Abstract
Addiction is a chronic relapsing disorder with devastating personal, societal, and economic consequences. In humans, early-life adversity (ELA) such as trauma, neglect, and resource scarcity are linked with increased risk of later-life addiction, but the brain mechanisms underlying this link are still poorly understood. Here, we focus on data from rodent models of ELA and addiction, in which causal effects of ELA on later-life responses to drugs and the neurodevelopmental mechanisms by which ELA increases vulnerability to addiction can be determined. We first summarize evidence for a link between ELA and addiction in humans, then describe how ELA is commonly modeled in rodents. Since addiction is a heterogeneous disease with many individually varying behavioral aspects that may be impacted by ELA, we next discuss common rodent assays of addiction-like behaviors. We then summarize the specific addiction-relevant behavioral phenotypes caused by ELA in male and female rodents and discuss some of the underlying changes in brain reward and stress circuits that are likely responsible. By better understanding the behavioral and neural mechanisms by which ELA promotes addiction vulnerability, we hope to facilitate development of new approaches for preventing or treating addiction in those with a history of ELA.
Substance use disorder (SUD) is characterized by loss of control over increasingly harmful substance use, often leading to physical dependence, as well as by persistent drug cravings and risk of relapse, which can last for years (Hasin et al., 2013). Addictive drugs are thought to hijack brain reward circuits that normally mediate seeking of and pleasure from natural rewards (Nesse & Berridge, 1997), thereby eliciting subjectively pleasurable experiences and continued recreational use (i.e. positive reinforcement) in some individuals. In others, initial drug use may instead result in relief of an underlying negative affective state (i.e. negative reinforcement), a process that is further exacerbated by subsequent escalating drug use and the affective dysregulation it causes (Koob & Le Moal, 1997). Moreover, continued chronic drug use may also promote excessive learning or inflexible drug habits (Berke & Hyman, 2000; Everitt & Wolf, 2002). The relative roles of positive or negative reinforcement to an individual’s drug use likely differ based on the abused drug of choice, specific drug availability, as well as one’s sex and heritable or environmental risk or resilience factors. In other words, there is more than one way to be at risk for addiction, and more than one manifestation of the disorder once it emerges (Fig. 1). Understanding how these complex factors interact to put individuals at risk of SUD and the contribution of ELA to these factors and mechanisms is essential for developing new ways to treat and prevent SUD.
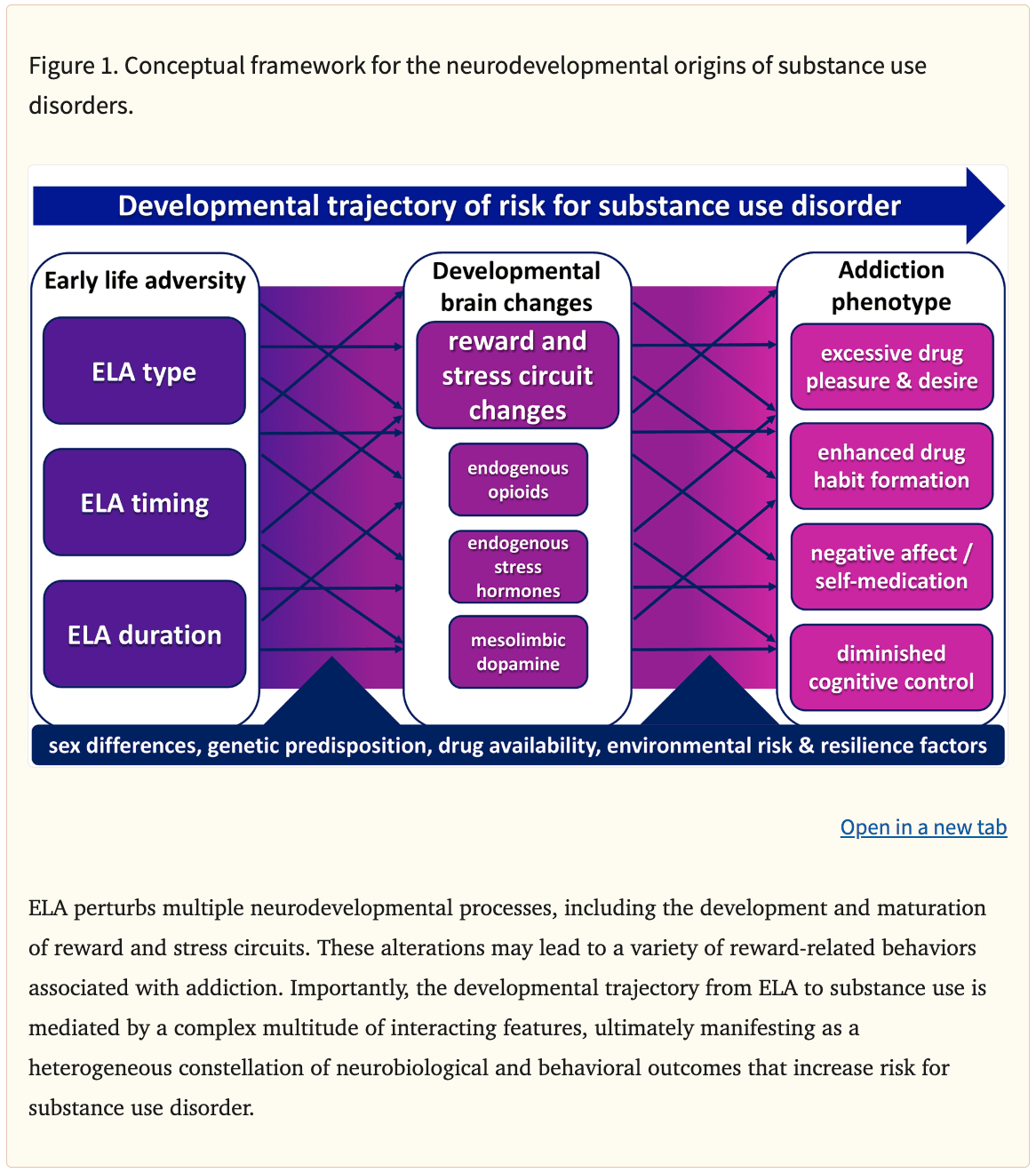
Many factors contribute to the risk for developing SUD, including developmental experiences such as stress or insecure social relationships, drug availability, genetic predisposition and biological sex differences (Schuckit, 2002; Dube et al., 2003; Sinha, 2008; Volkow et al., 2011; Kreek et al., 2012; Wright et al., 2014; Becker & Chartoff, 2019; Crist et al., 2019; Jiang et al., 2019). Moreover, these factors may also interact in important ways. Here we will focus on ELA, an important environmental risk factor for SUD. We will describe the association of ELA with addiction in humans, then concentrate on preclinical research showing long-lasting, causal effects of ELA on addiction-related behaviors and aim to elucidate the brain mechanisms that may be involved.
Association of ELA with Addiction in Humans
ELA related to poverty, trauma and chaotic environment affects over 30% of children in the U.S. (American Psychological Association, 2018). When adversity occurs during critical neurodevelopmental stages, it can impact cognitive and emotional processing long into adulthood (Callaghan & Tottenham, 2016; Chen & Baram, 2016; Short & Baram, 2019). A classic psychological mechanism by which this occurs involves potential disruption of the attachment of infants to their primary caregivers (Ainsworth, 1969; Bowlby, 1974; 2008); such disruption may have long-lasting effects on social and emotional development. From a neurobiological perspective, ELA perturbs numerous neurodevelopmental processes, including the development and maturation of brain circuits involved in cognition and emotion. In this review, we consider how adverse sensory signals from the environment (i.e., early life experiences), especially during critical developmental periods, can disturb synaptic strengthening or pruning in reward and stress circuits (Korosi et al., 2010; Singh-Taylor et al., 2017; Bolton et al., 2018a; Granger et al., 2020). By impacting the maturation of brain circuits, adverse early-life experiences lead to long-lasting changes in the function of these circuits, potentially impacting vulnerability to the addictive effects of drugs.
Indeed, adverse early life experiences are robustly associated with later-life substance addiction in humans (Nurco et al., 1996; Simpson & Miller, 2002; Dube et al., 2003; Widom et al., 2006; Gershon et al., 2008; Sinha, 2008; Enoch, 2011; Shand et al., 2011; Stein et al., 2017; Marsh et al., 2018; Levis et al., 2021). The landmark Adverse Childhood Experiences study found that in addition to increasing the likelihood of early initiation of drug use (Dube et al., 2003), a risk factor for addiction in itself (Grant & Dawson, 1997; McCabe et al., 2007; Chen et al., 2009), ELA increases risk for smoking up to 2-fold, alcoholism up to 7-fold, injected drug use up to 11-fold, and other illicit drug use up to 4-fold (Anda et al., 2006). Yet, ELA does not inevitably lead to substance use disorder in all individuals. Reasons for this may be that specific long-term outcomes of ELA vary based on type of adversity experienced (Sheridan & McLaughlin, 2014; Dennison et al., 2019), the age of exposure to adversity (Luby et al., 2020), individual variability in traits associated with resilience to stress (Fergusson & Horwood, 2003; Hartmann & Schmidt, 2020; Zinn et al., 2020; Méndez Leal & Silvers, 2021), and societal factors such as the availability of specific drugs (Wright et al., 2014) or access to supportive interpersonal relationships and community resources (Daskalakis et al., 2013; Gartland et al., 2019; Liu et al., 2020). Additionally, sex and gender differences may play a role in these outcomes. For example, in women, a history of neglect predicts a higher probability of opioid dependence, while dependence in men is instead better predicted by acute traumatic experiences and concomitant post-traumatic stress symptoms (Shand et al., 2011). In fact, women with a history of ELA appear to be particularly predisposed to substance use disorders relative to men and to individuals with no history of ELA (Widom et al., 1995; Najavits et al., 1997; Hyman et al., 2006; Gershon et al., 2008; Hyman et al., 2008; Lansford et al., 2010; Shand et al., 2011; Marsh et al., 2018; Peltier et al., 2019; Capusan et al., 2021). Notably, this sex-dependent relationship may be partially explained by the fact that girls and boys tend to be exposed to different types of adversities (Short & Baram, 2019; Haahr-Pedersen et al., 2020).
Although clinical studies strongly support an association of ELA with later-life SUD, it is challenging to establish causality in human studies. Therefore, animal models are essential for parsing the mechanisms by which ELA impacts neurodevelopment and characterizing the resulting differences in behavioral responses to drugs of abuse. In the following sections, we 1) describe two of the most commonly used rodent models of ELA, 2) overview common rodent tests used to model addiction-relevant behavioral processes, 3) describe how rodent ELA models impact addiction-relevant behaviors, and 4) discuss known effects of ELA upon brain reward and stress circuit development in rodents which may underlie this association.
Animal Models of Early Life Adversity
Several animal models have been developed to study the effects of ELA on brain development and behavior in rodents as well as nonhuman primates [For comprehensive reviews of these models see (Molet et al., 2014; Nishi et al., 2014; Doherty et al., 2017; Walker et al., 2017; Wakeford et al., 2018; Brenhouse & Bath, 2019)]. Here, we focus on two of the most commonly used rodent models; maternal separation (MS) and limited bedding and nesting (LBN). Notably, as in humans (Shand et al., 2011; Sheridan & McLaughlin, 2014; Strathearn et al., 2020), the outcomes of ELA in rodents varies based on such factors as the type and timing of adversity, as well as on the animal species and strain used, the outcome measures assayed, sex, and other factors (van Oers et al., 1998; Pryce & Feldon, 2003; Moffett et al., 2007; Schmidt et al., 2011; Kundakovic et al., 2013; Andersen, 2015; Di Segni et al., 2018; Bonapersona et al., 2019; Brenhouse & Bath, 2019; Walters & Kosten, 2019; Bath, 2020; Demaestri et al., 2020; Lundberg et al., 2020). It is important to be aware of this diversity and embrace the notion that different rodent models of ELA may lead to different neurodevelopmental changes and ultimately to distinct addiction-relevant phenotypes.
A number of studies examining the effects of ELA on reward-seeking behavior employ a version of the maternal separation (MS) procedure, first introduced by Seymore Levine (1957). Pups are separated from their mother daily during the first 1–2 weeks of life, for a period of time ranging between 15 minutes and 24 hours. This causes an acute, predictable daily stressor accompanied by transient corticosterone elevations during the period of separation (McCormick et al., 1998). Notably, the duration of separation period itself (minutes vs. hours) and resulting impact on maternal behavior is a crucial variable that determines the nature of long-term outcomes (Fenoglio et al., 2006; Korosi et al., 2010; Tractenberg et al., 2016; Orso et al., 2019). For example, following 15 minutes of separation, pups typically receive augmented care when returned to their mother (Pryce et al., 2001; Orso et al., 2019). Some have found that this augmented care following 15-minute separations (“handling”) promotes resilience and improve long-term outcomes (Levine, 1957; Korosi & Baram, 2009), whereas daily MS of three hours, the most common approach, tends to lead to more detrimental outcomes (Tractenberg et al., 2016; Bonapersona et al., 2019). However, some apparent contradictions in the literature exist, and others have observed that brief (minutes) or long (hours) maternal separation can in some cases result in reward-related outcomes that are similar to non-handled controls (Meaney et al., 2002; Schmidt et al., 2011; Nylander & Roman, 2013; Bian et al., 2015). These apparent inconsistencies highlight the complexities of the model, as well as the need for appropriate experimental controls (e.g., handled vs. non-handled conditions). In addition, the age of separation critically influences the outcomes of MS (van Oers et al., 1998; Peña et al., 2019). Relatedly, sex mediates MS effects on neurodevelopment in a manner that is still incompletely understood, but which may involve sex differences in neurodevelopmental sensitive periods, hormonal interactions, and other factors (Flagel et al., 2003; Bath, 2020).
A more recently developed model of ELA involves simulating chronic resource poverty by limiting bedding and nesting materials (LBN) from a postpartum rodent dam and her litter. This procedure has been adopted widely in rats and mice (Gilles et al., 1996; Wang et al., 2012; Molet et al., 2014; Bath et al., 2017; Walker et al., 2017) in original or modified formats (Walker et al., 2017; Opendak et al., 2019). In this model, most of the bedding and nesting materials are removed from the home cage environment, causing in the mother a mild, chronic stress that leads to unpredictable and fragmented maternal care (Ivy et al., 2008; Rice et al., 2008; Molet et al., 2014) and a transient increase in basal corticosterone levels in both dam and pups (Brunson et al., 2005; Ivy et al., 2008). Importantly, the total quantity of care received by pups is unaffected by LBN (Molet et al., 2016a). Instead, the quality of care is disrupted by LBN, such that dams more frequently and unpredictably switch between care elements (licking, feeding, etc). This chaotic patterning of care leads to pronounced long-term cognitive and affective deficits in rodents (Ivy et al., 2010; Molet et al., 2016a; Bolton et al., 2017; Short & Baram, 2019). Notably, unpredictable parental care is also a strong predictor of negative cognitive and emotional outcomes in humans and nonhuman primates (Rosenblum & Paully, 1987; Coplan et al., 1996; Davis et al., 2017; Wakeford et al., 2018; Davis et al., 2019; Glynn & Baram, 2019).
In both MS and LBN models of ELA, the developmental stage(s) at which adversity occurs is important for determining the long-term behavioral and neural outcomes. In rodents, early postnatal life, a period roughly analogous to the first year of life in humans (Avishai-Eliner et al., 2002; Birnie et al., 2020), seems to be a sensitive period for long-term negative outcomes of ELA. This may be because this period is especially important for organizing brain reward and stress circuits (Molet et al., 2014; Birnie et al., 2020; Luby et al., 2020), leaving them susceptible to perturbation by ELA. When adverse events or chaotic parental care occur during this sensitive period, circuits are impacted in a manner that may be irreversible once the sensitive window is closed, analogous to how sensory systems are shaped by appropriate environmental stimuli occurring at the necessary developmental stage (Hubel et al., 1977; Zhang et al., 2001; Hensch, 2004; Li et al., 2006; Espinosa & Stryker, 2012). Just as sensory systems require regular inputs at specific times during development to mature properly, these reward and stress circuits may be similarly “tuned” by factors like acute stressors or the predictability of parental care patterning (Hane & Fox, 2016; Davis et al., 2017; Andersen, 2018; Glynn & Baram, 2019), thereby permanently impacting reward and stress circuit function (Baram et al., 2012; Glynn & Baram, 2019; Birnie et al., 2020; Luby et al., 2020). Of note, the ability of brains to postnatally “tune” development of survival-critical reward and stress circuits may be an adaptive feature. By responding to environmental signals conveying information about the safety or predictability of the world in which one is born, circuits may develop in a manner that could promote survival and evolutionary fitness (Schmidt, 2011). Yet in our modern world, circuits developing under adversity seem too often to lead to unwanted adverse outcomes, such as vulnerability to SUD.
Modeling Behavioral Aspects of Addiction in Rodents
In order to understand how ELA may cause susceptibility to addiction-like outcomes later in life, it is essential to be precise about what is meant by “addiction-relevant behavior.” Drug addiction is a chronic, relapsing disorder characterized a heterogeneous set of maladaptive drug-seeking behaviors. It has been conceptualized as a “downward spiral” beginning with cycles of binging and intoxication motivated by positive reinforcement from pleasurable drug effects or by negative reinforcement due to drug-induced relief of negative affective states. Subsequently, drug abuse can transition into uncontrolled use, when discontinuation of use results in highly aversive withdrawal symptoms, drug cravings, persistent and invasive thoughts about drugs, and impaired cognitive control that can lead to relapse (Koob & Le Moal, 1997). However, trajectories through these stages are not uniform; individuals with SUD may present with different combinations of symptoms or reasons for seeking treatment, and relapse may be triggered by a variety of emotional, physiological, and environmental factors. Consideration of these potential individual differences will be important for understanding the neural mechanisms underlying the various factors associated with risk for SUD, as well as for developing effective prevention and treatment strategies. Accordingly, when using animal models to investigate the brain mechanisms by which ELA leads to SUD vulnerability, it is essential to consider the specific addiction-related behavioral processes being modeled.
The initial phases of SUD typically involve acute, repeated, pleasurable intoxication that is liable to be repeated (i.e. it is positively reinforcing). These reinforcing drug effects, as well as their potential alterations by ELA, can be assessed through several different behavioral measures. For example, conditioned place preference (CPP) models measure an animal’s ability to associate the pleasurable effects of a drug with a specific place, which can be recalled later in a drug-free state, causing the animal to return to that place. The reinforcing (or rewarding) effects of addictive drugs might also be inferred by measuring effects of acute drug exposure on intracranial self-stimulation (ICSS), or operant responding by an animal to receive increasingly intense patterns of rewarding electrical brain stimulation (Olds & Milner, 1954; Carlezon & Chartoff, 2007). Abused drugs tend to reduce ICSS threshold, which has been interpreted to result from the pleasurable effects of the drug substituting for pleasure derived from intracranial stimulation (Negus & Miller, 2014), though this interpretation has been questioned (Smith et al., 2010). Another behavioral model that may measure the addiction-promoting effects of drugs is locomotor sensitization, or an increase in the locomotor-activating effects of abused drugs after repeated experimenter administration. In addiction, desire for drugs increases markedly with repeated use (sometimes called incentive sensitization). Therefore, locomotor sensitization has been interpreted as a proxy for incentive motivational processes that fuel addiction-like drug seeking behaviors (Robinson & Berridge, 2008). In this manner, locomotor sensitization may model the excessive motivation to take drugs that characterizes addiction. ELA may impact any or all of these behavioral responses to experimenter-administered drugs, each of which may rely on distinct underlying brain circuits.
The aforementioned models involve non-voluntary administration of drug to experimental animals; yet, drug effects on the brain and behavior in both humans and rodents differ markedly based on whether they are experimenter- or self-administered (Robinson et al., 2002; Jacobs et al., 2003; Steketee & Kalivas, 2011). Researchers have therefore created models in rodents which measure voluntary drug use, for example via oral ingestion or intravenous self-administration. Voluntary consumption approaches can be used to model recreational drug-taking over short periods of time or escalating and compulsive use over more extended access periods (Markou et al., 1993; Ahmed et al., 2000; Ward et al., 2006; Rogers et al., 2008). ELA may therefore impact initial acquisition of drug taking, short-term “recreational” drug use, or escalation of use over extended periods, each implying impact on distinct neural substrates.
Drug self-administration models can also be adapted to measure several distinct types of drug intake, such as highly motivated, effortful drug seeking as opposed to drug taking under free access conditions. This is important, because these types of drug taking behaviors have different underlying neural mechanisms (Berridge & Robinson, 2003; Di Ciano & Everitt, 2005; Baldo & Kelley, 2007; Bentzley et al., 2013; Salamone et al., 2016; Volkow et al., 2017) that may be differentially affected by ELA. Analyses of high- versus low-effort drug seeking can capitalize on behavioral economic theory, which stipulates that consumption of any commodity is sensitive to increasing price, and that some commodities are more sensitive to price than others. This concept is referred to as “demand elasticity” (Hursh, 1980). Inelastic demand, or relative insensitivity to price, is a feature of SUD, in that addiction can be characterized as an excessively inelastic demand for a drug (Bickel et al., 2014). In other words, addicted individuals will pay higher prices (financial or in life consequences) for drugs than will non-addicted individuals. Importantly, demand elasticity for drugs is distinct from preferred drug intake when the drug is free or cheap, in which case consumption is governed instead by “hedonic setpoint” (Hursh & Silberberg, 2008; Bickel et al., 2014; Strickland et al., 2019). In rodents, demand elasticity and hedonic setpoint for abused drugs can be modeled by examining intake at different “prices,” operationalized as the amount of effort required to receive a unit of drug (Hursh & Silberberg, 2008; Oleson & Roberts, 2009). Recently, a version of this protocol was developed in which both demand elasticity and hedonic setpoint can be determined in a single ~2hr test session (Oleson & Roberts, 2008; Bentzley et al., 2013; Bentzley et al., 2014; Levis et al., 2019; Newman & Ferrario, 2020). Notably, the neural substrates of demand elasticity and hedonic setpoint for abused drugs including cocaine and opioids are distinct (Bentzley & Aston-Jones, 2015; Bolton et al., 2018b; Mahler et al., 2018; Salamone et al., 2018; Levis et al., 2019), meaning that ELA could alter one or both of these processes, leading to distinct addiction-relevant behavioral phenotypes.
Negative reinforcement, or reinforcement motivated by elimination of an unpleasant stimulus, is often endorsed by individuals with SUD, and likely contributes to addiction in several ways. One of these serves as the basis for “self-medication” theories of substance use, in which drugs are used to relieve pre-existing negative affective states. Self-medication likely plays a major role in addiction for some individuals (Khantzian, 1987; Markou et al., 1998), especially for pain-relieving or anxiolytic drugs like opioids or alcohol. Use of drugs to relieve negative states can be measured in animals, for example by examining how pain impacts seeking of analgesic opioid drugs (Martin & Ewan, 2008; Evans & Cahill, 2016). When drug use becomes chronic and escalating, negative reinforcement also underlies continued use in order to reverse withdrawal-induced sickness and negative affect. In rodents, somatic symptoms of acutely aversive withdrawal such as piloerection, “wet dog” shakes, and rapid weight loss can be measured (Gellert & Holtzman, 1978; Hildebrand et al., 1997; Becker, 2000), as can acute or persistent affective dysregulation occurring after cessation of drug exposure (Malin et al., 2000; Malin & Goyarzu, 2009; Rothwell et al., 2012). It is possible that ELA impacts one or more of these negative reinforcement processes, for example by inducing negative affective states that are relieved by initial drug use, by impacting physiological dependence upon drug with chronic use, or by influencing the severity of acute and/or protracted withdrawal symptoms.
Another important aspect of addiction is its chronic, relapsing nature. Indeed, risk for relapse often continues to be significant even after years of abstinence. Relapse is often precipitated by specific environmental triggers, such as experiencing drug-associated cues, acute stressors, or ingestion of small, “priming” doses of drug. Each of these factors can be modeled in rodents by imposing abstinence following a period of drug self-administration, then introducing one or more relapse triggers, causing animals to reinstate their drug seeking (Stewart & de Wit, 1987; Shaham et al., 2003). Adaptations of these models have also been recently developed in which animals voluntarily abstain from drug, a behavior that is characteristic of humans attempting to cease or curtail their drug use. This can be achieved in rodents by imposing punishments (e.g. shocks) along with drugs, or by forcing a choice between drugs and highly salient rewards such as palatable foods or social interactions (Panlilio et al., 2003; Ahmed, 2018; Farrell et al., 2018; Marchant et al., 2019; Venniro et al., 2019). Individual differences in these choice behaviors potentially represent one aspect of an individual’s risk for addiction that might be influenced by ELA, though choice behaviors have not been thoroughly examined in the context of ELA. Indeed, it is estimated that only a subset of outbred rats exhibit “compulsive” drug seeking (Belin et al., 2008; Flagel et al., 2009; Belin et al., 2011; George & Koob, 2017; Farrell et al., 2018), and it is possible that developmental environment manipulations such as ELA could alter this ratio. Given the variability in behavioral traits, ELA might therefore affect the manifestation of addiction-like drug-seeking behaviors by influencing reactivity to potential relapse triggers, the sensitivity to factors that suppress drug intake, or both.
It is also important to recognize that addiction-related behaviors in the models discussed above and their underlying neural substrates may vary based on the studied drug of abuse (Schuster & Thompson, 1969; Thompson & Pickens, 1970; Shalev et al., 2002; Meyer et al., 2016). Likewise, humans may have specific vulnerabilities only to certain drug classes (e.g. stimulants vs. depressants), and the mechanisms driving specific drug choices (beyond immediate drug availability) are not well understood. Furthermore, although some addiction-related drug effects are common to all major abused drugs (Wise & Rompré, 1989; Saal et al., 2003; Nestler, 2004; Scofield et al., 2016), there are also major differences in the neural mechanisms by which different classes of drugs act. Therefore, it is possible that the neurodevelopmental changes in brain reward and stress circuits caused by ELA will lead to susceptibility to addiction to specific classes of drug, and more work is required to test this possibility.
In sum, addiction is a heterogeneous disorder. Its multiple and interacting features and components can be impacted by ELA in complex ways. These facts necessitate sophisticated and precise modeling in rodents. Understanding exactly which addiction-relevant behaviors are affected by ELA will be essential for understanding the nature of the risk ELA imposes on individuals with SUD. In the next sections we review evidence that ELA in rodents leads to a variety of changes in addiction-relevant behaviors (summarized in Table 1), and discuss salient modulating factors including the specific ELA model, sex, and abused drug which contribute to these relationships.
Table 1Early Life Adversity Effects on Responses to Addictive Drugs
Effects of Maternal Separation ELA
Numerous studies have shown that MS impacts later-life responses to addictive drugs, and these effects may differ by drug class as well as sex.
While work on effects of ELA on opioid-seeking is still limited, evidence suggests that MS may augment opioid drug addiction-relevant behaviors. In male rats and mice, MS enhances morphine reward across multiple behavioral tests, including CPP, locomotor sensitization, and voluntary oral consumption (Kalinichev et al., 2002; Vazquez et al., 2005; Vazquez et al., 2006; Michaels & Holtzman, 2008). In females, effects of MS depend on the opioid response being measured. MS yields similar pro-opioid outcomes in females as in males on morphine CPP and oral intake tasks (Abad et al., 2016; Mohammadian et al., 2019), yet MS led to a heroin-induced increase in ICSS threshold in females at a dose of heroin that reduces ICSS threshold in controls, suggesting a potential MS-induced blunting of heroin’s hedonic effects in that sex (Matthews & Robbins, 2003).
Effects of MS on psychostimulant responses have been consistently reported, and these also appear to differ in males and females. In male rats and mice, MS increases oral and intravenous self-administration of the psychostimulants cocaine and methamphetamine, and both the locomotor sensitizing and place preference-inducing effects of stimulants are stronger in MS males than in females (Kosten et al., 2000; Brake et al., 2004; Marquardt et al., 2004; Kikusui et al., 2005; Zhang et al., 2005; Moffett et al., 2006; Lewis et al., 2013; Lewis et al., 2016; Castro-Zavala et al., 2020a; Castro-Zavala et al., 2020b). Indeed, some evidence suggests that MS females may in fact have blunted cocaine sensitization compared to control-reared females (Li et al., 2003). MS also enhances the “pro-hedonic” properties of amphetamine, as indicated by a larger reduction in ICSS threshold in male rats relative to control-reared males (Der-Avakian & Markou, 2010). However, the degree to which this psychostimulant-prone MS effect is specific to males is still unclear. Though some studies show a male-specific enhancement of psychostimulant responses by MS (Hensleigh & Pritchard, 2014; Ganguly et al., 2019; Castro-Zavala et al., 2020b), other reports suggest that MS also has similar effects in females (Matthews et al., 1999; Kosten et al., 2004), and others still show instead a blunting of psychostimulant reward in MS males relative to controls (Matthews et al., 1999; Matthews & Robbins, 2003; O’Connor et al., 2015), an effect also seen after short (15-minute) periods of MS (Campbell & Spear, 1999). The reason for these apparently conflicting findings is unclear, but could depend upon differences in the precise protocol used, timing of MS, species/strain differences, or other experimental differences. For example, Hensleigh and Pritchard (2014) and Ganguly et al (2019) separated pups individually, Castro-Zavala et al (2020b) included early weaning, whereas Matthews et al (1996; 1999; 2003) and O’Connor et al (2015) all separated pups in a group by litter. Investigation into whether these or other procedural differences might be causally related to the variability observed drug-related outcomes is needed.
MS also affects responses to alcohol in a persistent, and potentially sex-dependent manner. MS-reared male but not female rats, show greater voluntary oral alcohol consumption than their control counterparts (Ploj et al., 2003a; Roman et al., 2004), and MS increases preference for alcohol over water in male mice and rats (Huot et al., 2001; Cruz et al., 2008; Romano-López et al., 2012; Amancio-Belmont et al., 2020). Male MS mice also consume more alcohol when it is intermittently available in a “drinking in the dark” protocol (Portero-Tresserra et al., 2018). Although these effects were shown in male animals, other studies have shown that MS increases operant self-administration of alcohol in both male and female rats (Gondré-Lewis et al., 2016; Bassey & Gondré-Lewis, 2019), and MS increases the locomotor sensitizing effects of alcohol only in females (Kawakami et al., 2007).
In sum, MS clearly impacts behavioral effects of several classes of addictive drugs, potentially in a sex-dependent manner. Most likely, methodological differences such as duration and timing of the MS protocol, potential species and strain differences, drug of abuse tested, and the aspect of addiction-like behavior measured explains the complex pattern of findings using the MS ELA manipulation (Jaworski et al., 2005; van der Veen et al., 2008; Orso et al., 2019). More work is also required to understand how factors like sex, hormonal influences, and others affect how MS alters responses to addictive drugs.
Effects of Limited Bedding and Nesting ELA
Several groups have examined how chronic ELA in the limited bedding and nesting model affects later-life responses and addiction vulnerability to opioids, cocaine, and alcohol.
Our group has recently begun to examine how LBN affects opioid addiction-related behaviors. In female LBN rats, we found a striking increase in addiction-like behaviors in pursuit of opioid drugs (Levis et al., 2019). LBN-reared females had stronger reinstatement of heroin seeking triggered by either heroin priming injections or heroin cues than female controls. In addition, when we examined demand elasticity for the short-acting fentanyl-derivative opioid drug remifentanil, LBN females showed relatively inelastic, addiction-like demand, without measurable changes in hedonic setpoint. A similar decreased sensitivity to price of a highly palatable food reward was observed in LBN females, though no such effect was seen for a less palatable chow reward. Our recent unpublished observations indicate that these pro-opioid effects of LBN in females may not occur to the same extent in males. Notably, Ordoñes Sanchez et al. (2021) also observed sex differences in the effects of ELA on opioid addiction-like behaviors. In this study, male LBN rats self-administered less morphine and were less impulsive than their control counterparts, whereas females did not show LBN-induced changes in these behaviors. LBN also induced sex-specific changes in NAc gene expression. The differences in opioid reward-related effects of LBN between studies might involve procedural differences such as rat strain (Sprague-Dawley vs. Long-Evans), opioid drugs tested (heroin/remifentanil vs. morphine), or differences in prenatal handling procedures between the studies (shipping timed-pregnant dams vs. in-house breeding) (Levis et al., 2019; Ordoñes Sanchez et al., 2021). Regardless, the clear sex differences in ELA effects on susceptibility to OUD-related behaviors seem likely to have important implications for understanding human opioid use and addiction. Indeed, it is notable that women addicted to heroin have a much greater prevalence of adverse experiences during development than heroin-addicted men, and the association between ELA and substance abuse appears also to be stronger in women than in men (Hyman et al., 2006; Hyman et al., 2008; Shand et al., 2011).
In contrast to our opioid-related findings, our group recently found that LBN facilitates acquisition of cocaine self-administration in male rats, though stable intake of the drug was equivalent in LBN and controls. However, when we subsequently measured cocaine behavioral economic demand elasticity, we found no change in sensitivity of cocaine intake to price (elasticity), though there was a decrease in cocaine hedonic setpoint, or intake when price was very low (Bolton et al., 2018b). We interpreted this result as LBN-induced “anhedonia” for cocaine, similar to the reduced engagement with natural rewards like sucrose solution or social play observed in LBN males (Molet et al., 2016a; Yan et al., 2017; Bolton et al., 2018a). In male mice, LBN also leads to blunted cocaine locomotor sensitization (Mitchell et al., 2018), suggesting reduced cocaine reward. While LBN did not seem to increase addiction-like cocaine seeking in our study of male rats, this more general anhedonic phenotype could still impact addiction susceptibility, perhaps especially for other classes of drugs (like opioids) that could more effectively “self-medicate” this underlying affective dysregulation.
In a model of alcohol dependence in adult mice, males that have experienced LBN develop excessive alcohol drinking more rapidly than control-reared mice (as measured by escalation of voluntary alcohol consumption), an effect not seen in LBN females (Okhuarobo et al., 2020), suggesting that LBN may confer a specific vulnerability to alcohol reward in males. Further exploring sex differences in the effects of LBN on addiction and determining how LBN females respond to other classes of addictive drugs are important questions that remain open.
In summary, clinical and pre-clinical evidence, including some congruent findings from nonhuman primate models suggesting that ELA enhances drug abuse in adolescents (Wakeford et al., 2018), suggest that ELA can increase vulnerability to addiction to a wide range of drugs. This may occur by enhancing the rewarding or motivating effects of drugs themselves, by impacting factors like susceptibility to relapse triggers, or perhaps by inducing a state of affective dysregulation that may be self-medicated with certain drugs. Understanding the specific addiction-relevant behaviors which are most impacted by ELA may help elucidate causal mechanisms, such as changes in neural circuit structure and function caused by developmental adversity. We review some of these circuit and substrate-level ELA-induced changes in the following section.
Does ELA “Rewire” Brain Reward and Stress Circuits?
Considerable evidence links dysfunction of brain reward and stress circuits with addiction vulnerability and severity. These circuits undergo substantial maturation in the first weeks (rodents) or year (humans) of life, and mounting evidence supports the notion that ELA induces long-lasting developmental changes, leading to addiction-relevant neuroadaptations in brain reward and stress circuits and increased vulnerability to SUD (Koob, 2008; Sinha, 2008; Koob & Volkow, 2016; Ironside et al., 2018).
Here, we will focus specifically on the roles of specific brain systems for which a large body of evidence exists on the effects of ELA in mediating their function. We will first provide an overview of the behavioral functions of key reward-related systems, namely dopamine and opioid signaling molecules and receptors in mesolimbic circuits, as well as stress-related systems, specifically corticotropin releasing hormone (CRH) and dynorphin/kappa opioid receptors in extended amygdala. We then summarize findings about the specific changes in these molecules and circuits which may underlie ELA effects on addiction susceptibility. Notably, the effects of ELA are not limited to classical stress and reward circuits, as pronounced effects on memory-linked regions like hippocampus are also seen (Ivy et al., 2010; Chen & Baram, 2016; Molet et al., 2016b), which may lead to cognitive deficits or other psychiatric symptoms that may indirectly affect drug seeking. Likewise, the neural substrates altered by ELA that might mediate reward seeking are not limited to the dopamine and opioid systems (Forster et al., 2018).
Roles for Mesolimbic Opioids and Dopamine in “Reward Circuits”
Addictive drugs are thought to “hijack” neural circuits of reward, pharmacologically engaging the neural mechanisms responsible for registering pleasurable experiences, and generating motivation to pursue these rewards again in the future. These mechanisms normally operate in service of learning about and pursuing natural rewards like food, water, and sex, but repeated drug use may cause them to be specifically, and excessively, centered on drugs instead. The neural mechanisms by which drugs cause pleasurable states and/or states of compulsive seeking and desire are the subject of much study, and involve complex circuit, synaptic, and molecular mechanisms. Here we concentrate on two such mechanisms that are particularly strongly linked to drug reward: dopamine and opioids within “mesolimbic reward circuit” nodes like ventral tegmental area (VTA), prefrontal cortex (PFC), and nucleus accumbens (NAc).
Similar to other rewards, drugs of abuse are thought to generate pleasurable subjective states via actions in mesolimbic circuits. Reward-induced pleasure is complex, but a role for endogenous opioid signaling in nucleus accumbens seems to be particularly important. Endogenous opioid systems involve at least 3 opioid peptides (endorphin, enkephalin, and dynorphin), acting via three primary g-protein coupled receptors (mu, delta, and kappa opioid receptors) to modulate neural activity (Kieffer et al., 1992; Chen et al., 1993; Minami et al., 1993). Endogenous and exogenous ligands engage inhibitory intracellular signaling cascades, inhibiting neural firing postsynaptically, and suppressing neurotransmitter release from axon terminals (Mansour et al., 1995; Valentino & Volkow, 2018). Opioid receptors are densely expressed in NAc, where they are localized both pre- and post-synaptically (Mansour et al., 1994). Of particular relevance, opioid receptors in an anatomically segregated “hedonic hotspot” within the nucleus accumbens dorsomedial shell subregion play a major role in registering affective pleasure from food reward, in a manner suggesting that this restricted anatomical zone is of special importance for registering the pleasurable aspects of food or other types of rewards (Peciña & Berridge, 2005; Thompson & Swanson, 2010; Zahm et al., 2013; Castro & Berridge, 2014). Given this link between NAc opioids and pleasure, it is not surprising that addictive opioid drugs generate highly euphoric states. However, other major drugs of abuse also engage accumbens opioidergic signaling (Kreek, 1996; Olive et al., 2001; Gerrits et al., 2003; Yoo et al., 2012), which may likewise contribute to euphoric and pleasurable responses to these drugs.
Dopamine signaling within mesolimbic circuits, and especially in NAc, are another crucial mechanism by which drugs engage reward circuits to promote addiction. The mesolimbic dopamine circuit entails projections from VTA to NAc, PFC, and other forebrain limbic sites, which are thought to mediate multiple addiction-related behavioral processes (Kalivas & Volkow, 2005; Salamone et al., 2007; Kalivas, 2008). Addictive drugs, regardless of class and mechanism of action, engage the mesolimbic dopamine system, as do natural rewards of various types (Wise & Bozarth, 1987). The precise roles played by dopamine neurons is still debated, but it likely involves addiction-relevant psychological processes such as reward prediction (Schultz, 1998), inflexible habitual aspects of drug taking (Everitt & Robbins, 2005), and highly effortful drug seeking, especially when triggered by drug-associated cues (Shaham et al., 2003; Robinson & Berridge, 2008; Mahler et al., 2018).
In sum, mesolimbic opioids and dopamine play critical and nuanced roles within brain circuits that mediate pleasure, motivation, learning, and habits. Depending on precisely how ELA impacts these circuits, we may therefore see consequences on a range of SUD-relevant behaviors, any of which could lead to an addiction-vulnerable phenotype via distinct neural mechanisms.
“Stress Circuits” in Addiction
Stress also plays a key role in addiction, and ELA effects on stress circuits is likely to contribute to ELA-induced addiction risk. Physiologically, stress can be defined as activation of the hypothalamic-pituitary-adrenal (HPA) axis leading to release of CRH from the hypothalamus into the bloodstream, as well as directly into brain emotional systems via neural projections (Joëls & Baram, 2009; Koob & Zorrilla, 2010; McEwen & Gianaros, 2011). Brain circuits in which CRH is synthesized locally and acts to promote stressful and aversive states include extended amygdala regions such as central amygdala (CeA) and bed nucleus of the stria terminalis (BNST), as well as the dorsal raphe, the paraventricular and lateral hypothalamic nuclei.
Stress may impact addiction risk via the ability of some drugs to counteract negative emotional or affective states. Many users of anxiolytic drugs such as opioids, benzodiazepines, and alcohol report that when they began using these drugs, their underlying anxieties and negative emotions suddenly lifted. In this way, drug use may provide relief to an already suffering person, resulting in strong negative reinforcement, or “self-medication.” This may occur via direct or indirect recruitment by drugs of endogenous opioids such as enkephalin and beta-endorphin, which counteract neural responses to stress and help promote recovery from stressful events (Cohen et al., 1983; Curtis et al., 2001; Bowers et al., 2012; Valentino & Van Bockstaele, 2015; Valentino & Volkow, 2018). CRH and opioid receptors co-localize in regions related to stress and reward (Van Bockstaele et al., 2010; Williams & Milner, 2011; Reyes et al., 2017; Castro & Bruchas, 2019), and neuroadaptations induced by chronic opioid exposure in stress and reward regions appear to be modulated by glucocorticoids as well (García-Pérez et al., 2012), further supporting a link between opioid transmission and self-medication of negative affect with abused drugs.
Stress also plays an important role in maintaining compulsive substance use, particularly of drugs that cause physiological dependence and severe withdrawal symptoms, such as opioids and alcohol (Bruchas et al., 2010; George & Koob, 2017). Stress circuits and molecules play a key role in mediating these highly aversive acute withdrawal symptoms (Koob, 2008; Logrip et al., 2011; Gilpin & Roberto, 2012; Chartoff & Carlezon, 2014). For example, CRH is released in the extended amygdala structures CeA and BNST during alcohol withdrawal (Olive et al., 2002), and blockade of CeA CRH receptors prevents acute withdrawal-enhanced consumption of ethanol in dependent rats (Funk et al., 2006). Some have also described affective dysregulation that persists for extended periods after cessation of drug use, which may help promote relapse (Kenny & Markou, 2001; Aston-Jones & Harris, 2004). Moreover, protracted abstinence can also enhance the incentive salience of drug-associated cues upon drug re-exposure (Smith & Aston-Jones, 2014). Multiple brain circuits are involved in this excessive drug seeking seen even after persistent abstinence. For example, protracted withdrawal is associated with altered glutamate-dependent plasticity in the VTA and its afferent inputs such as the amygdala, BNST, lateral hypothalamus, VTA, and NAc (Aston-Jones & Harris, 2004), as well as altered function of the PFC that may be mediated by CRH (Zorrilla et al., 2014; Quadros et al., 2016; Blaine & Sinha, 2017). In addition, opioid withdrawal memories appear to promote opioid seeking via interactions between these stress and reward-related circuits (Frenois et al., 2005). Withdrawal-associated dysphoria and stress-induced reinstatement of drug-seeking is also thought to be mediated in part by the dynorphin/kappa opioid receptor system, likely by acting in concert with CRH (Land et al., 2008; Redila & Chavkin, 2008; Bruchas et al., 2010; Nygard et al., 2016).
In addition to withdrawal, acute stress in any form is thought be a major trigger for relapse in humans, and stress also potently induces reinstatement of drug seeking in rodents (Shalev et al., 2000; Sinha, 2001; Shaham et al., 2003; See & Waters, 2010; McReynolds et al., 2014; Mantsch et al., 2016). Specifically, activation of stress circuit nodes such as the BNST, CeA, BLA, and medial septum play a key role in stress-induced relapse, as do stress-linked transmitters like CRH and norepinephrine in these structures and elsewhere in the brain (Shaham et al., 2000; Koob & Zorrilla, 2010; Logrip et al., 2011). Notably, stressors may activate drug seeking via their recruitment of motivation circuits (Sarnyai et al., 2001; Yap & Miczek, 2008; Shalev et al., 2010; George et al., 2012; Lemos et al., 2019). For example, physical or psychological stressors elicit the release of CRH in the VTA, causing dopamine release in the NAc and leading to reinstatement of drug-seeking behaviors (Wang et al., 2005; Wang et al., 2007; Shalev et al., 2010; Ungless et al., 2010). CRH signaling within NAc itself may also play stress-independent roles in reward seeking, for example by increasing the incentive salience of reward-paired cues (Peciña et al., 2006; Baumgartner et al., 2021).
Clearly, stress is an important and multifaceted factor influencing SUD, and ELA-induced changes in stress circuits may impact initiation of drug use, maintenance of use/avoidance of withdrawal, and relapse risk in response to life stressors. In the next section, we review what is known about how ELA affects development of brain reward and stress circuits, and how this may influence the development of, and recovery from addiction.
Effects of ELA on Reward and Stress Circuits
Mounting evidence suggests that ELA causes profound, likely permanent changes in brain reward and stress systems, including mesolimbic and extended amygdala circuits, dopamine, endogenous opioids, and CRH. Given the importance of these systems to addiction, it is likely that these disruptions contribute to the ability of ELA to enhance addiction susceptibility in vulnerable individuals.
Adult function of stress-related circuits and molecules are profoundly impacted by ELA, and this may impact addiction propensity or severity. For example, ELA provoked enduring changes in the expression levels of several stress modulators. CRH expression is augmented in CeA (Dubé et al., 2015) and hippocampus (Ivy et al., 2010) of ELA rodents, leading to major changes in circuit functions (Brunson et al., 2005; Ivy et al., 2010). In the context of addiction, these changes in circuit function are evident from studies examining circuit activation in adult rodents that have experienced ELA. Thus, palatable food, social play, and acute cocaine rewards induce a stronger Fos response in CeA of LBN males than of control males, an effect accompanied by anhedonia-like behavioral responses to those same rewards (Bolton et al., 2018a; Bolton et al., 2018b). This may indicate a stress-like response to these normally rewarding stimuli following ELA. ELA also alters functional connectivity and microstructure of stress- and reward-related brain regions. For example, LBN males have increased adulthood amygdala-PFC structural connectivity relative to controls (Bolton et al., 2018a). Pre-weaning LBN males, but not females, have reduced BLA-PFC, and altered PFC-striatum resting state functional connectivity (Guadagno et al., 2018a; Guadagno et al., 2018b), a finding that persists into adulthood, accompanied by reduced sucrose preference and social interaction (Yan et al., 2017). Likewise, both MS and LBN disrupt early maturation of BLA-PFC connections (Brenhouse et al., 2013; Honeycutt et al., 2020; Manzano Nieves et al., 2020), further implicating this circuit in the effects of ELA. Notably, human studies suggest that ELA’s impact on amygdala development is essential for the resulting depression and anxiety (Callaghan & Tottenham, 2016; Fareri & Tottenham, 2016). The latter could set the stage for “self-medicating” drug use in vulnerable individuals (Kessler, 2004).
Mesolimbic dopamine system development is strongly impacted by ELA (Rodrigues et al., 2011; Ventura et al., 2013; Peña et al., 2014; Bonapersona et al., 2018), thereby potentially facilitating dopamine-dependent incentive motivational, learning, or habitual aspects of addiction. While it is clear that ELA affects the mesolimbic dopamine circuit, the precise changes are somewhat inconsistent across studies, and appear to be partially sex dependent. For example, MS females have more dopamine cells in the VTA than controls, and also show enhanced excitability of VTA dopamine neurons, whereas males appear instead to have more non-dopamine cells in VTA relative to control males, but no change in the number of dopamine cells there (Chocyk et al., 2011; Chocyk et al., 2015; Majcher-Maślanka et al., 2017; Spyrka et al., 2020). MS females, but not males, have increased dopamine turnover in prefrontal cortex, and turnover of other monoamines in the striatum also differ between sexes after ELA (González-Pardo et al., 2020). However, MS does appear to affect dopamine signaling in males in some cases. For example, in males, MS-enhanced sensitivity to amphetamine and cocaine are associated with decreased dopamine transporter expression in the NAc (Meaney et al., 2002; Brake et al., 2004). Others have found that alcohol self-administration in MS but not control male rats is negatively correlated with the number of dopamine neurons in VTA, a phenomenon that is also seen in genetically alcohol-preferring rats (Gondré-Lewis et al., 2016; Bassey & Gondré-Lewis, 2019). MS combined with limited nesting materials during the second week of life has also recently been found to alter histone modification and gene transcription in dopamine receptor type 2 (D2R) containing NAc medium spiny neurons more robustly in male mice than females, suggesting a sex-dependent change in function of those cells (Kronman et al., 2021). As mentioned above, differences in ELA protocols, age, and models used for addiction or quantification approaches may be responsible for this range of outcomes.
ELA-induced changes in dopamine receptor protein and mRNA expression are also observed across the mesolimbic circuit in both sexes following ELA. Consistent decreases in dopamine receptor expression in striatum are induced by MS in both males (Zhu et al., 2010; Romano-López et al., 2016) and females (Majcher-Maślanka et al., 2017), and striatal reductions of D2R correlate with the magnitude of MS-suppressed cocaine locomotor sensitization (Gracia-Rubio et al., 2016). However, others have observed that D2R and D3R in NAc are instead increased by MS in male rats, an effect associated with increased alcohol intake in male MS rats (Amancio-Belmont et al., 2020). Similarly, MS male mice have increased prefrontal cortex dopamine receptor gene expression relative to controls (Tractenberg et al., 2020).
Brain endogenous opioid systems are enduringly altered by ELA, a fact that may impact drug-induced pleasure or other addiction-relevant processes. MS persistently alters endogenous opioid peptides, as well as opioid and dopamine receptor expression in reward and stress related areas, including striatum, midbrain, hippocampus, and hypothalamus in both a sex- and ELA timing-dependent manner (Ploj et al., 1999; Ploj et al., 2001; Ploj & Nylander, 2003; Ploj et al., 2003a; Ploj et al., 2003b; Gustafsson et al., 2008). Specifically, long bouts of daily separation (360 minutes) lead to higher ethanol consumption in adulthood, whereas brief bouts (15 minutes) may be protective against chronic escalating ethanol consumption, even though both protocols lead to elevated expression of opioid peptides in the hypothalamus and pituitary (Ploj & Nylander, 2003; Ploj et al., 2003a; Ploj et al., 2003b), PFC, and VTA (Gustafsson et al., 2008). Additionally, 15-minute daily MS leads to higher delta opioid receptor density in amygdala, and enhanced dynorphin-mediated HPA-axis inhibition in males but not females, whereas females but not males have reduced dynorphin expression in PFC and amygdala (Ploj et al., 1999; Ploj et al., 2001). In a 12-hr MS model that led to enhanced ethanol consumption in male mice, mu opioid receptor gene expression was elevated in NAc (García-Gutiérrez et al., 2016). MS also alters the ability of addictive drugs to induce plasticity in opioidergic circuits. For example, MS male rats do not show the typical chronic ethanol-induced downregulation of delta, mu, and kappa opioid receptor gene expression in striatum (Granholm et al., 2017), potentially contributing to the excessive alcohol consumption seen in these animals. Clearly, MS leads to persistent changes in endogenous opioids, though more work is needed to link these changes to addiction-like behaviors.
Perhaps as a result of the above molecular circuit-development changes, ELA persistently alters neural responses to drugs of abuse themselves in a manner that may facilitate their rewarding effects via actions in limbic reward circuits. For example, in male rats, MS increases the sensitivity of limbic and striatal regions to ethanol-induced gene expression and DNA methylation changes (Vrettou et al., 2017) and alcohol exposure leads to MS-specific alterations in mesocorticolimbic dopamine and opioid receptor expression (Ploj et al., 2003a). In response to psychostimulants, MS increases cocaine-induced striatal c-Fos expression in both male and female rats after chronic cocaine exposure (Kohut et al., 2009) and potentiates methamphetamine-induced depletion of striatal dopamine transporter and tyrosine hydroxylase, but only in males (Hensleigh & Pritchard, 2015). Additionally, microdialysis experiments reveal enhanced dopamine release in NAc in response to d-amphetamine in MS males (Hall et al., 1999). LBN also increases cocaine-induced c-Fos in NAc core, lateral habenula, and CeA of male rats, which may be related to quicker acquisition of cocaine self-administration, but an eventual reduction of hedonic setpoint for the drug (Bolton et al., 2018b).
Finally, it is possible that ELA enhances addiction vulnerability by simultaneously altering both stress- and reward-related processes. For example, relative to controls, MS increases dopamine, endogenous opioid, and CRH expression simultaneously in NAc in male mice that also show increased ethanol consumption relative to controls (García-Gutiérrez et al., 2016). In male rats, the magnitude of MS-enhanced alcohol consumption is strongly correlated with HPA axis responses to startle stress (Huot et al., 2001). In female rats, MS also increases VTA neuron excitability, an effect that is accompanied by elevated peripheral stress hormones (Spyrka et al., 2020). These findings support the notion that an imbalance of stress and reward processes may play a mediating role in the effects of ELA on SUD vulnerability (Koob, 2008; Valentino & Van Bockstaele, 2015).
Conclusions
Both ELA and addiction are complex processes, yet it is clear that ELA is a predisposing factor to SUD. However, the link between ELA and addiction is intricate and remains poorly understood. ELA effects on brain reward and stress circuit development are likely contributors to this link, though other mechanisms certainly also contribute (Kim et al., 2017; Baracz et al., 2020). The effects of ELA may also differ based on the type of ELA, its timing, sex of the individual involved, and many other factors. In addition, the behavioral phenotypes caused by ELA are nuanced and can differ based on the drug of abuse and stage of the addiction process that is tested. Therefore, additional investigation is required to determine exactly how ELA impacts brain development, and how these resulting changes put individuals at risk for specific addiction-related behaviors that could all lead to SUD, possibly through a range of neural mechanisms. We propose that understanding the precise links between ELA and addiction-like outcomes opens the possibility of developing better strategies for preventing and reversing addiction in those predisposed by their history of ELA.
Acknowledgements:
This work is funded by the National Institutes of Health grants R01 MH073136 (TZB), P50 MH096889 (TZB), P50 DA044118 (SVM), F30 DA051137 (SCL), T32 GM 008620 (SCL), and the Tobacco Related Disease Research Program Grant T31IR1767 (SVM). The authors thank Joshua Nykamp for his contribution to the figure concept and design.
Abbreviations:
BNST
bed nucleus of the stria terminalis
CeA
central amygdala
CORT
corticosterone
CREB
cAMP-response element binding protein
pCREB
phosphorylated CREB
CPP
conditioned place preference
CRH
corticotropin releasing hormone
D2R
dopamine receptor type 2
D3R
dopamine receptor type 3
DAT
dopamine transporter
ELA
early life adversity
GluA1
AMPA glutamate receptor subunit A1
GluA2
AMPA glutamate receptor subunit A2
HPA axis
hypothalamic-pituitary-adrenal axis
ICSS
intracranial self-stimulation
LBN
limited bedding and nesting
MeCP2
methyl CpG binding protein 2
MS
maternal separation
NAc
nucleus accumbens
PFC
prefrontal cortex
SUD
substance use disorder
TH
tyrosine hydroxylase
VTA
ventral tegmental area