Abstract
Introduction: The Spot mobile clinic provides low-threshold buprenorphine integrated with clinical and social services in Baltimore City, MD. In 2021, The Spot modified practices to improve engagement including providing extended prescriptions, reducing frequency of toxicology testing, giving up to six months to stabilize on medication, offering maximum doses (up to 32 mg total) daily, and utilizing telemedicine. This study characterizes care retention by examining both the total time in care and the percentage of time with buprenorphine prescription coverage during these practice changes, and examines factors associated with retention. Methods: This retrospective cohort study includes patients (n = 341) who received a buprenorphine prescription who initiated care on The Spot mobile clinic from September 2021 to October 2022, with follow-up through October 2023. We utilized the Cox proportional hazards model and Kaplan-Meier survival analyses to assess differences in care retention by the factors of patient demographics and clinical characteristics. Additionally, we performed sensitivity analyses using Poisson regression to examine differences between patients with 80 % or greater time with active prescription coverage versus <80 % of time with active prescription coverage. Results: After practice setting changes, retention in care at 90 days was 60 %. Patients whose maximum daily buprenorphine dose was 28 to 32 mg were 80 % less likely to discontinue treatment over the study period than those prescribed ≤16 mg (adjusted hazard ratio of discontinuation: 0.2 [95 % CI: 0.1-0.3]). Engaging in wound care or hepatitis C treatment was associated with higher retention in care, and individuals experiencing homelessness remained engaged at rates comparable to stably housed patients. Conclusion: Practice changes aimed to improve access to patient-centered, low-threshold buprenorphine treatment may increase retention in care. Notably, higher doses of buprenorphine and integrated treatment with wound care and hepatitis C treatment were associated with increased retention. Due to gaps in patient care, retention metrics should incorporate total time in care as well as percentage of time with an active buprenorphine prescription.
1 Introduction
As overdose deaths in the United States reach a record high of over 100,000 per year (Ahmad et al., 2023), the urgency for expansion of accessible and effective treatment models increases. Buprenorphine unequivocally has been shown to be safe and reduce overdose mortality risk (Connery, 2015; Krawczyk et al., 2020; Larochelle et al., 2018; Sordo et al., 2017; Wakeman et al., 2020). However, access to buprenorphine remains limited, with studies showing that less than a quarter of patients receive buprenorphine after a non-fatal overdose (Barnett et al., 2023; Larochelle et al., 2018), and overall expansion of buprenorphine services has not kept up with the demand (Schuler et al., 2023). Furthermore, disparities in access persist, with persons of color and people experiencing homelessness having even lower rates of buprenorphine treatment (Amiri et al., 2024; Barnett et al., 2023; Hsu et al., 2023; McLaughlin et al., 2021; Nedjat et al., 2023).
Models of care including mobile treatment, care integrated with harm reduction programs, and low-threshold models have been successful at increasing buprenorphine uptake among patients who were Black, experiencing homelessness, and with a history of justice involvement (Bhatraju et al., 2017; Gibson et al., 2017; Jakubowski et al., 2022; Krawczyk et al., 2019; Lowenstein et al., 2023; Messmer et al., 2023; Pepin et al., 2023; Rosecrans et al., 2022; Stewart et al., 2023; Wakeman et al., 2022). In particular, low-threshold models have been associated with improved treatment outcomes compared to more restrictive structures (Bhatraju et al., 2017; Kourounis et al., 2016; Payne et al., 2019; Wakeman et al., 2022). Low-threshold programs are structured to reduce barriers to care engagement including offering short wait times to start treatment, flexible admission criteria and medication options, individualized treatment plans, tolerance for ongoing illicit substance use/relapses, allowing for take home therapies, and not requiring adjuvant psychological treatment (Kourounis et al., 2016). Qualitative research has shown that patients prefer programs that offer flexibility, rapid medication start, a harm reduction approach, and integrated social and health services (Grieb et al., 2022; Lowenstein et al., 2023).
With the COVID-19 pandemic, the structure of treatment programs for opioid use disorder necessarily changed to ensure access to life-saving medication for patients. Structural changes to already low-threshold programs including increased prescription lengths, reduced in-person visit requirements, reduced frequency of toxicology, and increased utilization of telemedicine were implemented safely with high rates of treatment retention (Harris et al., 2022; Nordeck et al., 2020). The experience during the pandemic has likely impacted programmatic practices moving forward, and the impact of these changes on treatment retention should be explored.
In response to high overdose rates in Baltimore City, the Baltimore City Health Department (BCHD) and Johns Hopkins University School of Medicine partnered in 2018 to initiate a mobile clinic called Healthcare on the Spot (“The Spot”), which provides low-threshold buprenorphine integrated with infectious disease services, wound care, and case management (Grieb et al., 2022; Rosecrans et al., 2022). Evaluation of the first 15 months of the program showed retention in buprenorphine treatment at three months (defined as having received a buprenorphine prescription between 91 and 106 days from intake regardless of gaps in care) was only 26 % (Rosecrans et al., 2022). During the beginning of the COVID-19 pandemic, the program paused in-person care and maintained a panel of patients on buprenorphine via telemedicine (Harris et al., 2022). Upon returning to in-person services in 2021, The Spot implemented practice changes that were intended to further decrease barriers to care. This is a retrospective cohort study of patients starting buprenorphine treatment on The Spot after return to in-person services which aims to: 1) characterize patient care across the study period, 2) examine retention in treatment based on total time in care and percentage of time with a buprenorphine prescription, and 3) to examine factors associated with retention in buprenorphine treatment.
2 Methods
2.1 The Spot model of care and practice changes
The Spot mobile clinic began in 2018 and operates through a public-private partnership between the Baltimore City Health Department and Johns Hopkins University School of Medicine. The first year and a half of the program was previously described (Rosecrans et al., 2022), and has continued to evolve in scope of services, locations, and programmatic practices and policies. The Spot offers low-barrier, integrated treatment for opioid use disorder along with other clinical and social services, to meet the needs of people who use drugs and people experiencing homelessness. Services include sublingual buprenorphine management, infectious disease treatment and prevention, wound care, depression treatment, vaccinations, case management, and syringe exchange services.
Beginning in September 2021, the Spot made the following programmatic changes to further reduce barriers and gaps in care, provide additional focus on harm reduction, and increase the program's ability to serve more patients:-More time between visits: The standard length of buprenorphine prescription and time between visits extended from one week to two weeks at initiation, along with quicker graduation from a two week prescription to two weeks with a refill, four weeks, or four weeks with refills once the patient stabilized on dose and demonstrated medication adherence.-More time between toxicology testing: Toxicology testing requirements reduced from every visit (previously weekly), to once at intake and then once every two to three months.-Extended time to show medication adherence: The program granted up to six months in to establish medication adherence in the form of a positive buprenorphine toxicology result, which was previously one month. Patients maintained a two week visit schedule until they had at least one toxicology showing metabolized buprenorphine. If a patient was not successful with demonstrating medication adherence during that initial period, the Spot referred them to other programs with the option to come back to The Spot to restart after four weeks. There was no limit to the number of times patients could reinitiate care.-Initiation of telemedicine: The program began utilizing telemedicine for inter-visit continuity. The Spot offered patients who had demonstrated stability on medication, including repeated toxicology showing metabolized buprenorphine and consistent visit attendance, the option of extended time between in-person visits up to six months. For patients on a six month in-person visit schedule, the program conducted a telemedicine visit at three months in between in-person visits.-Initiation of bridging prescriptions: The program utilized telemedicine to communicate with patients who had missed appointments or who had lost their medication or had it stolen, with prescriptions for buprenorphine written when possible to provide coverage until their next in-person visit to avoid gaps in medication.-Increase in maximum daily dosages of buprenorphine: The initial dose of buprenorphine was typically 16 to 24 mg total daily dose, which was dependent on the patients' prior experience with buprenorphine. The Spot titrated their dose in subsequent visits to minimize cravings and withdrawal symptoms. In July 2022, the program prescribed a limited number of patients a maximum of 28 to 32 mg total daily dose. This maximum dose became more routine practice starting in January of 2023, and the program determined appropriate dosing through shared decision-making with patients.
2.2 Study design
This retrospective cohort study includes 341 patients who received buprenorphine prescription and newly established care on The Spot in Baltimore City, MD, from September 21, 2021, to October 31, 2022. This study followed patient outcomes through October 31, 2023.
2.3 Data management and variable definitions
Our primary outcome was total time retained in care calculated for each patient by adding total days of prescription coverage during the study period. The study considered patients discontinued on the last date of their last active prescription; and administratively censored patients with active prescriptions on October 31, 2023. The study considered patients who were confirmed deceased during the study period (n = 11) as discontinued.
Our secondary outcome was the percentage of total study time with active prescription coverage. We used total time retained in care as the numerator. For the denominator, total study time, we needed to account for the fact that patients who initiated earlier on in the study period who were lost to follow-up would have artificially lower percent time in care, compared with patients who initiated later in the study. Patients initiating care later in the study period naturally had less overall follow-up time. Therefore, to calculate the denominator of this measure only, we censored follow-up time for patients lost to follow-up at 60 days after the last active prescription.
We collected relevant information including patient demographics and clinical characteristics, prescription coverage and dosing, toxicology results, using REDCap (Harris et al., 2009) hosted by BCHD. Patient demographic and clinical characteristics at baseline included age group, gender identity, race/ethnicity, housing status, injection drug use history, overdose history, wounds (at baseline or during follow-up), transactional sex/sex work, hepatitis C virus (HCV) antibody results, HCV treatment status, HIV status, and HIV treatment status. Total daily buprenorphine dose represented the maximum prescribed dose. Buprenorphine positive toxicology results were defined as buprenorphine positive results typical for metabolized buprenorphine (norbuprenorphine to buprenorphine ratio of >0.04) and not suspicious for adulteration; we calculated the proportion of all toxicology results that were buprenorphine positive for each patient.
The study created a timeline for each patient showing periods of time in care with an active buprenorphine prescription and gaps in care without an active prescription, as well as toxicology results that were buprenorphine positive versus not buprenorphine positive. These timelines are visual representations of some of the data contained in Table 1 and are descriptive in nature, without statistical testing.

2.4 Statistical analysis
We assessed differences in treatment discontinuation (time-to-event) across patient demographic and clinical characteristics using Kaplan-Meier survival analyses and univariable and multivariable Cox proportional hazards models with robust standard errors. We used a cutoff of P < 0.05 for statistical significance; however, we included potential risk factors for discontinuation in the multivariable models if a covariate was marginally statistically significant in univariable regressions (P < 0.1). Additionally, we included baseline age, gender, and race/ethnicity as covariates in the multivariable models as potential confounders. The study calculated variance inflation factors for multivariable models and identified no substantial collinearity. Data management and analyses were completed in R 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio 2023.06.1 (PBC, Boston, MA, USA). Johns Hopkins University Institutional Review Board and BCHD approved this study considered secondary research of routinely collected clinical data.
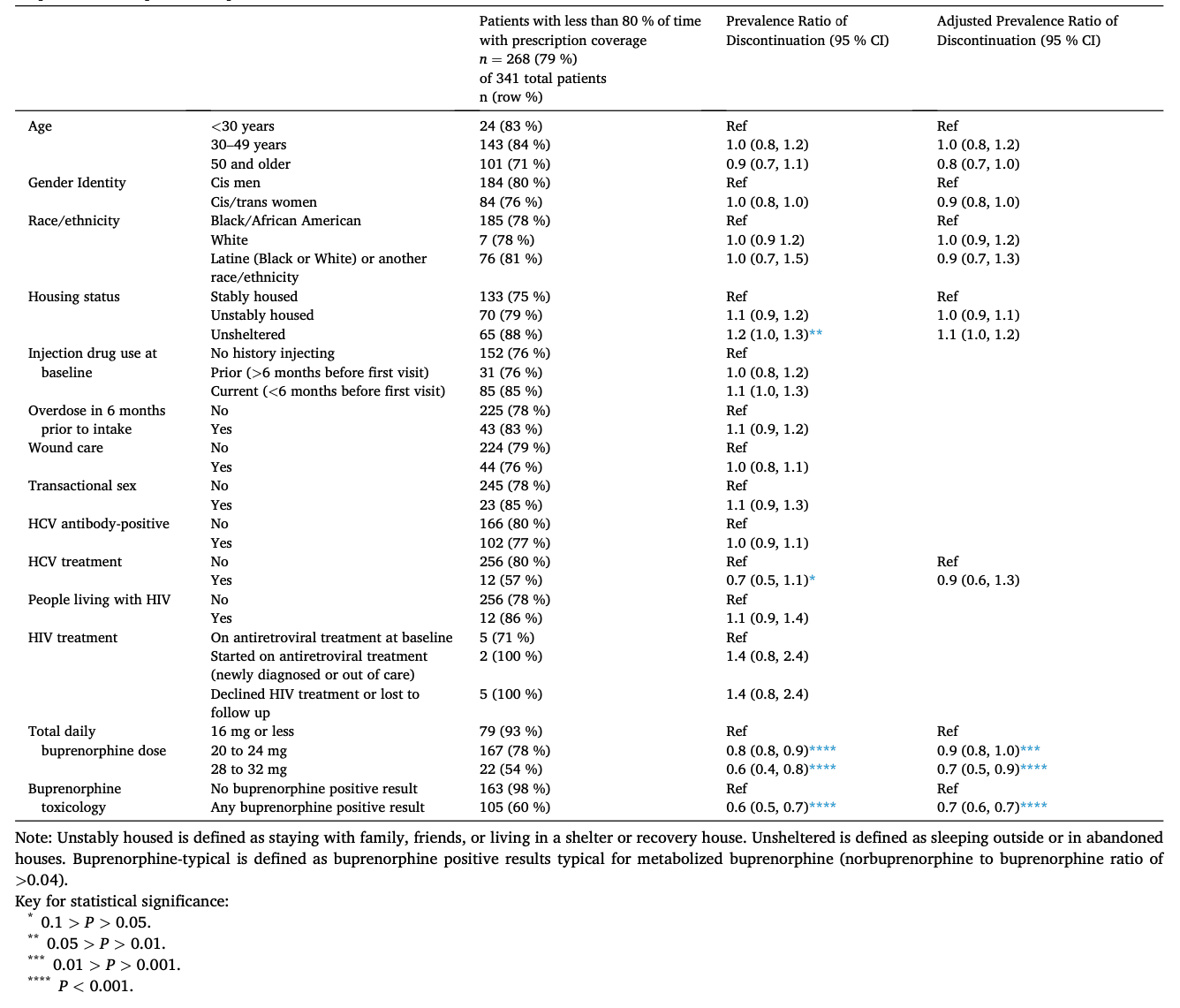
For a sensitivity analysis, we created a variable representing percentage of total study time with active prescription coverage; we then examined differences between patients with 80 % or greater time with active prescription coverage versus <80 % of time (binary outcome). We used univariable and multivariable Poisson regression with robust standard errors to correct for overestimation when binary outcomes are non-rare (Zou, 2004). The study chose the cutoff of 80 % because it represents a reasonable goal for medication coverage, and a recent large-scale analysis used a similar cutoff (Barnett et al., 2023).
3 Results
The Spot saw a total of 341 new patients for buprenorphine initiation from September 21, 2021, through October 31, 2022 (Table 1). In terms of patient factors, patients were primarily male (n = 231, 68 %), ages 30–49 (n = 170, 50 %), and Black/African American (n = 238, 70 %). Additional baseline characteristics include being unstably housed (n = 89, 26 %) or unsheltered (n = 74, 22 %); injection drug use in the last six months (n = 100, 29 %); recent overdose in the last six months (n = 52, 15 %); transactional sex (n = 27, 8 %); and active wounds (n = 58, 17 %). Most patients had an intake toxicology positive for fentanyl (n = 214, 63 %) and cocaine (n = 240, 70 %). Prevalence of hepatitis C antibody positivity was 39 % (n = 133) and among these patients, 21 started treatment for hepatitis C on The Spot during the study period. Prevalence of HIV was 4 % (n = 14); seven patients were on antiretroviral treatment at baseline, three were newly diagnosed, and three patients who were either out of care or newly diagnosed started on antiretroviral treatment during the study period.
For clinical factors, the majority of patients' maximum total daily buprenorphine dose was 20 mg to 24 mg total per day (n = 215, 63 %), and the program prescribed 41 (12 %) 28 mg to 32 mg per day (Table 1). At the end of the study period on October 31, 2023, 104 (30 %) patients were retained in care for <60 days; 105 (31 %) were in care from 120 to 364 days, and 75 (22 %) were in care for 365 days or longer. The median number of visits was eight (IQR: 3–14). Patients had an average of one care gap, with a median gap duration of almost two months (with an IQR between one month and over three months).
The median number of toxicology results was four (IQR: 2–7; Table 1). More than half of patients (n = 167, 51 %) ever had a toxicology test that was positive for buprenorphine, with 81 (24 %) having 50 to 100 % of their toxicology test buprenorphine positive. After three months total time in care, 135 patients (40 %) had at least one buprenorphine-positive toxicology result. Among patients with multiple toxicology results, the median amount of time between results was one-and-a-half months (with an IQR between one month and three months).
Fig. 1, which is divided into two panels for readability, displays individual patient care engagement timelines organized by increasing number of days in care (Y axis is patients 1 to 172 on panel 1 and patients 173 to 341 on panel 2; X axis is total days in care, 0 to 700).
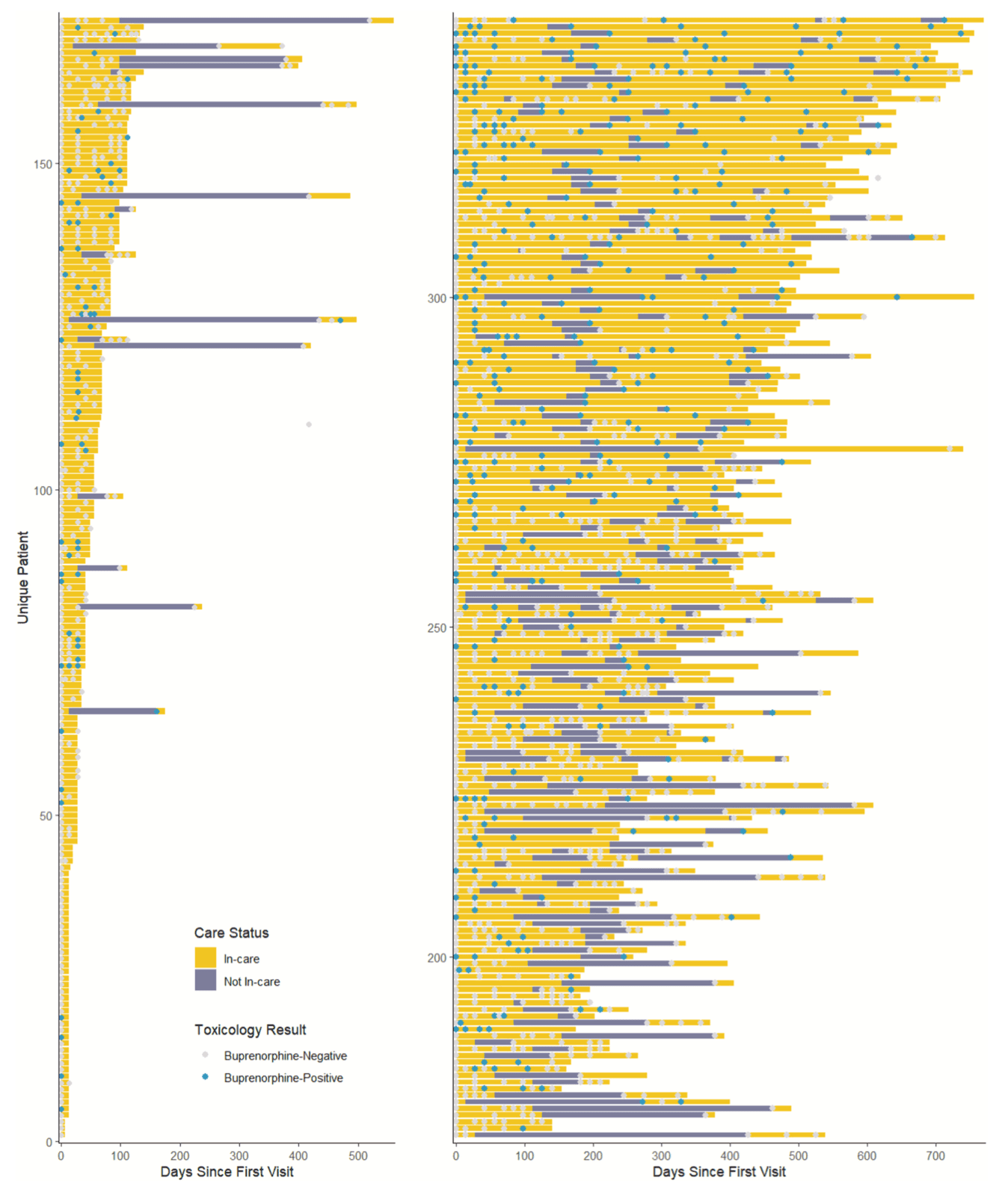
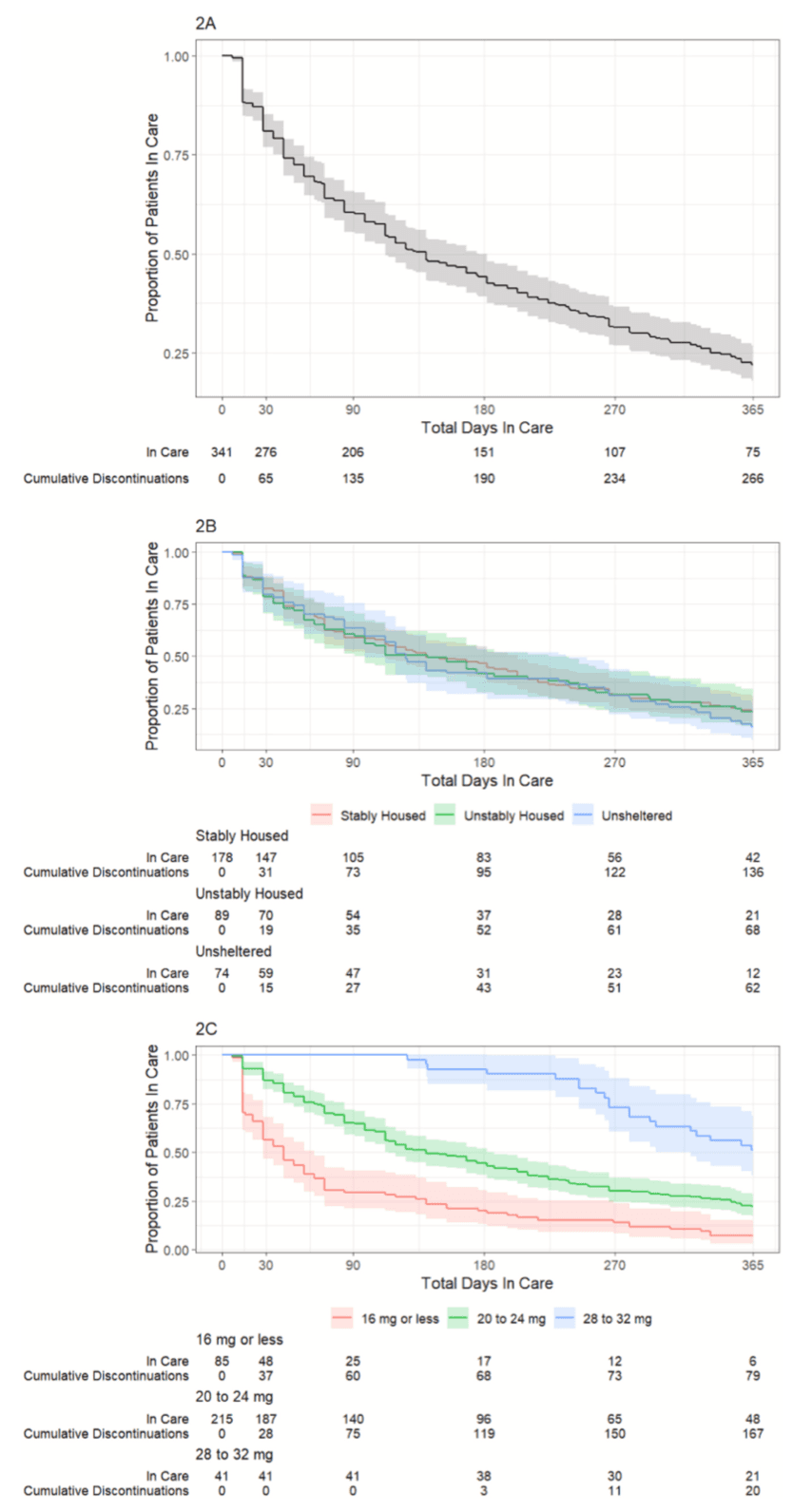
Fig. 2A shows a Kaplan-Meier curve for treatment discontinuation of all 341 patients over one year of follow up. At 90 days, 60 % of patients are retained in care and at 180 days, about 45 % of patients are retained. Fig. 2B demonstrates that there is no difference in discontinuation by patient housing status. Fig. 2C shows increased retention among patients with maximum daily buprenorphine dose of 20 mg or greater; among patients titrated up to 28 to 32 mg per day, over 50 % had one year of total time in care, compared with only 10 % of patients with 16 mg or less per day.
3.1 Factors associated with discontinuation
Using univariable Cox proportional hazards regression, our primary outcome of time-varying risk of discontinuation among patients initiating buprenorphine was not associated with age group, gender identity, race/ethnicity, housing status, recent overdose history, sex work, HCV status, HIV status, nor HIV treatment (Table 2).
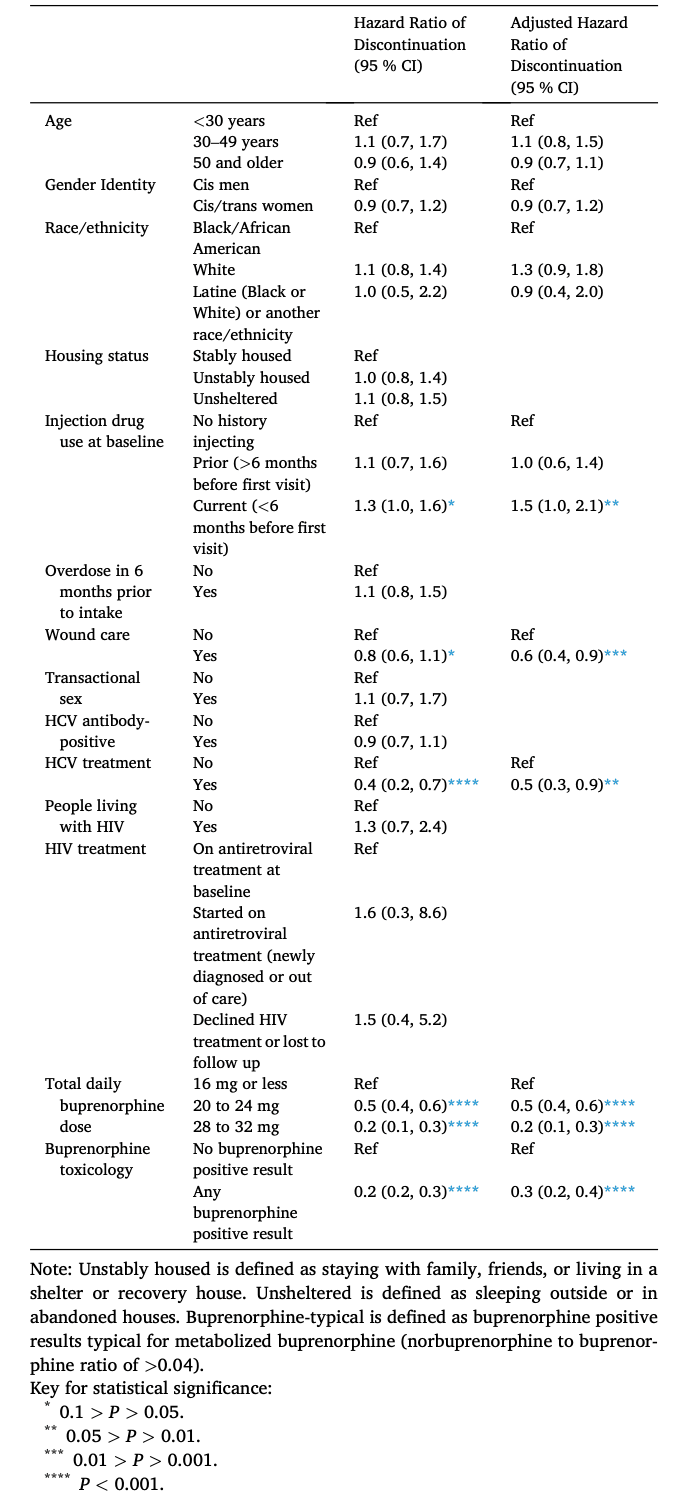
Injection drug use within six months of program initiation was associated with increased risk of discontinuation (Hazard Ratio [HR] 1.3, 95 % Confidence Interval [CI] 1.0–1.6), while the following factors were associated with program retention (i.e., hazard of discontinuation was lower): wound care at initiation or during follow-up (HR 0.8, 95 % CI 0.6–1.1), HCV treatment (HR 0.4, 95 % CI 0.2–0.7), maximum total daily buprenorphine dose (20 to 24 mg: HR 0.5, 95 % CI 0.4–0.6; 28 mg to 32 mg: HR 0.2, 95 % CI 0.1–0.3), and any buprenorphine positive result (HR 0.2, 95 % CI 0.2–0.3).
In the multivariable Cox proportional hazard analysis, the above associations remained essentially the same. Association with discontinuation strengthened slightly for injection drug use within six months of program initiation (Adjusted Hazard Ratio [aHR] 1.5, 95 % CI 1.0–2.1) and association with retention strengthened for wound care (aHR 0.6, 95 % CI 0.4–0.9; Table 2).
In sensitivity analyses (Table 3), only 73 (21 %) of patients had 80 % or more of time covered with an active buprenorphine prescription. Using this alternate, binary outcome for discontinuation, “less than 80% of time with prescription coverage”, we found similar results to our main analysis. In univariable regressions using the binary outcome and Poisson regression, we confirmed that age group, gender, race/ethnicity, recent overdose history, sex work, HCV status, HIV status, and HIV treatment were not associated with discontinuation. However, with the outcome of less than 80 % prescription coverage, we found a univariable association with discontinuation among patients experiencing unsheltered homelessness (Prevalence Ratio [PR] 1.2, 95 % CI 1.0–1.3), although this association did not remain significant in multivariable regression (Adjusted Prevalence Ratio [aPR] 1.1, 95 % CI 1.0–1.2). Compared with the main analysis using Cox proportional hazards models, models using <80 % prescription coverage as the outcome did not identify associations with discontinuation among injection drug use within six months of program initiation nor wound care treatment (Table 3). HCV treatment showed a marginal association with retention in univariable analysis (PR 0.7, 95 % CI 0.5–1.1), but this was not significant in multivariable regression (aPR 0.9, 95 % CI 0.6–1.3). Associations between <80 % prescription coverage and both buprenorphine-related factors confirmed results from Cox proportional hazards models, albeit at a smaller magnitude: maximum total daily buprenorphine dose (20 to 24 mg: aPR 0.9, 95 % CI 0.8–1.0; 28 mg to 32 mg: aPR 0.6, 95 % CI 0.4–0.8), and any buprenorphine-typical result (aPR 0.7, 95 % CI 0.6–0.7).
4 Discussion
The Spot mobile clinic modified programmatic policies to reduce barriers to engagement in buprenorphine services, and our findings indicate 90-day retention in care was 60 % in the setting of these modifications. Prior to these programmatic changes, retention in The Spot buprenorphine program was 27 % (Rosecrans et al., 2022), though the definition of retention in this prior study did not account for total time in care with an active prescription and is therefore not directly comparable. This adds to the growing literature that relaxing of programmatic requirements for buprenorphine may lead to favorable retention in care (Harris et al., 2022; Nordeck et al., 2020). The program implemented a number of changes together with the goals of moving our program to further reduce barriers, improve access to buprenorphine, and minimize gaps in medication coverage. National best practice guidance is needed to describe ideal components and policies of low-threshold models, and this study provides evidence that retention in care can be improved with modifications to a low-threshold mobile clinic model.
There is strong evidence that retention of patients in a methadone or buprenorphine program decreases mortality, and that the risk of mortality increases in the period after discontinuing medication for opioid use disorder (MOUD) (Krawczyk et al., 2020; Sordo et al., 2017; Wakeman et al., 2020). Our data show that patients engaged in our low threshold model have frequent gaps in care, but often reengage in care. Given frequent gaps in care, the time spent with an active buprenorphine prescription is an important metric for retention in treatment. This can be assessed by either cumulative time spent with an active prescription or by percentage of time from the start of care engagement with an active prescription. Our study shows that both methods resulted in similar results. Comparative effectiveness of different programmatic practices and models of care would be better informed with more standardized definitions of retention in care, including both total time in care and percentage of time with access to medication. The literature discussing retention in care for buprenorphine uses a range of definitions, but often focuses on the time from first to last appointment attended (Bhatraju et al., 2017; Nordeck et al., 2020; O'Gurek et al., 2021; Wakeman et al., 2022). One recent large study looking at access to buprenorphine after overdose used the definition of retention as receipt of a buprenorphine prescription for at least 150 out of 180 days (83 % of days) after an index overdose event (Barnett et al., 2023). While this is a high standard, it is a valuable metric to evaluate true engagement in care. Development of standardized definitions for these metrics would help guide comparison of program models and allow for monitoring programmatic progress.
We found that many people cycle in and out of care and sometimes have prolonged periods between engagement episodes, overall lowering the percent time of medication coverage. These gaps in care are especially concerning as time without a prescription and the time immediately after disenrollment from an MOUD program represent high risk periods for overdose (Krawczyk et al., 2020; Sordo et al., 2017). One study evaluating a similar low-threshold mobile model found that among the 27 % of people retained at five months, 75 % had lapses in care that averaged 1.5 weeks each (O'Gurek et al., 2021). One limitation of our mobile clinic schedule is that in order to accommodate more clinical sites across the city, we visited most sites only once every two weeks, which may have made it more challenging for patients who have fallen out of care to reconnect with our program. While many of our programmatic changes were intended to reduce the requirements of patients and to give the potential for more extended periods between visits, the changes to our schedule also reduced the frequency of our presence and accessibility to our program, which may have been a barrier to retention in care. While it is promising that so many Spot patients cycle back into care, more research is needed to better understand these gaps and how to reduce them.
A notable finding is the significant association of maximum buprenorphine dose and retention in care, with increased doses associated with significantly higher rates of retention over the study period. Compared to patients with maximum dose of 16 mg or less per day, patients with maximum dose 20 to 24 mg per day were 50 % less likely to discontinue; patients with maximum dose 28 to 32 mg per day were 80 % less likely to discontinue over the study period. However, given that doses up to 32 mg daily were not routinely offered to patients over the entire study period and that patients necessarily started at lower doses and titrated up only after a certain amount of time in care, or re-engagement in care, causal interpretation is not possible and this finding should be interpreted with caution. However, there has been increasing evidence that higher doses of buprenorphine may be needed in light of the prevalence of fentanyl in the drug supply, and can also improve retention, including that doses up to 32 mg may be necessary and beneficial for some patients (Coyle et al., 2023; Grande et al., 2023; Greenwald et al., 2023; Selitsky et al., 2023). Our data supports evidence that doses above 16 mg and up to 32 mg can be associated with increased retention and lends support to the call for an increased dose maximum on the FDA indication (Grande et al., 2023). Further research is needed to better understand the benefit of increased dosing and best practices for dose titration, particularly in these low-barrier settings.
Buprenorphine programs that meet the needs of people experiencing homelessness, particularly those who are unsheltered, are desperately needed. One scoping review found that people experiencing homelessness continue to be less engaged in buprenorphine services due to increased barriers, and that low-barrier care is key, particularly when paired with housing services (McLaughlin et al., 2021). Our study found that people who were unstably housed or unsheltered were as likely to be retained in care compared to those who were stably housed, which supports the evidence that mobile, low-threshold care is an effective model for this population (O'Gurek et al., 2021). However, additional housing support is needed, and our program has only recently begun offering housing case management. Additional investment is needed to provide low-barrier, harm reduction focused short- and long-term housing options, preferably integrated with buprenorphine and other clinical services.
Individuals receiving other services including wound care and hepatitis C treatment were more likely to be retained in care, providing further evidence for the value of integrated services. There is good evidence for the effectiveness of providing hepatitis C treatment with medication for opioid use disorder (Rosenthal et al., 2019, 2020; Socías et al., 2019), as well as integrating wound care into harm reduction programs (Castillo et al., 2020; Huyck et al., 2020; Robinowitz et al., 2014; Sanchez et al., 2021). With growing prevalence of wounds due to changing additives such as xylazine in the drug supply, offering wound care integrated with buprenorphine and other services can provide an entrance point into care. Anecdotally, patients often seek wound care with The Spot because this is a priority of theirs, and they subsequently engage in other services, including buprenorphine. Funding mechanisms should continue to support integration of behavioral health and medical services for people who use drugs.
Toxicology monitoring is required a minimum of 8 times per year at opioid treatment programs (The American Society of Addiction Medicine, 2020), but there are no guidelines for frequency of toxicology monitoring for buprenorphine management. With reduction in toxicology testing during the COVID-19 pandemic and the potential value of focusing on patient report rather than drug testing (Pytell & Rastegar, 2021), our program relaxed policies around toxicology testing so that patients did not need to submit a sample at each visit. Only 49 % of patients ever had a toxicology result that was typical for metabolized buprenorphine, and only 24 % of patients had 80 % or more of their toxicology tests with a result typical for metabolized buprenorphine. Anecdotally, our patients often report taking buprenorphine inconsistently, or ‘as needed’ to reduce illicit fentanyl or heroin use, despite not being ready or able to take buprenorphine daily. While this PRN dosing is not a guideline-based prescribing practice, continued engagement in regular visits and buprenorphine prescriptions may open the door for more frequent dosing when the patient is ready. Additionally, many patients have difficulty maintaining their supply of medication until their visit due to need or desire to use additional medication above the dose prescribed or having medication lost or stolen from them, which is a challenge particularly for unstably housed or unsheltered patients. While diversion of medication is a possibility, a scoping review showed that diversion in buprenorphine programs was overall low, with the highest reported at <5 %, and that motivations for using diverted buprenorphine are consistently reported to be management of withdrawal symptoms and to avoid illicit opioid use when buprenorphine treatment was inaccessible (Rubel et al., 2023). Furthermore, one recent modeling study demonstrated that buprenorphine diversion does not increase risk of opioid overdose at a population level, further calling for increased access to low- or no-barrier buprenorphine treatment (Adams et al., 2023). This evidence and our experience support continued prescribing of buprenorphine, at least for a period of time, while patients adjust to taking medication and work towards increased adherence. Our own practices continue to evolve, and more evidence is needed to guide best practices.
This study had several limitations. First, data were collected for clinical service delivery and not for research purposes, so data may be missing or incomplete. Second, many variables such as housing status, overdose, injection drug use, and transactional sex are self-reported by patients and may be subject to social desirability bias. Third, evaluation of factors associated with retention in care such as higher medication doses or engagement in hepatitis C treatment cannot establish direction of the relationship or causality. Fourth, this is a report of real-world experience with several programmatic changes in one mobile clinical setting and results may not be generalizable to other settings. Finally, although the programmatic changes were designed to be patient-centered, we were not able to include data on the patient experience in the program.
5 Conclusion
The Spot mobile clinic modified policies and practices to lower barriers to buprenorphine treatment, including giving extended prescriptions, reducing frequency of toxicology testing, giving extended periods to stabilize on medication, offering total daily doses above 24 mg, and utilizing telemedicine to minimize visits for stable patients and to bridge gaps for patients who missed appointments. In the setting of these changes, retention in care at 90 days was 60 %. Receiving higher buprenorphine doses and engaging in wound care or hepatitis C treatment were associated with higher retention in care, and individuals experiencing homelessness remained engaged at rates comparable to stably housed patients. Lastly, patient engagement timelines demonstrate frequent gaps in care for many patients, and assessing time with an active buprenorphine prescription should be a standard metric when assessing retention in care. More research is needed to inform best practices for low-threshold buprenorphine programs.