Abstract
"The mesofrontal dopaminergic circuit, which connects the midbrain motivation center to the cortical executive center, is engaged in control of motivated behaviors. In addition, deficiencies in this circuit are associated with adolescent-onset psychiatric disorders in humans. Developmental studies suggest that the mesofrontal circuit exhibits a protracted maturation through adolescence. However, whether the structure and function of this circuit are modifiable by activity in dopaminergic neurons during adolescence remains unknown. Using optogenetic stimulation and in vivo two-photon imaging in adolescent mice, we found that phasic, but not tonic, dopamine neuron activity induces the formation of mesofrontal axonal boutons. In contrast, in adult mice, the effect of phasic activity diminishes. Furthermore, our results showed that dopaminergic and glutamatergic transmission regulate this axonal plasticity in adolescence and inhibition of dopamine D2-type receptors restores this plasticity in adulthood. Finally, we found that phasic activation of dopamine neurons also induces greater changes in mesofrontal circuit activity and psychomotor response in adolescent mice than in adult mice. Together, our findings demonstrate that the structure and function of the mesofrontal circuit are modifiable by phasic activity in dopaminergic neurons during adolescence and suggest that the greater plasticity in adolescence may facilitate activity-dependent strengthening of dopaminergic input and improvement in behavioral control."
Introduction
The mesofrontal dopaminergic circuit, which comprises dopaminergic projection neurons in the ventral midbrain and target neurons in the frontal cortex (FC) (Björklund and Dunnett, 2007), is engaged in control of motivated behaviors (Tzschentke, 2001; Grace et al., 2007). In addition, deficiencies in this circuit are associated with adolescent-onset psychiatric disorders in humans (Chambers et al., 2003; Winterer and Weinberger, 2004; Casey et al., 2010). Developmental studies in nonhuman primates and rodents suggest that the mesofrontal circuit exhibits a protracted maturation through adolescence (Kalsbeek et al., 1988; Rosenberg and Lewis, 1995). However, little is known about whether dopamine neuron activity may elicit lasting effects on the structure and function of this circuit in adolescence.
Dopaminergic neurons fire action potentials in tonic or phasic modes (Grace et al., 2007). When animals are in a resting state, these neurons show tonic single-spike firing at frequencies lower than 10 Hz (Grace et al., 2007; Schultz, 2007). Phasic firing activities, which are bursts of spikes at frequencies >10 Hz, are correlated with rewarding, motivationally salient, or alerting signals (Schultz, 2007; Bromberg-Martin et al., 2010).
After the firing of dopamine neurons, dopamine is released from mesofrontal boutons, which are enlarged axonal structures containing a high level of the dopamine-synthesizing enzyme tyrosine hydroxylase (TH; Berger et al., 1991; Rosenberg and Lewis, 1995). Whereas the differentiation of frontal cortical layers and the adult-like topographic distribution of dopaminergic axons are established by the time of weaning (3 weeks) in rodents, dopaminergic boutons continue to develop through adolescence (Kalsbeek et al., 1988).
Mesofrontal dopaminergic input plays an important role in regulating the activity of frontal cortical neurons and psychomotor behaviors. Electrophysiologically, phasic stimulation of dopamine neurons in midbrain ventral tegmental area (VTA) evokes sustained neural activity in the adult FC (Lewis and O'Donnell, 2000; Lavin et al., 2005). Behaviorally, exposure to novel environments or psychostimulants such as amphetamine increases dopamine release and locomotor activity in rodents. Whereas activation of nucleus accumbens (NA) and its associated dopaminergic input facilitates this psychomotor response, activation of the mesofrontal circuit exerts an inhibitory effect (Tzschentke, 2001; Chambers et al., 2003).
To investigate whether dopamine neuron activity may modify the structure and function of the mesofrontal circuit, we used optogenetic methods to control the activity patterns of dopamine neurons. Our findings showed that, in adolescent mice, phasic dopamine neuron activity elicits lasting structural and functional changes in the mesofrontal circuit. Those changes include more boutons on dopaminergic axons in the FC, potentiated mesofrontal circuit activity, and suppressed psychomotor response, as revealed by in vivo two-photon imaging, electrophysiology, gene expression, and behavioral analysis. In contrast, in adult mice, even though VTA dopamine neurons are activated by optogenetic stimulation comparably to adolescent mice, the impacts of phasic activity on the mesofrontal circuit and psychomotor behavior are greatly diminished. These results reveal greater plasticity of the mesofrontal dopaminergic circuit in adolescence, which may facilitate activity-dependent strengthening of dopaminergic input and improvement in behavioral control.
Materials and Methods
Experimental Animals
Heterozygous TH-Cre (Gong et al., 2007) and Arc-GFP (Wang et al., 2006) mice in the C57BL/6 strain were used in this study. Mice were normally housed in groups (∼2–4 mice per cage) and maintained under a standard 12 h light/dark cycle. Adolescent [∼4–5 weeks old, postnatal days 28–42 (P28–P42)] and adult (∼2–4 months old) mice were used for experiments. Experimental protocols were approved by the National Institute of Mental Health Animal Care and Use Committee and were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Th Immunostaining in VTA and FC
Mice were perfused with 0.9% saline, followed by 4% paraformaldehyde (PFA) in PBS, and postfixed overnight in 4% PFA at 4°C. Then, 70-μm-thick coronal sections were incubated with blocking solution containing 10% normal goat serum (NGS), 0.3% Triton X-100 in PBS, pH 7.4, at room temperature for 1 h, followed by incubation in rabbit anti-TH antibody (1:10,000, catalog #AB152, RRID:AB_390204; Millipore; Di Salvio et al., 2010) with 5% NGS in PBS overnight at 4°C. Sections were then washed 3× (10 min each) with PBS, followed by incubation in Alexa Fluor 568-conjugated goat anti-rabbit IgG (1:100; Invitrogen) with 5% NGS in PBS for 2 h at room temperature. Sections were washed again and mounted for imaging with a confocal laser-scanning microscope (FV1000; Olympus).
Labeling of VTA Neurons with Tetramethylrhodamine Dextran
Tetramethylrhodamine (TRITC)-dextran (3000 MW, 10% in PBS) was loaded into a glass micropipette (tip size ∼10–20 μm), and iontophoretically injected (10 μA positive current, 200 ms pulse, 2 Hz, for 10 min) into the right VTA (4 injection sites, from bregma: AP −3.1, −3.3; ML 0.5; and DV 4.4, 4.5 mm). Mice were housed for 2–3 d to allow for recovery and anterograde labeling of the axons. For each animal, the VTA was sectioned and immunostained with TH antibody to confirm the location of the injection site. The frontal cortical region imaged by two-photon microscopy in vivo was marked with fluorescent beads (Lumafluor) and immunostained with TH antibody. Animals in which there were no TH-colabeled axons in the imaged frontal cortical region were not analyzed further.
Optogenetic Labeling of VTA Dopamine Neurons
The pAAV-EF1a double-floxed hChR2(H134R)-EYFP-WPRE-HGHpA plasmid was kindly provided by K. Deisseroth at Stanford University. Serotype 1 recombinant adeno-associated viruses (AAVs) were made by the University of North Carolina (Chapel Hill, NC) Vector Core at a concentration of 1.5 × 1012/ml. AAV1.CAG.FLEX.EGFP.WPRE.bGH viral vector was purchased through Addgene and University of Pennsylvania (Philadelphia, PA) Vector Core based on a plasmid established by the Allen Brain Institute. AAV (1 μl) was injected through a glass micropipette, which was connected to a 10 μl Hamilton syringe and a syringe pump, into a single site (from bregma: AP −3.2, ML 0.5, and DV 4.5 mm, for both preadolescent (∼P21) and adult mice). In addition, cannula guides were implanted over the same stereotaxic coordinates and secured with dental cement for optical fiber insertion. Animals were allowed to recover for ∼10–14 d for both age groups. For each animal, the VTA was sectioned after experiments to confirm the location of the cannula and the normal histology of labeled VTA cells. The extent of fluorescently labeled cells was typically ∼0.5 mm from the injection center in the anterior–posterior and medial–lateral directions and covered most of the VTA region. If the cannula tip went too deep and damaged labeled cells in the VTA or the tip was too far outside the VTA (> 1 mm above VTA or >1 mm lateral from the midline), those animals were not analyzed further.
Light Activation of VTA Dopamine Neurons
An optical fiber (200 μm in diameter; Thor Laboratories) was connected to a 473 nm solid-state laser diode (CrystaLaser) with ∼20 mW output from the fiber. To record light-evoked responses in VTA, the optical fiber was glued ∼0.5 mm above the tip of a tungsten microelectrode (∼0.5 MΩ). This optoelectrode was then lowered into the VTA (from bregma: AP −3.2, ML 0.5, DV 4.5 mm) of ChR2-EYFP AAV-injected TH-Cre mice under Avertin anesthesia. Tonic (1 Hz pulse train, 3 ms/pulse, 10 pulses/train, 1 train/min for 10 min) or phasic (50 Hz pulse train, 3 ms/pulse, 10 pulses/train, 1 train/min for 10 min) light pulses were delivered to those mice. Local field potential responses to optical stimulation were recorded with an amplifier (Molecular Devices). The signals were band-pass filtered between 0.1 and 100 Hz.
To determine c-Fos expression in the VTA after phasic optical stimulation, mice were perfused 1 h after stimulation and c-Fos immunostaining was performed. Sections were incubated in 50% ethanol in PBS for 30 min, 3% hydrogen peroxide for 10 min, blocking solution for 1 h at room temperature, and rabbit anti-c-Fos antibody (1:5000, catalog #PC38-100UL, RRID: AB_213663; Millipore; Deng et al., 2010) with 5% NGS in PBS overnight at 4°C. Sections were then incubated in biotinylated goat-anti rabbit secondary antibody for 2 h (1:1000; Jackson ImmunoResearch), streptavidin-HRP for 30 min (1:2500, TSA cyanine kit; PerkinElmer), and TSA plus Cy3 amplification reagent (PerkinElmer).
Two consecutive coronal sections (each 80 μm in thickness) containing the VTA region directly below the tip of optical fiber (from bregma: AP −3.2, ML 0.5, and DV 4.5 mm) in each animal were imaged using a 10× lens. Regions of interest covering the VTA (0.89 mm2 per section) were drawn in the imaged sections according to the stereotaxic atlas (Paxinos and Franklin, 2004) with the investigator blinded with regard to experimental conditions. c-Fos+ cell nuclei within these regions of interest were detected automatically by thresholding the images at 3 SDs above background, then selecting particles of 49–200 μm2 in size using ImageJ. To determine the percentage of ChR2-EYFP+ cells that are c-Fos+, a 20× [numerical aperture (NA) 0.95] lens at 2× zoom was used to take 4 adjacent image stacks (318 × 318 ×12 μm3) in each of these two brain sections. ChR2-YFP and c-Fos colabeling analysis was done manually by a human observer blinded to the experimental conditions. ChR2-EYFP labeling was on cell membrane and positive cells were identified in the green channel by searching through each slice of the 3D image stack. Each identified ChR2-YFP+ cell was then examined for c-Fos nuclei expression within adjacent slices in the red channel of the 3D image stack.
In Vivo Imaging of Mesofrontal Axons
Adolescent (∼4–5 weeks old) or adult (∼2–4 months old) male mice were anesthetized using Avertin (0.5 mg/g body weight) and placed on a stereotaxic apparatus. Cranial window surgery was performed similarly to a published protocol (Cao et al., 2013). A high-speed dental drill was used to thin the skull above the FC (from bregma: AP 1.0–3.0 mm, ML 0.3–1.3 mm, covering the M2/FrA region; Paxinos and Franklin, 2004). The thinned skull cap was removed with forceps to create a small (∼2 × 1 mm) cranial window. The cranial window was filled with 2% agarose and ACSF, covered with a glass coverslip, and sealed with dental cement. A two-photon microscope (FV1000; Olympus) was used to image the brain under the cranial window (excitation laser: 870 nm for TRITC-dextran, ∼15–75 mW; 920 nm for ChR2-EYFP and EGFP, ∼10–25 mW). A low-magnification image of the overall area containing labeled axons was first taken with a 20× water-immersion lens (NA 0.95). Multiple image stacks (∼30–50 slices, 159 × 159 μm2 per slice, 1 μm z-step) were then taken at 4× zoom at the center of this region (50–300 μm below pial surface) to follow all of the labeled axons continuously and in greater detail. The low-magnification image was used for realignment during the second imaging session and the high-magnification image stacks were taken again for comparison of axon morphology. For illustration purposes, 2D maximum-intensity projections of the 3D image stacks containing axonal segments of interest were used for all figures.
Axonal Dynamics Under Running Wheel and Home Cage Conditions
After the first two-photon imaging session, animals were allowed to recover from anesthesia. They were then placed in the home cage or exposed to a running wheel (Med Associates) for 2 h, anesthetized with isoflurane (1.5%), and imaged for the second time. To confirm the engagement of the FC in the running wheel task, heterozygous Arc-GFP mice were used in these experiments. A similar increase in mesofrontal bouton formation was also confirmed in wild-type adolescent mice (home cage: 2.6 ± 1.8%, running wheel: 14.9 ± 4.5%; p < 0.05, n = 4 mice for each group).
Axonal Dynamics Under Optogentic Stimulation and Conditions
An optical fiber was inserted into an internal cannula with ∼0.5 mm protrusion and glued into place. The internal cannula containing the optical fiber was then inserted into the implanted cannula guide in ChR2-EYFP AAV- or EGFP AAV-injected TH-Cre mice. After the first two-photon imaging session, the animals were either not stimulated, stimulated with a tonic pattern (1 Hz pulse train, 3 ms/pulse, 10 pulses/train, 1 train/min for 10 min), or stimulated with a phasic pattern (50 Hz pulse train, 3 ms/pulse, 10 pulses/train, 1 train/min for 10 min). These mice remained under constant isoflurane (∼1.5%) anesthesia for 2 h before being imaged for the second time.
To evaluate the effects of neurotransmission on axonal plasticity, D2 receptor agonist quinpirole (1 mg/kg) or antagonist eticlopride (0.5 mg/kg; Zhang et al., 2009) was administered intraperitoneally ∼10 min before phasic stimulation. The NMDA receptor antagonist CPP (3 mg/kg; Riekkinen et al., 1996) and the AMPA receptor antagonist CNQX (10 mg/kg; Bekar et al., 2008) were mixed together and administered intraperitoneally ∼20 min before phasic stimulation.
Analysis of in Vivo Two-Photon Images
To determine bouton dynamics in time-lapse in vivo two-photon images, axons and boutons at each time point were first labeled automatically using scripts written in Matlab (Mathworks) and NIH ImageJ, blind with regard to experimental conditions. Axons were detected using line filters based on the Hessian Matrix (Sato et al., 1998; FeatureJ plugin in ImageJ), and thresholded at 2 SDs above background. Boutons were detected as bright swellings along axons that are larger than 0.5 μm2 in size and have intensity values at least 2.5-fold brighter than the flanking axon backbone (Nishiyama et al., 2007; Holtmaat et al., 2009). The file names of each labeled images in a time series were randomly assigned, and a human observer blind to the imaging conditions aligned and verified the presence or absence of boutons in each image. The percentage of newly formed boutons is calculated as the number of boutons present at time point 2 but not at time point 1, divided by the total number of boutons present at time point 1. The percentage of eliminated boutons is calculated as the number of boutons present at time point 1 but not at time point 2, divided by the total number of boutons present at time point 1.
Electrophysiological Recording in the FC
A cranial window above the FC (from bregma: AP 1.0–3.0 mm, ML 0.3–1.3 mm, covering the M2/FrA region; Paxinos and Franklin, 2004) was opened in ChR2-EYFP AAV injected TH-Cre mice of either sex under isoflurane (∼1.5%) anesthesia. To record local field potentials in response to phasic stimulation of the VTA (Lewis and O'Donnell, 2000; Lavin et al., 2005), a glass micropipette (∼2 MOhm) was inserted into the FC (∼150–300 μm from the pial surface in layer II/III). The recorded signals were bandpass filtered between 0.1 and 100 Hz. The first session of VTA stimulation consisted of 10 trains of phasic light pulses (50 Hz pulse train, 15 ms/pulse, 10 pulses/train, 1 train/min for 10 min). Afterward, the animal was kept under constant isoflurane (∼1.5%) anesthesia for 2 h, and then received identical stimuli in the second session.
In each stimulation session, the LFP signals in 2 s windows before and after the start of each light train were averaged across 10 sweeps of recordings. In the poststimulation time window, the absolute value of LFP shifts below the baseline was summed up to indicate the total amount of LFP response. In addition, the peak amplitude and the duration (the period when LFP signals were lower than the baseline by >1.6× SDs) of negative LFP shifts were also examined.
To examine the higher frequency components in the LFP signals, we used Matlab (Mathworks) signal processing toolbox to calculate the spectrogram of the LFP signals in a period from 2 s before VTA stimulation to 3 s after. Based on the short-time Fourier-transform algorithm, spectral analyses were conducted for each 0.5 s sliding window (0.1 s step) in individual sweeps of LFP recordings. To summarize the long-lasting changes in high-frequency oscillations, the power spectral density calculated at 1 s post VTA stimulation was averaged over a high-frequency band (4–55 Hz, θ to γ oscillation range) for each subject and condition.
Home Cage Behavior and Psychomotor Responses
Male mice were randomly assigned to receive either ChR2-EYFP AAV or EGFP AAV injection in the midbrain. Mouse ID encoded conditions of viral injection. The same behavioral treatments were applied to all mice. Adolescent (∼4–5 weeks) and adult (∼2–4 months) mice expressing either virus were stimulated first with phasic light pulses (50 Hz pulse train, 3 ms/pulse, 10 pulses/train, 1 train/min for 10 min) under isoflurane (∼1.5%) anesthesia. The same stimulation was applied again ∼2 h later. Those mice were then returned to their home cage. This procedure was performed for three consecutive days. After the last stimulation on the third day, the animals were transferred to a new standard facility cage with food and water available ad libitum. Their behavior in this home cage was monitored using the HomeCageScan behavior monitoring system (Clever Systems) for 16 h. Infrared lights were used for illumination during the dark cycle. Automated video analysis of home cage behaviors was performed using HomeCageScan software blind to the experimental conditions. The amount of time the animal spent in all detected active behaviors (Rear up, Walk, Eat, Drink, Hang, Turn, Stretch, Jump, Dig, Forage, Groom, Sniff) was summed. During the light cycle one day after the last optogenetic stimulation session, the animals were placed in an open field arena (50 cm × 50 cm × 10 cm) for 10 min, then received d-amphetamine (2 mg/kg, i.p.) injection, and monitored by video camera for another 60 min in the arena. The animal's body position was automatically tracked and analyzed using the Limelight video tracking system (Actimetrics-Coulbourn Instruments) with the investigator blinded to the experimental conditions. The total distance traveled in 5 min bins of time was calculated from the tracked body positions. The percentage of time the animal spent in the center (30 × 30 cm) of the arena was determined from the position data. Rearing behavior was analyzed in the video by a human observer blinded to the experimental conditions. Starting at the 10th minute after amphetamine injection, the first minute of every 10 min of video was inspected visually. The number of rears that mice displayed during this time window was counted and these counts were summed for each animal.
F-Fos Expression in the FC and NA After Amphetamine Treatment
After the 60 min amphetamine test, the animals were perfused and c-Fos immunostaining in the FC and NA was performed. Two coronal sections (1.6 and 1.2 mm anterior from bregma) were imaged using a 10× lens. Regions of interest covering the FC and NA were drawn in the images according to the stereotaxic atlas (Paxinos and Franklin, 2004) with the investigator blinded with regard to experimental conditions. The total areas of the regions of interest are as follows: FC (M2, Cg, PrL, IL) = 5.05 mm2, NA (core and shell) = 3.80 mm2. Cell nuclei within these regions of interest were detected automatically by thresholding the images at 3 SDs above background and then selecting particles 49–200 μm2 in size using ImageJ.
Statistical Analysis
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported previously (Flores et al., 2005; Lavin et al., 2005; De Paola et al., 2006; Nishiyama et al., 2007). All data are expressed as the mean ± SE. Statistical differences between two groups were determined with two-sided Student's t test or Mann–Whitney test. Statistical differences among three groups or more were determined using one-way ANOVA, two-way ANOVA, or repeated-measures (RM) ANOVA, followed by Bonferroni's multiple-comparisons test. We found no major deviation from the assumptions of the ANOVA. For the cases in which normality or equal variance was questionable, the results of the ANOVA were confirmed by nonparametric tests (Kruskal–Wallis followed by post hoc Dunn's test).
Results
Wheel running enhances the formation of mesofrontal boutons in adolescent mice
Seeking to investigate activity-dependent modification of the mesofrontal circuit, we first conducted an exploratory experiment to examine potential changes in the mesofrontal axons in response to wheel-running behavior, which increases phasic dopamine neuron activity in the VTA (Wang and Tsien, 2011). We used in vivo two-photon microscopy to examine mesofrontal axons because this method can determine structural changes in the same axons before and after experimental manipulation. With this method, changes in axonal boutons are typically measured in proportion to the number of boutons sampled before experimental manipulation; bouton formation or elimination rates ranging from a few percentages at baseline level to tens of percentages hours after activity perturbation have been reported on cortical glutamatergic or GABAergic axons (Holtmaat et al., 2009; Fu and Zuo, 2011). However, the dynamics of mesofrontal axonal boutons have yet to be examined.
To label mesofrontal axons for imaging, we injected a fluorescent tracer, dextran-coupled TRITC, into the VTA (Fig. 1A–D). TH immunostaining of the FC (M2 region, layers I-III) revealed that 63 ± 4% of TRITC-labeled axons were TH-positive, and the rest might have been nondopaminergic axons originating from the VTA (Fields et al., 2007). We examined in vivo changes of TRITC-labeled boutons in response to 2 h of wheel running or home cage activities in adolescent (∼4–5 weeks) and adult (∼2–4 months) male mice. The proportion of newly formed boutons increased after wheel running in adolescence (from 1.7 ± 0.6% to 13.4 ± 1.9%) and this effect diminished in adulthood (from 1.9 ± 1.0% to 4.8 ± 1.7%; two-way ANOVA age-by-condition interaction, F(1,19) = 9.89, p < 0.005). By contrast, there was no significant difference in the elimination of boutons (two-way ANOVA age-by-condition interaction, F(1,19) = 0.04, p = 0.842; Fig. 1E,F, Table 1). Although wheel running is a complex behavioral treatment that does not specifically target dopamine neurons, these observations raise the possibility that, in adolescent mice, increasing phasic dopamine neuron activity may enhance the formation of mesofrontal boutons and that, in adult mice, this activity perturbation may be less effective.
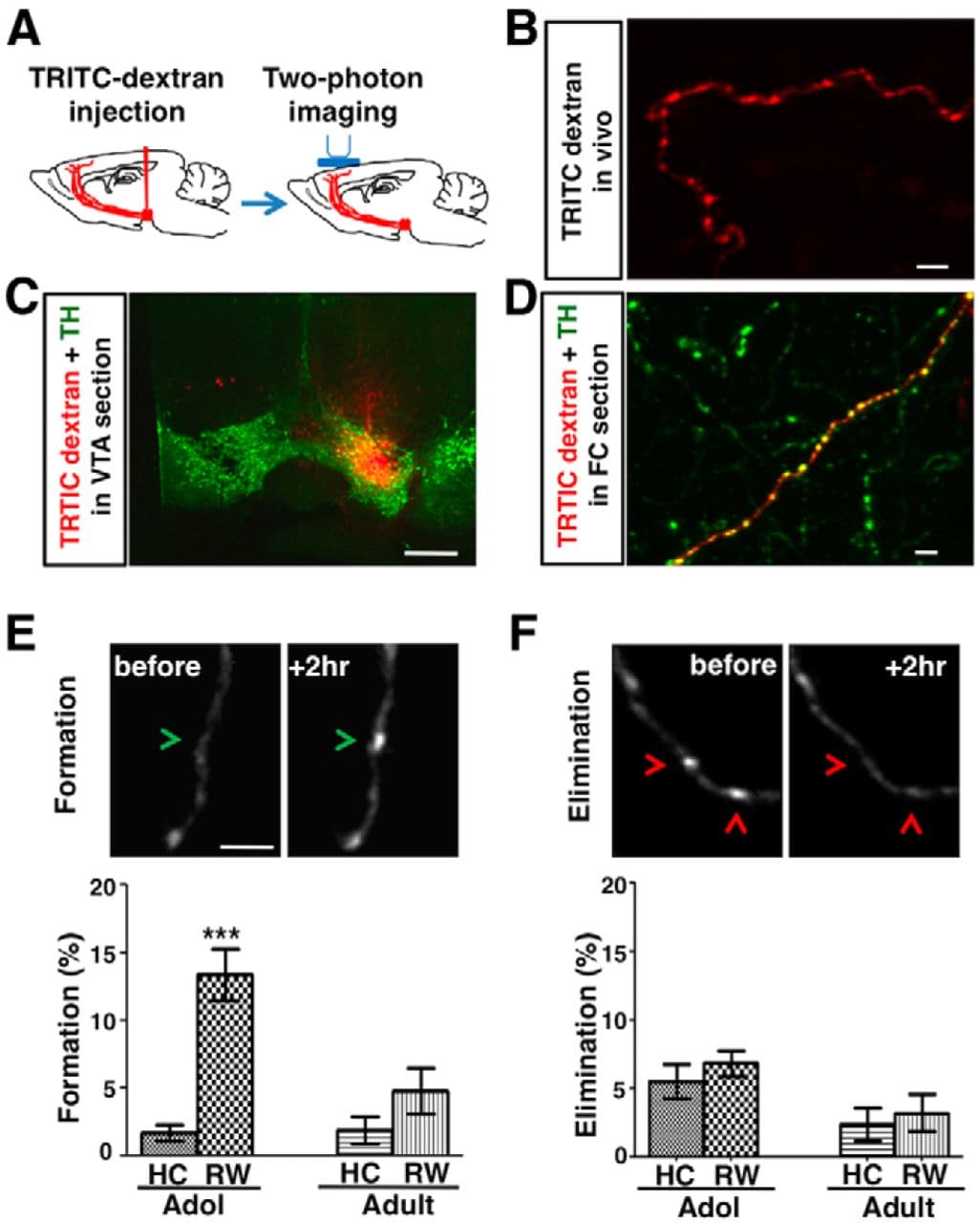
Figure 1. Wheel running enhances the formation of mesofrontal boutons in adolescent mice. A, Diagram showing experimental conditions. B, In vivo two-photon image of TRITC-dextran (red)-labeled axon in the frontal cortex (M2 region, layers I-III). C, Confocal image of coronal midbrain section showing the overlap of injected TRITC-dextran (red) with the VTA dopamine neurons labeled by TH immunofluorescence (green). Scale bar, 500 μm. D, Confocal image of TRITC-dextran (red) labeled axon in a frontal cortical section immunostained for TH (green). E, F, Top, In vivo two-photon images showing examples of bouton formation (E, green arrowheads) and elimination (F, red arrowheads). Bottom, Percentage of boutons formed (E) and eliminated (F) under home cage (HC) and running wheel (RW) conditions in adolescent (∼4–5 weeks) and adult (∼2–4 months) male mice heterozygous for an Arc-GFP gene expression reporter. For E, ***p < 0.001, Bonferroni's posttest; ANOVA age-by-condition interaction, F(1,19) = 9.89, p = 0.005. For F, ANOVA age-by-condition interaction, F(1,19) = 0.04, p = 0.842. n = 6 mice for each group except adult RW, where n = 5. Scale bars: B, D, E, F, 5 μm; C, 500 μm.
Table 1.
Percentages of boutons formed and eliminated under various experimental conditions in adolescent (∼4–5 weeks) and adult (∼2–4 months) male mice
Age | Label | Treatment | Bouton formation (%) | Bouton elimination (%) | Bouton no. | Mouse no. | ||||
Mean ± SE | p -value vs Ctrl | Mean ± SE | p
value vs Ctrl | |||||||
Parametric | Nonparametric | Parametric | Nonparametric | |||||||
Adolescent | TRITC-dextran | Home cage | 1.7 ± 0.6 | Ctrl | Ctrl | 5.6 ± 1.3 | Ctrl | Ctrl | 481 | 6 |
Wheel running | 13.4 ± 1.9 | 0.001 | 0.002 | 6.9 ± 1.0 | 0.434 | 0.788 | 344 | 6 | ||
Adult | TRITC-dextran | Home cage | 1.9 ± 1.0 | Ctrl | Ctrl | 2.4 ± 1.2 | Ctrl | Ctrl | 324 | 6 |
Wheel running | 4.8 ± 1.7 | 0.16 | 0.262 | 3.2 ± 1.4 | 0.659 | 0.784 | 262 | 5 | ||
Adolescent | ChR2-EYFP | No stim | 3.0 ± 1.6 | Ctrl | Ctrl | 0.4 ± 0.4 | Ctrl | Ctrl | 256 | 6 |
Tonic stim | 3.0 ± 1.0 | 0.999 | 0.805 | 3.7 ± 1.8 | 0.132 | 0.106 | 137 | 6 | ||
Phasic stim | 19.8 ± 4.0 | 0.003 | 0.004 | 1.9 ± 1.2 | 0.287 | 0.455 | 144 | 6 | ||
EGFP | Phasic stim | 3.2 ± 0.3 | 0.936 | 0.734 | 2.0 ± 0.8 | 0.107 | 0.106 | 384 | 6 | |
Adult | ChR2-EYFP | No stim | 1.0 ±0.6 | Ctrl | Ctrl | 3.5 ± 1.5 | Ctrl | Ctrl | 237 | 6 |
Phasic stim | 4.4 ± 2.1 | 0.163 | 0.318 | 0.9 ± 0.4 | 0.149 | 0.167 | 135 | 6 | ||
Adolescent | ChR2-EYFP | Saline + phasic stim | 24.4 ± 3.8 | Ctrl | Ctrl | 6.5 ± 2.5 | Ctrl | Ctrl | 235 | 6 |
Quinpirole + phasic stim | 5.0 ± 1.7 | 0.0009 | 0.002 | 2.1 ± 0.7 | 0.125 | 0.091 | 311 | 6 | ||
CPP/CNQX + phasic stim | 4.4 ± 1.8 | 0.0008 | 0.002 | 1.7 ± 0.5 | 0.091 | 0.041 | 202 | 6 | ||
Adult | ChR2-EYFP | Saline + phasic stim | 2.6 ± 1.4 | Ctrl | Ctrl | 1.0 ± 0.7 | Ctrl | Ctrl | 237 | 6 |
Eticlopride + phasic stim | 15.3 ± 2.8 | 0.002 | 0.002 | 1.8 ± 0.8 | 0.500 | 0.372 | 301 | 6 | ||
Eticlopride only | 5.2 ± 1.6 | 0.25 | 0.327 | 2.1 ± 1.1 | 0.434 | 0.437 | 311 | 6 |
Ctrl, Control; stim, stimulation.
p-values relative to the age-matched control groups are shown for both parametric (Student's t) and nonparametric (Mann–Whitney) tests. The total number of boutons and total number of mice are shown for each condition. Significant p-values are in bold. Data are shown as means ± SE.
Optogenetic Labeling and Activation of Dopaminergic Neurons
To investigate directly whether dopamine neuron activity affects the structure and function of the mesofrontal circuit, we adopted an optogenetic method to selectively label and activate dopaminergic neurons. We injected an AAV carrying a Cre recombinase-dependent expression construct for channelrhodopsin-2 fused with enhanced yellow fluorescent protein (ChR2-EYFP; Tsai et al., 2009) into the VTA of transgenic mice that expressed Cre under the control of TH promoter (Gong et al., 2007; Fig. 2A,B). ChR2-EYFP-labeled axons were detectable by in vivo two-photon microscopy in the FC (M2 region, layers I-III; Fig. 2C) and those axons and boutons were colabeled with TH immunofluorescence in brain sections (Fig. 2D).
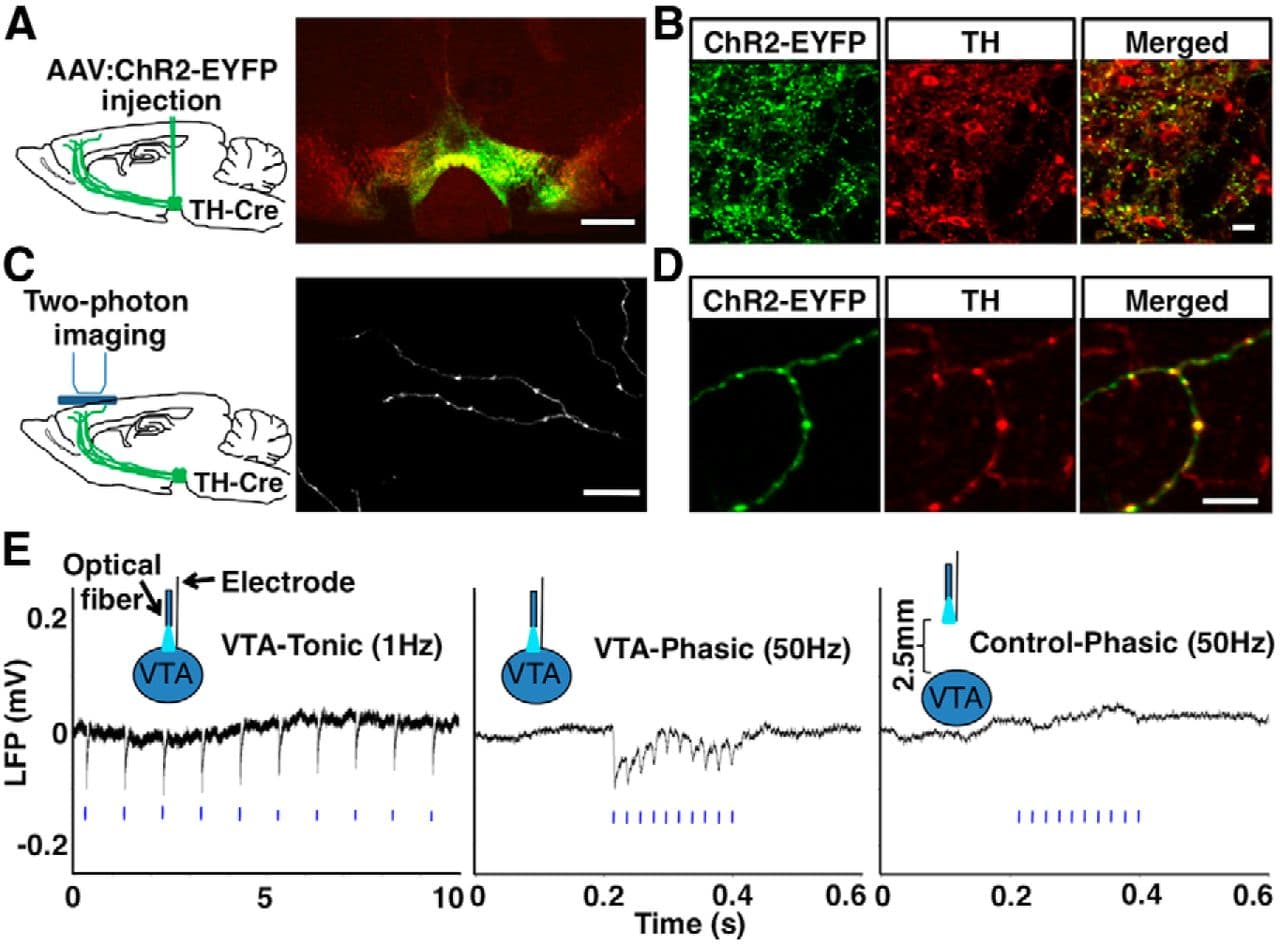
Figure 2. Optogenetic labeling and activation of dopaminergic neurons. A, B, Confocal images showing ChR2-EYFP (green) colocalized with TH-immunofluorescence (red) in the VTA of optogenetically labeled mice. ChR2-EYFP expression in dopaminergic neurons showed a high efficacy, because ∼90% (109/120) of TH-immunopositive cells in the injected region expressed ChR2-EYFP, and high specificity, because ∼96% (109/113) of ChR2-EYFP+ cells were TH+. (C) In vivo two-photon image of ChR2-EYFP+ axons in the FC. (D) Confocal images of FC sections showing colabeling of ChR2-EYFP (green) with TH immunofluorescence (red) in axons and boutons (repeated in 3 mice). E, LFP recordings showing VTA responses to tonic and phasic photostimulation (blue bars) in a ChR2-expressing mouse (P52). Each trace is averaged from 10 sweeps of recordings. No response was seen outside of the VTA where ChR2 was not expressed. The average number of LFP peaks (>3 SDs from baseline) time locked to the 10 light pulses was 9.4 ± 0.5 (n = 8 mice) for phasic (50 Hz), 10 ± 0 (n = 4 mice) for tonic (1 Hz), and 0 ± 0 (n = 6 mice) for ChR2− control (50 Hz) stimulations. Scale bars: A, 500 μm; B, C, 25 μm; D, 10 μm.
To activate dopaminergic neurons, we inserted an optic fiber over the VTA in ChR2-EYFP-expressing mice (Fig. 2E) and applied tonic (1 Hz) or phasic (50 Hz) blue light pulse trains (10 pulses/train) mimicking the natural activity patterns of those neurons (Grace et al., 2007; Tsai et al., 2009). To verify neuronal responses to light stimulation, we conducted local field potential (LFP) recordings, which sample the average activity of neurons locally (Lewis and O'Donnell, 2000; Lavin et al., 2005). We observed robust LFP responses to both tonic and phasic stimulation in the VTA and no response outside of the VTA in the absence of ChR2 expression (Fig. 2E). These results confirmed the specific activation of ChR2-expressing dopamine neurons by light.
Phasic Activation of Dopamine Neurons Promotes Mesofrontal Bouton Formation in Adolescence
To determine the effect of VTA activation on mesofrontal innervation, we imaged ChR2-EYFP-labeled dopaminergic axons by two-photon microscopy in the FC before and 2 h after VTA activation. We used near infrared laser (920 nm) to excite YFP under two-photon mechanism. ChR2 activation normally occurs under one-photon illumination by blue light (470 nm). Although ChR2 could in principle conduct a small amount of photocurrent in response to two-photon illumination by near infrared laser, previous studies using standard laser-scanning two-photon microscopy did not find evidence of neural activation, and this was attributed to limits in laser power, focal volume, and scanning duration (Zhang and Oertner, 2007; Rickgauer and Tank, 2009; Andrasfalvy et al., 2010; Papagiakoumou et al., 2010).
To control for any potential effect that might be contributed by two-photon imaging of ChR2-EYFP-labeled axons in our study, we included three groups of mice that all underwent the same extent of two-photon imaging in the FC, but received no stimulation, tonic (1 Hz) stimulation, or phasic (50 Hz) stimulation to the VTA (Fig. 3A). In addition, to control for any potential effect related to the phasic light itself, we transduced another group of mice with an EGFP-encoding AAV that produces no ChR2 expression and subjected these mice to the same phasic light stimulation and imaging procedures.
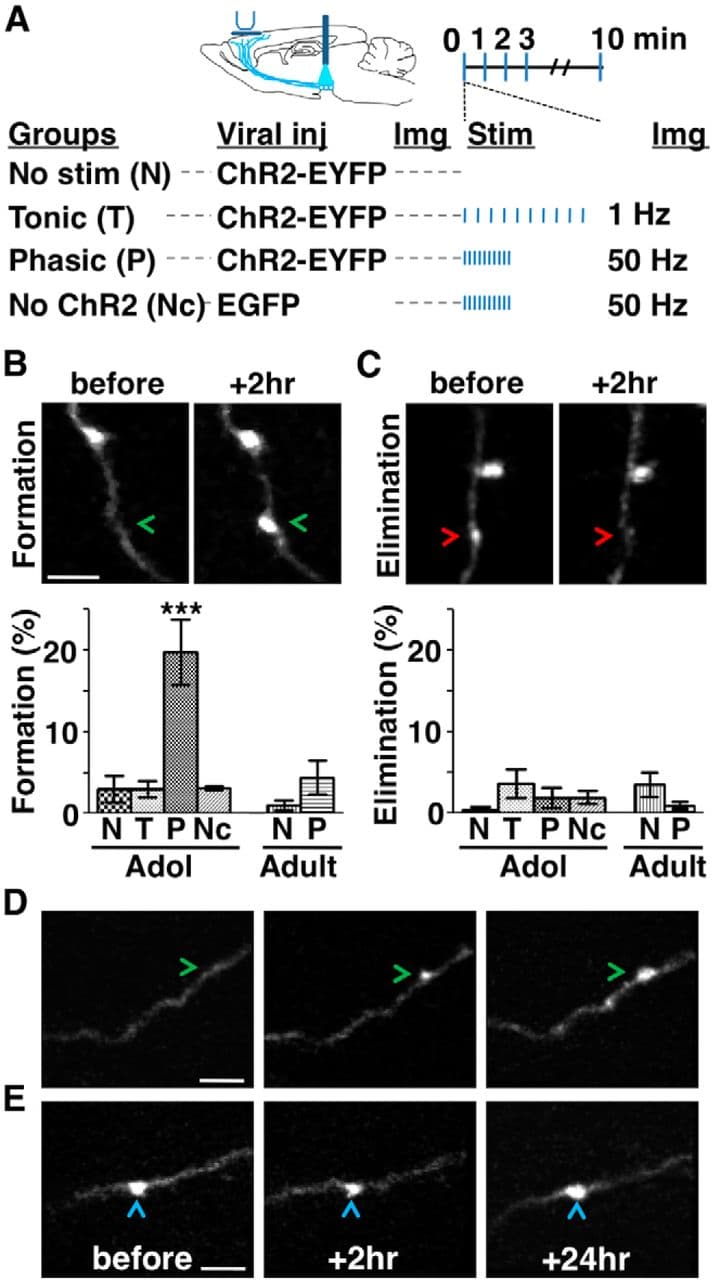
Figure 3. Phasic activation promotes mesofrontal bouton formation in adolescence. A, Experimental conditions. B, C, Top, two-photon images showing bouton formation (B, arrowheads) and elimination (C, arrowheads) on mesofrontal axons over a 2 h interval. Bottom, Percentage of boutons formed (B) and eliminated (C) in adolescent (∼4–5 weeks) and adult (∼2–4 months) male mice. For B, one-way ANOVA F(5,30) = 12.09, p < 0.0001; Bonferroni's posttest, ***p < 0.001, adolescent phasic group versus all other groups. For C, one-way ANOVA F(5,30) = 1.33, p = 0.279. n = 6 mice each group. Data are means ± SE. D, E, Two-photon images showing newly formed (D, arrowheads) and preexisting (E, arrowheads) boutons identified 2 h after phasic stimulation persisted after 24 h in adolescent mice (repeated in 9 mice). The majority (79%, 19/24) of new axonal boutons formed 2 h after phasic stimulation were still present 24 h later, comparable to the stability of boutons that existed before stimulation (88%, 109/124; p = 0.75, χ2 test). Scale bars, 5 μm.
We found that, in adolescent (∼4–5 weeks) male mice, the proportion of newly formed boutons increased in response to phasic, but not tonic, stimulation and this effect only occurred when ChR2 was expressed (no stimulation: 3.0 ± 1.6%, tonic stimulation: 3.0 ± 1.0%, phasic stimulation: 19.8 ± 4.0%, no ChR2: 3.2 ± 0.3%; one-way ANOVA, F(5,30) = 12.09, p < 0.0001; Bonferroni's posttests, only phasic stimulation differs from the other groups, p < 0.001; Fig. 3B, Table 1). Bouton elimination was not different among those groups (one-way ANOVA F(5,30) = 1.33, p = 0.279; Fig. 3C, Table 1). Because the same two-photon imaging procedure was applied to all the groups, the effect of phasic stimulation on bouton formation cannot be attributed to the two-photon laser. In addition, ChR2-negative axons exhibited similar bouton dynamics as ChR2-positive axons that received no VTA stimulation, further indicating that imaging ChR2-labeled axons with two-photon laser did not affect bouton dynamics. Together, these results suggest that phasic, but not tonic, activation of dopaminergic neurons is sufficient to induce mesofrontal bouton formation in adolescent mice.
Next, we investigated whether the axonal boutons formed after phasic activation would persist in the adolescent FC beyond the 2 h period thus far examined. We imaged mesofrontal axons before and 2 h after phasic stimulation; the imaged mice were then returned to their home cages and imaged again 24 h later. The majority (79%, 19/24) of new axonal boutons formed 2 h after phasic stimulation were still present 24 h later, comparable to the stability of boutons that existed before stimulation (88%, 109/124; p = 0.75, χ2 test; Fig. 3D,E). Therefore, phasic activation of dopaminergic neurons induced the formation of stable mesofrontal boutons in adolescent mice.
Effect of Phasic Activity on Mesofrontal Boutons Diminishes in Adulthood
To examine the effect of phasic activation on adult (∼2–4 months) mesofrontal axons, we applied the same phasic stimulation and imaging procedures. Several lines of evidence suggest that the imaging and stimulation conditions are comparable between adolescent and adult mice. First, at the gross anatomical level, there is little difference between adolescent and adult rodents in the midbrain and the frontal cortical regions (Kalsbeek et al., 1988; also see Figs. 4B, 8A). In our study, axonal boutons were sampled in the same cortical region and layers (M2, layers I-III) and bouton dynamics were measured as a percentage of preexisting structures similar to other two-photon imaging studies comparing different age groups (Holtmaat et al., 2009; Fu and Zuo, 2011). Second, we found that 50 Hz LFPs were evoked reliably by 50 Hz light stimulation in the VTA from adolescence to adulthood (Fig. 4A). Third, ChR2-EYFP expression was comparable between adolescent and adult VTA (p = 0.390, t test; Fig. 4E,F). Fourth, the numbers of VTA cells activated by phasic stimulation (RM-ANOVA: stimulation, F(1,6) = 27.80, p = 0.002; age, F(1,6) = 0.585, p = 0.474; stimulation-by-age interaction, F(1,6) = 0.034, p = 0.859; Fig. 4B–D), as indicated by the induction of immediate-early gene c-Fos (Tye and Deisseroth, 2012), and the percentages of ChR2+ cells that were c-Fos+ (p = 0.789, t test; Fig. 4E,G), were similar between adolescent and adult mice.
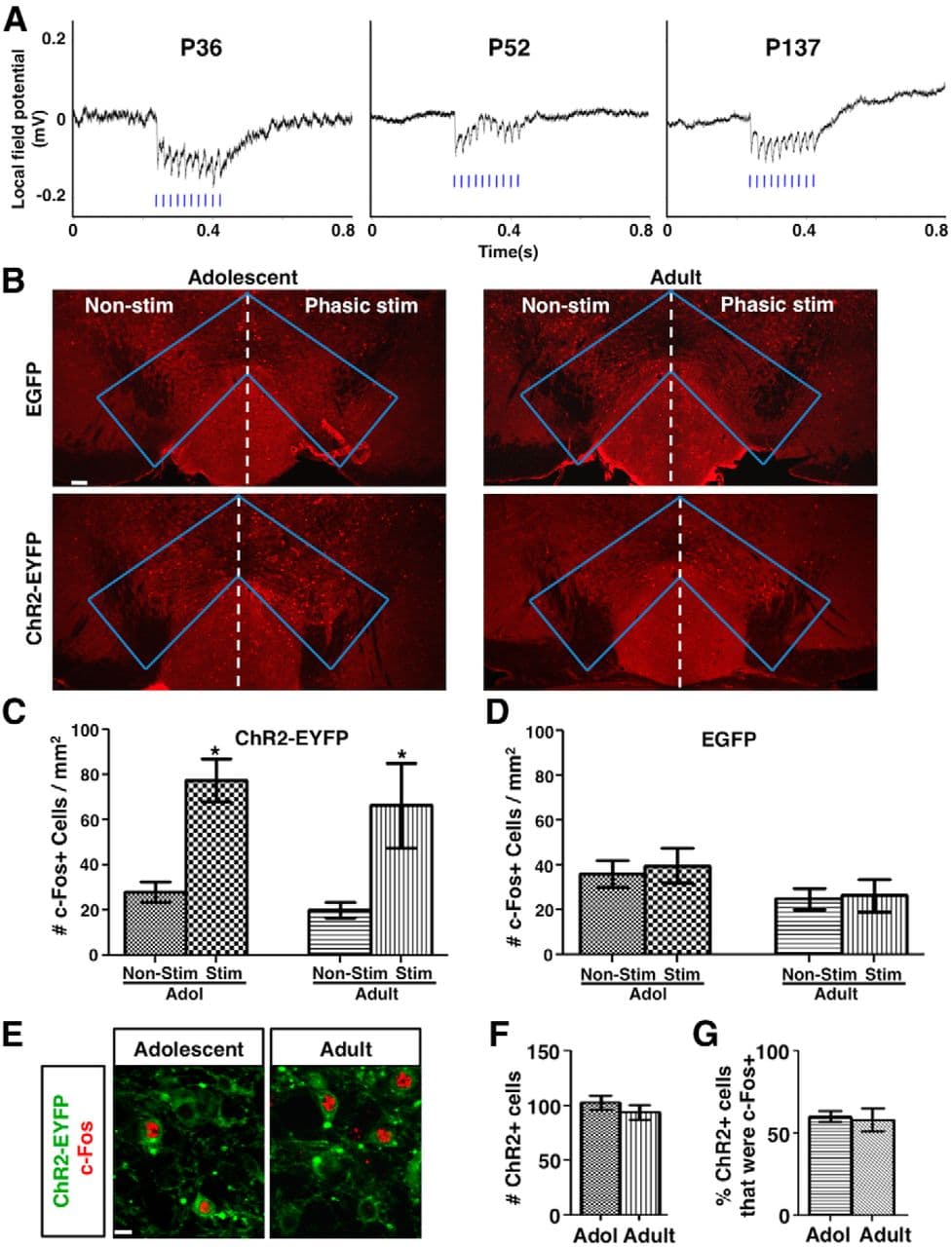
Figure 4. Phasic activation induces robust LFP response and comparable c-Fos expression in adolescent and adult mice. A, VTA LFP traces in response to phasic light stimulation of ChR2-expressing dopamine neurons in mice with ages ranging from adolescence to adulthood. 50 Hz LFP responses time locked to 50 Hz phasic light pulses (blue bars) were observed in all ages. Average of 10 sweeps of LFP recordings are shown for each condition. B, Confocal images of c-Fos immunofluorescence in the VTA of adolescent (∼4–5 weeks) and adult (∼2–4 months) mice that were transduced with either AAV:ChR2-EYFP or AAV:EGFP and unilaterally stimulated with phasic light pulses. C, In AAV:ChR2-EYFP-transduced mice, the density of c-Fos+ cells at the stimulated side was increased to a similar extent between adolescent (∼4–5 weeks) and adult (∼2–4 months) mice. RM-ANOVA: stimulation, F(1,6) = 27.80, p = 0.002; age, F(1,6) = 0.585, p = 0.474; simulation-by-age interaction, F(1,6) = 0.034, p = 0.859; nonstimulated versus stimulated, adolescent *p = 0.017, adult *p = 0.023, Bonferroni's posttests, n = 4 mice for each group. D, In AAV:GFP-transduced mice, no significant difference was observed. RM-ANOVA: stimulation, F(1,6) = 0.990, p = 0.358; age, F(1,6) = 1.91, p = 0.216; interaction, F(1,6) = 0.196, p = 0.674. n = 4 mice for each group. E, Confocal images of ChR2-EYFP (green) and c-Fos (red) labeled cells in the VTA of adolescent and adult mice after phasic stimulation. F, G, The number of ChR2+ cells (F) in 636 × 636 × 12 μm3 image stacks from two sections/mouse and the percentage of ChR2+ cells that were c-Fos+ (G) are not different between adolescent and adult mice. p = 0.390 (F) and 0.789 (G), t tests, n = 4 mice each. Scale bars: B, 100 μm; E, 10 μm. Data are means ± SE.
In adult mice, however, phasic activation did not lead to any significant change of bouton dynamics compared with the no-stimulation group (Fig. 3B,C, Table 1). The proportion of newly formed boutons in the stimulated mice was less in adulthood (4.4 ± 2.1%) than that in adolescence (19.8 ± 4.0%, p = 0.004, Bonferroni's posttest; two-way ANOVA age-by-stimulation interaction, F(1,20) = 7.8, p = 0.011). Therefore, phasic VTA activation has less effect on mesofrontal boutons in adult mice than in adolescent mice.
D2R Signaling Regulates Bidirectionally the Axonal Plasticity Induced by Phasic Activity
We next investigated what neurotransmitter may regulate phasic activity-induced bouton formation. In response to activation, dopamine and glutamate can be coreleased from VTA dopamine neurons (Sulzer et al., 1998; Lavin et al., 2005; Stuber et al., 2010), which might affect the structural plasticity of dopaminergic axons. Glutamatergic transmission through NMDA- and AMPA-type receptors has been implicated in the plasticity of excitatory synapses (McAllister, 2007; Fu and Zuo, 2011). For dopaminergic axons, it has been reported that genetic ablation of dopamine D2 receptor (D2R), but not D1 receptor (D1R), affects axonal structures. Consistent with the effects of genetic ablation, long-term pharmacological treatment with D2R antagonists resulted in sprouting of dopaminergic axons, whereas treatment with D2R agonists led to pruning of axon arbors in the striatum (Parish et al., 2001; Parish et al., 2002). Whether glutamatergic and D2R signaling regulates the plasticity of mesofrontal dopaminergic boutons, however, remains unknown.
We first tested whether acute pharmacological modulation of dopaminergic and glutamatergic transmission could block the increased bouton formation induced by phasic activity in adolescent mice. To avoid mechanical disruption of axonal structures potentially resulting from drug infusion into the FC, we administered pharmacological reagents by intraperitoneal injection minutes before phasic stimulation. We found that, in adolescent mice, D2R agonist (quinpirole, 1 mg/kg) or glutamate receptor antagonists (CPP for NMDA-type receptors, 3 mg/kg, administered together with CNQX for AMPA-type receptors, 10 mg/kg) suppressed the bouton formation induced by phasic stimulation (saline + stimulation: 24.4 ± 3.8%, quinpirole + stimulation: 5.0 ± 1.7%, CPP/CNQX + stimulation: 4.4 ± 1.8%; one-way ANOVA, F(2,15) = 18.77, p < 0.0001; Bonferroni's posttests, p < 0.001, saline + stimulation vs all other groups; Fig. 5A,B, Table 1). These results suggest that both dopaminergic and glutamatergic signaling regulate the structural changes induced by phasic activity in adolescence.
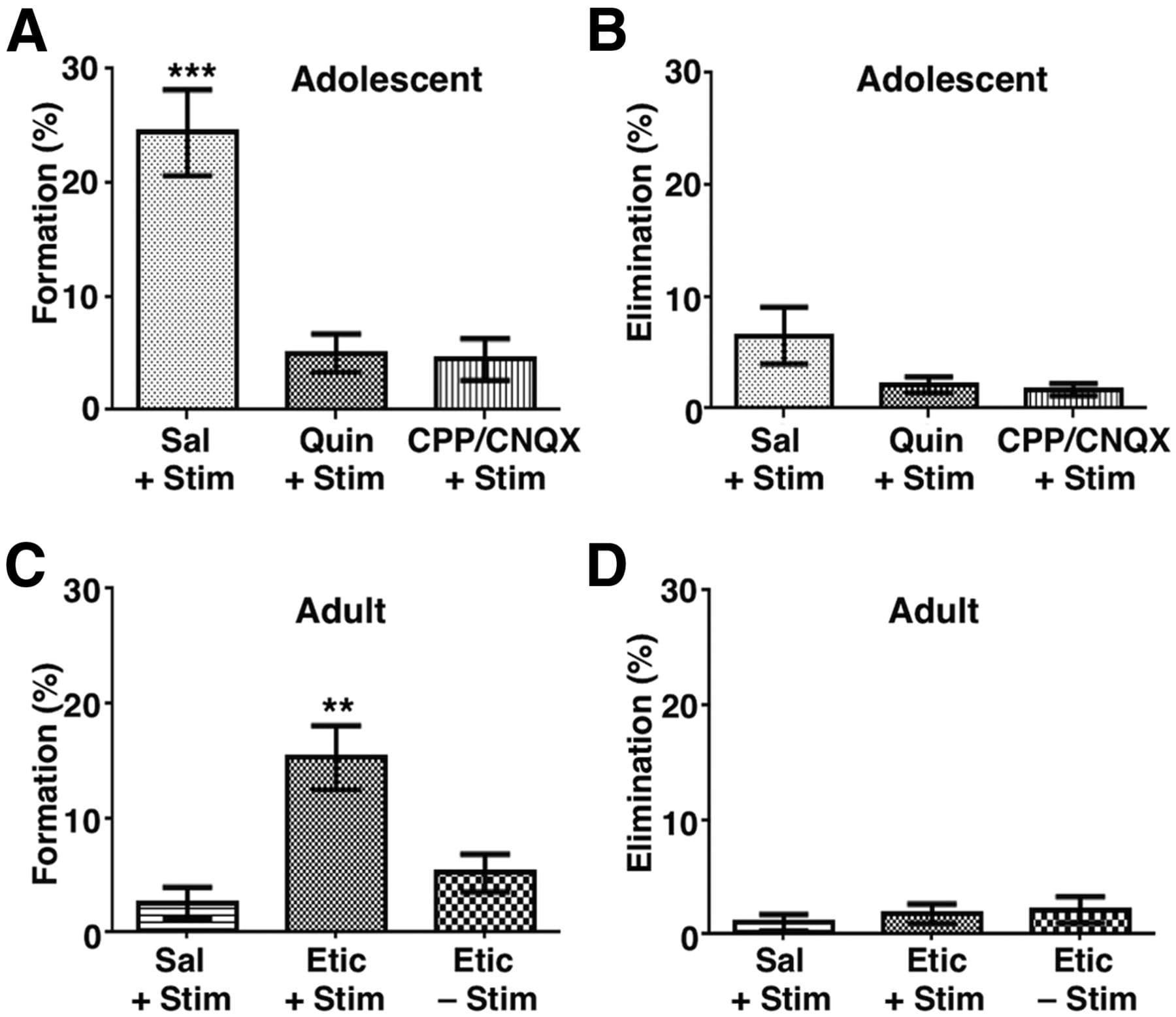
Figure 5. D2R signaling regulates bidirectionally the axonal plasticity induced by phasic activity. A, D2R agonist quinpirole (Quin, 1 mg/kg, i.p.) significantly suppresses phasic activation-induced bouton formation in adolescent mice. The NMDAR antagonist CPP (3 mg/kg) plus the AMPAR antagonist CNQX (10 mg/kg, i.p.) also led to significant suppression. One-way ANOVA F(2,15) = 18.77, p < 0.0001; Bonferroni's posttest, ***p < 0.001, saline (Sal) + stimulation (Stim) group versus all other groups. B, No significant difference is observed in the elimination of boutons in adolescent mice. One-way ANOVA F(2,15) = 2.98, p = 0.081. C, Although treatment with the D2R antagonist eticlopride (Etic, 0.5 mg/kg, i.p.) without phasic stimulation did not affect bouton formation, eticlopride treatment did enhance bouton formation in response to phasic stimulation in adult mice. One-way ANOVA, F(2,15) = 10.84, p = 0.0012; Bonferroni's posttest, **p < 0.01, Etic + Stim group versus all other groups. D, No significant difference is observed in the elimination of boutons in adult mice. One-way ANOVA F(2,15) = 0.380, p = 0.691. n = 6 mice each group. Data are means ± SE.
The suppressive effect of D2R activation on bouton formation raises the possibility that inhibiting D2R activity might restore the structural plasticity of mesofrontal axons in adult mice. Indeed, we found that whereas treatment with the D2R antagonist eticlopride (0.5 mg/kg, i.p.) alone without phasic stimulation did not affect bouton formation, eticlopride treatment did enhance bouton formation in response to phasic stimulation in adult mice (saline + stimulation: 2.6 ± 1.4%, eticlopride + stimulation: 15.3 ± 2.8%, eticlopride + no stimulation: 5.2 ± 1.6%; one-way ANOVA, F(2,15) = 10.84, p = 0.0012; Bonferroni's posttest, p < 0.01, eticlopride + stimulation group vs all other groups; Fig. 5C,D, Table 1). Together, our results suggest that D2R signaling regulates bidirectionally the axonal plasticity induced by phasic activity.
Phasic Activation of Dopamine Neurons Led to Elevated Mesofrontal Circuit Activity in Adolescence
Because phasic activation of VTA neurons altered the structure of adolescent mesofrontal circuit, we investigated whether this activation would also lead to functional changes consistent with the observed structural changes. Previous studies showed that phasic electrical stimulation of VTA evoked sustained neural activity outlasting the stimulus by more than hundreds of milliseconds in the adult FC. This prolonged activity depends on dopaminergic signaling and reflects enhanced local circuit activity in the presence of dopaminergic activation (Lewis and O'Donnell, 2000; Lavin et al., 2005; Onn and Wang, 2005). However, it is not yet known whether this functional characteristic of the mesofrontal circuit would be modified by the prior occurrence of phasic activity in dopamine neurons.
To address this question, we compared frontal cortical LFP responses (M2 region, layer II/III) to phasic optical stimulation of VTA between two recording sessions separated by a 2 h interval, as in our imaging experiments (Fig. 6A). We used LFP recording because it provides a robust index of the population activity in local neurons (Buzsáki et al., 2012). This method has been used to reveal the sustained frontal activity evoked by VTA stimulation (Lewis and O'Donnell, 2000; Seamans et al., 2003; Lavin et al., 2005) and high-frequency cortical oscillations (from θ to γ range) that are modulated by dopaminergic signaling (Costa et al., 2006; Gireesh and Plenz, 2008; Wood et al., 2012; Furth et al., 2013).
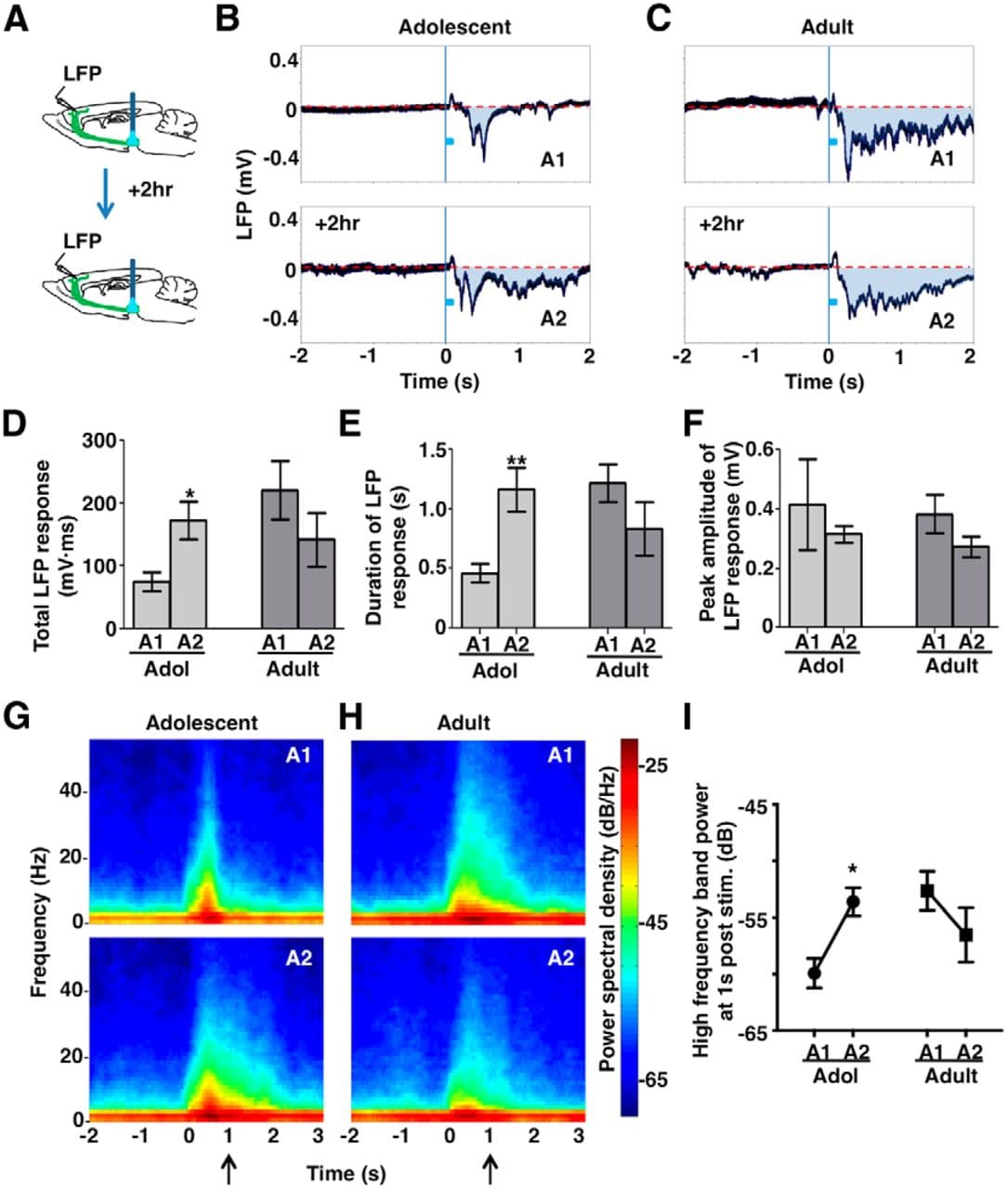
Figure 6. Phasic activation leads to elevated mesofrontal circuit activity in adolescence. A, Experimental conditions. B, C, Frontal cortical LFPs evoked by phasic VTA activation in an adolescent (B) and adult (C) mouse. The blue boxes indicate phasic stimuli (50 Hz pulses for 200 ms). The shaded areas (A1, A2) indicate total LFP responses induced by VTA stimulation. Each trace is averaged from 10 sweeps of recordings. D, Total LFP responses in session 1 (A1) and 2 (A2) in adolescent (∼4–5 weeks) and adult (∼2–4 months) mice. Two-way RM-ANOVA, age-by-session interaction, F(1,14) = 13.82, p = 0.002; Bonferroni's posttest, adolescent (Adol) A1 versus A2, *p = 0.023. E, Duration of LFP responses in session 1 (A1) and 2 (A2) in adolescent and adult mice. RM-ANOVA, age-by-session interaction, F(1,14) = 19.4, p = 0.0006. Bonferroni's posttest, Adol A1 versus A2, **p = 0.003. F, Peak amplitude of LFP responses in session 1 (A1) and 2 (A2) in adolescent and adult mice. No significant difference was observed in adolescent or adult mice. RM-ANOVA: age, F(1,14) = 0.146, p = 0.708; session, F(1,14) = 1.983, p = 0.181; interaction, F(1,14) = 0.004, p = 0.948. G, H, Spectrogram of the LFP signals in a period from 2 s before VTA stimulation to 3 s after in adolescent (G) and adult (H) mice. Spectral analyses were conducted for each 0.5 s sliding window (0.1 s step) in individual sweeps of LFP recordings. The graphs show the average of all the animals in each condition. I, To summarize the long-lasting changes in high-frequency oscillations, the power spectral density calculated at 1 s post VTA stimulation (arrows in G and H) was averaged over a high-frequency band (4–55 Hz, θ to γ oscillation range) for each subject and condition. RM-ANOVA, age-by-session interaction, F(1,14) = 11.83, p = 0.004; Bonferroni's posttest, Adol A1 versus A2, *p = 0.019. n = 8 mice for each group. Data are means ± SE.
Previous studies have shown that the shift of LFP trace below the prestimulation baseline indicates excitatory response to VTA activation (Lewis and O'Donnell, 2000; Seamans et al., 2003; Lavin et al., 2005). Using this measure, we found an enhanced total LFP response in the second session compared with the first session in adolescent mice, but not in adult mice (RM-ANOVA, age-by-session interaction, F(1,14) = 13.82, p = 0.002; Bonferroni's posttest, adolescent session 1 vs 2, p = 0.023; Fig. 6B–D). Rather than changes in peak amplitude (RM-ANOVA, age-by-session interaction, F(1,14) = 0.004, p = 0.948; Fig. 6F), this enhanced total LFP response was associated with an increase in response duration (RM-ANOVA, age-by-session interaction, F(1,14) = 19.4, p = 0.0006; Bonferroni's posttest, adolescent session 1 vs 2, p = 0.003; Fig. 6E).
To further examine the potential changes in high-frequency cortical oscillations, we analyzed the spectrogram of LFP signals around the time of VTA stimulation in each session (Fig. 6G,H). The spectrogram shows the distribution of signal power over frequency range in each short segment (0.5 s) of LFP signals. We found that, nested on top of the slow responses, phasic VTA stimulation also evoked higher frequency responses ranging from θ to γ (4–55 Hz) oscillations. Moreover, comparing the second session with the first session, phasic stimulation led to more prolonged increase of high-frequency power in adolescent mice, but not in adult mice (RM-ANOVA, age-by-session interaction, F(1,14) = 11.83, p = 0.004; Bonferroni's posttest, adolescent session 1 vs 2, p = 0.019; Fig. 6I).
Those changes in higher-frequency oscillations are consistent with what we observed in the slow shift of LFP response. Together, those findings indicate that phasic VTA activation potentiates mesofrontal circuit activity in adolescents, which is associated with enhanced dopaminergic input. Moreover, our results suggest that phasic activity can elicit lasting changes in both the structure and function of the mesofrontal circuit in adolescents and that these changes occur in an age-dependent manner.
Phasic VTA Activation Led to Suppressed Psychomotor Activity in Adolescence
The greater impact of phasic activity on the adolescent mesofrontal circuit raises the possibility that behaviors regulated by this circuit could be more readily altered in adolescent mice than in adult mice. Locomotor activity induced by exposure to novel environments or amphetamine is sensitive to frontal dopaminergic input (Tzschentke, 2001; Chambers et al., 2003). For example, increase of mesofrontal dopamine inputs by pharmacological or genetic manipulation is associated with suppression of psychomotor activity (Tzschentke, 2001; Flores et al., 2005; Niwa et al., 2013). Because phasic VTA stimulation promoted mesofrontal innervation more effectively in adolescent mice than in adult mice, we investigated whether this stimulation would also lead to suppressed psychomotor activity in adolescents, but not adults.
To evaluate the long-lasting effects of phasic VTA activation on psychomotor behavior, we delivered phasic light patterns twice per day for 3 d to adolescent (∼4–5 weeks) and adult (∼2–4 months) mice transduced with EGFP- or ChR2-encoding virus. After the last stimulation, those mice were video monitored in their home cages for ∼16 h, exposed to a novel open-field arena, and received amphetamine injection (2 mg/kg) to test psychomotor activity (Fig. 7A).
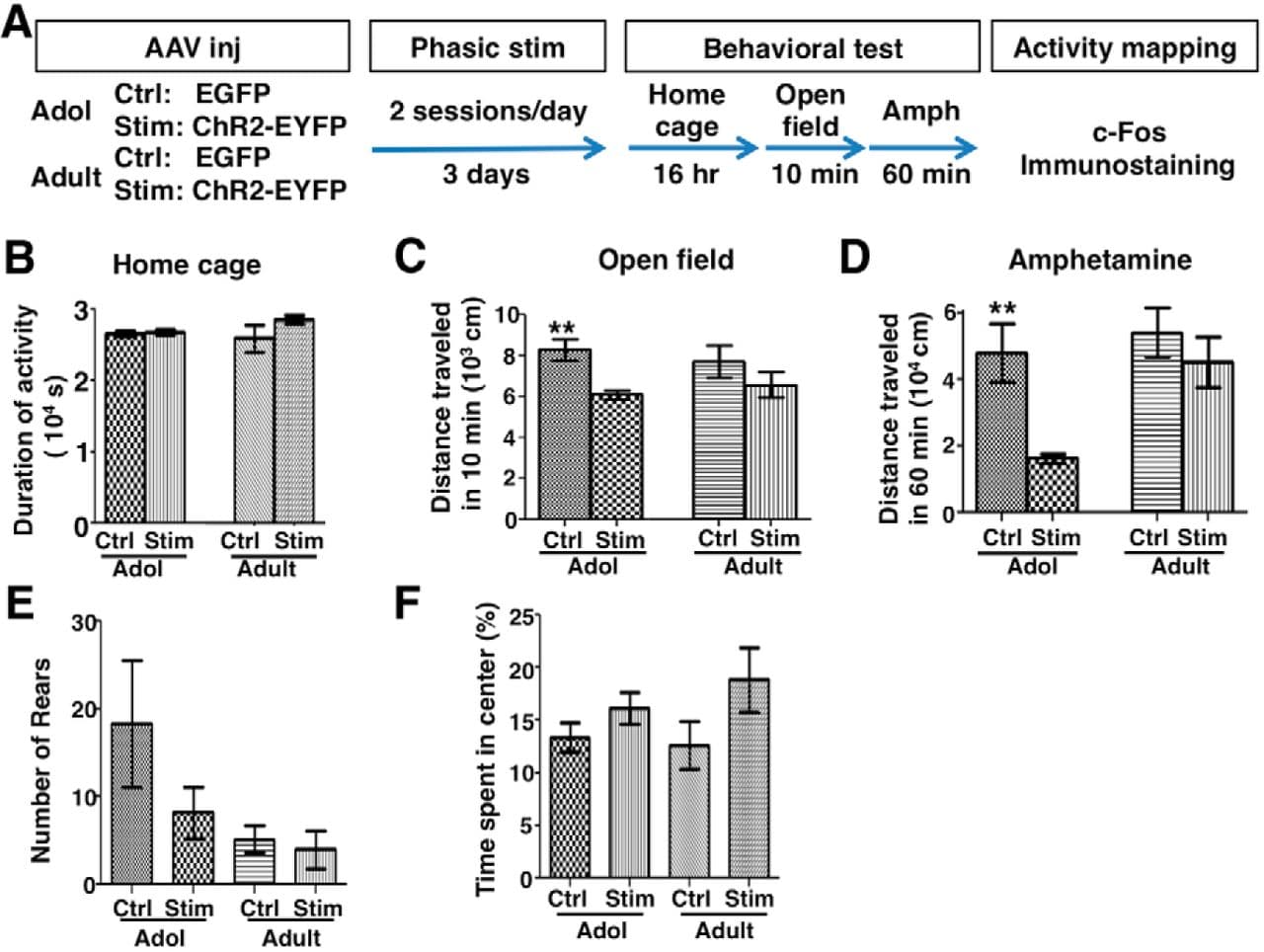
Figure 7. Phasic VTA activation led to suppressed psychomotor activity in adolescence. A, Experimental conditions. B, Duration of spontaneously active behaviors in home cages. One-way ANOVA, F(3,20) = 1.21, p = 0.332, n = 6 mice each. C, D, Total distance traveled by mice during the initial 10 min exposure to the open field (C, Kruskal–Wallis test, p = 0.032; Dunn's posttest, **p = 0.007) and the 60 min after amphetamine injection (D, Kruskal–Wallis test, p = 0.005; Dunn's posttest, **p = 0.008). n = 9 mice each, except adult controls, for which n = 10. E, Total number of rearing counts (sampled from 5 1 min video segments evenly distributed with 10 min interval after amphetamine exposure). Kruskal–Wallis test, p = 0.017; Dunn's posttests: adolescent control (Adol Ctrl) vs adolescent stimulated (Adol Stim), p = 0.795; Adult Ctrl vs Adult Stim, p = 0.351. n = 9 mice each, except Adult-Ctrl, for which n = 10. F, The percentage of time spent in the center of open field is not significantly different between the groups (one-way ANOVA, F(3,33) = 1.73, p = 0.181, n = 9 mice each, except Adult-Ctrl, for which n = 10). Data are means ± SE.
All groups of mice spent similar time in spontaneously active behaviors in their home cages (one-way ANOVA, F(3,20) = 1.21, p = 0.332; Fig. 7B). However, their activities differed under the psychomotor test (Fig. 7C,D). During the initial exposure to the novel test chamber, the stimulated adolescent mice traveled less than the control adolescent mice (Kruskal–Wallis test, p = 0.032; Dunn's posttest, p = 0.007; Fig. 7C). After amphetamine injection, the locomotor activity of the stimulated adolescent mice was also consistently reduced compared with that of the control adolescent mice (Kruskal–Wallis test, p = 0.005; Dunn's posttest, p = 0.008; Fig. 7D). This reduction in locomotion was not associated with an increase in stereotypic rearing behavior or avoidance of arena center (Fig. 7E,F). Therefore, these findings suggest that psychomotor response was suppressed in adolescent mice that received prior phasic stimulation compared with those that received no stimulation. In contrast, the same stimulation did not alter the locomotor activity in adult mice (Fig. 7C,D), suggesting that psychomotor activity in adult mice is less sensitive to the suppressive effect of prior phasic VTA stimulation.
Phasic VTA Activation Led to Enhanced c-Fos Expression in FC Versus NA in Adolescence
Previous studies have suggested competing roles of the FC and the NA in regulating psychomotor response. Although activation of NA and its associated dopaminergic input facilitates psychomotor response, activation of the mesofrontal circuit exerts an inhibitory effect on this response (Le Moal and Simon, 1991; Tzschentke, 2001; Chambers et al., 2003). To ascertain whether the suppression of psychomotor activity induced by phasic VTA stimulation is most consistent with activity changes in the FC, in the NA, or both, we performed c-Fos immunostaining in these two regions after the behavioral tests. Compared with the EGFP control group, c-Fos expression was increased in the FC, but not in the NA, of ChR2-stimulated adolescent mice (Fig. 8A,B), suggesting that the suppression of psychomotor activity induced by phasic VTA stimulation is more consistent with neural plasticity in the mesofrontal circuit than with that in the mesoaccumbens circuit. The increase of the FC-to-NA activation ratio due to VTA stimulation occurred only in adolescence, but not in adulthood (p = 0.0003, Bonferroni's posttest; ANOVA age-by-stimulation interaction, F(1,28) = 7.1, p = 0.013; Fig. 8C), suggesting again that the adult mesofrontal circuit is less sensitive to the effects of prior phasic VTA stimulation.
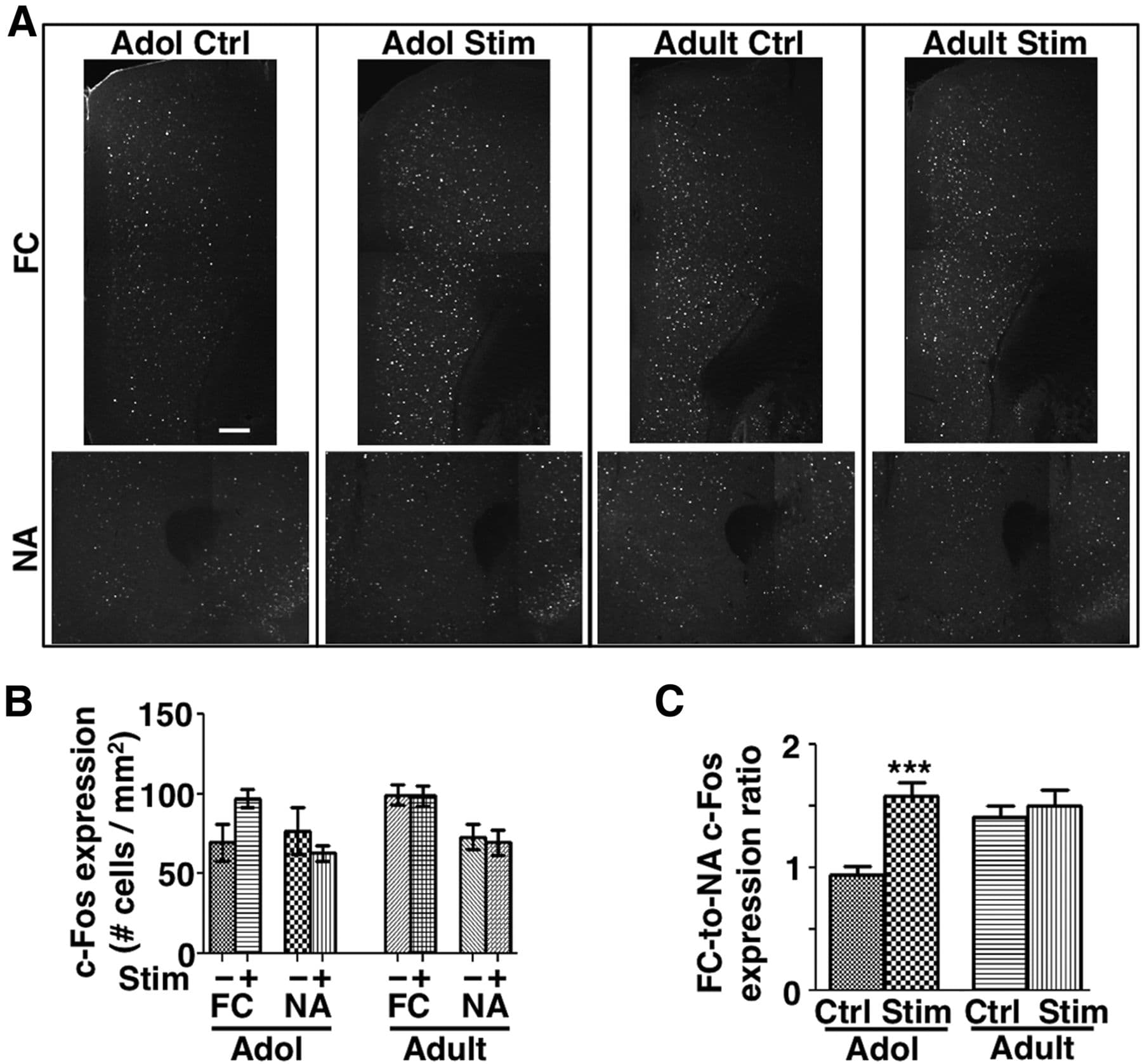
Figure 8. Phasic VTA activation led to enhanced c-Fos expression in FC versus NA in adolescence. A, Confocal images of c-Fos immunofluorescence in FC and NA from mice after the amphetamine test. B, The density of c-Fos+ cells in FC and NA in brain sections collected 60 min after amphetamine exposure. C, The ratio of the density of c-Fos+ cells in FC versus NA was increased in the stimulated (ChR2-EYFP) compared with the control (EGFP) adolescent mice. ***p = 0.0003, Bonferroni's posttest; 2-way ANOVA age-by-treatment interaction, F(1,28) = 7.06, p = 0.013. n = 8 mice each. Data are means ± SE. Scale bar, 200 μm.
Discussion
Here, we report that phasic dopamine neuron activity induces unique plasticity of the mesofrontal dopaminergic circuit in adolescence. Using optogenetic methods to control the activity of dopamine neurons directly, we found that, in adolescent mice, phasic activation of dopamine neurons elicits lasting changes in mesofrontal circuitry, including bouton changes detected by in vivo two-photon microscopy, electrophysiological changes detected by LFP recording, behavioral changes detected by psychomotor test, and brain activation pattern changes detected by c-Fos mapping. In contrast, in adult mice, although dopamine neurons are activated to comparable levels, the ensuing changes in the structure and function of mesofrontal circuitry diminish. Our study therefore provides direct evidence that the structure and function of the mesofrontal circuit is modifiable by phasic activation of dopamine neurons in adolescence. These adolescent changes are all causally linked to the phasic activity of dopamine neurons and consistent with each other. Together, they provide a multifaceted view of enhanced mesofrontal plasticity in adolescence across multiple biological levels.
At the structural level, this study delivers the first evidence that increasing the activity of dopamine neurons promotes the formation of mesofrontal boutons in adolescence. The rates of bouton dynamics we found, which are expressed as percentages of preexisting structures, are within the range (from a few percentages at baseline level to tens of percentage after activity perturbation) reported in previous in vivo imaging studies of glutamatergic or GABAergic axons (Holtmaat et al., 2009; Fu and Zuo, 2011). We did not find any significant effect on bouton elimination rates, although any reduction from the low baseline rate may require higher bouton sampling to detect. In contrast, our results revealed significantly increased bouton formation in response to phasic activation of dopamine neurons in adolescence. These findings suggest a causal role of phasic firing in strengthening the mesofrontal innervation during adolescence.
Phasic activity in dopamine neurons is naturally induced by reward-related or motivationally salient events, whereas tonic activity occurs spontaneously in these neurons (Grace et al., 2007; Schultz, 2007; Bromberg-Martin et al., 2010). Our initial findings showed that wheel running, a natural behavior that increases the phasic activity of VTA neurons (Wang and Tsien, 2011), promoted mesofrontal bouton formation in adolescence. We then used light pulses mimicking the natural activity patterns of dopamine neurons to examine the causal roles of those patterns in driving the structural changes of mesofrontal axons. In contrast to phasic activity, tonic activation of dopamine neurons with the same number of light pulses did not have any effect on the structure of adolescent mesofrontal axons. These results suggest that the temporal pattern of neural activity, rather than the total amount, is important for structural changes in this circuit. Moreover, our results imply a unique role of motivationally salient events in triggering structural changes in the mesoprefrontal circuit during adolescent development.
We found that the structural plasticity of mesofrontal axons induced by phasic dopamine neuron activity is significantly higher in adolescence than that in adulthood. Moreover, this plasticity can be modulated bidirectionally by D2R signaling. D2R has both presynaptic and postsynaptic localization and functions (Anzalone et al., 2012). However, in contrast to dopamine neurons projecting to other brain regions such as striatum, the mesocortical dopaminergic neurons in mouse VTA appear to lack D2R (Lammel et al., 2008). In our study, therefore, the effects of D2R agonist and antagonist may be mediated by D2R located in the FC. Interestingly, earlier electrophysiological studies have reported that D2R modulation of prefrontal neural activity becomes stronger in adults than in adolescents (Tseng and O'Donnell, 2007a, 2007b). Our results showed that, in adolescent mice, increasing D2R activation suppresses the high rate of bouton formation induced by phasic activity. In adult mice, conversely, decreasing D2R activation restores this structural plasticity. Those results suggest that age-related changes in D2R-signaling pathways may contribute to the difference in axonal plasticity between adolescents and adults.
The axonal changes we found here are long lasting and correlate with subsequent changes in frontal circuit activity. Previous studies have shown consistent associations between changes in dopaminergic axons and dopamine release in the FC. For example, the gradual development of dopaminergic axons in the FC correlates with a steady increase in dopamine levels (Niwa et al., 2010; Naneix et al., 2012), and genetic perturbations of dopaminergic innervation also lead to corresponding changes in frontal dopamine (Grant et al., 2009; Niwa et al., 2010; Manitt et al., 2011). The magnitude of structural change we found here (∼20% increase in bouton formation) is in the tens-of-percentage range observed in those earlier studies. Moreover, our results showed that the increased mesofrontal innervation is associated with a prolonged frontal LFP response containing high-frequency oscillations, which is characteristic of elevated dopaminergic signaling (Lewis and O'Donnell, 2000; Seamans et al., 2003; Gireesh and Plenz, 2008; Wood et al., 2012). In addition, the extent of c-Fos expression in response to amphetamine is also increased in frontal cortical neurons. Together, those findings suggest that the structural changes we observed in dopaminergic axons are likely translated into postsynaptic effects.
Our imaging and electrophysiological results demonstrate that phasic dopamine neuron activity during adolescence elicits unique structural and functional plasticity in the mesofrontal circuit compared with that during adulthood. Previous studies of phasic dopamine neuron activity have focused on its roles in eliciting reward- or aversion-related behaviors in adulthood (Tsai et al., 2009; Zweifel et al., 2009; Lammel et al., 2012; Chaudhury et al., 2013; Tye et al., 2013). Our findings showed that phasic dopamine neuron activity modulates behavioral patterns differently between adolescence and adulthood. In adolescent mice, phasic VTA activation led to increased mesofrontal activation and suppressed psychomotor response in subsequent behavioral tests, which is consistent with the inhibitory role of the mesofrontal circuit in psychomotor activity (Tzschentke, 2001; Flores et al., 2005; Niwa et al., 2013). In contrast, in adult mice, the impacts of phasic VTA activation on the mesofrontal circuit and psychomotor behavior both diminished. Our findings may provide an initial step toward elucidating the effects of adolescent neural perturbation on behavioral patterns relevant to neuropsychiatric disorders.
Separate populations of VTA dopamine neurons project to FC and NA, which may exert competing influences on animal behavior (Björklund and Dunnett, 2007; Lammel et al., 2008; Lammel et al., 2012). In addition, it has been suggested that FC and NA have different maturation trajectories (Casey et al., 2010; Wahlstrom et al., 2010; Naneix et al., 2012). In adolescent mice that received prior phasic VTA stimulation, we found a higher FC-to-NA c-Fos activation ratio than in those that received no stimulation. This observation raises the possibility that the mesofrontal dopaminergic circuit might be more malleable to the effects of phasic activation than the mesoaccumbens circuit in adolescence, which would be an interesting topic for future investigation using new optical imaging and modulation techniques to access both pathways (Deisseroth and Schnitzer, 2013).
Based on age-specific changes in behavior traits, body growth, sexual maturation, and neurobiological characteristics, an age range from ∼P28–P42 is suggested to represent the central adolescent period in rodents (Spear, 2000). Some adolescence-related ontogenetic changes, though, appear to emerge as early as P21 and last until P56 or so (Laviola et al., 2003; Ernst et al., 2009; Brenhouse and Andersen, 2011). In the mesofrontal circuit, dopaminergic innervation shows a protracted development from P21 to P56 (Kalsbeek et al., 1988; Niwa et al., 2010; Naneix et al., 2012) and dopamine's effects on frontal neural activity change rapidly over the late adolescence period (∼ P45–P50; Tseng and O'Donnell, 2005, 2007a). Because we aimed to investigate the differences in mesofrontal plasticity over adolescence, we chose a relatively early adolescent period (∼4–5 weeks, P28–P42) to provide a greater contrast with the adult period (2–4 months). Future studies including additional time points will provide a more detailed view of adolescent transitions.
Human studies suggest that dysfunctions in mesofrontal dopaminergic circuit are associated with adolescent-onset psychiatric disorders such as schizophrenia, ADHD, and addiction, and adolescent experience is proposed to be a critical factor (Lewis and Levitt, 2002; Chambers et al., 2003; Winterer and Weinberger, 2004; Casey et al., 2010). Neuropsychological studies have also suggested adolescent period as amendable for rapid changes in cognitive functions and motivational control (Chambers et al., 2003; Casey et al., 2010). Our analyses in mice show that the mesofrontal dopaminergic circuit exhibits unique plasticity in adolescence and is more susceptible to activity-dependent modification during this period than adulthood. Therapeutic brain stimulation strategies delivered at this age may therefore achieve potentially greater effect than that at adulthood. In addition, our findings show that combining brain stimulation with D2R antagonism, which is implicated in antipsychotic drug actions, can restore adult structural plasticity in the mesofrontal circuit. This result may help raise interest in exploring the joint effects of pharmacological and electrical treatment strategies. Our integrated optogenetic modulation and monitoring system may serve as a starting point for identifying molecular targets involved in activity-driven neurobiological changes during adolescence and for testing the potential impacts of therapeutic strategies on this circuit.