Abstract
Background: Few studies have investigated changes in brain structure and function associated with recovery from cocaine use disorder (CUD), and fewer still have identified brain changes associated with specific CUD treatments, which could inform treatment development and optimization. Methods: In this longitudinal study, T1-weighted magnetic resonance imaging scans were acquired from 41 methadone-maintained individuals with CUD (15 women) at the beginning of and after 12 weeks of outpatient treatment. As part of a larger randomized controlled trial, these participants were randomly assigned to receive (or not) computer-based training for cognitive behavioral therapy (CBT4CBT), and galantamine (or placebo). Results: Irrespective of treatment condition, whole-brain voxel-based morphometry analyses revealed a significant decrease in right caudate body, bilateral cerebellum, and right middle temporal gyrus gray matter volume (GMV) at post-treatment relative to the start of treatment. Subsequent region of interest analyses found that greater reductions in right caudate and bilateral cerebellar GMV were associated with higher relative and absolute levels of cocaine use during treatment, respectively. Participants who completed more CBT4CBT modules had a greater reduction in right middle temporal gyrus GMV. Conclusions: These results extend previous findings regarding changes in caudate and cerebellar GMV as a function of cocaine use and provide the first evidence of a change in brain structure as a function of engagement in digital CBT for addiction. These data suggest a novel potential mechanism underlying how CBT4CBT and CBT more broadly may exert therapeutic effects on substance-use-related behaviors through brain regions implicated in semantic knowledge.
1. Introduction
Cocaine use disorder (CUD) remains a serious public health concern associated with emergency room visits, overdose deaths, and physical health concerns including heart attacks and strokes (Schwartz et al., 2022, Simpson et al., 1999). At present, there are still no Food and Drug Administration-approved pharmacotherapies for CUD (Schwartz et al., 2022), and existing psychosocial interventions have variable effect sizes (Bentzley et al., 2021). Understanding the neurobiology of addiction treatments may provide insight into the mechanisms of behavior change associated with treatment, which can guide treatment development (Brody et al., 2007; Chung et al., 2016; Costello et al., 2010; DeVito et al., 2017; Houck et al., 2013; Kober et al., 2017; Potenza et al., 2013; Reese et al., 2021; Zilverstand et al., 2016) and identify neuromarkers that may predict treatment efficacy among individuals with addiction (Brewer et al., 2008, Garrison and Potenza, 2014, Gowin et al., 2019, Worhunsky et al., 2013, Xu et al., 2010, Yip et al., 2019). While there have been studies of brain structure and function associated with abstinence/recovery from CUD (Balodis et al., 2016, Bell et al., 2011, Jedema et al., 2021, Parvaz et al., 2017, Parvaz et al., 2022), few have identified changes in brain structure and function associated with specific pharmacological or psychosocial CUD treatments (DeVito et al., 2017, Reese et al., 2021).
Cognitive behavioral therapy (CBT) is a common psychosocial treatment approach for CUD given its wide evidence base and durability of effects (Bentzley et al., 2021, Schwartz et al., 2022). CBT for substance use disorders is a multifaceted treatment which includes cognitive components, such as identifying one’s thoughts, feelings and behaviors, and behavioral components, such as learning behavioral coping skills and avoiding high-risk situations (Carroll, 1998, McHugh et al., 2010). Effectively implementing and increasing accessibility of high-fidelity CBT remains a challenge and has led to the development of digital versions of CBT, such as computer-based training for CBT (CBT4CBT). CBT4CBT allows for standardized delivery of CBT skills targeting substance use and has been found to be efficacious as an adjunct to standard care in multiple clinical trials (Carroll et al., 2008, Carroll et al., 2014, Carroll et al., 2018), in part by improving the quality of coping skills (Kiluk et al., 2017, Kiluk et al., 2010).
The neural mechanisms of CBT in the context of addiction are less well-investigated compared to psychiatric disorders (Yuan et al., 2022), although theories have been proposed. For example, learning coping strategies to manage cravings may influence brain regions implicated in cue-induced drug craving such as the medial prefrontal cortex and anterior cingulate cortex (Potenza et al., 2011). Some longitudinal functional neuroimagingstudies have identified neural correlates associated with CBT in the context of addiction treatment (DeVito et al., 2017, DeVito et al., 2012, Naqvi et al., 2024, Srivastava et al., 2021). For example, one study found that individuals with CUD who completed more CBT sessions had greater reductions in Stroop-task-related activity in the precentral gyrus, inferior parietal lobule, and frontal gyrus (DeVito et al., 2017). We are not aware of any longitudinal structural neuroimaging studies that have investigated possible effects of CBT in the context of any substance use disorder or behavioral addiction.
In this study, we investigated longitudinal changes in brain gray matter volume (GMV) in a subset of participants who participated in a randomized controlled trial which investigated the efficacy of CBT4CBT and galantamine, a cholinesterase inhibitor that has been found in some studies to reduce cocaine use (Carroll et al., 2018, Sofuoglu and Carroll, 2011), over a 12-week period of outpatient treatment for individuals with CUD who were maintained on methadone for opioid use disorder (Carroll et al., 2018). Participants in the parent trial were randomly assigned to receive (or not) CBT4CBT and galantamine (or placebo), with the primary outcome measure being the percentage of days of self-reported abstinence from cocaine. The parent trial identified a significant main effect of each treatment over time on reducing cocaine use (Carroll et al., 2018).
Based on a prior study which found increased prefrontal and inferior frontal gyrus GMV in individuals with CUD who reduced their cocaine use (Parvaz et al., 2017), we hypothesized that independent of treatment condition, we would observe a similar overall increase in GMV after 12 weeks of treatment as compared to the start of treatment. Since different brain regions have been found to recover with abstinence depending on the substance (Parvaz et al., 2022), and the majority of participants in Parvaz et al. did not have other substance use disorders (Parvaz et al., 2017), we hypothesized that these increases in GMV would be correlated with measures of cocaine use (and not other substance use) during treatment. Finally, we explored whether there was a greater change in GMV in participants who received CBT4CBT versus participants who did not, and whether these differences were specific to CBT4CBT and not other treatment conditions.
2. Method
2.1. Overview of parent trial
Details of the randomized controlled trial and clinical outcomes can be found in the manuscript for the parent study (Carroll et al., 2018). Briefly, participants were recruited from an outpatient opioid treatment program in Connecticut between 2009 and 2015. At the time of enrollment, individuals were maintained on a stable dose of methadone for opioid use disorder, met criteria for current cocaine dependence or cocaine abuse as assessed by the Structured Clinical Interview for DSM-IV-TR, and provided at least one cocaine-positive urine test during screening. Eligible individuals provided written informed consent approved by the Yale School of Medicine institutional review board and were randomly assigned to one of four conditions using a masked, computerized urn randomization program.
2.2. Treatment conditions
The study treatment period was 12 weeks, during which all participants continued to receive standard methadone treatment, consisting of daily methadone plus individual and group counseling, with access to other program services. Methadone dose and frequency during treatment were recorded on a monthly and weekly basis, respectively. Frequency of individual and group counseling sessions attended during treatment were also documented. In addition, participants were randomized to CBT4CBT or not, and galantamine or placebo, in a 2×2 factorial design.
2.2.1. CBT4CBT
CBT4CBT is a web-based version of CBT designed for direct patient access that has been empirically validated in multiple randomized controlled trials (Carroll et al., 2008, Carroll et al., 2019, Carroll et al., 2014, Kiluk et al., 2016, Kiluk et al., 2018, Paris et al., 2018). CBT4CBT uses video vignettes, quizzes, and interactive exercises to teach and model effective use of skills and strategies for reducing substance use. Participants assigned to CBT4CBT accessed the program weekly on a dedicated computer in a private space within the treatment facility.
2.2.2. Galantamine
Participants were prescribed a maximum dose of 8 mg/day galantamine extended release. Study medication (galantamine or matched placebo capsules) was dispensed daily at the time of methadone dosing and observed by program nurses.
2.3. Assessments
2.3.1. Substance use
The frequency of substance use was quantified using the Timeline Followback method (Sobell and Sobell, 1992), which collected day-by-day self-report of substance use during a 4-week period (28 days) prior to study enrollment and during the 12-week study treatment period (84 days). The primary outcome measure in the parent trial was defined as percent days abstinent from cocaine per month (i.e., per 28 days). For ease of interpretation, two similar variables were used here: percent days of cocaine use 4 weeks before treatment and percent days of cocaine use during treatment (i.e., total number of days cocaine was used during treatment divided by 84 days). Percent days of other substance use (alcohol, cannabis, and opioids) before and during treatment were also quantified (Supplementary Table S1). In addition, we calculated a relative measure of cocaine use, operationalized as the number of days of cocaine use during the last week of study treatment (week 12) minus number of days of cocaine use during the week before randomization to treatment (week 0).
2.3.2. CBT engagement
The number of CBT4CBT modules completed out of a total of seven modules was quantified within the CBT4CBT software.
2.4. Structural brain data collection and pre-processing
Structural magnetic resonance imaging (MRI) scans were obtained at the beginning of study treatment (mean±SD 2.2±7.5 days after randomization) and after treatment (14.9±15.8 days after the 84-day treatment window) for a subset of participants interested in and eligible for MRI; the average duration between the two scans was 96.6±17.2 days (Supplementary Table S2). Participants underwent MRI scanning on Siemens Trio or Prisma3 T scanners (Siemens AG, Erlangen, Germany). Structural MRI data were acquired with a sagittal high-resolution T1-weighted 3D magnetization-prepared-rapid-gradient-echo (MPRAGE) sequence with the following parameters: repetition time=2530 ms, echo time=3.34 ms for Trio, 2.77 ms for Prisma, flip angle=7°, field of view=256 mm x 256 mm, matrix=256 x 256, 176 slices, 1 mm3 isotropic voxel resolution.
Voxel-based morphometry (VBM) analyses were performed using Computational Anatomy Toolbox (CAT12) (http://www.neuro.uni-jena.de/cat/ ), an extension toolkit of Statistical Parametric Mapping (SPM12), running in Matlab R2023a (Mathworks, MA, USA) (Ashburner and Friston, 2000, Gaser et al., 2022). Anatomical images were processed using the standard VBM preprocessing pipeline of CAT12: visual examination for structural abnormalities and artifacts related to head motion, bias correction to remove MRI inhomogeneities, segmentation into gray matter, white matter, and cerebrospinal fluid, registration to standard Montreal Neurological Institute (MNI) space which included a linear affine transformation and a non-linear deformation with diffeomorphic anatomical registration through exponentiated lie (DARTEL) algebra normalization, and spatial smoothing with an 8 mm full-width-half-maximum Gaussian kernel.
Of the 120 participants enrolled in the parent clinical trial, 45 had structural scans at both time points. Four participants’ scans were more than 2 standard deviations less correlated with other scans and were excluded after visual confirmation. The final sample included data from 41 participants at both timepoints with the following distribution across the four treatment conditions: CBT4CBT and galantamine n=6, CBT4CBT and placebo n=14; no-CBT4CBT and galantamine n=8, no-CBT4CBT and placebo n=13.
2.5. Structural analyses
Whole-brain VBM analyses were performed in SPM12. Since data were longitudinal, statistical correction for total intracranial volume and age was redundant, and those variables were not included as covariates. (These variables were not different between treatment conditions: Supplementary Tables S1 and S2). For second-level analyses, contrasts of interest were start-of-treatment<post-treatment and start-of-treatment>post-treatment to identify increases and decreases in GMV at the end of treatment relative to the start of treatment, respectively and independent of treatment condition.
To probe changes in brain structure related to CBT4CBT, two time-by-treatment-condition interaction contrasts were created: 1) no-CBT4CBT start-of-treatment>post-treatment and CBT4CBT start-of-treatment<post-treatment, to identify regions with relative GMV increases in CBT4CBT condition versus no-CBT4CBT condition at post-treatment relative to start-of-treatment, and 2) no-CBT4CBT start-of-treatment<post-treatment and CBT4CBT start-of>post-treatment, to identify regions with relative GMV increases in no-CBT4CBT condition versus CBT4CBT condition at post-treatment relative to start-of-treatment.
For other treatment variables, two time-by-treatment-condition interaction contrasts were created to probe the changes in brain structure related to galantamine: 1) placebo start-of-treatment>post-treatment and galantamine start-of-treatment<post-treatment, and 2) placebo start-of-treatment<post-treatment and galantamine start-of-treatment>post-treatment. For non-categorical variables, whole-brain multiple regression analyses were performed in SPM12 to identify regions that were significantly correlated with specific regressors. The following regressors were tested: “number of CBT4CBT modules completed”, “number of individual therapy sessions”, “number of group therapy sessions”, and “average methadone dose”. Since not all participants took galantamine or methadone on all 84 days, “galantamine frequency” (i.e., number of days galantamine was taken, only for participants assigned to the galantamine condition) and “methadone frequency” (i.e., number of days methadone was taken) were also included as regressors.
xjView (https://www.alivelearn.net/xjview ) was used to identify brain regions corresponding to significant voxels. Regions of interest (ROIs) were defined using MarsBar (Brett et al., 2002) in SPM12. A 7 mm-radius sphere was created around the peak voxel of each significant cluster to create one ROI per cluster. Start-of-treatment and post-treatment GMV values within each sphere were extracted; changes in GMV were calculated as post-treatment GMVs minus start-of-treatment GMVs. These values were imported into SPSS for subsequent analyses.
2.6. Data analytic plan
T-tests or Mann-Whitney tests and chi-squared tests were performed in SPSS to identify differences in demographic, clinical, treatment, and neuroimaging variables between treatment conditions. Whole-brain VBM results were performed in CAT12 and were two-tailed, false-discovery-rate-corrected (pFDR<0.05), with an extent threshold set at 100 contiguous voxels, as has been done previously (Farokhian et al., 2017, Zhe et al., 2020). A MANOVA with CBT4CBT/no-CBT4CBT as the fixed factor and dependent variables (change in GMV of identified ROIs) was conducted in SPSS to identify differences in changes in GMV in ROIs between treatment conditions.
Since most variables related to treatment and cocaine use in this study were not normally distributed, Spearman’s rho values were calculated in SPSS to quantify correlations between treatment engagement variables (e.g., number of CBT4CBT modules completed, days of galantamine use) and changes in GMV. To control for multiple comparisons, Bonferroni correction was used to determine significance of correlations, where p-values were calculated as 0.05 divided by the number of ROIs identified. A MANOVA with galantamine/placebo as the fixed factor and dependent variables (change in GMV of identified ROIs) was conducted in SPSS to identify differences in changes in GMV in ROIs between treatment conditions. Graphs were plotted in Prism 8.
3. Results
3.1. Participant characteristics
In general, participants were majority men who had used cocaine for about 8 years (Table 1). Across participants, cocaine use frequency decreased by about 50 % during treatment relative to pre-treatment levels (Table 1), consistent with the parent trial reporting a significant main effect of time on cocaine use (Carroll et al., 2018). There were no significant differences in demographic or baseline clinical variables between participants assigned to CBT4CBT versus not (Supplementary Table S1). Data on treatment engagement, drug use during treatment, and neuroimaging measures segregated by treatment condition is provided in Supplementary Table S2.
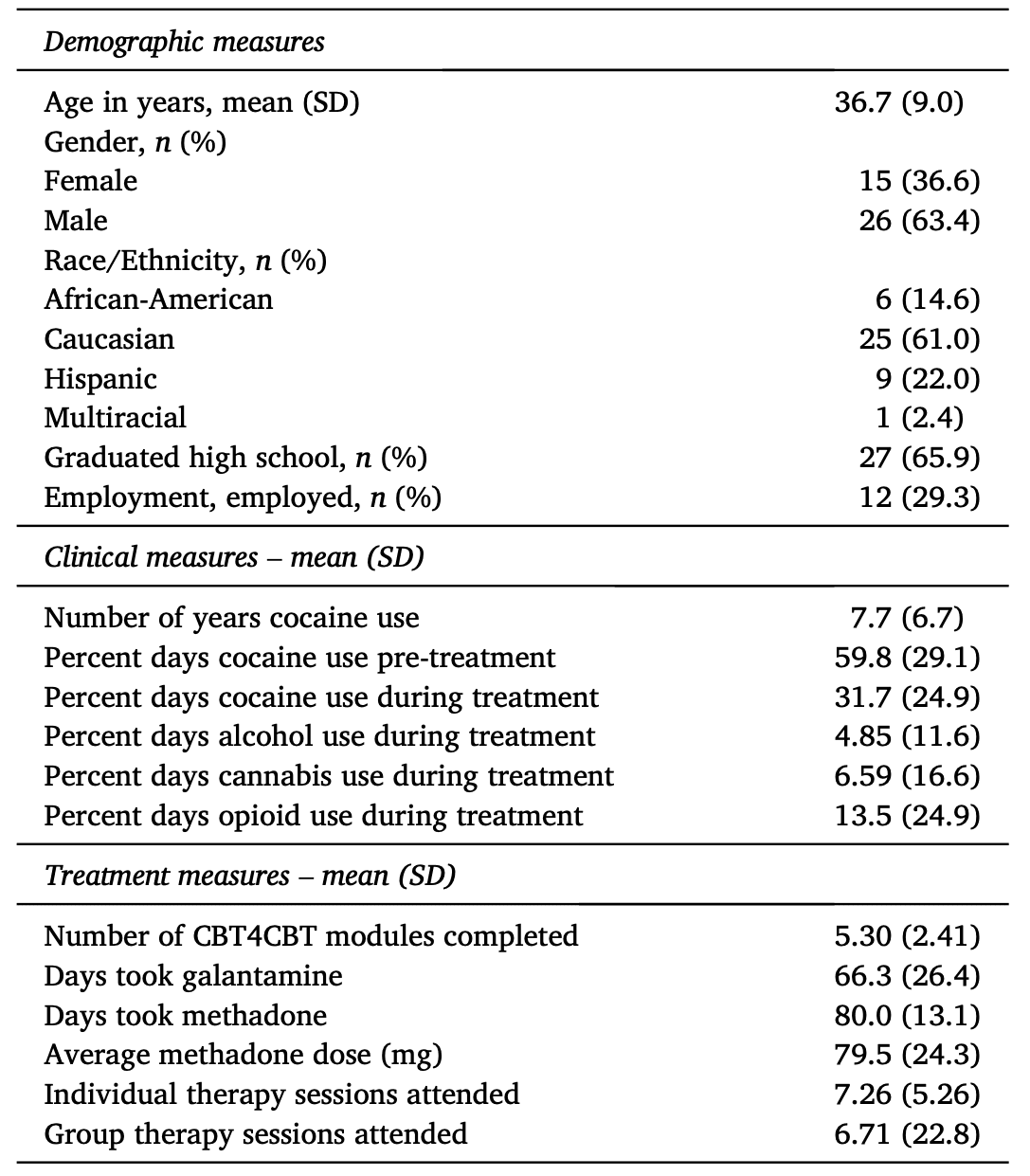
3.2. Treatment-independent changes in GMV
Independent of treatment condition, whole-brain comparison of start-of-treatment and post-treatment structural scans revealed that the right caudate body, bilateral cerebellum, and right middle temporal gyrus (MTG) were significantly smaller at post-treatment relative to start-of-treatment (Fig. 1). ROI analyses revealed a significant negative correlation between percent days of cocaine use during treatment and change in cerebellar GMV (Fig. 2A), where higher cocaine use during treatment was associated with a greater reduction in GMV. Cocaine use during treatment and change in right caudate (rho=-0.30, p=0.06) and right MTG GMV (rho=-0.24, p=0.1, n=41) were not significantly correlated. This relationship between frequency of substance use during treatment and change in bilateral cerebellar GMV was specific to cocaine, as there were no correlations between alcohol, cannabis, or opioid use and change in GMV (Supplementary Table S3).
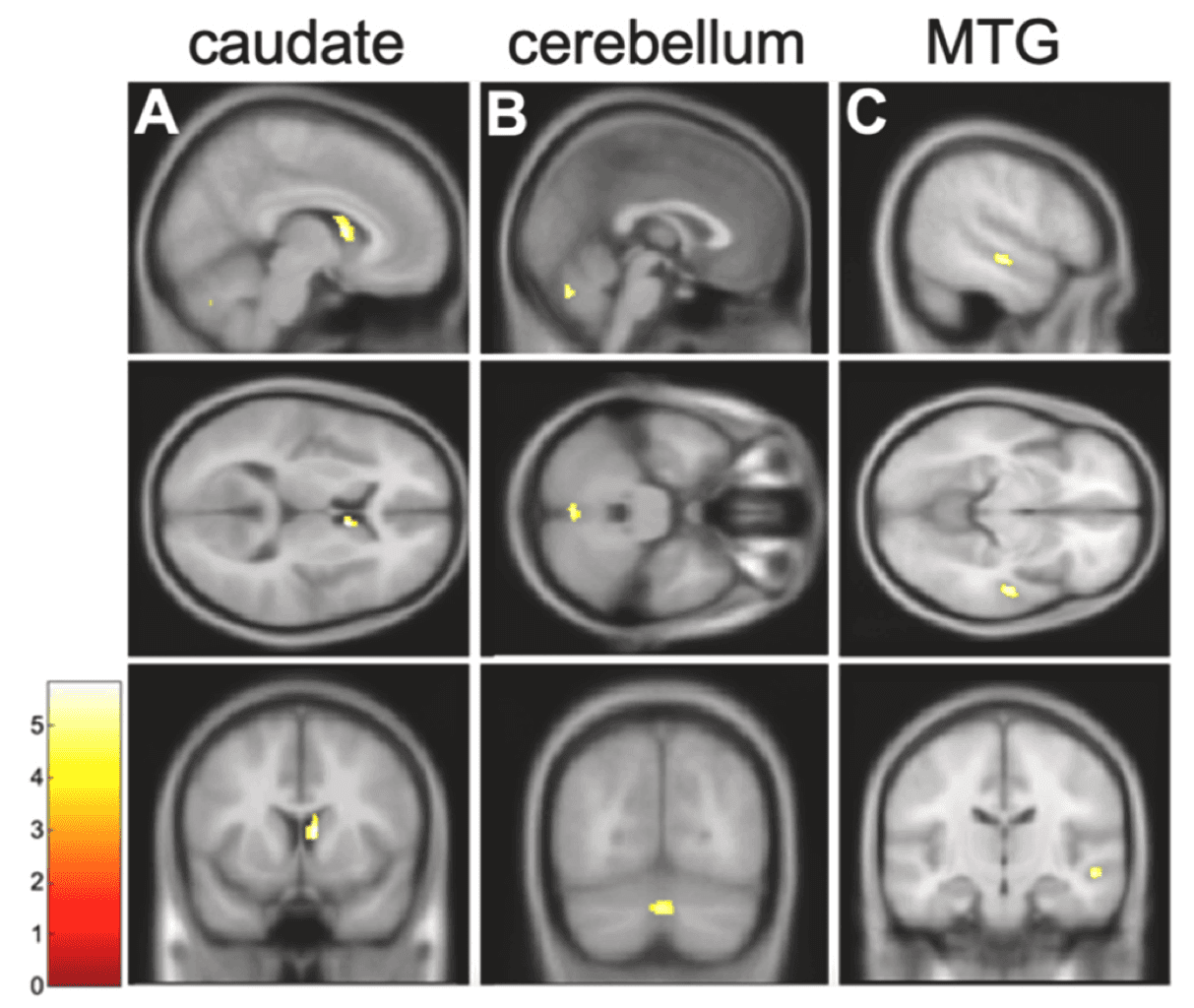
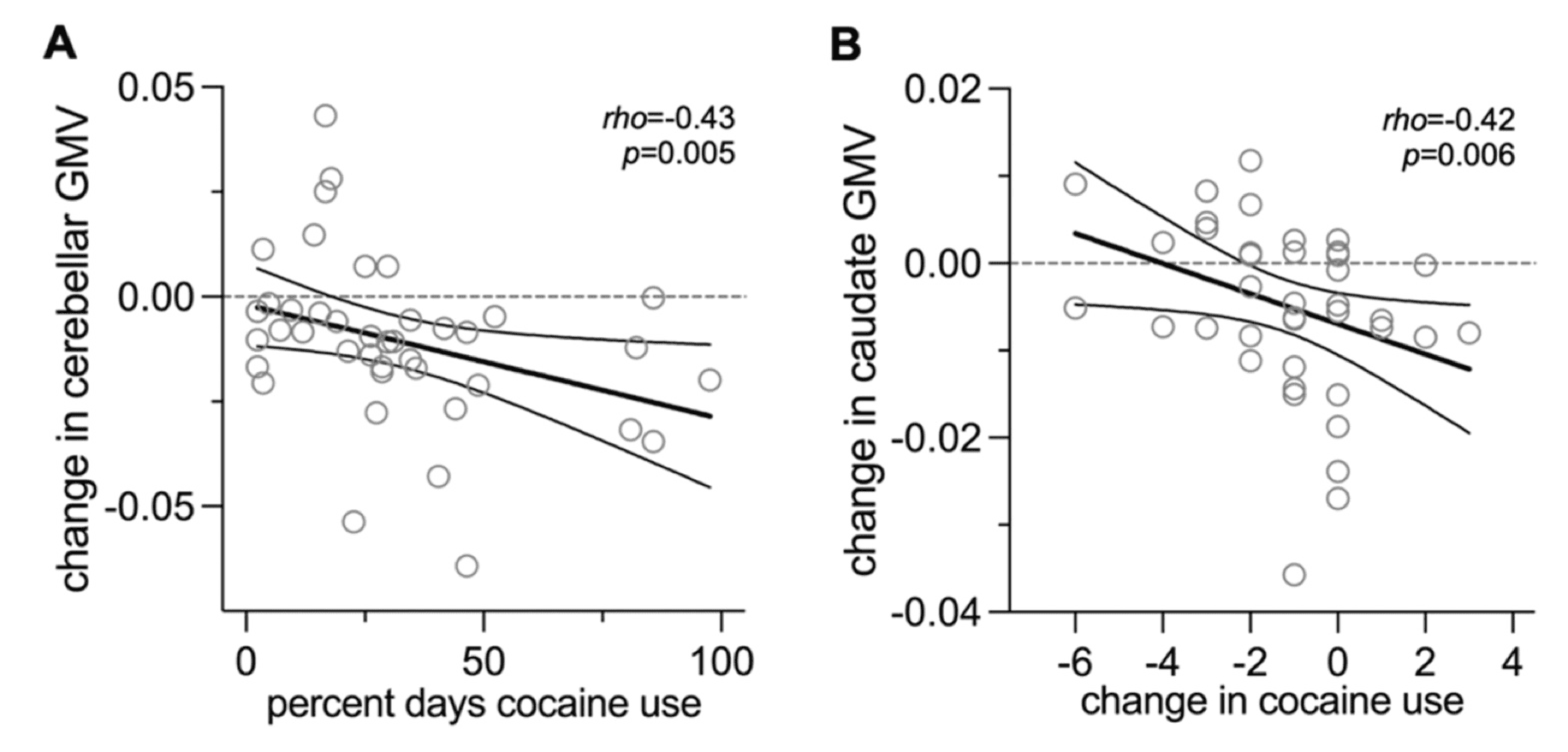
Since our brain variable of interest is a change in GMV (as opposed to absolute GMV), we considered whether change in cocaine use frequency during treatment was associated with changes in GMV. There was a significant correlation between change in cocaine use and change in right caudate GMV, where greater cocaine use at the end of treatment relative to the start of treatment was associated with a greater reduction in right caudate GMV (Fig. 2B). This was not true of the relationship between change in cocaine use and change in bilateral cerebellar GMV (rho=-0.24, p=0.1) or right MTG GMV (rho=0.19, p=0.2).
3.3. CBT4CBT-specific change in GMV
There were no significant differences in whole-brain comparison of start-of-treatment versus post-treatment scans between participants who were assigned to CBT4CBT versus no-CBT4CBT (pFDR>0.05). Consistent with this, ROI analyses in the three ROIs identified earlier revealed no differences in change in GMV between participants assigned to CBT4CBT versus no-CBT4CBT (Supplementary Table S2; Supplementary Fig. S1).
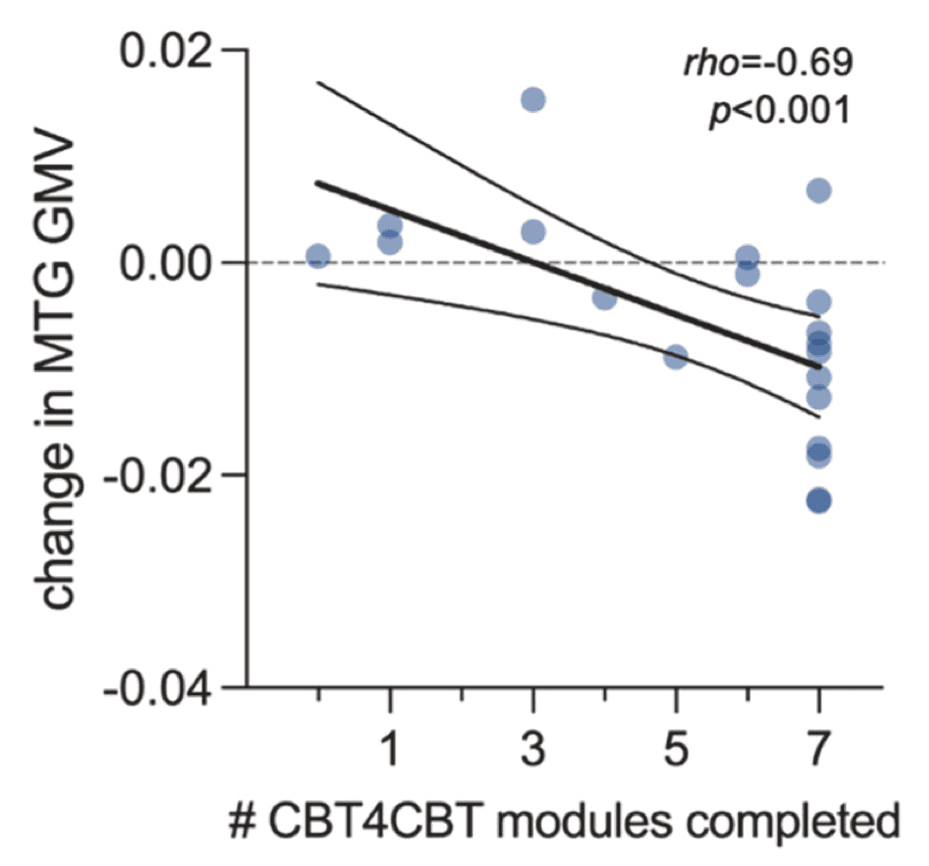
We next considered whether there was a relationship between CBT4CBT treatment engagement and change in GMV. There were no voxels at the whole-brain level that were significantly correlated with number of CBT4CBT modules completed (pFDR>0.05). However, ROI analyses revealed that among participants assigned to CBT4CBT (n=20), there was a significant dose-response relationship between number of modules completed and change in right MTG GMV, where more CBT4CBT modules completed was associated with a greater reduction in right MTG GMV (Fig. 3). This relationship was not observed for change in right caudate (rho=-0.21, p=0.4) or bilateral cerebellar (rho=0.004, p=0.9) GMV.
3.4. Associations between other treatment-specific variables and changes in GMV
There were no significant differences in whole-brain comparison of start-of-treatment versus post-treatment scans between participants who were assigned to galantamine versus placebo (pFDR>0.05). Consistent with this observation, ROI analyses involving the three brain regions identified earlier revealed no differences in change in GMV between participants who were assigned to galantamine versus placebo (main effect of galantamine: F3,37=1.47, p=0.2). There were no significant associations between other treatment variables such as galantamine frequency, methadone frequency, methadone dose, or therapy sessions attended and changes in GMV at the whole-brain level or in any of the three ROIs (Supplementary Table S4).
4. Discussion
This study assessed change in brain structure associated with treatment among individuals with CUD maintained on methadone for opioid use disorder participating in a clinical trial of computerized CBT and galantamine. Independent of study treatment condition, right caudate body, bilateral cerebellum, and right MTG GMV were significantly smaller at post-treatment relative to start-of-treatment. Higher absolute and relative cocaine use during treatment were associated with greater reductions in bilateral cerebellar and right caudate GMV, respectively. CBT4CBT engagement was associated with a greater reduction in right MTG GMV. Thus, volumetric changes in different brain regions were associated with distinct treatment variables and outcomes.
4.1. Associations between caudate and cerebellar GMV and cocaine use
The caudate and cerebellum are brain regions involved in several facets of cocaine addiction, including compulsive use, craving, and return to use (Miquel et al., 2020, Ranjbar et al., 2021, Volkow et al., 2006, Yager et al., 2015). In our study, there was a decrease in caudate and cerebellar GMV over the course of treatment. These findings add to seemingly contradictory results regarding changes in brain structure as a function of cocaine use and abstinence. On one hand, cross-sectional studies have found larger striatal GMV among individuals with CUD (Allen et al., 2023, Ersche et al., 2011, Fein et al., 2002, Jacobsen et al., 2001, Mackey and Paulus, 2013, Vaquero et al., 2017), suggesting that decreased GMV could be a sign of recovery and be associated with better treatment outcomes. On the other, increases in caudate GMV occur after months of abstinence from cocaine (Barrós-Loscertales et al., 2011, Jedema et al., 2021), and decreased GMV in this circumstance may suggest deleterious effects. Likewise, cross-sectional studies have found that individuals with CUD have lower cerebellar GMV than individuals who do not use cocaine (Moreno-López et al., 2015, Sim et al., 2007). Since decreases in caudate and cerebellar GMV in this study were correlated with higher relative and absolute levels of cocaine use during treatment, respectively, we propose that the decreases in GMV observed in this study may reflect maladaptive responses to cocaine use, although more study is warranted.
4.2. MTG and CBT4CBT engagement
The MTG is a brain region broadly associated with a semantic network, which underlies several cognitive processes, such as multisensory integration of visual and auditory information into semantic representations and conceptual associations, and integration of novel conceptual knowledge shaped by prior semantic knowledge (Cabeza and Nyberg, 2000, Chao et al., 1999, Ren et al., 2020, Tranel et al., 1997). CBT4CBT is designed to teach CBT skills and strategies using content presented in a range of formats, including graphic illustrations, video-based vignettes, verbal instructions, audio voiceovers, interactive assessments, and practice exercises, which requires audiovisual integration to extract and update one’s semantic knowledge. As such, we propose that one way in which CBT4CBT may exert its therapeutic effects is via the MTG, based on our findings that decreases in MTG GMV were correlated with more CBT4CBT modules completed.
4.3. Biological significance of GMV changes
Our neuroimaging results suggest that decreases in right caudate and bilateral cerebellar GMV reflect maladaptive responses to increased/high-frequency use of cocaine, while decreases in right MTG GMV reflect therapeutic benefits of engagement with a digital CBT program. These results highlight the complexity in interpreting the biological significance of changes in GMV, as the neural mechanisms underlying GMV changes with cocaine use may be distinct from those underlying treatment- or abstinence-induced changes. For example, maladaptive increases in striatal GMV may occur as a response to illicit stimulantuse possibly via increased activation of dorsal striatum medium spiny neurons and may reflect compensatory mechanisms in response to toxicity (Berman et al., 2008, Pando-Naude et al., 2021), while adaptive increases in striatal GMV have been observed as a result of stimulant medications for treatment of attention-deficit/hyperactivity disorder (Nakao et al., 2011) and electroconvulsive therapy for depression (Bouckaert et al., 2016, Camilleri et al., 2020), possibly reflective of neuroplasticity involving dendritic branching and synaptogenesis (Zatorre et al., 2012). In some circumstances, increases (via glial proliferation) and decreases (via neuronal loss) in GMV may even occur within the same brain region (Petzold et al., 2022). Overall, the biological mechanisms underlying GMV changes can be multifaceted. This complexity highlights the importance of collecting behavioral measures and possibly other biological measures (e.g., assessing synaptic density (Angarita et al., 2022)) in parallel to better understand and interpret GMV data.
4.4. No associations between other treatment variables and changes in GMV
In this study, change in right MTG GMV was associated with CBT4CBT engagement but not other psychosocial treatment components such as number of individual or group sessions attended. We propose that this finding may reflect the multimedia style of teaching cognitive and behavioral skills in CBT4CBT. Future studies could directly compare digitally delivered CBT (i.e., CBT4CBT) to clinician-delivered CBT as well as clinician-delivered non-CBT treatment to better understand the neural underpinnings of CBT-specific content and delivery format.
Galantamine, a cholinesterase inhibitor, has been found to reduce cocaine use (Carroll et al., 2018, Sofuoglu and Carroll, 2011) and opioid use (Carroll et al., 2019) in methadone-maintained individuals with CUD, possibly by improving sustained attention and working memory (Sofuoglu et al., 2011), though not in individuals with CUD who were not taking methadone for opioid use disorder (DeVito et al., 2019). We did not observe any structural brain changes associated with taking galantamine versus placebo or correlations between amount of galantamine taken during treatment and changes in brain structure. Future studies are needed to probe how galantamine affects the brain to confer its therapeutic effects, perhaps with an emphasis on functional brain changes. We also did not observe any significant GMV changes as a function of methadone medication. Some studies have identified effects of methadone on the brain, some interpreted as adaptive (e.g., increased functional coupling between salience and executive control networks (Chen et al., 2022)) and others as maladaptive (e.g., white matter integrity damage and smaller GMVs including in the caudate and MTG (Li et al., 2016; Zhu et al., 2021)). We found a negative correlation between methadone dose and MTG GMV that trended towards significance, and future studies could investigate whether methadone dose may moderate the relationship between CBT4CBT modules completed and changes in MTG.
4.5. Limitations
While the longitudinal design of the study permitted important within-subject analyses, the absence of a group of healthy control participants to serve as matched comparisons prevented controlling for variance induced by repeated measurements. There are also limitations regarding generalizability. Our study population included individuals with CUD who were maintained on methadone for opioid use disorder, so it is unclear whether these findings also hold true for individuals with CUD who do not have opioid use disorder and/or who are not taking methadone for opioid use disorder. Further research using a wider clinical population is required to replicate and extend these findings. We are also cautious to generalize our findings to CBT broadly, as CBT4CBT is not traditional therapist-delivered CBT. Future studies could investigate longitudinal brain effects associated with therapist-delivered CBT versus CBT4CBT to identify differential effects associated with CBT4CBT and to determine generalizability of these findings (Kiluk et al., 2018).
5. Conclusions
This study provides the first evidence of changes in brain structure associated with engagement with a digital CBT program in the context of addiction. The findings suggest a role for semantic brain regions in how CBT4CBT may exert therapeutic effects. Future studies should probe the effects of CBT and changes in right MTG GMV on other post-treatment outcomes to identify the impact that these changes may have on quality of life for individuals in treatment for CUD. Overall, this study adds to the scarce literature that investigates brain changes associated with psychosocial and digitally delivered treatment of addictions and introduces new evidence about brain changes associated with digitally delivered treatment of addictions.