Abstract
Objective To systematically investigate if there is a significant association between markers of autonomic functioning and emotional dysregulation (ED) in children and adolescents.
Method Based on a preregistered protocol (PROSPERO: CRD42021239635), PubMed, Web of Knowledge/Science, Ovid MEDLINE, Embase, and APA PsycInfo databases were searched until April 21, 2021, to identify empirical studies reporting indices of autonomic nervous system (ANS) functioning in youths meeting DSM (version III, IV, IV-TR, 5 or 5-TR) or International Classification of Diseases (ICD) (version 9 or 10) criteria for any psychopathological/neurodevelopmental condition and assessed for ED with a validated scale. Eligible outcomes included correlation coefficients between ED and ANS measures or differences in ANS measures between youths with and without ED. Study quality was assessed with the Appraisal tool for Cross-Sectional Studies (AXIS) and the Newcastle-Ottawa Scale (NOS) for cohort studies. Random-effects meta-analyses were used for data synthesis.
Results There were 12 studies (1,016 participants) included in the descriptive review and 9 studies (567 participants) included in the meta-analyses. No evidence of a significant association between ED and altered cardiac or electrodermal functioning was found. However, exploratory meta-regressions suggested a possible association between reduced resting-state cardiac vagal control and increased ED.
Conclusion This study did not find evidence of an association between ED and autonomic dysfunction. However, preliminary evidence that reduced vagal control at rest might be a transdiagnostic marker of ED in young people was found. Additional studies comparing autonomic measures in youths with and without ED are needed and should also assess the effects of interventions for ED on ANS functioning.
Being able to self-regulate (both behaviorally and physiologically) is an important ability that children and adolescents acquire throughout development. Specifically, emotion regulation entails any kind of strategies aimed at monitoring, assessing, and modulating emotional contents and suppressing related maladaptive behaviors.1, 2, 3 Emotion regulation may occur via engagement with the emotional content (eg, cognitive reappraisal—changing the cognitive connotation of a situation and consequently its emotional valence) or disengagement (eg, expressive suppression—voluntary suppression of facial expressions and behaviors).3,4 Being able to efficiently regulate emotions is essential to human functioning, as it also relates to the ability to suppress aversive (eg, violent) behaviors toward self or others. Indeed, emotion regulation is a robust predictor of adaptive behaviors including successful school and work functioning, prosocial interactions, and promotion of health and well-being.5, 6, 7
By contrast, the failure to regulate one’s own emotions, ie, emotional dysregulation (ED), may lead to a variety of problematic behaviors in several situations of everyday life as well as physiological dysfunction. For example, exaggerated emotional reactivity and impulsivity, severe irritability and temper tantrums, low tolerance to frustration, low reactivity threshold and hyperarousal, inappropriate emotional expression with excessive intensity and slow renormalization, rapid changes in energy and motivation, and increased sensitivity to negative events are signs of ED that may be evident from an early age.8, 9, 10 Especially in childhood, adolescence, and young adulthood, ED may significantly and negatively impact school functioning and professional outcomes, social adjustment and acceptance, and quality of life.11, 12, 13, 14
Despite its relevance in terms of developmental outcomes and prognostic implications, the heterogeneity of presentations and different definitions of ED proposed in the last decades have made this construct a diagnostic challenge.9,10,15 For example, distinct terms are used interchangeably to define ED (eg, affective lability, mood instability), though the term emotional dysregulation is currently the most widely accepted.8,9 Moreover, several tools have been validated to clinically assess ED in both adults16 and youths,17 but are mainly based on self- or parent-report. Currently, the Dysregulation Profile of the Child Behavior Checklist (CBCL-DP)18,19 is one of the most used instruments,20, 21, 22 along with the Strength and Difficulties Questionnaire (SDQ),23 Behavior Rating Inventory of Executive Function (BRIEF),24 Emotion Regulation Checklist (ERC),25 Emotion Regulation Questionnaire (ERQ),26 Difficulties in Emotion Regulation Scale (DERS),27 and Affective Reactivity Index (ARI).28
Although these tools are currently used in clinical practice, most of them do not finely capture the multidimensional nature of ED and are limited to individual components of its construct. For instance, the ARI is specifically focused on the irritability dimension, which is conceptualized as an excessive susceptibility to emotionally salient stimuli with disproportionate intensity of angry responsiveness. Other measures assess the presence of emotional lability—as a pattern of volatile and changeable exaggerated negative emotions—or affective instability—as a cyclic pattern of rapid mood swings of opposite polarities. All these narrow phenomena are often considered interchangeably, though representing subtle variations (or subdimensions) of the broader and complex construct of ED, whose definition is still debated. Although novel tools are being developed to disentangle the different components of ED (eg, the recently validated Reactivity, Intensity, Polarity and Stability questionnaire),29,30 these do not capture the physiological component of ED because they are usually based on parent- and self-report.
Being a transdiagnostic construct, ED may characterize several psychiatric conditions in childhood, including major depressive disorder (MDD), disruptive mood dysregulation disorder, oppositional defiant disorder, conduct disorder (CD), anxiety disorders, and early manifestations of borderline personality disorder (BPD). Moreover, it is also a co-occurring feature of many neurodevelopmental disorders, such as attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), intellectual disability, and Tourette’s syndrome.21,31, 32, 33 Notably, the Research Domain Criteria initiative34,35 has recently proposed emotion regulation as a core dimension of human functioning,36,37 for which specific neurobiological and physiological correlates are expected to be identified as clinical biomarkers that could serve for diagnostic or treatment purposes.
Therefore, we refer here to ED as a transnosographic dimension that can be described across psychiatric conditions regardless of the diagnostic category and, most importantly, their possible etiologies, including both genetic and environmental factors and their complex reciprocal interactions. Specifically, in our view, ED may be conceptualized as a transdiagnostic developmental trait that in some cases may become a (rather stable) specifier, similar to callous unemotional trait, which was initially conceived as a specifier of CD, but is now considered a transdiagnostic trait38 that can vary over time39 and is associated with autonomic and cardiac dysregulation, especially in children and young people.40 Moreover, ED is usually conceptualized as a continuous feature and therefore is assessed dimensionally on a continuum. However, by applying cutoff scores from validated clinical measures of ED (including the scales/tools presented above), it is possible to construct a binary variable to discriminate between young people with and without ED.
From this perspective, we advocate the importance of a rigorous clinical assessment of ED through validated instruments, and thus we decided to include in the present systematic review only studies that provided measures of ED in a clinical population and that were grounded on the definition of ED we provided above. Of note, this approach, which is more focused on the phenomenology of psychiatric conditions and less on the etiopathology underlying ED, has the advantage of preventing a priori exclusion of any possible theoretical framework in relation to the unresolved controversy on the role of nature (eg, individual susceptibility) vs nurture (eg, maltreatment, trauma, and stress in early life).
In the past 2 decades, measures of functioning of the autonomic nervous system (ANS)—a core component of the arousal system that is known to regulate bodily functions including heart rate, perspiration, and pupil dilation—have emerged as potentially reliable transdiagnostic biomarkers of ED that are more strongly related to self-rated reports of ED than several other common biomarkers.41, 42, 43 For instance, accelerations and decelerations of heart rate reflect the activity of the sympathetic and parasympathetic branches of the ANS, and this can be measured in terms of fluctuations in heart rate over time, or heart rate variability (HRV). HRV in the high-frequency band generally corresponds to respiratory sinus arrhythmia (RSA), which reflects the cyclic respiratory gating of autonomic control by afferent inputs from lung receptors through vagal inhibitory efferences.44,45 RSA is thus considered a peripheral index of parasympathetic regulation and cardiac vagal control, coordinated by prefrontal systems, limbic structures, and the amygdala. Interestingly, HRV not only has been found to be abnormal in specific clinical groups (eg, CD),46 but also is considered a transdiagnostic, psychophysiological marker of general psychopathology, or at least of a general vulnerability to psychopathology.47 Among children and adolescents, low baseline RSA and/or excessive RSA withdrawal in response to emotionally salient stimuli have been found to be associated with symptoms of both internalizing and externalizing psychopathology and have been observed in mood disorders and nonsuicidal self-injury, disruptive behavior disorders, and neurodevelopmental conditions, especially ASD and ADHD.47
Similarly, electrodermal activity (EDA), an index of sympathetic activation, refers to the measurement of changes in conductance through sweat produced by skin surface glands innervated by sympathetic terminals; thus changes in the skin conductance level and skin conductance responses reflect changes in ANS activity—predominantly sympathetic cholinergic sudomotor fibers—that regulates this pattern. While tonic skin conductance level varies greatly between and within subjects in different psychological states, gradually decreasing at rest, phasic skin conductance responses are small waves superimposed on the tidal drifts of skin conductance level and occur after the presentation of unexpected significant or aversive stimuli.44 It has been proposed that exaggerated EDA could be a marker of internalizing symptoms, eg, anxiety, which may lead to behavioral inhibition, eg, social withdrawal in response to negative events or punishment.48 Behavioral inhibition has been shown to predict reduced emotion regulation and social competence in children,49 and it has been demonstrated that increased EDA is associated with ED in several neurodevelopmental conditions,50,51 suggesting an association between emotion and arousal dysregulation, internalizing symptoms, and behavioral inhibition. However, a tendency toward reduced EDA has also been observed, eg, in people with ADHD.52
To date, a comprehensive synthesis of quantitative evidence of the autonomic correlates of ED across diagnostic categories in children and adolescents is missing. The present study was planned to fill this gap by conducting a systematic review and a series of meta-analyses of empirical studies measuring markers of ANS functioning in relation to ED in children and adolescents regardless of their clinical diagnosis. We expected to find significant differences in ANS functioning between people with and without ED, although the exact directionality of this effect (eg, hyperfunctioning or hypofunctioning of the ANS associated with ED) could not be predicted. However, based on the available literature, we speculated we would observe associations between ED and reduced vagal control. Findings from our study have the potential to lead to further understanding of the pathophysiological correlates of ED and to inform future research aimed at tracking the outcomes of pharmacological and nonpharmacological interventions by analyzing objective indices of autonomic arousal in support of clinical judgment, including the implementation of noninvasive real-time systems for early detection of emotional outbursts based on the recording of physiological signals.53
Method
This systematic review and meta-analysis followed the 2020 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines54 (see PRISMA Checklist in Supplement 1, available online). The protocol was preregistered on PROSPERO (CRD42021239635).
Search Strategy
We systematically searched PubMed, Web of Knowledge/Science, Ovid MEDLINE, Embase, and APA PsycInfo databases until April 21, 2021, with no language/type of document limits. The full search strategy included keywords and terms associated with the following semantic areas: ED and measures of ANS functioning (including pupil size, heart rate, and EDA) (see Supplement 2, available online).
Selection Criteria
Studies were considered eligible for inclusion when they met the following criteria: empirical studies (case studies and previous systematic or narrative reviews were not included, but their reference lists were searched by hand to identify any additional eligible studies); including youths (up to 20 years old) meeting DSM (version III, IV, IV-TR, 5 or 5-TR) or International Classification of Diseases (ICD) (version 9 or 10) diagnostic criteria for any psychopathological or neurodevelopmental condition; reporting at least one index of ANS functioning (including, but not limited to, heart rate, EDA, respiratory rate, or pupillometry); using any scale assessing ED; reporting, as outcome, correlation coefficients between ED and ANS measures or means and SDs of ANS measures in participants with and without ED.
Data Selection, Extraction, and Coding
Titles and abstracts of studies retrieved from the searches were screened independently by 2 authors (A.B. and G.S.) to identify studies that potentially met inclusion criteria; disagreements were resolved through discussion. The full text of each article marked as eligible for inclusion was then assessed to confirm eligibility. Data from each half of the retained studies were extracted by one author (A.B. or G.S.) using standardized forms and then cross-checked by the other author for accuracy. Extracted information included study design, sample characteristics (size, age, sex, racial/ethnic background, intellectual ability), clinical characteristics (clinical diagnoses, presence of co-occurring conditions), and outcome measures (ED scale used, ANS measure investigated and method, main results and effect sizes with CIs, when provided). Data not available from the published report(s) of the study were requested from corresponding, first, or senior authors via e-mail request.
Outcomes and Assessment of Study Quality
We planned to consider any index of ANS functioning, such as EDA, heart rate, or pupillometry. Study quality was rated by 2 authors (A.B. and G.S.) with the Appraisal tool for Cross-Sectional Studies (AXIS)55 for cross-sectional studies and with the Newcastle-Ottawa Scale (NOS)56 for cohort studies (see Supplement 3, available online).
Data Synthesis and Analysis
First, a descriptive synthesis was performed for all studies included in the systematic review. Then, meta-analyses were conducted in R 4.0.257 to primarily estimate the pooled effect size across studies for each domain of ANS functioning (eg, cardiac, EDA), whenever at least 2 studies reporting on the same outcome for each domain were available. For studies that reported mean and SD of ANS measures in groups of children with and without clinically significant ED, Fisher z-score was calculated (via the R package esc58), together with its standard error and variance. For studies that reported correlations between indices of ED and measures of ANS functioning, we converted Pearson r coefficients into Fisher z scores, calculated the standard error and variance of Fisher z scores (via the R package esc58), and used these as a measure of effect size in the meta-analyses.59
Multilevel random-effects meta-analytic models were fitted to the data in metafor60 with effect sizes nested within studies for those that reported multiple effect sizes for the same component to account for nonindependence of data. The restricted maximum likelihood estimator was used with the Knapp-Hartung CI adjustment.61 The Cochran Q test (p value < .05) was calculated to assess the presence of significant heterogeneity. Publication bias was assessed visually using funnel plots (for small studies) and quantitatively with the rank correlation test for funnel plot asymmetry62 (a version of the Egger test,63 which is suitable for multivariate meta-analytic models). On identification of significant heterogeneity, we planned to carry out exploratory meta-regressions to investigate potential factors that may have affected the pooled effect size, eg, clinical diagnosis or experimental condition during which autonomic functioning was assessed. Supplement 4, available online, reports the changes/additions to the original preregistered protocol, with reasons for the changes. Raw data and R codes are available on request to the corresponding author.
Results
Of 5,739 references initially retrieved as possibly relevant, after excluding 5,659 references based on the title/abstract and 68 further references based on full text (list of articles excluded at full text screening and reasons of exclusion are reported in Supplement 5, available online), 12 studies met inclusion criteria and were included in the review (1,016 total participants; 65.6% with a clinical diagnosis), most of which assessed ED dimensionally on a continuum (Figure 1, Table 1). Of these studies, 5 investigated ED in ADHD (252 participants with a diagnosis of ADHD; 149 control participants)43,50,64, 65, 66; 3 investigated ED in ASD (107 participants with a diagnosis of ASD; 45 control participants)51,67,68; and 1 each investigated ED in internalizing disorders (63 participants with a diagnosis of anxiety disorder or MDD; 62 control participants),69 in CD and MDD (30 participants with a diagnosis of CD; 28 participants with a diagnosis of MDD; 80 participants with a diagnosis of CD+MDD; 69 control participants),70 in oppositional defiant disorder (63 participants with a diagnosis of oppositional defiant disorder; 25 control participants),71 and in a cohort of 43 youths with BPD.72 Specifically, 8 studies reported cardiac measures,43,50,64, 65, 66, 67, 68,70,72 4 studies reported EDA measures,50,51,66,71 1 study reported respiratory rate measures,69 and 1 study reported data on cortisol (Table 1).68 All 12 studies were included in the descriptive review, and 9 studies (including a total of 567 youths) were included in the meta-analyses (Figure 1).
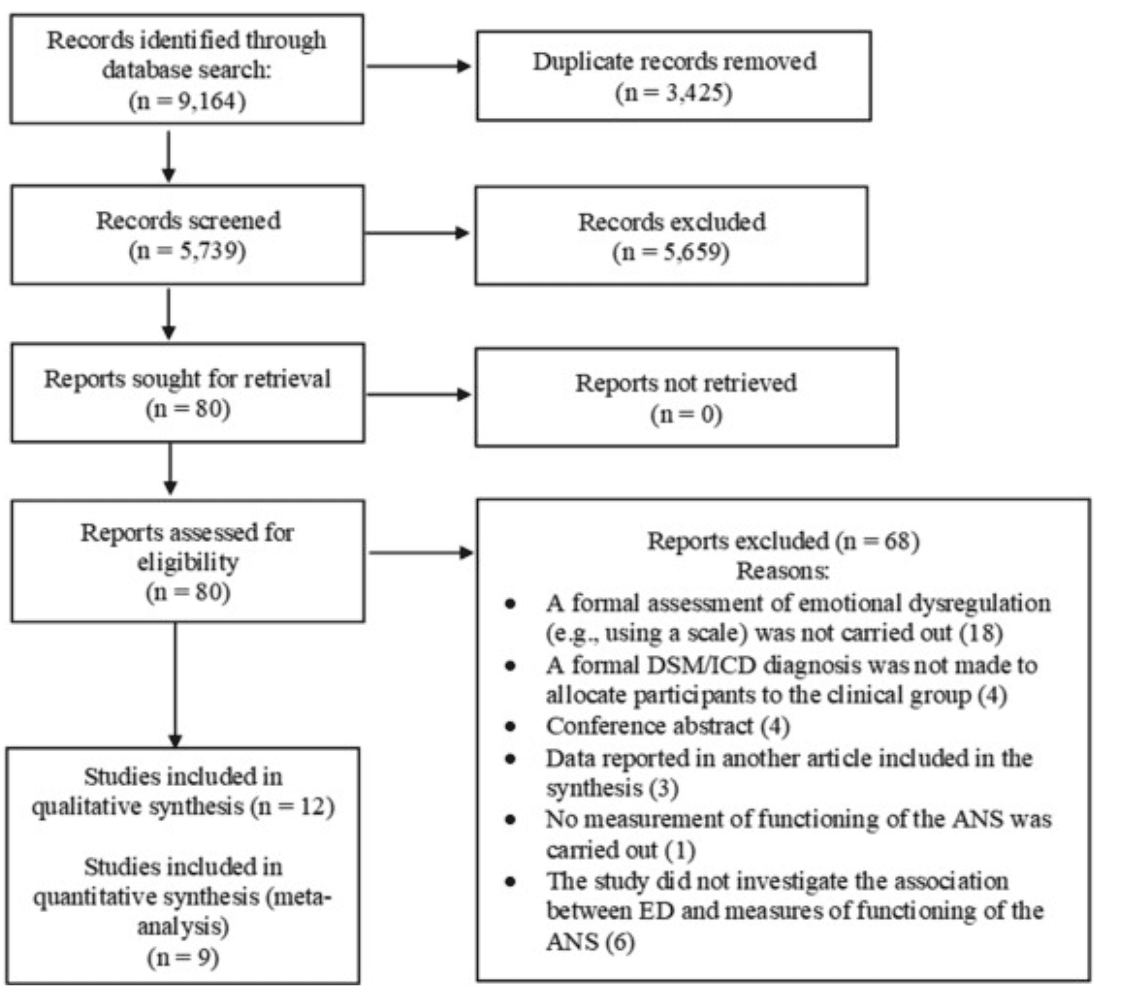
Figure 1. PRISMA Flowchart
Note: ANS = autonomic nervous system; ED= emotional dysregulation. Adapted from Page et al.54 (For more information, visit http://www.prisma-statement.org/ . Published under Creative Commons license CC BY: https://creativecommons.org/licenses/by/4.0 ).
Table 1. Studies Investigating Autonomic Nervous System (ANS) Functioning in Youths With Emotional Dysregulation (ED)
First author, year | Clinical group(s) | Sample size | Mean age, y | Sociodemographic background | ED scale | ANS measure(s) | Main findings |
|---|---|---|---|---|---|---|---|
Beauchaine, 201343 | ADHD | 99 clinical | 5.36 | Preschoolers attending schools in Washington area; no data reported on race/ethnicity | SCS–Emotion Regulation | RSA | Reduced RSA during resting state was associated with increased ED (Pearson correlation) |
Bunford, 201764 | ADHD | 48 clinical; 56 controls | Clinical: 9.01; controls: 9.01 | Children attending private or public schools in Ohio; 47.9% African American/Black, 52.1% White | ERC–Lability Negativity and ERC–Emotion Regulation | RMSSD | Reduced HRV during resting state was associated with increased ED (Pearson correlation) |
Fenning, 201851 | ASD | 46 clinical | 6.39 | Autistic children from California; 9% African American, 11% Asian American, 24% Hispanic, 44% non-Hispanic, 11% other, 68% White | Standardized coding from videotape using Dysregulation Coding System | EDA | EDA during coregulation and independent regulation tasks was not associated with emotional dysregulation (Pearson correlation) |
Guy, 201467 | ASD | 14 clinical; 22 controls | Clinical: 12.27; controls: 13.12 | Children and adolescents recruited from the community in Philadelphia, Pennsylvania; no data reported on race/ethnicity | BRIEF–Emotional Control | RSA, R-R intervals and HR | Shorter R-R intervals (increased HR) during resting- state were associated with increased ED; RSA was not associated with ED (Pearson correlation) |
Henje Blom, 201469 | MDD and AD | 63 clinical; 62 controls | Clinical: 16.80; controls: 16.50 | Adolescent girls living in the center of Stockholm, Sweden, surrounding suburbs, or in smaller towns nearby; no data reported on race/ethnicity | SDQ-Emotional | Respiratory rate | Higher respiratory rate during resting state was associated with increased ED (Pearson correlation) |
Kvadsheim, 202065 | ADHD | 34 clinical; 33 controls | Clinical: 14.34; controls: 14.62 | Adolescents recruited in Bergen, Norway; no data reported on race/ethnicity | DERS | HF peak and HF power | Lower cardiac vagal control (HF power) during resting state was associated with increased ED (Pearson correlation) |
McQuade, 201750 | ADHD | 23 clinical; 38 controls | Clinical: 11.62; controls: 11.62 | Children and adolescents recruited in Massachusetts; 5% Asian, 7% other or mixed race, 8% Hispanic or Latino, 85% White; median household income $100,000; average parent education level 16 y | ERC–Lability Negativity, ERC–Emotion Regulation, and ERC–Emotional Control | RSA reactivity and SCL reactivity | Increased RSA reactivity in response to an experience of social rejection (but not during an impossible puzzle task) was marginally associated with increased ED; SCL reactivity was not associated with ED (Pearson correlation) |
Mikita, 201568 | ASD | 47 clinical; 23 controls | Clinical: 12.8; controls: 13.9 | Autistic boys recruited in London and the southeast of United Kingdom; no data reported on race/ethnicity | ARI | Cortisol and HR | Parent-reported and self-reported ED was associated with reduced cortisol response and reduced HR during psychosocial stress test |
Taskiran, 201866 | ADHD | 48 clinical; 22 controls | Clinical: 8.43; controls: 8.26 | Children recruited in Ankara, Turkey; no data reported on race/ethnicity | CBCL–Dysregulation Profile and SDQ-Emotional | EDA and HR | Children with ADHD and ED showed lower HR reactivity than controls (but not ADHD without ED) in response to unpleasant IAPS visual stimuli; children with ADHD (with or without ED) and healthy controls did not differ on EDA reactivity to IAPS pictures (between-group effects; ED vs non-ED) |
Tonacci, 201971 | ODD | 63 clinical; 25 controls | Clinical: 9.00; controls: 9.00 | Children recruited from the community in Pisa, Italy; no data reported on race/ethnicity | CBCL–Dysregulation Profile | SCR | Children with ODD and ED showed increased SCR to emotional visual stimuli displaying anger and sadness compared with children with ODD without ED and healthy controls (between-group effects; ED vs non-ED) |
Vasilev, 200970 | CD and MDD | 138 clinical; 69 controls | Clinical: 10.00; controls: 10.00 | Children recruited from urban Seattle, Washington; 6% Asian, 12% Black, 20% other, 62% White; annual income below $70,000 (below median) for 77% of families | DERS | RSA | No association between RSA and ED, either at rest or after a sadness-inducing video (Pearson correlation) |
Weise, 202072 | BPD | 43 clinical | 15.50 | Adolescents with diagnosis of BPD recruited in Heidelberg, Germany; no data reported on race/ethnicity | LPI-ED and self-reported affective instability | HR and RMSSD | Higher HR (but not HRV) during resting state was associated with increased ED (based on LPI, but not on self-reported measure) in adolescents with BPD (Pearson correlation) |
Note: AD = anxiety disorder; ADHD = attention-deficit/hyperactivity disorder; ARI = Affective Reactivity Index; ASD =autism spectrum disorder; BPD = borderline personality disorder; BRIEF = Behavior Rating Inventory of Executive Function; CBCL = Child Behavior Checklist 6–18; CD = conduct disorder; DERS = Difficulties in Emotion Regulation Scale; EDA = electrodermal activity; ERC = Emotion Regulation Checklist; HF = high frequency; HR = heart rate; HRV = heart rate variability; IAPS = International Affective Picture System; LPI = Life Problems Inventory; MDD = major depressive disorder; ODD = oppositional defiant disorder; RMSSD = root mean square of successive differences between inter-beat intervals; R-R interval = peak-to-peak interval; RSA = respiratory sinus arrhythmia; SCL = skin conductance level; SCR = skin conductance response; SCS = Social Competence Scale–Parent Report; SDQ = Strengths and Difficulties Questionnaire.
Association Between ED and Cardiac Measures
Of 9 studies examining the association between cardiac functioning and ED, 6 studies43,50,64,65,67,72 provided sufficient information for effect sizes to be computed and were included in the meta-analysis. Meta-analysis conducted on 14 effect sizes showed that ED was not significantly associated with cardiac measures in children and adolescents with ADHD, BPD, or ASD (Fisher z = −0.1750, SE = 0.0933, 95% CI [−0.3764, 0.0265], t = −1.8760, p = .0833) (Table 2, Figure 2A). Between-study heterogeneity was significant (Q = 43.4794, p < .0001), while no publication bias was detected (Kendall τ = −0.0597, p = .7779) (see Supplement 6, Figure S1, available online).
Table 2. Studies Investigating the Association Between Emotional Dysregulation (ED) and Cardiac Measures
Studies included in meta-analysis
First author, year | Clinical group(s) | Experimental task/condition | Fisher z | [95% CI] |
|---|---|---|---|---|
Beuchaine, 201343 | ADHD | Resting state | −0.30 | [−0.50, −0.10] |
Bunford, 201764 | ADHD | Resting state | −0.01 | [−0.20, 0.19] |
ADHD | Resting state | 0.08 | [−0.12, 0.27] | |
ADHD | Resting state | −0.00 | [−0.20, 0.19] | |
ADHD | Resting state | −0.28 | [−0.48, −0.09] | |
Guy, 201467 | ASD | Resting state | −0.23 | [−0.58, 0.11] |
ASD | Resting state | −0.38 | [−0.72, −0.04] | |
Kvadsheim, 202065 | ADHD | Resting state | −0.30 | [−0.54, −0.05] |
McQuade, 201750 | ADHD | Impossible puzzles | 0.12 | [−0.14, 0.38] |
ADHD | Impossible puzzles | 0.11 | [−0.15, 0.37] | |
Social rejection task | 0.20 | [−0.05, 0.46] | ||
Social rejection task | 0.27 | [0.01, 0.52] | ||
Weise, 202072 | BPD | Resting state | −0.50 | [−0.81, −0.19] |
BPD | Resting state | −0.28 | [−0.59, 0.03] |
Studies included in review
Author, year | Clinical group(s) | Experimental task/condition | Descriptive summary of findings |
|---|---|---|---|
Mikita, 201568 | ASD | Psychosocial stress test | Parent-reported and self-reported ED (ARI) was associated with reduced HR in response to stress |
Taskiran, 201866 | ADHD | IAPS pictures presentation | Children with ADHD and ED (CBCL–Dysregulation Profile and SDQ-Emotional) showed lower HR reactivity than controls (but not ADHD without ED) in response to unpleasant visual stimuli |
Vasilev, 200970 | CD and MDD | Resting state and sadness-inducing video | No association between RSA and ED (DERS), either at rest or after sadness-inducing video |
Note: ADHD = attention deficit/hyperactivity disorder; ARI = Affective Reactivity Index; ASD =autism spectrum disorder; BPD = borderline personality disorder; CBCL = Child Behavior Checklist 6–18; CD = conduct disorder; DERS = Difficulties in Emotion Regulation Scale; HR = heart rate; IAPS = International Affective Picture System; MDD = major depressive disorder; RSA = respiratory sinus arrhythmia; SDQ = Strengths and Difficulties Questionnaire.
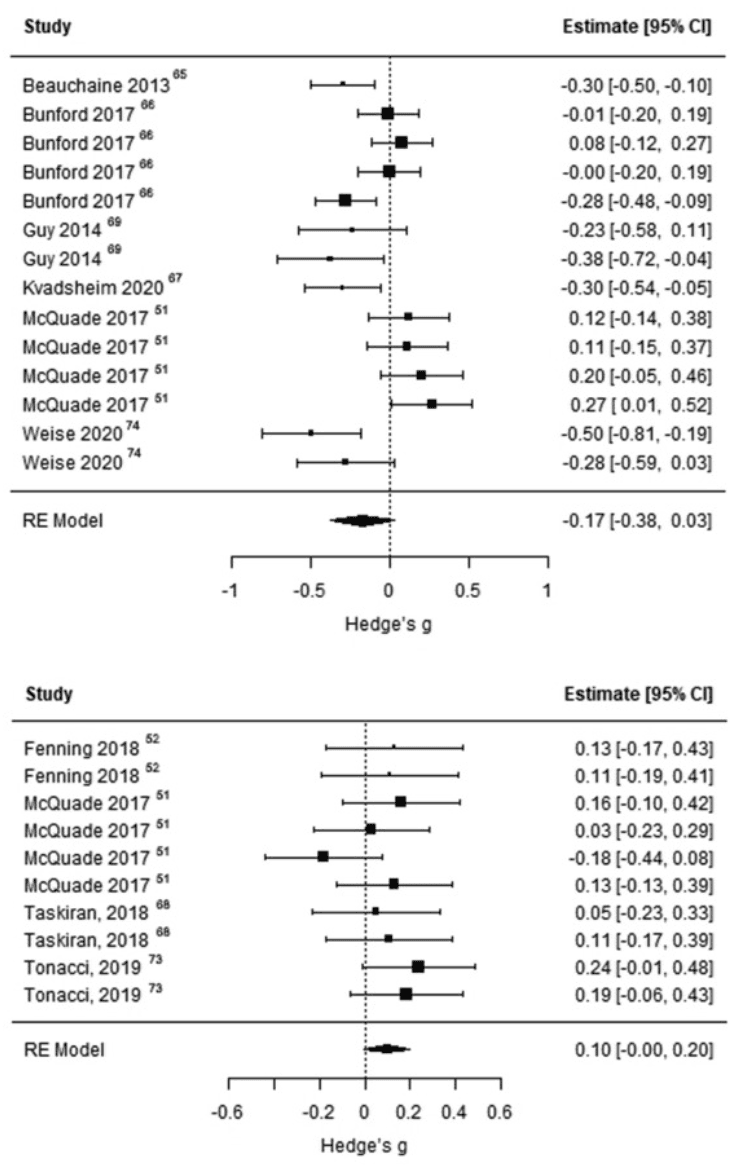
Figure 2. Forest Plots
Note: Plots report effect sizes for studies investigating the association between emotional dysregulation and cardiac measures (A) and the association between emotional dysregulation and electrodermal activity measures (B).
Considering that significant heterogeneity was detected, we conducted a series of post hoc meta-regressions to investigate if the clinical diagnosis of participants and the experimental condition during which autonomic functioning was measured affected the main findings of the meta-analysis. Clinical diagnosis was not a significant moderator of the pooled effect size (F1,12 = 1.9259, p = .1904), indicating that results were the same across the different diagnostic groups. However, we found that the experimental condition was a significant moderator of the pooled effect size (F1,12 = 6.9599, p = .0216). Specifically, as highlighted in Figure S3 (available online), the meta-analytic model was significant for resting-state cardiac measures43,64,65,67,72 (Fisher z = −0.2403, SE = 0.0751, 95% CI [−0.4103, −0.0703], t = −3.1983, p = .0109), but not for cardiac measures collected during an active task50 (Fisher z = 0.1750, SE = 0.0657, 95% CI [−0.0340, 0.3839], t = 2.6650, p = .0760) (see Supplement 7, Figure S2, available online). Although still significant, cross-study heterogeneity was lower for the meta-analysis on resting-state cardiac measures compared with the initial meta-analysis (Q = 21.7456, p = .0097), and it was not detected for the meta-analysis on task-related cardiac measures (Q = 0.9395, p = .8159). Overall, these findings suggest that reduced resting-state cardiac vagal control is associated with increased ED, while no association between ED and task-related cardiac measures was detected.
We could not obtain data for 3 studies, which therefore were included in the descriptive review only (Table 2). One study reported a significant association between higher ED and reduced heart rate in response to stress during a psychosocial stress test in autistic youths,68 while 2 others did not find any association between ED and heart rate reactivity66 or RSA70 in youths with ADHD and youths with CD and MDD, respectively.
Association Between ED and EDA
The meta-analysis included 4 studies examining the association between EDA and ED.50,51,66,71 The meta-analytic model including 10 effect sizes was not significant (Fisher’s z = 0.0982, SE = 0.0445, 95% CI [−0.0024, 0.1987], t = 2.2075, p = .0547) (Table 3, Figure 2B). Cross-study heterogeneity was not significant (Q = 6.9286, p = .6446), and publication bias was not detected (Kendall τ = −0.2418, p = .3518) (see Supplement 8, Figure S3, available online). Overall, we did not find evidence of an association between ED and altered EDA.
Table 3. Studies Investigating Association Between Emotion Dysregulation (ED) and Electrodermal Activity (EDA) and Other Autonomic Nervous System (ANS) Measures
Studies included in meta-analysis investigating association between EDA and ED EDA
First author, year | Clinical group(s) | Experimental task/condition | Fishers z | [95% CI] |
|---|---|---|---|---|
Fenning, 201851 | ASD | Coregulation task | 0.13 | [−0.17, 0.43] |
ASD | Independent regulation task | 0.11 | [−0.19, 0.41] | |
McQuade, 201750 | ADHD | Social rejection task | −0.16 | [−0.42, 0.10] |
ADHD | Social rejection task | 0.03 | [−0.23, 0.29] | |
ADHD | Impossible puzzles | 0.18 | [−0.08, 0.44] | |
ADHD | Impossible puzzles | 0.13 | [−0.13, 0.39] | |
Taskiran, 201866 | ADHD | IAPS pictures presentation | 0.05 | [−0.23, 0.33] |
ADHD | IAPS pictures presentation | 0.11 | [−0.17, 0.39] | |
Tonacci, 201971 | ODD | Emotional pictures presentation | 0.24 | [−0.01, 0.48] |
ODD | Emotional pictures presentation | 0.19 | [−0.06, 0.43] |
Other measures of ANS functioning
Henje Blom, 201469 | MDD and AD | IAPS pictures presentation | Higher respiratory rate was associated with increased ED (SDQ-Emotional) |
Mikita, 201568 | ASD | Psychosocial stress test | Parent-reported and self-reported ED (ARI) was associated with reduced cortisol response to stress |
Note: AD = anxiety disorder; ADHD = attention-deficit/hyperactivity disorder; ARI = Affective Reactivity Index; ASD = autism spectrum disorder; IAPS = International Affective Picture System; MDD = major depressive disorder; ODD = oppositional defiant disorder; SDQ = Strengths and Difficulties Questionnaire.
Association Between ED and Other Measures of ANS Functioning
Two studies that investigated the association between other measures of ANS functioning and ED were not included in meta-analyses, but were included in the descriptive review (Table 3). The study investigating ED in relation to cortisol68 reported that higher ED (measured with parent-reported and self-reported ARI) was associated with reduced cortisol response to stress, while the other study69 found that higher respiratory rate was associated with increased ED (measured with the SDQ emotional symptoms).
Discussion
This is, to our knowledge, the first systematic review with meta-analysis investigating, transnosographically, the association between markers of ANS functioning and ED in children and adolescents with a variety of clinical diagnoses. Although we did not find a direct association between cardiac measures and ED (as reported in the main meta-analysis), we observed significant between-study heterogeneity, and after assessing the role of clinical diagnosis or experimental condition as possible moderators of such an association, we found a significant association between indices of cardiac vagal control and ED, but only when these were measured at rest (and not during an active task), indicating that reduced vagal control is associated with increased ED. This finding emerged from studies that included children and adolescents with ADHD, BPD, and ASD, indicating how reduced vagal control may be a transdiagnostic marker of ED in youths. The meta-analytic model investigating the association between ED and EDA measures was marginally not significant, suggesting that additional studies will contribute to better clarifying the possible role of increased EDA as a biomarker of ED.
Our findings are in line with previous studies that focused on general psychopathology (but not ED, specifically), which showed that reduced vagal control is associated with internalizing and externalizing symptoms,47 and with previous evidence showing that weak arousal regulation may be a key factor in the pathophysiology of ADHD.52 Irritability and temper tantrums, low tolerance to frustration, and low reactivity threshold in children and adolescents may be an indirect index of autonomic dysregulation, negatively impacting global functioning. Only a limited number of studies specifically assessing RSA reactivity to emotionally salient stimuli were retrieved and thus could be meta-analyzed. Nonetheless, the importance of assessing ANS correlates of ED in a transdiagnostic way is also highlighted by the findings of a recent study (which was published after the search for this systematic review and meta-analysis was conducted) reporting an association between irritability and reduced HRV.73
If replicated in future larger meta-analyses, the results of our study suggest the potential relevance of assessing ANS functioning in patients with ED. Indeed, indices of arousal regulation may represent reliable biomarkers that might serve (on being found cost-effective) as screening tools in clinical settings, adopting a dimensional approach to psychopathology.34,35 This is also supported by recent efforts to implement noninvasive real-time systems for the early detection of emotional outbursts in individuals with ASD based on physiological signals.53 Moreover, such biomarkers may also help clinicians for treatment purposes, as one might expect interventions aimed at improving ED to be associated with changes on measures of ANS functioning. For example, both selective serotonin reuptake inhibitors and tricyclic antidepressants are known to alter HRV in adults and adolescents74,75 as well as psychostimulants, and interventions that increase HRV also improve sleep quality and stress-related cortisol responses.76,77
The present study has several limitations. First, we could include only a limited number of studies in the meta-analyses, especially in relation to EDA. As such, the pooled effect sizes for these should be considered preliminary until further larger meta-analyses can be conducted. However, the effect sizes we calculated are based on all the available data in the existing literature to date. Moreover, it is important to note that, as highlighted in Supplement 3 (available online), a substantial proportion of studies (more than 60%) had methodological flaws, including no justification for the sample size recruited for the study (eg, no power analysis reported), a sample not fully representative of the population, and no description of how much data were missing or how many participants were included in final analyses compared with the initially recruited samples. These limitations impact the confidence in the strength of our results; therefore, we suggest that future studies be carefully designed to avoid reporting results of dubious quality and to improve transparency in reporting the main results and sample characteristics in full (either in the main article or in supplementary materials).
Second, cross-study heterogeneity was significant for the meta-analysis on cardiac measures, probably due to differences in study design and experimental conditions. In this regard, it should be noted that detailed recommendations on how to assess HRV in studies of arousal systems in psychopathology have been recently proposed.35 These include careful baseline recording with accurate RSA estimation, standardized electrocardiogram sampling rates and recording epoch lengths, and preferred quantification algorithms. Standardized approaches in the assessment of HRV are expected to reduce heterogeneity across future studies, thus making evidence synthesis more compelling. Third, even though we planned to explore ED regardless of the clinical diagnosis, most studies included in the meta-analyses were carried out with children and adolescents with ADHD. This is not surprising considering the prevalence of ED in this population.78,79 Alternatively, as current criteria for ADHD do not include ED as a core symptom (despite the large clinical and research evidence), additional and specific evaluation of ED is usually needed for this condition, in contrast to others (eg, mood disorders) where ED is a core feature and is therefore not explicitly assessed. Fourth, although we had planned to investigate if interventions aimed at improving ED were associated with changes on measures of ANS functioning, we did not find any study that adopted this empirical approach. Fifth, the results present in this review are valid at the group level, but cannot be informative at the individual patient level. Sixth, our review was exclusively focused on clinical samples, which can be seen as a choice limiting the scope of the study. However, the decision to restrict our search to studies on clinical samples was intended to limit the heterogeneity of the included studies, identify correlates of clinical conditions, and inform further research that can be ultimately translated in daily clinical practice. Finally, our broad conceptualization of ED, especially from samples not drawn from the general populations, makes it challenging to distinguish between the effects of ED itself or simply the presence of any psychopathology.
The systematic overview of the available studies allowed us to make some suggestions for future research in the field. We suggest that future research studies should measure ED and ANS functioning in both laboratory and naturalistic settings to improve external validity of findings from laboratory settings. Our results indicate that, among different indices of autonomic functioning, cardiac measures of vagal/parasympathetic regulation at rest can prove particularly helpful in objectively assessing the severity of ED in clinical samples of youths compared with electrodermal measures or cardiac measures obtained during cognitive, attentional, or distressing tasks. However, to make this conclusion more solid, further studies are needed, for example, to examine if cardiac measures (eg, RSA and HRV) can be used to predict increases in ED severity over time and the onset of potentially harmful behaviors, such as self-harm or suicide attempts. From this perspective, larger samples, including broader arrays of diagnostic categories, should be recruited, and dimensional research frameworks should be adopted to further investigate the neurobiological and neurocognitive correlates of ED in patients with a variety of neurodevelopmental and psychiatric conditions. In line with this approach, studies should also be conducted to understand if an association between ED and reduced vagal control can also be found in nonclinical populations. Whereas we focused on ED at the phenomenological level, further studies should assess to which extent the relationship between ED and autonomic dysregulation is moderated by specific causal/contributing factors, including childhood traumatic experiences and individual susceptibility to ED.
Further research is also needed to clarify if pharmacological and/or psychological interventions aimed at treating ED have a significant impact on ANS functioning. We did not find any study that planned to investigate whether interventions aimed at improving ED were associated with changes on measures of ANS functioning. Research so far has shown that cognitive and/or behavioral interventions for posttraumatic stress disorder,80 ADHD,43 and externalizing behavioral problems81 lead to positive effects on cardiac measures of parasympathetic regulation (ie, those we found altered in youths with ED in our study). However, these studies also seem to suggest that baseline autonomic functioning or dysregulation is likely to impact the effectiveness of such interventions, with worse outcomes for young people displaying indices of autonomic dysregulation or weaker arousal regulation (eg, reduced RSA) before starting the intervention. Therefore, we suggest that further randomized trials should be conducted to assess the impact of interventions for ED (eg, dialectical behavioral therapy) on measures of autonomic functioning (specifically, measures reflecting parasympathetic regulation). Moreover, investigating if markers of dysregulated autonomic arousal at baseline contribute to predict treatment outcomes should be a priority for the field.
Future studies should also focus on the association between ANS functioning and ED in adult samples, assessing if developmental changes in ANS functioning and arousal regulation are associated with changes in emotional regulation throughout different stages of life. Several lines of research on specific conditions could be developed. For instance, it should be explored whether these putative biomarkers may help detect hypersensitive individuals who may be more prone to develop full-blown psychiatric disorders, similar to what has already been reported for posttraumatic stress disorder after exposure to social and environmental stresses of different degrees.82 Lastly, as also suggested by a recently published systematic review and meta-analysis on task-based functional magnetic resonance imaging studies in young people with irritability,83 researchers and clinicians should work toward developing valid and reliable measures to assess ED components, such as irritability.
In conclusion, we found preliminary evidence that dysregulated autonomic arousal—more specifically, reduced resting-state vagal control—may be associated with increased ED. Weaker autonomic arousal regulation is likely to be a transdiagnostic marker of psychopathology and behavioral dysregulation in children and adolescents; however, further research is still needed to understand whether medical and nonmedical interventions aimed at treating ED have some effects on ANS functioning or if, in a complementary fashion, interventions aimed at increasing arousal regulation and vagal control could improve emotion regulation during childhood and adolescence.