Abstract
Empathy, the ability to perceive the affective state of another, is a complex process that is integral to many of the prosocial behaviors expressed in humans and across the animal kingdom. Research into the behavioral and neurobiological underpinnings of empathic behaviors has increased in recent years. Growing evidence suggests changes in empathy may contribute to a myriad of psychiatric illnesses, including substance use disorder (SUD). Indeed, both clinical and preclinical research in SUD demonstrates a strong relationship between drug taking or relapse events and changes to empathic behavior. Further, there is significant overlap in the underlying neural substrates of these complex behaviors, including the insula, paraventricular nucleus of thalamus (PVT), and the paraventricular nucleus of the hypothalamus (PVN). In this review, we will discuss our current understanding of the interplay between empathic behaviors and SUD. We will also examine the underlying neurobiology that may regulate this interaction, focusing specifically on the insula, PVT, and PVN. Finally, we discuss the biologic and therapeutic importance of taking empathic processes into consideration when discussing SUD.
1. Introduction
Empathy can be understood broadly as the ability to share emotional states with others, as well as the ability to adopt similar emotional points of view (Preston and de Waal, 2017). It is a multidimensional concept that helps guide many complex societal norms such as cooperation towards common goals and social bonding, which are imperative for forming and maintaining relationships (Decety, 2011; Decety et al. 2015; Sivaselvachandran et al., 2016; de Waal and Preston 2017; Adriaense et al., 2020). It was once believed that empathy was reserved for humans and non-human primates. However, evidence spanning from the mid-twentieth century suggests even rats are capable of forgoing personal reward to aid a distressed conspecific (Church, 1959), or assist another independent of operational training (Rice and Gainer, 1962). Since then, an expanding library of research has begun to elucidate the evolutionarily conserved components and underlying neurobiology of empathic behavior in rodents. Already, there have been several reviews regarding the evolution of empathy (Decety, 2011; Decety et al., 2012), cross-species comparisons (Panksepp and Panksepp, 2013; Perez-Manrique and Gomila, 2018); and its neural correlates (Bernhardt and Singer, 2012; Decety 2015; Keysers and Gazzola 2018; Marsh 2018). The improvement of our understanding of the behavior through the use of rodent models has begun to advance our translational understanding of the neurobiology of empathy as well.
The study of empathy has also gained momentum recently due to the growing evidence that empathic processes contribute to a variety of psychiatric disorders defined in the Diagnostic and Statistical Manual (DSM-V), including Substance Use Disorder (SUD). SUD is a complex continuum of biological, psychological, and social factors culminating in chronic relapsing events. Given its social nature, it is not surprising that decrements of empathic processes are beginning to be recognized as part of the cognitive sequelae of SUD. Only a handful of primary studies have considered the relationship between empathy and SUD (for a review examining the emotional processing and social cognition in alcohol use disorder (AUD), see Le Berre, 2019), but in those few studies, the consistency of results across drug class is striking. Stimulant users, polydrug users, and those with AUD all show diminished empathy (McCown, 1989; McCown, 1990; Massey et al., 2018; Robinson et al., 2018). Many individuals with SUD may devalue the consequences of the disorder on the welfare of their families and continue to use without regard to the pain and distress their actions are causing others. However, one current conceptual framework posits that deficits in empathy may not only be a consequence of SUD but may be a precursor to the development of an addiction phenotype (Massey et al., 2018). Further, empathic processes may be a treatment-modifiable risk factor for maintaining drug-free abstinence (Massey et al., 2018; Robinson et al., 2018).
The goal of this review is to highlight what is known about the relationship between SUD and empathy. In the sections below, we will briefly discuss theoretical models often used to understand the complexities of empathy. Then we review what is known about the intersection of addiction and empathy in both human and preclinical animal models, followed by introducing some research elucidating the neurobiological overlap between the two constructs. We will conclude with a discussion on the importance for considering empathic processes as a critical feature of addiction.
2. Theoretical models of empathy
Despite its ubiquity and importance, there has been extensive debate regarding the theoretical framework that best describes the complex nature of empathy (Preston and de Waal, 2017; Sivaselvachandran et al., 2016; Yamamoto, 2017; Uysal et al, 2019). Historically, much of the debate has been centered around the distinction of emotional and cognitive empathy and the role each plays in prosocial behaviors (discussed in Preston and de Waal, 2002 and Cox and Reichel, 2021). Emotional empathy, sometimes referred to as emotional contagion, has been thought to primarily involve subcortical, phylogenetically conserved neural substrates (Mogil 2012; Sivaselvachandran et al., 2016; Lahvis, 2017; Meyza et al., 2017) that afford creatures the ability to match the emotional state of another (Panksepp and Panksepp, 2013; Keysers and Gazzola, 2018). In contrast, cognitive empathy is often considered a primarily cortically driven process by which one is able to infer the emotional state of another (Lamm, et al., 2007; Shamay-Tsoory et al., 2009; Lamm and Majdandžić, 2015; Barrett et al., 2016). Until recently, cortical perspective taking afforded by the concept of cognitive empathy was thought to be reserved for non-human primates (de Waal, 2009; Yamamoto and Takimoto, 2012; Perez-Manrique and Gomila, 2017) and humans (Singer et al., 2004; Shamay-Tsoory, 2011; Bernhardt and Singer, 2012; Zaki and Ochsner, 2012). However, evidence from newer models of targeted helping intimates cognitive empathy, to some degree, is present in rodents as well (Ben-Ami Bartal et al., 2011; Sato et al., 2015; Bartal et al., 2016; Karakilic et al., 2018; Cox and Reichel, 2020). Therefore, it is likely describing empathy as emotional or cognitive is an oversimplification. These two forms of empathy, once thought to be distinct from one another, are interconnected neurobiologically and seen to varying degrees across species (de Waal and Preston, 2017). There continue to be debates regarding the merits of various theoretical models of empathy, and their use often depends on the research question being studied. The following section will focus on the prominent frameworks used to understand empathic behaviors in clinical and preclinical studies.
2.1: Dualistic Theories of Empathy
Research focused on understanding human empathy often maintains a stringent distinction between emotional and cognitive empathy within its theoretical models. One such model is the Self-Other Model of Empathy (SOME), which is used to understand how affective and cognitive systems allow empathy to be experienced (Bird and Viding, 2014). Briefly, the SOME is made of multiple subsystems of empathy, all of which are necessary to achieve an empathic process. These subsystems include: 1) the situation understanding system that provides information on other’s emotional states; 2) the affective representation system, which represents the self’s affect; 3) the affective cue classification system that allows for emotional pattern matching; 4) the Theory of Mind system, which represents the mental states of self and others (see Bird and Viding, 2014). This theory argues that emotional contagion is possible once affective states are matched between individuals, but empathy is only possible once a self-other switch is manifested. This switch occurs when, through Theory of Mind, the individual that is empathizing tends to the affective state of another more than their own. This model has been helpful in understanding the complex changes that occur to empathic processes in certain psychiatric disorders. For example, Saito et al. (2016) demonstrated that enhancing self-other distinction in individuals with alexithymia potentiates their ability to use cognitive empathy, but not emotional empathy.
A different clinical model that maintains a strong distinction between emotional and cognitive empathy for the purposes of elucidating the neural substrates of each is known as the Dual Route Model of Empathy (Yu and Chou, 2018). In this model, empathy has both a low and high road (LeDoux, 2003). The low road is the shorter latency route via subcortical regions that is subconscious and automatic, while the high road is slower and comprised of conscious cortical processes (Bernhardt and Singer, 2012; Decety 2015; Marsh, 2018). The two roads have anatomical distinction, but intersect along several key features, including social experience (Levine et al., 2005; Han and Northoff, 2008; Serino et al., 2009; Liew et al 2011; Lamm et al., 2011; Meyer et al., 2012,). Further, both are critical for empathic behaviors, as activity in substrates associated with emotional (e.g., amygdala, anterior insula) and cognitive empathy (e.g., dorsolateral prefrontal cortex, anterior cingulate cortex) can positively predict the willingness to perform prosocial behaviors in fMRI studies (Singer et al., 2008; Rameson et al., 2012; Waytz et al., 2012; Keysers and Gazzola, 2018).
2.2: Perception-Action Model of Empathy
A model that has extensive utility in the translational understanding of empathic behaviors is Preston and de Waal’s Perception Action Model (PAM) of empathy (Preston and de Waal, 2002; de Waal, 2012; de Waal & Preston, 2017; Preston & de Waal, 2017). It concludes that the affective state of a conspecific can, via evolutionarily conserved abilities of the nervous system to map affective states of others onto our own emotional state (Preston & de Waal, 2017), automatically activate the representations of the state of the other (Preston and de Waal, 2002). Emotional transfer between a distressed target and an observer occurs and causes a shared affect. An observer must regulate their affective state to perform an action that reduces the distress of the target and, by extension, their own (de Waal & Preston, 2017). The authors liken empathic processes to a Russian nesting doll; complex behaviors like perspective-taking and targeted helping that necessarily require additional capabilities like emotional self-regulation are the outermost layers of the doll, while simpler behaviors, like emotional contagion and motor mimicry, are nearer to the center. However, all of these abilities are reliant and are built upon the PAM (Preston & de Waal, 2002; de Waal, 2012; de Waal & Preston, 2017). This model is uniquely structured to describe how affective transfer may promote helping behavior (Bartal et al., 2011; Meyza et al., 2017). Importantly, it acknowledges both a cognitive and emotional component of empathy, but concludes this distinction is less definitive than previously hypothesized. This theory is supported by research in multiple species (Perez-Manrique and Gomila, 2007), including rodents (reviewed in Cox and Reichel, 2021), making it a critical tool and widely accepted model used to understand the evolutionarily conserved and distinct components of empathic behaviors.
While the PAM is an incredibly useful framework, some in the field believe it may not adequately capture the full complexity of empathic behaviors in all situations (Hollis and Nowbahari, 2013; Yamamoto, 2017; Adriaense et al., 2020). In an expansion of the PAM, Yamamoto intimates the prosocial behaviors of many creatures may not be able to be interpreted in a “linearly developing model” (Yamamoto, 2017; Adriaense et al., 2020). Chimpanzees, for example, are easily able to take the perspective of another, but they rarely actively help another chimpanzee unless they are reacting to begging (Yamamoto et al., 2009 a, b). The authors assert, therefore, that it is incorrect to assume complex empathic behaviors necessarily build from more simplistic and common ones. Instead, behaviors are organized into a combinatorial “three factor” model: 1) matching with others, 2) understanding of others, and 3) prosociality, can be present individually or in combination with one another.
Overall, empathy is a multimodal, complex process that can be measured and understood in myriad ways based on the research question being explored. While dualistic models of empathy can be useful, they may create an arbitrary distinction of empathy types. Further, the PAM’s simplicity for the translational understanding of prosocial behaviors makes it one of the most commonly used theory to describe empathy.
3. Behavioral Interaction between Empathy and Addiction
3.1: Current Clinical Research
Data are emerging from clinical research with patients diagnosed with substance use disorders (SUD) that suggest deficits in empathic processing may interfere with successful abstinence. Further, empathy may be a modifiable factor that can prolong abstinence duration. Across numerous drugs of abuse, including opioids, alcohol, cocaine and other stimulants, as well as polysubstance users, empathy is diminished in behavioral tasks and self-report (McCown 1989; McCown, 1990; Massey et al., 2018; Robinson et al., 2018; Carlyle et al., 2020). In a meta-analysis of the literature evaluating the empathic deficits that contribute to emotional dysregulation and interpersonal deficits seen in alcohol use disorder (AUD) (Le Berre, 2019), empathy was blunted in AUD patients compared to controls across numerous studies using multiple theory of mind tasks. For example, impairments in decoding facial expressions of others across a spectrum of emotional valence was consistently reported in individuals with AUD, demonstrating either overestimation or frank misinterpretation of the valence (Massey et al., 2018; Le Berre 2019). In contrast, cognitive empathy function was preserved (Maurage et al., 2011). It has been hypothesized that empathic deficits in this population could compromise interpersonal relationships and promote social isolation for which heavy drinking could be a maladaptive coping strategy (Le Berre, 2019). Empathy levels also may vary throughout the different stages of SUD. For example, empathy was reduced in alcohol dependent compared to abstinent men; however, levels of empathy (as measured by the Basic Empathy Scale for Adults) increased from precontemplation to action stages of addiction (Nachane et al., 2021). Further, there was a negative correlation with empathy scores and the number of relapses. These results suggest improving empathy at specific points in the motivation cycle (contemplation to action) may assist treatment and reduce relapse rates (Nachane et al., 2021).
Opioid users have reported impairments in empathy overall (Strange et al., 2017), but the chronicity of use may be another variable that must be considered. In a recent study, patients with opioid use disorder (OUD) taking substitution medication were divided into “intoxicated” (replacement therapy taken day of testing) and “non-intoxicated” (medication taken >12 hours prior to testing) (Carlyle et al., 2020). The authors found a reduced ability to perceive the emotions of others, based on questionnaire answers, and greater anger after social exclusion in non-intoxicated OUD patients compared with the intoxicated users and drug-naïve controls (Carlyle et al., 2020). The ability to spontaneously share the emotions of others was reduced in “non-intoxicated” users, specifically positively valent emotions (Kroll et al., 2018; Carlyle et al., 2020). It was hypothesized the equivalent rates of empathy between the intoxicated users and controls could indicate some remediating effects of acute opioids (Carlyle et al., 2020).
Recreational and dependent cocaine users also demonstrate decreased empathic concern compared to drug-naïve controls (Preller et al., 2014), as well as a diminished ability to perceive emotions of others (Fox et al., 2011). In a study assessing the deficits of empathic concern using an intentional vs. accidental harms task, concern for those being intentionally harmed is significantly blunted in those using smoked crack cocaine compared to control and those using cocaine hydrochloride (Baez et al., 2021). This empathic deficit directly correlated with significantly attenuated gray matter volumes in regions associated with social cognition, as well as lower functional connectivity between empathy and social cognition networks in the brains of smoked crack cocaine users (Baez et al., 2021). Additionally, in a study looking at patients with polysubstance abuse that had undergone chemical detox, deficits in emotional empathy, assessed using the Empathy Quotient (EQ) questionnaire, were present, but no difference was noted compared to control patients in cognitive empathy or social skills per the EQ (Ferrari et al., 2014).
Much of the clinical research evaluating dysregulated empathy in SUD is in adults, but a unique study evaluated the role of empathic concern and perspective-taking on binge drinking in adolescents (Laghi et al., 2019). They found that low levels of empathy were positively correlated with binge drinking, while lower perspective-taking was associated with more binge drinking in those adolescents with a lower perceived ability to withstand peer pressure (Laghi et al., 2019). These results suggest emotional dysregulation with substance use, like ethanol, is not limited to chronic users, and screening and assessment of empathic processes may be useful for identifying at-risk individuals for the development of SUD.
With a stronger impact on the serotonin system and significantly lower addiction potential than the drugs of abuse discussed previously, 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) has been widely reported to cause increased levels of subjective empathy, closeness, and prosocial feelings towards others. It has thus been labelled an ‘empathogen,’ and has begun to be used in psychotherapy. However, the effects of MDMA on empathy and cooperative behavior are inconsistent. While MDMA has been shown to increase emotional empathy (Kuypers et al., 2014; Hysek et al., 2014), evidence points to it having no, or even negative effects on cognitive empathic processes such as inferring emotional valence from viewing complex emotional faces, particularly regarding negatively valent emotions (Hysek et al., 2014; Kuypers et al., 2014; Schmid et al., 2014; Dolder et al., 2018). In a recent double-blind placebo-controlled experiment that examined the effects of MDMA on multiple tasks assessing empathy, subjects reported MDMA increased self-reported closeness to others acutely, but it did not significantly alter task-based empathic tasks or cooperation with others (Borissova et al., 2021). Further research is needed to understand this potential disconnect between subjective and objective changes in empathy caused by MDMA.
Although the evidence of empathic dysregulation following drugs of abuse is strong, there is also evidence that targeting empathic processes may be a treatment-modifiable risk factor for maintaining drug-free abstinence. For example, while a decreased ability to take the perspective of others was positively correlated with frequency of drinking and poor drinking outcomes in patients with AUD (Robinson et al., 2018), higher levels of empathy, as evaluated by questionnaire, in patients participating in twelve step addiction programs was correlated with more program involvement and prolonged abstinence periods (McCown, 1989). These findings, alongside evidence that social support and maintenance of personal relationships via empathy improves the overall success of treatment outcomes in SUD, suggest the potentiation of empathic behaviors in individuals suffering from SUD may improve treatment outcomes and reduce the chance of relapse (Massey et al., 2018; Robinson et al., 2018).
3.2: Preclinical Studies of Empathic Dysregulation in Addiction
The preclinical evaluation of the interplay between prosocial behaviors and drug self-administration is far less robust, but this nascent field has begun to corroborate many clinical studies. Some of the first evidence in rodent models to demonstrate a strong connection between these behaviors was a series of studies performed by Bruce Alexander and colleagues, known as “rat park,” that showed rats given the opportunity for social interaction would forego morphine-laced water for plain water, even in the context of withdrawal symptoms (reviewed in Pickard, 2012). Group housing has since repeatedly proven to decrease drug self-administration (SA), reinstatement, and conditioned place preference (CPP) compared to single-housed rats (Solinas et al., 2008; Bardo et al., 2013; Lacey et al., 2016). Additionally, placing a drug-naïve conspecific in the operant chamber during testing decreases cocaine self-administration in the testing rat compared to rats with no conspecific present (Smith, 2012; Strickland and Smith, 2014). Until recently, however, it was unclear if prosocial actions, per se, attenuated drug self-administration.
This question has recently been examined using a model of volitional social interaction during SA (reviewed in Venniro and Shaham, 2020). In brief, this operant model affords rats opportunity to press levers for either drug or for social interaction with a conspecific. Using this model, they were able to show that when both SA for drug and social reward was available as a series of mutually exclusive choices, independent of sex, drug class (tested in methamphetamine and heroin) or dose, length of self-administration or drug abstinence length, the rate of abstinence in animals approached 100% (Venniro et al., 2018). Self-administration of the drug resumed only when the social interaction choice was delayed or punished. Further, operant social SA significantly reduced incubation of meth and heroin craving (Venniro et al., 2019). These studies were some of the first to show the choice of social contact will significantly attenuate drug self-administration, drug craving, and reinstatement, which highlights the importance, according to the authors, of incorporating social factors into neuroscientific studies of addictive behaviors (Venniro et al., 2018).
The only published reports to date that have tested the impact of drug self-administration directly on empathic behaviors were performed by Tomek and colleagues (2019; 2020). Rats’ empathic behavior was tested using a trapped conspecific model of targeted helping in which an animal learns to release a distressed conspecific from a Plexiglas restraint tube (Ben-Ami Bartal et al., 2011; 2016; 2021; Tomek et al., 2019). Briefly, a designated trapped rat was placed in a plastic restrainer with a removable door, and the restrainer was placed in an operant conditioning chamber for testing. The designated rescuer rat was then placed in the operant chamber; a two-week baseline level of targeted helping was established by the assessment of the occurrence of and latency to rescue the trapped rat. Rats were afforded a maximum time initially of one hour, which was subsequently reduced to 45 min and ultimately 30 min over the course of the two weeks of testing (Ben-Ami Bartal et al., 2011). Rats failing to release the trapped rat across the two weeks of baseline testing were removed from the study. Rats were then assigned to SA either heroin or saline, and, if assigned to the heroin group, underwent catheter surgery and recovery. Following recovery, rats underwent 6 hour daily self-administration sessions. During trials, following entry into the active nose poke, heroin was delivered at a dose of 0.06 mg/kg per infusion for two seconds, or, for animals in the sucrose group, the nose poke resulted in delivery of a 45 mg sucrose pellet. Each infusion or pellet was accompanied by a light + tone complex for 2 seconds. Self-administration was conducted on a fixed ratio 1 (FR1) schedule of reinforcement and performed 7 days/week for 14 consecutive days (see Tomek et al., 2019 for full methods). Following self-administration, release behavior was re-examined while being allowed concurrent access to their respective reinforcer (Tomek et al., 2019). Rats with a history of heroin SA continued to self-administer the drug and had significantly diminished helping behavior compared to baseline. In contrast, rats trained to self-administer sucrose received as many reinforcers as the heroin group during testing, but also maintained rescue behavior similar to baseline levels (Tomek et al., 2019). A unique caveat to this study was in animals whose catheters lost patency during the trial and therefore were unable to receive heroin during final testing. These rats also rescued distressed conspecifics at rates similar to baseline (Tomek et al, 2019). This is the first series of studies in animal models to suggest heroin intake, particularly if the presence of continued access, may directly interfere with empathic behaviors.
Our lab has also begun to explore the interplay of empathic behaviors and addiction using a three-chamber soaked conspecific model (described in Cox and Reichel, 2020). Rats (“Observers”) learn to release a cagemate (“Targets”) placed in a pool of 100 m of water. To do so, Observers must execute a chain pull response, thereby activating a guillotine door that releases the Target onto a dry platform in a separate chamber from the Observer. This model eliminates social interaction as motivation for the Observer’s release behavior. Using this procedure, we have shown that rats learn to aid a distressed conspecific in the absence of social reward, retain the task over time, and previous experience increases the rate of task acquisition (Cox and Reichel, 2020). Additionally, we validated the specificity of this new model to targeted helping, as well as characterized the importance of the target’s level of distress on the Observer’s behavior. Further, we showed that Observers’ level of familiarity with the target also modulates helping behavior (Cox and Reichel, 2020).
Using this task, our lab evaluated the impact of heroin self-administration (SA) on targeted helping (see the schematic in Figure 1). Male Sprague Dawley rats (Observers) had intravenous catheter implantation surgery and subsequent recovery. They were then given 15 daily sessions of heroin self-administration (40 ug/infusion) for 3 hours/day. The first 5 days were on a fixed ratio (FR) one schedule (FR1) of reinforcement, followed by 3 days on an FR3, and finishing with 7 days on an FR5. Each heroin infusion was accompanied by a light + tone stimulus complex. Control animals self-administered saline rather than heroin. Following heroin SA, rats spent seven days in home cage abstinence with the Target cagemates. They then underwent the targeted helping task (Cox and Reichel, 2020) as described in the Methods section. Latency to chain pull was taken as an index of helping behavior. Trials (2 per day) were conducted daily, spaced 1.5 hours apart from one another during the rats’ dark cycle and lasted a total of 300 s (5 min). Figures 1A and 1B depicts an increased number of nose pokes over time in the heroin group and a greater number of infusions compared to saline control Observers. Figure 1C demonstrates that saline Observers decreased the latency to release the Target over days, whereas heroin animals failed to reduce the release rate over time. Data are converted into a difference score with the following calculation: Day 1 latency – Day 10 latency. Positive values indicate a reduction in latency over time whereas negative values indicate no change or increased latency. We conclude from these data that a history of heroin SA blunts targeted helping during abstinence.
Figure 1. Rats undergoing heroin abstinence demonstrate diminished targeted helping.
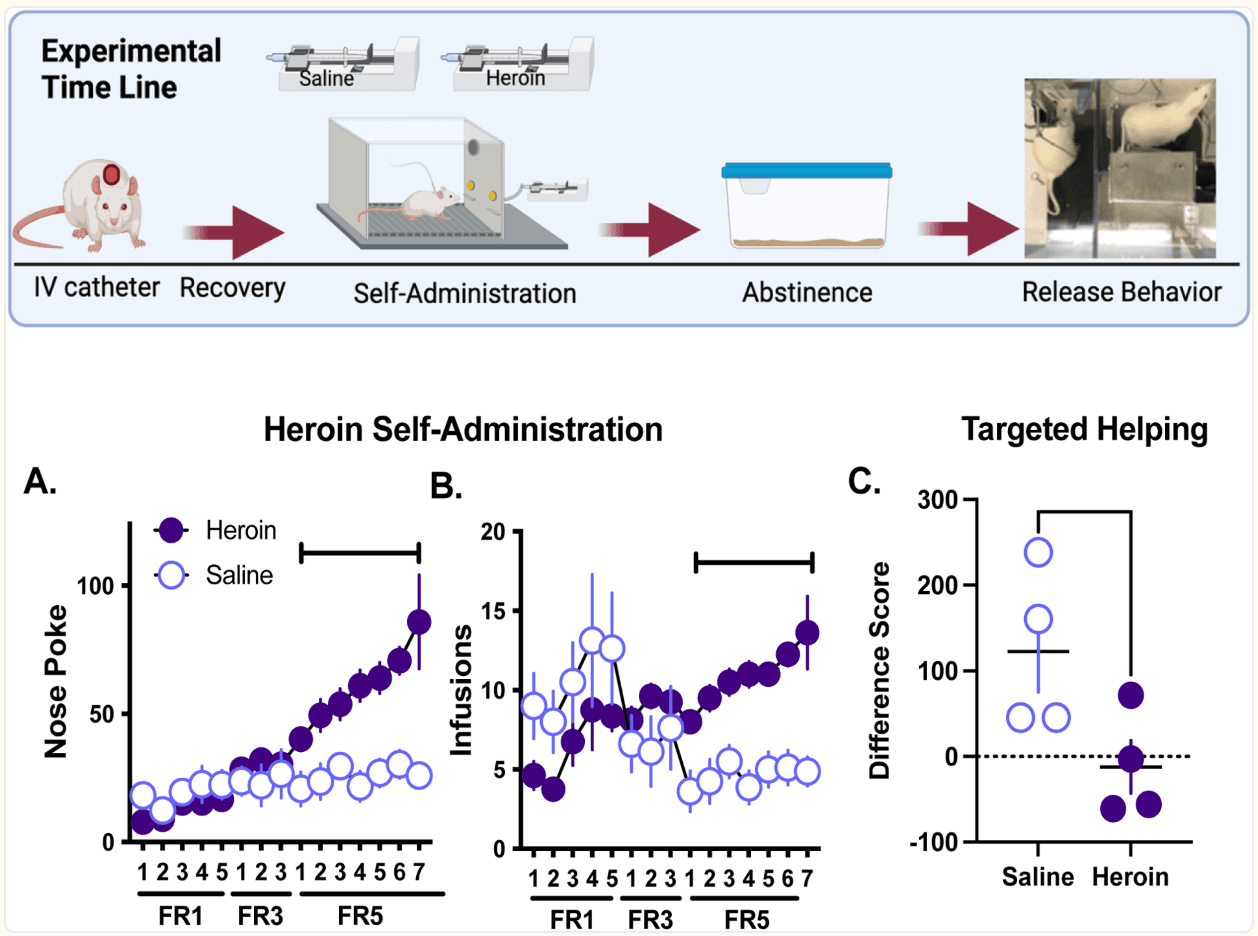
The experimental timeline depicts that male Sprague Dawley Observer rats (n=4/group) underwent intravenous jugular catheter surgery, 15 days of self-administration (SA) of either heroin or saline, 7 days of abstinence in their home cages with their Target cagemate, and then performed the three-chamber targeted helping task (as described in Cox and Reichel, 2020; Cox et al., 2022a; 2022b). A-B). Total nose pokes (A) and infusions (B) per session were recorded for each day of SA. Rats given the opportunity to SA heroin had significantly more total nose pokes and infusions compared to the saline control group (self-administration day X group interaction, F(14,196)=8.77, p<0.0001, post hoc Holm’s Sidak, P<0.05) and a greater number of infusions (self-administration day X group interaction, F(14,196)=6.46, p<0.0001, post hoc Holm’s Sidak, P<0.05) compared to saline control Observers. C). Following abstinence, Observers underwent 10 days of testing in the targeted helping task. Chain pull release latency was recorded and a difference score between the first and last days of testing were calculated as: day 1 latency - day 10 latency. Saline Observers demonstrated significantly higher difference scores versus the heroin group, indicating only the saline group had a change in latency over time [t(6)=2.39, p<0.027]. These data have not been published elsewhere.
*p<0.05, significant difference between saline and heroin.
Taken together, the limited animal research exploring the complex interaction between empathy and addiction is congruent with human studies and demonstrates that prosocial behaviors can be a powerful deterrent in several facets of addiction-like behaviors, including SA, craving and relapse. However, previous experience with, or continued access to drugs of abuse may also attenuate targeted helping.
4. Shared neurobiology of empathic behavior and addiction
The current understanding of the neural substrates involved in addiction (Feltenstein and See, 2008; Koob and Volkow, 2010; 2016) and empathy (Singer and Lamm, 2009; Singer et al., 2008; Sivaselvachandran et al., 2016; Meyza et al., 2018) have been extensively reviewed separately elsewhere and is beyond the scope of this review. Research into addiction has started to push past the canonical circuitry involving the medial prefrontal cortex to the nucleus accumbens to include regions like the insula and the paraventricular nucleus of the thalamus (PVT) and hypothalamus (PVN) as critical substrates for drug taking and relapse behaviors. It is only recently, however, that these regions have begun to garner interest in the roles in prosocial behaviors. There is a dearth of knowledge about these regions as possible nodes for interplay of both empathic and addictive behaviors. Therefore, this review will focus on describing the current research on the role the of insula, PVN, and PVT in addiction, empathy and the possible overlap between these behaviors.
4.1: Insula
In a preclinical model of drug self-administration (SA), using several drugs of abuse have demonstrated the insula to be critical in drug-seeking and cued-reinstatement (Cosme et al., 2015; Pushparaj et al., 2015). Further, in two separate conditioned place preference (CPP) experiments in both rats and mice, the preference for the environment paired with nicotine (Scott and Hiroi, 2011) and amphetamines (Contreras et al., 2012) was attenuated after lesioning the insula, suggesting it plays a role in motivation or decision-making surrounding drug taking. A recent article studying the anterior insular cortex in a murine model of binge drinking found that in male but not female mice, glutamatergic inputs of the insula to the dorsolateral striatum were dysregulated (Haggerty et al., 2022). In addition, stimulation of the insula to dorsolateral striatum glutamatergic neurons in male mice significantly attenuated binge drinking, suggesting alcohol-mediated changes at insular inputs may be directly responsible for the behaviors that maintain binge drinking (Haggerty et al., 2022). The impact of the insula on addictive behaviors have been largely mirrored in clinical research. Neuroimaging studies in humans taking multiple drugs of abuse, including opioids, show reduced grey matter volume in the insula compared to controls (Gardini and Venneri, 2012; Koob and Volkow, 2016). Interestingly, the degree of volume reduction seems to be dependent on the duration of drug use (Ersche et al., 2011). Diminished functional connectivity between the insula and other limbic structures, namely the amygdala, has also been observed in this population (Droutman et al., 2015). Further, tobacco smokers with insular damage were able to quit smoking more easily and with fewer cravings compared to smokers with non-insula lesions (Naqvi et al., 2007), indicating a causal link between the insula and specific behavioral components of SUD.
The insula, known for its capacity to integrate interoceptive and affective information (Craig, 2009; Bernhardt and Singer, 2012), has also garnered strong attention as it relates to empathic behaviors (Fan et al., 2011; Decety, 2015). Functional neuroimaging studies demonstrate an overlapping activity pattern in the insula when personally receiving a painful stimulus and the vicarious pain experienced by watching another receive the same stimulus (Singer et al., 2004; Saarela et al., 2007; Singer and Leiborg, 2009). Human lesion studies corroborate the role of the insula in prosociality; patients with epilepsy that have undergone insular resection had impairments in the ability to recognize others’ facial expressions (Boucher et al., 2015; Uddin et al., 2017). In addition, aberrant insular activity plays a role in the dysregulation of empathic behaviors seen in patients with ASD (Gu et al., 2015). In animal studies, the insula has been implicated in prosocial behaviors, including social approach and avoidance (Rogers-Carter et al., 2018). More specifically to empathy, it has been shown to be active during targeted helping and suggested to participate in processing the distress of others (Ben-Ami Bartal et al., 2021). Further, inactivation of the insula reverses hyperalgesia induced by emotional contagion of pain (Zaniboni et al., 2018) and attenuates targeted helping (Cox et al., 2022a). What is less clear is the role the insula plays in the interplay between empathy and addiction. In a model of alcohol-induced social hyperalgesia, “bystander mice” developed equivalent levels of hyperalgesia as the primary mice that were undergoing forced abstinence from voluntary two bottle choice alcohol self-administration (SA), simply by environmental cues (Smith et al., 2017). The experimenters found the social induced hyperalgesia in bystander mice correlated with significantly elevated c-fos levels in the insula compared to controls who were exposed to alcohol-naïve mice (Smith et al., 2017). A more recent series of studies worked to elucidate the direct effects of the insula on targeted helping in the setting of heroin SA (Tomek et al., 2019; 2020). As mentioned above, Tomek and colleagues assessed the impact of concurrent heroin (or sucrose) SA on rescuing a distressed conspecific and discovered that the sucrose group continued to rescue the distressed conspecifics, but heroin rats chose to self-administer heroin and significantly reduced their rescue behavior (Tomek et al., 2019). Interestingly, they found in a separate study that chemogenetic activation of the insula restores this heroin-induced attenuation of targeted helping, but insular inhibition had no behavioral effect (Tomek et al., 2020). These are the first studies to demonstrate a direct relationship between insula activity and restoration of prosocial behaviors in the setting of drug use. This overlap at the level of the insula could make it a potential therapeutic target to ameliorate both empathic dysregulation and relapse propensity in those suffering from SUD.
4.2: Paraventricular Nucleus of the Thalamus
Another region that is anatomically and functionally well-placed to contribute to the reinforcing effects of drugs of abuse, as well as drug seeking, and relapse is the paraventricular nucleus of the thalamus (PVT). It has been established that PVT neurons are recruited, as measured by c-fos activity, during SA of multiple drugs of abuse (reviewed in Millan et al., 2017). Disruption of PVT neural activity attenuated drug, cue- or context-induced reinstatement in rats trained to SA cocaine (James et al., 2010; Browning et al., 2014; Matzeu et al., 2015). Moreover, it sends and receives projections to regions critical in SA or relapse, including the prefrontal cortex, nucleus accumbens (NAc), and amygdala (James & Dayas, 2013; Khoo et al., 2016). In an eloquent study, Zhu et al., (2016) demonstrated spontaneous or naloxone-precipitated opiate withdrawal-induced c-fos expression in PVT neurons, specifically those that projected to the NAc. When this circuit was inhibited optogenetically or chemogenetically, the expression of somatic opiate withdrawal symptoms and conditioned place aversion (CPA) caused by naloxone injection in opiate-dependent mice was significantly reduced (Zhu et al., 2016).
The role of the PVT in prosocial behaviors has only recently started to be elucidated. A recent paper (Zeng et al., 2021) explored the role of the PVT in consolation behaviors between mice. In this study, cagemates had either undergone right common artery exposure surgery or were surgery naïve. Mice exhibited increased allo-grooming (a proxy of consolation behavior) towards mice that had recently undergone surgery, and less time grooming mice that were surgery naive (Zeng et al., 2021). Further, consolation behaviors correlated with increased c-fos concentrations in the PVT. This study went on to show that inhibition of PVT activity, either with the neurotoxin ibotenic acid or chemogenetically, abolished consolation behaviors toward mice that recently underwent surgery, suggesting a causal relationship between PVT activity and consolation behaviors (Zeng et al., 2021).
As it relates to targeted helping (Cox et al., 2020), we discovered there is potentiated activity (as measured by c-fos activity) in the PVT (Cox et al., 2022b) following the rescue of a distressed conspecific. This increase was seen specifically in the initial trials of behavior acquisition, a measurement for an initial helping response. Interestingly, the activity in the PVT positively correlated to the slower release latency in the early trials, but not the faster release latency in later trials (Cox et al., 2022b). These data suggest thalamic activity may drive initial emotional contagion in early acquisition but may not be necessary for the prolonged maintenance of targeted helping behavior (Cox et al., 2022b).
4.3: Paraventricular Nucleus of the Hypothalamus
Finally, the paraventricular nucleus of the hypothalamus (PVN), specifically via oxytocin (Oxy) release, has been associated with changes in drug taking. For example, systemic administration of oxytocin attenuated methamphetamine CPP (Carson et al., 2010) and methamphetamine SA (Baracz and Cornish, 2012). And in a randomized, double-blind placebo-controlled clinical trial, males with opioid dependence that received intranasal Oxy had reduced craving and withdrawal scores (Moeini et al., 2019).
Of course, the PVN has been extensively studied clinically due to the perceived role of Oxy on a broad array of social behaviors including empathy (reviewed in Shamay-Tsoory and Abu-Akel, 2016; Barchi-Ferreira and Osório, 2021). As it relates to preclinical evidence specifically, it has been shown neurons from the PVN project to cortical regions that regulate complex prosocial behaviors (Donaldson and Young, 2008; Liao et al., 2020). Oxytocin neural levels have been associated with differences in emotional contagion (Laviola et al., 2017), observational fear learning, and socially transmitted fear (Guzman et al., 2014; Pisansky et al., 2017). Our laboratory found increased activity of Oxy neurons within the PVN during targeted helping. Male Observer rats were trained to release a distressed conspecific for ten days. The following day, animals either remained in their home cage (Controls, “Con”) or performed the targeted helping task (Observers, “Obs”). Ninety minutes following the targeted helping session, rats were transcardially perfused and their brains were processed for immunohistochemical detection of the immediate early gene c-fos and oxytocin in the PVN (see Methods section). While the number of Oxy+ neurons between Observers and control rats were comparable (Figure 2A), Observers had significantly more Fos+ cells (Figure 2B), as well as a higher percentage of Fos+/Oxy+ cells within the PVN compared to controls (Figure 2C), strengthening the evidence of the PVN and Oxy playing a role in empathic behaviors even in the absence of direct social contact.
Figure 2. Oxytocin neurons in the PVN are active during targeted helping.
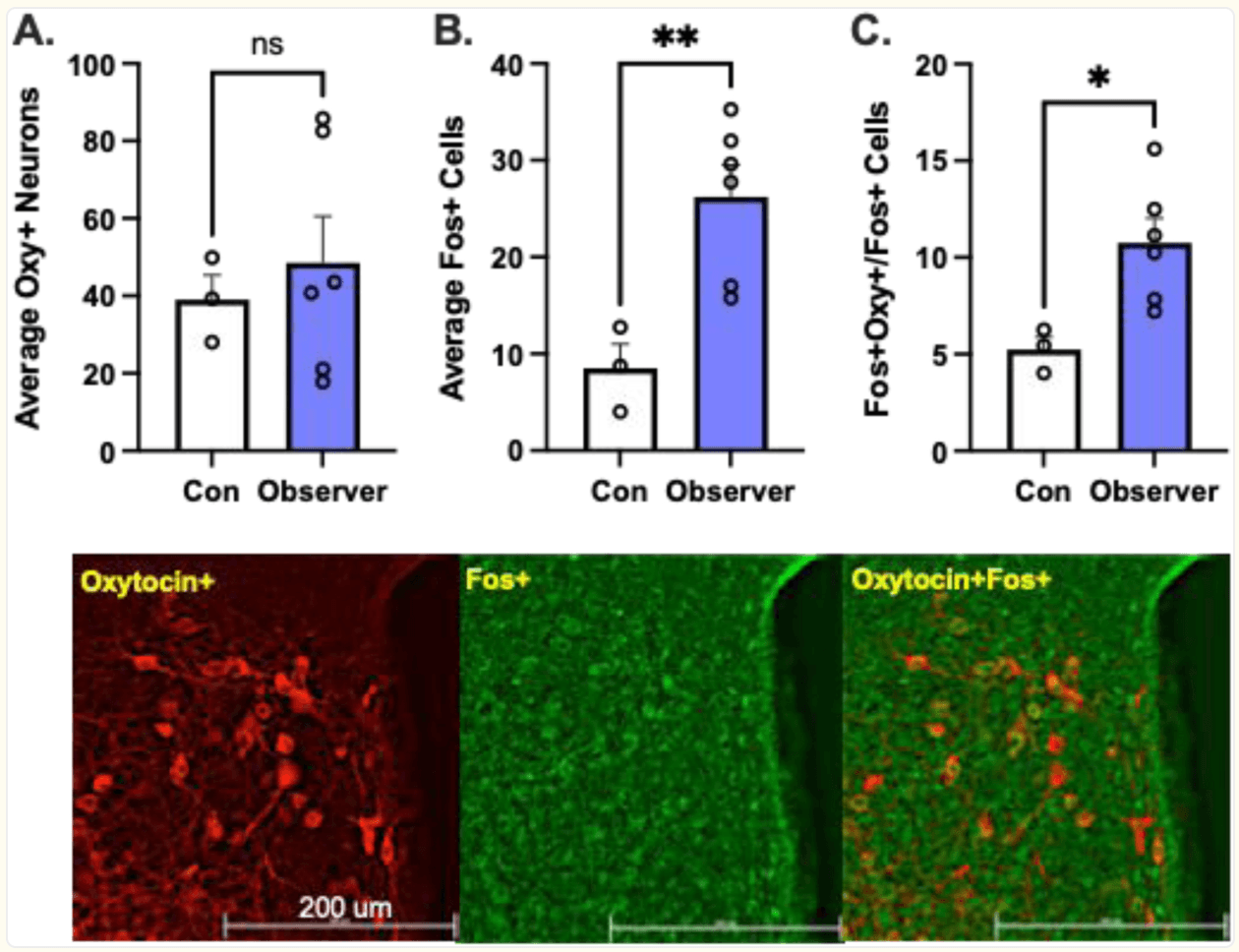
Male Sprague Dawley rats (n=3–6/group) were trained to release a distressed conspecific in a three chamber targeted helping task. On test day, animals either remained in their home cage (Controls, “Con”) or performed the targeted helping task (Observers, “Obs”). Ninety minutes following the targeted helping session, rats were transcardinally perfused, and their brains were processed as previously described (Cox et al., 2022b). A) The average number of Oxy+ cells per animal within the PVN did not differ between the two groups [t(7)=0.52, p>0.05)]. B) However, the number of Fos+ cells in the PVN of Obs animals was significantly higher [t(7)=3.45, p<0.005)]. C) Further, the number of Oxy+/Fos+ cells, an indication of active Oxy neurons, within the PVN, were also higher in the Obs groups [t(7)=2.9, p<0.0114)], suggesting a higher level of activity within the PVN of animals that performed the task.. D) Representative images of immunohistochemical detection of oxytocin and c-fos, as well as an overlay indicating Oxy+/Fos+ cells. These data have not been published elsewhere.
*p<0.05, **p<0.01, significant difference between control and observers
However, a unique experiment evaluated the importance of social context on Oxy’s impact of prosocial behaviors by comparing the effect of Oxy injection on time to release a distressed conspecific (Sato et al., 2015) in rats that were either pair or solo housed (Yamagishi et al., 2020). Rats were injected with 1mg/kg Oxy or saline intraperitoneally for 5 days prior to starting the targeted helping task and were then evaluated on the latency to release either their cagemate (pair housed) or a stranger rat (solo housed) (Yamagishi et al., 2020). Solo housed rats that receive Oxy injections learned the rescue behavior faster than pair housed helper rats and demonstrated significantly faster latency to release a distressed stranger per trial (Yamagishi et al., 2020). The authors concluded the effects of Oxy administration on helping behavior are dependent on the social context, suggesting a far more nuanced effect than previously hypothesized.
5. Importance of considering empathic processes as a critical feature of addiction
Substance abuse continues to be a significant issue worldwide, with over 70,000 deaths attributed to substances in the United States in 2019 alone, according to the CDC (Ahmad et al., 2022). Successful treatments remain elusive, with up to 60% of individuals with Substance Use Disorder (SUD) experiencing at least one relapse. Preclinical and clinical research alike continues to find an overlap between the underlying behavior and neural substrates that regulate both empathy and addiction. Treatment programs that include strong social support networks are more effective, and individuals within the programs that have diminished empathic capabilities or reduced social structure have more relapse events and increased frequency of drinking or drug use (Maurage, et al., 2011). Familial support and peer relationships are an important component of success in treatment and recovery for patients with opioid use disorder (OUD) (Griffith et al., 1998) and functional impairments in empathy during withdrawal from opioids may contribute to the erosion of perspective-taking and interpersonal relationships in individuals suffering from OUD (Robinson et. al, 2018). Preclinical data in drug-exposed animals converge with these clinical findings, however the direction of this interaction is poorly understood. Some research has found potentiated empathic behaviors to be protective against substance use, while others have focused on the diminished prosocial ability in patients with SUD. The limited animal research available has corroborated clinical evidence supporting a direct relationship between drug-taking or craving and diminished helping behaviors (Tomek et al., 2019; 2020), but it has yet to assist in our understanding of the direction of the relationship in a significant way.
The interplay between the neurobiology of these complex behaviors is still unclear. While some neural substrates like the insula, PVT and PVN may be a part of converging circuits that affect both empathic and addictive behaviors and could prove to be translationally relevant targets for intervention, there is still more work to be done before these findings can improve therapeutic outcomes. Although there are still myriad questions that need exploring, the clinical benefit of pursuing the answers is clear. The potential to enhance empathic behaviors in patients with SUD through targeted psychopharmacology or as a component of therapy may allow for successful reconstruction of interpersonal relationships and social support with the goal of having greater chances of reaching and maintaining sobriety.
Materials and Methods
Animals.
Male Sprague Dawley rats weighing 250–275g were pair-housed on a 12-hour reversed light cycle (lights on at 1800). Animals were given food and water ad libitum until behavioral testing, when they were then switched to a daily stable intake (20g) of rat chow (Harlan). Following arrival, rats were given at least 5 days to acclimate to their cage mate. Afterward, one rat was randomly selected to be the “Observer” and the other the “Target”. Animals were handled and weighed for 2 days, 5 min/day before the behavioral assessment. For all behavioral evaluations, rats were transported to the experiment room and left undisturbed for 5 minutes. All experimental procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and approved by the IACUC of the Medical University of South Carolina (IACUC-2021–00551 and −00451).
Social Contact-Independent Targeted Helping.
Social contact-independent helping behavior was evaluated using a custom (Med Associates; Fairfax, VT, USA) operant box with three chambers, as described previously (Cox and Reichel, 2020). Briefly, Targets were placed in 100 mm of water in the ‘wet’ compartment, while Observers were placed on a dry platform with access to a chain that, when pulled, opened an automated door and released the Target into a dry compartment separate from the Observer, preventing any opportunity for social interaction after the chain pull. Latency to chain pull was taken as an index of helping behavior. Trials (2 per day) were conducted daily spaced 1.5 hours apart from one another during the rats’ dark cycle and lasted a total of 300 s (5 min) regardless of the chain pull latency to reduce the likelihood that removal from the apparatus was a motivating factor for the behavior. If the Observer did not pull the chain within the allotted time, the experimenter ended the trial and released the Target followed by the Observer.
Jugular Catheter Surgery and Heroin Self-Administration.
Male Sprague Dawley Observer rats (n=4/group) performing heroin self-administration (SA) underwent intravenous jugular catheter surgery as previously described (Carter et al., 2020). Briefly, rats were anesthetized with IP injections of ketamine (66 mg/kg; Vedco Inc, St. Joseph, MO, USA), xylazine (1.3 mg/kg; Lloyd Laboratories, Shenandoah, IA, USA), and equithesin (0.5 ml/kg; sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution). Ketorolac (2.0 mg/kg, IP; Sigma Chemical, St. Louis, MO, USA) and cefazolin (0.2 g/kg, Patterson Veterinary, Saint Paul, MN, USA) were given before surgery as an analgesic and antibiotic, respectively. Catheters (constructed with Silastic tubing, Dow Corning Corporation, Midland, MI, USA) were inserted 4 cm into the right jugular vein and secured with silk sutures. The opposite end of the tubing ran subcutaneously and exited through a small incision on the back below the shoulder blades where an external port was exposed. Rats were given 5 days for recovery from the procedure. Rats then underwent 15 days of self-administration (SA) of either heroin or saline. Heroin (Research Triangle Institute Intl., Research Triangle Park, NC, USA) was diluted in saline to the dose of 40 μg/infusion. SA procedures were conducted for 3 hours during the rat’s light cycle in SA chambers (30 × 20 × 20 cm) that were enclosed in sound reducing compartments with a ventilation fan (Med Associates, Fairfax, VT, USA) and connected to a computerized data collection program (MED PC, Med Associates). Each chamber had two retractable levers with a white stimulus light above each lever, house light, and tone generator. The infusion tubing was enclosed in steel spring leashes (Plastics One Inc., Roanoke, VA, USA) that connected to the external infusion port and a weighted swivel apparatus (Instech, Plymouth Meeting, PA, USA). The swivel was suspended above the box to allow the rat unrestricted movement throughout the chamber. Heroin SA was conducted 5 days per week and performed in the following increasing fixed ratio (FR) schedule; 5 days of FR1, 3 days of FR3, and 7 days of FR5 (see Carter et al., 2020). Observers next had 7 days of abstinence in their home cages with their Target cagemate. Finally, both heroin and saline Observers were tested in the targeted helping task as described above (see schematic of experimental timeline in Figure 1).
Immunohistochemistry.
A separate group of male Sprague Dawley rats (n=3–6/group) were trained to release a distressed conspecific in the targeted helping task (see above) for 10 days. On test day, animals either remained in their home cage (Controls, “Con”) or performed the targeted helping task (Observers, “Obs”). Ninety minutes following the targeted helping session, rats were transcardinally perfused and their brains were processed as previously described (Cox et al., 2022b). Briefly, rats were anesthetized with Equithesin and then transcardially perfused with 150–200 mL cold 0.9% saline followed by 400–500 mL of 10% buffered formalin. Brains were removed and postfixed in 10% formalin for 24 h, submerged in 20% sucrose/0.1% sodium azide solution for 48 h, and then sectioned into 50-μm tissue sections. For immunohistochemical detection of both c-fos (Fos) (Synaptic Systems Cat# 226 008, RRID:AB_2891278) and oxytocin (Oxy) (Millipore-Sigma: AB911; RRID:AB_2157629), 50-μm tissue slices were permeabilized and blocked in 2% normal goat serum (NGS) and 2% Triton X-100 in PBS and were incubated in a rabbit anti-Fos primary (1:1000) and mouse anti-Oxy (1:1000) overnight at 4°C primary with 2% NGS. Tissue was then incubated at room temperature for 5h while protected from light in goat anti-rabbit Alexa 488 (Invitrogen, 1:1000; RRID# AB_143165) and goat anti-mouse Alexa 594 (Abcam, 1:1000; RRID#AB_2650601). Slices were mounted and cover slipped with Prolong Gold, and representative images of the PVN were taken at 10 × magnification using a fluorescent microscope. PVN location was determined with the assistance of the the stereotaxic atlas (Paxinos, 2007). An average of 3 adjacent slices per animal were analyzed. Animals were excluded from the analysis if a minimum of 3 representative slice of the PVN were not intact. Total Fos+, Oxy+, and Oxy+/Fos+ cells were quantified by an experimenter blind to the animals’ experimental group. All images were quantified using ImageJ software (NIH).
Statistical Analysis.
Nose pokes and infusions are the primary dependent measures for heroin self-administration. During targeted helping the primary dependent measure is latency to chain pull. To assess acquisition of the task, chain pull release latency was recorded and a difference score between the first and last days of testing were calculated as: day 1 latency - day 10 latency. Two-way repeated measures analysis of variance (ANOVA) was used to evaluate lever responses and infusions with group (heroin or saline) and days as the between and repeated measure, respectively. Difference scores were analyzed by t-tests between heroin and saline groups. Cell counts were also analyzed by t-test between control and observer rats. Significance was set at p<0.05.