Abstract
Substance use often begins, and noticeably escalates, during adolescence. Identifying predictive neurobehavioral vulnerability markers of substance use and related problems may improve targeted prevention and early intervention initiatives. This review synthesizes 44 longitudinal studies and explores the utility of developmental imbalance models and neurobehavioral addiction frameworks in predicting neural and cognitive patterns that are associated with prospective substance use initiation and escalation among young people. A total of 234 effect sizes were calculated and compared. Findings suggest that aberrant neural structure and function of regions implicated in reward processing, cognitive control, and impulsivity can predate substance use initiation, escalation, and disorder. Functional vulnerability markers of substance use include hyperactivation during reward feedback and risk evaluation in prefrontal and ventral striatal regions, fronto-parietal hypoactivation during working memory, distinctive neural patterns during successful (fronto-parietal hyperactivation) and failed response inhibition (frontal hypoactivation), and related cognitive deficits. Structurally, smaller fronto-parietal and amygdala volume and larger ventral striatal volume predicts prospective substance misuse. Taken together, the findings of this review suggest that neurobehavioral data can be useful in predicting future substance use behaviors. Notably, little to no research has empirically tested the underlying assumptions of widely used theoretical frameworks. To improve the reliability and utility of neurobehavioral data in predicting future substance use behaviors, recommendations for future research are provided.
1. Introduction
Substance use often begins, and noticeably escalates, during adolescence (Johnston et al., 2020). Alcohol is the most frequently used substance, with 27% of adolescents around the globe consuming alcohol in the previous month (World Health Organization, 2018). Experimentation with other substances, particularly cannabis, is also relatively common. By the eighth grade, approximately 11% and 20% of Australian and US youth, respectively, have tried cannabis or an illicit drug, and 30% and 50% have done so by the tenth grade (Guerin and White, 2020; Johnston et al., 2020). Earlier substance use initiation is related to poorer outcomes, such as elevated risk of mental health problems and increased risk of subsequent alcohol and other substance use disorders (SUDs) (Spear, 2018; Substance Abuse and Mental Health Services Administration, 2019). The risk associated with early onset substance use initiation is related to complex interactions between biological (neurocircuitry, genetics, epigenetics) and environmental factors (sociocultural, psychopathology, trauma). Identifying vulnerability markers for early and escalating substance use has been an important line of investigation to detect at-risk individuals and guide prevention efforts. Here, the current review focuses on neurobehavioral vulnerability markers that prospectively predict substance use, including functional neuroimaging indices (resting-state, task-based [reward processing, decision making, working memory, response inhibition]), structural neuroimaging indices (volume, area, thickness, gray matter density, white matter integrity), and cognitive measures (inhibition, impulsivity, delay discounting, cognitive flexibility, working memory, attention, short-term memory, learning, word knowledge, abstract reasoning, computation skills, visuospatial functioning). Neurobehavioral vulnerabilities included in this review include both premorbid heritable, trait-based markers that remain unchanged with substance use progression as well as non-genetic, state-based markers that change with severity of substance use (Gottesman and Gould, 2003; Kwako et al., 2018). See Bickel et al. (2014) and Mackillop (2013) for detailed examples of this concept using delay discounting as a behavioral marker for substance use. In order to provide a broader context of the neural and cognitive markers associated with prospective substance use, this review begins with an overview of adolescent neurodevelopmental processes and theoretical frameworks for predicting adolescent risk behaviors and addiction, followed by a synthesis of human longitudinal studies investigating neurobehavioral markers associated with early and escalating substance use and SUDs.
2. Adolescence and young adulthood are critical neurodevelopmental periods
The brain is already 90% of its total size by the age of six; however, important structural and functional developments continue to occur throughout adolescence and into adulthood, reaching maturational asymptote by the early 30s (Giedd, 2004; Giedd et al., 1999; Gogtay et al., 2004; Tamnes et al., 2017). Gray matter volume follows a curvilinear growth pattern. After increasing from infancy to childhood, gray matter volume and cortical thickness decreases throughout the second and third decades of life, beginning with posterior and inferior structures and proceeding anteriorly and superiorly throughout the brain (Shaw et al., 2008). This process has been described as synaptic pruning of superfluous neuronal connections and reductions in glial cells (Shaw et al., 2008). Synaptic pruning is proposed to result in more specialized functional networks and more efficient processing of information (Blakemore, 2008; Giorgio et al., 2010). Concurrently, white matter volume, which consists of myelin-coated axons, and white matter integrity increase over this period in a mostly linear pattern (Giedd, 2004; Østby et al., 2009). Myelination is thought to increase distributed brain connectivity between distant brain regions, such as cortico-subcortical regions, relative to more local connectivity, like cortico-cortical regions (Baker et al., 2015; Dennis et al., 2013; Fair et al., 2009). These connectivity-based shifts are thought to reflect a brain that is becoming more efficient in its within-network communication and more integrated in its between-network communication (Fair et al., 2009). Furthermore, ontogenic changes in neurotransmitter systems occur, including peaks in dopamine receptors throughout the mesocortical and mesolimbic systems, which are fundamental in the neural processing of motivation and rewards (Ernst and Luciana, 2015). These systems include projections from the ventral tegmental area in the midbrain to the dorsal striatum, prefrontal cortex (PFC), thalamus, and parietal regions (mesocortical system) and to the amygdala, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and nucleus accumbens (NAcc) (mesolimbic system)(Beaulieu and Gainetdinov, 2011; Björklund and Dunnett, 2007). Refinements in brain structure, function, and neurotransmitter systems parallel the complex integration of cognitive processing and socioemotional regulation that strongly influence decision making, peer affiliation, behaviour, and wellbeing (Crone and Dahl, 2012).
2.1. The ‘imbalance hypothesis’ of neurodevelopment
The neural changes described have been summarized in neurodevelopmental models which suggest that adolescent motivation is particularly sensitive to rewarding stimuli, such as alcohol and other substances. According to dual systems models which describe the imbalance hypothesis, salience of rewarding stimuli results from a developmental imbalance of two neural systems: a rapidly developing socioemotional neural system that increases salience and motivation to pursue rewards (i.e., brain regions involved in the dopaminergic mesolimbic system) and a gradually developing self-regulating cognitive control system that restrains impulses, which includes the PFC, lateral parietal regions, and ACC (Casey et al., 2008; Luna et al., 2015; Steinberg, 2010). During adolescence, the reactivity of the socioemotional neural system is thought to be particularly sensitive and may prevail over controlled responses in emotionally-salient contexts, such as peer influence or when there is potential to obtain an immediate reward. Notably, these models are not biologically deterministic, rather they emphasize the context in which decision-making takes place. Other theoretical frameworks incorporating the imbalance hypothesis include the triadic model which additionally hypothesizes that a weak and less developed amygdala (i.e., harm-avoidant system) may independently contribute to a hypersensitive reward salience/socioemotional system in the ventral striatum (includes the NAcc, caudate, putamen) (Ernst et al., 2006). In relation to adolescent substance use experimentation, models describing the imbalance hypothesis postulate that the magnitude of the imbalance between the developing socioemotional reward and cognitive control systems should predict the propensity for engaging in risky behaviors (Meisel et al., 2019).
More recent variants of dual systems models increasingly acknowledge the complexity of neurobehavioral changes across adolescence and young adulthood. These models highlight the importance of neural connectivity within and between socioemotional and cognitive control neural circuits (Casey, 2015; Casey et al., 2016), as well as the interplay between neurobiological underpinnings with changing social contexts (i.e., greater independence, change from parents to peers as primary influence) (Shulman et al., 2016) as crucial to understanding adolescent vulnerabilities in decision making and risk taking behaviors. Pubertal development has also been considered a critical factor (Crone and Dahl, 2012); increasing testosterone during adolescence promotes pubertal maturation of the ventral striatum, which has been related to reward-seeking behaviors, including substance use during adolescence (Silvers et al., 2019).
Overall, models describing the imbalance hypothesis – which have been characterized as a heuristic device, generative across multiple fields – consider adolescence and young adulthood to be a critical neurodevelopmental period for socioemotional and cognitive control refinement and suggest that neurobiological, social, and pubertal changes increase sensitivity towards rewarding experiences. The degree of imbalance between developing neural systems is thought to predict the likelihood of engaging in risky behaviors, such as substance use and misuse.
3. Pathways to substance use disorders
Neurobehavioral theories of addiction across the lifespan commonly posit that mesolimbic and mesocortical reward processing and cognitive control pathways are central to the development of psychological dependence and recurrent relapse (Bjork et al., 2012, 2010; Blum et al., 2000; Buckholtz et al., 2010; Cloninger, 1987; Feil et al., 2010; Koob and Volkow, 2010; Robinson and Berridge, 2008). Reward processing and cognitive control in individuals with vulnerabilities towards addictive behaviors, including substance use, has been extensively studied by measuring neural response to rewards and neural activation during cognitive tasks using functional magnetic resonance imaging (fMRI). The standard variable of interest is blood-oxygen-level dependent (BOLD) signal which measures the regional differences in cerebral blood flow and volume to delineate regional neural activity and connectivity. Some theoretical frameworks of addiction primarily implicate the cortico-striatal reward pathway which includes the ventral striatum and medial PFC (Bjork et al., 2012, 2010; Blum et al., 2000; Buckholtz et al., 2010; Cloninger, 1987; Robinson and Berridge, 2008) while other frameworks have also described the role of cognitive control-related circuitry in the addiction cycle, which includes more widespread brain regions from mesolimbic and mesocortical systems (Feil et al., 2010; Koob and Volkow, 2010).
3.1. Reward processing pathways to substance use disorders
Dominant theoretical frameworks in this space include the reward deficiency syndrome theory, the impulsivity theory of addiction, and the incentive sensitization theory; each framework highlights aberrant functioning of the ventral striatum. According to the reward deficiency theory, individuals with vulnerabilities towards addictive behaviors have a general deficiency in their ability to recruit and activate neural reward pathways which results in hypoactivation of these circuits and reduced pleasure from rewards. From this perspective, it is hypothesized that substance use, among other addictive behaviors such as gambling, are initiated to compensate for this reward deficiency and to stimulate underlying neural circuitry, including the ventral striatum (Blum et al., 2000; Cloninger, 1987). This pathway to SUD has also been referred to as the ‘internalizing’ or ‘anhedonic’ pathway because blunted ventral striatal response to reward cues is also observed in individuals with depression (Epstein et al., 2006; Nikolova et al., 2012; Stringaris et al., 2015; Wacker et al., 2009). This pattern of activity (i.e., hypoactivation of the ventral striatum) may therefore have predictive utility among young people who go on to use substances to cope with internalizing symptoms (Hussong et al., 2011; Lees et al., 2020c).
In contrast, the impulsivity theory of addiction posits that hyperactivation of the ventral striatum to reward cues may lead to escalating and problematic substance use through a pathway of externalizing behaviors, including high sensation seeking, impulsivity, and motivation to obtain potentially rewarding stimuli (Bjork et al., 2012, 2010; Buckholtz et al., 2010). From this perspective, concurrent blunted medial PFC response during reward anticipation and feedback is thought to reflect reduced prefrontal regulation of reward processing (Swartz et al., 2020). Finally, the incentive sensitization theory argues that individuals with a bias towards substance-related cues are at heightened risk of escalating substance use, owing to acquired incentive salience of such cues (Robinson and Berridge, 2008). Therefore, individuals with this vulnerability would show relatively greater ventral striatal response to substance use cues and relatively lower ventral striatal response to nonsubstance use cues.
3.2. Cognitive control and impulsivity pathways to substance use disorders
While the previously described theories primarily implicate the ventral striatum in reward sensitivity and vulnerability to SUD (and the medial PFC to a lesser extent), the PFC-striatothalamic dysfunction model (Feil et al., 2010) and three-stage cycle of addiction framework (Koob and Volkow, 2010) also emphasize the role of other prefrontal and limbic regions in regulating inhibitory control over substance use.
The PFC-striatothalamic dysfunction model concentrates on three circuits which include projections from the dorsolateral PFC (DLPFC), OFC, and ACC, respectively, to the globus pallidus, basal ganglia, thalamus, and caudate nucleus (Alvarez and Emory, 2006; Bradshaw, 2001; Tekin and Cummings, 2002). Early initiation, escalating levels of use, and SUD are thought to be related to aberrations in these circuits. From the PFC-striatothalamic dysfunction model perspective, an aberrant DLPFC circuit (indexed by poor response inhibition and working memory (Blasi et al., 2006; Garavan et al., 2002; Kelly et al., 2004)) modifies a young person’s ability to integrate information and this results in selection of risky behavioral choices, such as consuming substances. These risky behavioral choices may also occur due to a delayed or abnormal ACC circuit (indexed by poor error correction and sustained attention (Tekin and Cummings, 2002)), which results in inordinate assessment of the potential positive and negative outcomes of substance use. Additionally, dysregulation of the OFC circuit (indexed by poor response inhibition and impulsivity (Olausson et al., 2007; Tanabe et al., 2009)) is hypothesized to result in poor decision-making with low capacity to inhibit compulsive, socially-influenced behaviors (Feil et al., 2010). Therefore, individuals with aberrant development of these fronto-striatal circuits are considered to have lowered sensitivity to the risk of substance use initiation, and because of low aversion to the consequences, are thought to be vulnerable to substance use escalation and SUD.
Finally, the three-stage cycle of addiction framework argues that discrete networks underlie the pathway to SUD, which involves disrupted inhibitory control and impulsivity (Koob and Volkow, 2010). The three stages of addiction include (1) binge/intoxication, (2) withdrawal/negative affect, and (3) cravings. Transitions through these stages are mediated by aberrant structure and function of the ventral tegmental midbrain area and ventral striatum (binge/intoxication stage), the amygdala (withdrawal/negative affect stage), and the OFC circuit, PFC, hippocampus, and insula (craving stage). From this perspective, aberrant development of the cingulate, DLPFC, and inferior frontal cortex (indexed by poor inhibitory control and high impulsivity) are postulated to be critical to general vulnerability for developing and maintaining addiction (Koob and Volkow, 2010). Overall, this heuristic framework implicates widespread brain regions from the mesocortical and mesolimbic systems in vulnerability for substance use initiation and escalation.
In summary, neurobehavioral theories of addiction implicate ventral striatal (including NAcc, caudate, putamen), prefrontal, and limbic circuits involved in reward processing and cognitive control in substance use behaviors and addiction. While the reward deficiency syndrome theory, impulsivity theory of addiction, and incentive sensitization theory posit that functional differences will be observed in response to reward anticipation or feedback processes, the PFC-striatothalamic dysfunction model and three-stage cycle of addiction framework also hypothesize that structural aberrations, indexed by cognitive deficits, could identify individuals with increased risk of problematic substance use behaviors. These aberrations may be related to genetic and epigenetic factors, prenatal exposures, puberty, or as a result of a developmental imbalance within the circuits, among other factors (Casey et al., 2008; Luna et al., 2015; Steinberg, 2010).
All of these models (i.e., reward deficiency syndrome theory, impulsivity theory of addiction, incentive sensitization theory, PFC-striatothalamic dysfunction model, three-stage cycle of addiction) originate from the addiction field and aim to explain the chronic course of use among individuals (often adults) with SUD and recurrent relapse. The following review will explore the utility of addiction models and developmental models (i.e., imbalance hypothesis) in predicting regions associated with prospective substance use initiation and escalation among young people. In the following section, we summarize the rapidly expanding literature of human longitudinal neuroimaging and cognitive studies that aims to identify neurobehavioral features that predate substance use or misuse and may be associated with increased SUD vulnerability. Predictive neural and cognitive markers can be examined through prospective, longitudinal studies that begin prior to substance use initiation or heavy use and assess individuals repeatedly over time as patterns of substance use emerge and potentially escalate. This design allows for examination of typical developmental neural trajectories in youth who have never used alcohol or other substances and compares their brain maturation to youth who transition into substance use.
4. Neurobehavioral vulnerabilities associated with future substance use
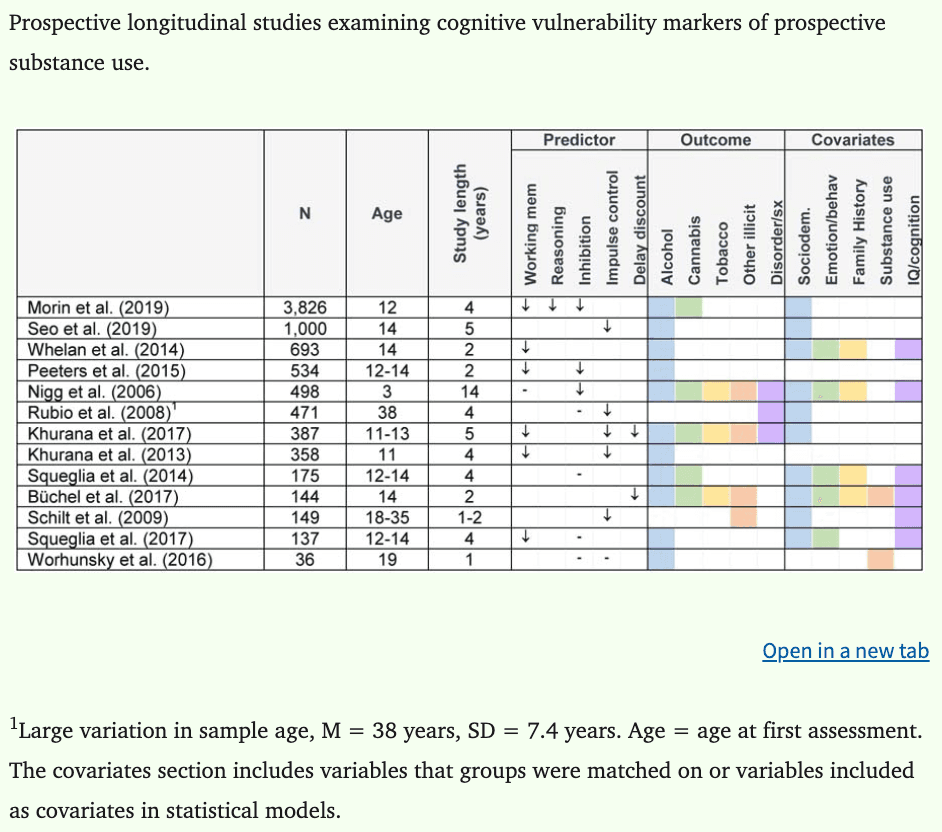
Literature searches using PubMed were conducted on 10 June 2020 using combinations of the following search terms: (i) neurobiological, brain, neural, neuroimaging, cognitive; (ii) substance use, alcohol use, drug use; (iii) youth, adolescent, young adult; (iv) vulnerability, risk factor, predisposing, precursor. In total, 44 longitudinal neurobehavioral studies predicting substance use were identified and have been reported in this review. Of the 44 studies identified, 20 report on functional neuroimaging indices, 21 report on structural brain indices, and 13 report on cognitive deficits that may increase risk of later substance use and misuse. While there was insufficient data to conduct a meta-analysis (Eickhoff et al., 2016), a quantitative synthesis was conducted by computing and charting effect sizes for all available data in each individual study. Cohen’s d was used as the measure of effect size, where 0.2 = small, 0.5 = medium, and 0.8 = large (Cohen, 1988; Lakens, 2013), and confidence intervals were calculated where possible. When data were not available to calculate effect sizes, we report the key findings in text. In cases where low scores on measures indicated better performance, data were adjusted so that a negative d statistic indicated worse performance. A total of 234 effect sizes from 36 studies were calculated. Relevant data were not available to calculate effect sizes for eight studies (Camchong et al., 2017; Khurana et al., 2013; Mahmood et al., 2013; Morales et al., 2019; Morin et al., 2019; Nikolova and Hariri, 2012; O’Brien and Hill, 2017; Peeters et al., 2015). For these studies, standardized betas are reported where available. Neuroimaging and cognitive study characteristics are reported in Tables 1 and 2, respectively.
Table 1:
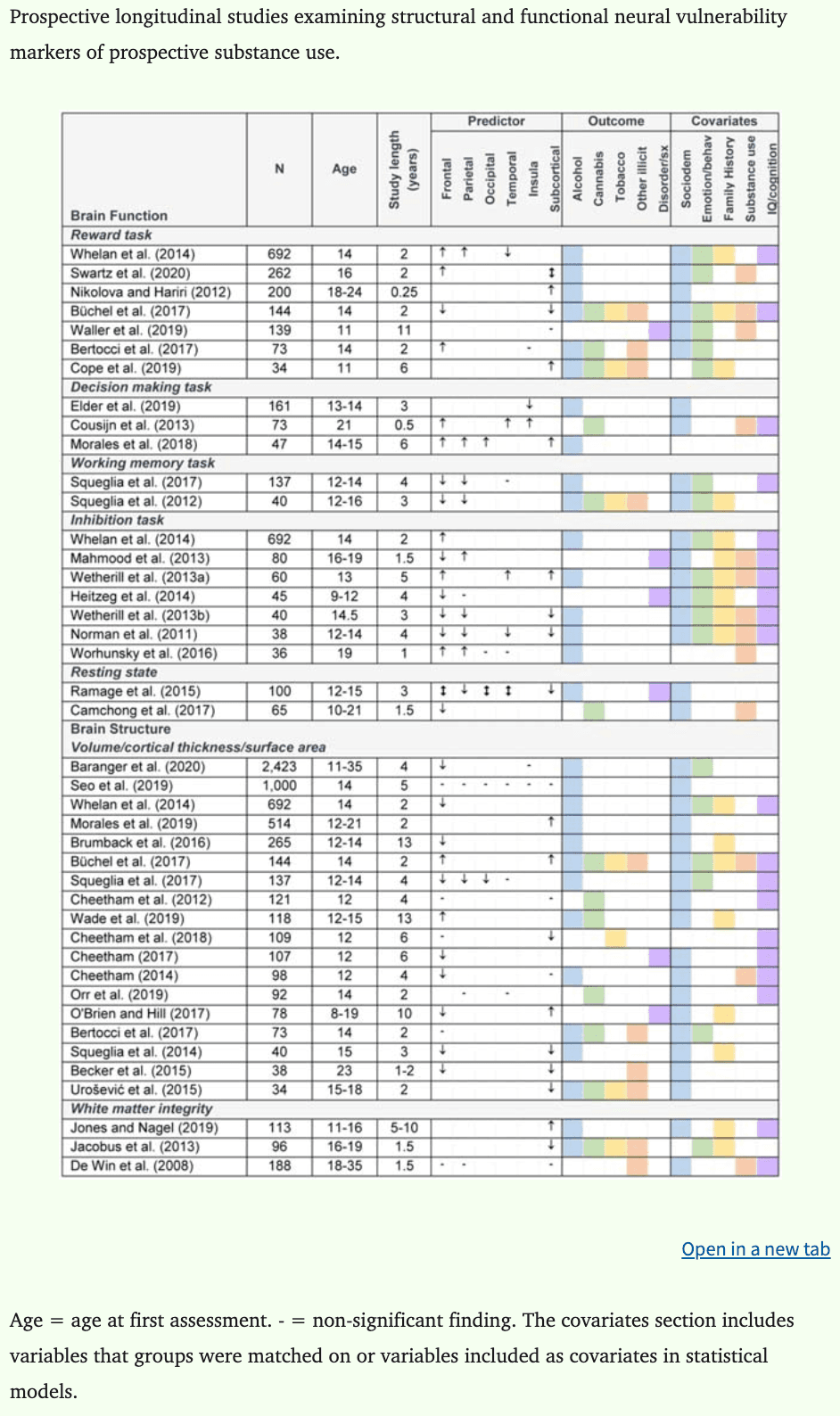
Table 2:
4.1. Functional neural features
From 20 fMRI studies, 95 effect sizes were calculated. Four studies did not report data required to calculate d (Camchong et al., 2017; Mahmood et al., 2013; Nikolova and Hariri, 2012; Whelan et al., 2014). To date, the predominance of studies have identified altered neural signatures during tasks of reward or cognitive control before substance initiation among those who go on to use and misuse substances.
4.1.1. Reward processing.
Data were available to generate effect sizes for five of seven studies examining neural response to reward processing (Figure 1), where the average d was −0.18 during reward gain anticipation (k (number of effect sizes) = 13, range = −1.05 to 0.97) and 0.11 during reward gain feedback (k = 6, range = −0.15 to 0.48).
Figure 1:
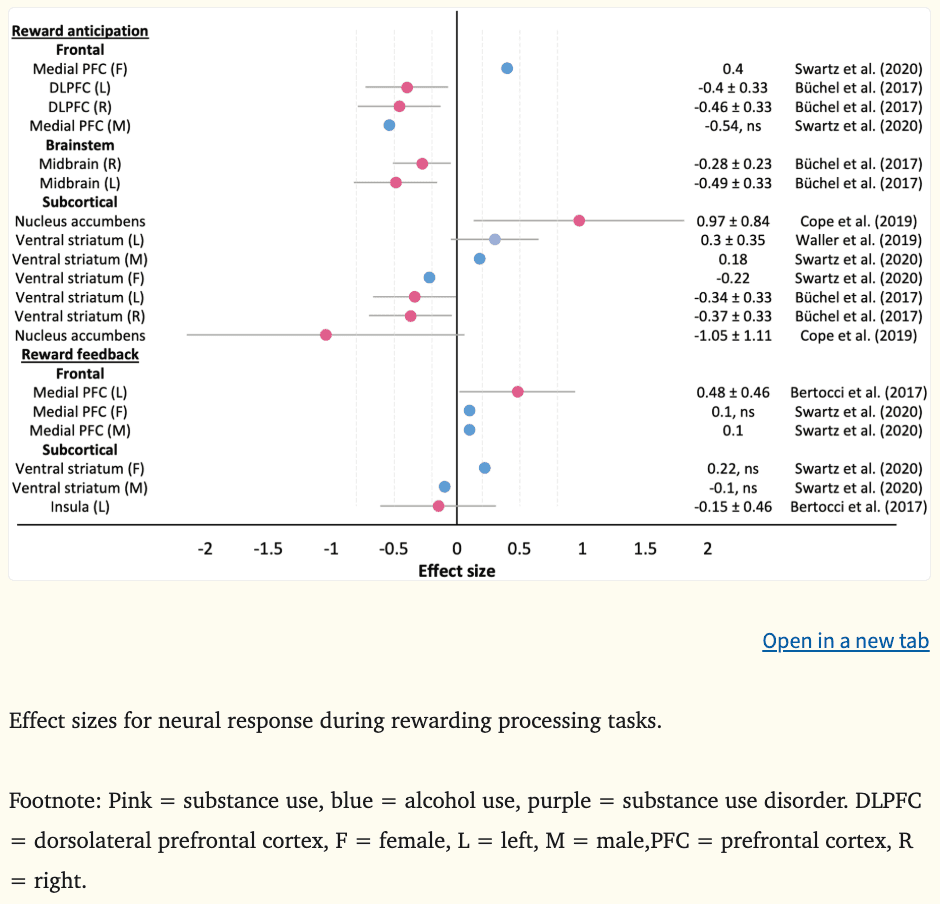
Reward anticipation.
The reward anticipation contrast is thought to reflect motivational and impulsive processes, resulting from relevant cues whose incentive value is either innate or learned from positive associations (Schultz, 2001). Among high novelty seeking adolescents, hypoactivation in the bilateral DLPFC (|ds|=0.40–0.46) and mesolimbic regions (ventral striatum [|ds|=0.34–0.37] and midbrain [|ds|=0.28–0.49]) during reward gain anticipation at age 14 prospectively predicted escalating substance misuse two years later (Büchel et al., 2017). Furthermore, hypoactivation in the bilateral ventral striatum at age 16 in females predicted escalating alcohol use two years later, although impulsivity at age 18 did not mediate this effect, nor did depression symptoms at age 17 (|d|=0.22) (Swartz et al., 2020). These findings are consistent with a recent meta-analysis reporting striatal hypoactivation during reward anticipation among individuals with SUDs (Luijten et al., 2017). The direction of effects are also consistent with the reward deficiency theory (Blum et al., 2000; Cloninger, 1987). However, there was no strong evidence to indicate that hypoactivation in the DLPFC or ventral striatum during reward anticipation in youth reflected an internalizing or anhedonic pathway to substance use, with no psychopathology baseline differences observed in the highly impulsive cohort recruited by Büchel et al. (2017) and no depression-related mediation effects observed in Swartz et al. (2020) prior to substance use escalation.
In contrast, greater medial PFC activity (including the OFC and ACC) during reward gain anticipation at age 16 predicted increases in alcohol use among females at age 18 (|d|=0.40) (Swartz et al., 2020). Similarly, heightened reward gain anticipation in the ventral striatum during childhood and adolescence has had predictive utility for alcohol, tobacco, and illicit substance use initiation and use two to six years later (|ds|=0.18–0.97), over and above early externalizing behaviors and family history of SUD (Cope et al., 2019; Swartz et al., 2020). The prospective alcohol association reported by Swartz and colleagues was only observed among males and neither impulsivity or depression symptoms mediated associations between neural response and future alcohol use behaviors. Hyperactivity of the ventral striatum is consistent with the impulsivity theory of addiction, however heightened medial PFC activity among females deviates from the theory, where blunted response is hypothesized.
Reward feedback.
The reward feedback contrast is thought to be relevant for learning processes (Miller et al., 2014; Redish, 2004). Greater bilateral prefrontal reward feedback in the medial PFC and temporally proximate regions (superior frontal, precentral gyrus) at age 14 predicted binge drinking (βs|≥0.13) (Whelan et al., 2014) and lifetime substance use (|d|=0.48)(Bertocci et al., 2017) by age 16, above and beyond other clinical and sociodemographic variables, such as depression, mania, and age. Likewise, heightened ventral striatal activity during young adulthood predicted future stress-related problem drinking when individuals also exhibited lower threat-related amygdala reactivity (Nikolova and Hariri, 2012). Swartz and colleagues (2020) also observed interactions between increased ventral striatal activity and stress during reward anticipation; however, it was not a predictor of interest in their analyses, so they did not explore associations any further. Hyperactivation during reward feedback is consistent the findings reported in the recent meta-analysis of individuals with SUD (Luijten et al., 2017) and provide support for the impulsivity theory of addiction (Bjork et al., 2012, 2010; Buckholtz et al., 2010).
Overall, each of the currently dominant reward processing theories of addiction cannot fully account for the neural activation patterns and associated psychopathology and personality traits observed among young people who go on to use and misuse substances. It is likely that several other factors are contributing to the conflicting findings observed during reward anticipation. For example, there was evidence of unique neural markers for increases in alcohol use among males (i.e., higher ventral striatal activity) and females (i.e., higher medial PFC activity, lower ventral striatal activity) in the study by Swartz and colleagues (2020). There was also preliminary evidence of complex interactions with stress (Nikolova and Hariri, 2012; Swartz et al., 2020). This finding highlights the protective role of threat-related amygdala activity against prospective substance use in individuals with exaggerated responsiveness to reward, as hypothesized by the triadic model (Ernst et al., 2006). Furthermore, it’s also probable that psychopathology, changing social contexts, and pubertal development interact with neural responses and reward responsiveness, as hypothesized by more recent renditions of the developmental imbalance models (Crone and Dahl, 2012; Shulman et al., 2016). Consistent with conclusions drawn from previous reviews of neurodevelopmental models (Meisel et al., 2019), the imbalance hypothesis was not directly examined in the studies included in this review (e.g., predictive utility of functional connectivity patterns between fronto-striatal regions during reward processing). Further theoretical conceptualizations of addiction pathways in adolescence and empirical testing of potential mediating and moderating factors involved in prospective prediction of substance misuse is required. Of note, no prospective longitudinal studies to date have directly examined the incentive sensitization theory among young people, identifying a significant gap in the existing literature base.
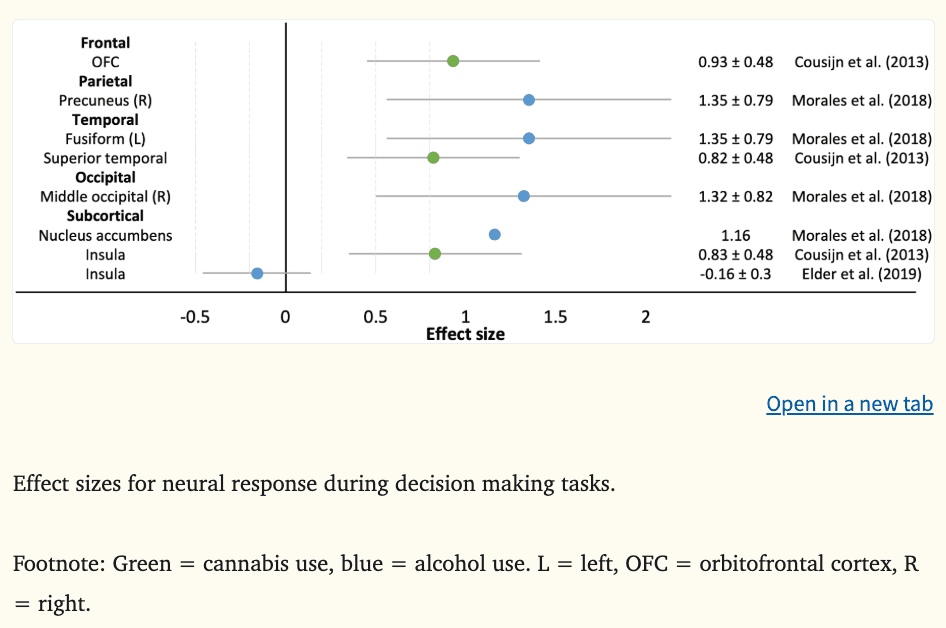
4.1.2. Risk-based decision making.
Three fMRI studies examined neural response during risk-based decision making where the average effect was 0.95 (k = 8, range = −0.16 to 1.35), see Figure 2. During a win evaluation decision making task, greater neural response in the OFC, superior temporal gyrus, and insula at age 21 was related to greater cannabis use six months later (|ds|=0.82–0.93) (Cousijn et al., 2013). During high risk choices, greater neural response in cortical regions (precuneus, fusiform, middle occipital gyrus) and the NAcc at ages 14 to 15 years has been associated with prospective onset of binge drinking six years later (Morales et al., 2018). Large effect sizes were reported in both studies. Insula activation was examined in a third study (Elder et al., 2019). While activation was not directly associated with alcohol use three years later, there was a significant indirect effect for males where insula activity during risk processing predicted future externalizing symptoms, which in turn predicted higher alcohol use frequency. Greater insula, NAcc, and OFC activity during decision tasks evaluating risk align with the impulsivity theory and the three-stage cycle of addiction framework. Considering the small number of studies examining decision making ability and prospective substance use, and the exploration of disparate brain regions, further research is required to understand whether neural response during these tasks can be considered a reliable vulnerability marker of future substance use behaviors.
Figure 2:
4.1.3. Cognitive control.
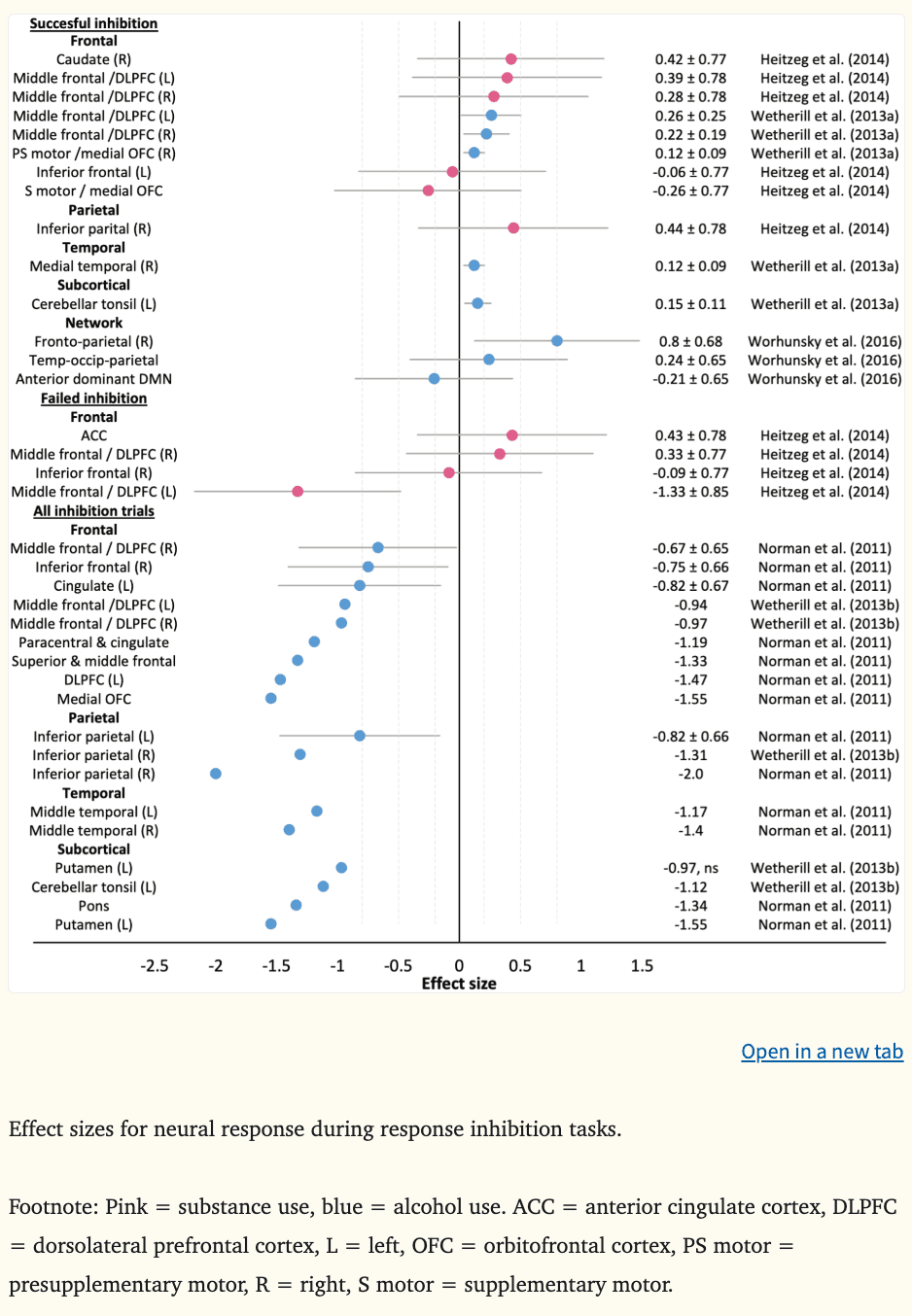
Effect sizes could be calculated for the two working memory fMRI studies (Figure 3) and five of seven inhibition fMRI studies (Figure 4), where the average d was −0.44 during working memory (k = 9, range = −1.09 to −0.20), 0.21 during successful inhibition (k = 14, range = −0.26 to 0.80), −0.17 during failed inhibition (k = 4, range = −1.33 to 0.43), and −1.19 across inhibition trials (k = 18, range = −2.00 to −0.67).
Figure 3:
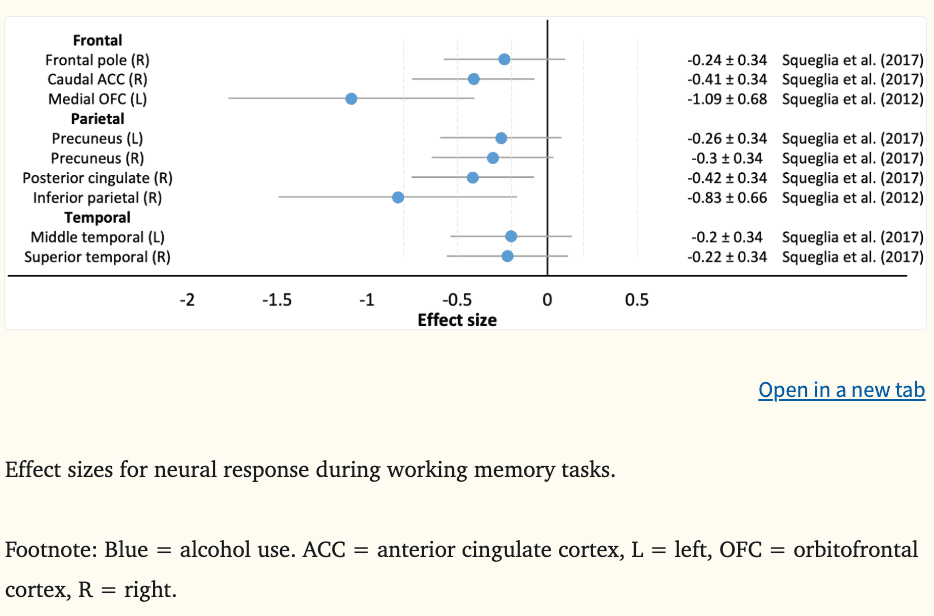
Figure 4:
Working memory.
In two studies, lower fronto-parietal neural response during a visual working memory task at ages 12 to 16 years was predictive of alcohol, tobacco, and other illicit substance use three to four years later, however, no overlap in regions between the studies was observed (Squeglia et al., 2017, 2012). Relevant to addiction frameworks implicating cognitive control regions in prospective substance use behaviors (i.e., PFC-striatothalamic dysfunction model, three-stage cycle of addiction framework), medium to large effect sizes were observed in the left medial OFC (|d|=1.09), the right caudal ACC (|d|=0.41), and right posterior cingulate (|d|=0.42). Hypoactivation of fronto-parietal regions during working memory tasks is thought to reflect a less efficient information processing and cognitive control system (Squeglia et al., 2012), modifying a young person’s ability to inhibit impulsive, rewarding behaviors and detect the possibility of negative consequences. In contrast, activation in temporal regions did not appear to prospectively predict substance use in adolescence (Figure 3).
Inhibition.
At age 13, greater neural response during successful inhibition in part of the bilateral DLPFC (middle frontal gyrus) (|ds| = 0.22–0.26), right medial OFC (|d|=0.12), right medial temporal lobule (|d|=0.12), and left cerebellar tonsil (|d|=0.15) was predictive of alcohol-induced black outs in binge drinking youth five years later, above and beyond externalizing symptoms (Wetherill et al., 2013a). Additionally, greater neural response during successful inhibition throughout the fronto-parietal network at age 19 prospectively predicted escalating binge and heavy drinking one year later (|d|=0.80) (Worhunsky et al., 2016). Conversely, hypoactivation of the middle frontal gyrus during failed inhibitory control at ages 9 to 12 prospectively predicted substance use problems five years later, when controlling for externalizing symptoms in childhood (|d|=1.33) (Heitzeg et al., 2014) (Figure 4).
When studies have examined neural response across all inhibition trials during adolescence (i.e., both successful and failed inhibition), more widespread global hypoactivation has prospectively predicted subsequent binge and heavy drinking three to four years later (Norman et al., 2011; Wetherill et al., 2013b) and substance use and SUD symptoms 18 months later (Mahmood et al., 2013). At ages 12 to 14 years, lower activation in areas of the DLPFC (|d|=0.67–1.47) and medial OFC (|d|=1.55), as well as the right inferior frontal gyrus (|d|=0.75), cingulate (|ds|=0.82–1.19), bilateral inferior parietal (|ds|=0.82–2.00) and temporal lobules (|ds|=1.17–1.40), the left putamen (|d|=1.55), left cerebellar tonsil (|d|=1.12), and pons (|d|=1.34) predicted subsequent binge and heavy drinking among youth by age ~18 with limited substance use histories at baseline (Norman et al., 2011; Wetherill et al., 2013b). At ages 16 to 19 years, lower activation in the medial OFC (|βs|=0.23–0.39) and greater activation in the angular gyrus (parietal region) (|βs|=0.13–0.48) has predicted escalating substance use behaviors, particularly among adolescents who were heavy users at baseline (Mahmood et al., 2013). Many of these findings were above and beyond other predictors such as age, sex, socioeconomic status, externalizing behaviors (i.e., hyperactivity, impulsivity, aggression, oppositionality), and family histories of SUDs. Contrasting directionality in parietal regions between studies could reflect differences in substance use characteristics among adolescents at baseline.
Consistent with developmental models of the imbalance hypothesis and cognitive control frameworks of addiction, the largest effects were observed in the DLPFC and OFC (and temporally proximate fronto-parietal regions) and ventral striatum (i.e., putamen) (see Figure 4). The findings generally provide support for theoretical models emphasizing the role of cognitive control in substance use behaviors (Feil et al., 2010; Koob and Volkow, 2010). The fronto-parietal network has been linked to response inhibition as well as attention, memory, and decision making (Laird et al., 2011). Therefore, hyperactivation during successful inhibition may reflect a global cognitive impairment with the correct response requiring increased coordination of goal-directed executive functioning and attentional processes. Blunted DLPFC activation during performance errors and more widespread fronto-parietal hypoactivation across inhibition trials could reflect underlying difficulties in adapting behavior appropriately (Feil et al., 2010; Koob and Volkow, 2010). Collectively, greater neural exertion to inhibit behavior and difficulties in modifying behavioral choices may increase susceptibility to engage in substance use.
4.1.4. Resting state.
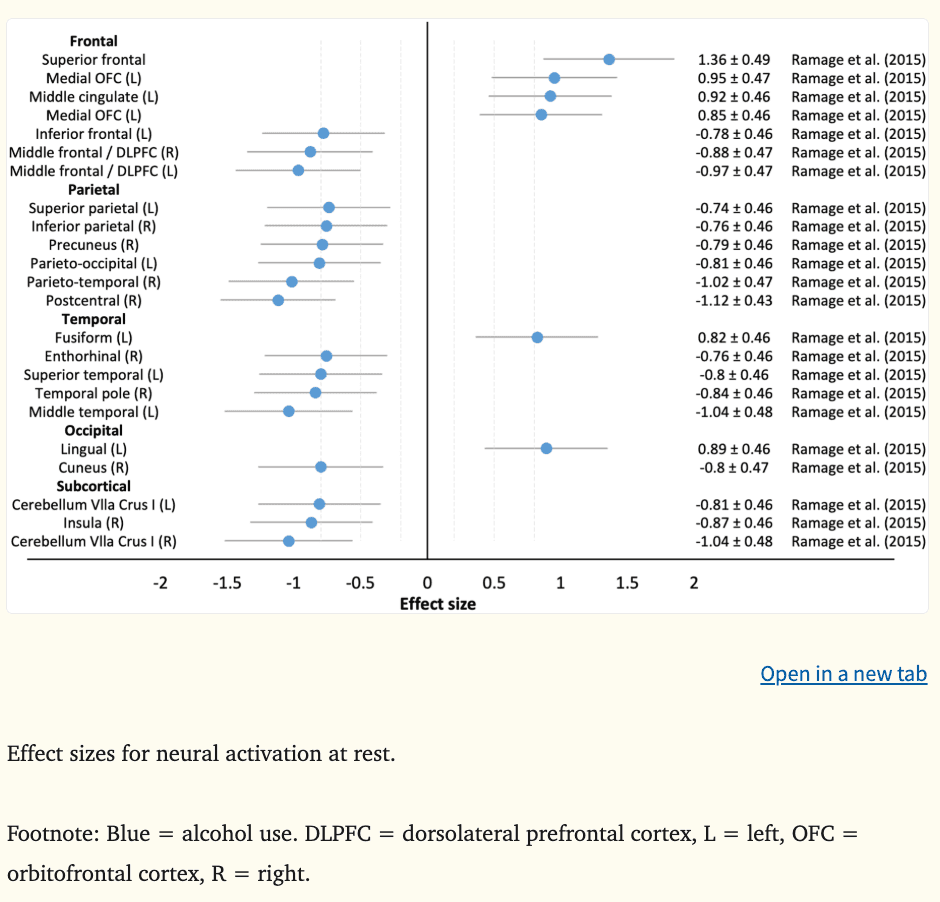
Resting state fMRI is a type of fMRI that is collected in the absence of a task or stimulus and measures synchronous activations between neural regions (Biswal et al., 1995). One of two studies had available resting-state data to calculate effect sizes, where the mean d was −0.39 (k = 23, range = −1.12 to 1.36) (Figure 5). There was preliminary evidence to suggest that aberrant regional blood flow among substance-naïve 12 to 15 year olds in prefrontal regions, including the OFC, DLPFC, and cingulate (|ds=0.85–0.95|), as well as parieto-temporo-occipital (|ds|=0.74–1.12), insula (|d|=0.87), and cerebellar (|ds|=0.81–1.04) regions predicts alcohol initiation and heavier use three years later (Ramage et al., 2015). Lower functional connectivity between the ACC and OFC among youth aged 10 to 21 has predicted increased cannabis use over the following 18 months, which was linked to lower IQ and slower cognitive performance (Camchong et al., 2017). While regions associated with the PFC-striatothalamic dysfunction model and three-stage cycle of addiction framework exhibited altered blood flow, and this was associated with cognitive deficits among youth who went on to initiate and escalate their substance use, greater evidence is required before resting state indices could be considered as vulnerability markers of future substance use and related problems.
Figure 5:
4.2. Macrostructural features
Structural magnetic resonance imaging (MRI) captures static images of the brain and provides macrostructural indices such as brain volume, area, and cortical thickness, as well as microstructural indices, such as white matter integrity and connectivity through diffusion tensor imaging techniques (DTI). A total of 83 effect sizes measuring macrostructural features from 16 structural MRI studies were calculated (see Figure 6 for cortical thickness, area, and gray matter density and Figure 7 for brain volume). Four studies did not report data required to calculate effect sizes (Morales et al., 2019; O’Brien and Hill, 2017; Seo et al., 2019; Whelan et al., 2014). Overall, the mean effect sizes (d) were −0.25 for brain volume (k = 52, range = −1.32 to 0.52), −0.24 for cortical thickness (k = 21, range = −0.87 to 0.30), 0.13 for surface area (k = 4, range = 0.00 to 0.28), and 0.42 for gray matter density (k = 6, range = 0.00 to 0.56). Morphometric indices were associated with prospective alcohol use, amphetamine use, and SUDs, with less evidence for the predictive value of cannabis and tobacco use (only one study examined tobacco use).
Figure 6:
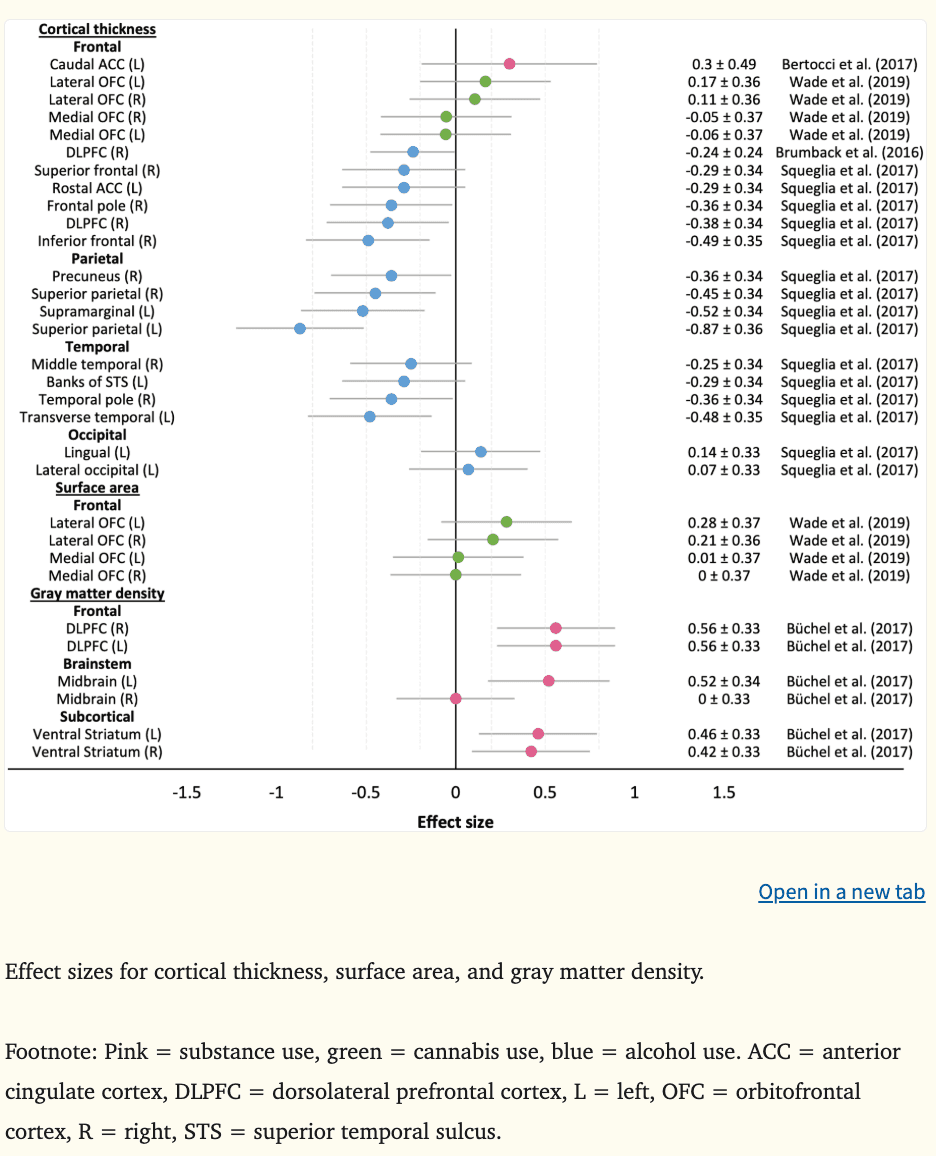
Figure 7:
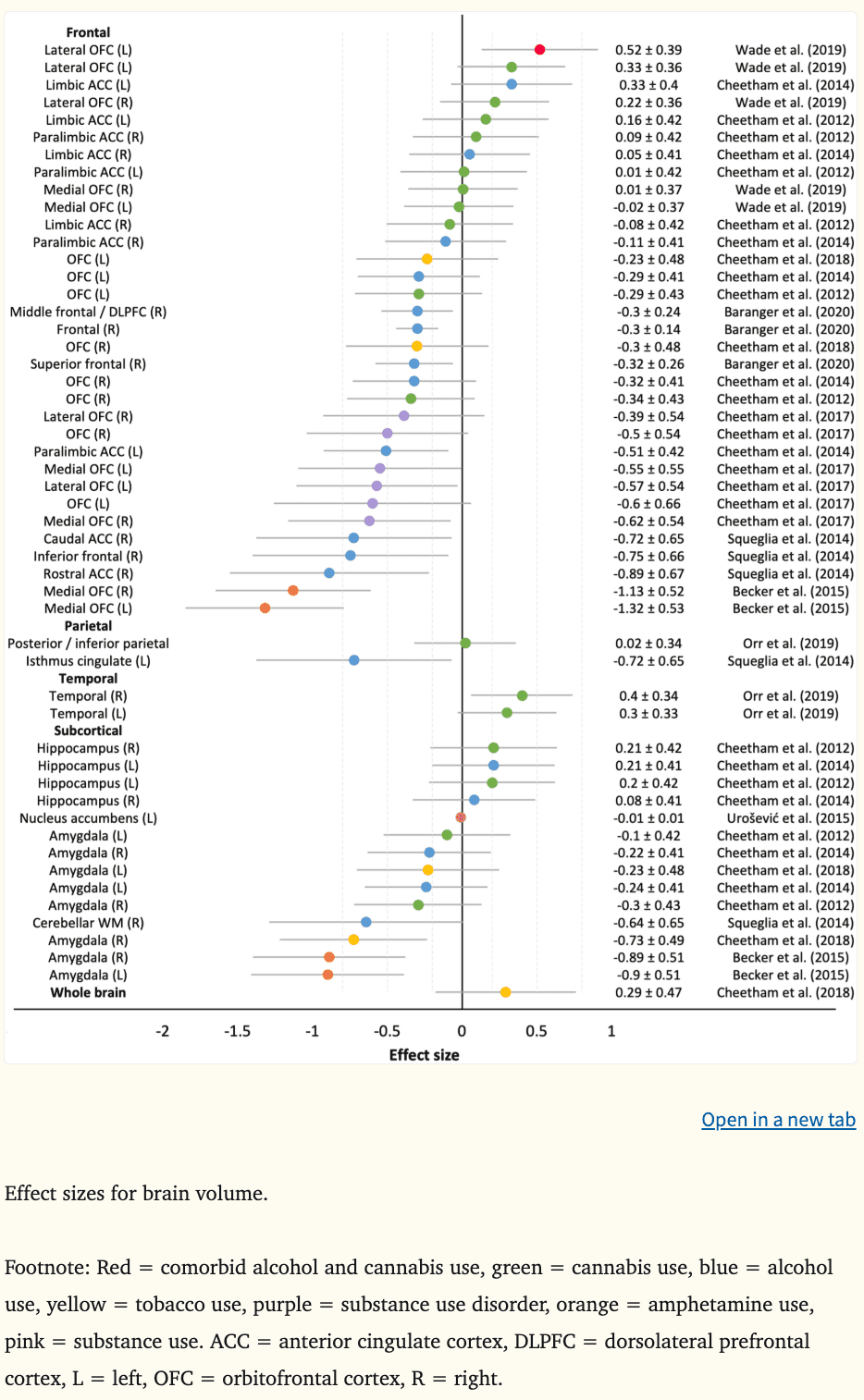
4.2.1. Prefrontal cortex.
Aligned with theoretical frameworks implicating the DPLFC and medial PFC (OFC, ACC) in addictive behaviors (Bjork et al., 2012, 2010; Buckholtz et al., 2010; Feil et al., 2010; Koob and Volkow, 2010), there was evidence that a smaller and thinner right DLPFC (|ds|=0.24–0.38) and smaller bilateral ACC (|ds|=0.51–0.89) predicted earlier alcohol initiation (Squeglia et al., 2017) and binge and heavy drinking (Brumback et al., 2016; Squeglia et al., 2014); however, greater bilateral DLPFC gray matter density at age 14.5 has predicted escalating substance use at age 16 in highly impulsive youth (|ds|=0.56) (Büchel et al., 2017). Total OFC volume has not been a consistent predictor of prospective alcohol use, cannabis use, tobacco use, or SUDs (Cheetham et al., 2018, 2017, 2014, 2012). Although, when OFC volume was considered in relation to amygdala size, adolescents with lower ratios were more likely to meet criteria for SUDs up to 10 years later (O’Brien and Hill, 2017); a finding that aligns with the imbalance hypothesis. Predictive value has been observed when medial and lateral OFC volumes have been examined separately. For example, smaller bilateral medial OFC volume was predictive of adolescent SUDs (|ds|=0.55–0.62) and transitions from occasional to escalating amphetamine use in young adults (|ds|=1.13–1.32), with medium to large effect sizes reported (Becker et al., 2015; Cheetham et al., 2017). Likewise, smaller left lateral OFC volume at age 12 was a medium-sized predictor of earlier onset of adolescent SUDs by age 18 (|d|=0.57) (Cheetham et al., 2017). In contrast, another study reported a medium effect size where larger left lateral OFC volume at ages 12 to 15 was predictive of comorbid alcohol and cannabis use by age 28 (|d|=0.52) (Wade et al., 2019). Inconsistencies in directionality may relate to variation in baseline ages between the studies. On average, participants enrolled in the studies by Wade and Büchel and their colleagues were one to two years older than those enrolled in the other studies. Given that prefrontal volume peaks around ages 10.5–11 years for girls and 11.5–12 years for boys prior to declining (Lenroot et al., 2007; Pfefferbaum et al., 2016), proponents of imbalance models would argue that the disparities reflect a delayed OFC developmental trajectory among youth who go on to use substances and/or meet criteria for SUD, reaching peak volume later (i.e., smaller volume than controls as observed in Brumback et al., 2016, Cheetham et al., 2017, and Squeglia et al., 2017) and declining in volume later than healthy controls (i.e., greater volume and density as observed in Büchel et al., 2017 and Wade et al., 2019).
The PFC-striatothalamic dysfunction model and three-stage cycle of addiction framework posits that aberrant OFC structure is involved in poor control of impulsive behaviors (Feil et al., 2010; Koob and Volkow, 2010). Interestingly, OFC volume was shown to mediate the relationship between effortful cognitive control and SUD (Cheetham et al., 2017) and be moderately correlated with reward responsiveness prior to substance use initiation (Wade et al., 2019), providing support for the role of OFC circuitry in reward-related impulsivity and substance use.
In temporally proximate regions to those described above, there was evidence that a smaller and thinner right inferior frontal gyrus (|ds|=0.49–0.75), smaller right superior frontal gyrus (|d|=0.32), and thinner right frontal poles (|d|=0.36) predicted future alcohol use behaviors three to four years later, with small to large effect sizes reported (Baranger et al., 2020; Squeglia et al., 2017, 2014). Collectively, these findings align well with the neurodevelopmental imbalance models of adolescent risk-related behaviors and the neurobehavioral models of addiction implicating regions involved in cognitive control processes.
4.2.2. Parietal lobe.
Consistent with neurodevelopmental models describing the imbalance hypothesis which links the parietal lobe to impulse control, there was preliminary evidence to indicate that a smaller left isthmus cingulate (|d|=0.72) and thinner bilateral superior parietal lobules (|ds|=0.45–0.87) and left supramarginal gyri (|d|=0.52) predict alcohol initiation and subsequent heavy drinking three to four years later (Squeglia et al., 2017, 2014).
4.2.3. Ventral striatum and subcortical regions.
In older adolescence, a smaller NAcc, which makes up part of the ventral striatum, was related to greater illicit substance use two years later, although the effect size was small (|d|=0.01) (Urošević et al., 2015). In contrast, larger NAcc size across adolescence in females directly predicted alcohol use two years later, while there was an indirect effect for males via youth sensation seeking (i.e., greater NAcc volume was related to higher sensation seeking, which in turn was related to greater prospective alcohol use) (Morales et al., 2019). Greater NAcc volume among substance-using participants aligns with prior cross-sectional work (Gilman et al., 2014; Howell et al., 2013; Thayer et al., 2012). The discrepant finding by Urošević et al. (2015) may be explained by the small sample size (14 participants initiated substance use over two years) where potential confounding factors may not have been adequately addressed. The sex differences observed by Morales and colleagues (2019) may be attributable to pubertal development differences (Crone and Dahl, 2012; Herting et al., 2014), or perhaps the findings indicate that the structural development of other brain regions could be useful for explaining differences in sensation seeking and prospective alcohol use in females. Further exploratory whole brain analyses are required to determine the predictive utility of other structural regions in prospective impulsiveness and future substance use.
Smaller bilateral amygdala volume in young adults has been a large predictor of escalating amphetamine use two years later (|ds|=0.89–0.90) (Becker et al., 2015) and smaller right amygdala volume among 12 year olds has also predicted daily smoking behaviors six years later (|d|=0.73), mediating the association between externalizing symptoms and smoking outcomes (Cheetham et al., 2018). However, amygdala volume at age 12 was not predictive of alcohol-related problems or cannabis use initiation at age 16 (Cheetham et al., 2014, 2012). In adolescence, alcohol-related harms, such as violence and risky sexual activity, are strongly influenced by interpersonal (e.g., parental and peer substance use, violence exposure) and attitudinal factors (e.g., media, religion), independent of frequency and quantity of alcohol consumption (Grigsby et al., 2016; Little et al., 2013), which may explain the null association. Overall, these results may indicate that smaller amygdala volume underpins an impulsive or externalizing temperament and could be one pathway in which susceptibility to escalating substance use, rather than initiation, is conferred.
Thus far, data from samples ranging in size from 40 to 121 adolescents do not indicate that hippocampal volume or cerebellar white matter volume are predictive of heavy drinking or cannabis use behaviours (Cheetham et al., 2014, 2012; Squeglia et al., 2014).
Overall, the results indicate that the structure of the ventral striatum and amygdala are associated with prospective substance use and may underlie impulsive paths towards SUD. The mechanisms underlying the contrasting direction of effects between the NAcc and amygdala among substance-using individuals remains unknown and requires further investigation.
4.3. Microstructural features
Three studies have examined white matter integrity and prospective substance use, reporting on directional preference of diffusion (i.e., fractional anisotropy) and molecular diffusion rate (i.e., mean diffusivity, apparent diffusion coefficient). The average effect size (d) was 0.03 for fractional anisotropy (k = 9, range = −1.12 to 0.72), 0.54 for mean diffusivity (k = 2, range = 0.54 to 0.54), and 0.03 for apparent diffusion coefficient (k = 5, range = −0.17 to 0.19) (Figure 8).
Figure 8:
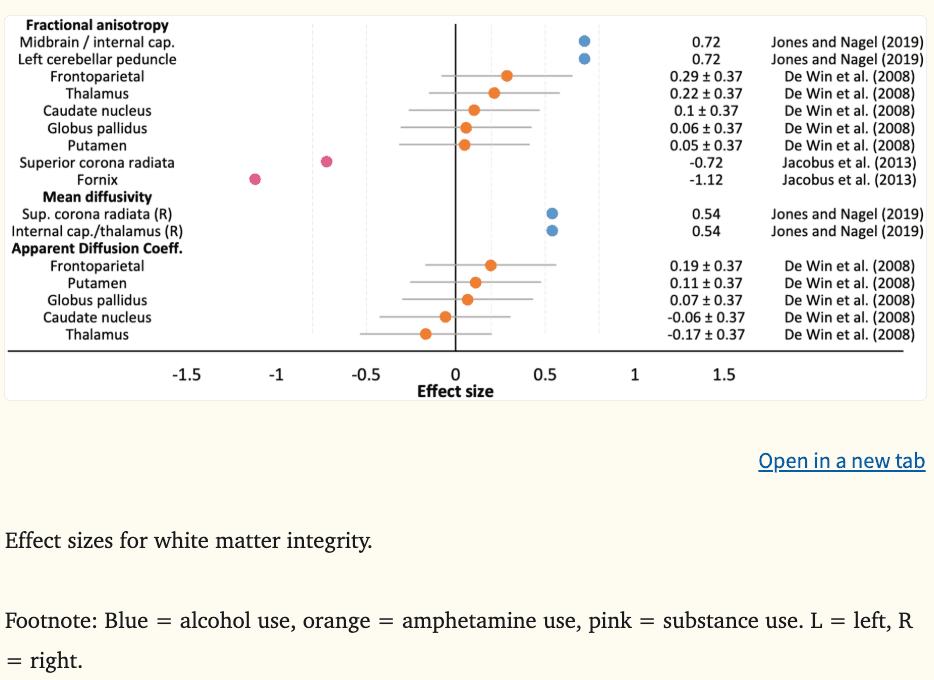
Greater fractional anisotropy and mean diffusivity in reward-related regions of the internal capsule (|ds|=0.54–0.72), midbrain (|d|=0.72), and cerebellum (|d|=0.72) among substance-naïve youth aged 11 to 16 years predicted binge drinking at age 21, with medium to large effects reported (Jones and Nagel, 2019). In contrast, lower fractional anisotropy in cortico-limbic tracts (e.g., superior corona radiata, fornix) among substance-using older adolescents aged 16 to 19 years has predicted substance use behaviors 18 months later, including alcohol, tobacco, cannabis, and illicit drugs (|ds|=0.72–1.12) (Jacobus et al., 2013). Greater white matter integrity along major reward processing tracts may reflect a more mature or dominant reward circuit, representing a greater vulnerability to engage in future substance use, while lower integrity may result from substance use and further escalate risk behaviors, perhaps representing a regression or deviation from usual neurodevelopmental trajectories. Interestingly, when microstructural indices were examined by subcortical segmentation, rather than by tract, no significant differences were observed (De Win et al., 2008). These interpretations are made with caution as further research is needed to understand microstructural features associated with prospective substance use initiation and escalation.
4.4. Cognitive vulnerabilities
Given that there appears to be neural features that predate substance use, it is important to review related cognitive markers that may prove useful alongside psychological and behavioral assessments in clinical, community, or school settings in identifying at-risk youth for targeted prevention efforts aimed at reducing substance-related problems and the prevalence of SUDs.
Effect sizes could be calculated for nine of 13 studies (k = 40). There was evidence to suggest that poor executive functioning performance and greater impulsivity may constitute a common vulnerability to substance misuse (Table 2). The mean effect size (d) was −0.21 for inhibitory control (k = 8, range = −0.56 to 0.22), −0.33 for impulsivity (k = 6, range = −0.47 to 0.20), −0.28 for delay discounting (k = 3, range = −0.38 to −0.17), and −0.23 for working memory (k = 8, range = −0.47 to −0.08), where lower scores indicate poorer performance/greater impulsivity (Figure 9). Inhibitory deficits and high impulsivity in childhood and adolescence have predicted escalating cannabis (|β|=1.19) (Morin et al., 2019) and amphetamine use (|d|=0.40) (Schilt et al., 2009), comorbid substance use (|d|=0.37) (Nigg et al., 2006), and SUD symptoms and problems (|d|=0.37) (Nigg et al., 2006) by ages 18 to 20, in addition to alcohol use disorders in adulthood (|d|=0.56) (Rubio et al., 2008). These results are consistent with a previous cross-sectional meta-analysis which reported deficits in behavioral inhibition among individuals with SUD (Smith et al., 2014). In contrast, inhibition performance does not appear to predict alcohol initiation, nor has performance consistently predicted escalating alcohol use. In some instances performance at ages 11 to 14 has prospectively predicted alcohol use two to five years later (|βs|=0.02–0.59) (Khurana et al., 2013; Morin et al., 2019; Peeters et al., 2015; Seo et al., 2019); however, in other cases, performance at ages 14 and 19 has not been associated with alcohol use one to two years later (Whelan et al., 2014; Worhunsky et al., 2016). These studies did not adjust for psychopathological symptoms, family history of SUD, or IQ, which are known to be associated with cognition, and may be confounding results. Further, it may be the case that the time of measurement (i.e., younger vs. older adolescence) may be a better predictor of future consumption. Consistent with greater impulsivity, steeper discounting of future rewards at ages 11 to 14 was associated with escalating substance use (|ds|=0.28–0.38) (Büchel et al., 2017; Khurana et al., 2017) two to five years later. Aligned with developmental imbalance models and addiction models implicating impulsivity and cognitive control in prospective substance use behaviors, these findings implicate poor cognitive control in substance use behaviors consistent with SUD trajectories, such as comorbid, problematic substance use.
Figure 9:
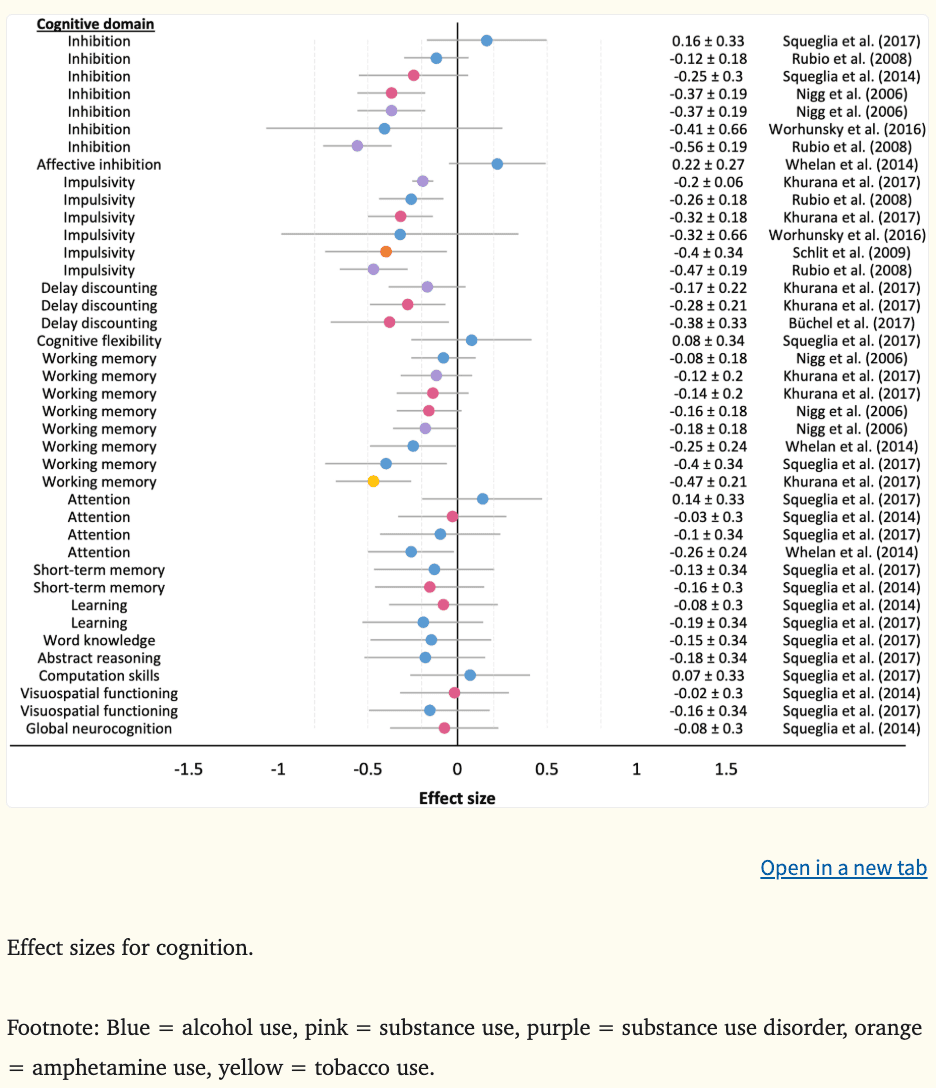
Associations between working memory performance and future substance use are inconsistent. Studies utilizing large samples (Khurana et al., 2017, 2013; Morin et al., 2019; Peeters et al., 2015; Whelan et al., 2014), machine learning techniques (Whelan et al., 2014), and survival analyses (Peeters et al., 2015) have identified that working memory deficits at ages 11 to 14 predict alcohol use initiation (|βs|=0.02–0.68; |d|=0.40) (Khurana et al., 2013; Morin et al., 2019; Peeters et al., 2015), binge and heavy drinking (|β|=0.68) (Peeters et al., 2015; Whelan et al., 2014), cannabis use (|βs|=0.51) (Morin et al., 2019), and tobacco use disorder symptoms (|d|=0.47) (Khurana et al., 2017) by ages 14 to 18. Many of these associations were observed above and beyond other predictors, such as sociodemographic factors, externalizing behaviors, academic achievement, and family history of SUDs. While other studies have reported null results for prospective alcohol and other substance use (Figure 9). Likewise, a recent meta-analysis reported a non-significant association between binge drinking and working memory performance among adolescents and young adults (Lees et al., 2019). Thus, working memory performance may not be a consistent vulnerability marker of future substance use behaviors.
In terms of other cognitive domains which are not theorized to largely influence SUD vulnerability, the mean effect sizes were −0.06 for attention (k = 4, range = −0.26 to −0.03), −0.14 for short-term memory (k = 2, range = −0.16 to −0.13), −0.14 for learning (k = 2, range = −0.19 to −0.08), −0.15 for word knowledge (k = 1), −0.18 for abstract reasoning (k =1), 0.07 for computation skills (k = 1), and −0.09 for visuospatial functioning (k = 2, range = −0.16 to −0.02). Current evidence indicates that these cognitive domains are not associated with prospective substance use behaviors (Figure 8).
Taken together, executive functioning deficits, particularly inhibitory control deficits and high levels of impulsivity, may represent common vulnerabilities to problematic substance use trajectories. These findings are consistent with neurodevelopmental imbalance models, as well as the PFC-striatothalamic dysfunction model and three-stage cycle of addiction framework.
5. Conclusions and future directions
Overall, several neurobehavioral aberrations appear to consistently predate substance use initiation or escalation and are associated with increased vulnerability to problematic substance use and related problems, including SUDs. In response to reward feedback and during risk-based decision making, greater prefrontal (including medial and dorsolateral) and ventral striatal reactivity represent common vulnerabilities to substance misuse. When completing cognitive control tasks, youth exhibiting hypoactivation in medial PFC and parietal regions during working memory, widespread hyperactivation during successful inhibition and hypoactivation across inhibitory trials (particularly in fronto-parietal and ventral striatal regions), and related cognitive deficits are more likely to escalate their substance use. Structurally, smaller fronto-parietal and amygdala volume and larger NAcc volume predicts prospective substance misuse. In some instances, mediating associations between neural indices, behavioral measures (such as impulsivity or externalizing symptoms), and substance use outcomes have been observed. Consistent with theoretical frameworks of addiction, the largest effects have generally been observed in parts of the DLPFC, medial PFC, and ventral striatum. In contrast, the predictive utility of neural responses during reward gain anticipation for prospective substance use behaviors remains limited, with conflicting results observed that do not align well with current reward processing theoretical frameworks (Bjork et al., 2012, 2010; Blum et al., 2000; Buckholtz et al., 2010; Cloninger, 1987).
This review highlights that brain regions implicated in neurodevelopmental models (Casey et al., 2008; Luna et al., 2015; Steinberg, 2010) and cognitive control-based addiction models (Feil et al., 2010; Koob and Volkow, 2010) are relevant vulnerability markers of substance use initiation and escalation. These models postulate that aberrant development of the socioemotional/mesolimbic and cognitive control neural systems can prospectively predict increased risk of early and escalating substance use, and in some cases, SUDs. However, there has been little to no direct investigation of the proposed neurobehavioral mechanisms underlying substance use vulnerability described in each of these models. One study investigated the theorized developmental ‘imbalance’ between prefrontal and limbic regions, providing preliminary support for the predictive utility of fronto-striatal size ratios in SUD diagnoses over a 10-year period (O’Brien and Hill, 2017). Meanwhile, no studies reported in this review directly investigated the three-stage cycle of addiction framework or the circuits described in the PFC-striatothalamic dysfunction model. Further work is required to determine the role of the ventral striatum, amygdala, and OFC/PFC in the binge/intoxication, withdrawal/negative affect, and craving stages of addiction, respectively, and the implications of aberrant DLPFC, OFC, or ACC circuits for prospective substance use behaviors. This review also demonstrates that dominant reward processing theories of addiction (i.e., reward deficiency syndrome theory, impulsivity theory of addiction) cannot fully account for the neural activation patterns and associated psychopathology and personality traits observed among young people who go on to use and misuse substances. Furthermore, no prospective longitudinal studies to date have directly examined the incentive sensitization theory among young people, identifying a significant gap in the existing literature base. Overall, further theoretical conceptualizations of addiction pathways in adolescence as well as direct empirical testing of these models and potential mediating and moderating factors involved in prospective prediction of substance misuse is urgently required.
It should also be noted that the predominance of studies have utilized a selective ‘regions-of-interest’ approach, focusing investigations on cognitive control and reward processing paradigms, and implicated neural structures. The few studies that have examined brain parcellations more broadly, or conducted a whole-brain multivariate analysis, have found that other areas, such as regions within the occipital and temporal lobes, are predictive of alcohol use initiation (Ramage et al., 2015; Squeglia, 2017), binge and heavy drinking (Norman et al., 2011; Whelan et al., 2014), heavy cannabis use (Cousijn et al., 2013), and SUDs (Ramage et al., 2015). Future prospective research which employs whole-brain analysis approaches and utilizes alternative fMRI tasks (including resting state and substance-related cues) are required to provide more reliable vulnerability neural markers of substance misuse among young people. Large-scale neuroimaging studies currently underway (e.g., Adolescent Brain Cognitive Development [ABCD] Study (Luciana et al., 2018; Volkow et al., 2018), National Consortium on Alcohol and Neurodevelopment in Adolescence [NCANDA] (Brown et al., 2015), IMAGEN study (Schumann et al., 2010)) will help address these limitations and could potentially provide more nuanced prospective prediction of neurobehavioral features contributing to substance-related problems.
This field of research, which is dependent on observational human studies, has been limited by difficulties in establishing causality and directionality. This review aimed to explore possible predictive neurobehavioral vulnerability markers of substance use and misuse by summarizing prospective, longitudinal studies that repeatedly assess individuals over time as patterns of substance use emerge and escalate. However, reliably identifying causal mechanisms in observational studies without randomization is challenging, with the principal concern being confounding (i.e., are causal associations real, or entirely or partly confounded by other variables?). As shown in Tables 1–2, studies widely diverge on covariates included in statistical models and it is likely that confounding factors have contributed to the inconsistent findings and conflicting direction of effects observed (Hyatt et al., 2020). Numerous methods, mainly from econometrics, have been developed in recent years in response to the confounding problem in observational data (Marinescu et al., 2018). These methods, seldom used in this field thus far, include complex models that aim to adjust for all confounders (e.g., Granger causal models, structural equation models, Bayesian networks, state-space models) and quasi-experimental causality models (e.g., regression discontinuity design, difference-in-differences approach, instrumental variable approaches, including Mendelian randomization). These techniques have the ability to improve causal understanding and should be utilized in future analyses of large-scale cohorts to delineate causal neurobehavioral markers of substance misuse and to ultimately inform prevention efforts aimed at reducing rates of SUDs and related harms.
Furthermore, the previous decade has seen increasing attention to the reproducibility crisis, with concerns raised from failed replication studies and null meta-analytic results. Within neuroimaging, there has been a particular focus on issues of analytic variability, poor measurement reliability (particularly when using task fMRI), statistical power, and test-retest reliability (Elliott et al., 2020; Poldrack et al., 2019). Moving forward, researchers investigating predictors of substance use should consider conducting replication studies (for example, using cross-validation approaches), utilizing larger samples to accurately detect small effect sizes, applying machine learning regression methods to predict substance use outcomes, and appropriately accounting for variance in neurodevelopmental stage (Jollans et al., 2019; Poldrack et al., 2019).
Altered neurodevelopment of self-regulatory processes related to problematic substance use could stem from a multitude of factors including sociodemographic characteristics, early-onset puberty, genetic polymorphisms, prenatal exposures, childhood adversity, and psychopathology, among other important factors (Jordan and Andersen, 2017; Lees et al., 2020a). Greater exploration of possible interactive effects and underlying mechanisms of neurobehavioral markers are necessary to improve identification of youth who may be at risk of future problematic substance use. Likewise, observed neurodevelopmental trajectories associated with prospective substance use are also likely to be related to other psychological and behavioral outcomes. There is a growing body of literature investigating broad dimensional spectra of psychopathology, including internalizing, externalizing (e.g., substance use), and thought disorder symptoms, which provides evidence of some common and unique neurobiological features (Goodkind et al., 2015; Lees et al., 2020b). For example, a study of 1,778 adolescents reported that prefrontal hyperactivation and ventral striatal hypoactivation during successful inhibition was a common feature across all externalizing psychopathology (i.e., attention deficit hyperactivity disorder, conduct disorder, substance misuse), while OFC hyperactivation and inferior frontal hypoactivation during reward anticipation were specific to substance misuse (Castellanos-Ryan et al., 2014). Further longitudinal prospective examination of common and unique markers of various forms of psychopathology and substance misuse will provide greater nuance and specificity for identifying individuals who are at greater risk of transitioning into SUD and preventing related problems.
Understanding the relative prediction power and diagnostic ability of neural features for future substance use behaviors as compared with other personal, interpersonal, and environmental factors is important when considering the clinical and practical utility of incorporating neuroimaging measures into clinical assessments. For example, the fMRI reward processing study by Bertocci and colleagues (2017), reported that neural features explained 14% of future substance use variance while clinical and sociodemographic variables explained 46% of future use. In a separate reward processing study by Büchel and colleagues (2017), they reported that the model with the greatest predictive power of problematic substance use combined neural and personality measures (explained 20% of variance), when compared to neural-only (explained 15%) or personality-only models (explained 7%). Currently, model fit comparisons and Receiver Operator Characteristic curves of various predictor variables are seldom reported in this field. Future longitudinal analyses should report fit comparisons and Receiver Operator Characteristic curves to enable researchers and practitioners to determine whether the incorporation of neuroimaging assessments would add significant value in identifying individuals at risk of SUD.
Overwhelmingly, the predominance of studies thus far have examined vulnerabilities related to substance use initiation and normative levels of use (i.e., state-based neurocognitive markers). More research is required to determine predictive neural markers for SUD risk (i.e., genetic, enduring trait-based endophenotype markers). Future studies also need to make concerted efforts to enroll more individuals with diverse backgrounds, as vulnerabilities to substance-related problems may not generalize across ethnicities and cultures (most research to date has been in Caucasian youth from upper middle class families (Wendt et al., 2019), both sexes, various family structures, or psychopathology profiles. This knowledge will benefit practitioners working with children, adolescents, and young adults and hopefully will inform future substance use prevention efforts aimed at reducing the prevalence of SUDs.
Targeting shared vulnerabilities that increase risk of early and escalating substance use by strengthening executive functioning could be helpful in avoiding the onset of SUDs. Cognitive training treatment strategies have demonstrated success in reducing alcohol use (Bowley et al., 2013) and SUDs (Keshavan et al., 2014). However, research into the effectiveness of online cognitive training as a prevention tool to delay or avoid early and escalating adolescent substance use has found null effects (Mewton et al., 2020). Future research should explore whether cognitive training supplemented with substance use prevention materials and emotion regulation techniques can reduce the prevalence of problematic substance use. Additionally, mindfulness-based activities, such as meditation or yoga, show promise in improving inhibitory control, attention, and emotion regulation, as well as increasing brain activity and cortical thickness in prefrontal regions (Jordan and Andersen, 2017). Preliminary evidence demonstrates these activities have some success in treating SUDs (Bowen et al., 2009; Witkiewitz et al., 2005), however, more research is needed to examine the utility of these activities as effective prevention measures.
Furthermore, school-based psychoeducational programs targeting cognitive control and risk-taking behaviors have been found to reduce rates of alcohol and other substance use, and substance-related harms by approximately 50% in adolescents, with sustained effects for up to three years (Edalati and Conrod, 2019). Likewise, pilot studies of neuroscience-informed psychoeducational programs targeting high-risk adolescents have also shown promise in reducing substance-related harms (Debenham et al., 2020; Meredith et al., 2020), with randomized controlled trials currently underway.
Taken together, the findings of this review suggest that neurobehavioral data can be useful in predicting future substance use behaviors. Particular attention should be given to youth who show altered neurodevelopment in prefrontal and ventral striatal regions, exhibit executive functioning deficits, and are highly impulsive. The review will contribute to the refinement of developmental and addiction neurobehavioral models in explaining the relationship between neural features and prospective substance use behaviors. Analysis of large-scale data (e.g., ABCD, NCANDA, and IMAGEN studies) will provide important opportunities for replication to examine the robustness of identified vulnerability markers, as well as provide critical new insights to help disentangle the complicated picture of substance use uptake and progression to SUDs.