Abstract
Addiction, the most severe form of substance use disorder, is a chronic brain disorder molded by strong biosocial factors that has devastating consequences to individuals and to society. Our understanding of substance use disorder has advanced significantly over the last 3 decades in part due to major progress in genetics and neuroscience research and to the development of new technologies, including tools to interrogate molecular changes in specific neuronal populations in animal models of substance use disorder, as well as brain imaging devices to assess brain function and neurochemistry in humans. These advances have illuminated the neurobiological processes through which biological and sociocultural factors contribute to resilience against or vulnerability for drug use and addiction. The delineation of the neurocircuitry disrupted in addiction, which includes circuits that mediate reward and motivation, executive control, and emotional processing, has given us an understanding of the aberrant behaviors displayed by addicted individuals and has provided new targets for treatment. Most prominent are the disruptions of an individual’s ability to prioritize behaviors that result in long-term benefit over those that provide short-term rewards and the increasing difficulty exerting control over these behaviors even when associated with catastrophic consequences. These advances in our understanding of brain development and of the role of genes and environment on brain structure and function have built a foundation on which to develop more effective tools to prevent and treat substance use disorder.
Drug overdoses claimed more than 63,300 American lives in 2016 (1), and excessive alcohol use and tobacco use are estimated to contribute to 88,000 (2) and 480,000 (3) deaths each year, respectively. More than 20 million Americans suffer with substance use disorder, and overdose deaths represent only a fraction of the resultant health consequences. For example, the rate of emergency department visits from opioid-related overdoses or accidents is 20 times greater than the rate of opioid overdose death (4). Of every 1,000 babies born, six have neonatal abstinence syndrome (5) and up to nine have fetal alcohol spectrum disorders (6). Yearly there are approximately 30,500 new cases of hepatitis C (7), a virus transmitted by injection drug use. The combined consequences of drug use and addiction also have enormous economic consequences, which include an estimated cost of $249 billion from alcohol (8), $300 billion from tobacco (3), and $193 billion from other drugs (9). And while research has identified many evidence-based prevention and treatment strategies that could help reduce alcohol use and drug use and their consequences, these interventions are highly underutilized and not effective for everyone.
Our deepening understanding of the neurobiological, genetic, epigenetic, and environmental mechanisms underlying addiction is helping researchers identify new targets for prevention and treatment interventions. In addition, advancing technologies—from gene sequencing and manipulation, to increasingly more sensitive imaging technologies, to brain stimulation devices, to information technologies and mobile health tools—are producing unprecedented capacity to interrogate addiction and its causes. This growing knowledge base offers unique opportunities for translation of substance use disorder prevention and treatment strategies.
Neurobiology Of Addiction Risk
The risk for addiction is related to complex interactions between biological factors (genetics, epigenetics, developmental attributes, neurocircuitry) and environmental factors (social and cultural systems, stress, trauma, exposure to alternative reinforcers). Research has started to uncover how psychological traits, emotions, and behaviors are encoded in the brain; how environmental factors influence brain circuits and subsequent behavior; and how genetic and epigenetic factors influence the development and functioning of the brain, all of which are of relevance to addiction risk and resilience.
Genetic factors account for approximately half of the risk for addiction (10). Thus far, most genes implicated in this risk largely influence an individual’s biological response to substances of abuse or their metabolism (11). Genetic findings have also provided new insight into the neurobiology of addiction. For example, a variant in the gene encoding for the alpha 5 subunit of the nicotinic receptor, which is highly expressed in the habenula, has been consistently associated with higher vulnerability to nicotine addiction (12, 13). This discovery brought attention to the importance of the midbrain habenula-interpeduncular axis in nicotine dependence and withdrawal (14). Further, studies in transgenic mice have documented that the alpha 5 polymorphism influences the firing pattern of pyramidal neurons in the prefrontal cortex in a way that could help explain the greater vulnerability for smoking among patients with schizophrenia (15). Ongoing research aims to identify how genes mediate the development of the human brain and the subsequent sensitivity of the brain to environmental factors that influence the risk for substance use disorder.
Epigenetic factors, which orchestrate gene expression and silencing, have been implicated in the long-lasting neuroplastic changes associated with drug taking in animal models of addiction (16), regulating pathways through which environmental risk factors, such as stress, influence biological drivers of substance use and addiction (17). For example, early life stress can influence the development of the hypothalamic-pituitary-adrenal (HPA) axis, leading to increased reactivity to stress and susceptibility to addiction. Interestingly, preclinical studies have identified transgenerational epigenetic effects of parental drug taking prior to conception on addiction-related behavior in offspring (18, 19), though such transgenerational effects have not been demonstrated yet in humans.
Characterization of human neurodevelopment has allowed us to understand why adolescents are more likely than adults to experiment with drugs and to develop substance use disorders. This understanding in part reflects the fact that the adolescent brain has not completed its development and is more neuroplastic than the adult brain. The human brain continues to develop until the early to mid-20s, and the rate of development differs across neuronal circuits, with development occurring faster for reward/motivation and emotional circuits than for prefrontal top-down control circuits, which are among the last to develop (20). As a result, during adolescence, the striatal reward/motivation and limbic-emotional circuits are hyperactive, leading to greater emotional reactivity and reward-seeking behaviors. Moreover, the prefrontal cortex cannot fully self-regulate, leading to more impulsivity and risk taking (21). Early exposure to drugs of abuse may further impair the development of the prefrontal cortex, increasing the long-term risk for addiction (16). The increased neuroplasticity of the adolescent brain explains why addiction develops faster in an adolescent than in an adult (22), and it also explains the greater sensitivity of adolescents to environmental stimuli, such as stress, that influence drug taking (23, 24). Similarly, studies have started to assess the effects of social stressors on development of the human brain, and these studies are relevant for understanding why social stressors increase the risk for substance use disorder and other mental illnesses. For example, studies evaluating the effects of social deprivation during infancy and early childhood have reported delayed maturation that results in impaired brain connectivity, which could underlie increased impulsivity in these children (25). Importantly, preliminary studies have reported that interventions that provide social support and care may be able to reverse some of these impairments (26).
Multiple psychological traits have also been shown to influence risk for addiction, including impulsivity, novelty and sensation seeking, and stress reactivity (27). Neuroscience has started to delineate the brain circuits that mediate these traits. For example, trait impulsivity is associated with dysregulation of corticostriatal circuits as well as altered activation of, and functional connectivity between, the anterior cingulate cortex and amygdala (28). High sensation seeking has been associated with reduced thickness of the anterior cingulate cortex and middle frontal gyrus (29), as well as altered midbrain volumes (30). Stress reactivity is correlated with prefrontal corticolimbic regulation of HPA axis activity (31). Understanding the neurobiological basis of traits that influence risk for substance use disorder may lead to development of biomarkers that can be used to target prevention interventions for individuals at high risk. Furthermore, this knowledge will facilitate the development of strategies to strengthen specific circuits to improve resilience and support recovery.
Neurobiology Of The Addicted Brain
Brain imaging research has helped map the neuronal circuits that mediate the relapsing pattern of addictive behaviors, including rewarding responses during intoxication, conditioning to the drug and its cues, negative mood and increased stress reactivity during drug withdrawal, and drug craving during exposure to cues or stressors (32).
During intoxication, the drug stimulates large bursts of dopamine in the mesolimbic reward system (the nucleus accumbens and dorsal striatum) that reinforce drug taking (bingeing) (33) and strengthen conditioned associations, which link stimuli that precede drug consumption with the expectation of reward (34). Counterintuitively, in a person suffering from addiction, the drug-induced dopamine increases are attenuated, an effect that has been observed in both human subjects and animal models (35–37). In humans, the attenuated dopamine response to the drug is associated with reduced subjective experience of reward during intoxication (37). While major emphasis has been placed on the dopaminergic system in explaining the rewarding and reinforcing effects of drugs, it is also clear that other neurotransmitters, including opioids, cannabinoids, GABA, and serotonin—to a greater or lesser extent, depending on the pharmacological characteristics of the drug—contribute to the pleasurable or euphorigenic responses to drugs and to the neuroadaptations that result in addiction (38).
As the intoxicating effects of a drug wear off, an addicted individual enters the withdrawal phase, which is associated with negative mood, including anhedonia, increased sensitivity to stress, and significant dysphoria and anxiety. Such a response is not typically observed in an individual with short drug exposure history, and the duration of exposure needed for a response to emerge varies for the different types of drugs, with opioids producing these effects particularly rapidly. The circuits underlying the withdrawal phase comprise basal forebrain areas, including the extended amygdala as well as the habenula, and implicate neurotransmitters and neuropeptides such as corticotropin-releasing factor (CRF), norepinephrine, and dynorphin (39, 40). Increased signaling in these circuits triggers aversive symptoms that render the individual vulnerable to cravings and preoccupation with taking the drug as means to counteract this aversive state. In parallel, the dopamine reward/motivation system is hypofunctional, contributing to anhedonia and the aversive state during withdrawal (41).
During the craving stage, the conditioned stimuli (drug cues) themselves elicit dopamine release in the striatum, triggering the motivation to seek and consume the drug (42). This phase also involves prefrontal circuits, including the orbitofrontal and anterior cingulate cortex, which underlie salience (or value) attribution (43), as well as circuits in the hippocampus and amygdala, which mediate conditioned responses (44). Glutamatergic projections from these regions to the ventral tegmental area and striatum modulate the sensitivity and reactivity to cues and to adverse emotions that trigger the urgent motivation for, and preoccupation with, drug taking (32).
In a brain not affected by addiction, the circuits controlling desire for a drug are held in check by prefrontal cortical regions that underlie executive functions, which support making rational, healthy decisions, and that regulate emotions. Thus, the awareness that a drug will provide an immediate reward is balanced by consideration of long-term goals, and the individual is able to make a reasonable choice and carry it through. However, when the prefrontal cortical circuits underlying executive functions are hypofunctional—as a result of repeated drug exposure or from an underlying vulnerability—and the limbic circuits underlying conditioned responses and stress reactivity are hyperactive—as a result of drug withdrawal and long-term neuroadaptations that downregulate sensitivity to nondrug rewards—the addicted individual is at a tremendous disadvantage in opposing the strong motivation to take the drug. This explains the difficulty addicted individuals face when trying to stop taking drugs even when they experience negative consequences and have become tolerant to the drug’s pleasurable effects.
The delineation of the various brain circuits and neurotransmitters (dopamine, glutamate, dynorphin, enkephalin, GABA, serotonin) that contribute to addiction has helped identify potential targets for addiction treatment (see below). For example, in animal models, interventions to enhance dopamine signaling through D2 receptors, which are downregulated in addiction and are associated with impaired prefrontal activity, reduce compulsive drug taking (41). In addition, interventions that counteract the enhanced reactivity of glutamatergic projections from the prefrontal cortex and amygdala to the ventral tegmental area and striatum have been shown to prevent drug taking following exposures to cues or stressors (45), and those that counteract the negative mood during the withdrawal state (CRF or kappa antagonists) can prevent escalation of drug use (46, 47).
In developing new strategies to prevent and treat substance use disorder, it is also important to recognize the high rate of comorbidity with mental illness (48–50). Patients with these comorbidities often have more severe and treatment-resistant disorders compared with patients who have either disorder alone (49, 51). Many overlapping brain regions and circuits (49, 50, 52, 53)—including those that mediate reward, executive function, and emotions—and neurotransmitter systems—including dopamine (54–56), serotonin (57, 58), glutamate (59, 60), GABA (61), and norepinephrine (56, 62, 63)—have been implicated in substance use disorder and other mental illnesses. There is also overlap in the genetic and environmental risk factors for these disorders. Genes that influence stress reactivity, risk taking, and novelty-seeking behaviors can influence the initiation of substance use as well as the development of substance use disorder and other mental illnesses. And environmental factors such as chronic stress, trauma, and adverse childhood experiences increase risk for both substance use disorder and mental illness (49, 64).
Accelerating Development Of New Prevention Interventions
There are several evidence-based prevention interventions for substance use disorder that have been developed on the basis of epidemiological data identifying factors that increase risk for or provide resilience against substance use disorder (Table 1).
TABLE 1. Strategies for Prevention of Substance Use Disordera
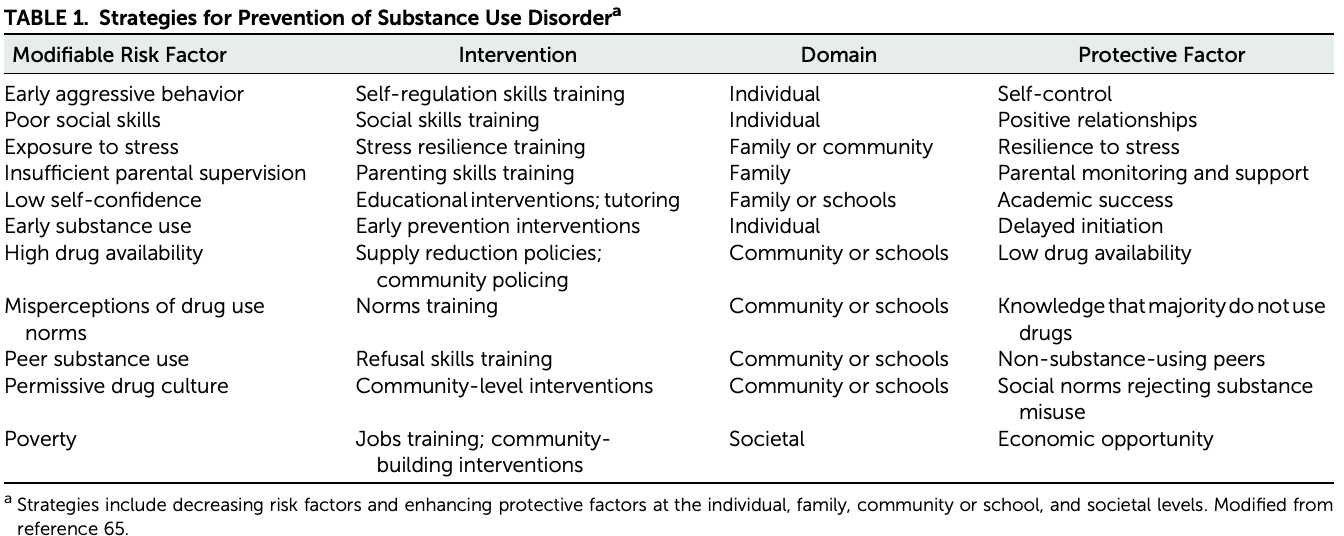
Our increased understanding of the effects of substance use on normal brain development, the deleterious effects of adverse environments, and the role of innate vulnerabilities will allow for the development of personalized intervention to reverse or mitigate some of these deficits. For example, adverse social environments during early childhood can result in delayed prefrontal limbic connectivity (25), which is associated with impulsivity (66). In turn, impulsivity predicts greater vulnerability for substance use disorder (67). However, children can be trained to improve their self-regulation and hence control impulsivity (68). Furthermore, social isolation and exposure to social environments with limited support are associated with reduced dopamine D2 receptor expression in the striatum, which is linked with greater vulnerability for impulsivity and compulsive drug use (69–71). Research is also starting to identify changes in brain development triggered by early exposure to drugs, including alcohol and marijuana (72, 73). Future access to standardized measures of brain development will support the development of early interventions to mitigate developmental vulnerabilities or counteract negative neuroadaptations. In this respect, the recently launched Adolescent Brain Cognitive Development Study, which aims to study 10,000 children with brain imaging, genotyping, and deep phenotyping across the transition from childhood into adulthood, will provide valuable data for determining normal human variability in brain development and how it is disrupted by drug use and mental illnesses (74). Similarly, the Baby Connectome Project, a study of brain development in children from birth through 5 years of age (75), will provide insight into the early development of the human brain at a stage when it is most sensitive to adverse environmental effects, such as neglect and abuse.
Accelerating Development Of New Treatments
The substance use disorder treatment field has seen some important successes, but therapeutic options are still limited. Although the Food and Drug Administration (FDA) has approved medications for the treatment of opioid, alcohol, and tobacco use disorders (Table 2) (77), which represent meaningful advances in therapeutics for addictions, they are not effective for all patients. Similarly, while evidence-based psychosocial treatments (e.g., cognitive-behavioral therapy, contingency management interventions) are available for substance use disorder, their effectiveness is also limited (78).
TABLE 2. Medications for Addiction Treatmenta
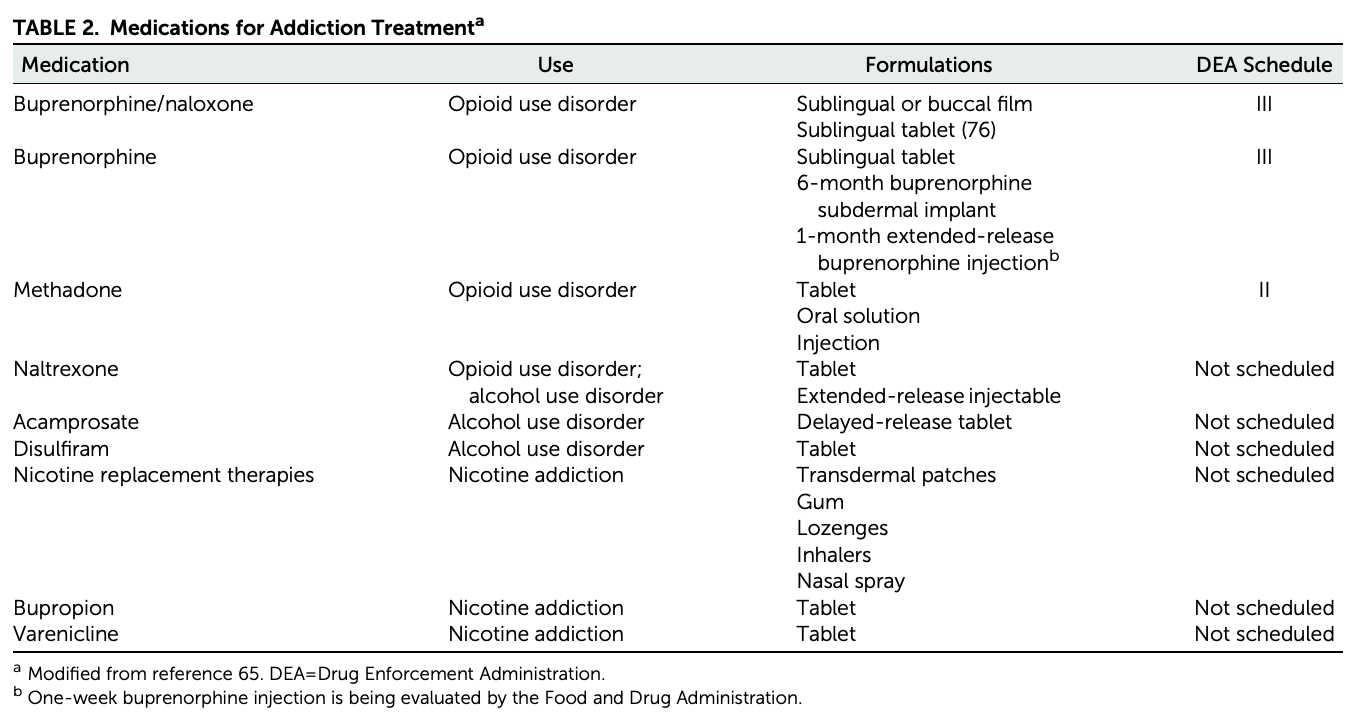
Most pharmaceutical companies have been reticent to invest in addiction due in part to stigma (79) as well as to perceptions that the market is small, that executing clinical trials in patients with substance use disorders is difficult (because of frequent comorbidities, criminal or legal problems, and poor adherence to treatment protocols), and that the regulatory bar required for FDA approval is too high (i.e., abstinence—discussed below [80]). These factors represent a major challenge in medication development. Consequently, the National Institute on Drug Abuse (NIDA) has focused on partnering with industry to encourage drug development by identifying promising targets and funding research to lessen risks associated with drug development. Examples of such partnerships include helping in the development of medications to treat opioid use disorders, such as a buprenorphine/naloxone combination (Suboxone) (81), a 1-month extended-release naltrexone (Vivitrol), a 6-month buprenorphine subdermal implant (Probuphine) (82), and a “user-friendly” intranasal opioid overdose naloxone formulation (Narcan Nasal Spray) (83). The current opioid crisis has further highlighted the urgency for greater participation of industry in medication development (84).
In parallel, research is ongoing to translate basic knowledge about the molecular pathways and brain circuits involved in substance use disorders into new treatments. A promising strategy explores the use of pharmacotherapies that target endophenotypes associated with addiction—for example, using cognitive enhancers to improve impulse control, planning, and decision making (85–87), and using medications to reduce stress reactivity and dysphoria to prevent relapses (88, 89). As discussed above, various neurotransmitter systems are involved in such cognitive and emotional functions and represent potential therapeutic targets.
Another strategy involves “repurposing” medications already approved for other indications. Because there is overlap in the neuropathology and symptomatology between substance use disorder and other mental illnesses (see the section “Neurobiology of the Addicted Brain”), and many of the medications used to treat mental illnesses bind to multiple therapeutic targets (90), some of these may be beneficial for substance use disorders. The medications’ existing safety profiles and pharmacology data shorten the timeline for obtaining FDA approval if the drugs are found effective for treating substance use disorders. A notable example is bupropion, which was originally developed as an antidepressant and was subsequently found to be clinically useful in smoking cessation (91).
Although there is only one approved drug combination for substance use disorders (i.e., the buprenorphine/naloxone combination), the few studies evaluating other drug combinations have shown promising results. For example, the combination of buprenorphine with naltrexone not only decreased cocaine use in individuals with dual cocaine and heroin use disorders (92) but also reduced heroin use to a greater extent than buprenorphine alone (93). Similarly, the combination of lofexidine and dronabinol reduced symptoms of cannabis withdrawal significantly more than either medication alone (94).
Research is also ongoing to define endpoints other than abstinence for measuring treatment efficacy, such as clinically meaningful reductions in drug use associated with improvements in health outcomes (95). Such adjustments could reduce the regulatory bar for obtaining FDA approval for new therapeutics. For example, research has indicated that reduced use of cocaine led to decreased endothelial dysfunction, a marker of heart-disease risk associated with chronic cocaine use (96). Importantly, there is precedent for alternative endpoints: the FDA has approved the use of percentage of subjects with no heavy drinking days as an outcome for alcohol use disorder. This measurement is sensitive for detecting differences between medication and placebo, and allowing some days of consumption increases the effect size (97). In the case of opioid use disorders, a relevant endpoint could be overdose prevention; recently, a study on parolees found reduced overdoses were associated with extended-release naltrexone (98).
Promising Pharmacological Targets
The preclinical literature includes a wide range of promising strategies for substance use disorders that aim either at the main protein target of specific drugs, at modulators of the brain reward system, or at modulators of downstream circuits disrupted in addiction (Figure 1).
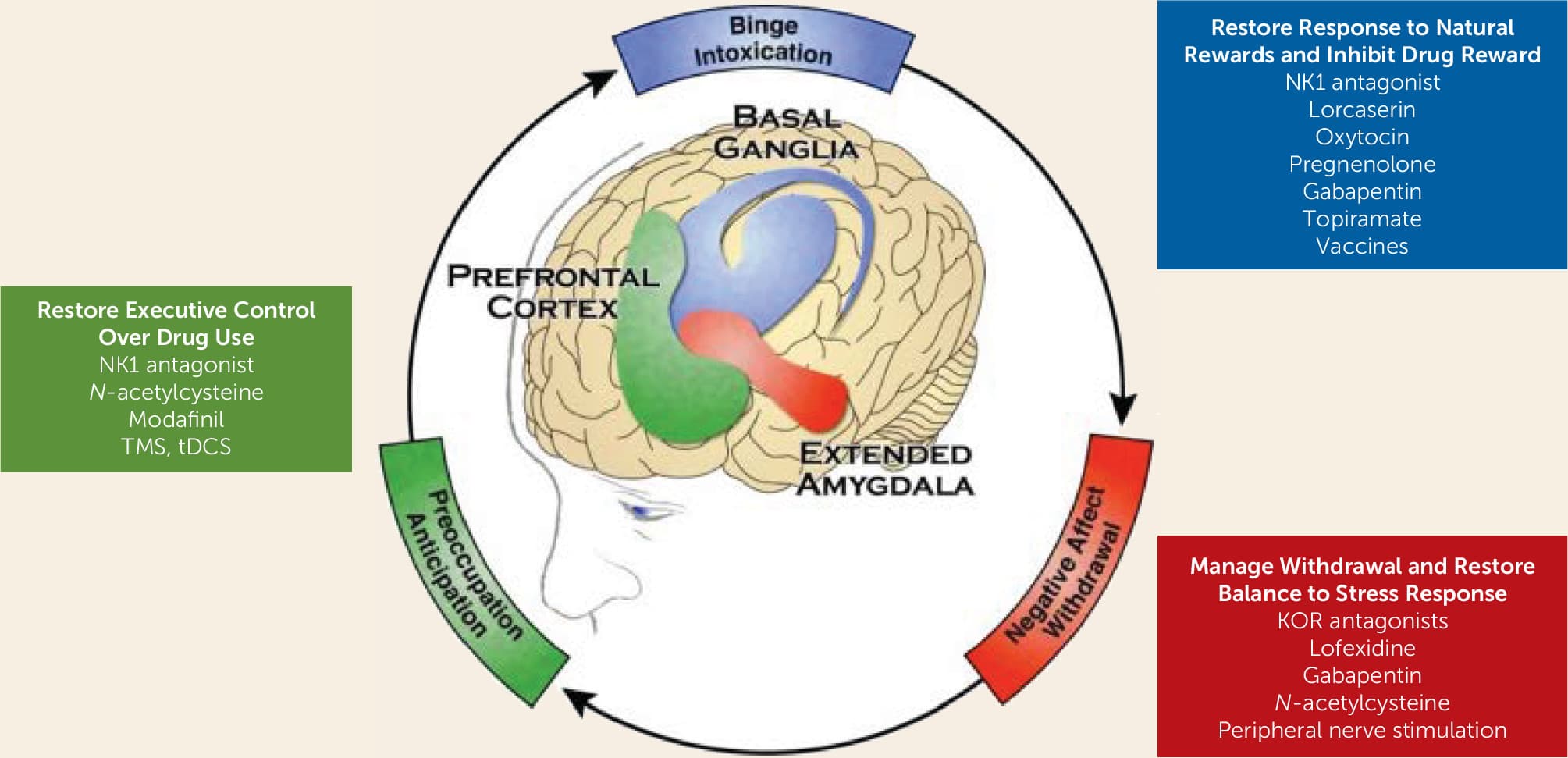
FIGURE 1. The Three Stages of the Addiction Cycle and the Main Brain Regions Implicateda
a Potential medication targets and therapeutic strategies that target each stage are listed. KOR=kappa-opioid receptor; NK1=neurokinin 1; tDCS=transcranial direct current stimulation; TMS=transcranial magnetic stimulation. Modified from reference 65.
Therapeutic strategies that target the mu-opioid receptor have been most effective for opioid use disorders and are being pursued in the development of nonaddictive analgesics, which could help reduce the risk of addiction as an unintended consequence of pain management (84). In this respect, the recent identification of the structure of the mu-opioid receptor has provided novel insights into mechanisms of tolerance and is facilitating development of medications that target specific intracellular signaling pathways of the mu-opioid receptor, referred to as biased agonists. Biased opioid agonists that are developed as analgesics target the G-coupled protein “Gi” intracellular pathway, which is believed to underlie analgesia, while not engaging the β-arrestin pathway, which is associated with tolerance and the respiratory-depressing effects of opioid agonists (99). Phase 3 clinical testing of a mu-opioid receptor biased agonist (TRV130) is under way (100, 101). In addition, the orvinol analogue BU08028, a compound similar to buprenorphine, has been shown to be a safe opioid analgesic without abuse liability in nonhuman primates (102). Research into these and other opioids (103) is poised to lead to improved treatments for opioid use disorders and pain.
Novel pharmacological approaches for treating opioid use disorders that do not involve the mu-opioid receptor include strategies to modulate the reward circuit via antagonism of the neurokinin 1 receptor (104) and to counteract withdrawal via antagonism of the kappa-opioid receptor (89). Lofexidine, an α2A-adrenergic receptor agonist originally developed as an antihypertensive, also decreases opioid withdrawal symptoms and is undergoing NIDA-funded trials (105, 106). Another promising medication is lorcaserin, a selective 5-HT2C receptor agonist already FDA-approved for weight loss that reduces opioid seeking in rodent models (107).
Oxytocin, a neuropeptide known for its role in social bonding, is also of interest for substance use disorder. Oxytocin-expressing neurons project to brain regions implicated in reward (including the ventral tegmental area and nucleus accumbens) (108, 109) and stress (including the amygdala and hippocampus) (108, 109). Preclinical studies have shown that oxytocin decreases self-administration of heroin (110), cocaine (111, 112), methamphetamine (113), and alcohol (114–116) and also alleviates nicotine withdrawal (117). Oxytocin treatment during adolescence also reduced methamphetamine (118) and alcohol (119) seeking in adult rodents. In addition, oxytocin reduced reinstatement of drug seeking in rodents in response to triggers of drug craving for methamphetamine (120–122) and cocaine (112, 123, 124). In humans, intranasal oxytocin reduces cue-induced craving for nicotine (125), stress-induced craving for marijuana (126), and withdrawal and anxiety symptoms.
Cannabinoids may also be useful for treating substance use disorders, and identifying medications that target the endocannabinoid system without producing cognitive impairment and rewarding effects could lead to new treatments for substance use disorders. For example, dronabinol (a synthetic tetrahydrocannabinol [THC] formulation approved for AIDS-related anorexia and chemotherapy-related nausea) reduced withdrawal symptoms associated with cannabis use disorder (127), and nabilone (a synthetic cannabinoid similar to THC) reduced cannabis withdrawal- and relapse-related measures in human laboratory studies (128). In addition, a study of nabiximol (an oral mucosal spray containing a THC-to-cannabidiol [CBD] ratio of 1:1) found reductions in severity and duration of cannabis withdrawal and increased retention in treatment (129). Finally, an ongoing randomized clinical trial will evaluate whether CBD (Epidiolex), when added to medical management, can improve treatment outcomes for cannabis use disorders (130).
The body’s endogenous cannabinoids (anandamide and 2-arachidonoylglycerol, or 2-AG, which interact with cannabinoid receptors CB1R and CB2R) optimize the inhibitory and excitatory balance in the brain in a state-dependent manner (131), so side effects might occur with orthosteric ligands that either activate (e.g., dronabinol) or block (e.g., rimonabant) CB1R broadly. For example, rimonabant, which showed efficacy in treating obesity and inhibiting the rewarding effects of cannabis, was also associated with negative affect and suicidal ideation (132). Instead, the manipulation of cannabinoid receptors (CB1R or CB2R) via allosteric modulators, which simply enhance or inhibit receptor responses to endocannabinoids, maintaining the state dependence of the endocannabinoid system, may be more promising for medication development. Although the development of CB1R allosteric modulator medications is in its infancy (133), the negative allosteric modulator pregnenolone appears to protect against the intoxicating effects of THC (134) and is being evaluated for the treatment of cannabis use disorder. In addition, the positive allosteric CB1R modulator ZCZ011 has antinociceptive effects without being reinforcing (135) and thus holds potential as a nonaddictive analgesic.
Other endocannabinoid system modulators being evaluated for cannabis use disorder include specific inhibitors of fatty acid amide hydrolase or of monoglycerol lipase, which slow the breakdown of endocannabinoids, as these compounds may reduce withdrawal symptoms and/or may lead to new nonaddictive analgesics (136, 137).
Stimulant use disorders have been among the most challenging for therapeutics development. The most investigated strategy has been the use of longer-acting stimulant medications (e.g., methylphenidate and amphetamines) for cocaine addiction. Many of these studies have failed to reach significant positive outcomes, except in individuals with comorbid attention deficit hyperactivity disorder. However, a meta-analysis reported evidence of mild benefit for the use of amphetamines in cocaine addiction (138). Similarly, modafinil, a medication used for narcolepsy that has mild stimulant properties (139), has been shown by some studies to be beneficial for the treatment of cocaine addiction (140), though not by others (141). A different approach, based on studies documenting that enhanced glutamatergic signaling from limbic and ventral prefrontal regions can drive cue-induced cravings and relapse (142), targets medications that can help restore balance to these glutamatergic projections. For example, N-acetylcysteine, which helps modulate glutamate signals by activating the cystine-glutamate exchange and thereby stimulating extrasynaptic metabotropic glutamate receptors (143), decreases cocaine seeking in animal models (144). N-acetylcysteine is well tolerated in cocaine-dependent individuals and may reduce cocaine-related withdrawal symptoms and craving (145). A clinical trial did not find N-acetylcysteine to be effective for patients actively using cocaine; however, N-acetylcysteine reduced cravings and prevented relapse in patients who had achieved abstinence (146). The efficacy of N-acetylcysteine is limited by low bioavailability and poor permeability of the blood-brain barrier. Similar compounds with greater potency and bioavailability, such as N-acetylcysteine amide, may prove more effective (147).
Strategies to deliver degradative enzymes have also been proposed for cocaine use disorders, and research is ongoing to develop stable, long-lasting forms of cocaine-degrading enzymes, including cocaine esterase, cocaine hydrolase (148–151), and butyrylcholinesterase (152). Preclinical evidence has shown that these fusion proteins increase cocaine’s metabolism (153, 154). However, clinical studies have failed to show efficacy (152).
Antiepileptic drugs have also been evaluated in the management of substance use disorder. A proposed mechanism for their benefit is that in cocaine use disorders, and in other substance use disorders, dysfunction of GABA-ergic signaling contributes to drug taking (155, 156). Gabapentin, a widely prescribed anticonvulsant and pain medication, has shown some benefits in the treatment of alcohol use disorders (157) and might reduce cannabis use and withdrawal symptoms in cannabis use disorders. Topiramate is an antiepileptic medication (158) for which there is preliminary clinical evidence of reduced cocaine use and improved outcomes in cocaine-dependent individuals (159). In methamphetamine users, topiramate reduced the amount of drug taken by patients with an active use disorder and reduced relapse rates among those in recovery (160).
Promising Nonpharmacological Therapies
Alternative nonpharmacological strategies include vaccines and other biologics (i.e., monoclonal antibodies and gene-delivery strategies); neural stimulation technologies, such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), deep-brain stimulation, and peripheral stimulation devices; and behavioral interventions.
Biological therapeutics.
Vaccines and passive immunization with antibodies work by binding to the drug in the blood and preventing it from entering the brain. Preclinical studies have shown encouraging results for vaccines against prescription opioids, heroin, and fentanyl, inducing high-titer antibody responses to opioids (161–163). Clinical studies both with cocaine and nicotine vaccines have resulted in insufficient antibody titers in humans, and further work is needed (164–166).
A similar approach involves passive immunization, or treating patients with monoclonal antibodies. Monoclonal antibodies can be delivered in high concentrations, and the dosing can be more precisely controlled than with vaccines. An anti-methamphetamine monoclonal antibody (ch-mAb7F9) was found to be safe and well tolerated in phase 1 trials (167). Research is ongoing to develop monoclonal antibodies for fentanyl, nicotine, and cocaine (168, 169). Production of long-lasting monoclonal or polyclonal antibodies against very potent synthetic opioids (e.g., fentanyl and its analogs) could be beneficial to prevent relapse and help prevent overdoses, which are currently driving increases in fatalities in the United States (1, 170).
Brain and peripheral stimulation therapeutics.
The identification of the neuronal circuits affected in addiction can also suggest therapeutic targets for brain stimulation strategies, such as TMS and tDCS. Examples include the strengthening of prefrontal activity to improve self-regulation or the modulation of limbic pathways to reduce incentive salience and cravings.
TMS uses repetitive pulses of a magnetic field, and tDCS uses weak electrical currents through electrodes placed on the scalp to modulate the activity of targeted brain regions. Preliminary clinical trials have reported positive results with TMS for the treatment of nicotine, alcohol, and cocaine use disorders and with tDCS for nicotine, alcohol, cocaine, methamphetamine, opioid, and cannabis use disorders, with reductions in cravings, use, or both reported (171–173). Most studies have targeted the dorsolateral prefrontal cortex, although some studies have targeted the anterior cingulate cortex, the insular cortex, the frontal-parietal-temporal area, and the ventromedial prefrontal cortex. The small sample sizes, short durations, and heterogeneity in technical protocols (e.g., frequency, duration, hemisphere targeted) limit the conclusions that can be drawn. However, existing research suggests these technologies have significant potential for the treatment of addiction.
Other nonpharmacological strategies include peripheral nerve stimulation and neurofeedback strategies. An example of a peripheral nerve stimulator is the BRIDGE, an FDA-approved device for the treatment of pain, which is being explored as a treatment to reduce opioid withdrawal symptoms and facilitate induction on opioid use disorder medications (174). Neurofeedback strategies train patients to regulate their own brain activity using real-time feedback from functional magnetic resonance imaging or EEG. These strategies can be used in combination with behavioral interventions to help improve executive function in addicted individuals (175). A few small studies have found positive results with EEG neurofeedback in individuals with alcohol and cocaine use disorder (176, 177). Although these treatments are still in their infancy, these studies are promising and may lead to treatment advances.
Behavioral therapies.
There is also a significant body of research describing the efficacy of behavioral interventions for substance use disorder. Currently, these represent the only interventions available to treat stimulant, cannabis, and hallucinogen use disorders. Understanding the neurobiological mechanisms that underlie their efficacy is important for guiding the refinement of behavioral treatment strategies.
The Promise Of Basic Research
Basic research is the foundation for future advancements in addiction prevention and treatment. A sustained focus in this area will help researchers define the pathways from gene variation to molecular profile, neuron function, brain-circuit activity, and ultimately to disordered behavior, revealing new targets for prevention and treatment interventions.
While research in animal models has contributed to the development of medications for alcohol, opioid, and tobacco use disorders, it often fails to predict efficacy in clinical trials. This may reflect the reliance on abstinence as the primary endpoint in clinical trials for substance use disorder. The use of alternative outcomes may lead to greater correlation of findings. In parallel, researchers are incorporating more complex social environments into experiments testing medications in animal models that might increase their predictive validity.
The prevention and treatment of substance use disorders would also benefit from biomarkers to help classify individuals into biologically based categories that are reproducible and have predictive validity (178). Biomarkers for the detection of drug exposures in body fluids are valuable but can be used to corroborate only acute or relatively recent drug use. Thus, research is needed to develop and validate biomarkers that reflect chronic drug exposure and that predict disease trajectories and treatment responses. Advances in genetic, epigenetic, and brain imaging tools and technologies offer unprecedented opportunities for the development of such biomarkers.
The same neuroimaging tools that have expanded our understanding of the structural and functional deficits underlying addiction may one day be deployed to monitor, optimize, and personalize addiction treatment (175). An individual’s environment, experience, and biology combine to determine his or her risk for developing a substance use disorder, the trajectory the substance use disorder will take, and the interventions that will be most effective for treating it. Large, national investments in basic research, including the Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative (179), the Adolescent Brain Cognitive Development study (74), and the Precision Medicine Initiative (180), a prospective study that aims to genotype and phenotype one million Americans, are poised to bridge the gap between neuroscience, genetics, behavioral research, and personalized interventions for the prevention and treatment of substance use disorder.
Conclusions
Scientific advances have revolutionized our understanding of the biological and psychosocial drivers of addiction. There is tremendous potential to translate this vast knowledge base into meaningful advances in the prevention and treatment of substance use disorder that will benefit not only addiction medicine but the multiplicity of health conditions that are triggered or exacerbated by drug use (181).