Abstract
Background Trauma is a risk factor for developing maladaptive alcohol use. Preclinical research has shown that stress alters the processing of midbrain and striatal reward and incentive signals. However, little research has been conducted on alterations in reward-related neurocircuitry posttrauma in humans. Neuroimaging markers may be particularly useful because they can provide insight into the mechanisms that may make an individual vulnerable to developing trauma-related psychopathologies. In this study, we aimed to identify reward-related neural correlates associated with changes in alcohol use after trauma exposure. Methods Participants were recruited from U.S. emergency departments for the AURORA study (n = 286; 178 female). Trauma-related change in alcohol use at 8 weeks posttrauma relative to pretrauma was quantified as a change in 30-day total drinking per the PhenX Toolkit Alcohol 30-Day Quantity and Frequency measure. Reward-related neurocircuitry activation and functional connectivity were assessed 2 weeks posttrauma using functional magnetic resonance imaging during a monetary reward task using region of interest and whole-brain voxelwise analyses. Results Greater increase in alcohol use from pretrauma to 8 weeks posttrauma was predicted by 1) greater ventral tegmental area, 2) greater cerebellum activation during gain > loss trials measured 2 weeks posttrauma, and 3) greater seed-based functional connectivity between the ventral tegmental area and lateral occipital cortex and precuneus. Conclusions Altered ventral tegmental area activation and functional connectivity early posttrauma may be associated with reward seeking and processing, thereby contributing to greater alcohol use posttrauma. These data provide novel evidence of neural correlates that underlie increased alcohol use early posttrauma that may be targeted via early interventions to prevent the development of maladaptive alcohol use.
Little is known about the mechanisms that place an individual at an increased risk for developing alcohol use disorder (AUD) after experiencing trauma. Uncovering vulnerability factors that contribute to increased alcohol use and ultimate disorder posttrauma will be critical for creating early interventions and improving current treatments. To this end, neuroimaging markers may be particularly useful because they can provide insight into the underlying mechanisms that make an individual more vulnerable to developing trauma-related psychopathologies, such as AUD.
According to a recent national survey, 29.5 million people in the United States had an AUD in the past year. Risk factors that increase the likelihood of developing an AUD include exposure to fatal/catastrophic events, such as natural disasters and events that pose a physical threat to one’s life. Similarly, in the United States, up to 70% of Americans will experience at least 1 traumatic event during their lifetime. Unfortunately, frequent alcohol use, used to alleviate stress-related symptoms, increases the risk of dependence. One proposed theoretical mechanism through which AUD may develop following trauma exposure is associated with stress due to its anxiolytic effects (i.e., self-medication hypothesis).
Many preclinical animal and clinical human studies provide empirical evidence supporting the self-medication hypothesis. In preclinical models, stress exposure, especially in early life, can produce increased alcohol self-administration in adulthood. In humans, longitudinal studies highlight the onset of mood and anxiety disorders developing first and alcohol and substance use disorders developing second. Thus, a robust literature supports the development of alcohol use problems upon trauma exposure. However, few studies have tested whether neurobiological mechanisms identified in animal studies translate to stress-related alcohol use in human populations.
Reward neurocircuitry has been thoroughly explored, highlighting dopaminergic pathways, including the mesocorticolimbic pathway and the nigrostriatal pathway. The mesocorticolimbic pathway contains dopaminergic projections from the ventral tegmental area (VTA) to the medial prefrontal cortex and nucleus accumbens (NAcc). Alcohol use and anticipation of consumption release dopamine in this pathway, which strengthens the rewarding effects, ultimately leading to compulsive drug seeking. The nigrostriatal pathway connects the midbrain to the dorsal striatum, including the globus pallidus, caudate, and putamen. Previous studies have found a significant role of the dorsal striatum in compulsive alcohol use.
Chronic and acute stress can directly influence alcohol use via morphological changes in the brain. For example, exposure to chronic stress, such as social defeat stress and restraint stress, can exert excitotoxic cascades that lead to the loss of VTA dopaminergic neurons. Furthermore, rats who underwent single restraint stress showed greater alcohol self-administration due to the excitation of GABA (gamma-aminobutyric acid) neurons in the VTA. Studies with trauma-exposed humans have also shown alterations in the function of this circuit. Thus, alterations have been found in neural reward-related neurocircuitry with stress, although these studies were largely cross-sectional and did not specifically examine stress-related changes in alcohol use. Longitudinal studies would allow scientists to determine when maladaptive increases in alcohol use arise, potentially leading to the development of targeted, time-sensitive interventions to prevent the escalation of maladaptive alcohol use.
Additionally, while rates of AUD have historically been higher in males, excessive alcohol use in females has risen substantially. This increase in alcohol use is alarming given the amplified detrimental health effects that females face from alcohol, including developing liver cirrhosis more rapidly and having a greater risk of developing alcohol-related cancers than males. Furthermore, females show a more rapid escalation of drug taking to addiction, possibly mediated by estradiol-induced dopamine release on the striatum. Even though males are more likely to experience trauma during their lifetime, females are twice as likely to develop trauma-related psychopathology. Furthermore, females report that the development of trauma-related psychopathology predates the onset of substance use, consistent with findings that females endorse more coping motives for use than males. Thus, it is imperative to determine whether sex differences exist in reward neurocircuitry that could promote the development of maladaptive alcohol use by sex.
In this investigation, we used a reward reactivity task during functional magnetic resonance imaging (fMRI) to measure reward-related brain activation among recently trauma-exposed participants to identify neural correlates that may predict change in alcohol use posttrauma. We hypothesized that in the early aftermath of trauma, 1) greater response to monetary reward in the midbrain and striatal regions, including the VTA, NAcc, globus pallidus (external and internal), caudate, and putamen, would predict increased alcohol use from pre- to posttrauma and 2) greater task-based functional connectivity (FC) between reward-related brain regions and the ventral medial prefrontal cortex and hippocampus would negatively predict an increase in alcohol use from pre- to posttrauma. We also investigated whether differences in reward neurocircuitry influenced trauma-related changes in alcohol use by sex. Finally, to ensure that we did not miss any potential brain regions associated with increased alcohol use, we conducted exploratory whole-brain voxelwise analyses of regions whose reward response may be linked with alcohol use posttrauma.
Methods and Materials
Participants
Participants were recruited from 22 U.S. emergency departments (EDs) as part of a multisite longitudinal study (AURORA). Participants had to have experienced a traumatic event within 72 hours of their ED visit. MRI scanning procedures were performed at 5 sites near multiple enrolling EDs. MRI exclusion criteria included metal or ferromagnetic implants, unwillingness to complete the MRI, a history of seizures or epilepsy, a history of Parkinson’s disease, dementia (inclusive of Alzheimer’s disease), having endured a moderate to severe traumatic brain injury, and current pregnancy. None of the participants in the current study sustained moderate to severe traumatic brain injury based on readings of computed tomography scans ordered during the ED admission. All participants provided written informed consent approved by each study site’s institutional review board. Of the full sample (N = 2625), a subsample of participants was able to complete neuroimaging procedures (n = 286). Sample characteristics for the full sample can be found in Table S1; sample characteristics for the neuroimaging sample can be found in Table 1. The total and neuroimaging samples exhibited similar demographic characteristics, including biological sex, race/ethnicity, employment, income, trauma type distributions, and alcohol use patterns.Table 1. Demographic and Clinical Information, n = 286
Variable | n
(%) or Mean (SD) |
Sex | |
Female | 178 (62.2%) |
Male | 108 (37.8%) |
Age, Years | 33.75 (12.61) |
Race/Ethnicity | |
Black, Non-Hispanic American | 123 (43%) |
Hispanic/Latin American | 45 (15.73%) |
Other | 14 (0.5%) |
White, Non-Hispanic American | 102 (35.66%) |
Employment | |
Employed | 174 (60.84%) |
Retired | 6 (0.21%) |
Homemaker | 7 (0.24%) |
Student | 12 (0.42%) |
Unemployed/disabled/other | 53 (18.53%) |
No response | 34 (11.89%) |
Income | |
<$19,000 | 71 (24.83%) |
$19,001–$35,000 | 77 (26.92%) |
$35,001–$50,000 | 36 (12.59%) |
$50,001–$75,000 | 27 (9.44%) |
$75,001–$100,000 | 18 (6.29%) |
>$100,000 | 21 (7.34%) |
No response | 36 (12.59%) |
Chance of Dying | 5.15 (3.42) |
Trauma Type | |
Motor vehicle collision | 202 (70.63%) |
Sexual assault | 3 (1.05%) |
Physical assault | 32 (11.19%) |
Mass incident | 1 (0.35%) |
Fall <10 feet or unknown height | 16 (5.59%) |
Fall ≥10 feet | 4 (1.40%) |
Nonmotorized collision | 11 (3.85%) |
Animal related | 9 (3.15%) |
Burns | 1 (0.35%) |
Other | 7 (2.45%) |
CTQ-SF Total | 10.39 (10.47) |
Mild Traumatic Brain Injury | 81 (28.32%) |
Medication | |
Anticholinergics | 15 (5.24%) |
Beta blockers | 6 (2.10%) |
Opioids | 16 (5.59%) |
Benzodiazepines | 7 (2.45%) |
Serotonin and norepinephrine reuptake inhibitors | 11 (3.85%) |
Selective serotonin reuptake inhibitors | 18 (6.29%) |
Alcohol Use Score | |
Pretrauma | 16.61 (53.65) |
2 weeks | 10.16 (23.35) |
8 weeks | 17.99 (51.07) |
3 months | 17.37 (52.40) |
6 months | 18.57 (64.71) |
CTQ-SF, Childhood Trauma Questionnaire-Short Form.aMild traumatic brain injury was defined using the American College of Rehabilitation Medicine 2015 criteria.
Measures
Once enrolled in the study, participants completed questionnaires measuring demographics and current trauma characteristics at multiple time points. Demographic data collected included race/ethnicity, sex assigned at birth, marital status, income, education level, and employment status. Participant demographic and clinical information is presented in Table 1 for individuals with neuroimaging data. Participants’ reported alcohol use was obtained at the following time points: pretrauma (assessed in the ED at enrollment), weeks 2 and 8, and months 3 and 6. Trauma severity was measured via participants’ subjective ratings of their chance of dying. Given the previous findings that childhood trauma was associated with reward-related neurocircuitry, childhood trauma was assessed via the Childhood Trauma Questionnaire Short Form, a 28-item scale used to examine exposure to traumatic experiences during childhood.
Alcohol quantity and frequency were assessed using the PhenX Toolkit Alcohol 30-Day Quantity and Frequency measure, a 2-item questionnaire. To assess alcohol frequency, we asked, “During the ‘reference period,’ how many days did you have at least one drink of any kind of alcohol, not including small tastes or sips?” For alcohol quantity, we asked an additional question: “On that day/those days, about how many drinks of alcohol did you have/usually have, on average, per day?” Our primary alcohol use outcome of interest was the product of frequency and quantity for the 30 days, as measured by the difference in this product at 8 weeks posttrauma minus pretrauma (ED enrollment). Thus, this score examines the difference in alcohol use from pretrauma to 8 weeks posttrauma (the earliest posttrauma time point showing an increase in drinking). The reference period for all assessments was the past 30 days, except for the 2-week time point, where data was assessed in the past 14 days. To facilitate longitudinal analysis, we multiplied this variable by 2 to create a rate of drinking that more closely matched the other time points (see Table 1).
fMRI Task Procedures
Table S2 displays key data acquisition parameters obtained at the 5 neuroimaging sites. While inside the scanner, participants completed a brief validated version of Delgado’s monetary reward task, which only includes reward or loss receipt without allowing an anticipation period or including neutral trials. During this task, participants viewed a card with a question mark. They were asked to guess correctly via button press whether the card’s value would be higher or lower than 5 when the real value was revealed (see the Supplement for more details). The contrast gain > loss was used to measure reward processing.
Data Analysis
fMRI Data Analysis
See the Supplement for details regarding fMRI data analysis. Data on blood oxygen level–dependent activation in response to the monetary reward task were available for 368 participants. Of these 368 participants, 82 were excluded for the following reasons: excessive motion (movement exceeding 1 mm for more than 15% of volumes, n = 35), technical issues during scan collection (n = 21), poor task response (responding to 65% of trials or less, n = 18), issues with anatomical scan quality (n = 7), and missing demographic information (n = 1). The remaining 286 participants were used in the final analysis. Hypotheses were tested as contrasts where linear compounds of the model parameters were evaluated using t statistics, which were then transformed into z scores. Voxelwise gain > loss contrasts were computed for each participant (first level).
Region of Interest Analyses
Hypothesis-driven regions of interest (ROIs) were extracted using the Reinforcement Learning Atlas (https://osf.io/JKZWP/ ). Our ROIs included the bilateral VTA, NAcc, globus pallidus (external and internal), caudate, and putamen. A mean of all voxels within these anatomically defined ROIs was extracted for contrast estimates from gain > loss contrast. To determine whether greater reward-related activation was associated with a change in alcohol use from 8 weeks versus pretrauma, we used an alpha of .05 and a Bonferroni correction for multiple comparisons (6 ROIs, p < .008).
Whole-Brain Voxelwise Analyses
Whole-brain group-level maps were created for reward processes and their correlation with alcohol use change scores, and dummy variables for age, MRI site, sex, and reward-related region × sex interaction terms were included. A primary threshold of p < .005 combined with a familywise error (FWE) cluster-level correction was applied to correct for multiple comparisons.
FC Analysis
The Conn toolbox (http://web.mit.edu/swg/software.htm ) was used for task-based FC analysis. For each voxel within the whole-brain mask, covariance with each ROI during responses to gain was contrasted with covariance with each respective ROI during responses to loss trials. Scrubbing, realignment, cerebrospinal fluid, global signal, and white matter time courses were first-level covariates. Age, MRI site, and sex were included as second-level covariates. We examined the effect of change in alcohol use from pretrauma to 8 weeks posttrauma on FC values.
Statistical Analysis
We used the Hmisc R package to impute data missing for alcohol use. Data imputation was conducted using predictive mean matching, and imputation uncertainty was accounted for by bootstrapping. We examined descriptive statistics and the distributional properties of each variable at each time point (see the Supplement). Given that alcohol use was positively skewed across all time points, we applied square-root transformation to each time point. Alcohol change scores were created after imputation and square-root transformation. For the extracted gain > loss values within the ROIs (with the inverse being loss > gain) and change in alcohol use from pretrauma to 8 weeks posttrauma, when extreme outliers (z score > 3.29) were present, scores were winsorized to match the next z score value < 3.29. Winsorization is known to preserve information and power while removing outliers.
Using the total sample, a 5 (time: pretrauma, 2 weeks, 8 weeks, 3 months, 6 months) × 2 (sex: female, male) analysis of variance with sex as an independent factor and time point as a within-subjects factor was performed to determine whether statistically significant differences existed in alcohol use over time and differed between males and females and whether significant differences existed in alcohol use between sexes across time points. Because we were interested in exploring alcohol use before and after trauma exposure, we created a change score between pretrauma alcohol use and the earliest posttrauma time point, showing an increase in drinking to be used in subsequent analyses.
To test whether reward-related brain fMRI measures predicted change in alcohol use after a trauma, we conducted hierarchical linear regression analyses. In step 1, we included age, site, race/ethnicity, the chance of dying, and Childhood Trauma Questionnaire-Short Form scores to determine the variance that these demographic and clinical variables accounted for in alcohol use change. Previous studies have highlighted the importance of controlling for age, and it is customary to control for site given the differences in scanners used across data collection sites. Race/ethnicity is particularly important in predicting clinical symptoms posttrauma. In step 2, we separately added reward-related brain activation to the model for each ROI (i.e., 6 separate regression models). Lastly, in step 3, a term for the interaction between reward-related brain activation at 2 weeks posttrauma and sex for each ROI (i.e., 6 separate regression models) was included to determine whether brain activation contributed significant variance in predicting clinical symptoms above and beyond the variance contributed by demographic and clinical variables and whether sex contributed significantly to the model.
Results
Change in Alcohol Use Over Time
A significant main effect of time was found (F4,13115 = 13.33, p < .001). Bonferroni-corrected post hoc tests were performed to identify pairwise changes in alcohol use between time points. Alcohol use decreased from pretrauma to 2 weeks posttrauma (pretrauma: mean = 2.13, SD = 2.87; 2 weeks: mean = 1.97, SD = 2.67; padjusted = .02) and increased from pretrauma to 8 weeks (mean = 2.47, SD = 3.32; padjusted < .001), 3 months (mean = 2.34, SD = 3.27; padjusted = .004), and 6 months (mean = 2.45, SD = 3.26; padjusted < .001) posttrauma. There was also a significant increase from 2 weeks posttrauma to 8 weeks (padjusted < .001), 3 months (padjusted < .001), and 6 months (padjusted < .001) posttrauma. A significant main effect of sex was also found (F1,13115 = 184.87, p < .001), wherein males exhibited significantly greater alcohol use than females across all time points (Figure 1). We removed the imputed data, and the results remained significant (ps < .001).
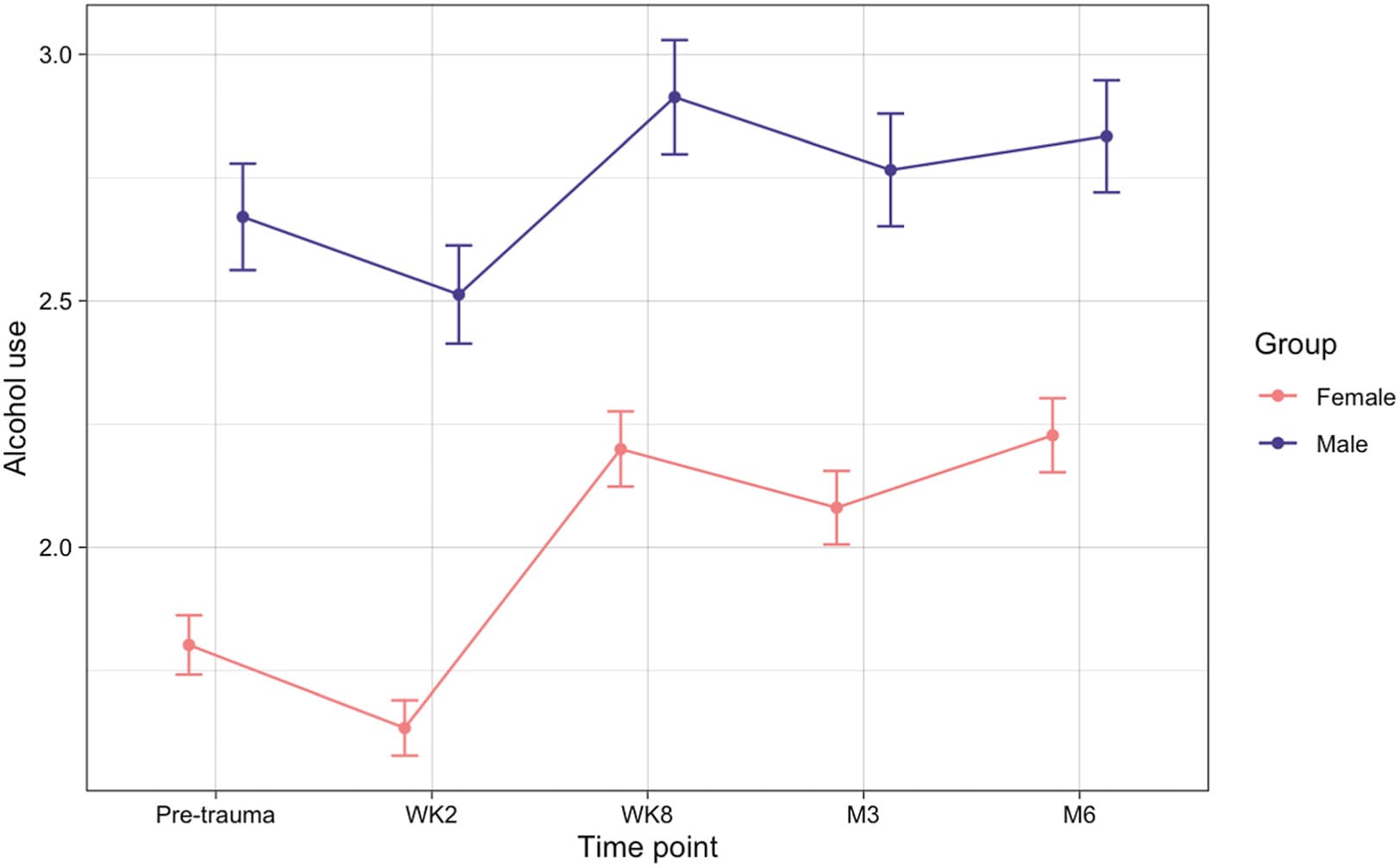
Figure 1. Mean alcohol use across 5 time points, separated by sex across all participants. Dots indicate mean quantity × frequency (square-root transformed) within each group. Error bars represent standard error. M, month; WK, week.
ROI Analyses
In the neuroimaging sample, 99 participants (35%) exhibited increased alcohol use from pretrauma to 8 weeks posttrauma. When constructing the predictive model for trauma-related alcohol use, step 1, which contained demographic and clinical variables, was nonsignificant (F5,235 = 0.95, p = .45, R2 = 0.02). Including VTA activation gathered at 2 weeks posttrauma in step 2 predicted alcohol change scores above and beyond covariates of age, MRI site, race and ethnicity, self-reported chance of dying, and childhood trauma exposure (ΔR2 = 0.05, b = 1.06, t234 = 3.67, p < .001) (Figure 2A). Holding our covariates constant, 1.06 was the expected increase in alcohol use from pretrauma to 8 weeks posttrauma given a 1 unit higher score in VTA activation. In step 3, the VTA activation × sex interaction effect did not significantly predict alcohol change scores above and beyond covariates of age, MRI site, the chance of dying, childhood trauma exposure, or VTA activation (b = 0.40, t232 = 0.67, p = .51). No additional regression model for the other reward-related ROIs was significant (ps > .15) (see Table 2). We removed the imputed and winsorized data; the results are presented in the Supplement.
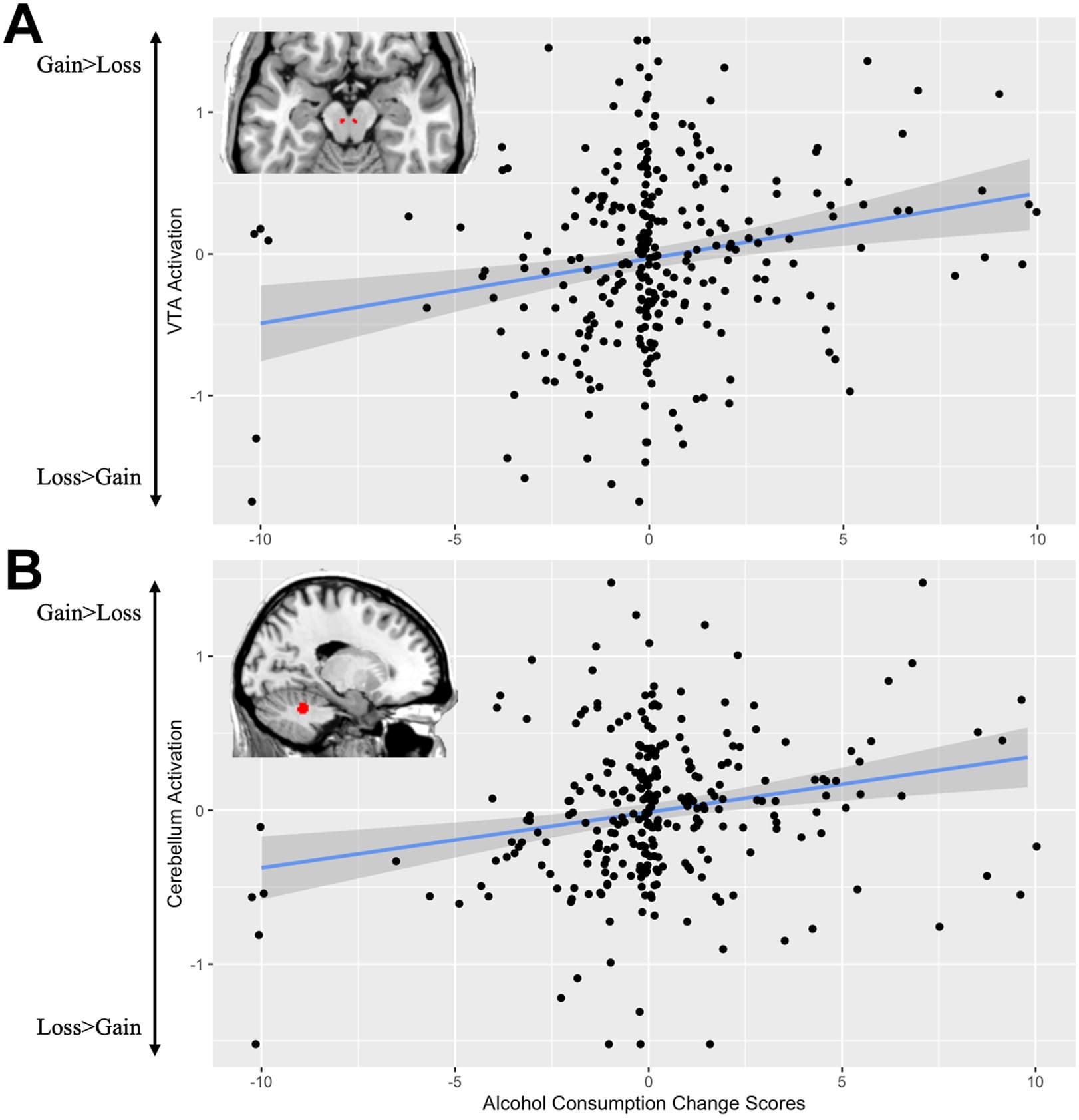
Figure 2. (A) Positive correlation with ventral tegmental area (VTA) activation during gain > loss and alcohol change scores from pretrauma to week 8; (B) positive whole-brain voxelwise correlation with spinocerebellum activation (Montreal Neurological Institute coordinates x = −16, y = −48, z = −28) during gain > loss and alcohol change scores from pretrauma to week 8. Activation was extracted using REX.
Table 2. Predicting Alcohol Change Scores From Pretrauma to 8 Weeks Posttrauma From Reward-Related ROIs
Brain Region | Step 2 | Step 3 | ||||
B ROI | t | p | B ROI × Sex | t | p | |
Week 8 − Pretrauma Alcohol Change Scores | ||||||
Ventral Tegmental Area | 1.06 | 3.67 | .000296 , , | 0.40 | 0.67 | .51 |
Putamen | 0.60 | 1.15 | .25 | 1.39 | 1.26 | .21 |
Globus Pallidus (Internal) | 0.59 | 1.12 | .26 | −1.26 | −1.18 | .24 |
Globus Pallidus (External) | 1.24 | 2.18 | .03 | 1.36 | 1.11 | .27 |
Nucleus Accumbens | 0.69 | 2.18 | .03 | 0.14 | 0.20 | .84 |
Caudate | 0.32 | 0.66 | .51 | −1.12 | −1.08 | .28 |
Month 6 − Pretrauma Alcohol Change Scores | ||||||
Ventral Tegmental Area | 0.59 | 2.35 | .02 | 0.76 | 1.47 | .14 |
Putamen | 0.44 | 0.99 | .32 | 1.66 | 1.77 | .08 |
Globus Pallidus (Internal) | 0.18 | 0.39 | .70 | −0.86 | −0.94 | .35 |
Globus Pallidus (External) | 0.29 | 0.59 | .56 | −0.16 | −0.15 | .88 |
Nucleus Accumbens | 0.11 | 0.38 | .70 | −0.19 | −0.31 | .76 |
Caudate | 0.27 | 0.65 | .52 | 0.15 | 0.17 | .86 |
Step 2: alcohol change scores ∼ age + MRI site + race/ethnicity + CTQ + chance of dying + ROI. Step 3: alcohol change scores ∼ age + MRI site + race/ethnicity + CTQ + chance of dying + ROI × sex.
CTQ, Childhood Trauma Questionnaire; MRI, magnetic resonance imaging; ROI, region of interest.
a. p < .001.
b. Significant (p < .05) overall regression model.
c. Met Bonferroni correction of p = .008.
d. p < .05.
Whole-Brain Voxelwise Analyses
Whole-brain voxelwise correlations to the gain > loss contrast were conducted with alcohol use change from pretrauma to 8 weeks and its correlation with alcohol use change scores. A statistically significant positive correlation was observed in the functional subdivision of the cerebellum called the spinocerebellum (Montreal Neurological Institute (MNI) coordinates x = −16, y = −48, z = −28; FWE-corrected p = .03; z = 5.46; KE [number of voxels in a cluster] = 184) (Figure 2B). No statistically significant negative correlations were found. A term was included for the interaction between alcohol use change from pretrauma to 8 weeks posttrauma and sex. No statistically significant positive or negative correlations were found after we included this interaction term.
FC Analyses
The alcohol change score was positively associated with FC between the VTA seed and the right lateral occipital cortex (LOC) (MNI coordinates x = 28, y = −76, z = 50; FWE-corrected p < .001; z = 4.33; KE = 607) (Figure 3A) and left precuneus (MNI coordinates x = −20, y = −60, z = 18; FWE-corrected p = .014; z = 3.93; KE = 290) (Figure 3B). This finding suggests that functional integration of the VTA and right LOC and left precuneus in response to monetary reward was positively associated with change in alcohol use early posttrauma. No regions showed statistically significant negative correlations between alcohol change scores and their connectivity with the VTA seed.
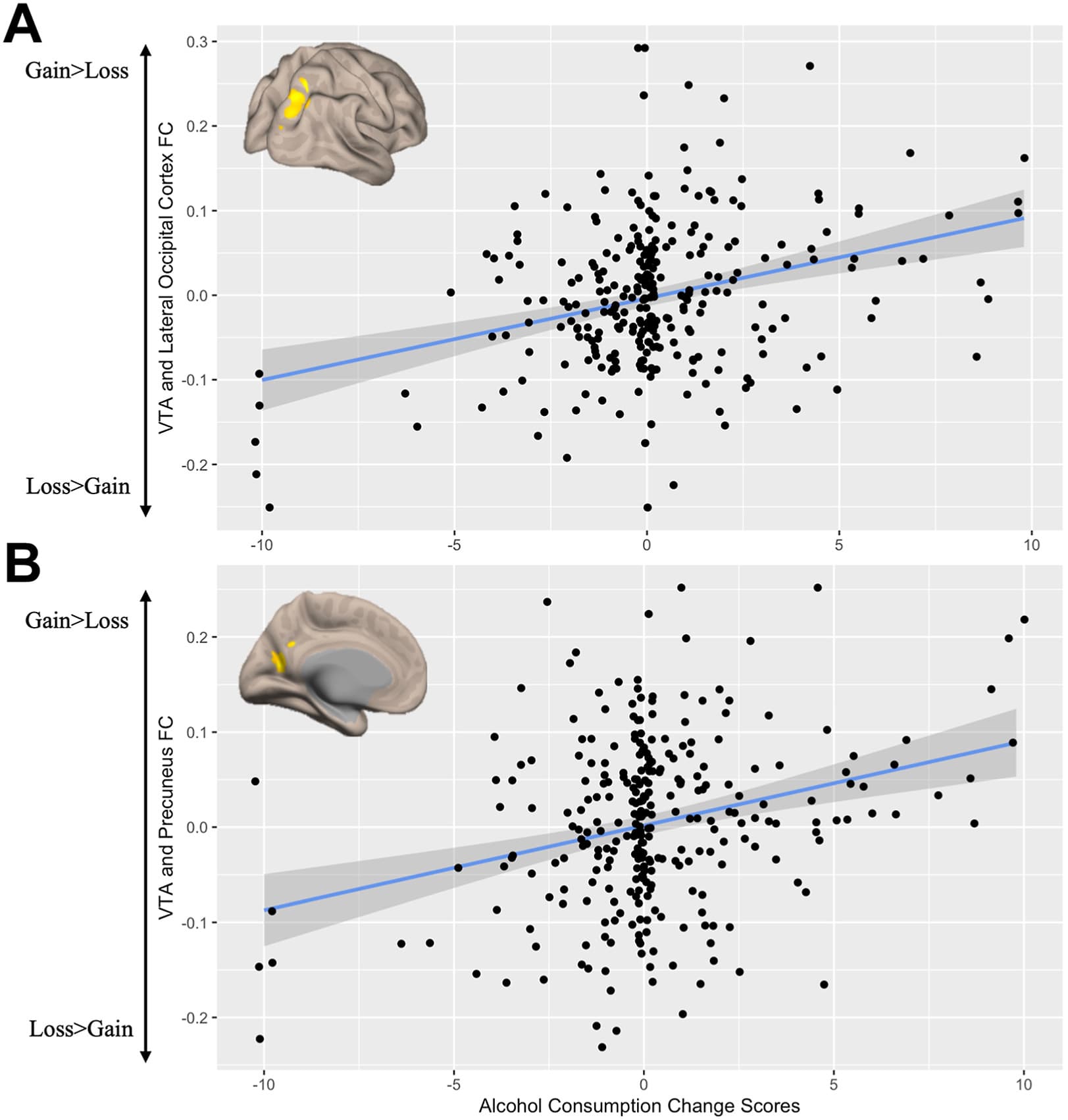
Figure 3. Alcohol change scores and functional connectivity (FC) between the ventral tegmental area (VTA) seed and (A) the lateral occipital cortex (Montreal Neurological Institute coordinates x = 28, y = −76, z = 50; z = 4.33; K = 607) and (B) the precuneus (Montreal Neurological Institute coordinates x = −20, y = −60, z = 18; z = 3.93; KE = 290). Clusters that survived the familywise error–corrected threshold were extracted using REX.
Discussion
This investigation is one of the first to utilize a longitudinal design to uncover reward-related brain activation associated with increased alcohol use in trauma-exposed participants, a group that is more at risk for the development of AUD. The findings partially support our first hypothesis: greater response to monetary reward in the VTA predicted increased alcohol use from pretrauma to 8 weeks posttrauma. The results did not support our second hypothesis of lower task-based FC between the reward-related brain regions and the ventromedial prefrontal cortex and hippocampus. Instead, we found greater task-based FC between the VTA and LOC, and the precuneus was associated with increased alcohol use from pre- to posttrauma. Lastly, we found no statistically significant differences in associations between brain activation and alcohol change scores based on sex.
The VTA is a key region in the mesolimbic pathway that is important in the development and progression of an AUD. The neural processes that underlie the progression of alcohol use to the development of an AUD are defined by an increase in dopamine release in the VTA to the NAcc during the initial consumption of alcohol; however, upon consumption of alcohol for an extended time, dopamine levels decrease, and motivation for use to relieve or prevent withdrawal leads to disorder. In this investigation, the positive association between VTA activation and trauma-related increase in alcohol use may reflect an acute-phase poststressor increase in dopamine signaling from the VTA. Preclinical studies have found that alcohol directly stimulates dopamine firing in the VTA, increasing dopamine release in the NAcc and ultimately mediating the rewarding effects of alcohol. For our participants, secondary rewards might have caused greater dopamine release. On a mechanistic level, alterations in the mesolimbic pathway caused by stress may contribute to increases in alcohol use posttrauma. Preclinical studies have highlighted the role of chronic and acute stress in alcohol use mediated by many factors, including corticotropin-releasing factors, glucocorticoids, and other stress-related neuropeptides. One study found that adrenalectomy caused decreased alcohol drinking in alcohol-preferring rats, while intracerebroventricular infusion of corticosterone increased alcohol intake. Our findings provide preliminary evidence that measuring VTA responsivity to reward is a potential biomarker for predicting increased drinking early posttrauma. This makes sense given the role of glucocorticoids in potentiating dopamine release within the VTA. Our study results are important for potentially developing preventive interventions such as neuromodulation, psychoeducation, and psychopharmacological avenues. However, future research must provide more detailed mechanistic validation of our findings, especially using preclinical studies to manipulate dopaminergic pathways early poststressor.
Our whole-brain voxelwise findings highlighted the potential importance of cerebellar reward responsivity in predicting alcohol use posttrauma. The cerebellum is a hindbrain structure responsible for motor coordination, cognitive processing, and sensory discrimination and has connections to the VTA. Cerebellum activation has been associated with alcohol craving, especially during the presentation of alcohol cues, and encodes reward-related information. While our fMRI task did not use alcohol-related stimuli, it is possible that the cerebellum is responding to the presentation of the reward-related cue (i.e., a green check mark in this study that corresponded to a $1 monetary value received).
The seed-based FC findings highlight the importance of the VTA’s functional coupling with the LOC and precuneus. While dopamine receptors are sparse in the visual cortex, previous studies have found modulation of this region in response to reward-related task performance. Greater FC between the VTA seed and LOC could contribute to an elevated visual attentional bias to reward cues. Previous studies have reported that participants who exhibited a greater change in alcohol use over time were generally biased toward directing attention to reward-related cues. However, our study did not measure the time an individual fixated on reward-related cues or subjective feelings to reward, so this interpretation should be evaluated further. Given the lack of dopamine receptors in the LOC, alternative interpretations should be investigated. Previous studies have also shown that the LOC is involved in numeric representation and activation, and activation from this area was significantly correlated with the reaction time that it took to for participants to respond to numbers versus object names. Thus, it could be that the presentation of numbers activates the LOC. These findings highlight that greater activation in visual regions may be related to greater attention to stimuli associated with reward-related cues to help determine how to respond to subsequent trials to achieve the same positive outcome. It will be important to replicate our findings and conduct more research on the role of the LOC in alcohol use given that this area could be a good target for neuromodulation techniques that are designed to change VTA-cortical circuits. Neuromodulation techniques have shown promise in targeting the occipital cortex and treating maladaptive alcohol and substance use.
The precuneus is important for self-monitoring and processing internal states and has dopamine D1 and D2/D3 receptors, making it easily modulated by dopamine. In individuals with alcohol dependence who had recently relapsed, precuneus activation in response to script-driven imagery was correlated with alcohol craving. In our study, greater FC between the VTA and precuneus was found [as was found in a previous study in trauma-exposed children] and associated with greater trauma-related change in alcohol use. One interpretation is that given the role of the precuneus in self-monitoring and the processing of internal states, trauma-exposed participants might have been more conscious about the strategy they used that ended in their receiving a reward and ruminated on how that reward made them feel. This also incorporates the VTA-LOC findings because it incentivizes the participant to expend attentional resources on the trials in which they received rewards to succeed in future trials.
We did not find significant associations between clinical symptoms and reward-related activation (see Table S3). This is interesting because many studies have provided empirical evidence for the self-medication hypothesis (i.e., individuals use alcohol to relieve clinical symptoms experienced after trauma exposure) (11). We may have failed to find evidence supporting the self-medication hypothesis given methodological differences (i.e., differences in the timing of when assessments were gathered and the assessments used). While our findings highlight the variability that exists in trauma-related alcohol use, it should be emphasized that none of our participants had an AUD.
We found sex differences in alcohol use; males drank more than females, even when we controlled for pretrauma alcohol use scores. However, sex did not significantly influence brain activation to predict alcohol use. Previous preclinical studies have highlighted a potentially important role of estradiol in modulating reward responses in the VTA. For example, one study found that when estradiol levels were highest in female rats, VTA neurons were more sensitive to alcohol excitation as mediated by the activation of the estrogen receptor alpha. Thus, it is interesting that we did not identify neural correlates that mediated the different trends for females versus males. However, this lack of significant findings in our sample could be related to the stimuli used to examine reward. The preclinical studies described examined sex differences in response to alcohol administration, while our study utilized a monetary reward task, a secondary reinforcer. Thus, a broader examination outside the context of monetary reward is warranted. Furthermore, our sample shows a divergence between males and females in alcohol use from 3 to 6 months. Given that the current study was focused on the early aftermath of trauma, future studies should examine neural correlates that mediate these longitudinal changes in alcohol use between sexes.
Limitations
Although our investigation had many strengths, including its longitudinal design, large sample size, recruitment of participants from multiple, geographically diverse EDs that increase its external validity, a standardized posttrauma timeline, and examination of sex differences, our study also has some limitations. One limitation of our work is that we relied on self-report data to measure our factors and outcomes of interest. Furthermore, we asked participants to recall alcohol use for the previous 14 to 30 days, introducing the potential for recall bias. Future longitudinal prospective studies that follow participants before they experience trauma would be ideal for gathering less potentially biased baseline assessments of alcohol use behavior, and the use of more ecologically valid forms of measurement, such as daily mobile surveys or diary-keeping of alcohol use behavior, could be used to measure alcohol use. Additionally, our reward task did not include a neutral condition, which would have allowed us to remove potential confounds inherently caused by the task. Specifically, we may be missing activation that is present for both gain and loss trials given that the activation is excluded when the gain > loss contrast is calculated. Future studies should incorporate a neutral condition to examine the relationship of overlapping activation separately for gain and loss trials with alcohol use change scores in trauma-exposed individuals. Lastly, we did not assess AUD directly, which limits conclusions regarding severity and consequences that may inform intervention efforts.
Conclusions
This investigation is one of the first to utilize a longitudinal design to uncover reward-related brain activation associated with increased alcohol use in trauma-exposed individuals. Our findings highlight that even at 2 weeks posttrauma, VTA function in response to monetary gain can help guide decisions about which trauma-exposed individuals are at greatest risk for greater alcohol use. We have also identified a neural pathway (VTA and LOC) as a potential neural circuit–based target for future neuromodulation techniques for intervention. This work highlights the importance of measuring reward-related neurocircuitry, specifically VTA activation, early posttrauma to gain insight into those who are most at risk for greater alcohol use.