Abstract
The present review focuses on potential neural mechanisms underlying recovery from psychiatric conditions characterised by impaired impulse control, specifically substance use disorders, gambling disorder, and internet gaming disorder. Existing treatments (both pharmacological and psychological) for these addictions may impact brain processes, and these have been evaluated in neuroimaging studies. Medication challenge and short-term intervention administration will be considered with respect to treatment utility. Main models of addiction (e.g., dual process, reward deficiency syndrome) will be considered in the context of extant data. Additionally, advanced analytic approaches (e.g., machine-learning approaches) will be considered with respect to guiding treatment development efforts. Thus, this narrative review aims to provide directions for treatment development for addictive disorders.
Introduction
Substance use disorders (SUDs) and behavioural addictions (such as gambling disorder (GD) or internet gaming disorder (IGD)) often include complex clinical courses involving cyclical patterns of relapse, treatment, and recovery (El-Guebaly, 2012). Recovery is a complex construct that has been associated with abstinence in addictions (El-Guebaly, 2012). Although recovery can occur naturally, numerous pharmacological and psychological interventions have been tested to promote recovery. However, brain mechanisms underlying specific treatments for addictions remain underexplored and poorly understood. Therefore, we review existing knowledge regarding brain-based factors related to recovery and treatment outcomes or potential mechanisms of change gleaned from neuroimaging studies of addictions, and specifically SUDs, GD, and IGD. In this process, we will provide current conceptual frameworks for these disorders, consider existing treatments facilitating recovery, and review neurobiological underpinnings implicated in recovery processes.
Substance use disorders
Substance use disorders conceptualisation
Substance addictions are termed SUDs in the Diagnostic and Statistical Manual (DSM-5) (APA 2013). SUD criteria exist for alcohol, tobacco, cannabis, hallucinogens, inhalants, opioids, sedatives, hypnotics or anxiolytics, caffeine, and other unknown substances. Diagnostic criteria for SUDs currently include using larger amounts than intended, persistent desires to cut down or quit, significant time spent taking or obtaining substance, cravings, failure to fulfil obligations, continued use despite negative consequences, reduced social or recreational activities, use in physically hazardous situations, use despite knowledge of harms, tolerance, and withdrawal. Of note, craving was added as a criterion for SUDs, although for other addictive disorders (GD, IGD), there is no formal craving criterion. Nonetheless, cravings/urges represent important treatment targets across addictive disorders.
Substance use disorders treatment
Multiple drugs have support in the treatment of SUDs. For example, for alcohol use disorder, naltrexone and acamprosate have been tested as first-line agents in maintenance therapy, while disulphiram has been considered a second-line agent, and all have formal indications (Klein 2016). In the case of a tobacco use disorder, nicotine replacement therapy, varenicline and bupropion are considered first-line agents. For opioid use disorders, methadone, buprenorphine and naltrexone are considered first-line agents (Klein 2016). For cocaine use disorders, the Clinical Research Efficacy Screening Trial (CREST) program of the US National Institute on Drug Abuse supported phase II controlled clinical trials of four medications (cabergoline, reserpine, sertraline and tiagabine) (Gorelick et al. 2004; Kampman et al. 2005; Leiderman et al. 2005). Modafinil also appears to be effective in the treatment retention and reduction of cocaine use, although more controlled studies supporting its efficacy are lacking (Gorelick et al. 2004). Several recent articles have reviewed novel pharmacotherapies for cocaine use disorder (Angarita et al. 2021; Hadizadeh, Flores, Mayerson, et al. 2022; Hadizadeh, Flores, Nunes, et al. 2022), and further testing of their efficacies, tolerabilities and mechanisms of action appear warranted. Regarding cannabis use disorder, buspirone has demonstrated efficacy in a controlled clinical trial (Weinstein and Gorelick 2011). Additional research is warranted to examine the efficacy of buspirone and other drugs such as gabapentin and N-acetylcysteine as treatments for cannabis use disorder (Gorelick 2017). Finally, despite many unsuccessful clinical trials, there are some promising medications, such as modafinil, bupropion, and naltrexone, which have shown positive results in reducing amphetamine or methamphetamine use, along with agonist replacement medications like d-amphetamine and methylphenidate, although none have formal indications (Karila et al. 2010). To sum up, the currently U.S. Food and Drug Administration (FDA) approved drugs for alcohol use disorder include disulphiram, naltrexone, and acamprosate (Akbar et al. 2018); for tobacco cessation include nicotine replacement therapy, bupropion sustained-release, and varenicline (Krist et al. 2021); for opioid use disorder include methadone, naltrexone and buprenorphine (Shulman et al. 2019); and for cocaine and cannabis use disorders, there are no FDA-indicated drugs.
With regard to psychological interventions, cognitive behavioural therapy (CBT) has demonstrated efficacy for SUDs, both as monotherapy and in combination with other therapeutic approaches (Kathryn McHugh et al. 2010). Alternatives to CBT also exist, and these include practical group counselling (Costello et al. 2010), motivational enhancement (DiClemente et al., 2008), 12-step facilitation (Project MATCH Research Group 1997), mindfulness-based relapse prevention (Korecki et al. 2020), extinction-based therapies (Vollstädt-Klein et al. 2011), cognitive bias modification training (Wiers et al. 2015), and virtual reality (Son et al. 2015), among others.
Finally, neuromodulation strategies, both invasive and non-invasive, are beginning to be tested for the treatment of SUDs. These approaches include repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), and deep brain stimulation (DBS) (Mahoney et al. 2020).
Neural mechanisms underlying substance use disorders and recovery
SUDs have been associated with lower grey-matter volumes (GMVs) regionally, especially in the prefrontal cortex (PFC). Increased GMV in these areas have been linked to treatment responses (Moeller and Paulus 2018). Nonetheless, whether changing GMVs in people with SUDs may lead to better outcomes is unclear. Recently, synaptic density has been reported as being reduced in PFC regions in individuals with cocaine use disorder (Angarita et al., 2022). These findings raise the intriguing and currently speculative notion that interventions promoting synaptic density (e.g., ketamine) may have potential treatment utility, and perhaps particularly in individuals with co-occurring depression (Angarita et al. 2021). Regarding subcortical regions such as the striatum, amygdala or hippocampus, there are insufficient studies to draw strong conclusions regarding treatment-GMV relationships, with some studies suggesting different directions of associations when linking GMVs to treatment outcomes (Moeller and Paulus 2018). Additionally, in contrast to the PFC findings in cocaine use disorder, reduced synaptic density has been observed in the hippocampus in cannabis use disorder (Souza et al. 2021), indicating the importance of considering targeted interventions differentially across SUDs.
Pharmacological treatments
Naltrexone
Treatment with naltrexone, an opioid receptor antagonist, is indicated in the treatment of alcohol use disorder (Myrick et al. 2008). The combination of naltrexone and ondansetron hydrochloride reduced cue-related activation in the ventral striatum (VS) during functional magnetic resonance imaging (fMRI) and craving (Myrick et al. 2008). These findings suggest that naltrexone may reduce VS activations to alcohol cues, consistent with its effects on reducing alcohol-related cravings.
Modafinil
Modafinil has stimulant properties linked to actions on the dopamine, glutamate and GABA systems. Modafinil seems to block dopamine transporters and elevate dopamine levels in the human brain, particularly in the nucleus accumbens (Volkow et al. 2009). In people with alcohol use disorders, modafinil administration was associated with greater functional connectivity (rsFC) between the superior frontal gyrus (SFG) and VS and less impulsive choices on a delay-discounting task (Schmaal et al. 2014). These findings suggest that modafinil may act by increasing cortical control over subcortical regions implicated in promoting drug use, consistent with dual process models of addictions (Kober et al. 2010; Lannoy et al. 2014). The administration of modafinil to patients with methamphetamine use disorder generated increased activation in the anterior cingulate cortex (ACC) and bilateral insula/ventrolateral PFC, reflecting a greater activation of these areas during cognitive and learning processing associated with the fMRI task and suggesting a role for cognitive-enhancement processes in treating SUDs (Ghahremani et al. 2011). Finally, modafinil does not seem to be an effective treatment option for cocaine use disorder (Karila et al. 2016).
Bupropion
Buproprion has stimulant properties and impacts bioaminergic systems, blocking noradrenergic and dopaminergic reuptake. Culbertson et al. (2021) administered bupropion or placebo for 8 weeks to 30 individuals with tobacco use disorder. Participants who were treated with bupropion showed, when actively resisting craving, reduced posttreatment activation in the bilateral ACC, right medial orbitofrontal cortex (OFC), and left VS, compared with control subjects, as well as reduced craving. These findings suggest that this drug may help individuals with tobacco use disorder resist craving by reducing drug-reward-cue-related neural activations.
Amisulpride
Amisulpiride inhibits D2-like dopamine receptors. Ten abstinent men with alcohol use disorder showed reduced cue-induced activation in the right thalamus after amisulpride administration compared to the pre-administration fMRI scan, although there were no changes in self-reported craving (Hermann et al. 2006). After administration, no significant differences were observed in cue-elicited blood oxygenation level dependent (BOLD) responses compared to controls men without alcohol use disorder. Thus, mechanisms underlying amisulpiride treatment warrant additional investigation.
Baclofen
After administration of the GABAergic drug baclofen, patients with cocaine use disorder showed less activation to subliminal cocaine cues in the amygdala, VS, ventral pallidum, midbrain, and OFC, compared to patients receiving a placebo. This study suggests that baclofen may impact drug-reward-cue processing in reward circuitry at levels below consciousness (Young et al. 2014).
Methadone
Neural mechanisms underlying treatment with methadone, a long-acting agonist at opioid receptors, may involve the following: (a) increased activity in people with opioid use disorder receiving methadone maintenance treatment, in comparison with controls, in visuospatial attention and mesolimbic reward circuits (Wang et al. 2014); (b) greater activations in the cerebellum and right lingual gyrus in patients undergoing methadone maintenance treatment, in comparison to people with opioid use disorder undergoing opiate abstinence (Tabatabaei-Jafari et al. 2019); and, (c) increased activation to drug-related cues in the insula, OFC, and left hippocampal complex (Langleben et al. 2008). Additionally, methadone-related outcomes have been explored using connectome-based predictive modelling (Lichenstein et al. 2021), as described below.
Varenicline
Varenicline, a partial nicotinic agonist, has a formal indication for treating tobacco use disorder, and it has been observed that a more positive smoke-food difference score for executive control predicted a greater response to the drug, compared to placebo, suggesting that brain reactivity in default mode network (DMN) to palatable cues may be associated with tobacco use disorder (Wilcox et al. 2018). These findings are consistent with the notion that difficulties disengaging the DMN are linked to psychopathology including other SUDs (Worhunsky et al. 2021), and altering such tendencies/abilities (e.g., through drugs or other interventions like mindful meditation) (Brewer et al. 2011) may represent effective treatments for SUDs.
Another study of varenicline observed increased activation of the lateral OFC, ACC, posterior cingulate cortex (PCC), SFG and dorsolateral prefrontal cortex (dlPFC), as well as reduced activation of the VS and medial OFC among people with tobacco use disorder when exposed to tobacco cues (Franklin et al. 2011), consistent with dual process models of addictions that suggest increasing control-related activity over subcortically driven motivations may reflect a mechanism underlying effective treatments for SUDs. Among individuals with alcohol use disorder, although varenicline did not reduce drinking behaviour, it reduced cue-induced OFC activation bilaterally, consistent with findings in tobacco use disorder with respect to reducing addiction-cue-related activation in reward circuitry (Schacht et al. 2014).
Psychological treatments
Cognitive behavioural therapy
Few studies have analysed the effects of CBT using neuroimaging (Cabrera et al. 2016). Among them, some have evaluated associations between pre-treatment brain activation and treatment outcome. For example, Brewer et al. (2008) studied treatment-seeking participants with cocaine use disorder and administered an event-related fMRI Stroop color-word interference task before patients started treatment. The different treatments investigated included treatment as usual, computer-assisted CBT, or therapist-delivered CBT in conjunction with one of four conditions: (a) placebo, (b) disulphiram, (c) contingency management and placebo, and (d) contingency management and disulphiram. Activation of the ventromedial prefrontal cortex (vmPFC), right striatum, and left PCC correlated with longer durations of self-reported abstinence and striatal activation correlated with percent cocaine-free urine toxicologies. These findings suggest that more robust activation of control circuitry activation were associated with drug abstinence. Subsequent studies (described below) suggest that neural processing related to cognitive control may become more efficient following treatment (DeVito et al. 2012, 2017, 2019; Yip et al. 2014). In contrast, VS activation during anticipation of monetary rewards, typically blunted in people with SUDs, may increase during treatment, suggesting a corrective action on blunted non-drug-reward-anticipation-related VS activation in individuals with SUDs (Balodis et al. 2016; Garrison et al. 2017; Luijten et al. 2017). Given similar findings of blunted reward-anticipation-related VS activation in individuals with binge eating disorder that have been linked to treatment outcomes (Balodis et al. 2013, 2014), the findings suggest a possible transdiagnostic mechanism for recovery, consistent with models (e.g., reward deficiency syndrome) proposed for SUDs and related disorders (Blum et al. 2000).
Xu et al. (2014) evaluated relationships between cocaine use before and during CBT and hippocampal and amygdalar volume in 23 patients with cocaine use disorder. More days of cocaine use before starting CBT were associated with larger hippocampal volumes which were also related to treatment response. Moreover, hippocampal volumes mediated the association between cocaine use before CBT and treatment response.
Yip et al. (2014) analysed pre-treatment structural and functional fMRI data and associated them with treatment responses to contingency management, CBT or both among individuals with cannabis use disorder. Relative to comparison subjects, those with cannabis use disorder who did not achieve 21 d of consecutive abstinence showed relatively increased activation within the striatum while processing losing outcomes on the monetary incentive delay task. The abstinent cannabis-use-disorder group, however, did not show differences with comparison subjects without, and therefore striatal involvement in associative reward-related learning may be a mechanism linked to treatment outcome. Another study suggested that greater Stroop-related activity in the dorsal ACC (dACC) was related to less cannabis use during and following treatment, again suggesting that greater control-related activation prior to treatment was related to better treatment outcome (Kober et al. 2014).
Other studies have also included post-treatment fMRI. For example, DeVito et al. (2012) explored changes in brain activity in individuals with SUDs before and after CBT. Patients showed decreased Stroop-related BOLD signal in the inferior frontal gyrus (IFG), ACC, and midbrain following CBT, in comparison to pre-treatment. Changes in BOLD signal in the subthalamic nucleus, midbrain and surrounding regions showed greater treatment-related changes in the SUD group as compared to pre-test and post-test in control comparison subjects. Therefore, CBT may have an impact on reducing SUD symptomatology via making neural systems related to cognitive control more efficient. Subsequent studies similarly have found relatively reduced Stroop-related cortical, thalamic and midbrain activations post-treatment, consistent with initial preliminary results (DeVito et al. 2012, 2017, 2019; Yip et al. 2014).
More recent studies have investigated functional connectivity (FC) and neural circuitry linked to treatment-related outcomes in individuals with SUDs receiving CBT. For example, a model based on connectome-based predictive modelling results proposed that during reward processing, within-network coordination of attentional and control networks (frontoparietal and medial frontal networks) and within-network coordination of salience encoding and reward responding (salience, sensory-motor and subcortical networks) predicted cocaine abstinence, with between-network segregation of these network groups also implicated (Yip et al. 2019). In contrast, opioid abstinence was predicted by greater within-network motor/sensory connectivity and reduced connectivity between the motor/sensory network and medial-frontal, default-mode and frontoparietal networks (Lichenstein et al. 2021).
Other approaches have provided further insight. For example, intrinsic connectivity has suggested that individuals with cocaine use disorder show increased subcortical connectivity in a manner linked to treatment outcome (Mitchell et al. 2013). These findings suggest that greater connectivity within regions implicated in emotionally ‘hot’ processes during the performance of a ‘cold’ cognitive-control task link to poorer treatment outcomes. Another approach, independent component analysis, implicated five brain networks linked to Stroop performance that were differentially associated with cocaine use disorder and treatment retention and cocaine abstinence (Worhunsky et al. 2013), consistent with and extending prior regional-activation-based findngs (Brewer et al. 2008). More recently, this approach was used to investigate potential recovery from cocaine use disorder (Morie et al. 2021). Relatively decreased engagement of frontoparietal, amygdala-striatal, and middle-frontal networks during Stroop performance was observed following treatment, with less change in engagement of the amygdala-striatal associated with more years of lifetime cocaine use and engagement of the frontoparietal network related to within-treatment abstinence. These findings resonate with prior regional-activation studies that suggest more efficient neural processing during cognitive control following treatment, with greater engagement of executive-functioning circuitry linking to drug abstinence.
Regarding comorbidity, Cornelius et al. (2013) focused on cannabis use disorder with co-occurring major depression. Six participants studied received CBT/motivational enhancement therapy and, in addition, were randomised to receive fluoxetine or placebo. When performing a reward task, both before and after treatment, activation of the insula and prefrontal and striatal regions was observed. However, less activation was detected after treatment compared to pre-treatment. The small number of subjects suggests the need for more rigorous examination of these preliminary results.
Practical group counselling
Costello et al. (2010) used resting F-fluorodeoxyglucose-positron emission tomography (FDG-PET) to analyse the effects of bupropion HCl, practical group counselling (PGC) and placebo in 54 participants with tobacco use disorder. Both bupropion HCl and PGC (although PGC at a greater lever) were linked to reductions in glucose metabolism in the posterior cingulate gyrus, compared to placebo. In addition, the authors found a positive correlation between glucose metabolism and daily cigarette use in both the parietal-temporal junction and left occipital gyrus. Both bupropion HCl and PGC may therefore reduce neural activity in a similar way as a goal-oriented task does in the DMN, and thus promote the brain to be in a more goal-oriented state, and this possibility warrants additional investigation.
Mindfulness
Mindfulness-based therapies (MBTs) have been studied, especially in people with tobacco use disorder (Tang et al. 2013; Westbrook et al. 2013). Decreased right inferior frontal connectivity (within the anterior DMN) has been described in patients with opioid use disorder receiving mindfulness-based therapy, compared to those receiving treatment as usual. In addition, decreased right superior frontal cortex connectivity has been observed after treatment in patients receiving mindfulness-based therapy. MBTs have been associated with FC changes in the DMN (Fahmy et al. 2019). Thus, MBTs may strengthen the same networks that other cognitive interventions address, although the mechanisms involved may be distinct (Zilverstand et al. 2016).
Cue-exposure-based extinction training (CET)
In their randomised controlled trial, Vollstädt-Klein et al. (2011) assigned 30 abstinent participants with alcohol use disorder to a CET group or control group. The CET group, in addition to detoxification sessions, health education and supportive therapy, received, for 3 weeks, 9 sessions of CET, in which individuals were exposed to their preferred alcoholic beverages. The fMRI cue-reactivity reduction between pre- and post-treatment (especially in the insula, anterior cingulate gyrus, and limbic and frontal areas) was greater in the CET group compared to control participants. Furthermore, after treatment, the CET group showed lower activation in the left VS compared with control participants. Therefore, CET may have specific effects on brain areas implicated in reward processing and attentional focus towards alcohol-associated cues.
Cognitive bias modification training (CBM)
In a double-blind placebo-controlled study, Wiers et al. (2015) assigned 26 patients with alcohol use disorder to either a CBM or placebo group. Over 3 weeks, participants performed the Approach Avoidance Task before and after training. Both groups showed an alcohol approach bias-related activation of the medial PFC (MPFC) before training. However, after training, the CBM group showed more significant reductions in MPFC activation compared to the placebo group, and these reductions correlated with reductions in approach bias scores. Therefore, CBM could alter neural processes associated with automatic alcohol approach biases, including in regions implicated in craving. In the same vein, Wiers et al. (2015) observed that in the CBM group, a reduction of right amygdala activity correlated with a reduction in craving. These findings suggest that CBM could impact the motivational salience of drug cues through specific neural mechanisms involving reward circuitry.
Virtual reality
Son et al. (2015) evaluated with FDG-PET 12 individuals with alcohol use disorder exposed to a 10-session virtual reality therapy protocol. These individuals, compared to controls, had at baseline higher metabolism in the right temporal lobe and the right lentiform nucleus and lower metabolism in the left anterior cingulate. After treatment, participants with alcohol use disorder showed reduced brain metabolism in the right temporal lobe and lentiform nucleus compared to baseline. Therefore, virtual reality therapy may have a regulating effect on corticolimbic circuits.
Neuromodulation
Repetitive TMS has been investigated in individuals with alcohol, tobacco, and cocaine use disorders. While the number of clinical studies is currently limited, the results are promising (Antonelli et al. 2021). Shen and Ward (2021) reviewed 6 studies that explored TMS and neuroimaging in individuals with cocaine use disorder, of which three studies observed intervention-related reduction in cue reactivity (Hanlon et al. 2017; Kearney-Ramos et al. 2018, 2019). Among individuals with tobacco use disorder, Newman-Norlund et al. (2020) observed that excitatory theta-burst repetitive TMS improved inhibitory control, whereas inhibitory theta-burst repetitive TMS impaired it. Moreover, tDCS may show specific effects on cravings, and multiple sessions can further enhance its impact, suggesting potential therapeutic use for smoking cessation and modifying stimulus-induced behaviours (Boggio et al. 2009). A systematic review in tobacco use disorder suggests that tDCS targeting dlPFC activity may be an effective option for reducing symptoms of tobacco use disorder (Kang et al. 2019). Among individuals with alcohol use disorder, dACC activation may be altered by high-frequency repetitive TMS (HF-rTMS) treatment in a rate-dependent manner: the lower the dACC activation was at baseline, the more dACC activation increased after treatment (Herremans et al. 2016). Moreover, in individuals with severe alcohol use disorder, bilateral tDCS over the dlPFC may decrease the likelihood of relapse and lead to enhanced quality of life (Klauss et al. 2014).
Gambling disorder
Gambling disorder conceptualisation
The DSM-5 defines GD as a persistent, recurrent pattern of gambling that is associated with substantial distress or impairment (APA 2013). GD includes criteria for tolerance, withdrawal, repeated unsuccessful attempts to control or stop gambling, preoccupation, gambling when distressed, chasing losses, lies to conceal gambling, jeopardising or losing significant relationships, jobs, or other opportunities because of gambling, and financial bailouts (APA 2013). Separate criteria have been introduced in the eleventh revision of the International Classification of Diseases, with the central elements including persistent or recurrent gambling that features impaired control, increasing priority given to gambling over other activities, and continued gambling despite adverse consequences (World Health Organization 2018).
Gambling disorder treatment
Although no medications have formal approval for the treatment of GD, different psychotropic agents have been proposed for GD treatment: opioid-receptor antagonists (such as naltrexone and nalmefene), monoaminergic drugs (e.g., olanzapine and bupropion), and glutamatergic agents (e.g., N-acetylcysteine). Likewise, different psychosocial interventions have been employed, such as CBT, cognitive therapies, motivational interventions, or self-help groups (Potenza et al. 2019).
Neural mechanisms underlying gambling disorder and recovery
MRI studies of GD suggest possible reductions in white matter integrity and modest differences in regional GMV (Yip et al. 2013, 2017, 2018; Potenza et al. 2019). At the functional level, studies have reported dysregulation in reward-related circuitry, especially in the MPFC and VS (Balodis et al. 2012; Luijten et al. 2017; Clark et al. 2019).
Pharmacological treatments
Few studies have evaluated associations between treatment response and neuroimaging in GD (Potenza et al. 2013).
Bupropion
Bae et al. (2018) evaluated brain changes in 15 patients with internet-based GD after 12 weeks of bupropion treatment. Increased FC within a cognitive control network was observed in these patients, as well as a reduction of FC within the posterior DMN. Dopamine stimulation triggered by the pharmacodynamic activity of bupropion could promote the activity of the top-down circuitry and have an impact on the cognitive control network and promote more advantageous decision-making.
Fluvoxamine
Fluvoxamine is a serotonin reuptake inhibitor. A case study explored associations between fluvoxamine treatment, treatment outcome and brain activations (Chung et al. 2009). The individual with GD showed, upon presentation of playing cards, frontal, occipital, and parietal activations at baseline. However, a decrease in the activated brain regions was observed after treatment with fluvoxamine, which was associated with a reduction in the desire to gamble. However, these results should be interpreted with caution, as this is a case report.
Tolcapone
Grant et al. (2013) tested the efficacy of oral tolcapone, a catechol-O-methyl transferase inhibitor, over 8 weeks (100 mg/d titrated to 100 mg thrice/day) in 12 individuals with GD. After treatment, a reduction in GD symptomatology was observed, and this was associated with an increase in planning-related frontoparietal activation during an executive-functioning task, with responses particularly particularly robust in individuals with genetic variations linked to more efficient COMT functioning. These findings suggest that improving executive-functioning deficits in GD individuals with specific genetic features linked to drug mechanism of action may represent an inroad towards personalised interventions.
Lithium
The efficacy of lithium, a mood-stabilizing salt, has been tested in individuals with GD and co-occurring bipolar-spectrum diagnoses. PET has been used to explore treatment effects (Hollander et al. 2008; Pallanti et al. 2010). Lithium may increase the relative glucose metabolic rate (rGMR) in the ventral caudate, albeit not to a statistically significant level, in individuals with GD and bipolar-spectrum disorders. In addition, lithium may increase rGMR in the OFC, dlPFC and PCC (Hollander et al. 2008). The extent to which these changes in rGMR may relate to the functioning of these regions and circuits during specific processes warrants additional study.
Psychological treatments
Potenza et al. (2013) presented a proof-of-concept fMRI study to explore the response to treatment for GD. Seven treatment-seeking individuals with GD and co-occurring tobacco use disorder conducted the Stroop task during fMRI before initiating treatment for GD (6 weeks of CBT involving imaginal desensitisation motivational interviewing, smoking cessation instruction and either the amino-acid dietary supplement N-acetyl cysteine or placebo). An association was observed between pre-treatment brain activations and GD severity. These preliminary findings should be considered cautiously.
Neuromodulation
tDCS seems to enhance the functional connectivity of a frontoparietal circuit in individuals with GD, particularly in those with a larger dlPFC volume, reinforcing the connectivity of a brain network linked to cognitive control (Bouchard et al. 2021). However, more evidence is needed.
Internet gaming disorder
Internet gaming disorder conceptualisation
IGD was suggested as a mental disorder in Section III of the DSM-5 (APA 2013) and gaming disorder has been included in the International Classification of Diseases 11th Revision (ICD-11) (World Health Organization 2019). DSM-5 IGD shares clinical characteristics similar to the DSM-5 criteria for GD, and gaming disorder, like GD in the ICD-11, is characterised by impaired control, an increasing priority given to gaming, and continued involvement in gaming despite negative consequences (World Health Organization 2018). To meet a diagnosis, the maladaptive gaming pattern needs to generate psychological distress or significant impairment in personal, family, social, professional, and/or other important areas of functioning.
Internet gaming disorder treatment
Different therapeutic options have been proposed for the management of IGD. Drugs used for treating conditions that frequently co-occur with IGD such as depression (bupropion and escitalopram) and those used for attention-deficit/hyperactivity disorder (methylphenidate and atomoxetine) have been evaluated in the treatment of IGD (Han et al. 2010; Park et al. 2016; Zajac et al. 2017, 2020; Bae et al. 2018). Currently no medication has a formal indication for the treatment of IGD. Among psychotherapies, CBT has arguably the most empirical support (King et al. 2017; Zajac et al. 2017, 2020; Stevens et al. 2019; Han et al. 2020). A craving behavioural intervention (CBI) has also been developed that includes psychoeducation on subjective craving, identification of erroneous cognitions about craving, strategies to regulate craving and the emotions associated with it, ocialization tools, skills training, and time management. In addition, each session includes some mindfulness training. As compared to a test/re-test group, CBI has been associated with reductions in cue-elicited craving, weekly gaming time and severity of internet addiction (Wang et al. 2022). Other approaches have been proposed, such as the programs ‘Online-based motivational intervention to reduce problematic internet use and promote treatment motivation in internet gaming disorder and internet use disorder’ (OMPRIS) (Dieris-Hirche et al. 2021) and ‘Programa Individualizado Psicoterapéutico para la Adicción a las Tecnologías de la información y la comunicación’ (PIPATIC) (Torres-Rodríguez, Griffiths, Carbonell, Oberst 2018; Torres-Rodríguez, Griffiths, Carbonell 2018), family therapy (Zajac et al. 2017; Bonnaire et al. 2019; Kim and Noh 2019; Nielsen et al. 2021), mindfulness (Li et al. 2017, 2018), therapeutic (self-discovery) residential camps for adolescents (Sakuma et al. 2017; Zajac et al. 2017), equine-assisted activities and therapies (Kang et al. 2018), virtual reality therapy (Zhang and Ho 2017), tDCS (Lee et al. 2021; Wu et al. 2021), high-frequency repetitive TMS (Cuppone et al. 2021), among others, often with limited empirical support.
Neural mechanisms underlying internet gaming disorder and recovery
A meta-analysis suggested that individuals with IGD have relatively decreased GMVs in regions including the vmPFC and ACC that have been implicated in decision-making, emotional regulation and cognitive control (Yao et al. 2017). The same meta-analysis suggested between-group differences in regional activations during ‘hot’ and ‘cold’ executive tasks in individuals with and without IGD.
Exposure to relevant stimuli may activate the reward system differentially people with and without addictive disorders like IGD. Like individuals with SUDs and GD (Luijten et al. 2017), individuals with IGD show relatively blunted VS activation during the processing of monetary rewards (Dong et al. 2017). Individuals with IGD compared to those with regular game use show greater lentiform activation to gaming stimuli, similar to the cue reactivity observed in SUDs (Dong et al. 2019). Individuals recovered from IGD without formal intervention have shown decreased gaming cue-related activations in the lentiform nucleus and vmPFC/OFC (Dong et al. 2019). Less neural reactivity in the lentiform nucleus has been associated with less subjective cue-elicited craving responses and, therefore, possibly less motivation to perform gaming behaviours, promoting recovery. Further, cue-elicited activation in the lentiform nucleus has been associated with the emergence of IGD among individuals with regular game use (Dong et al. 2020). Therefore, gaming-cue-elicited lentiform activity may have considerable relevance to both IGD recovery and emergence. Additionally, relatively diminished activation of the ACC has been described in individuals recovered from IGD, possibly suggesting more efficient cognitive control (Dong et al. 2019).
Pharmacological treatments
Bupropion
Bae et al. (2018) observed that, after 12 weeks of buproprion treatment (150–300 mg/d), the IGD group (consisting of 16 subjects) showed a reduction in FC in the posterior DMN and between the DMN and cognitive control network after treatment. Increased FC in the DMN has been associated with elevated levels of impulsivity, attentional deficits, and risky decision-making processes. Therefore, decreasing FC within or to the DMN may reduce impulsive behaviours and excessive gaming, with findings raising the possibility that more efficient disengagement of the DMN may be advantageous. Han et al. (2010) explored the effects of bupropion after 6 weeks of sustained-release treatment in 11 individuals with IGD. These individuals, compared to control subjects (n = 8), showed in response to game cues greater brain activation in the left parahippocampal gyrus, left occipital lobe, cuneus, and left dlPFC. In addition, after pharmacological treatment, a reduction in total gaming time, craving for online video games and cue-induced brain activity in the dlPFC was observed in the group of individuals with IGD.
Psychological treatments
Cognitive behavioural therapy
Han et al. (2018) explored the possible effects of CBT in individuals with IGD using resting-state fMRI. When comparing the group of individuals with IGD (n = 26) with the controls (n = 30) before the start of treatment, it was observed that the former showed relatively increased amplitude of low-frequency fluctuation (ALFF) values in the right medial OFC, bilateral putamen, left postcentral gyrus, bilateral supplementary motor area (SMA), and left ACC. Following CBT, ALFF values were significantly reduced in the left putamen and left superior OFC. In addition, the FC between them increased significantly after therapy. The authors suggested that CBT could be an effective approach to regulate abnormal ALFFs that individuals with IGD demonstrate in prefrontal-striatal areas, in turn improving symptomatology.
Family therapy
Han et al. (2012) studied possible changes in brain activation patterns associated with gaming following a brief (3-week) family therapy in 15 adolescents with IGD and family dysfunction. Prior to therapy, and in comparison with 15 control subjects, participants with IGD presented decreased activity within the caudate, middle temporal gyrus, and occipital lobe in response to pictures depicting parental affection. Moreover, this group showed relatively increased activity of the middle frontal and inferior parietal cortices in response to scenes from on-line games. After the 3 weeks of treatment, increases in the activity of the caudate nucleus in response to affectionate stimuli were associated with improvements in perceived family cohesion, which was also inversely associated with changes in on-line game playing time. These findings suggest that increasing striatal responses to non-addiction-related rewards may underlie recovery from IGD, as has been suggested for SUDs.
Craving behavioural intervention
The mechanisms linked to CBI treatment have been investigated. Wang et al. (2022) included 23 individuals with IGD who received CBI over a 6-week period. The authors identified, through multi-voxel pattern analysis (MVPA), a pattern of high prediction ability in relation to treatment response (specifically weekly changes in gaming time) through cue-related activations in the SFG, right middle frontal gyrus, supramarginal gyrus, left postcentral gyrus, and anterior/posterior lobe of the cerebellum. In addition, the authors observed a negative correlation between changes in weekly gaming time and activations in the SFG, right middle frontal gyrus, supramarginal gyrus, left postcentral gyrus, and anterior lobe of the cerebellum. Zhang et al. (2018) applied the same intervention in 20 individuals with IGD, finding decreases in time spent on online gaming. They also described a significant interaction of intervention (CBI vs. no CBI) and session (pre-test vs. post-test) on resting-state FC (rsFC) between VS and the left inferior parietal lobule (lIPL). Specifically, the strength of VS-lIPL rsFC in the group that received CBI was reduced, in comparison with the group that did not receive CBI, suggesting that the intervention may reverse partially the altered connectivity. As such, VS-lIPL may represent a biomarker for IGD interventions such as CBI. Similarly, Zhang et al. (2016) included 36 individuals with IGD in their study, of whom 20 received six weekly 2.5-h sessions of CBI. Compared to the control subjects (n = 19), individuals with IGD showed increased rsFC between the dlPFC and the PCC. Moreover, these participants exhibited decreased amplitude of low fluctuation in the PCC and OFC. On the other hand, compared to individuals with IGD who did not receive CBI, those who received the intervention showed reduced rsFC between the PCC and the SMA and precentral and postcentral gyri. In addition, they evidenced reduced rsFC between the orbital frontal cortex and hippocampus/parahippocampal gyrus. Therefore, individuals with IGD may have abnormal resting-state neural activity in different networks (reward-related, executive control, and DMN), and CBI may be effective in reducing interactions between regions in the different networks.
Zhang et al. (2016) delivered CBI to 23 of 40 individuals with IGD. After CBI, reductions in weekly gaming time, IGD severity and cue-elicited craving were observed. Further, increased cue-elicited activation of the anterior insula, and decreased FC between the insula and lingual gyrus and precuneus were seen. CBI could, therefore, alter insula activation as well as connectivity with brain areas involved in attention, visual processing and craving.
Liu et al. (2021) suggested that IGD may be associated with alterations in brain connectivity and that these may be sensitive to therapeutic interventions. Specifically, the authors observed that CBI was associated with increased brain connectivity within areas in the DMN and salience networks.
Natural recovery
Finally, some studies have explored natural recovery in IGD (the reduction of IGD symptomatology without the use of formal/professional interventions). For example, Dong et al. (2019) highlighted that individuals with IGD in recovery show diminished cue-elicited responses both at neural and subjective levels. In addition, the authors observed decreased gaming cue-related activations in reward-circuit-related brain regions (lentiform, vmPFC/OFC) after IGD recovery. Similarly, Dong et al. (2019) observed that individuals recovered from IGD showed decreased dlPFC activation to gaming cues at pre- and post-gaming assessments, compared to the 20 participants who, after one year, still met diagnostic criteria for IGD.
Conclusion
In recent years there has been an effort to explore by neuroimaging responses to treatment for SUDs, GD and IGD. In multidisciplinary work related to psychopharmacology, psychology, cognitive science and neuroscience (Chung et al. 2016), both pharmacological and psychological treatments may be associated with changes in specific neural regions and circuits. Some themes have emerged from studies, and some of these patterns are schematised in Figure 1. For example, greater regional brain activations during the performance of cognitive-control tasks prior to treatment have been linked to better outcomes, and more efficient control-related neural processing following treatment has been associated with better outcomes. Further, reducing addiction-cue-related activations also appears important in recovery processes. Additionally, increasing VS activity during the processing of non-addiction-related rewards appears associated with recovery.
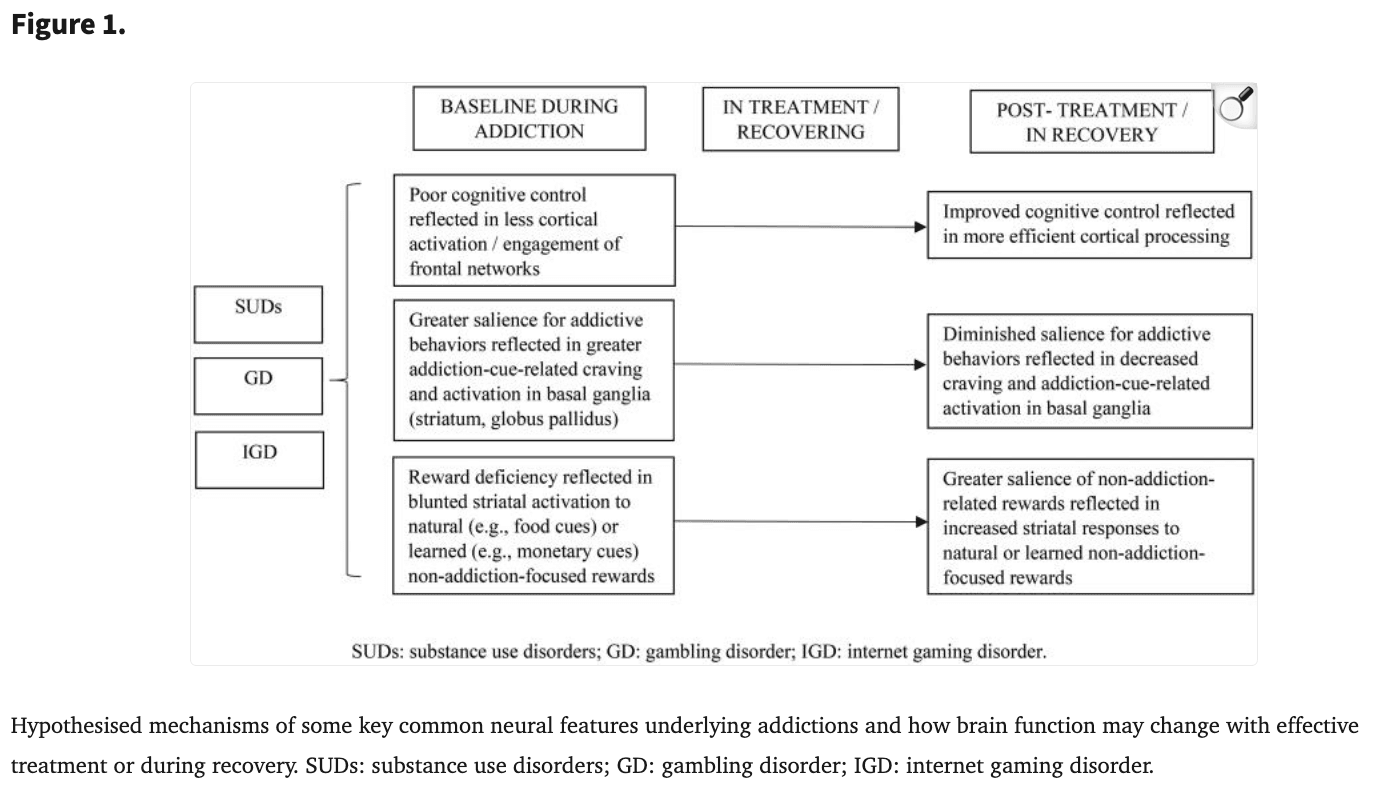
More specifically with respect to Figure 1, we note that to date there have been limited studies that have typically involved small samples assessed over limited periods of time. To date, some of these findings have been partially supported, For example, blunted ventral striatal activation that has been observed in youth with tobacco use disorder (Peters et al. 2011) has been found to increase after treatment (Garrison et al. 2017). Similar findings have been reported in adults with cocaine use disorder (Balodis et al. 2016; Luijten et al. 2017). However, in the case of cognitive control, such patterns do not seem to ‘reverse,’ per se, with findings instead perhaps reflecting more of a within-group effect of more efficient processing with treatment in conjunction with less activation in people with substance use disorders prior to treatment (DeVito et al. 2012, 2017, 2019). In IGD, interactive effects between individuals receiving a craving behavioural intervention have been reported implicating changes in insular activation across the active (craving behavioural intervention) and control (test-retest) groups in response to gaming cues (Zhang et al. 2016).
As more advanced analytic methods become applied to studies incorporating neuroimaging measures into treatment trials for people with SUDs and disorders due to addictive behaviours, the hypotheses displayed in Figure 1 may become more refined. For example, circuit-based analyses may complement region-based analyses in supporting or refining these themes. While Figure 1 highlights some key findings and themes, it does not extensively cover findings (e.g., cortical changes related to craving responses as highlighted in the text of the manuscript). While the highlighted findings are consistent with multiple models of addiction (e.g., dual process (Lannoy et al. 2014), reward deficiency (Blum et al. 2000) and the interaction of person, affect, cognition and execution (I-PACE) (Brand et al. 2016, 2019), among others), additional research is needed to understand other relevant domains (e.g., related to stress and emotional processing).
Specific targeting of aspects associated with addictive disorders could lead to specific changes in brain circuitry (e.g., in cortical and subcortical regions underlying reward processing and cognitive control or other relevant processes), which could mediate responses to treatment. Imaging is a promising tool to explore mechanisms underlying different treatments, including with respect to psychotherapies, pharmacotherapies and neuromodulation (Zilverstand et al. 2016; Wu et al. 2020). More studies are needed to further investigate the efficacies of treatments, the neural mechanisms associated with them and new brain-based treatment targets. Further, improved understanding of neural processes related to the emergence of addictive processes (e.g., through studies of the Adolescent Brain Cognition and Development (ABCD) data) should help develop more effective prevention strategies.