Abstract
Background: Stimulus-reactive neuronal populations are groups of neurons that become activated by environmental stimuli. These sparsely activated neuronal assemblies are implicated in encoding associations between environmental contexts and subjectively rewarding or aversive experiences that regulate behavior. How positive or negative hedonic states are represented in brain neurocircuits is a fundamental question relevant for understanding the processing of emotionally meaningful stimuli that drive appropriate versus maladaptive behavior. It is well known that animals avoid noxious stimuli and experiences. However, little is known about how the conditioning of environmental stimuli to behavior that leads to amelioration of dysphoric states establishes powerful associations that lead to compulsive maladaptive behavior. Methods: Here, we sought to identify stimulus-reactive neurons that may mediate the conditioned effects of environmental stimuli associated with the reversal of dysphoric alcohol withdrawal states using a dependent withdrawal-related learning (WDL) experimental condition (DEP-WDL) (N = 13) and 3 controls: nondependent WDL (NDEP-WDL) (N = 12), dependent no-WDL (DEP-NWDL) (N = 9), NDEP-NWDL (N = 9). Results The results document a role for clusters of neurons in the paraventricular nucleus of the thalamus (N = 8), the central nucleus of the amygdala (N = 8), and the dorsal striatum (N = 9) in this conditioned negative reinforcement process. Conclusions: These findings suggest that associations between reversal of negative hedonic states and environmental contexts are encoded in distinct neuronal populations that may serve as a neural substrate of compulsive alcohol seeking and vulnerability to relapse associated with reward dysregulation and hedonic allostasis.
The conditioning of environmental stimuli or contexts with rewarding or aversive events represents a fundamental learning process that becomes encoded in neurocircuits and subsequently drives appropriate behavior. Animals and humans perceive stimuli as rewarding or aversive; they can also learn through associative processes that avoiding or removing negative stimuli ameliorates unpleasant experiences. In subjects with drug dependence, for example, learned associations between contextual stimuli and a drug include associations linked to the reversal of the adverse withdrawal state by drug use. The ability to process meaningful stimuli related to this negative reinforcement learning is a vital neural function that is essential for maintaining stability, well-being, and survival. Therefore, how stimuli that drive behavior are represented in neurocircuits is a fundamental question.
Exposure to motivationally relevant stimuli elicits sparse patterns of neuronal activation known as neuronal ensembles or cellular assemblies, which can occur without coactivation of synaptically connected partners. Because learned associations between stimuli and subjectively rewarding or aversive experiences are a major factor in the chronically relapsing nature of compulsive alcohol seeking and use, a withdrawal-related learning (WDL) procedure was used to identify stimulus-reactive neurons that mediate the motivating effects of negative reinforcement learning. In the WDL procedure, environmental stimuli conditioned to reversal of aversive alcohol withdrawal states by alcohol self-administration (i.e., negative reinforcement) acquire conditioned incentive value and may represent a significant major factor in substance craving and relapse, leading to reward dysregulation and pathological hedonic allostasis.
The formation of sparse and distributed neuronal assemblies that encode learned associations that then mediate appetitively motivated behavior including drug-seeking responses is thought to be the basic unit of acquired learning. However, the neuronal substrates that specifically mediate the motivating effects of stimuli associated with the reversal of negative hedonic states such as dysphoria, anxiety, and sensitivity to stress after excessive or long-term substance use remain to be understood. The relevance of this understanding is illustrated by findings of a parallel behavioral study in which alcohol seeking induced by contextual stimuli associated with the reversal of adverse withdrawal states by alcohol (WDL) was qualitatively and quantitatively different from that in rats without a dependence and WDL history. Specifically, alcohol seeking in rats with WDL experience was stronger overall, and more importantly, it was compulsive in nature (i.e., resistant to punishment and increased effort requirements), whereas alcohol seeking in nondependent rats with a social drinking history was not. Therefore, using a rat reinstatement model, we sought to 1) identify stimulus-reactive neurons recruited by environmental stimuli linked to alcohol availability and consumption during withdrawal episodes following the development of alcohol dependence (negative reinforcement) versus alcohol availability in the nondependent state (positive reinforcement) and 2) establish whether alcohol seeking in rats with WDL experience recruits different neurons than in rats without this experience as well as the nature of these differences.
Methods and Materials
Animal Use and Care
All procedures were conducted in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Scripps Research. All animals were adult male Wistar rats (Charles River Laboratories) weighing approximately 450 grams.
Brains were obtained from rats trained and tested under the conditions described below in Behavioral Training.
Behavioral Training
The WDL and all other behavioral procedures were conducted as previously described. These procedures established compound contextual stimuli (i.e., both contextual stimuli and response-contingent discrete stimuli) to response-contingent alcohol availability during withdrawal in dependent rats versus alcohol availability in both nondependent and postdependent rats (Figure 1). The purpose of the WDL experimental condition (DEP-WDL, N = 13) (Figure 1, top row) was to establish the effects of these stimuli on reinstatement in the presence of motivational and environmental challenges (i.e., punishment by footshock and effort manipulations). Following ethanol (EtOH) self-administration training, rats were subjected to alcohol vapor inhalation or remained nondependent. After 3 weeks, rats were transiently removed from the vapor (or control) chambers, and after 8 hours of withdrawal, they were given the opportunity to operantly self-administer EtOH in the presence of the compound contextual stimuli and conditioned stimuli in 30-minute sessions. EtOH self-administration conditioning continued for 9 sessions, separated by 1 to 2 days during which rats remained undisturbed in the vapor chambers (or room air). Three control/comparison groups were included. First, a nondependent group (NDEP-WDL, N = 12) provided a comparison between the effects of a history of dependence and withdrawal (DEP-WDL) versus a history of only nondependence (NDEP-WDL) on stimulus-induced alcohol seeking, with all other conditions remaining equal. A no WDL (NWDL) group served the purpose of providing a control for the effects of a dependence history alone without alcohol reinforcement during withdrawal. Second, a dependent (DEP-NWDL, N = 9) control group served as a control for the effects of a history of dependence and withdrawal alone (without WDL) to establish whether such a history alone rather than WDL experience (DEP-WDL) accounts for the observed effects on stimulus-induced alcohol seeking. Third, a nondependent and no WDL (NDEP-NWDL, N = 9) group was tested in parallel with the DEP-NWDL group to provide a final comparison between the effects on alcohol seeking with a history of dependence and withdrawal alone (DEP-NWDL) versus a history of nondependence, with all other conditions remaining equal. Following dependence induction, DEP-NWDL rats were withdrawn from alcohol for 1 week and then given the opportunity to operantly self-administer EtOH in the presence of compound contextual stimuli. Paralleling the procedures for the WDL group, conditioning was conducted in 9 sessions, separated by 1 to 2 days during which rats remained undisturbed in the home cages. After completion of procedures in the dependence induction phase, rats remained in their home cages for 1 week, followed by daily reexposure to the self-administration operant chamber under extinction conditions. Subsequent tolerance of increased effort, resistance to punishment, and simple reinstatement tests were conducted across 14 days.
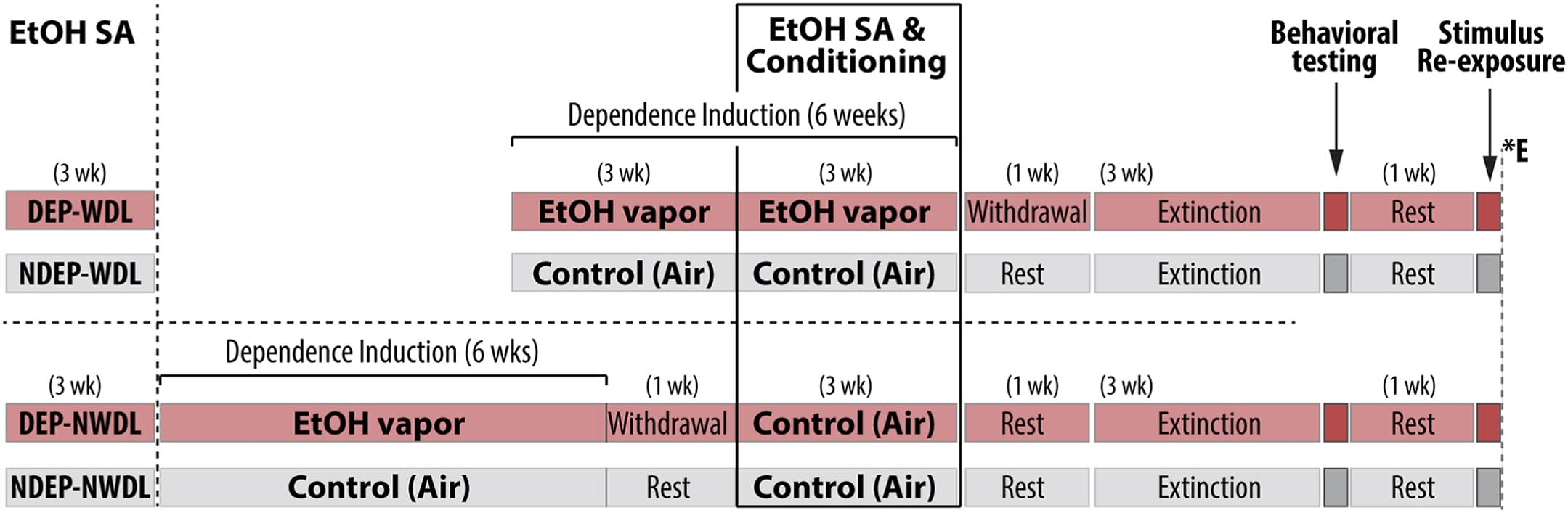
Figure 1. Illustration of the design including the WDL procedure. Rats were divided into 4 groups (DEP-WDL, NDEP-WDL, DEP-NWDL, NDEP-NWDL), 2 of which were subjected to alcohol dependence (DEP-WDL and DEP-NWDL) induction while the other 2 remained nondependent (NDEP-WDL and NDEP-NWDL). DEP-WDL (experimental group): history of alcohol SA, dependence, and withdrawal—WDL via repeated exposure to stimuli associated with alcohol availability during withdrawal states in the training phase. NDEP-WDL (control group 1): alcohol nondependent rats, tested in parallel with DEP-WDL rats; history of alcohol SA but no history of alcohol dependence/withdrawal; exposed to the same alcohol-associated stimuli as the DEP-WDL group but during alcohol availability in the nondependent state (i.e., no WDL experience). DEP-NWDL (control group 2): history of alcohol SA, dependence, and withdrawal—but no WDL experience (i.e., no history of stimulus presentation during alcohol availability in the training phase). NDEP-NWDL (control group 3): alcohol nondependent rats, tested in parallel with DEP-NWDL rats; history of alcohol SA; no history of alcohol dependence/withdrawal; no WDL but exposed to the same alcohol-associated stimuli as the DEP-NWDL group but during alcohol availability in the nondependent state. Control group 1 provided a comparison between the effects of a history of dependence and WDL (DEP-WDL) vs. a history of only nondependence (NDEP-WDL) on stimulus-induced alcohol seeking, with all other conditions remaining equal. Control group 2 served as a control for the effects of a history of dependence and withdrawal alone (without WDL) to establish whether such a history alone rather than WDL experience (DEP-WDL) accounts for the observed effects on stimulus-induced alcohol seeking. Control group 3 provided a final comparison between the effects on alcohol seeking of a history of dependence and withdrawal alone (DEP-NWDL) vs. a history of nondependence, with all other conditions remaining equal. DEP, dependent; ∗E, euthanasia; EtOH, ethanol; NDEP, nondependent; NWDL, no WDL; SA, self-administration; WDL, withdrawal-related learning.
Tissue Preparation
Brains from our previous study were used to analyze neuronal activation in the current study. All rats were sacrificed with CO2 and transcardially perfused (4% paraformaldehyde in 0.1-mM sodium tetraborate) 90 minutes after reexposure to contextual stimuli in the operant chamber (without alcohol availability). This time frame allows for the visualization of c-Fos protein expression in activated neurons. Then, brains were harvested and placed in 30% sucrose before cutting 40 to 50 μm thick tissue sections using a Leica microtome. In this study, whole brains were sectioned at the same thickness, and all tissue sections for this study were collected together into appropriate wells in a 24-well plate for immunostaining. Immunolabeling was achieved with an anti-c-Fos antibody (#2250 c-Fos [9F6] rabbit monoclonal antibody; 1:5000; Cell Signaling). The c-Fos family of nuclear oncogenes includes c-Fos, c-FosB, FRA1, and FRA2. Here, c-Fos antibody was used that detects endogenous levels of total c-Fos protein. The antibody does not cross-react with other c-Fos proteins, and it was characterized by Western blot analysis. A donkey anti-rabbit Alexa Fluor 488 was used to visualize c-Fos-positive neurons (#A21206; Life Technologies). First, tissue sections were incubated in blocking solution (5% donkey serum, 0.25% triton, and 0.05% sodium azide in 0.01M phosphate-buffered saline [PBS]) for 1 hour before being left to incubate in primary antibody for 72 hours. Next, sections were rinsed 6 times in 1× PBS and then allowed to incubate in the secondary antibody (1:800) for 4 hours. Final rinses and DAPI (1:1000; Thermo Fisher Scientific) staining were achieved before the tissue was mounted on glass slides and cover slipped.
Quantitative Analysis
Imaging details are provided in the Supplement. Representative sampled neuroanatomical regions are delineated in Figure 2A–F, with Figure 2D, F showing c-Fos-expressing cortical-, amygdalar-, and paraventricular thalamic-activated neurons. c-Fos-expressing neurons within contoured regions were detected automatically with the cell detection function in NeuroInfo-rat (MBF Bioscience) (Figure 3 and Supplement). Using the c-Fos dataset from the current study, NeuroInfo-rat was built specifically for c-Fos-expressing cells in rat tissue. All automated cell detections were validated and edited by an experimenter before finalization. Three independent experimenters edited automated cell detections, and all 3 finalized cell count values were averaged. Experimenters were blinded to the treatment conditions. c-Fos-positive cell counts were normalized over the total surface area of each region of interest to obtain c-Fos density (c-Fos counts/mm2) as a proxy for neuronal activation.
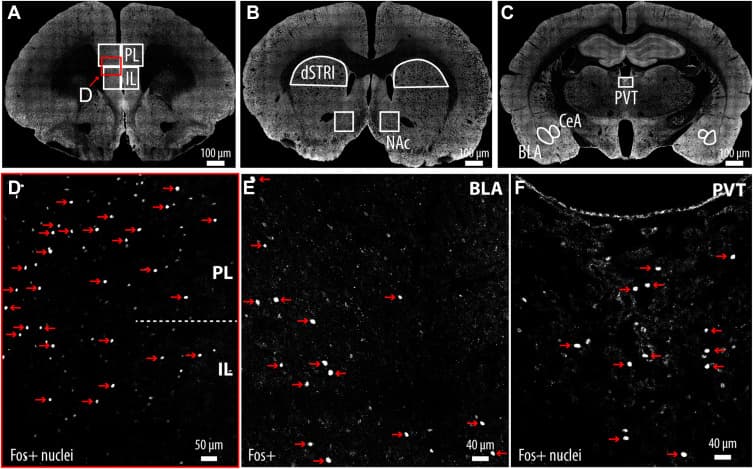
Figure 2. Representative confocal images showing brain regions analyzed for c-Fos-positive stimulus-reactive neurons. (A) Low-magnification coronal section depicting the PL and IL, with outlined areas used for quantification of c-Fos-positive nuclei. (B) Low-magnification image indicating regions sampled in the dSTRI and NAc. (C) Low-magnification image showing regions sampled in the PVT, CeA, and BLA. (D) Higher magnification view of the PL and IL showing c-Fos-positive nuclei. Representative c-Fos-positive nuclei (red arrows) with approximate diameters of 10 μm in the PL/IL (E) and PVT (F). Nuclei smaller than 7 μm were excluded from quantification via automated thresholding in NeuroInfo-rat (MBF Bioscience), and thus, any small speckle was excluded from the counts. All c-Fos-positive neurons were visualized with an Alexa Fluor 488 antibody and pseudocolored for improved contrast and visibility. BLA, basolateral amygdala; CeA, central amygdala; dSTRI, dorsal striatum; IL, infralimbic cortex; NAc, nucleus accumbens; PL, prelimbic cortex; PVT, paraventricular nucleus of the thalamus.
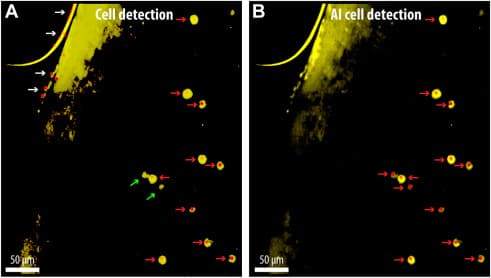
Figure 3. Automated detection of c-Fos-positive cells using conventional and AI-enhanced methods. (A) c-Fos-expressing neurons identified using a standard cell detection algorithm available in NeuroInfo-rat (MBF Bioscience). Details are provided in the Supplement. (B) Improved detection using an AI-based method incorporating machine learning, which reduces false positives from edge artifacts (white arrows) and enhances detection of low-intensity (dimmer) c-Fos-positive nuclei. Note the 2 green arrows in (A) pointing to c-Fos-positive cells (>7 μm) that were not detected by the standard cell detection algorithm but were captured using AI-assisted image analysis algorithm integrated within the NeuroInfo-rat software platform, which incorporates machine learning principles for cell recognition based on pixel intensity, morphology, and pattern recognition in (B). Red arrows represent c-Fos-positive neurons, which were visualized by an Alexa Fluor 488 antibody and pseudocolored yellow for improved contrast and visibility. AI, artificial intelligence.
Statistical Analysis
The design consisted of 4 experimental groups: 1) DEP-WDL (N = 13), 2) NDEP-WDL (N = 12), 3) DEP-NWDL (N = 9), and 4) NDEP-NWDL (N = 9) as described above. To study the effects of the respective contextual stimulus exposure on neuronal activity, c-Fos density was used as the dependent variable. While the assumptions that underlie parametric tests were met for independent random sampling, normal distributions and homogeneity of variance were not observed in these data. The Shapiro-Wilk test indicated that the distribution of c-Fos-positive values in the WDL group deviated significantly from normality (W = 0.90, p ≤ .001) (see histograms in the Supplement). Moreover, Levene’s test for homogeneity of variance revealed a significant difference in variances across groups (F3,39 = 3.32, p ≤ .029). For these reasons, the treatment effects on c-Fos density were analyzed using nonparametric tests, and, where appropriate, these were followed by post hoc comparisons with Bonferroni corrections.
Results
Overall Neuronal Activation Associated With Alcohol Seeking Is Increased in the DEP-WDL Group Relative to Nondependent Groups
To examine whether WDL stimulus exposure had an effect on neuronal activity, c-Fos density was quantified in key brain regions with an established role in alcohol seeking and craving. A Kruskal-Wallis test was conducted to examine the differences in c-Fos density among the following 4 groups: DEP-WDL, NDEP-WDL, DEP-NWDL, and NDEP-NWDL. The results of this test indicated a significant difference in c-Fos density among the treatment groups (H3 = 7.5, p ≤ .040). Post hoc analysis using pairwise comparisons revealed an increased overall density of activated neurons in the DEP-WDL group compared with both nondependent groups (Dunn’s test with Bonferroni correction, DEP-WDL vs. NDEP-WDL p ≤ .014, DEP-WDL vs. NDEP-NWDL p ≤ .024) (Figure 4A). No significant difference was found between the DEP-WDL and DEP-NWDL groups (p > .05, nonsignificant [n.s.]) or between the DEP-NWDL and NDEP-NWDL groups (p > .05, n.s.).
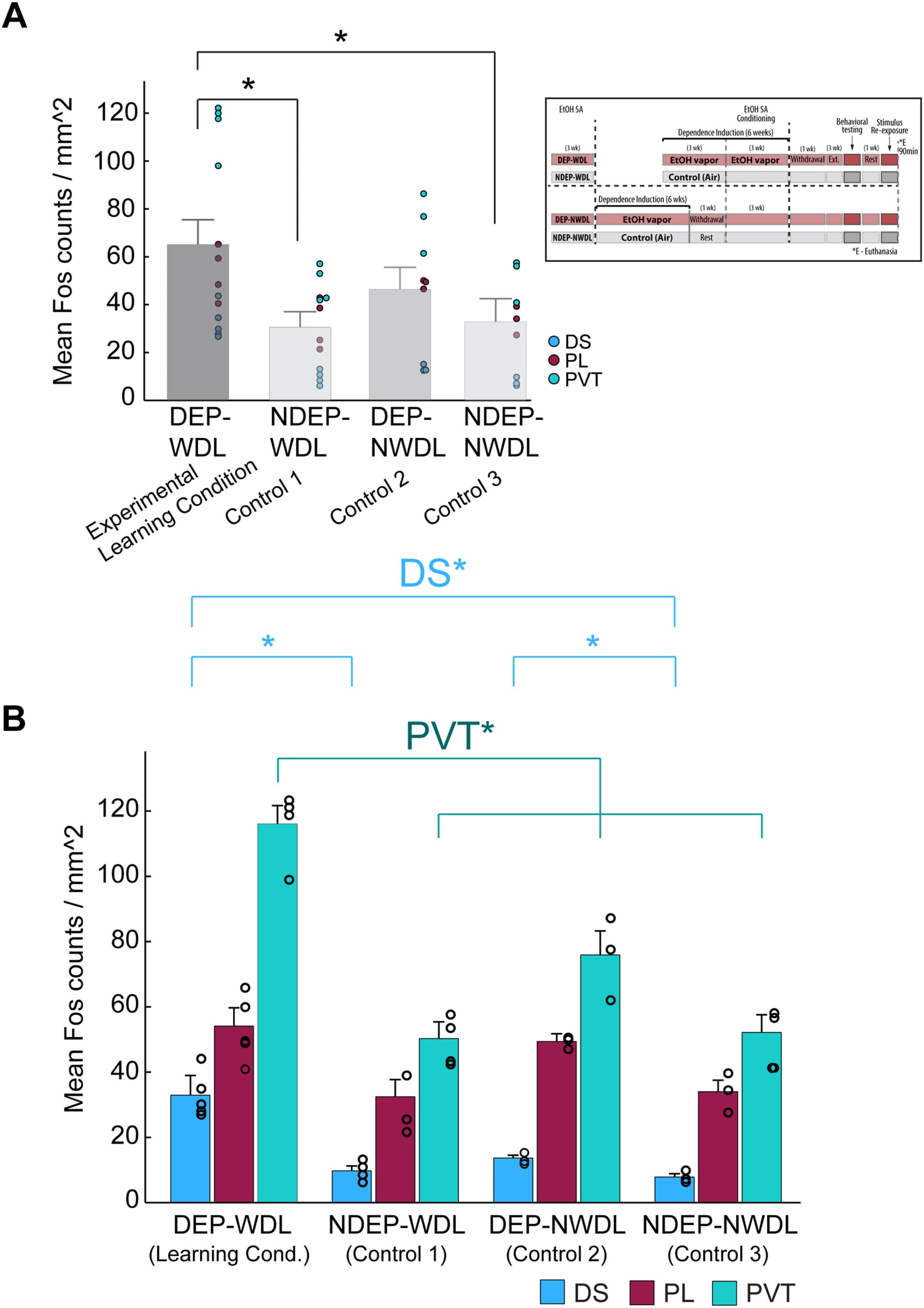
Figure 4. (A) Plot summarizing the overall c-Fos-activated neurons for all 4 experimental groups: DEP-WDL (N = 13), NDEP-WDL (N = 12), DEP-NWDL (N = 9), and NDEP-NWDL (N = 9). Inset shows group conditions. (B) Measurement of c-Fos activity in the DS (N = 15), PL (N = 14), and PVT (N = 15) revealed an increased number of activated neurons in the PVT of DEP-WDL rats (Kruskal-Wallis, Dunn’s test with Bonferroni correction p ≤ .019). Increased neuronal activity in the DS was also present in both dependent groups (DEP-WDL, DEP-NWDL) relative to the nondependent (NDEP-WDL, NDEP-NWDL) groups (Kruskal-Wallis, Dunn’s test with Bonferroni correction p ≤ .046). Bars represent mean and SEM. Asterisks denote statistical significance. DEP, dependent; DS, dorsal striatum; EtOH, ethanol; NDEP, nondependent; NWDL, no WDL; PL, prelimbic cortex; PVT, paraventricular nucleus of the thalamus; SA, self-administration; WDL, withdrawal-related learning.
Withdrawal Learning Experience–Dependent Neuronal Activation Is Increased in the Paraventricular Nucleus of the Thalamus and Dorsal Striatum
To better understand the neuroanatomical localization of these stimulus-reactive neurons, c-Fos density was initially analyzed in 3 brain regions with an established role in alcohol/drug addiction: the dorsal striatum (DS), prelimbic cortex (PL), and paraventricular nucleus of the thalamus (PVT). A Kruskal-Wallis test was conducted to examine the differences in neuronal activation among the 4 treatment groups and across the 3 brain regions. The results revealed a significant treatment group difference in c-Fos density in the DS and PVT (DS: H3 = 8.5, p ≤ .010; PVT: H3 = 10.5, p ≤ .012) but not in the PL (H3 = 4.4, p > .05, n.s.). Multiple pairwise comparisons using Dunn’s test with Bonferroni correction revealed that the DEP-WDL group showed an increased density of activated neurons specifically in the PVT compared with all other treatment groups (DEP-WDL vs. NDEP-WDL p ≤ .003; DEP-WDL vs. DEP-NWDL p ≤ .045; DEP-WDL vs. NDEP-NWDL p ≤ .011) (Figure 4B). In addition, increased neuronal activation was observed in the DS of DEP-WDL rats relative to rats in the nondependent groups, NDEP-WDL and NDEP-NWDL (p ≤ .008 and p ≤ .003, respectively); however, no difference was found between the DEP-WDL and DEP-NWDL groups for this striatal region (Figure 4B). Because these data revealed that WDL experience was a critical factor in the observed neuronal activity increases, especially in the PVT, we focused the remaining analyses on activity-dependent neuronal changes between the DEP-WDL and NDEP-WDL groups.
For a more comprehensive analysis across different neuroanatomical brain regions, c-Fos counts were extended to 4 additional brain regions with an established role in drug addiction including the infralimbic cortex (IL), nucleus accumbens (NAc), central nucleus of the amygdala (CeA), and basolateral amygdala (BLA) (Figure 5). To gain further insights into the functional changes across neuroanatomical regions and the recruitment of c-Fos-positive neurons as a result of the respective learning experience during the withdrawal state in dependent animals versus nondependent controls, a Mann-Whitney U test was used to compare neuronal activity in the DEP-WDL and NDEP-WDL groups across all 8 brain regions: the DS, PL, PVT, IL, NAc, CeA, BLA, and lateral hypothalamus (LH). The results revealed a significant increase in the density of activated neurons between the DEP-WDL and NDEP-WDL groups following WDL experience in the PVT, DS, and CeA (PVT: U = 1, p ≤ .021; DS: U = 0, p ≤ .014; CeA: U = 0, p ≤ .021) but no difference in c-Fos density for the PL, IL, NAc, BLA, or LH between the DEP-WDL and NDEP-WDL groups (Figure 5).
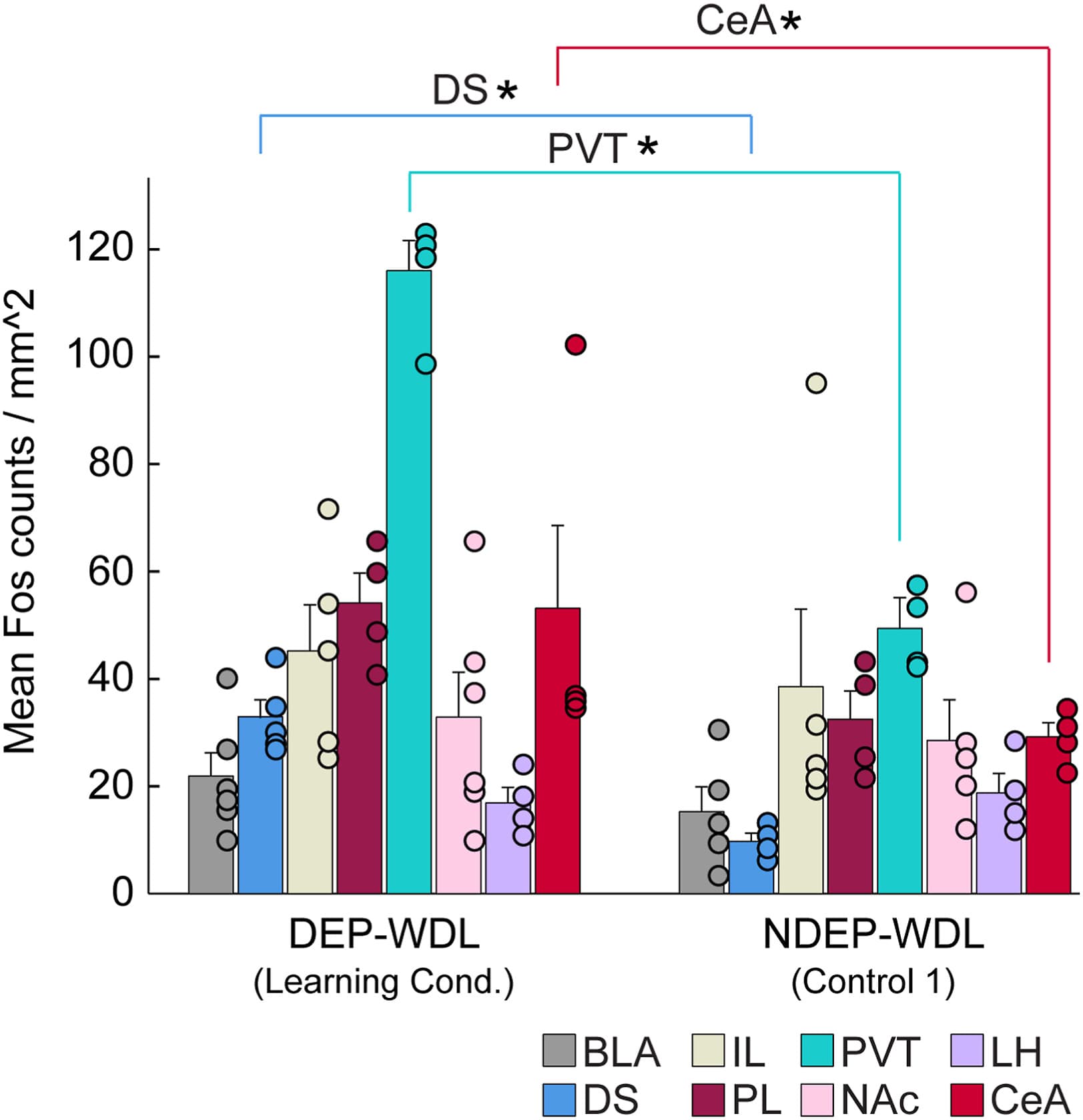
Figure 5. Recruitment of c-Fos-positive neurons in the DS (N = 9), PVT (N = 8), and CeA (N = 8), but not in the IL (N = 9), PL (N = 8), NAc (N = 11), BLA (N = 11), or LH, following WDL (Mann-Whitney U; DS p ≤ .016, PVT p ≤ .029, CeA p ≤ .29). Asterisks denote statistical significance. BLA, basolateral amygdala; CeA, central amygdala; DEP, dependent; DS, dorsal striatum; IL, infralimbic cortex; LH, lateral hypothalamus; NAc, nucleus accumbens; NDEP, nondependent; NWDL, no WDL; PL, prelimbic cortex; PVT, paraventricular nucleus of the thalamus; WDL, withdrawal-related learning.
Discussion
Differential Neuronal Recruitment in Alcohol-Seeking Rats With Versus Without a History of WDL
Our previous behavioral findings established that environmental stimuli conditioned specifically to amelioration of withdrawal (i.e., the negative reinforcing effects of alcohol) exert more powerful control over alcohol seeking than stimuli conditioned only to the positive reinforcing effects of alcohol. More specifically, reinstatement of alcohol seeking in rats with a history of WDL was not only greater but also impervious to motivational challenges including punishment of responding by electrical footshock and increased effort requirements than behavior induced by stimuli conditioned to alcohol in the nondependent state. The current findings extend these behavioral observations to the neuroanatomical level and implicate a role for clusters of neurons in the PVT, CeA, and DS in the potent motivating effects of WDL.
The findings confirm significant differences in c-Fos density across brain regions activated during context-induced compulsive alcohol seeking in rats with a WDL history versus rats without such a history that do not show compulsive behavior. More specifically, c-Fos-positive neurons were recruited in the PVT, DS, and CeA of WDL animals in which contextual stimuli were associated with the reversal of adverse withdrawal effects (i.e., negative hedonic states) (Figure 4, Figure 5, Figure 6). In contrast, exposure to stimuli associated with the hedonically positive aspects of alcohol consumption (i.e., in nondependent or postdependent rats without WDL experience) did not recruit the same number of c-Fos-positive neurons in the PVT and produced only mild, although significant, activation in the DS of dependent (DEP-NWDL) compared with nondependent (NDEP-NWDL) animals. These observations support the hypothesis that the conditioned effects of contextual stimuli associated with the reversal of withdrawal distress are differentially represented in the brain compared with the effects of stimuli associated with all other learning conditions in both alcohol-dependent and nondependent rats.
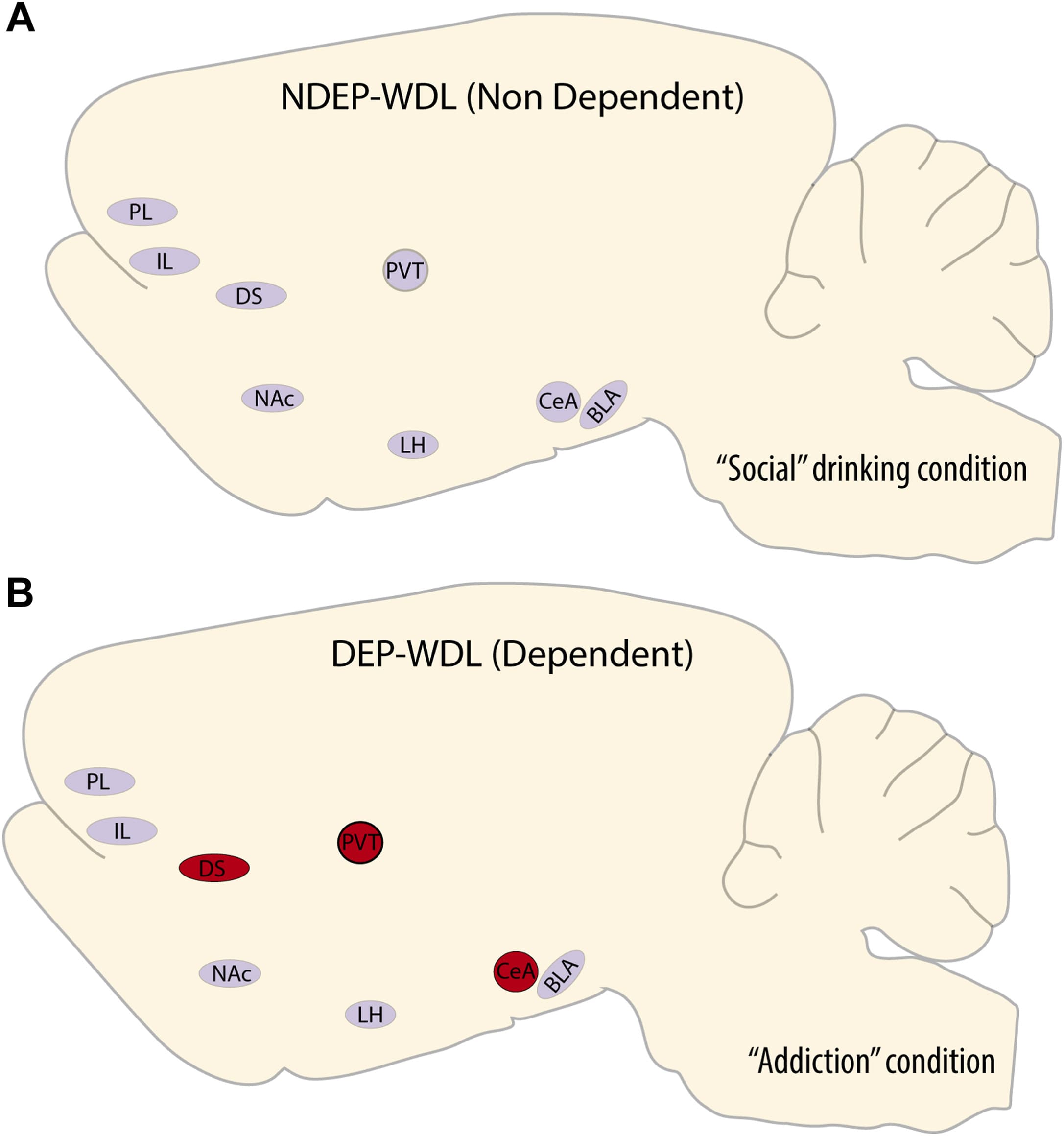
Figure 6. Neuroanatomical summary illustration of the recruitment of active neurons associated with context-induced alcohol seeking in rats with WDL experience and dependent drinking (B) vs. alcohol seeking induced by the same context in nondependent rats with a social drinking history (A). BLA, basolateral amygdala; CeA, central amygdala; DEP, dependent; DS, dorsal striatum; IL, infralimbic cortex; LH, lateral hypothalamus; NAc, nucleus accumbens; NDEP, nondependent; PL, prelimbic cortex; PVT, paraventricular nucleus of the thalamus; WDL, withdrawal-related learning.
In the PVT, increased c-Fos density was observed only in the WDL group but not in 3 relevant control groups (NDEP-WDL, DEP-NWDL, NDEP-NWDL). This prominent neuronal activation pattern found exclusively in the PVT of the WDL group suggests that this nucleus plays an important role in 1) the learning or acquisition of the negative contingency between alcohol consumption and the dysphoric effects of withdrawal and 2) the resulting development of compulsive drug seeking (Figures 4B, 5, and 6). The PVT is a key hub for neural circuits implicated in drug addiction. Moreover, this nucleus has an established role in emotional responses to anxiety and stress. Stress increases neuronal activity in the PVT, and the PVT was shown to be critical for stress-induced reinstatement of oxycodone seeking. The dysphoric effects of alcohol withdrawal are strongly associated with stress. Therefore, this finding is consistent with a major role of withdrawal stress in WDL learning and compulsive drug seeking following WDL acquisition. The stress-sensitive PVT area projects axonal fibers to the CeA, a region associated with negative emotion, stress, alcohol dependence, and particularly the aversive effects of alcohol withdrawal. Not unexpectedly, therefore, exposure to the WDL-associated stimulus context resulted in enhanced recruitment of c-Fos-positive neurons in the CeA, suggestive of increased CeA neuronal activity compared with NDEP-WDL rats, in which the stimulus context was associated only with the hedonically positive experience of alcohol consumption (Figure 5). Given that chemogenetic inhibition of the PVT to CeA projection alleviates stress responses, the current findings confirm a possibly major role of the CeA-projecting PVT system in behavioral responses to stress.
c-Fos-Positive Neurons Are Recruited During Alcohol Seeking in the DS of Dependent Animals
Independent of the WDL experience, exposure to stimuli conditioned to the hedonically negative aspects of alcohol withdrawal in dependent rats resulted in increased striatal c-Fos density relative to nondependent rats. The development of dependence is associated with the emergence of habitual behavior that is thought to be mediated by striatal brain regions, in particular the DS . This enhanced activation of neurons in the DS of the DEP-WDL group may be explained by a progressive engagement of dorsal striatal regions in habit formation that emerges during the development of substance dependence as proposed by the spiraling hypothesis [i.e., neural processes through which the ventral striatum comes to exert control over dorsal striatal processes mediated by so-called spiraling striato-nigro-striatal circuitry]. A pattern of activated neurons in the DS was also noted in the brains of postdependent (DEP-NWDL) animals (Figure 4B). These rats had a history of dependence but without WDL experience and were trained to associate the stimulus context with alcohol availability following completion of alcohol withdrawal. The neuronal activation in the DS of these DEP-NWDL animals was smaller than that in the WDL history group but significantly different from nondependent controls (NDEP-NWDL). Thus, DS neurons were recruited and became activated to some degree not only as a consequence of the WDL experience or negative reinforcement learning but also other dependence associated or experiential factors independent of WDL.
Addiction as a State of Reward Dysregulation and Hedonic Allostasis
It can be assumed that rats in all nondependent (NDEP-WDL) groups experienced a positive hedonic state during alcohol self-administration. In contrast, rats in the WDL group (DEP-WDL) experienced a profound negative hedonic state during withdrawal. Upon stimulus reexposure, increased neuronal activation occurred exclusively in the PVT of DEP-WDL animals (Figure 4B). Stimuli-reactive neurons in the PVT were associated specifically with WDL experience and likely directly linked to the reversal of the stressful aspect of alcohol withdrawal in rats with WDL experience. By contrast, the DS ensemble of active neurons, although differential, was observed in both dependent animals with WDL experience (DEP-WDL) and animals with a history of dependence but not WDL (DEP-NWDL). Considering our previous observations, these new findings suggest that the PVT may have a broader role in behavior motivated by hedonically negative states beyond alcohol-seeking behavior by providing a neuroanatomical hub for the development of hedonic allostasis, a chronic deviation from normal hedonic homeostasis and a state associated with reward dysregulation, stress responses, abnormal motivation, and addiction.
The current conditioning regimen that elicited behavior motivated by both positive and negative hedonic states may provide a tool for investigating aspects of the opponent process hypothesis of motivation and the development of hedonic allostasis. According to these hypotheses, the experience of a positive hedonic state results in a transient opposing negative hedonic state until equilibrium or hedonic homeostasis is restored. Over time, repeated experience of hedonically positive stimuli or substances (e.g., alcohol, opioids, sugar, skydiving, sexual activity, nicotine, gambling) leads to an allostatic state in which both the initial positive and resulting opponent negative processes still occur but eventually drop below the baseline level of normal hedonic homeostasis into negative hedonic territory, i.e., hedonic allostasis. Although these hypotheses of motivation are widely accepted, the corresponding neuronal mechanisms that mediate these processes remain to be established. Based on the current findings, one may speculate that the PVT plays a significant role in opponent processes and the development of hedonic allostasis. The prominent activation of the PVT following WDL stimulus exposure implicates this nucleus in mediating the powerful motivating effects of stimuli linked to alcohol (or other reinforcers) that reverse negative hedonic states—negative reinforcement—and thereby drive further alcohol consumption, exacerbating the negative hedonic process that ultimately conveys incremental hedonic valence to alcohol, with eventual progression toward an allostatic state. Given that marked neuronal activation in the PVT was associated exclusively with alcohol seeking elicited by the WDL-paired stimuli, this neuroanatomical region may provide a prime target to study the neuronal mechanisms that mediate opponent processes and the development of hedonic allostasis.
Limitations and Their Implications
The results are limited to information from male rats and require extension to female rats. The study was also somewhat constrained by the amount of tissue available from the original behavioral study. Finally, neuronal activation to identify stimulus-reactive neurons was measured by c-Fos protein expression, a marker for recent neuronal activity. However, populations of c-Fos-positive neurons can become activated by contextual stimuli through a mechanism that involves the expansion of multisynaptic boutons independently of the coactivation state of postsynaptic partners. That is, stimulus-reactive presynaptic boutons of projection neurons may recruit other neurons that were not engaged during the learning task to drive subsequent behavior. Such a mechanism could contribute to enhanced network-level responsiveness in the PVT of DEP-WDL rats by recruiting additional neurons, thus extending activation beyond traditional coactive engram ensembles. This activation might also have been influenced by the animal’s previous behavioral experiences, resulting in spontaneous c-Fos expression around the time of compound contextual stimulus presentation. Therefore, the stimulus-evoked signal is likely superimposed on a background level of c-Fos activity shaped by the animal’s unique behavioral trajectory throughout the experiment.
Conclusions
The findings suggest that the activated neurons identified here serve as a neural substrate for compulsive drug seeking resulting from 1) negative reinforcement learning associated with hedonic allostasis (PVT), 2) the development of habitual behavior over the progression of dependence (DS), and 3) stress memories associated with the stimulus context in which withdrawal and reversal of withdrawal were experienced (CeA). To confirm a role for these stimulus-activated neurons specifically in mediating WDL experience, confirmatory experiments will be required such as the silencing of each reactive group of neurons with cell type–specific methods. Finally, these findings undoubtedly have implications for maladaptive behavior linked to reward dysregulation and the processing of emotionally salient stimuli that drives behavior beyond substance use disorders. Research to establish the neurobiological alterations that link the conditioned incentive value of substance-associated contexts and their role in exacerbating drug seeking will also require extension to other classes of maladaptive behavior. These include, but are not limited to, systems that regulate fear conditioning, anxiety disorders, traumatic avoidance learning, and possibly predatory behavior.