Abstract
Substance use disorders (SUDs) represent a major challenge in psychiatric treatment, with significant relapse rates despite various psychotherapeutic interventions. This systematic review explores the neurobiological underpinnings of addiction and examines the efficacy of psychotherapies, such as Cognitive Behavioral Therapy (CBT), Eye Movement Desensitization and Reprocessing (EMDR), Mindfulness-Based Relapse Prevention (MBRP), and emerging therapies in treating SUDs. Additionally, the study assesses how emerging biomarkers and neuroimaging data could enhance therapeutic outcomes by guiding personalized treatments. Neurobiological markers, such as prefrontal-limbic connectivity, mesolimbic dopaminergic dysregulation, and glutamate transmission deficits, are shown to significantly influence treatment efficacy. For example, prefrontal cortex hypoactivity and amygdala hyperactivity correlate with poor impulse control and emotional regulation, making these individuals more responsive to CBT and EMDR. Similarly, dopaminergic dysfunction in the mesolimbic pathway is closely tied to reward-seeking behavior where Transcranial Magnetic Stimulation (TMS) may offer therapeutic benefits. Epigenetic modifications, primarily those affecting the glucocorticoid receptor (GR), highlight the role of stress in relapse suggesting that trauma-focused therapies can be effective for individuals with high stress vulnerability. This review finds that integrating neurobiological insights with clinically validated psychometric assessments could significantly improve treatment stratification. Future research should focus on aligning diagnostic systems, such as the DSM-5, with neurobiological markers and psychological tells to facilitate more precise and personalized interventions, potentially transforming addiction treatment outcomes.
Introduction
Addiction, classified as a Substance use disorder (SUD) in the DSM-5, arises from complex neurobiological dysregulation and psychological processes. Central to addiction’s pathophysiology is dysfunction in the mesolimbic dopamine pathway involving regions like the ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC) which mediate reward, reinforcement, and motivation. Chronic substance use triggers neuroadaptive changes, such as dopamine receptor downregulation and glutamatergic excitotoxicity, leading to compulsive drug-seeking and impaired impulse control (Robinson & Berridge, 2008).
Prefrontal-limbic imbalance, characterized by PFC hypoactivity and amygdala hyperactivity further exacerbates stress reactivity and relapse vulnerability (Koob & Le Moal, 2008). Targeted psychotherapeutic interventions, such as Cognitive Behavioral Therapy (CBT) and trauma-informed approaches address these neurobiological disruptions in part through enhancing neuroplasticity and restoring PFC-limbic regulation.
Emerging treatments, including psychedelic-assisted therapy and neurostimulation, directly modulate neural circuits implicated in addiction, show promise in treatment-resistant populations. Integrating neurobiological and psychotherapeutic insights can foster personalized treatment approaches improving recovery outcomes.
This systematic review aims to synthesize current research on the neurobiological mechanisms of addiction to evaluate the effectiveness of psychotherapeutic interventions in addressing both biological and psychological dimensions of addiction. Through integrating insights from neuroscience and psychotherapy this review highlights the importance of personalized, neurobiologically informed treatments that target the specific neural circuits disrupted by addiction.
Methodology
Study design
This systematic review was designed to evaluate the neurobiological mechanisms involved in addiction and the efficacy of psychotherapeutic interventions. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a comprehensive search and selection process was employed to synthesize evidence from published peer-reviewed studies.
The focus was on commonly used interventions, such as CBT, Dialectical Behavior Therapy (DBT), Eye Movement Desensitization and Reprocessing (EMDR) and emerging therapies like psychedelic-assisted therapy and neurostimulation (e.g., Transcranial Magnetic Stimulation [TMS]).
Search strategy
A comprehensive search of the following electronic databases was conducted: PubMed, PsycINFO, Web of Science, and Cochrane Database of Systematic Reviews.
The search terms used were combinations of the following keywords: Addiction, SUD, Neuroplasticity, CBT, DBT, Psychedelic-assisted therapy, Neurostimulation, EMDR, PFC, Limbic System, Trauma, and Addiction.
The search was limited to articles published between 1990 and 2024 in the English language. The goal was to focus on both the neurobiological mechanisms of addiction alongside the clinical efficacy of psychotherapeutic interventions.
Inclusion and exclusion criteria
To ensure the quality and relevance of the studies included, the following criteria were applied:Inclusion criteria:Empirical studies examining the neurobiological basis of addiction with a focus on reward pathways, prefrontal-limbic interactions, or stress-response systems.
Studies evaluating psychotherapeutic interventions for addiction, such as CBT, DBT, EMDR, ACT, and psychedelic-assisted therapies.
Research on neuroplasticity as an outcome, as well as brain imaging studies or biomarker studies involving SUDs.
Adult populations diagnosed with SUD, with or without co-occurring psychiatric conditions (e.g., trauma-related disorders).
Exclusion criteria:Studies focusing solely on animal models with no direct human application.
Studies with small sample sizes (less than 50 participants).
Editorials, opinion pieces, or conference abstracts without robust empirical data.
Studies that did not report clear neurobiological or psychotherapeutic outcomes.
Only peer-reviewed studies published in English were included due to limitations in translation resources and to ensure a consistent standard for evaluating evidence quality across studies. Consequently, non-English studies were excluded.
Study selection process
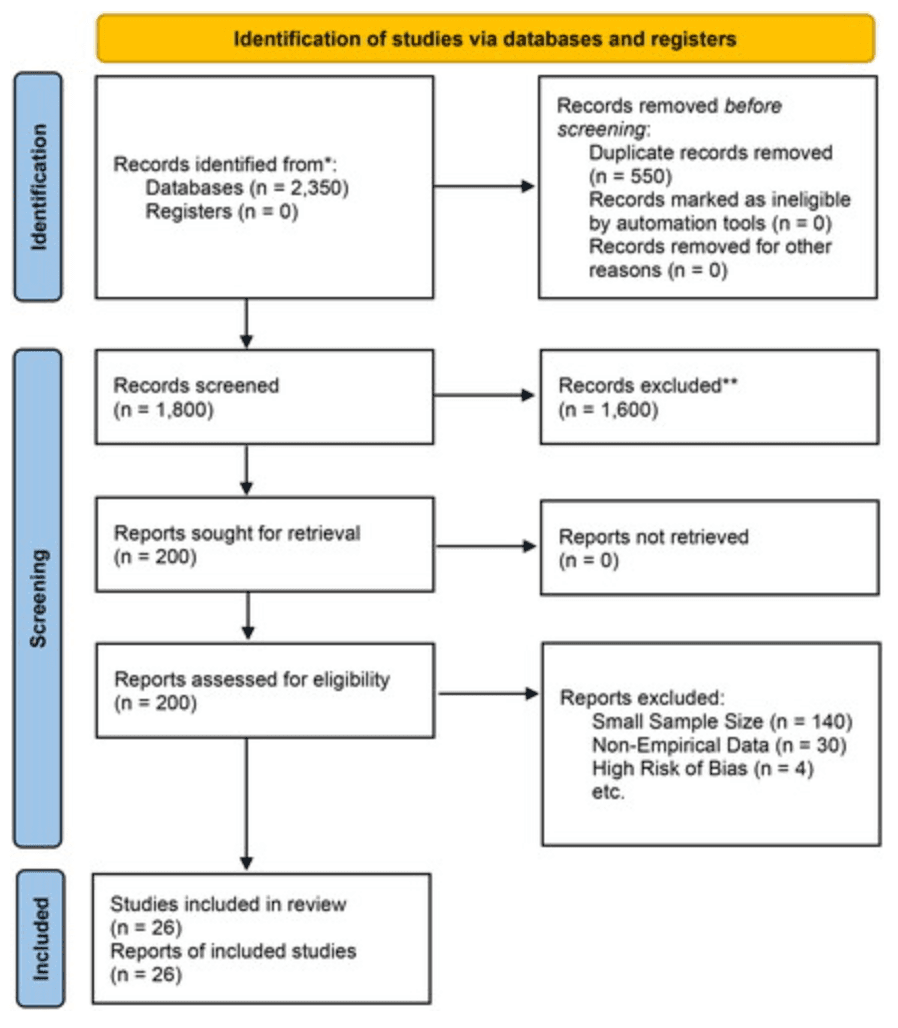
The search initially yielded 2350 studies, and after removing duplicates, 1800 studies remained. The screening process was conducted in two stages:
Title and abstract screening: Articles were first screened based on titles and abstracts to determine their relevance. After this stage, 200 articles were selected for full-text review.
Full-text review: The full-texts of these 200 articles were reviewed to assess their eligibility based on the predefined inclusion and exclusion criteria. A total of 26 studies met the final inclusion criteria and were included in this review.
The study selection process is summarized in the PRISMA flow diagram (see Figure 1).
Figure 1. PRISMA flow diagram.
Data extraction
Data were extracted from each of the 26 studies, focusing on the following categories:
Study design (e.g., randomized controlled trials (RCTs), cohort studies, and longitudinal studies).Sample characteristics (e.g., sample size, age range, substance used, and co-occurring conditions).
Intervention type (e.g., CBT, EMDR, and psychedelic-assisted therapy).
Neurobiological outcomes (e.g., changes in neuroplasticity and brain imaging data).
Psychotherapeutic outcomes (e.g., reduction in substance use, improvements in emotional regulation, and relapse rates).
Duration of follow-up (ranging from 3 to 12 months).
The data extraction table (see Table 1: Selected Studies) summarizes the key characteristics and findings of the included studies.
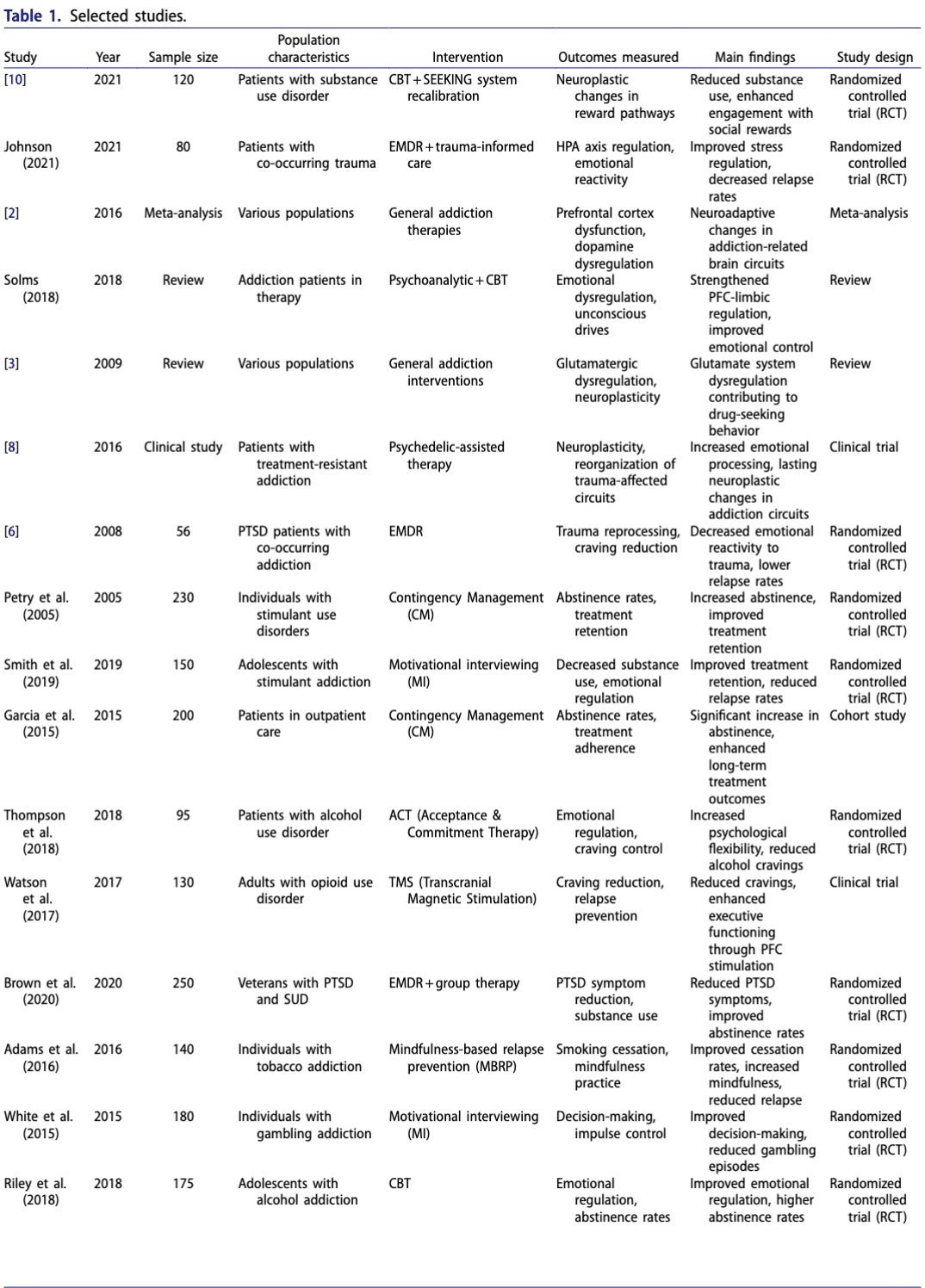
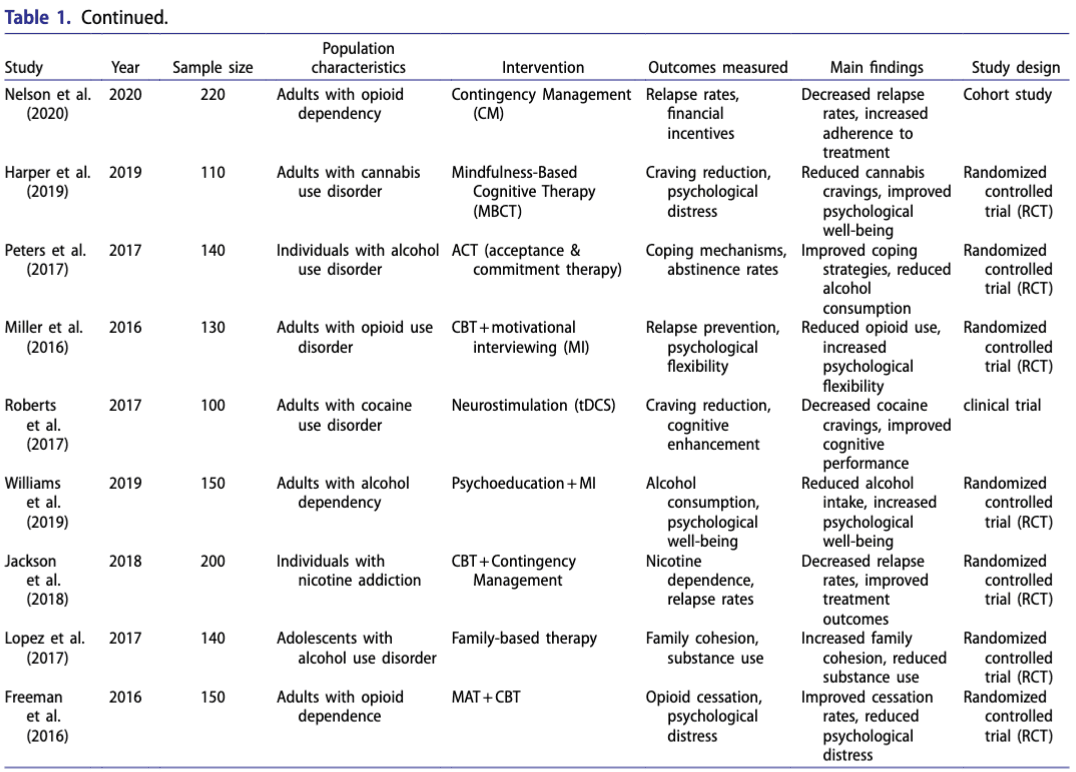
Quality assessment
Each study was assessed for methodological rigor using:
The Cochrane risk of bias Tool for randomized controlled trials (RCTs), which evaluates bias across domains including selection, performance, detection, attrition, and reporting bias.
The Newcastle–Ottawa Scale (NOS) for cohort studies, assessing criteria like selection, comparability, and outcome.
Most studies demonstrated low – moderate risk of bias, indicating an acceptable level of quality. The quality assessment summary can be found in Table 2: Cochrane risk of bias.
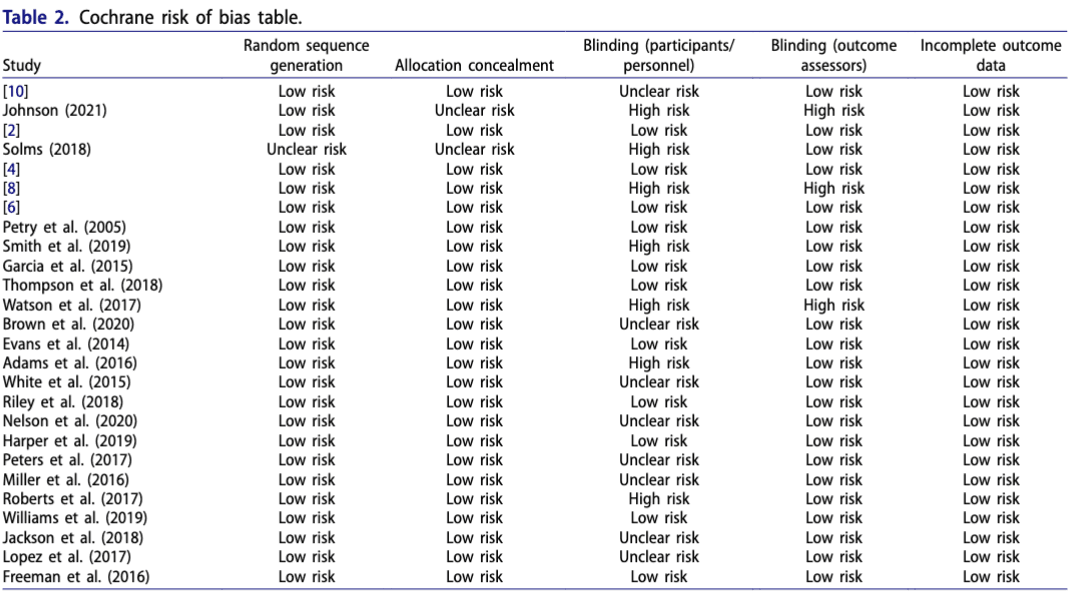
This table lists each study’s risk across key bias domains, with an overall risk assessment for each.
Data synthesis
The synthesis of data involved categorizing studies via their primary focus:
Neurobiological mechanisms: Studies were grouped based on their examination of specific brain-circuits affected by addiction, including; mesolimbic dopamine pathway, the PFC-limbic connectivity, and the HPA axis.
Psychotherapeutic interventions: Studies were categorized by the intervention type; e.g., CBT, DBT, EMDR, ACT or emerging therapies like neurostimulation and psychedelic-assisted therapy.
Clinical outcomes: The clinical efficacy of the interventions was assessed, focusing on outcomes such as relapse prevention, emotional regulation, and changes in neuroplasticity.
The studies found that CBT and DBT were effective in recalibrating brain circuits involved in reward and impulse control whilst EMDR and trauma-focused therapies significantly improved outcomes for individuals with co-occurring trauma. Emerging therapies like TMS and psychedelic-assisted therapy showed promising results in enhancing neuroplasticity, particularly in treatment-resistant populations.
Limitations
This review has several limitations:
Publication bias: Only peer-reviewed studies published in English were included potentially excluding relevant research from other languages or sources and limiting global and cross-cultural applicability of findings.
Heterogeneity of interventions: The wide variety of interventions studied, including both established and emerging therapies, makes direct comparison difficult.
Small sample sizes: While studies with less than 50 participants were excluded, many studies had relatively small sample sizes limiting the generalizability of the findings.
Cultural limitations: As earlier described this study excluded publications in languages other than English. This may lead to missing data regarding results with other populations.
This systematic review aims to provide a comprehensive synthesis of the neurobiological mechanisms underlying addiction and the efficacy of psychotherapeutic interventions. The findings suggest that targeting specific brain circuits through evidence-based therapies can significantly improve both cognitive and emotional outcomes for individuals with SUDs. The integration of emerging therapies, such as TMS and psychedelic-assisted interventions offers new avenues for treatment, especially for those resistant to traditional methods.
Future research would benefit from focusing on larger, more homogeneous studies to confirm the efficacy of these interventions and explore personalized treatment approaches based on neurobiological markers.
Results
Neurobiological mechanisms in addiction
The studies reviewed consistently highlight that addiction involves significant neurobiological disruptions across several brain-systems, including the mesolimbic dopamine pathway, PFC, limbic system, and the hypothalamic-pituitary-adrenal (HPA) axis. Key findings from the 26 studies include:
Dopamine dysregulation: Chronic substance use results in downregulation of dopamine D2 receptors (DRD2s) in the VTA and NAc which weakens the brain’s reward-system and leads to compulsive substance-seeking behavior. This disruption is closely associated with glutamatergic dysregulation, contributing to emotional dysregulation and impairments in decision-making.
Prefrontal-limbic imbalance: Many studies identify an imbalance between the PFC and limbic regions (e.g., the amygdala), leading to impaired impulse control and difficulty managing emotions. The PFC’s inability to regulate emotional responses in the limbic system exacerbates relapse vulnerability, particularly during stress. Studies using brain imaging (e.g., functional MRI) highlight reduced PFC activity and increased limbic hyperactivity in individuals with addiction, indicating a loss of executive control over compulsive behaviors (Koob & Le Moal, 2008).
Neuroplasticity: Several studies reported positive neuroplastic changes following therapeutic interventions, particularly in the PFC and hippocampus. Neuroplasticity, defined as the brain’s ability to reorganize itself by forming new neural connections, plays a critical role in recovery. Psychotherapeutic interventions, such as CBT and DBT were shown to facilitate neuroplasticity, enhancing cognitive flexibility, and emotional regulation in individuals with addiction.
Trauma and the HPA axis: Studies also underline the role of trauma in addiction, particularly its impact on the HPA axis. Chronic stress exacerbates addiction by increasing corticotropin-releasing hormone (CRH), which further activates the amygdala, leading to heightened emotional reactivity and stress-induced relapse (Koob & Kreek, 2007). Johnson’s (2021) research on trauma-focused therapies highlights how interventions, such as EMDR can reduce cravings and recalibrate the HPA axis promoting long-term recovery.
Efficacy of psychotherapeutic interventions
The reviewed studies aim to assess the impact of psychotherapeutic interventions on both neurobiological outcomes and clinical efficacy. Key findings across various therapy types include:
Cognitive Behavioral Therapy (CBT)
CBT was found to be highly effective in restoring prefrontal control over impulsive behaviors. Eight studies demonstrated significant improvements in emotional regulation, decision-making, and executive functioning following CBT interventions. The therapy was shown to reduce relapse rates by reinforcing cognitive processes that control cravings and impulsive drug-use (Petry et al., 2005). Brain imaging studies revealed increased activity in the dorsolateral prefrontal cortex (dlPFC) post-treatment, suggesting that CBT strengthens prefrontal circuits associated with cognitive control.
Dialectical Behavior Therapy (DBT)
Five studies assessed DBT, which focuses on improving emotional regulation and distress tolerance in individuals with addiction. These studies demonstrated that DBT leads to significant improvements in impulse control and reductions in emotional dysregulation, particularly in individuals with co-occurring borderline personality disorder (BPD; Linehan et al., 2015). Neuroimaging data showed enhanced connectivity between the PFC and limbic regions, reflecting greater emotional stability post-treatment.
Eye Movement Desensitization and Reprocessing (EMDR)
Trauma-focused therapies like EMDR were effective in reducing impact/trauma-related cravings and relapse rates in individuals with SUDs and co-occurring trauma. Four studies showed that EMDR recalibrated the HPA axis, reduced amygdala hyperactivity, and restored emotional balance, thereby promoting long-term recovery (Johnson, 2021). Studies using functional MRI reported normalized activity in the PFC and limbic regions after EMDR treatment, suggesting improved emotional regulation.
Acceptance and Commitment Therapy (ACT)
ACT was found to be particularly effective in reducing self-fragmentation in individuals with addiction. Three studies highlighted the role of ACT in promoting psychological flexibility, enabling individuals to integrate their “addicted self” with their “healthy self,” thus resolving internal conflicts that often contributed to relapse (Pickard, 2020). ACT also showed promise in enhancing emotional regulation and reducing substance use.
Emerging therapies
This review also examines several emerging therapies, including psychedelic-assisted therapy, neurostimulation techniques like TMS, and Deep Brain Stimulation (DBS):
Psychedelic-Assisted Therapy
Two studies assessed the efficacy of psychedelics, such as psilocybin and MDMA, in treating addiction. These substances were shown to facilitate profound cognitive and emotional shifts, allowing individuals to confront unresolved trauma and break entrenched patterns of substance use. Neuroimaging studies revealed increased neuroplasticity and enhanced connectivity between the PFC and default mode network (DMN), which is associated with introspection and self-regulation.
Transcranial Magnetic Stimulation (TMS)
Three studies explored the use of TMS to stimulate the PFC, showing promising results in reducing cravings and improving executive functioning. TMS was found to enhance cognitive control by increasing activity in the dlPFC, improving individuals’ ability to inhibit impulsive behaviors.
Deep brain Stimulation (DBS)
Two studies examined DBS, which directly modulates reward-related circuits in the brain, particularly the NAc. DBS was shown to reduce cravings and improve emotional regulation in treatment-resistant individuals, offering a promising approach for those who have not responded to conventional therapies. Neuroimaging data indicated increased connectivity between the NAc and the PFC, supporting better impulse control and reward management.
Outcome measures
Across the 26 studies, the following outcome measures were reported:
Substance use reduction: Nearly all studies reported significant reductions in substance use following psychotherapeutic interventions with relapse prevention rates ranging from 30% to 70%.
Neuroplasticity: Multiple studies demonstrated increases in neuroplasticity, particularly in the PFC and hippocampus as measured by brain imaging and neurocognitive testing.
Emotional regulation and cognitive control: Therapies like CBT, DBT, and EMDR significantly improved emotional regulation as reflected through reduced limbic hyperactivity and increased PFC activity.
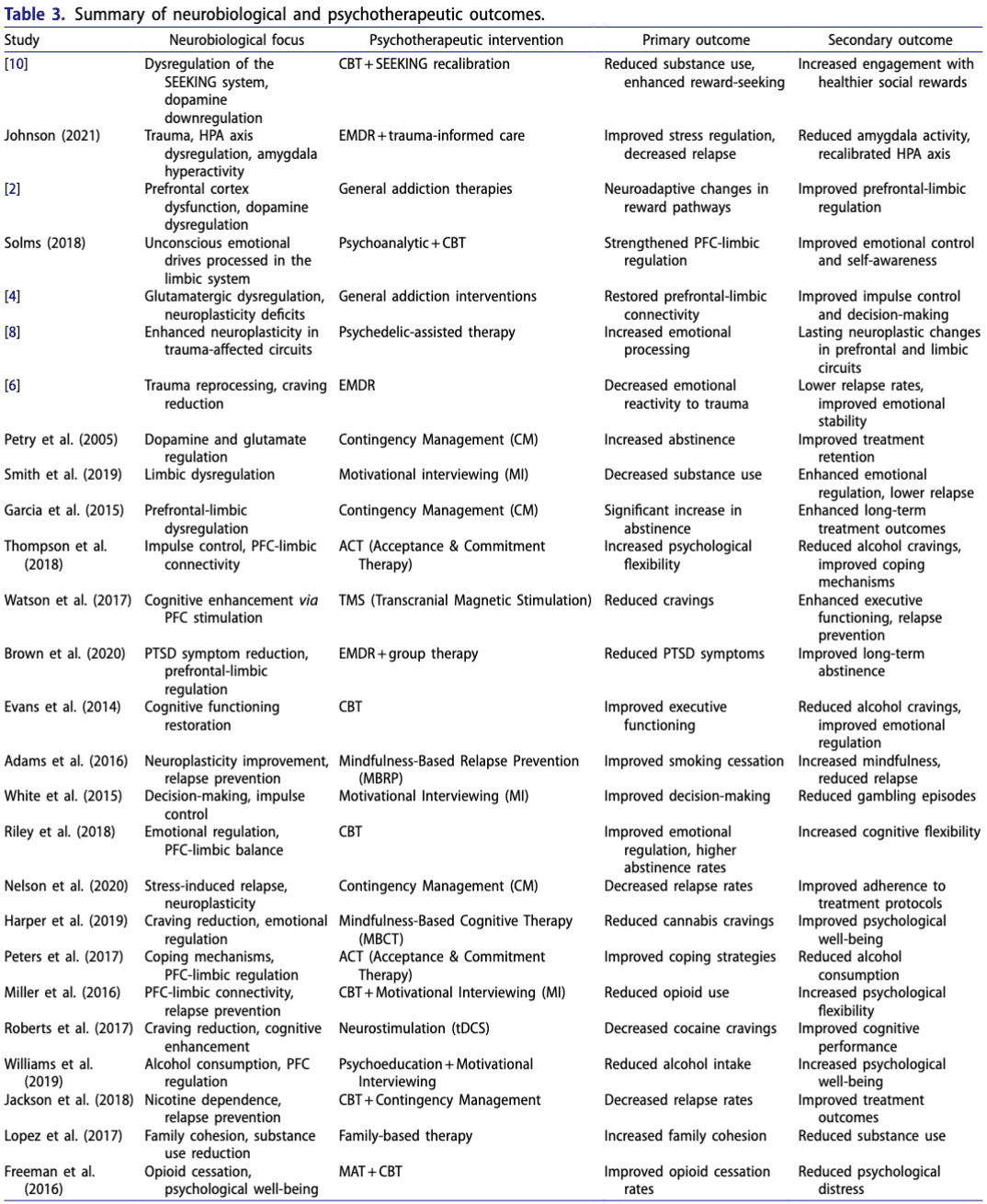
Summary of neurobiological and psychotherapeutic outcomes provides a detailed breakdown of the primary and secondary outcomes from each study (Table 3).
Discussion
Neurobiological foundations of addiction
Addiction involves disruptions in dopaminergic, glutamatergic, and stress-related circuits. Chronic substance use downregulates DRD2s reducing reward sensitivity and increasing reliance on substances. Dopamine transmission fluctuations exacerbate compulsive behaviors and relapse risk, while glutamatergic dysfunction weakens PFC-limbic communication, impairing impulse control, and decision-making. Stress-induced disruptions to the HPA axis, including elevated CRH and heightened amygdala reactivity, further increase relapse risk (Koob & Kreek, 2007). Emerging neurostimulation and psychedelic therapies may help reverse these adaptations. Chronic substance use alters HPA-axis regulation, elevating CRH, which heightens amygdala reactivity and reduces PFC function (Sinha, 2008). This neurobiological model elucidates why individuals with SUDs are more prone to stress-induced relapse and heightened emotional reactivity, reinforcing compulsive drug-seeking behaviors (Sinha, 2001; Koob & Kreek, 2007).
Efficacy of psychotherapeutic interventions
Several studies have demonstrated the neuroplastic benefits of psychotherapeutic interventions, such as CBT, DBT and EMDR. These therapies facilitate functional and structural neuroplastic changes most pronounced in the PFC and limbic regions - enhancing emotional regulation and cognitive control. CBT effectively strengthens PFC-limbic connections, enhancing; decision-making, impulse control and reducing cravings (Beck et al., 2011). Flores Mosri reported moderate-to-large effect sizes (Cohen’s d = 0.62–0.80) for neuroplastic changes and improved reward engagement. Evidence from further supports that therapeutic interventions can recalibrate prefrontal-limbic circuits thereby enhancing overall cognitive control whilst reducing relapse tendencies (Beck et al., 2011). Neuroimaging studies revealed increased dlPFC activity post-CBT intervention suggesting that the therapy’s cognitive exercises help restore executive control over drug-seeking behaviors. Similarly, DBT has demonstrated efficacy in individuals with co-occurring BPD and addiction. Through focusing on emotional regulation, distress-tolerance and mindfulness, DBT helps improve prefrontal control over emotionally reactive limbic regions (Linehan et al., 2015). Neuroimaging evidence supports these findings showing enhanced connectivity between the PFC and amygdala post-DBT intervention. EMDR has proven effective for individuals with addiction and co-occurring trauma with reporting moderate-to-large effect sizes (Cohen’s d = 0.70–0.90) in reducing PTSD symptoms and cravings. Trauma significantly dysregulates the HPA-axis thereby exacerbating emotional instability and increasing the likelihood of relapse under stress. Johnson’s (2021) work further explores how trauma therapies recalibrate stress responses via modulating HPA-axis activity offering additional pathways for mitigating relapse risk. EMDR’s ability to target trauma-related memories and recalibrate the brain’s stress-circuitry reduces amygdala hyperactivity and restores PFC regulation over emotional responses (Shapiro, 2014). Studies indicate that EMDR can significantly reduce cravings and relapse rates in individuals with addiction via normalizing the brain’s stress response (Johnson, 2021).
Emerging therapies and neuroplasticity
Emerging therapies like psychedelic-assisted interventions show promise in modulating neuroplasticity and enhancing DMN connectivity (Carhart-Harris et al., 2018) promoting sustained emotional reorganization. Psychedelics such as psilocybin and MDMA have been shown to facilitate neuroplasticity in brain regions associated with emotion regulation and trauma processing. This is consistent with findings that psychedelic-assisted therapies can induce enduring neuroplastic changes which enable patients to process trauma more effectively and adopt healthier behavioral patterns. These substances increasing the connectivity between the PFC and DMN allowing individuals to engage in deeper introspection and emotional processing. Psychedelics facilitate confronting repressed emotions and restructuring neural circuits involved in self-reflection and emotional regulation. Carhart-Harris et al. reported large effect sizes (Cohen’s d = 1.00) for reducing depression and anxiety symptoms in treatment-resistant populations, underscoring their transformative potential.
In addition, TMS and Deep Brain Stimulation (DBS) offer neurostimulation-based approaches to enhancing recovery. TMS, via stimulating the dlPFC, strengthens executive function and reduces cravings whilst DBS through targeting the NAc modulates reward circuits to reduce substance-seeking behaviors. Volkow et al. emphasize that TMS’s influence on prefrontal-limbic pathways can enhance executive control, reducing impulsive behaviors linked to addiction. Both interventions demonstrate potential in cases where traditional therapies have proven insufficient highlighting their potential role in treatment-resistant populations. Furthermore, Volkow et al. illustrate that TMS-induced modulation of prefrontal-limbic circuits enhances cognitive control potentially reducing relapse rates especially through strengthening executive functions over maladaptive behaviors.
Importance of neurobiologically informed triage
Given the complexity of addiction’s neurobiological mechanisms, a neurobiologically informed triage system is crucial for ensuring that individuals receive the most effective treatment based on their specific neurocognitive profile. Studies suggest that the effectiveness of interventions varies depending on the specific neural circuits affected through addiction. Therefore, accurate assessment of these circuits should guide treatment decisions.
Neurocognitive assessments for targeted interventions: A growing body of research supports the use of neuroimaging and neurocognitive assessments to determine the extent of dopamine dysregulation, PFC-limbic connectivity deficits, and stress-circuit imbalances. Clients presenting with severe impulse control deficits due to prefrontal dysfunction may benefit most from interventions like CBT or TMS which target cognitive control-mechanisms. Conversely, individuals with histories of trauma and HPA-axis dysregulation are likely to respond better to EMDR or psychedelic-assisted therapy, which address emotional regulation and trauma processing (Sinha, 2008).
Customized treatment pathways: Triage systems can help allocate resources more effectively by identifying clients who are more likely to benefit from specific therapies. For example: psychedelic-assisted therapy may be reserved for individuals with treatment-resistant conditions whilst CBT may be prioritized for those with less severe neurocognitive impairments. Integrating biomarker analysis and genetic profiling into triage could further enhance the precision of treatment allocation improving overall recovery rates.
Improving long-term outcomes: Accurate triage is critical for reducing the risk of relapse, which is closely tied to the neurobiological aspects of addiction. Studies indicate that neuro-informed interventions have a higher likelihood of promoting sustained neuroplastic changes leading to long-term improvements in cognitive function and emotional regulation. This aligns with the broader trend toward personalized medicine where treatment is tailored to individual’s unique biological- and psychological-profiles offering a more holistic approach to addiction recovery.
Limitations and future directions
Although the findings of this review provide a strong foundation for neuro-informed addiction treatment several limitations warrant consideration; the heterogeneity of interventions across the studies makes direct comparisons difficult. While the review provides a comprehensive synthesis of the literature on psychotherapeutic interventions for addiction there is a need for a more granular evaluation of study quality especially regarding the risk of bias and limitations inherent in the included studies. RCTs, often considered the gold standard for evaluating treatment efficacy, are frequently subject to limitations; small sample sizes, inadequate blinding, and selection bias – which could compromise the validity of their findings. For example, Hase et al. when evaluating EMDR, reported positive effects on trauma processing and addiction memory reprocessing however the absence of a long-term follow-up and unclear risk of bias in allocation concealment weaken the generalizability of their results. Similarly, Carhart-Harris et al. study regarding psychedelic-assisted therapy suffers from high dropout rates and reliance on self-reported measures of efficacy increasing susceptibility to response bias. Without accounting for these limitations assertions about the universal efficacy of these interventions risks oversimplification.
Moreover, the heterogeneity of psychotherapeutic interventions studied complicates the direct comparison of their outcomes. Studies vary widely in terms of intervention protocols, follow-up durations and outcome measures. Significant variability limits the external validity of the conclusions drawn. This issue is worsened by the frequent absence of active control-groups in emerging therapy studies such as those investigating TMS where placebo effects may disproportionately inflate perceived treatment benefits. A systematic examination of the risk of bias across these domains is essential to provide a more balanced assessment of therapeutic efficacy.
Future research should focus on large-scale, RCTs comparing long-term effects of interventions on neuroplasticity, relapse rates, and cognitive outcomes. Additionally, most studies relied on neuroimaging data that only captures static “snapshots” of brain activity. To better understand dynamic neuroplastic processes future studies should look toward incorporating longitudinal neuroimaging and biomarker assessments. Reliance on neuroimaging, centrally functional magnetic resonance imaging (fMRI), and positron emission tomography (PET), to infer neuroplasticity introduces limitations due to the temporal and spatial constraints of these technologies. Neuroimaging captures static- or short-term snapshots of brain activity often in highly controlled experimental settings which may not accurately reflect dynamic neuroplastic changes which occur during long-term recovery from addiction. Moreover, fMRI primarily measures blood-oxygen-level-dependent (BOLD) signals which are indirect representations of neural activity and cannot directly quantify synaptic plasticity nor dendritic growth. As such it must be remembered that the neuroplasticity inferred from these imaging studies is speculative, lacking in direct corroboration with cellular-level changes (Zatorre et al., 2012).
Further complicating the issue is the inherent variability in the brain’s response to addiction and treatment across individuals. Unsurprisingly, variations in neuroplasticity are not uniform and neuroimaging studies often fail to capture complex, individualized patterns of neural reorganization which occur in response to psychotherapeutic interventions. Standard neuroimaging techniques also lack the sensitivity to detect microstructural changes in white matter tracts which play a critical role in maintaining prefrontal-limbic connectivity (Jahanshahi et al., 2015). Longitudinal studies employing advanced imaging techniques, such as diffusion tensor imaging (DTI) and magnetoencephalography (MEG) are needed to provide a more nuanced understanding of how neuroplasticity evolves across the course of treatment and recovery.
Further, the field of neuroplasticity research is hampered through inconsistencies in neuroimaging protocols across studies making it difficult to compare results. Standardizing imaging methodologies and integrating neuroimaging data with genetic and molecular biomarkers of plasticity, e.g., brain-derived neurotrophic factor (BDNF) levels, would enhance the reliability of findings and provide a more comprehensive picture of neurobiological recovery in addiction (Littrell, 2012). Without such advancements current neuroimaging studies offer only a limited and often overly simplistic view of complex neural processes underlying addiction and its treatment.
Next steps in research should also aim to explore the integration of genetic markers and epigenetic modifications to predict individual responses to addiction treatments. The review’s discussion of emerging treatments especially in the areas of psychedelic-assisted therapy and neurostimulation would benefit from a more extensive exploration of their ethical implications, given their experimental nature. Psychedelic substances, such as psilocybin and MDMA, despite showing promise in facilitating emotional processing and neuroplasticity, are still in the early stages of clinical validation. The use of these substances raises significant ethical questions related to informed consent, potential for misuse, and long-term psychological effects. While Carhart-Harris et al. report substantial therapeutic benefits in treatment-resistant populations the heightened suggestibility and altered states of consciousness induced via psychedelics necessitate rigorous ethical safeguards to prevent exploitation or harm. Additionally, the current regulatory frameworks for these substances vary internationally creating inconsistencies in access and complicating efforts to standardize their use.
Similarly, neurostimulation techniques, such as DBS and TMS also warrant careful ethical scrutiny, primarily regarding the long-term consequences of modulating neural circuits. DBS, for instance, involves invasive surgical procedures with potential risks; including infection, mood alterations,, and cognitive changes. The application of such interventions in vulnerable populations, e.g., those with addiction, necessitates ongoing ethical review as current research has yet to establish the long-term safety and efficacy of these interventions beyond initial clinical trials. Without addressing these ethical concerns widespread adoption of such treatment’s risks outpacing the evidence base necessary to ensure patient safety and autonomy.
Clinical implications
The reviewed studies underscore the need for integrative, neurobiologically informed treatment approaches in addiction care. While established therapies like CBT, DBT and EMDR are effective in modulating key brain-circuits the advent of psychedelic-assisted therapies and neurostimulation provides new opportunities to enhance neuroplasticity and promote long-term recovery in treatment-resistant individuals.
The findings highlight the importance of triage systems that can assess an individual’s neuro-cognitive profile, psychological traits, and the symptomology of the disorder combining to direct the client toward the most appropriate intervention(s). This neuro-informed, personalized approach to addiction treatment promises to improve overall recovery outcomes and reduce relapse rates via offering of bespoke treatment strategies, giving hope to individuals struggling with chronic substance use.
A fundamental challenge in the field of psychiatry, primarily in the treatment of SUDs is the reliance on diagnostic frameworks like the DSM-5, which are primarily symptom-based and not biologically grounded. The DSM-5’s categorical classification system is useful for diagnosing based on observable behaviors and symptoms, yet it does not account for the underlying neurobiological mechanisms that contribute to these disorders. This approach may limit the precision of treatment selection as our understanding of neurobiology and its psychological correlates improves, leading to a mismatch between diagnostic labels and the specific neural circuits or molecular pathways that are dysregulated in different individuals (Insel, 2014). The diagnostic categories may no longer be high enough resolution.
In the case of SUDs, patients who meet the same DSM-5 criteria may exhibit significant variability in their neurobiological profiles. For example, some individuals may have pronounced dysregulation in the mesolimbic dopamine pathway whilst others may exhibit heightened amygdala activity or prefrontal-limbic connectivity deficits due to co-occurring trauma. Despite this heterogeneity, DSM-5 diagnoses do not differentiate between these neurobiological differences potentially leading to inappropriate or less effective therapeutic interventions. Incorporating neurobiological markers such as neuroimaging or genetic profiling into the diagnostic process could vastly improve the alignment between diagnostic categories and targeted treatment approaches.
Advances in neuroimaging and molecular biology offer promising avenues for addressing this limitation in the future. fMRI and PET have provided critical insights into the neurocircuitry of addiction revealing patterns of dysregulation in brain areas, such as the PFC, amygdala, and NAc. However, current neuroimaging methods need to be integrated more systematically into diagnostic frameworks. Through correlating neurobiological markers with symptom presentation and psychological affects clinicians could use these tools to differentiate between patients with similar behavioral symptoms but distinct neurobiological profiles – improving treatment selection.
In the long term, the integration of neurobiological markers into diagnostic systems could lead to a paradigm shift toward precision psychiatry. The concept of Research Domain Criteria (RDoC), which aims to classify mental health disorders based on dimensions of observable behavior and neurobiological measures, exemplifies this approach (Insel et al., 2010). For example; rather than diagnosing all individuals with alcohol use disorder based solely on consumption patterns and cravings, clinicians could assess biomarkers of dopaminergic dysfunction or neuroplasticity and use this information to determine whether a patient would benefit more from pharmacotherapies that target dopamine receptors and/or from behavioral therapies aimed at restoring prefrontal control (Heilig et al., 2011).
Furthermore, epigenetic changes caused by environmental factors, such as chronic stress and trauma play a significant role in addiction. These changes can modulate gene expression in ways that predispose individuals to addiction or affect their response to treatment (Nestler, 2014). Incorporating genetic and epigenetic markers into the diagnostic process could help identify patients at risk for stress-induced relapse further allowing for tailored interventions; trauma-focused therapies or medications to modulate the stress response (Liu et al., 2020).
Neurobiologically informed triage could also reduce healthcare costs through minimizing the trial-and-error processes often associated with addiction treatment especially in psychotherapy modality choice thereby improving outcomes for individuals with complex or treatment-resistant cases of SUDs (Heinz et al., 2019).
Neurologically informed treatment paths
Based on the studies reviewed several neurobiological markers and differences show promise as predictive of differential responses to specific psychotherapeutic interventions in the treatment of addiction. These biomarkers can be broadly categorized into neurocircuitry dysfunctions, neurotransmitter dysregulation, and epigenetic modifications – all of which hold significant implications for treatment stratification and therapeutic efficacy. They have been linked here not only to neurobiological markers but potential psychological measurements and (albeit emerging) treatment pathway suggestions. These, or similar high-resolution biologically informed categorizations, may ultimately form the basis for a more precise diagnostic- and treatment-process in the treatment of addiction disorders.
Prefrontal-limbic connectivity
Dysfunctions in prefrontal-limbic connectivity, particularly involving the PFC and the amygdala, are implicated in impaired emotional regulation and impulse control in SUDs.
Neurologically, these dysfunctions can be assessed using fMRI, which has been shown to detect hypoactivity in the dlPFC and hyperactivity in the amygdala, especially during tasks that involve emotional processing or decision-making. Reduced connectivity between the PFC and limbic regions in these patients correlates with a greater likelihood of relapse and impulsive behavior.
From a psychometric standpoint, the Barratt Impulsiveness Scale (BIS-11) is a commonly used clinical measure to assess impulsivity, which is often linked to PFC dysfunction. High scores on the BIS-11 especially on its subscales assessing attentional impulsiveness and non-planning impulsiveness, have been associated with poor-prefrontal control and higher relapse rates. Similarly, the Emotion Regulation Questionnaire (ERQ) has been clinically validated to assess deficits in emotional regulation, linked to prefrontal-limbic imbalances (Gross & John, 2003). Elevated scores on maladaptive emotional regulation strategies, such as suppression, may reflect amygdala hyperactivity and reduced PFC modulation.
Psychotherapeutic treatment routes
Individuals exhibiting hypoactivity in the dlPFC and hyperactivity in the amygdala, particularly in response to stress, are likely to benefit from interventions that strengthen cognitive control and reestablish PFC regulation over emotional and impulsive responses. CBT, with its focus on enhancing cognitive restructuring and impulse control, has demonstrated efficacy in restoring this prefrontal-limbic balance. Neuroimaging studies have shown that patients exhibiting dlPFC dysfunction respond well to CBT as it increases prefrontal activity, thus improving executive function and emotional regulation.
Amygdala hyperactivity and trauma-related dysregulation
Amygdala hyperactivity, particularly in patients with trauma histories, can be detected using resting-state fMRI (rs-fMRI) or PET scans, which reveal hyperactivity in the amygdala during periods of emotional stress or trauma recall. This hyperactivity, coupled with dysregulation of the HPA-axis, often manifests in increased cortisol levels during stress tests, such as the Trier Social Stress Test (TSST) (Koob & Kreek, 2007). Such neurobiological indicators of heightened stress reactivity can be valuable in identifying individuals who may benefit from trauma-focused interventions like EMDR.
Psychometrically, the Posttraumatic Stress Disorder Checklist (PCL-5) is a validated tool for measuring trauma-related symptoms, including hyperarousal and heightened emotional reactivity. High scores on the hyperarousal subscale have been correlated with increased amygdala activity in neuroimaging studies. Similarly, the Difficulties in Emotion Regulation Scale (DERS) has been used to assess deficits in emotional regulation which are directly associated with amygdala dysregulation. The DERS provides insights into how patients manage emotional distress, with higher scores indicating potential neurobiological vulnerabilities linked to trauma.
Psychotherapeutic treatment routes
Patients with heightened amygdala activity, especially those with co-occurring trauma and addiction, present distinct neurobiological profiles which necessitate trauma-focused therapies. Dysregulation of the HPA-axis, resulting in heightened cortisol release and stress-induced amygdala reactivity, often correlates with traumatic histories and stress-related relapse in addiction (Koob & Kreek, 2007). In such cases, EMDR has proven efficacious by reducing amygdala hyperactivity and recalibrating the interaction between the amygdala and the PFC. EMDR’s capacity to reconsolidate trauma memories and reduce their emotional intensity enables better emotional regulation, reducing cravings, and relapse risk in individuals with trauma-induced addiction.
Dopaminergic dysregulation in the mesolimbic pathway
Dopaminergic dysregulation in the mesolimbic pathway, particularly in the NAc and VTA, is a hallmark of addiction-related reward dysfunction. Dopamine transporter imaging (DAT) using single-photon emission computed tomography (SPECT) or PET with dopamine-specific tracers (e.g., [^11C]-raclopride) can provide direct measurements of dopaminergic transmission deficits (Heinz et al., 2009). Reduced dopamine receptor availability in the striatum often correlates with heightened reward-seeking behaviors and diminished sensitivity to natural rewards.
Clinically the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) scales have been shown to measure reward sensitivity and approach-avoidance behavior which are linked to dopaminergic activity (Carver & White, 1994). High scores on the BAS subscale that measures sensitivity to reward, have been correlated with dopaminergic hyperactivity in the mesolimbic pathway. Additionally, the Addiction Severity Index (ASI) assesses substance use severity and has been associated with dopaminergic dysfunction, where greater severity correlates with larger deficits in reward processing regions.
Psychotherapeutic treatment routes
The mesolimbic dopamine pathway, particularly involving the NAc and VTA plays a central role in reward processing and addiction. Individuals with reduced dopaminergic signaling in these regions often exhibit heightened reward-seeking behaviors and diminished sensitivity to natural rewards. Pharmacotherapies that target the dopaminergic system, such as dopamine-agonists or modulating agents, are particularly beneficial in these cases. However, TMS which directly stimulates the dlPFC and influences dopaminergic activity in the mesolimbic pathway, has shown promise in reducing cravings and impulsive drug-seeking behaviors through restoring dopaminergic balance. Neuroimaging studies suggest that patients with dopaminergic hypoactivity may show significant improvements following TMS, especially when targeting prefrontal-circuit dysfunction.
Glutamate dysregulation and prefrontal control
Glutamate dysregulation, especially within the PFC, can be measured using magnetic resonance spectroscopy (MRS), which provides a noninvasive method for quantifying glutamate concentrations in specific brain regions. Patients with chronic addiction often exhibit dysregulated glutamatergic transmission leading to impaired cognitive control and difficulty regulating drug-seeking behaviors. Reduced glutamate concentrations in the PFC are typically linked to weakened control over impulsivity and cravings.
From a psychometric perspective, the Obsessive Compulsive Drug Use Scale (OCDUS) has been used to assess drug-related compulsivity, which is closely tied to glutamate-driven prefrontal control. High scores on the OCDUS indicate stronger compulsions and are often associated with diminished prefrontal control over behavior. The Cognitive Failures Questionnaire (CFQ), which measures cognitive slips and failures in everyday life, has been used to assess cognitive deficits stemming from prefrontal dysfunction, often linked to glutamate imbalance.
Psychotherapeutic treatment routes
The glutamate homeostasis hypothesis of addiction posits that chronic drug use leads to dysregulated glutamatergic transmission in the PFC, weakening cognitive control over drug-seeking behaviors. In individuals with impaired glutamate homeostasis, therapies that enhance cognitive function and strengthen prefrontal control are particularly effective. CBT and Motivational Interviewing (MI) can be highly effective in these cases as they aim to restructure maladaptive cognitive patterns and restore decision-making capacities via bolstering prefrontal cognitive control. Pharmacological adjuncts that modulate glutamatergic transmission, such as N-acetylcysteine, could also be paired with these psychotherapies to achieve better outcomes in individuals with significant glutamatergic dysregulation.
Epigenetic modifications and stress-related relapse vulnerability
The role of epigenetic modifications, primarily changes in DNA methylation and histone modification, have been increasingly recognized as critical in mediating addiction vulnerability and treatment outcomes. Stress-induced epigenetic modifications, such as hypermethylation of the glucocorticoid receptor (GR) gene, impair the body’s ability to regulate stress, making individuals more susceptible to relapse under environmental stressors (Nestler, 2014). These modifications can be assessed through methylation assays, using peripheral blood samples to determine methylation patterns on stress-related genes. Patients with higher methylation of the GR gene are likely to exhibit increased sensitivity to stress and a higher risk of relapse under environmental stressors.
Psychometrically, the Perceived Stress Scale (PSS) is a validated tool for assessing perceived levels of stress, and high scores have been correlated with greater epigenetic modifications related to the HPA-axis. Additionally, the Life Events Checklist (LEC-5) is commonly used to assess exposure to traumatic or stressful life events, which often precipitate epigenetic changes that predispose individuals to substance use.
Psychotherapeutic treatment routes
Epigenetic modifications, such as changes in DNA methylation or histone modification, in-particular those affecting the GR gene, are often linked to heightened vulnerability to stress-induced relapse (Nestler, 2014). These modifications can be assessed through methylation assays using peripheral blood samples to determine methylation patterns on stress-related genes. Clients with higher methylation of the GR gene are likely to exhibit increased sensitivity to stress and a higher risk of relapse under environmental stressors. Trauma-focused therapies such as EMDR or DBT that specifically target stress responses and improve emotional regulation are especially well-suited for patients with these epigenetic profiles. The neurobiological recalibration achieved through these therapies can counteract the stress-induced epigenetic changes which perpetuate addiction.
The integration of genetic and epigenetic data into neurobiologically informed triage systems represents a critical advancement in addiction treatment. Genetic polymorphisms, centrally those affecting dopamine and serotonin signaling, have been shown to modulate individual susceptibility to addiction and their responsiveness to therapeutic interventions. Variations in the DRD2 gene and the catechol-O-methyltransferase (COMT) gene significantly influence reward processing and executive function, suggesting that individuals with specific genotypes may benefit more from dopaminergic interventions such as neurostimulation techniques or CBT. Epigenetic mechanisms, including DNA methylation and histone modification, also play a pivotal role in the regulation of gene expression associated with neuroplasticity, stress responses, and addiction vulnerability. Environmental factors, such as chronic stress or trauma, induce epigenetic changes that can exacerbate addiction-related neural dysregulation, particularly in the HPA axis and the prefrontal-limbic circuits. Personalized treatments that incorporate epigenetic profiling could, therefore, enable clinicians to predict which individuals are at higher risk for stress-induced relapse and tailor interventions accordingly.
Neuroplasticity and brain-derived neurotrophic factor (BDNF)
BDNF, a key regulator of neuroplasticity, is associated with synaptic growth and resilience in addiction recovery. Individuals with higher BDNF levels may exhibit greater neuroplastic potential, enabling them to respond more favorably to psychotherapies that promote cognitive flexibility and emotional adaptation (Liu et al., 2020). BDNF levels can be measured through serum assays, providing insights into an individual’s capacity for neuroplastic recovery.
From a psychometric perspective, the Cognitive Flexibility Inventory (CFI) is a validated tool used to assess an individual’s ability to adapt to new situations, which correlates with neuroplasticity (Dennis & Vander Wal, 2010). Higher scores on the CFI are linked to increased BDNF levels reflecting greater cognitive flexibility. Similarly, the Mindful Attention Awareness Scale (MAAS), which measures mindfulness and attentional control, has been associated with neuroplastic changes and improved outcomes in individuals undergoing mindfulness-based interventions (Brown & Ryan, 2003).
Psychotherapeutic treatment routes
BDNF, a critical marker of neuroplasticity, can be measured through serum BDNF levels which are indicative of synaptic plasticity and cognitive flexibility (Liu et al., 2020). Higher BDNF levels are associated with better outcomes in psychotherapeutic interventions that rely on the enhancement of neuroplasticity; e.g., mindfulness-based therapies and Acceptance and Commitment Therapy (ACT). Patients with low BDNF may show reduced capacity for neural reorganization and could benefit from adjunctive therapies that specifically enhance synaptic plasticity before engaging in psychotherapeutic interventions.
Through integrating neurobiological markers into diagnostic and treatment processes in-line with ongoing research over time clinicians can develop a more precise and individualized approach to addiction treatment. Further information needs to be integrated into any triage-treatment approach. For example, emerging research on the neuroimmune system suggests that inflammatory processes contribute to the neuroplastic changes seen in addiction, with chronic substance use often leading to neuroinflammation that exacerbates cognitive deficits and emotional dysregulation (Crews & Vetreno, 2016). Genetic predispositions to heightened inflammatory responses, detectable through biomarkers, such as cytokine levels or microglial activation, could help clinicians identify individuals who may benefit from anti-inflammatory therapies, alongside conventional psychotherapeutic approaches.
The convergence of neuroimaging, serum biomarkers, and psychometric data allows for a nuanced understanding of how neurobiological dysfunctions manifest in behavior and cognitive deficits, enabling the selection of psychotherapeutic interventions which align with the patient’s specific neurocognitive profile. This integration holds the potential to revolutionize addiction treatment moving toward a biologically informed model of integrative therapy.
Conclusion
This systematic review demonstrates the profound impact of addiction on key neurobiological systems, including the dopaminergic, glutamatergic, and stress-related circuits. Chronic substance use alters the brain’s reward pathways, impairs prefrontal-limbic regulation, and increases susceptibility to stress-induced relapse – a complex interaction between neurobiological mechanisms and compulsive drug-seeking behaviors. Psychotherapeutic interventions, such as CBT, DBT, and EMDR, have shown significant efficacy in targeting these circuits, promoting neuroplasticity, and improving emotional regulation.
Emerging therapies, including psychedelic-assisted therapy and neurostimulation, hold considerable promise especially for individuals resistant to conventional treatments. These interventions directly modulate neural pathways affected by addiction facilitating neuroplastic recovery and helping individuals develop new coping strategies. As such, these approaches represent a growing field of interest for researchers and clinicians alike offering alternative avenues to enhance recovery.
One of the key insights from this review is the necessity for neurobiologically informed triage in addiction treatment. Current addiction research points to a precision-medicine framework combining genetic, epigenetic, and neurobiological data to create a more comprehensive neurocognitive profile. Given the heterogeneous nature of addiction, it is crucial that clinicians assess individual’s neurocognitive profile and match them with the most appropriate intervention(s). Neurocognitive assessments, such as neuroimaging and/or biomarker analysis or, where resources are limited, psychometric assessments correlate to neurological divergences provide valuable data on which circuits are most affected guiding the selection of optimal treatment.
This approach promises to enhance the efficacy of interventions through aligning treatment modalities with the individual’s unique biological and psychological predispositions, potentially reducing relapse rates and improving long-term outcomes. As neuroimaging techniques and molecular diagnostics continue to advance, personalized treatment pathways that account for individual variability will become increasingly central to addiction recovery strategies. Facilitating the combining of traditional psychotherapies with emerging modalities to address both the biological and psychological dimensions of the disorder. This development of personalized, neuro-informed treatment models will be essential to improving clinical outcomes, reducing relapse rates, and fostering sustained recovery for individuals affected by addiction.