Abstract
Substance use disorder is a chronic and relapsing disease that burdens both the individual and the society. In addition to psychosocial treatment approaches, currently there are approved pharmacological treatment options for opioid, alcohol and tobacco use disorders, but only symptomatic treatment can be offered to patients with other substance use disorders. Advances in neuroscience and a better understanding of the addiction process offer an opportunity to create new treatment options. There is a wide range of studies, ranging from the use of drugs with different indications to the development of new pharmacological treatments, and from vaccine studies to neuromodulation techniques. Establishing novel treatment goals in addition to complete abstinence and individualizing treatment by focusing on endophenotypes may increase the treatment alternatives and the efficacy of these treatments for SUD.
INTRODUCTION
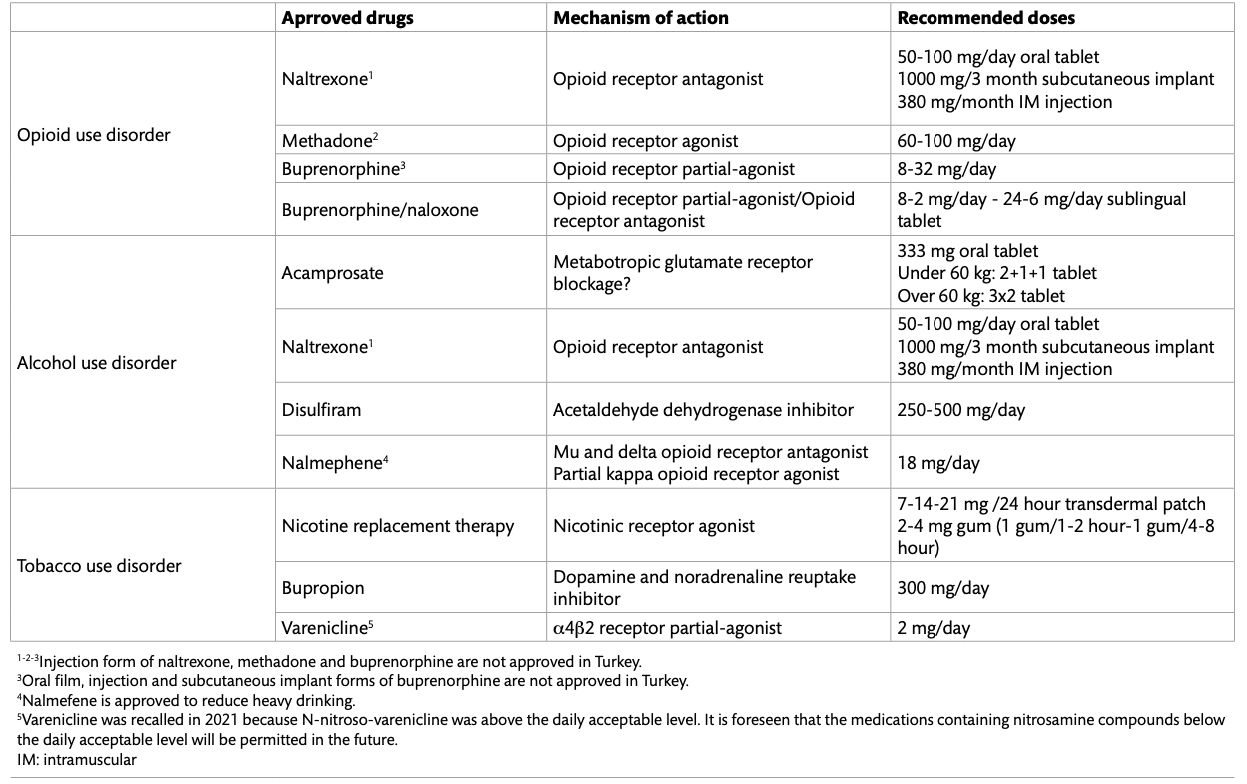
Substance use disorder (SUD) is a chronic relapsing disorder, characterized by repetitive substance use and substance-seeking behaviors, despite negative consequences. Despite its burden on public health, only a small number of pharmacological treatment strategies are available for SUD (Table 1). Although these treatment strategies have been shown to reduce substance-related mortality, existing pharmacotherapies are approved for only some of the substances and they are only effective for a subset of patients. Moreover, the effect of these treatment strategies is often limited and short term. As our knowledge of the neurobiological, genetic, epigenetic, and environmental mechanisms involved in the addiction process improves, new targets for prevention and treatment interventions are identified. In addition, emerging technologies such as neuroimaging and brain stimulation or mobile health interventions offer new opportunities for treatment. These developments may introduce new targets for treatments and facilitate personalized treatment approaches. However, current data suggests that only a small number of novel medications have been approved for SUD. Legal problems, high psychiatric comorbidity, stigma, high relapse rates that generally accompany SUD and high treatment goals represent a major challenge for research conducted in this area. Currently approved pharmacological treatments, promising new studies in the field of addiction, and their neurobiological mechanisms will be discussed in this article.
Neurobiological Basis of Addiction
Addiction can be conceptualized as a disease related to the brain’s reward system. The reward system is responsible for directing attention, motivation, and behavior towards survival goals. Substance of abuse eventually hijacks the brain reward system and exerts considerable dominance over rationale thought. This leads to the progressive loss of control over substance use despite medical, occupational, and interpersonal hazards. After consumption, the substance stimulates large bursts of dopamine in the reward system that reinforce substance use and strengthens conditioned associations, which links the stimulus that precede drug consumption with the expectation of a reward. Euphoria promotes the repeated use of the substance. A while after regular substance use, the substance-induced dopamine release is attenuated and subjective experience of reward during intoxication is diminished. Other neurotransmitters, including opioids, cannabinoids, gama aminobutyric acid (GABA), and serotonin beyond dopamine also contribute to the euphorigenic effects of substances and to the neuroadaptations that result in addiction.
Withdrawal phase is characterized by dysphoria, anxiety, anhedonia and increased sensitivity to stress. Basal forebrain areas, including the extended amygdala as well as the habenula and neurotransmitters, and neuropeptides such as the corticotropin-releasing factor, norepinephrine, and dynorphin are responsible from the withdrawal symptoms. Increased signaling in these circuits triggers aversive symptoms that make the individual more vulnerable to craving. Neuroadaptations that occur as a result of chronic exposure to substances such as cocaine, alcohol or opioids leads to hypoactivity in the dopaminergic reward system, and this hypoactivity contributes to anhedonia and increased aversive state during withdrawal.
Craving is known to be one of the most important factors in relapse. Substance related cues lead to robust activation of limbic structures through conditioned learning. Neuroimaging studies have revealed that the activation of limbic structures is correlated with the intensity of craving. Cues elicit dopamine release in striatum and triggers the motivation for seeking substance. Prefrontal circuits like orbitofrontal and anterior cingulate cortex are also involved in salience attribution while hippocampus and amygdala mediates conditioned responses. Especially glutamatergic projections from these areas to the striatum and ventral tegmental area modulate the sensitivity to substance related cues.
In an individual with a SUD, chronic substance use or an underlying vulnerability leads to hypofunction in the prefrontal cortex. On the other hand, limbic structures become hyperactive as a result of substance withdrawal or long-term neuroadaptations. The hypofunctional prefrontal cortex, together with the hyperactive limbic system prevents the individual from stopping substance use despite long-term negative consequences.
Current pharmacological treatment options in substance use disorder
Principles of pharmacologic treatment approaches in SUD are fundamentally based on 3 strategies: 1) Blocking the target of the substance, 2) Mimicking the effects of the substance, and 3) Intervening in the process of addiction formation. The aim is to prevent the substance from being clinically effective even if it is used by blocking the receptors targeted by the substances. The best-known example of this strategy is naltrexone used in opioid use disorder (OUD). Vaccines for SUD that are still in the research phase similarly aim to prevent substances from binding and acting on receptors. The aim of mimicking the effects of the substance is to reduce withdrawal and craving symptoms, as with methadone, buprenorphine and nicotine replacement therapy (NRT). Long-acting medications stimulate the brain areas in a sustained manner by avoiding the repeated rapid on-off phases seen in SUDs. Although it is thought that dopamine receptor agonists may be useful in stimulant use disorder, their effectiveness has not been demonstrated. Apart from these, although it is predicted that glutamate receptors or postsynaptic signal proteins may change the addiction process, there is no approved treatment method in this field yet.
Approved Pharmacological Treatments for Opioid Use Disorder
Naltrexone is a mu-opioid receptor antagonist that prevents opioids from binding to the opioid receptors and that blocks the euphoria induced by an opioid agonist. Although the use of naltrexone in opioid use disorder (OUD) seems to be advantageous, it is seen that drug compliance is very poor in clinical practice. To overcome the adherence problem, long-acting injectable and implantable forms have been manufactured. In brain imaging studies, extended-release naltrexone increased functional magnetic resonance imaging (fMRI) response in the precuneus and the medial frontal gyrus, and decreased fMRI response in the left amygdala, right caudate, precentral gyrus and cuneus to substance related cues among patients with OUD. Extended-release naltrexone treatment was reported to be associated with significantly higher rates of abstinence, decreases in cravings, and higher retention rates compared to placebo. On the other hand, naltrexone may interfere with the natural reward system by blocking endogenous opioids, and may cause negative affective states. One obstacle for naltrexone treatment is the fact that the patient must be completely opioid-free before starting the treatment, and the other is the high cost of extended-release naltrexone injection.
Another approach in OUD treatment is to mimic substance effects. The best example of this treatment approach is methadone, a long-acting opioid receptor agonist used in OUD. At the proper dose, patients on methadone have a low level of sustained opioid receptor activation which prevents them from opioid craving, withdrawal and substance related harms. Methadone is one of the most strictly controlled drugs in the world and is not approved for OUD in Turkey. A high affinity partial opioid receptor agonist buprenorphine is another treatment of choice in OUD. As a partial agonist, buprenorphine exerts similar clinical efficacy to full agonists such as morphine or methadone, but it is safer at high doses. Buprenorphine is often formulated in combination with naloxone to prevent intravenous abuse. For buprenorphine or buprenorphine/naloxone treatment, the main issue is the retention of treatment. The rate of retention and quitting the use of illicit drugs were higher in methadone therapy compared to low-dose or flexible-dose buprenorphine treatment. However, fixed, medium, or high buprenorphine doses were equivalent to methadone in terms of rates of retention in treatment and prevention of illicit drug use. Therefore, prescribing sufficient doses of buprenorphine is important, especially at the beginning of treatment. Extended-release formulations of buprenorphine have been developed to improve treatment adherence, ease of use, and reduce abuse rates. Although buprenorphine implants have been shown to be as effective as sublingual buprenorphine, there is still no evidence that any of the extended-release forms are superior to the sublingual form.
Approved Pharmacological Treatments for Alcohol Use Disorder
Naltrexone is an FDA-approved drug for the treatment of OUD as well as Alcohol Use Disorder (AUD). Opioid peptides released by alcohol intake are associated with alcohol-related reward through their activity on mu receptors, and naltrexone blocks this cascade. Naltrexone prevents relapse in AUD by reducing the number of days drinking alcohol, heavy drinking and craving, but its overall effect is moderate. Naltrexone’s effectiveness is influenced by individual characteristics such as being male, having a strong craving for alcohol, having a family history of alcohol use disorder, and an early-onset drinking problem. Nalmefene is another opioid antagonist approved for preventing heavy alcohol intake in Europe. Unlike naltrexone, it is a partial agonist of the kappa opioid receptor and has a longer half-life. Reduction of heavy drinking in patients who cannot achieve complete abstinence reduces alcohol-related mortality and morbidity.
The homo-taurine analog acamprosate is approved for the treatment of AUD but its mechanism of action is not clear. Long-term exposure to alcohol results in an up-regulation of N-methyl D-aspartate (NMDA) receptors and voltage-dependent calcium channels. Withdrawal from alcohol induces an increase in glutamatergic activity and conversely, acamprosate promotes the release of taurine which is one of a major inhibitor neuromodulator/neurotransmitter in the brain. It is also suggested that acamprosate exerts its function by transporting calcium ions to the central nervous system. Thus, each of these changes produced by acamprosate may contribute to the decreased neuronal hyperexcitability. Acamprosate is effective in maintaining abstinence and preventing relapse in AUD, but its effectiveness is low-medium.
Disulfiram is the oldest drug that approved for AUD treatment. Disulfiram is an aldehyde dehydrogenase inhibitor and its mechanism of action is peripheral. It cannot alleviate craving and treatment compliance is low unless administered under supervision. Considering the side effects and risks of disulfiram, it is recommended as a second-line therapy in most AUD treatment guidelines.
Approved Pharmacological Treatments for Tobacco Use Disorder
Another example of mimicry approach is nicotine replacement treatment (NRT) in tobacco use disorder. Nicotine patch, gum, spray or drops are being used to alleviate nicotine withdrawal symptoms and to prevent relapse. Nicotine patch provides a slow release of nicotine for 16-24 hours, and NRT products that are used when needed produce a much slower nicotine increase than cigarettes. A second possible mechanism of action for NRT is positive reinforcement, particularly for arousal and stress-release effects. The degree of positive reinforcement is related to the rapidity of absorption and the peak plasma nicotine level achieved. It is more relevant for short-acting formulations like nicotine gums rather than nicotine patches. A third possible mechanism of action is the desensitization of α4β2 nicotinic receptors that results in a reduced effect of nicotine and decreased satisfaction from smoking. NRT was reported to increase the rate of quitting by 50-60%.
Bupropion is a dopamine noradrenaline reuptake inhibitor and has an antagonistic effect on α4β2 receptors. Bupropion mimics nicotine activity in the brain, increasing noradrenaline and dopamine levels while somewhat blocking nicotine receptor activity, halting the reinforcing properties of smoking. Another FDA-approved treatment option to quit smokingis varenicline. However, varenicline was recalled in 2021 because N-nitroso-varenicline, which has carcinogenic potential, was above the acceptable daily level. It is predicted that drugs containing nitrosamine compounds below the daily acceptable level will be approved for reuse. Varenicline is an α4β2 receptor partial agonist and an α7 agonist, and it is able to reduce withdrawal symptoms and cravings possibly through the elevation of dopamine levels. Because varenicline is a partial agonist, by blocking the effects of nicotine, it can reduce the reinforcing effects of smoking.
New Treatment Strategies in Substance Use Disorder
Research is ongoing to reflect our neurobiological information about addiction into treatment models and to develop new treatment approaches. Some of these studies are conducted to evaluate the effectiveness of drugs approved for other indications in MUD. Because most of the medications used in psychiatry have multi-model activity and because there is an overlap between SUD and other mental illnesses, this strategy may be useful as it was with bupropion. Since the dysfunction of the GABA-ergic system is associated with substance use, a number of studies are also conducted on the effectiveness of antiepileptic drugs. Gabapentin and topiramate are recommended as first and second line treatment options for AUD in some treatment guidelines. There are also preliminary clinical evidence that topiramate reduces cocaine and metamphetamine use. On the other hand, combination therapies may be another option for SUD. It was reported that buprenorphine/naloxone combination decreased heroin use to a greater extent than buprenorphine on its own and similarly, the combination of lofexidine and dronabinol reduced cannabis withdrawal symptoms more than either medication on their own.
Identification of new treatment endpoints, such as a clinically considerable reduction in substance use other than complete abstinence while assessing treatment efficacy may lower the regulatory bar for FDA approval in SUD. In Europe, nalmefene has been approved for preventing heavy drinking in AUD. It has been reported that a decrease in cocaine use also reduces endothelial dysfunction, which is a predictor of heart disease risk, and thus harm reduction in cocaine use may also be accepted as a treatment goal. For OUD, preventing opioid overdoses with extended-release naltrexone may be accepted as a relevant treatment goal to decrease mortality. Another treatment strategy is to target endophenotypes associated with SUD. For example, using cognitive enhancers to improve decision making, planning or impulse control can be a useful strategy. These strategies provide an opportunity for personalizing treatment.
A wide range of studies are ongoing for SUD treatment that aim either main protein targets of specific substances, modulators of the brain reward system or modulators of downstream circuits involved in addiction. Recently identified mu-opioid biased opioid receptor agonists are safer opioid agonists that target the G-coupled protein intracellular pathway associated with analgesia without activating the β-arrestin pathway, which is associated with tolerance and respiratory-depressing effects. Opioid bias agonists may serve as a safer treatment option for pain and may be a novel treatment option for OUD. In addition to biased mu-opioid agonists, another novel pharmacological approach is modulating the reward system with neurokinin 1 receptor agonists. Lofexidine is another treatment option that does not directly involve opioid receptors. It is an antihypertensive with alpha-adrenergic receptor agonist activity and has been shown to relieve opioid withdrawal symptoms.
Oxytocin, a neuropeptide that plays a role in social bounding, learning and memory, also has anxiolytic properties. It reduces immune and inflammatory responses, and it is thought to interfere with the addiction process. Oxytocin projection neurons target the forebrain, limbic and reward systems that are involved with social and substance related rewards. Preclinical studies showed that oxytocin administration reduces drug seeking, self-administration and tolerance for different substances like cocaine, heroin or methamphetamine. Oxytocin is predicted to be a neuropeptide that may restore balance in the central nervous system in people with SUD.
Endocannabinoid system is also involved in reward and addiction and drugs targeting this system may be useful in the treatment of SUD. Studies are ongoing to identify whether cannabinoid agonists/partial agonists can work as a substitution therapy in cannabis use disorder. So far, CB1 agonists such as dronabinol, nabilone, and nabiximols have been showed to reduce cannabis withdrawal symptoms in human studies. CB1 receptor modulation may be still effective for substances whose primary target are not CB1 receptors. For example, CB1 antagonism could potentially alter the reinforcing properties of some substances. Smoking cessation trials have demonstrated encouraging results with rimonabant. However, as rimonabant was associated with negative emotions and suicidal thought, modulation of CB1 and CB2 receptors via allosteric modulators, which enhance or inhibit receptor responses to endocannobinoids while maintaining the main function of the endocannobinoid system may be more promising for SUD treatment. For example, pregnenolone, a negative allosteric CB1 receptor, seems to be protective against intoxicating effects of cannabis.
No treatment has yet been approved for stimulant use disorder. Although positive results were reported in a small number of studies conducted with longer-acting stimulant drugs (methylphenidate or amphetamines) or other drugs with stimulant effects such as modafinil, no significant changes related to addiction were reported in most of them. A different approach for stimulant use disorder is based on N-acetylcysteine (NAC). NAC helps to modulate glutamatergic activity through cysteine-glutamate exchange and thereby stimulating glutamate receptors. NAC seems to be useful for preventing relapse and reducing craving in abstinent cocaine users while ineffective for active users.
Vaccines
Another way of blocking substance effect is the administration of vaccines against substances like cocaine, heroin or nicotine. Vaccines bind to the substances in the blood circulation and prevent them from acting on brain. Addictive drugs are small molecules that are not highly immunogenic and the immune system does not readily produce antibodies directed against these substances. So, generally the addictive substances are conjugated to adenovirus capsid proteins, which are highly immunogenic, to stimulate immune system in vaccine studies. Vaccines for opioids show promise in preclinical studies but nicotine and cocaine vaccines failed to produce sufficient antibody titers in humans. Monoclonal antibodies are a way of passive immunization that can be administered in higher doses than vaccines. Studies are ongoing to develop monoclonal antibodies for nicotine, opioids, methamphetamine and cocaine. Long-lasting monoclonal antibodies can prevent people from substance overdoses or relapses and may be a treatment of choice for appropriate patients in the future.
Neuromodulation
Identification of the neurobiological mechanisms that involve in addiction suggests new therapeutic targets for brain neuromodulation practices. Basic principle of brain stimulation in SUD is to strengthen prefrontal activity to improve self-regulation and to modulate limbic system in order to reduce craving. Although there are promising results with transmagnetic stimulation (TMS) and direct current stimulation (DCS) in SUD, scientific evidence is not relevant enough to recommend brain stimulation as a standard treatment for addiction. Deep brain stimulation (DBS) directly reaches the subcortical structures that are involved in addiction unlike TMS, but it is a disadvantage that it requires a surgical intervention and has more side effects. Although DBS reduced craving and prolonged abstinence in people with SUD in studies, it is still early to recommend it as a treatment option since there are no double-blind controlled studies in this area. The heterogeneity in brain stimulation studies (e.g. different brain regions, different stimulation frequencies and durations) makes it difficult to evaluate their effectiveness in SUD. Percutaneous nerve field stimulation, a specific auricular nerve stimulation method, has been approved by the FDA to reduce opioid withdrawal symptoms. It has been reported that this method can provide or at least facilitate the transition to long-acting naltrexone without the use of buprenorphine. However, it should be noted that the FDA approval is based on a retrospective and uncontrolled study. The brain is not directly stimulated in electroencephalography (EEG) or fMRI-neurofeedback, instead these strategies are based on the training of patients to regulate their own brain activity. Although a few small studies have promising results with EEG-neurofeedback, these treatments are still in their early stages.
CONCLUSION
Although pharmacological treatment methods and treatment approaches such as cognitive behavioral therapy are beneficial in SUD, the efficacy of all these treatments is limited. Research is ongoing to increase treatment compliance with long-acting drug formulations, to develop new pharmacological agents, and to identify the effectiveness of drugs that have indications for other diseases in addiction. However, since addiction is a multifactorial disease with psychological, neurobiological and sociological factors, individualization of treatment, appropriate psychotherapy and pharmacological treatments with different neural targets should be used together for an effective treatment. In addition, adoption of new treatment goals as part of harm reduction strategies rather than complete abstinence in SUD may lead to more diverse treatment options.