promotes the expression of genes that regulate synaptic plasticity and spur dendritic growth. The mechanism is thought to involve stimulation of 5-HT2A and AMPA receptors, which triggers a positive feedback loop, enhancing the release of brain-derived neurotrophic factor and promoting dendritic growth. As a treatment, it is thought that psilocybin (like ketamine) helps restore the balance between the striatum and the PFC, increasing the ability to enact change.
Only one randomized controlled trial has been conducted in SUD with psilocybin. It compared psilocybin to diphenhydramine in participants with AUD (all participants received intensive motivational enhancement therapy and CBT). The results showed that both groups significantly decreased drinking, although this effect was greater in the psilocybin group.
TMS, which uses electromagnetic fields to stimulate the brain, is FDA cleared for the treatment of major depression, obsessive-compulsive disorder, and tobacco use disorder. The mechanism underlying TMS has been linked to its ability to induce synaptic plasticity through the electric field generated in the brain. Animal studies show that electrical stimulation (including electroconvulsive therapy) enhances the excitability of cortical regions with increased brain-derived neurotrophin levels and strengthens glutamatergic synapses. This has the ability to improve neuronal survivability and growth while promoting an increase in neuronal receptor density.
With respect to SUD, only TMS for tobacco use disorder has FDA clearance, based on a clinical trial showing improvement in smoking cessation. However, additional research suggests that TMS may serve as a treatment for other types of SUD. In cocaine use disorder, high-frequency TMS (to the PFC) was shown to reduce craving and cocaine use in a series of clinical trials, although there remains a need for definitive sham-controlled clinical trials.
Similar results have been reported for TMS in AUD, although fewer studies have been conducted. Studies of TMS targeting the dorsolateral prefrontal cortex (DLPFC) showed modest effects in reducing alcohol craving, but further investigation is needed to determine the optimal stimulation parameters for treatment. Two recent imaging studies showed that TMS directed at the midline fronto-cortical brain regions reduced reactivity to alcohol cues and progression of white matter changes in AUD while improving abstinence.
In participants with opioid use disorder, high-frequency TMS to the DLPFC was shown to inhibit craving. Early studies investigating cannabis use disorder showed that TMS reduced craving and use, especially when multiple sessions are delivered over weeks.
Taken together, the research shows that interventions using ketamine, psilocybin, or TMS have the potential to improve outcomes in SUD. These modalities are thought to induce plasticity, which could spur patients’ ability to change behavior. Thus, one approach to improving treatment response in SUD would be to combine these interventional approaches with the therapies described above (pharmacotherapies and behavioral treatments) that address craving, intoxication, withdrawal, and stress.
DISCUSSION
In the United States, SUD is prevalent. Tobacco and alcohol use disorders affect 11.5% and 10.6% of Americans, respectively, and cannabis use disorder is seen in 5.8%. SUD also has an impact on morbidity and mortality: overdose is the primary cause of death in young adults, tobacco kills about 480,000 adults per year, and rates of alcohol-induced cirrhosis continue to rise.
Fortunately, treatment for SUD is effective: it improves quality of life, reduces fatalities, and improves health disparities. SUD is also quite responsive to treatment. Studies show that the recurrence rates of SUD are similar to those of illnesses like diabetes, asthma, and hypertension. However, there is a need for more clinicians trained to treat SUD: at present, there are about 3,000 physicians with the required expertise, while 21 million Americans struggle with this disorder.
Patients with psychiatric disorders are particularly vulnerable to SUD. Research shows that about half of individuals who experience mental health problems during their lives will also have SUD. This includes practically every psychiatric disorder: anxiety disorders, mood disorders, psychosis, personality disorders, and more. For example, about 30% of patients with major depressive disorder have SUD, as do 46% of those with posttraumatic stress disorder and over 50% of those with attention deficit hyperactivity disorder.
Thus, there is a need to integrate SUD treatment into all types of psychiatric care. While neuroscience and clinical trials provide the scaffolding for evidence-based treatments, clinicians are needed to implement these discoveries and address patients’ needs.
A number of organizations provide educational opportunities for physicians interested in treating SUD, including the following:
APA provides training through its website (https://education.psychiatry.org/). This includes on-demand webinars on office-based management of SUD, behavioral treatments, and the emerging use of psychedelics for SUD. Practice guidelines on the treatment of AUD are also provided.
The American Academy of Addiction Psychiatry (https://www.aaap.org) and the American Society of Addiction Medicine (https://www.asam.org) provide courses that range from introductory webinars to advanced training on addiction psychopharmacology (board certification in addiction psychiatry is not required for these trainings). The AAAP/ASAM also provides practice guidelines on stimulant use disorder.
The Providers Clinical Support System (https://pcssnow.org), which is funded by the Substance Abuse and Mental Health Services Administration, offers a wide range of free training on treatments for SUD for all clinicians. This includes webinars, training modules, and access to mentorship from an addiction psychiatrist (with no charge).
The CDC provides a smoking cessation tool kit for practitioners, including guidelines, tutorials, and patient resources (https://www.cdc.gov/tobacco/hcp/patient-care/clinical-cessation-tools.html).
Both the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism provide free educational opportunities on the management and treatment of SUD. These include continuing medical education training and webinars that review a number of topics, from the neuroscience of SUD to addressing stigma. (These can be accessed at https://nida.nih.gov/nidamed-medical-health-professionals/health-professions-education/cmece-activities and https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/free-cme-and-ce-credits-general-information.)
Practice guidelines and resources for the treatment of different types of SUD are available from professional organizations, as described in Tables 1–5. They provide updates on pharmacotherapy and describe the strength of the evidence supporting these treatments.
Through these resources, clinicians can choose the type of SUD and level of severity that they feel comfortable treating. For example, a clinician could choose to learn more about the management of mild or moderate AUD while providing referrals to patients with a severe disorder. Additionally, these professional organizations can provide psychiatrists with a network of dedicated clinicians working to address this pervasive, but treatable, unmet need.