Abstract
Importance: The prevalence of and mortality associated with methamphetamine use has doubled during the past 10 years. There is evidence suggesting that methamphetamine use disorder could be the next substance use crisis in the United States and possibly worldwide. Observation: The neurobiology of methamphetamine use disorder extends beyond the acute effect of the drug as a monoaminergic modulator and includes intracellular pathways focused on oxidative stress, neurotoxic and excitotoxic effects, and neuroinflammation. Similarly, the clinical picture extends beyond the acute psychostimulatory symptoms to include complex cardiovascular and cerebrovascular signs and symptoms that need to be identified by the clinician. Although there are no pharmacologic treatments for methamphetamine use disorder, cognitive behavioral therapy, behavioral activation, and contingency management show modest effectiveness. Conclusions and relevance: There is a need to better understand the complex neurobiology of methamphetamine use disorder and to develop interventions aimed at novel biological targets. Parsing the disorder into different processes (eg, craving or mood-associated alterations) and targeting the neural systems and biological pathways underlying these processes may lead to greater success in identifying disease-modifying interventions. Finally, mental health professionals need to be trained in recognizing early cardiovascular and cerebrovascular warning signs to mitigate the mortality associated with methamphetamine use disorder."
Introduction
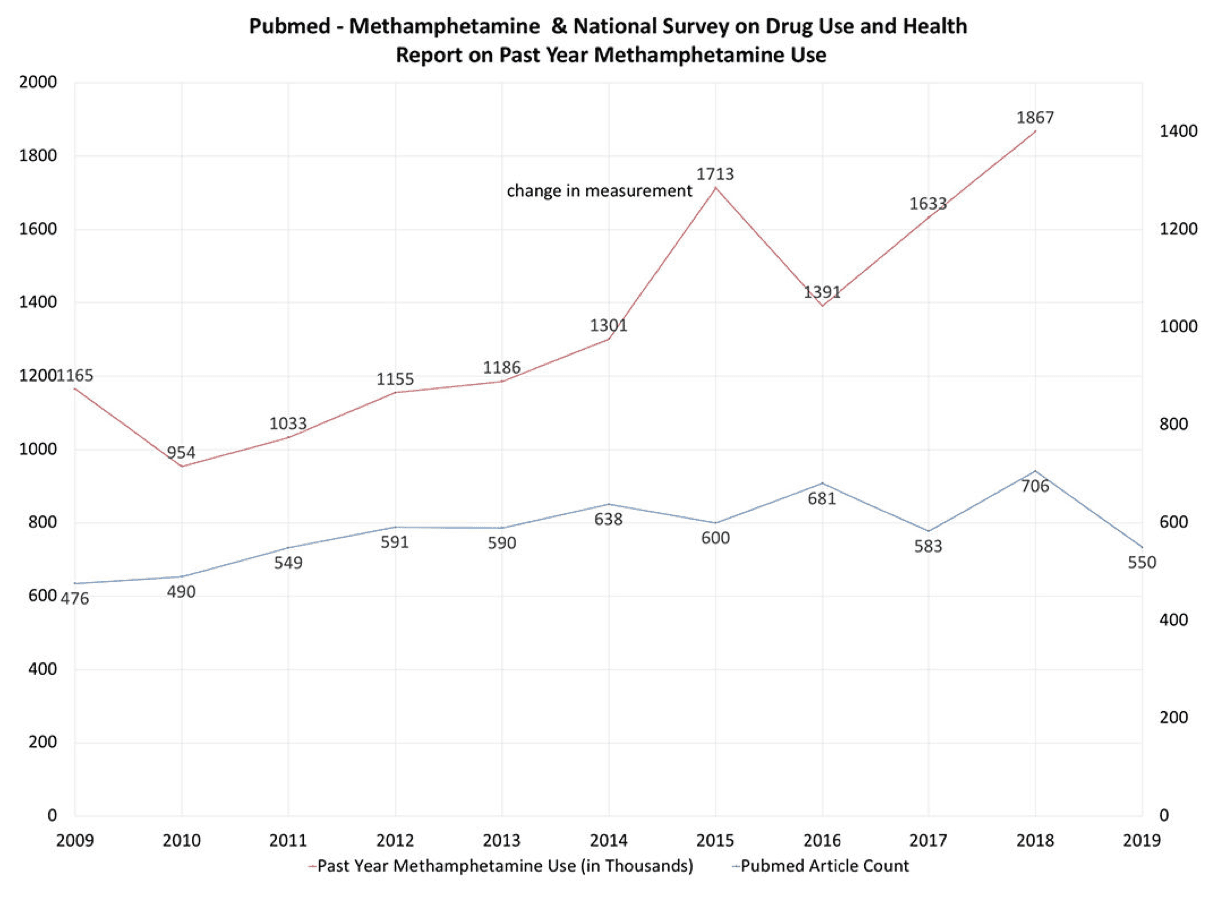
In the wake of the opioid crisis, methamphetamine has re-emerged as a challenge to mental health providers and researchers alike. Methamphetamine is now available in different forms such as ice, powder, and pills with different pharmacokinetic characteristics that make them popular among certain types of users . Recent seizure data suggest that its production and trafficking is spreading into new areas of the globe . According to the “Automation of Reports and Consolidated Orders System”, methamphetamine consumption increased 4-fold between 2015 and 2016 and total stimulant usage doubled in the last decade . From 2011 through 2016, the age-adjusted rate of drug overdose deaths involving methamphetamine more than tripled . Moreover, drug overdose deaths involving cocaine, amphetamines, or both substances combined increased 42.4% from 12,122 in 2015 to 17,258 in 2016 . Based on the most recent data from the National Survey on Drug Use and Health, 12-month prevalence of individuals age 12 and above reporting methamphetamine use has increased by 195% from its low in 2010 to 2018 (Figure 1) and it is estimated that 1.86 million Americans used methamphetamine in 2018. These numbers underline the importance of paying attention to the possibility of the next substance use crisis. However, whereas opioid use disorder can be treated pharmacologically and behaviorally, there are significant challenges for the treatment of methamphetamine use disorder (MUD). This review will focus on three specific aspects of MUD. First, the neurobiology of methamphetamine is more complex than the traditional view of it as a monoaminergic modulator. Second, the clinical presentation is not limited to the symptoms associated with use disorder but extend to medical presentations, most notably the cardio- and cerebrovascular systems. Third, pharmacological interventions focused on modulating the monoaminergic pathways have largely failed and new pharmacological approaches are necessary to focus on novel treatment targets. In the final section several suggestions will be proposed for both clinicians and researchers to advance the understanding of MUD.
Biological Pathways, Neural Basis and Cognition
Methamphetamine has been conceptualized primarily as a releaser of dopamine, serotonin, noradrenaline, and adrenaline from nerve terminals in the central and peripheral nervous system, which occurs via a number of different mechanisms, including: (1) redistributing catecholamines from synaptic vesicles to the cytosol; (2) reversing the plasma membrane transport of neurotransmitter; (3) blocking the activity of monoamine transporters; (4) decreasing the expression of dopamine transporters at the cell surface; (5) inhibiting monoamine oxidase activity; and (6) increasing the activity and expression of tyrosine hydroxylase, the critical enzyme for synthesizing dopamine. However, there has been a substantial expansion of methamphetamine-driven neurobiological targets over the past decade. Methamphetamine modulates at least three different molecular cascades, which have been described as oxidative stress, neuro- and excitotoxicity, and neuroinflammation (Figure 2). For example, mitochondria are the are primary site of oxidative metabolism and are organized in a tubular, dynamic network that undergoes continuous remodeling via fusion or fission. Methamphetamine induces changes in morphology of mitochondria in neurons and microglia, which disturbs the mitochondrial homeostasis, morphology, and oxidative stress metabolism towards an increase in oxidative burden conducive to neurodegeneration. Methamphetamine also causes single and double strand breaks in DNA due to reactive oxygen species, leading to persisting mutations at the chromosomal level at blood concentrations that are observed in users. Methamphetamine-induced neuroinflammation is partially mediated by direct binding to the toll-like receptor 4 (TLR4) transmembrane protein within the ventral tegmental area, which has the downstream effect of elevating dopamine in the nucleus accumbens shell. The inflammatory changes in the brain occur largely in microglia, i.e. the primary cells of active immune defense in the central nervous system. The inflammasome is a molecular system that consists of the sensing molecule NLRP3, the adaptor apoptosis-associated speck-like (ASC) protein, and the executive enzyme caspase-1. Methamphetamine upregulates caspase-1 and ASC aggregation, which promotes inflammasome-mediated interleukin 1 beta maturation and secretion mediating microglia-induced neurotoxicity. This process also occurs in a number of conditions ranging from neurodevelopmental disorders to neurodegenerative disorders. Importantly, the methamphetamine induced cellular dysregulation in neurons and microglia can affect neural processing, altered reward motivation due to sickness behavior, and reduced prefrontal control, which – together - may contribute to the development and maintenance of drug-taking behavior. Methamphetamine-induced neurotoxicity has been hypothesized to be the result of interdependent mechanisms including: (1) excessive dopamine, resulting in an increased production of reactive oxygen species such as peroxides that can damage cell structures; (2) ubiquitin-proteasome system dysfunction, activating intracellular degradation systems leading to autophagy; (3) protein nitration, leading to an increase in radical nitric oxide with subsequent cytotoxic effects; (4) endoplasmic reticulum stress, leading to increased apoptosis; (5) increased tumor protein p53 expression in the striatum, altering DNA repair, arresting cell cycles, and dysregulating the expression of stress response genes; (6) inflammatory cytokines, leading to inflammatory activation in the brain; (7) activation of the dopamine D3 receptor, resulting in hyperthermia; and (8) microtubule deacetylation, disrupting the blood brain barrier. Together, these neurobiological cascades of oxidative stress, neuro- and excitotoxicity, and neuroinflammation result in a unique metabolic state of the brain, which has been termed the Warburg effect, i.e. when cells favor metabolism via glycolysis rather than the much more efficient oxidative phosphorylation. Thus, methamphetamine use acutely, and possibly chronically, places the brain in a different metabolic state characterized by: (1) a quicker but less efficient availability of energy; (2) an increased rate of biosynthesis; (3) acidification of the microenvironment; and (4) altered cell signaling via reactive oxygen species, which promotes an oncogenic and degenerative cell environment. In summary, MUD does not just reflect a dopamine dysregulation but represents an altered brain state that is consistent with those observed in degenerative central nervous system diseases. These complex molecular dysregulations provide an opportunity to identify modifiable drug targets to develop novel pharmacological interventions for MUD.

Others have proposed that compulsive drug taking is the result of an imbalance between an orbitofrontal cortex-dorsomedial striatal “go” circuit and an opposing dorsolateral frontal-striatal “stop” circuit. Numerous studies have focused on examining evidence of structural and functional alterations within these circuits. For example, methamphetamine users show widespread gray and white matter alterations, particularly affecting the frontostriatal system as well as prominent reductions in the left superior temporal gyrus and the right inferior parietal lobe, which provide contextual information to the dorsolateral frontal circuits. Moreover, abnormalities include deficits in markers of dopaminergic and serotonergic neurotransmitter systems, differences in glucose metabolism and deficits in gray matter. Chronic methamphetamine users show aberrant patterns of brain connectivity and function within both orbitofrontal-striatal and dorsolateral frontal-striatal systems when engaged in cognitive tasks and at rest. Functional neuroimaging studies have shown that methamphetamine users show changes in orbitofrontal cortex during empathic processing, in salience and dorsolateral frontal functioning areas during decision-making, and in both dorsolateral and inferior frontal areas during inhibitory processing. Interestingly, lower cortico-striatal connectivity as measured by resting state functional magnetic resonance imaging (fMRI) has been associated with higher concentration of peripherally measured cytokines, which may provide evidence for the link between neuroinflammation and brain processing changes in MUD. Although functional brain activation differences during various behavioral tasks among MUD individuals have been used to predict relapse, none of these measures have thus far been clinically useful, i.e. have been able to aid in the diagnosis, prognosis or treatment of the disorder.
The impact of methamphetamine on cognition has been heavily debated and a dearth of longitudinal studies makes it difficult to assess whether the observed cognitive dysfunctions are pre-existing, a consequence of the exposure, or a consequence of behaviors that are associated with substance use disorders in general. Nevertheless, several recent studies that provide a more cohesive picture of cognitive problems that exist both shortly after cessation of use and to some extent after longer periods of abstinence. For example, MUD subjects in early abstinence but post-acute withdrawal show poorer performance on tasks examining motor and processing speed, verbal fluency, and attention. Even following prolonged abstinence, individuals with MUD perform more poorly than matched comparison subjects on learning efficiency, visual-spatial processing, comprehension knowledge, retrieval fluency, processing speed, and psychomotor speed. In addition, dysfunctions of impulsivity have been associated with greater of severity of use and earlier age of use onset. Global assessments of cognitive function support the idea that more than 2/3 of individuals with MUD show cognitive impairment, the extent of which is linked to older age, longer duration and higher frequency of use. Aside from providing an objective assessment of the impact of methamphetamine use, neuropsychological assessment can also be used as a prognostic indicator. For example, cognitive measures such as problems with sustained attention can predict reduced treatment motivation and different forms of impulsivity predict poorer 6-week outcomes in treatment. Taken together, methamphetamine is associated with a moderate dysfunction of a number of cognitive processes, limiting the degree to which individuals with MUD are able to focus attention on goal-directed activity away from methamphetamine use in early abstinence. Given that neuropsychological function has some predictive utility for treatment retention and success, more work needs to be done to determine whether any of these cognitive dysfunctions can be remediated by targeted interventions.
Clinical Presentation
The acute behavioral effects of methamphetamine include increased energy and alertness, decreased need for sleep, euphoria, increased sexuality, excessive talking, weight loss, sweating, tightened jaw muscles, grinding teeth, and loss of appetite. Symptoms exacerbated by methamphetamine can be divided into three factors: (1) positive psychotic symptoms such as suspiciousness, unusual thought content, hallucinations, and bizarre behavior; (2) affective symptoms including depression, suicidality, guilt, hostility, somatic concern, and self-neglect; and (3) psychomotor symptoms, such as tension, excitement, distractibility, and motor hyperactivity. The clinical picture can be complex and mimics a variety of psychiatric disorders. The transition from casual to compulsive methamphetamine use can be rapid and some have reported that it takes on average about 50 days from the onset of drug use to the first drug craving, 60 days to regular use, and 85 days to compulsive use. Although most methamphetamine-associated psychoses are brief lasting hours to days, in some cases psychotic episodes may persist for longer than 6 months and can reoccur during periods of abstinence from the drug. On average, 36.5% of methamphetamine using individuals, regardless of age or sex, report psychotic symptoms, but if one takes lifetime symptoms into account this number increases to 42.7%. Some have suggested that self-reported psychotic illness is more prevalent among users of crystal methamphetamine than other forms of methamphetamine, which may be related to its purity and the self-selection of individuals who use this form of the drug. The psychiatric comorbidity of MUD is complex because there is evidence for both pre-existing factors that increase risk for psychiatric disorder, e.g. a prevalence of 44% of moderate to severe childhood abuse or neglect. Moreover, early lifetime adversity such as emotional or sexual trauma may also increase the likelihood of MUD due to fact that some individuals use methamphetamine as a coping method. In addition, there are other co-existing psychiatric disorders such as mood disorders (16.0%), psychotic disorders (13.0%) and anxiety disorders (7.0%). Both early life trauma and psychiatric comorbidity can adversely affect both age of first use as well as treatment success.
The path to using methamphetamine involves at least two trajectories. First, younger users take methamphetamine primarily for recreational and performance enhancement purposes, whereas those initiating at a later age may use to “self-medicate” e.g. stressful life events. This is consistent with the observation that the rate of females, who are much more likely than males report using methamphetamine for weight-related issues, is higher among adolescent relative to adult users. Second, there is emerging evidence of individuals using methamphetamine as an opioid substitute, to obtain a synergistic high, or to balance out the effects of opioids. Recent longitudinal evidence suggests that increase of cannabis use among adolescents may increase the probability of initiating other illicit drugs such as methamphetamine via both biological and social processes, providing some evidence for the “gateway hypothesis”. Similar to many other substance use disorders, the course of MUD is often characterized by repeated periods of intense use with intermittent sobriety and relapse. Those who do not engage in treatment only show 5-year remission rates up to 30% and of those who engage in treatment, 61% relapse within the first 12 months and another 14% relapse during years 2-5. These findings underscore that MUD is a chronic, relapsing and possibly degenerative condition, which is consistent with the profound molecular changes induced by methamphetamine.
The most severe medical problems and the leading cause of death associated with MUD are cardiovascular and cerebrovascular disease. Methamphetamine-related strokes have been on the rise, occurring most often in young men, and are primarily hemorrhagic in nature. Methamphetamine is associated with vasoconstriction, pulmonary hypertension, atherosclerotic plaque formation, cardiac arrhythmias, and cardiomyopathy. Methamphetamine-associated cardiomyopathy is characterized by left ventricular dilatation and impaired left ventricular ejection fraction as well as elevated tissue markers of inflammation and fibrosis. On the electrocardiogram, these individuals frequently show tachyarrhythmias, right axis deviation, left ventricular hypertrophy, P pulmonale pattern, inferior Q waves, lateral T wave inversion, and longer QTc interval. Symptoms preceding death attributed solely to methamphetamine toxicity include collapse, breathing difficulty, and hyperthermia, which may be a consequence of acute abnormal enlargement of the heart. Methamphetamine also contributes significantly to opioid mortality statistics, as the drug is also present in 63% of opioid deaths. It is important to emphasize that in individuals who present with acute intoxication with methamphetamine, symptoms of dyspnea, angina, palpitations, cough, and hemoptysis should prompt the clinician to closely monitor the medical status to prevent mortality.
Interventions
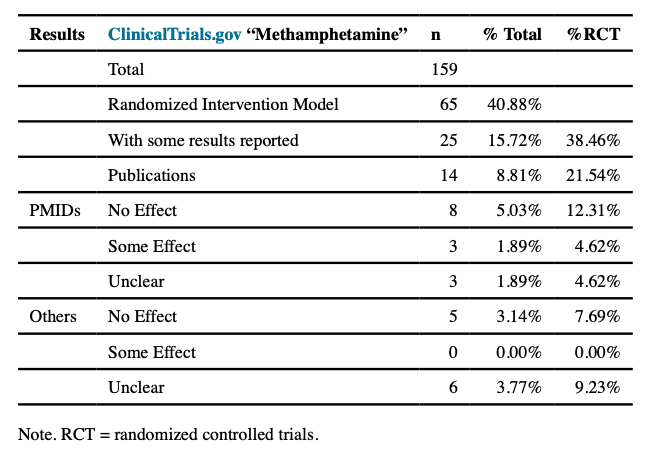
There are very limited pharmacological options for which there is sufficient efficacy data to treat MUD. Table 1 summarizes all intervention trials registered at http://clinicialtrials.gov that contained the term “methamphetamine”. Of the 159 registered studies, 65 represented randomized clinical trials (RCTs); of those, 25 reported results, of which 14 resulted in publications with identifiable PMIDs. Examining these publications, 8 reported no effect, 3 reported some effect, and 3 reported effects that did not speak to the efficacy of the intervention. Moreover, reports submitted to http://clinicaltrials.gov were either mostly unclear with respect to efficacy or reported null results (see supplemental table). This short summary is consistent with conclusions by others who conducted meta-analyses. Specifically, examining 49 studies investigating 20 potential pharmacotherapies, a total of 35 studies related to 33 Phase II quality, i.e. efficacy studies, RCTs. For the 5 medications that were subject to multiple RCTs, 4 of these— methylphenidate, bupropion, modafinil, and naltrexone— demonstrated some limited evidence of benefit for reducing methamphetamine use. The authors concluded that none of these drugs showed sufficient and consistent evidence of effectiveness to support its use in routine treatment. This assessment is similar to another study, which concluded that (1) no agent demonstrated a broad and strong effect in achieving methamphetamine abstinence in Phase II trials and (2) there was not sufficient evidence available for dopamine analogue treatment after the initial withdrawal-period. Studies of anticonvulsants, antipsychotics, opioid antagonists, varenicline, and atomoxetine provided either low-strength or insufficient evidence of no effect on the outcomes of interest, i.e. abstinence, defined as 3 or more consecutive weeks with negative urine drug screens. Immunotherapy has been suggested as an alternative form of treatment for drug abuse; however, none of the anti-drug immunotherapies have reached phase III clinical trials so far. Although some have reported that that the combination of pharmacological treatments aimed at treating psychiatric target symptoms and brief cognitive behavioral treatment in a research setting outperformed control conditions, there is no sufficient evidence that pharmacological interventions by themselves are useful for the treatment of MUD.
Results from studies using behavioral interventions to treat MUDs are more encouraging. In a recent network meta-analysis, compared to treatment-as-usual, only contingency management, i.e. a procedure that aims to alter drug use by systematically arranging consequences which are designed to weaken drug use and strengthen abstinence, plus community reinforcement, i.e. adjusting an individual’s environment such that abstinence is more rewarding than using the drug, increased the number of abstinent MUD patients at the end of treatment. Others reported that brief cognitive behavioral therapy (CBT) resulted in significant reductions in frequency of methamphetamine use, MUD severity, and number of days of methamphetamine use at weeks 4 and 12, findings consistent with a systematic review that finds weak evidence for increased percentage of abstinent days in 90 days and reduced MUD symptoms. Similarly, behavioral activation, which aims to maximize activities that are not drug-related but are positively valued by the individual, had a beneficial effect on abstinence in alcohol, tobacco, opioid, and methamphetamine use in 7 of the 8 reviewed studies, and improved depression over time in six studies. Finally, several studies demonstrate beneficial effects of exercise on reducing MUD symptoms. For example, an aerobic exercise program provided benefits for methamphetamine-associated craving and improved inhibitory control in individuals with MUD. Moreover, exercise by methamphetamine users reduced levels of depression and anxiety over an 8-week period compared to a health education control group. Taken together, there is some evidence that contingency management, CBT, behavioral activation, and exercise help to maintain abstinence. There is also encouraging evidence for computer-delivered interventions and app-based approaches. Nevertheless, there are two significant shortcomings. First, intervention programs for methamphetamine are plagued by high discontinuation rates. For example, in one large program 51% dropped out within the first two weeks and the average number of days retained was only 60 days. Second, there is little understanding as to: (1) how these behavioral interventions affect the underlying neurobiology of MUD; and (2) whether these interventions improve neural processing and cognitive dysfunctions within these individuals.
Conclusion and Relevance
MUD is re-emerging as a significant public health burden, a challenge for the clinician, and a difficult problem to solve for the researcher. First, MUD can develop rapidly, has a complex course characterized by episodes of intense use and intermittent abstinence, has profound medical consequences, is a difficult to treat condition, and results in significant long-term cognitive and neurological deficits. Second, clinicians faced with the presentation of an individual with acute methamphetamine intoxication should examine the patient for evidence of cardiovascular and cerebrovascular signs and symptoms, which are the primary reason for deaths due to methamphetamine. Third, there are several next steps to consider for pragmatically focused program of research. Modifiable biological targets should be examined in MUD individuals that focus on dysregulation of oxidative stress, neuro- and excitotoxicity, and neuroinflammation. Neuromodulatory approaches appear promising in ameliorating impairments associated with MUD. For example, electroencephalography (EEG) neurofeedback targeting the beta frequency band has increased, among other outcomes, periods of abstinence in methamphetamine users. Moreover, repetitive transcranial magnetic stimulation (rTMS) targeting frontal regions has resulted in decreased methamphetamine craving and/or increased cognitive-emotional function. Real-time fMRI neurofeedback, which demonstrates a beneficial effect on reduction of depressive symptoms, may also be helpful in reducing dysphoria present in methamphetamine users. Understanding the neurobiology of exercise-induced craving reduction in MUD may help to delineate novel disease-relevant targets. Fourth, preventative behavioral interventions focused on factors such as childhood trauma and dysregulated negative affect processing that increase the likelihood of initiating or continuing methamphetamine use may help to reduce future use. Finally, the neurobiology of this disorder is almost entirely derived from cross-sectional studies, which provide very little mechanistic insights. Thus, longitudinal assessments of brain-related changes are necessary to determine what brain-based treatment targets are modifiable and what brain processes put individuals at high risk for relapse. Taken together, given the limited evidence-based intervention options, it will be critically important to develop an implementation framework such that behavioral interventions can be delivered with high fidelity to maximize treatment effects and help individuals overcome MUD.