Abstract
The increase of cannabis use, particularly with the evolution of high potency products, and of cannabis use disorder (CUD) are a growing health care concern. While the harms of adult use and potential medicinal properties of cannabis continue to be debated, it is becoming evident that adolescent cannabis use is a critical window for CUD risk with potential lifelong mental health implications. Herein, we discuss mental health consequences of adolescent cannabis use, factors that contribute to the risk of developing CUD, and what remains unclear in the changing legal landscape of cannabis use. We also discuss the importance of preclinical models to provide translational insight about the causal relationship of cannabis to CUD-related phenotypes and conclude by highlighting opportunities for clinicians and allied professionals to engage in addressing adolescent cannabis use.
Adolescent Cannabis Use
In 2022, cannabis use had an estimated lifetime prevalence of 38% among 12th grade students in the United States, a 17% increase compared to 1992. Additionally, while the prevalence of use remained relatively steady among youth aged 12–17 years over the past decade, rates have continued to rise among older youth and emerging adults aged 18–25 years. These older youth also have the highest prevalence of cannabis use compared to other age groups. Although not all individuals who consume cannabis develop a CUD, broadly conceptualized as a pattern of continued cannabis use despite the development of clinically significant problems, a significant number do. Epidemiologic data of the prevalence of CUD in youth are limited and largely predate broad adoption of medical and recreational marijuana laws in the United States. However, a recent meta-analysis which included youth and adult studies reported that among individuals who used cannabis, 22% met criteria for CUD (95% CI 18%–26%). CUD was most prevalent in young adults, with the highest risk of CUD (41.1%, 95% CI 38.4%–43.8%) among the cohort of 21-year-old emerging adults.
The complex biological properties of cannabis and cannabinoid products and their potential medicinal or adverse effects and their relation to the developing brain are still actively being explored. Of the more than 500 chemicals in the cannabis plant, Δ9-tetrahydrocannabinol (THC) is known to be the most abundant intoxicating cannabinoid. Although most individuals who consume cannabis use full-spectrum cannabis products, THC has been shown to be associated with adverse mental health outcomes. Moreover, the higher the THC potency, the greater the risk of developing CUD and poorer mental health outcomes. The potential of THC to impact neurodevelopment is thought to be mediated through its direct effects on the endocannabinoid system. This modulatory system plays a vital role in regulating neural differentiation and migration, axon guidance, synaptogenesis, and myelination, as well as neurotransmitter system development. Consequently, cannabis exposure during neurodevelopment, whether through exposure in early life (prenatal or childhood) or adolescent use, has the potential to alter the endocannabinoid system. Such exposure could thus impact the development of neural pathways that mediate reward; emotional regulation; and multiple cognitive domains including executive functioning and decision making, learning, abstraction, and attention, all processes central to substance use disorder and other psychiatric disorders.
Growing concerns regarding adolescent-onset cannabis use relates to its association with the increased prevalence and severity of mental health disorders, including psychosis, depression, anxiety, bipolar disorder, and other substance use disorders. Youth who use cannabis are also more likely to endorse suicidal behavior including suicide attempts. Of the various mental health challenges, significant attention has focused on the co-occurrence of cannabis use and psychosis. Though significant debates remain regarding their causal relationship, the literature has highlighted factors of cannabis use, including frequency, potency, and earlier age of onset, as risk factors for psychosis. Moreover, a recent study of over 6 million individuals in Denmark showed that CUD was a major risk factor for schizophrenia, particularly among young males. While Hjorthoj et al., were not able to establish causality or conclude CUD was a modifiable risk factor, the authors estimated that as many as 30% of cases of schizophrenia among men aged 21–30 years might be prevented by averting CUD. The relationship between cannabis use and mental health is likely bidirectional, with shared common predisposing risk factors, neurobiological perturbations and overlapping genetics that may contribute to high rates of comorbidity.
Cannabis Use Disorder Risk
In addition to psychiatric comorbidities, several risk factors for the development of CUD have been identified, including social factors, environmental conditions, and personality traits (Figure 1). However, large-scale studies consistently report two main factors associated with CUD risk. The first is age, both for the onset and frequency of use at younger age. Similar to a number of other psychiatric conditions, CUD risk peaks in adolescence, with most CUD cases becoming evident between ages 18–30 years. Those who start using cannabis prior to age 16 years are at the highest risk of developing CUD. Moreover, youth who initiate use before the age of 18 years are significantly more likely to develop CUD, with substance-related problems continuing into adulthood, and to experience adverse psychiatric and personal outcomes. The risk of developing CUD also increases significantly among youth who use cannabis at least weekly, with the highest prevalence among youth who use cannabis daily. One large-scale study reported increased use frequency associated with an 8–17-fold increased risk for developing CUD.
FIGURE 1. Early predictors of cannabis use and presentations and outcomes of cannabis use disorder
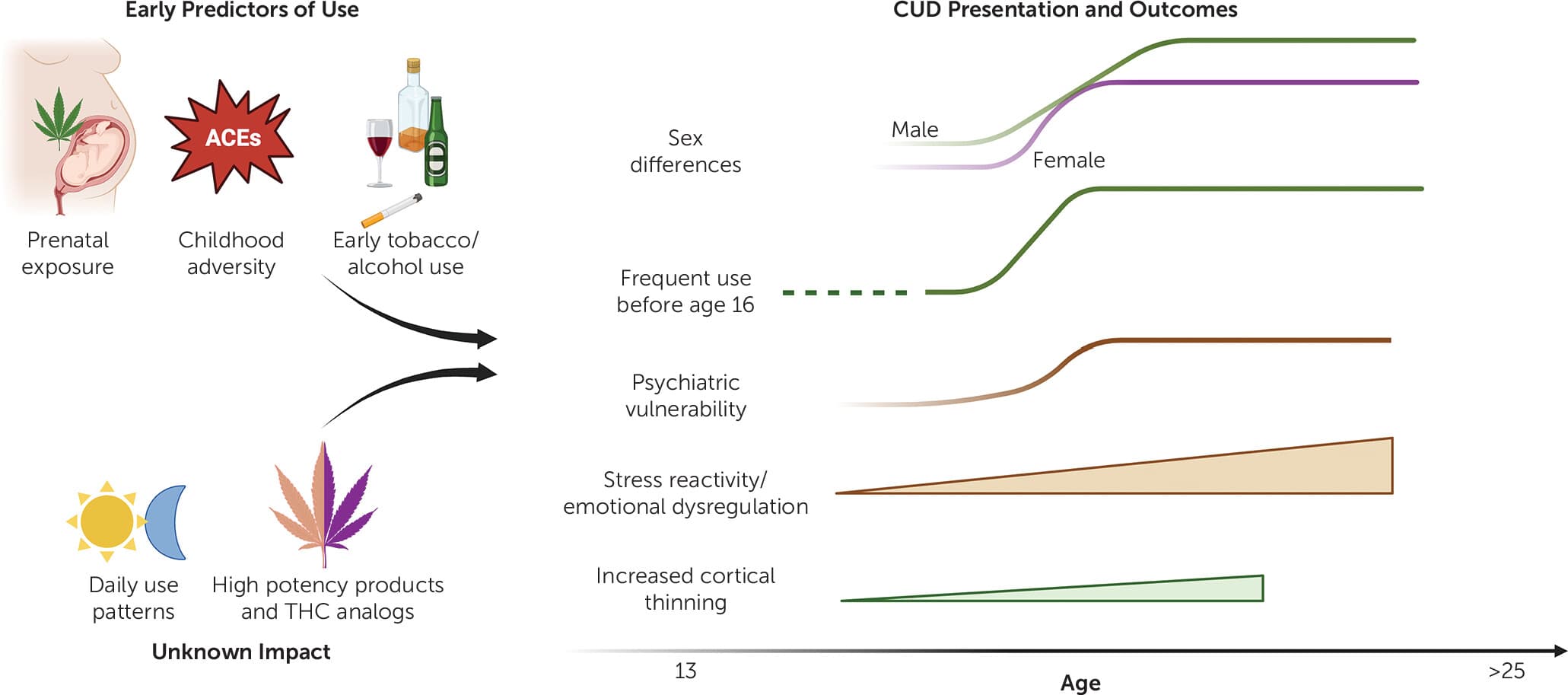
As noted above, the increased vulnerability to CUD following early use has implicated developmental perturbations in mesocorticolimbic brain regions, which mediate reward and emotion processing as well as cognitive control. Prospective longitudinal neuroimaging studies from the European IMAGEN consortium of teens from age 14 revealed that adolescent cannabis use is associated with accelerated cortical thinning, which was correlated with impulsive behavior. This finding of cortical thinning is consistent with a number of cross-sectional neuroimaging studies. Furthermore, amygdala reactivity during adolescence prospectively predicts cannabis use and CUD. Neuroimaging studies in adult individuals diagnosed with CUD also report similar mesocorticolimbic alterations. More recent ongoing longitudinal neuroimaging efforts includes the long-term Adolescent Brain Cognitive Development (ABCD) study that has tracked youth from age 9–10 years. Emerging data has so far revealed that early initiation of cannabis use and CUD is predicted by early childhood adversity, early initiation of tobacco and alcohol use, and maternal prenatal cannabis use. However, the causal relationship between these factors and cannabis use remains challenging to establish.
The second factor consistently associated with the risk of CUD is biological sex. CUD rates are normally higher among male individuals, but marked gender differences in use patterns, motivations, and CUD presentation are noted. For example, male individuals are more likely to be diagnosed with CUD and show higher frequency of use. However, when controlling for frequency of use, CUD incidence does not differ between genders. Female individuals show faster CUD progression, referred to as telescoping. Similarly, female individuals with CUD may be more likely to show increased withdrawal symptoms, comorbidity with anxiety or mood disorders, and interpersonal difficulties. These data suggest that cannabis and certain products may have different subjective and physiological effects in male and female individuals, which ultimately may influence the development of CUD. Importantly, the sex gap for CUD is narrowing, which might be due to higher consumption by young females or the higher potency of products used today.
A Changing Product and Use Pattern in the Cannabis Landscape
Commercialization of cannabis products in legal markets has led to a sharp rise in THC potency, as well as availability and utilization of high-THC products, such as dab pens, wax, or shatter, among youth. Though recent studies have shown that high THC potency may be associated with increased risk of developing CUD, the neurodevelopmental impact of using current THC concentrates during adolescence remains understudied. To date, the integration of research findings has also been compromised by diverse and inconsistent measures of exposure. This is in part due to the wide array of cannabis products, with many individuals regularly using more than one type of product. Moreover, very limited information is known about the type of cannabis and cannabinoid products being used including a recently identified rare but extremely potent cannabinoid, tetrahydrocannabiphorol (THC-P), now widely available commercially. Information is also lacking regarding the proliferation of hemp-derived products that circumvented state and federal laws in manufacturing cannabinoids such as Δ8-THC other THC analogs (e.g., Δ10-THC and hexahydrocannabinol [HHC]), through the chemical conversion of cannabidiol, a non-intoxicating cannabinoid. The same challenges are evident with precursor products such as THC-acid (THCA) which converts to Δ9-THC upon heating. Though adolescents and young adults often think that these popular new THC-analogs are “healthier,” they can produce cannabimimetic effects similar or greater than Δ9-THC. The mental health implications expected with these new THC analogs requires significant monitoring and research attention.
Another factor critical for CUD is the developmental pattern of cannabis use relevant to severity of use. Most epidemiologic studies query the prevalence of cannabis use within a set time frame, most often past 30-day, past year, or lifetime. As noted above, the frequency of cannabis use is associated with increased risk of developing a CUD, but some clinicians misapply frequency of use as a measure of CUD severity. Consideration of factors used with identifying alcohol use disorder may yield new insights into high-risk patterns of cannabis use and the development of CUD. For instance, cannabis use in the morning (e.g., “eye opener,” “wake and bake”) may be more indicative of problematic use. Such information is, however, not often considered within screening and diagnostic constructs of CUD. Similarly, binge patterns of cannabis use have not been characterized, and the impact of episodic consumption of large amounts of high potency THC on the development of CUD is unknown. Alternatively, improved characterization of an individual’s use beyond timeline follow-back may be accomplished via boarder adoption of subjective measures of cannabis use, though further studies are needed to validate such measures and establish a consensus guideline for future research.
Insights from Preclinical Models
Multidisciplinary efforts are required to address the critical need to understand the neurodevelopmental impact of the proliferating diverse cannabis and cannabinoid products. Animal models therefore remain a critical resource to interrogate the causal impact of cannabinoids on the developing brain that may be relevant to the genesis of CUD. Preclinical studies to date have provided unique insights demonstrating that prenatal and adolescent THC exposure increases anxiety behavior, deficits in sociality, increased depressive-like behavior, addiction vulnerability, and cognitive deficits. Deficits are tied to perturbations in mesocorticolimbic (prefrontal cortex, nucleus accumbens, and amygdala), gene expression, protein, and cell morphology. For example, rodent models of adolescent THC exposure demonstrate reduced morphological complexity of pyramidal cortical neurons, which would be in line with cortical thinning seen in human adolescent studies. These animal models have also elucidated unique neurobiological underpinnings associated with high potency THC during adolescence on brain and cognitive behavior relevant to CUD risk.
There are still, however, substantial translational gaps between existing animal models and the current cannabis landscape. For example, the majority of preclinical studies utilize parenteral administration of cannabinoids to determine the impact on behavioral, physiological, and molecular phenotypes. This is due to the challenge that rodents do not readily self-administer THC through traditional intravenous preclinical “addiction” methods and often find THC aversive. Although injections of THC have revealed important relationships between drug and outcomes, human users mainly smoke, vape, or consume edible cannabis products. Novel rodent data indicate that vaporized THC produces different peak plasma and brain concentrations, metabolism profiles, molecular, and behavioral outcomes compared to injected THC. Furthermore, vaporized cannabis extracts are self-administered by rats and adolescent animals will volitionally consume edible THC gelatin. These novel translational models create new inroads to better understand how developmental cannabis exposure and self-administration impact the trajectory of brain processes and behavior relevant to CUD risk.
To maximize the potential of novel translational models, both the clinical and preclinical fields need to standardize metrics of key outcomes. This includes determining fundamental pharmacological metrics (e.g., peak plasma concentrations, metabolite profile) to better compare the potency impact in animal models versus humans, as well as setting standard translationally relevant behavioral outcomes that recapitulate phenotypes observed in humans. Integration of longitudinal designs should test behaviors across development into adulthood using doses and routes of administration relevant to the current landscape seen in human cannabis consumption. These preclinical efforts will accelerate our mechanistic understanding as to how developmental THC and cannabinoids causally influence phenotypes relevant to psychiatric and CUD risk.
Addressing Adolescent Cannabis Use
Another important factor in tackling the changing cannabis landscape is treatment. There is currently an unfortunate disparity between the estimated prevalence of CUD and the number of youths who receive evidence-based treatment. Treatment strategies are currently limited and consist mainly of motivational enhancement and cognitive behavioral therapies. Given that the increased potency of cannabis and cannabinoid products is expected to increase CUD risk, it is disturbing that less than 10% of youths who meet the criteria for a substance use disorder, including CUD, receive treatment. More recently, there has been a decline in treatment admissions for CUD among youths across the United States, including in states with recreational marijuana laws.
Even when treatment is available, adolescents often do not engage due to lack of perceived need for treatment. With the expansion of recreational laws and statutory classification as “medicinal” at the state level, perceived harmfulness of cannabis use continues to decline. In fact, some studies suggest that youths perceive concentrated THC products, particularly vapes or dab pens, as less harmful than combustible plant-based products. This is further complicated by the perception that cannabis use is helpful for mental health problems that may be exacerbated by cannabis use. Broad education efforts are needed, but educating youths about cannabis is complicated by the extensive amount of information and misinformation available online and via social media. Individualized interventions may be better targeted by primary care and mental health professionals, who can address individual and family factors that often contribute to comorbid mental health problems as well.
Challenges in treatment provision also exist. Of the few evidence-based interventions currently used to treat CUD, their availability and efficacy remain limited. This is paired with potential lack of insight into cannabis-related problems. For example, self-reported physiologic changes consistent with tolerance and withdrawal are often not recognized as problems related to cannabis use. Insufficient clinical screening and unrecognized substance-related problems may also result in clinicians missing problematic cannabis use entirely or inaccurately classifying adolescent cannabis use as misuse rather than a CUD. Further, clinicians may not screen for substance use problems because of a lack of available resources or programs to which youth may be referred.
A multifaceted approach is required to address this gap in care, including broader implementation of universal and selective interventions. Risk and protective factors for the onset of youth cannabis use can be conceptualized using the socio-ecological model, which posits that factors at multiple levels, including individual and peer, family, school, and community, contribute to cannabis use. Using this framework, current evidence supports the broad implementation of universal and selective interventions that enhance protective and reduce risk factors. This may include implementation of evidence-based interventions at the institutional (e.g., school) or community level. Integration of behavioral interventions into primary care, social work, and school-based settings presents a significant opportunity to leverage current infrastructure and provide treatment where youth are already engaged in other services. Moreover, as states vie to leverage tax dollars from the growing cannabis industry, a significant portion of such funds must be used for early intervention/prevention strategies to reduce the impact of cannabis on the developing brain.
Conclusions
The relationship between developmental cannabis, the impact of high potency products, and increased risk of developing CUD and mental health problems must be taken seriously, especially in light of the current mental health crisis. The plasticity of the developing brain offers windows of opportunity for prevention and early intervention to change that trajectory. Clearly new treatment strategies are needed to address the mounting challenge of CUD risk in teens and young adults. While data accumulated over the past decades about the effects of now “low dose” THC has been very valuable, significant research efforts in preclinical models are needed, focused on THC potency relevant to today’s products. Additionally, longitudinal studies such as ABCD should be able to provide important insights about factors related to resilience that may also help guide the development of intervention strategies. Altogether, the combined longitudinal, clinical and preclinical efforts will help provide unprecedented knowledge to mitigate the trajectory of CUD and related psychiatric disorders, both of which have a strong neurodevelopmental etiology.