Abstract
Opioid use disorder (OUD) rarely presents as a unitary psychiatric condition, and the comorbid symptoms likely depend upon the diverse risk factors and mechanisms by which OUD can arise. These factors are heterogeneous and include genetic predisposition, exposure to prescription opioids, and environmental risks. Crucially, one key environmental risk factor for OUD is early life adversity (ELA). OUD and other substance use disorders are widely considered to derive in part from abnormal reward circuit function, which is likely also implicated in comorbid mental illnesses such as depression, bipolar disorder, and schizophrenia. ELA may disrupt reward circuit development and function in a manner predisposing to these disorders. Here, we describe new findings addressing the effects of ELA on reward circuitry that lead to OUD and comorbid disorders, potentially via shared neural mechanisms. We discuss some of these OUD-related problems in both humans and animals. We also highlight the increasingly apparent, crucial contribution of biological sex in mediating the range of ELA-induced disruptions of reward circuitry which may confer risk for the development of OUD and comorbid neuropsychiatric disorders.
Introduction
Opioid use disorder (OUD) is a growing epidemic in the United States and globally. To mitigate the rise in opioid-related morbidity and mortality, effective strategies are urgently needed to prevent the onset of opioid addiction by identifying individuals at high risk for developing OUD. Notably, OUD often occurs with psychiatric comorbidities such as depression, bipolar disorder, and schizophrenia (Brooner et al., 1997), all of which involve dysfunctional reward processing. Therefore, studying the basis for this disruption will provide greater understanding and insight into treating both OUD and its comorbidities.
The risk factors for OUD are numerous and complex, and genetics (Kreek et al., 2012; Crist et al., 2019; Jiang et al., 2019), drug availability (Volkow et al., 2011; Wright et al., 2014), and environmental factors such as early life adversity (ELA; Dube et al., 2003; Sinha, 2008; Kreek et al., 2012) all play a role. ELA related to poverty, trauma and chaotic environment affects over 30% of children in the U.S. (American Psychological Association, 2018). ELA is linked to numerous long-term negative health consequences including obesity, heart disease, respiratory illnesses, as well as cognitive and emotional problems (Felitti et al., 1998), and it is associated with several affective problems that indicate dysfunction of the brain’s reward circuitry (Kessler et al., 1997, 2010; Anda et al., 2006; Green et al., 2010; Pechtel and Pizzagalli, 2011; Novick et al., 2018). While a variety of the physical and mental health outcomes following ELA may lead to enhanced risk for OUD and its many comorbidities, here we focus on the effects of ELA on reward-related behaviors and underlying circuitry and propose that disrupted reward processing is a common developmental mechanism by which OUD and its comorbidities may arise following ELA. We also highlight the contribution of biological sex to the range of outcomes related to ELA-induced aberrations in reward circuitry.
Normal Reward Circuit Development Involves An Early-Life Sensitive Period
Reward circuitry in the brain is a network comprised of cortical and subcortical forebrain structures that regulate reward seeking. This circuitry is evolutionarily adapted to drive the acquisition of natural rewards, such as food, water, and reproduction. However, the maladaptive function of this circuitry can also lead to psychiatric manifestations such as mood disorders and addiction.
Whereas the reward circuitry has been extensively studied in the adolescent and mature brain, its function and developmental trajectory in infancy and early childhood are less well-known. The ventral tegmental area (VTA), nucleus accumbens (NAc), and amygdala, major nodes of the reward circuit, begin to appear in the first trimester in humans and around the second week of gestation in rodents, and continue to undergo significant maturation postnatally (Birnie et al., 2020). Behavioral manifestations of the reward function, such as responsivity to sucrose (Desor et al., 1973; Vigorito and Sclafani, 1988) and appetitive learning (Johanson and Hall, 1979; Hayne et al., 1986), emerge within the first months of life in humans and within the first postnatal days in rodents. These developmental timelines suggest that reward circuitry in a rodent in its first week of life might approximate that of a human neonate (Birnie et al., 2020).
The development of these circuits that occurs early in postnatal life suggests a possible sensitive period during which time aberrant environmental signals, such as parental abuse or neglect, may shape their developmental trajectories (Baram et al., 2012; Glynn and Baram, 2019; Luby et al., 2020). Analogous influences of critical environmental signals on network maturation are known for other circuits, including the visual and auditory (Zhang et al., 2001; Li et al., 2006). Just as these systems require predictable sensory inputs at specific times during development to mature properly, parental signals may provide important stimuli for the maturing reward system (Hane and Fox, 2016; Davis et al., 2017; Andersen, 2018; Glynn and Baram, 2019). Thus, understanding how the early environment alters reward circuitry will be critical for developing future interventions against OUD and other mental health problems.
Dysfunction of Reward Circuits: A Common Thread for Oud and Its Comorbidities?
The high prevalence of multiple diagnoses in patients with OUD (Kessler, 2004) supports shared or overlapping underlying processes and has led to searches for common genetic mechanisms (Carey et al., 2016). OUD is often diagnosed in patients who have other mental health problems (Brooner et al., 1997; Conway et al., 2006; Farrugia et al., 2011; Danovitch, 2016). Dysfunction of reward circuitry has been implicated in many of these other mental health diagnoses, such as depression and bipolar disorder (Russo and Nestler, 2013; Pizzagalli, 2014; Whitton et al., 2015), post-traumatic stress disorder (PTSD; Nawijn et al., 2015), personality disorders (Lawrence et al., 2010; Murray et al., 2018), and schizophrenia or psychosis (Kapur et al., 2005; Radua et al., 2015; Whitton et al., 2015). The specific comorbidities present with OUD also appear to be mediated by gender (Brooner et al., 1997; Conway et al., 2006). While women with OUD are more likely to also have a diagnosis of mood, anxiety, and eating disorders, men are more likely to have a diagnosed personality disorder (Brooner et al., 1997).
Notably, the prevalence of dual diagnoses is particularly high among patients who have experienced ELA, suggesting that ELA may impact a shared substrate involved in OUD and its comorbidities. In a study of patients admitted for chemical dependency treatment, those who reported a history of childhood abuse were also more likely to show symptoms of other reward-related comorbidities such as depression, bipolar, and anxiety disorders (Ellason et al., 1996). Another study found a very high co-incidence of PTSD and opioid abuse among women that was explained by a history of childhood trauma (Najavits et al., 1997). The risk for schizophrenia and psychosis is also increased by ELA (van Os et al., 2010; Bentall et al., 2014), which are highly comorbid with substance use disorder (Schmidt L. M. et al., 2011; Li et al., 2020). Palatable food cravings and disordered eating are strongly associated with ELA (Halmi, 2009; Dallman, 2014; Osadchiy et al., 2019), and these cravings are commonly observed in individuals with OUD (Morabia et al., 1989; Pelchat, 2002; Mysels and Sullivan, 2010; Canan et al., 2017; McDonald and Laurent, 2019; Nolan, 2019). This high co-incidence of multiple reward-related problems suggests a common underlying mechanism by which disruption of reward circuitry may lead to a variety of poor mental health outcomes.
Developmental Origins: Ela Leads to Poor Neuropsychiatric Health Outcomes
Numerous studies have linked ELA to poor cognitive (Lupien et al., 2009; Pechtel and Pizzagalli, 2011; Chen and Baram, 2016; Short and Baram, 2019) and emotional health (Heim and Nemeroff, 2001; Anda et al., 2006; Smyke et al., 2007; Maccari et al., 2014; Callaghan and Tottenham, 2016; Hane and Fox, 2016; Krugers et al., 2016; Strathearn et al., 2020). For example, ELA is associated with lower educational achievement (Shonkoff et al., 2012) and poorer executive functioning abilities (McDermott et al., 2012). Evidence from clinical and epidemiological literature demonstrate links between adverse childhood experiences and increased risk for depression, anxiety, PTSD, eating disorders, and psychosis (Felitti et al., 1998; Chapman et al., 2004; Whitfield et al., 2005; Anda et al., 2006; Bale et al., 2010). The specific psychiatric outcomes resulting from ELA also vary by gender (Humphreys et al., 2015), with women more frequently diagnosed with anxiety and depression (Hammen et al., 2000; Heim and Nemeroff, 2001; Davis and Pfaff, 2014), whereas men are more likely to be diagnosed with personality disorders after ELA (Anda et al., 2006), the same pattern seen among those with comorbid OUD (Brooner et al., 1997).
Adverse childhood experiences are also robustly associated with later-life substance addiction (Nurco et al., 1996; Simpson and Miller, 2002; Dube et al., 2003; Widom et al., 2006; Gershon et al., 2008; Sinha, 2008; Enoch, 2011; Shand et al., 2011; Stein et al., 2017; Marsh et al., 2018). Results from the Adverse Childhood Experiences study show that ELA can increase the risk for injection drug use up to 11-fold (Anda et al., 2006) and that ELA increases the likelihood of early initiation of drug use independent of availability or changes in social attitudes towards drugs (Dube et al., 2003), suggesting a specific effect of adverse experiences on addiction liability. Additionally, individuals with a history of ELA are more likely to be prescribed opioid pain medications (Anda et al., 2008). This effect was mediated by an increased likelihood to experience other health and psychosocial problems, which highlights the interplay among the numerous physical and mental health problems associated with ELA, and the challenges in discerning causal mechanisms.
Interestingly, women appear to be particularly predisposed to OUD following ELA (Gershon et al., 2008; Lansford et al., 2010; Shand et al., 2011; Marsh et al., 2018). For example, although men have higher rates of overall substance dependence diagnoses, women who have experienced ELA are overrepresented among heroin and nonmedical prescription opioid users (Shand et al., 2011; Marsh et al., 2018). Women diagnosed with OUD are also two to three times more likely to have a history of PTSD related to ELA than men with OUD (Najavits et al., 1997). While this could be accounted for by the fact that girls tend to experience more childhood trauma than boys (Felitti et al., 1998), the magnitude of difference suggests a mediating role of sex. The type of adversity experienced may also interact with biological sex to affect outcomes. For example, Shand et al. (2011) found that emotional neglect during childhood predicted drug dependence in women, whereas PTSD predicted drug-related diagnoses for men. Again, the presence of other comorbidities varied by sex; men were more likely to display antisocial behaviors, whereas women were more likely to be diagnosed with anxiety and depression. These differences suggest divergent mechanisms by which ELA may alter reward circuit development between sexes, resulting in psychiatric outcomes that differ between men and women.
Anhedonia and Oud, Each Manifestations of Reward Circuit Dysfunction, Arise After Ela
The paragraphs above suggest a strong association between ELA and malfunction of the reward circuit, which can manifest as OUD or other problems in reward-related behaviors. Many of these are common across several mental illnesses and may share common biological substrates. Anhedonia defined broadly as an inability to experience pleasure is a feature of substance use disorder in some individuals (Ahmed and Koob, 1998; Koob and Moal, 2001; Janiri et al., 2005; Hatzigiakoumis et al., 2011; Sussman and Leventhal, 2014; Kiluk et al., 2019; Brenner et al., 2020) and of other psychiatric diagnoses that are comorbid with addiction (Gorwood, 2008), such as depression (Loas, 1996; Blanchard et al., 2001; Pizzagalli et al., 2008; Martinotti et al., 2012), schizophrenia and psychosis (Andreasen and Olsen, 1982; Blanchard et al., 2001; Martinotti et al., 2012), PTSD (Risbrough et al., 2018), eating disorders (Davis and Woodside, 2002; Halmi, 2009), and other “high-risk” behaviors (Franken et al., 2006).
Indeed, the concept of anhedonia serves as a distinct useful transdiagnostic construct for understanding the role of altered reward processing in the etiology of psychiatric conditions (Bedwell et al., 2014; Lake et al., 2017). In line with the Research Domain Criteria (RDoC) framework put forth by the NIH, the ability to define a neurobiological basis of anhedonia, along with empirical behavioral measures both in humans and animal models, makes anhedonia a useful translational construct for studying reward circuit dysfunction and related behavioral disorders such as those seen after ELA (Cuthbert and Insel, 2013). Furthermore, the ubiquity of anhedonia as a feature of many of the psychiatric outcomes of ELA provides evidence that a mechanism by which ELA may impact cognitive and emotional health outcomes is through disruption of reward circuit development (Birnie et al., 2020). There are multiple domains of anhedonic behaviors that can be measured in humans and animal models which may have distinct neural processes (Der-Avakian and Markou, 2012; Shankman et al., 2014; Zald and Treadway, 2017). For example, anhedonia may represent a deficit in either anticipatory or consummatory reward, motivation, can be manifest for some reinforcers but not others (e.g., social vs. food rewards), and is also described as a feature of flat affect (for review, see Shankman et al., 2014). The neural substrates that govern these different forms of anhedonia have been explored (Gorwood, 2008; Der-Avakian and Markou, 2012; Treadway and Zald, 2013; Pizzagalli, 2014), and the specific effects of ELA on distinct types of anhedonic behaviors as well as their potentially dissociable neural substrates is an important area of continued investigation.
How Does Ela Provoke Anhedonia, Oud, and Comorbidities? A Need for Animal Studies
While studies in humans offer important insights into the effects of ELA on reward circuitry, one cannot dissociate the influence of early-life experiences on reward circuitry function from other genetic and environmental variables that may mediate the links between ELA, OUD, and other comorbidities. Animal models provide a method for investigating the effects of these environmental factors in isolation.
In animal studies, several different models of ELA have been used to isolate the effects of adversity on brain development from other genetic and environmental variables. These methods, such as maternal separation (MS), limited bedding and nesting (LBN), fostering by abusive caregivers, and others, have been extensively described elsewhere (Molet et al., 2014; Doherty et al., 2017; Walker et al., 2017; Wakeford et al., 2018; Brenhouse and Bath, 2019). In rodents and non-human primates, numerous studies have demonstrated that ELA results in behavioral phenotypes that suggest underlying dysfunction in reward-related brain regions (Molet et al., 2014; Andersen, 2015, 2018; Wakeford et al., 2018; Bonapersona et al., 2019; Birnie et al., 2020). The particular behavioral outcomes of ELA in animal models can vary depending on the type, timing, and duration of the paradigm, the species and strain of animal, and the timing and type of behavioral assays (Schmidt M. V. et al., 2011; Molet et al., 2014; Andersen, 2015; Walker et al., 2017; Brenhouse and Bath, 2019; Demaestri et al., 2020; Lundberg et al., 2020), as well as sex (Kundakovic et al., 2013; Bath, 2020). While this poses a challenge for interpreting this vast literature, the variability also mirrors human experience; indeed, ELA in humans can take many different forms, such as poverty, trauma, physical or sexual abuse, and neglect, and these, in combination with other environmental and biological factors, likely contribute to individual differences in clinical outcomes (Shand et al., 2011; Daskalakis et al., 2013; Sheridan and McLaughlin, 2014; Strathearn et al., 2020), highlighting the sensitivity of the brain to different types of stressors during these developmental periods.
Given that anhedonia has been associated clinically with many of the psychiatric outcomes of ELA, establishing whether ELA can actually cause anhedonia seems useful for determining neurobiological mechanisms that may ultimately underlie ELA-associated OUD and its comorbidities. Thus, we will highlight some animal studies that have focused specifically on anhedonia. The expression of anhedonia in animal models appears to be mediated by interactions between the ELA paradigm, biological sex, and testing parameters (Matthews and Robbins, 2003; Rüedi-Bettschen et al., 2005; Der-Avakian and Markou, 2010; Leussis et al., 2012; Lukkes et al., 2017; Di Segni et al., 2019). For example, in male rodents, ELA imposed via rearing for 1 week (P2-P9) in cages with limited bedding and nesting materials (LBN) leads to enduring anhedonia for both natural and drug rewards. This includes blunted sucrose and palatable food preference, reduced interest in social play, and decreased low-effort cocaine consumption (Molet et al., 2016; Bolton et al., 2018a, b). In contrast, such anhedonia is not observed in female rats after LBN (Levis et al., 2019). Yet, others have identified an age-dependent reduction of sucrose preference and depressive-like behaviors in female mice (Goodwill et al., 2019). Using a MS model of ELA, both male and female rats have reduced sucrose preference later in life (Matthews et al., 1996; Leventopoulos et al., 2009; Coccurello et al., 2014). Anhedonia has been reported also in nonhuman primates exposed to maternal deprivation and maltreatment (Rosenblum and Paully, 1987; Paul et al., 2000; Pryce et al., 2004; Kaufman et al., 2007; Glynn and Baram, 2019), such as reduced sucrose preference (Paul et al., 2000) or interest in social interaction (Coplan et al., 1996). However, others have found increased sucrose drinking in juvenile males (Nelson et al., 2009).
In contrast to natural reward anhedonia, other studies have demonstrated increased sensitivity to drug-related rewards (Andersen, 2018) as well as addiction-related behavioral traits (Hynes et al., 2018) after ELA. Although this may appear contradictory, these findings support the notion that the behavioral expression of altered reward circuitry by ELA depends on reward type and testing paradigm; thus, anhedonia and reward-seeking are not necessarily mutually exclusive.
While effects of ELA on increased alcohol and cocaine-seeking have been extensively studied and reviewed (Andersen, 2018), considerably less work has been done to model the effects of ELA specifically on opioid addiction vulnerability. Some evidence exists that MS increases morphine seeking in both male and female adult rats (Abad et al., 2016; Mohammadian et al., 2019) while others observed morphine preference only in MS males (Kalinichev et al., 2002; Vazquez et al., 2005, 2006; Michaels and Holtzman, 2008; Vey et al., 2016). Holtzman and colleagues show that male rats that have experienced MS demonstrate a greater place preference for morphine than their control counterparts (Michaels and Holtzman, 2008) and increased locomotor sensitization to repeated morphine, a measure of the psychoactive properties of the drug (Kalinichev et al., 2002). However, others have found attenuated sensitivity to the rewarding properties of heroin in MS females (Matthews and Robbins, 2003). Using the LBN model of ELA, we have demonstrated that, while males develop anhedonia for natural rewards like social play, palatable food, and sucrose (Molet et al., 2016; Bolton et al., 2018a, b), females developed a strikingly different phenotype (Figure 1). LBN females exhibit a marked increase in addiction-like seeking for opioid drugs (Levis et al., 2019). These rats were resistant to the extinction of opioid-seeking behavior, had stronger cue-induced and heroin-primed reinstatement responses, and increased motivation to self-administer the opioid remifentanil in a translationally relevant task measuring economic demand (Treadway et al., 2009; Bentzley et al., 2013; Bickel et al., 2014), demonstrating a motivation to obtain the drug even at a very high cost. Motivation for consuming palatable food was also significantly higher in LBN females, concurrent with the marked increase in addiction-like seeking for opioid drugs. Notably, this same phenomenon has been observed among patients seeking treatment for OUD (McDonald and Laurent, 2019).
Figure 1. Early life adversity (ELA) augments opioid-seeking behaviors and increases the demand for opioid drugs and highly palatable food.
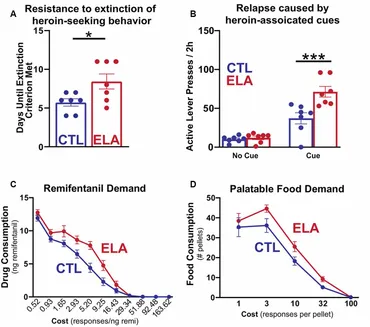
Together, the findings in rodents and non-human primates suggest that ELA disrupts the maturation of reward circuits, and the resulting behavioral manifestations may vary by the timing, duration, and nature of the ELA and be further modulated by sex. Whereas deficits in reward-seeking behaviors are observed in males, such deficits are not commonly found in females. Rather, in females, the prevailing phenotype includes the enhanced consumption of opioids (and other drugs of abuse) and palatable food. The mechanisms underlying this phenotype are poorly understood and may involve ELA-induced changes in both reward and stress circuits. Support for this notion is provided by studies showing that female rats that have experienced stress tend to engage in more pro-hedonic consumption of palatable food (Dallman et al., 2003, 2005; Pecoraro et al., 2004; Jahng, 2011, 2014; Tomiyama et al., 2011; Machado et al., 2013; Kim et al., 2015), and that this may be specifically associated with anhedonia (Jahng et al., 2012; Jahng, 2014). Much information is needed to gain insight into the bases of palatable food craving as sex-dependent comorbidity of OUD.
Furthermore, the variable consequences of ELA on distinct assays of reward-seeking behaviors in animal models demonstrate that reward processing is not a singular phenomenon; rather, individuals may express different and dissociable phenotypes that suggest potentially discrete mechanisms of reward circuit disruption. Thus, further investigation into how ELA alters specific aspects of reward processing and underlying neural substrates will be critical for understanding the biological processes that contribute to the risk for OUD and comorbid disorders.
How Might Ela Lead to Oud and Related Disorders? Evidence from Clinical Imaging Studies
Evidence from human imaging studies suggests impaired development of specific reward-related brain regions and circuits after ELA that impose a risk for substance abuse and related comorbidities. Many studies have demonstrated functional and neuroanatomical effects of ELA on brain regions involved with reward and reward-learning, such as the hippocampus, amygdala, medial prefrontal cortex, and striatal areas including nucleus accumbens (Bremner, 2003; Hackman and Farah, 2009; Rao et al., 2010; Pechtel and Pizzagalli, 2011; Gee et al., 2013; Boecker et al., 2014; Callaghan and Tottenham, 2016; Teicher et al., 2016; Miguel et al., 2019; Herzberg and Gunnar, 2020). Childhood maltreatment is associated with blunted activation of these brain regions during reward processing tasks (Dillon et al., 2009; Mehta et al., 2010; Goff et al., 2013; Novick et al., 2018), a potential functional mechanism explaining the presence of anhedonia among individuals who have experienced ELA. Of these, the striatum appears to be especially important in mediating the link between reduced reward reactivity and ELA (Dillon et al., 2009; Goff et al., 2013; Goff and Tottenham, 2015; Egerton et al., 2016; Kamkar et al., 2017; Dennison et al., 2019). The ventral striatum in particular seems to be a key mediator between ELA, anhedonia, and substance abuse. Corral-Frías et al. (2015) report that reduced reward reactivity in the ventral striatum predicts ELA-associated anhedonia and structural equation modeling revealed that this relationship also predicts substance-related coping behaviors, such as self-medication. This finding highlights a possible common mechanism by which ELA can lead to OUD and its comorbidities. The type of adversity experienced may also mediate the striatal response to reward (Dennison et al., 2019; Herzberg and Gunnar, 2020), as ELA in the form of childhood poverty, specifically, is associated with increased reactivity to reward in the striatum (Gonzalez et al., 2016), especially in girls (Romens et al., 2015). These sex- and experience-dependent differences are consistent with the observed variability of mental health outcomes in humans and behavioral phenotypes in animals.
Ela Causes Functional and Anatomical Changes in Reward-Related Brain Regions: Evidence from Animal Models
Building on clinical evidence, studies using animal models provide tools for identifying mechanisms that underlie disruptions in reward circuitry after ELA. In analogy to human literature, these outcomes appear to be partially mediated by sex. In males, our group has previously shown that anhedonia after LBN is associated with altered functional connectivity between the amygdala and mPFC in rats that may be mediated by CRH expression in the amygdala (Bolton et al., 2018a). This is supported by evidence that depressive-like behaviors and natural reward anhedonia following LBN are associated with disrupted amygdala-PFC and PFC-striatal functional connectivity (Yan et al., 2017). Additionally, Walker et al. (2017) have observed morphological and functional changes in the basolateral amygdala (BLA) and reduced functional connectivity between BLA and PFC in LBN-exposed male rats (Guadagno et al., 2018a, b). MS-induced ELA alters the development of PFC→NAc projections and dopamine (DA) signaling within the pathway in male rats (Brenhouse et al., 2013). In females, MS induces early maturation of the BLA-PFC circuit (Honeycutt et al., 2020), and early life social stress alters resting-state functional connectivity in NAc, hippocampus, and PFC (Nephew et al., 2017). In nonhuman primates, maltreatment during infancy leads to increased amygdala volume (Howell et al., 2014) and altered connectivity in regions implicated in mood disorders (Howell et al., 2013). c-Fos mapping studies measuring neuronal activity further suggest specific ELA-induced alterations in reward circuit function (Rincón-Cortés and Sullivan, 2016; Bolton et al., 2018a, b; Di Segni et al., 2019). Specifically, ELA leads to reduced NAc c-Fos activation in response to typically-rewarding stimuli like a social interaction (Rincón-Cortés and Sullivan, 2016), or aberrant over-activation of other regions associated with stress and reward (Bolton et al., 2018a, b).
Molecular mechanisms mediating the effects of ELA on OUD and related comorbidities may involve alterations in neurotransmitter and neuromodulator systems. Whereas a comprehensive discussion of this important topic is beyond the scope of this review article, a few salient points are mentioned: A vast literature documents the role of DA signaling in motivated and reward-seeking behaviors. Altered DA signaling is an important mediator of drug-seeking (Koob, 1992) as well as other psychiatric problems associated with ELA such as mood disorders (Diehl and Gershon, 1992) and psychosis (Kapur et al., 2005) and has been implicated in the expression of anhedonia (Willner et al., 1992; Pizzagalli, 2014). ELA has been extensively linked to dysfunction of the DA system in rodents, especially in the striatum (for a comprehensive review of this literature, see Bonapersona et al., 2018), and this may be mediated by alterations in other stress and reward-related transmitter systems (Forster et al., 2018). Additionally, the effects of early life experiences on DA signaling may be more pronounced in females (Camp et al., 1984; Chocyk et al., 2011). It is therefore tempting to speculate about the role of ELA-provoked deficits in DA signaling as involved in ELA-related OUD and its comorbidities.
Endogenous opioids play an important role in mediating hedonic processes (Smith and Berridge, 2007; Mahler and Berridge, 2009, 2012; Mitchell et al., 2018) as well as social attachment early in life (Panksepp et al., 1980), so the endogenous opioid system might also represent an important link between ELA and reward-related outcomes later in life. Alterations in opioid receptor mRNA have been observed in both males and females after ELA, although differentially between the sexes. Chang et al. (2019) show female-specific increases in NAc mu and delta-opioid receptor mRNA levels in mice after early life predator odor exposure. Nylander and colleagues have found long-term alterations in endogenous opioid peptides and opioid and DA receptor expression in reward-associated areas that vary both by sex and by the duration of MS (Ploj et al., 1999, 2001, 2003a,b; Ploj and Nylander, 2003; Gustafsson et al., 2008). Opioid receptors are known to modulate striatal DA signaling (Mulder et al., 1984; Johnson and North, 1992), an effect that may be potentiated by ELA (Karkhanis et al., 2016). Thus, disturbances in endogenous opioids might also mediate ELA-induced alterations of striatal DA signaling leading to aberrant reward-related behaviors. These ELA-induced opioids and DA-related disruptions suggest a mechanism by which ELA may lead simultaneously or in parallel to psychiatric disorders and enhanced consumption of opioids (Khantzian, 1987; Dallman et al., 2005; Kim et al., 2015; Lovallo et al., 2018).
Together with evidence from human subjects, these findings demonstrate that ELA alters important reward-related circuit nodes to provoke vulnerability to poor psychiatric outcomes. Establishing causality between network- and molecular-level changes induced by ELA and resulting reward-related deficits remains an important area of investigation to cure OUD and its psychiatric comorbidities.
Conclusion
Evidence across species suggests that ELA during sensitive developmental periods alters the developmental trajectory of reward circuitry. The precise nature of ELA, the potentially disparate consequences of different types of ELA, and the mechanisms underlying the aberrant maturation of reward circuits remain topics of much-needed investigation. The resulting maladaptive reward processing is likely a mechanism common to OUD and its comorbidities. As both animal and human studies demonstrate, the manifestations of this aberrant reward circuit function are varied and depend on the type and extent of adversity, biological sex, and later life experiences. However, functional, anatomical, and molecular disruptions in reward-related brain regions such as the medial PFC, striatum, and amygdala have been described across multiple paradigms and several species, suggesting a common developmental origin. Likewise, anhedonia may be an important behavioral biomarker of disturbed reward processing that links ELA, OUD, and other mental health problems. Further investigation into the neurobiological basis for ELA-induced reward circuit disruptions will provide key insights into the origins of OUD and its comorbidities and may uncover new interventions that will be successful in treating both.