Abstract
The Triple Network Model of psychopathology identifies the salience network (SN), central executive network (CEN), and default mode network (DMN) as key networks underlying the pathophysiology of psychiatric disorders. In particular, abnormal SN-initiated network switching impacts the engagement and disengagement of the CEN and DMN, and is proposed to lead to the generation of psychosis symptoms. Between-network connectivity has been shown to be abnormal in both substance use disorders (SUD) and psychosis. However, none have studied how SUD affects connectivity between sub-networks of the DMN, SN, and CEN in early stage psychosis (ESP) patients. In this study, we collected data from 113 ESP patients and 50 healthy controls to investigate the effect of SUD on between-network connectivity. In addition, we performed sub-group analysis by exploring whether past SUD vs current SUD co-morbidity, or diagnosis (affective vs non-affective psychosis) had a modulatory effect. Connectivity between four network-pairs, consisting of sub-networks of the SN, CEN, and DMN, was significantly different between ESP patients and controls. Two patterns of connectivity were observed when patients were divided into sub-groups with current vs past SUD. In particular, connectivity between right CEN and the cingulo-opercular salience sub-network (rCEN-CON) showed a gradient effect where the severity of abnormalities increased from no history of SUD to past+ to current+. We also observed diagnosis-specific effects, suggesting non-affective psychosis patients were particularly vulnerable to effects of substance use on rCEN-CON connectivity. Our findings reveal insights into how comorbid SUD affects between-network connectivity and symptom severity in ESP.
Introduction
Psychotic disorders are among the most debilitating mental illnesses, and are characterized by structural and functional abnormalities in multiple brain areas involving several distinct brain systems. Dysconnectivity in functional networks, a failure of integration or altered connectivity between brain networks, has been suggested to underlie the pathophysiology of psychosis. Menon et al. (2011) proposed a Triple Network Model of psychopathology which highlights abnormal organization, functioning of, and interactions between the Salience Network (SN), Default Mode Network (DMN), and Central Executive Network (CEN) as a framework for understanding the pathophysiology of psychiatric disorders. In this model, the SN is suggested to play a key role in initiating network switching, thus resulting in the engagement/disengagement of attention to external events (CEN) and internal events (DMN). This model highlights the importance of studying between-network connectivity, as dysfunction in one of the three networks is likely to result in abnormalities in the other two. In psychosis, structural and functional abnormalities have been observed in all 3 networks. Supekar et al. computed a network interaction index (NII), posited to capture the extent to which the SN is engaged with the CEN and decoupled from the DMN, and showed that it was significantly lower in schizophrenia patients. The NII was also significantly correlated with positive symptom severity. This finding suggests a shift toward inappropriate allocation of resources to internal events (ie reduced suppression of the DMN) away from external events, implicating SN-based switching mechanisms in the generation of psychosis symptoms.
Recent work has suggested that the DMN, SN, and CEN are heterogeneous systems that consist of distinct sub-networks, linked to separate processes and functions, that co-activate or interact with each other. The DMN is thought to comprise of at least 3 functionally-distinct components, including a dorsal medial pre-frontal sub-network (linked to mentalizing) and a mesial temporal lobe sub-network (linked to cognitive functions and episodic memory). The SN has also been associated with 2 distinct sub-networks that reflect a dorsal-ventral organization of salience network to synchronize with externally and internally-oriented networks, respectively. The cingulo-opercular salience network (CON) is a transitional ventral-insula network linking cognition with emotion and interoception that is also involved in functions such as tonic alertness and the maintenance of task mode, while the prefrontal-dorsal insula salience network (Sal) is linked to orchestrating task-switching. Finally, the CEN is thought to show hemisphere lateralization, with both left and right lateralized CEN being associated with cognitive processing. However the left CEN is more strongly associated with language functions and the right CEN is more strongly associated with reasoning, attention, inhibition, and memory.
Substance use, particularly during adolescence or early adulthood, is associated with a consistent increase in the risk of developing psychosis. A growing body of evidence has shown associations between substance use disorder (SUD) and psychotic disorders – substance use co-occurs at a far higher rate in psychosis patients compared to the general population, patients with SUD have greater impairments (eg lower functioning) and poorer outcomes (eg higher unemployment) than patients without. In addition, the effects of SUD co-morbidity appear to be diagnosis-dependent, where clinical outcomes were observed to be worse in patients diagnosed with schizophrenia compared to patients diagnosed with affective psychosis. Importantly, SUD has also been shown to affect between-network connectivity in similar regions to psychosis, particularly between the SN-CEN. Altogether, these findings suggest that substance use may mediate the relationship between psychosis symptom severity and between-network connectivity.
The early stage of psychosis (ESP) – the initial years following the first episode of psychosis (FEP) – is considered a critical period during which effective intervention may help slow functional decline and promote favorable outcomes. However, the key influencing factors and alterations in the brain that render sub-groups of patients vulnerable to symptom exacerbation and poor recovery are not well understood. Also, the actual impact of SUD on brain connectivity in ESP patients is not well studied. While lifetime prevalence of SUD is high in psychosis patients, studies have also reported that patients significantly reduce substance use after FEP. To date, few studies differentiate between past and current SUD. Thus, the persistence of connectivity abnormalities resulting from SUD is unclear.
Studies have examined substance use prevalence and between-network connectivity in psychosis patients independently, but to our knowledge none have looked at them together, especially during the critical ESP period. Furthermore, previous research has mostly focused on the DMN, SN, and CEN as a whole and not studied between-network connectivity in the Triple Network framework at the finer granularity of sub-networks. Also, given that SUD and psychosis appear to affect similar resting state networks (RSNs), understanding (1) whether SUD results in independent or additive/gradient effects, (2) whether SUD effects on between-network connectivity are persistent, and (3) the impact of diagnosis, would have significant potential to guide better treatment. In this study, we collected neuroimaging and clinical data from a cohort of transdiagnostic ESP patients and healthy controls to investigate the effect of SUD and diagnosis specificity on between-network connectivity in ESP patients. We first assessed differences between ESP patients and controls in connectivity between sub-networks of the SN, DMN, and CEN. We then examined whether histories of past or current SUD co-morbidity were associated with differences in between-network connectivity in ESP patients. We also investigated whether observed differences in between-network connectivity were affected by diagnosis. Finally, we explored whether SUD mediated the connectivity between networks and symptom severity. We hypothesized that between-network connectivity of sub-networks in the Triple Network Model would be abnormal in ESP patients; patients with co-morbid SUD would show a greater dysregulation of between-network connectivity than those without; and that patients with non-affective psychosis and SUD co-morbidity would show greater impairments than those with affective psychosis and SUD co-morbidity, particularly between sub-networks of the SN and CEN.
Methods
Participants
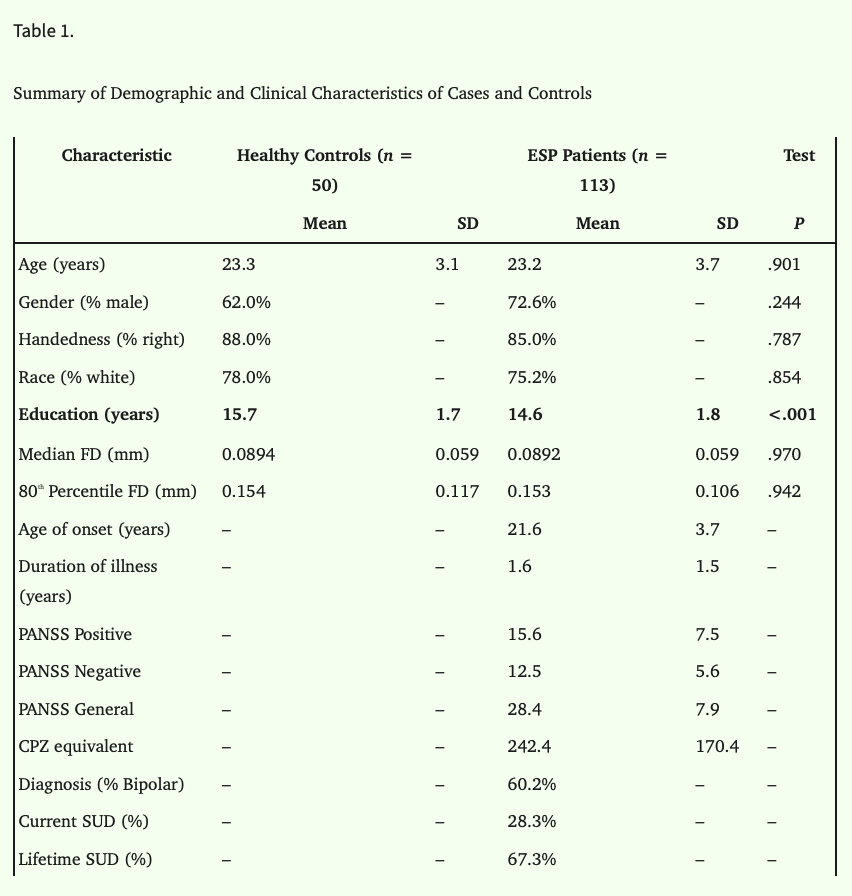
163 participants (ESP = 113, healthy controls [HCs] = 50) were recruited for this study. Patients were recruited from the First Episode Psychosis Clinic at McLean Hospital and met DSM-IV criteria for Schizophrenia, Schizoaffective disorder, Bipolar I Disorder with psychotic feature, or psychosis not otherwise specified. Supplementary material S1 presented exclusion criteria for ESP patients including substance-induced psychotic disorder as listed in the DSM. ESP at the time of entry to the clinic was defined as having an onset of psychotic symptoms within the past five years. Age-matched HCs were recruited from the community. This study was approved by the McLean Hospital Institutional Review Board. All participants provided written informed consent.
Clinical Assessments
Patients with psychosis were assessed for lifetime (past + current) substance abuse or dependence using the DSM-IV Module E. Those meeting criteria for either abuse or dependence were categorized as having a substance use disorder. Patients were determined to have current SUD co-morbidity if they met criteria in the past month. Most patients had a diagnosis of alcohol and/or cannabis SUD (supplementary material S2).
Psychotic symptom severity was assessed using the positive, negative, and general subscales of the Positive and Negative Syndrome Scale (PANSS). Medication information was collected; antipsychotic medication dosages (97% second-generation) were converted into chlorpromazine equivalents (CPZE) based on the recommendations of Gardner et al.
MRI Image Acquisition and Neuroimaging Data Pre-processing
Imaging data were acquired using a Siemens 3T Tim Trio scanner with a 12-channel phased-array head coil. Two runs of resting state fMRI sensitive to blood oxygenation level-dependent (BOLD) contrast were collected: 6.2 min (124 time-points), TE/TR/Θ = 30 ms/3000 ms/85°, 3 mm isotropic voxels. A T1-weighted image (1.2 mm iso) was also collected for co-registration.
Functional and structural imaging data were converted from raw DICOM images to the BIDS specification format, and pre-processing of each run was performed using the fMRIPrep 1.4.0 pipeline (figure 1B) based on Nipype 1.2.0. Automated removal of motion artifacts using independent component analysis (ICA-AROMA) was performed on the preprocessed BOLD on MNI space time-series after removal of non-steady state volumes and spatial smoothing with an isotropic, Gaussian kernel of 6mm FWHM (full-width half-maximum). To ensure rigorous denoising of subject movements, ICA-AROMA components were visually assessed, and erroneous labels were manually edited. fMRI data were non-aggressively denoised to remove artifacts. Additionally, noise regressors were generated to implement aggressive denoising (fsl_regfilt) in runs that exceeded motion exclusion criteria (Maximum framewise displacement [FD] exceeding 3 mm; or 80th percentile FD of all volumes in a scan exceeding 0.3mm). Finally, data underwent high pass temporal filtering (sigma = 25.0). (Additional details in supplementary material S3)
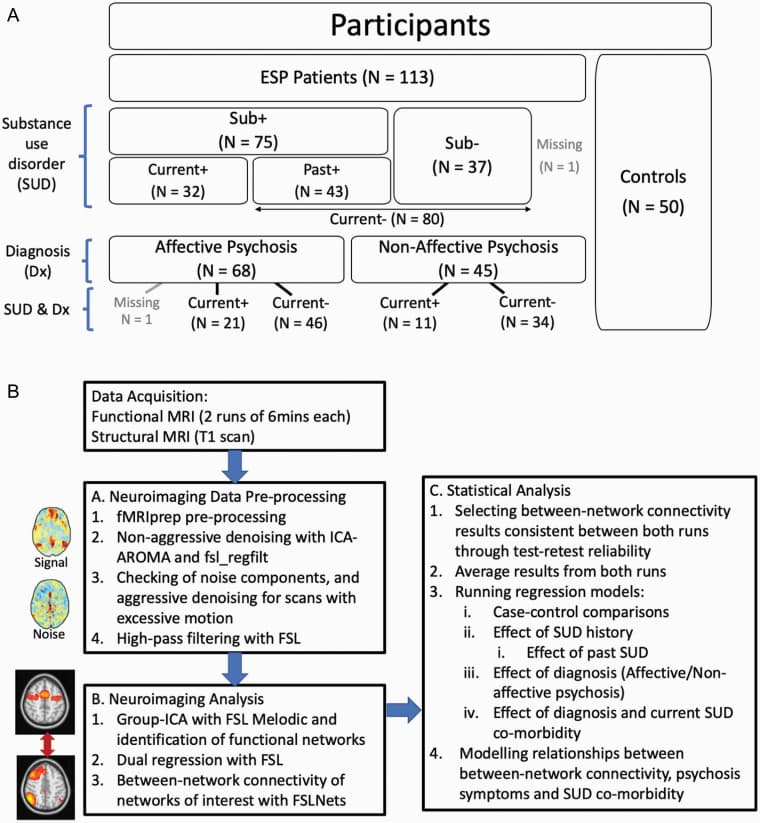
Neuroimaging Analysis
Group-ICA with Dual Regression.
Group-ICA (GICA) with different model orders (30, 35, 40, 45) was performed with FSL MELODIC for both runs of all subjects (326 scans). GICA maps were visually inspected to determine the optimal number of components that parcellated the brain into sub-networks most closely resembling published network maps; a 40-component model was selected for good separation of signals into sub-networks of the DMN, SN, and CEN. By deriving a data-driven parcellation using our data rather than a published scheme from the literature, we were able to implement a more fine-grained parcellation of sub-networks, and also able to identify and remediate sources of study-specific noise.
Eight components corresponding to the Triple Network framework were identified from the GICA for assessment of between-network connectivity: 4 DMN sub-networks (anterior DMN, ventral-medial DMN, posterior DMN, mesial temporal lobe DMN [tempDMN]); 2 CEN sub-networks (left CEN [lCEN], right CEN [rCEN]); and 2 SN sub-networks (Cingulo-Opercular network [CON], prefrontal-dorsal insula salience network [Sal]). Dual regression analysis (in FSL) was performed as previously described to generate network-specific timecourses for each run for each subject.
Between-Network Connectivity.
The FSLNets Toolbox was used to compute functional connectivity between networks as the partial correlation (via Ridge Regression with rho = 0.1) between each pair of network timecourses (28 network-pairs) for each BOLD run. Partial correlations were then Fisher z-transformed.
Statistical Analysis
Statistical analyses conducted are summarized in figure 1B.
Selection of Network Pairs of Interest.
To focus on meaningful network-pairs in addressing our study aims, test–retest reliability between the two runs was calculated for each network-pair (supplementary material S4). Network-pairs that had consistent connectivity values across both runs were selected for further analyses. Four network-pairs (rCEN–CON, rCEN–tempDMN, Sal–CON, and rCEN–lCEN) survived the reliability threshold. Thus, all further statistical analyses were based on these four network-pairs, where partial correlation values between each network-pair from both runs were averaged and used as dependent variables. The remaining 24 network-pairs were not further analyzed.
Group and Sub-Group Differences in Between-Network Connectivity.
Between-network connectivity differences between HCs, ESP patients as a whole, and ESP patient sub-groups (split by SUD co-morbidity and/or diagnosis; figure 1A) were assessed separately with linear regression models (LM) (StataIC 15.1). In all models, age, gender, and years of education were included as co-variates of no interest as SUD is generally more prevalent in younger males with lower education. In models with only patients, medication (CPZE) and duration of illness were also included as co-variates.
Patient-Control Comparisons
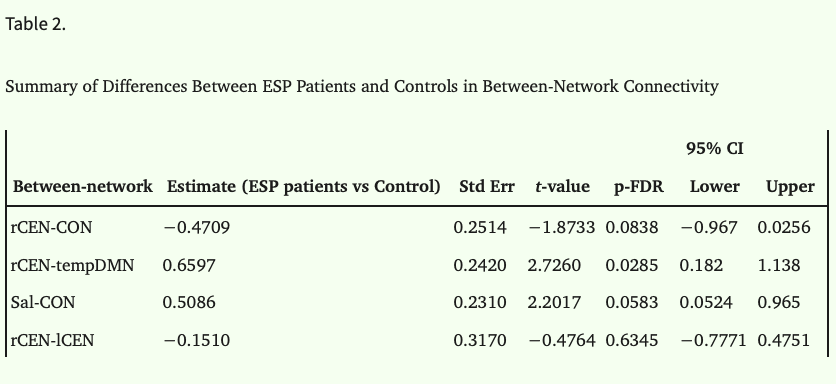
One LM model was ran for each of the 4 network-pairs with between-network connectivity as the dependent variable, and group (ESP vs control) as the independent variable. Significance level was P < .05 after correction with FDR (Benjamini–Hochberg method) across the four network-pairs.
Patients Split Into Sub-Groups
Given the small sample size in each patient sub-group, multiple comparison corrections were applied for pairwise sub-group comparisons (specified below) for each network-pair, but not across the different network-pairs to retain sensitivity. Significance level was set at P < .05 for all analyses (after corrections where relevant). Therefore, for analyses involving sub-groups, each network-pair was regarded separately with independent hypotheses (following analyses repeated across all 4 network-pairs).
Effect of SUD
Two separate LM models were ran with between-network connectivity as the dependent variable in patients alone, and (1) history vs no history of SUD, or (2) past vs current SUD as the independent variables. To determine if a particular sub-group was more vulnerable to connectivity abnormalities, exploratory analysis comparing patient sub-groups with controls was ran with Dunnett’s correction applied over the 3 sub-group comparisons (eg no history of SUD / past SUD / current SUD vs controls).
Effect of Diagnosis
The effect of diagnosis was investigated in two ways. First, between-network connectivity in patients diagnosed with affective (AP) vs non-affective psychosis (NAP) were compared with controls where Dunnett’s multiple comparison correction was applied for 2 sub-group comparisons (eg AP vs controls and NAP vs controls). Second, for the network-pair that showed a significant current SUD effect (rCEN–CON), a LM was ran in patients alone with between-network connectivity as the dependent variable, and the interaction between diagnosis (AP/NAP) and current SUD (with/without) as the independent variable. The difference in connectivity in NAP patients with and without current SUD, and AP patients with and without current SUD was assessed.
Relationships Between Symptom Severity, Current SUD, and Between-Network Connectivity.
In patients alone, associations between the 3 PANSS sub-scales and between-network connectivity were assessed using Pearson’s correlation (StataIC 15.1) for network-pairs that showed a significant current SUD effect (rCEN–CON). Structural equation modelling with maximum likelihood method was used to model the relationships among psychosis symptoms, SUD co-morbidity, and between-network connectivity. Model fit was assessed with seven goodness-of-fit statistics indices (details in supplementary material S9).
Results
Demographics Data
The average age of controls and patients was 23 years (HCs: 23.3 ± 3.1; ESP: 23.2 ± 3.7). Males comprised of 62% of the HC sample and 72.6% of the patient sample. On the whole, demographics were similar between patients and controls except that controls had, on average, 1 year more education than patients (table 1). Characteristics of the sub-groups are provided in supplementary materials S5 and S6 respectively.
Case-Control Comparison
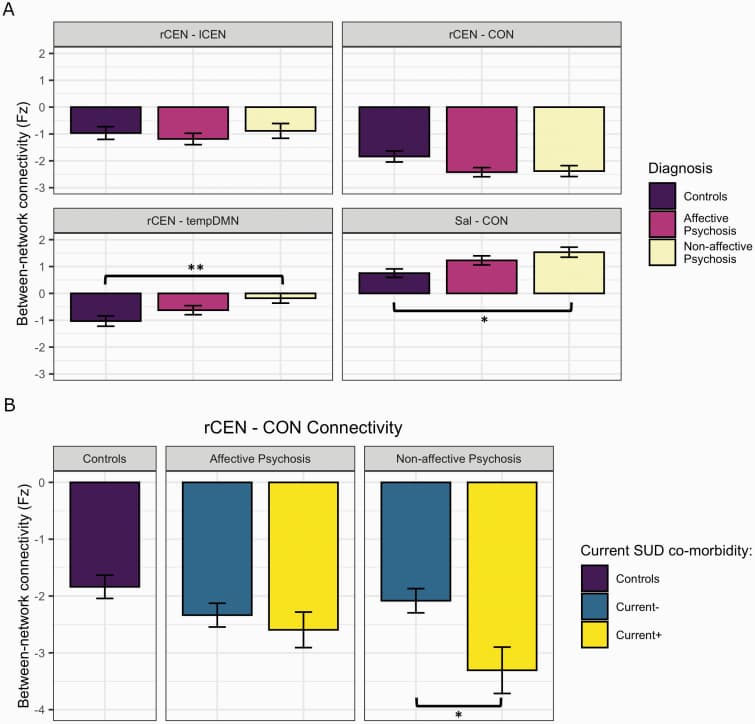
Results showed that rCEN-tempDMN connectivity was decreased in patients (p-FDR < 0.05; table 2). In this case, although the estimated difference (ESP vs control) was positive, figure 2 shows that controls have, on average stronger negative connectivity between these two networks. Connectivity between rCEN-CON, Sal-CON, and rCEN-lCEN was not significantly different between patients and controls. Supplementary material S7 shows spatial maps of the networks.
Effect of Substance Use Disorder (SUD) on Between-Network Connectivity
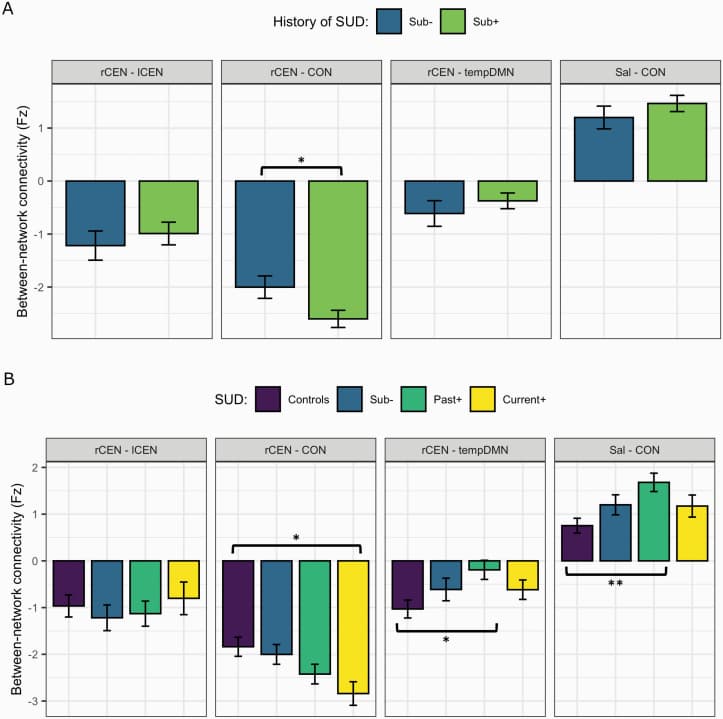
History of SUD. To test the effect of SUD on between-network connectivity, ESP patients were split into two groups: patients with (sub+; n = 75) and without (sub-; n = 37) a history of SUD.
rCEN-CON connectivity was significantly more negative in sub+ compared to sub-group (−0.596, t = −1.99, P = .049, 95% CI = −1.190 to −0.0026). There were no significant differences between sub+/- in the other between-network connections of interest (figure 2A).
Current vs Past SUD. To test if past SUD had persistent effects on connectivity, patients with a history of SUD were split into past SUD (past+; n = 43) and current SUD (current+; n = 32).
There were no significant differences between the two sub-groups for the between-network connections, which may be due to a true null difference, or a difference that was not detected due to our small sample sizes. An assessment of power shows that we are likely underpowered to detect small effects, with ~100 participants per sub-group needed to achieve a power of 0.8.
An exploratory analysis comparing patient sub-groups (sub-, past+, current+) with controls showed the past+ group had increased connectivity between Sal-CON (0.884, t = 3.21, P = .013) and weaker negative connectivity between rCEN-tempDMN (0.852, t = 2.88, P = .005) than controls. Current+ differed from controls for rCEN-CON, with increased negative connectivity (−0.925, t = −2.86, P = .013) (figure 2B). No differences between patient sub-groups and controls were observed for rCEN-lCEN.
This analysis suggests: (1) a gradient SUD effect in connectivity between rCEN-CON where the severity of abnormalities increased from no history of SUD to past+ to current+ (figure 2B, 2nd Panel); (2) a persistent effect of SUD where abnormalities in connectivity between rCEN-tempDMN and Sal-CON were most severe in past+ group (figure 2B, 3rd and 4th panel).
Effect of Diagnosis on Between-Network Connectivity
To test the effect of diagnosis on between-network connectivity, ESP patients were split into two groups: affective psychosis (AP; n = 68) and non-affective psychosis (NAP; n = 45) and compared to controls separately.
The NAP group differed from controls in two networks: rCEN-tempDMN and Sal-CON (figure 3A, supplementary material S8). For rCEN-CON and rCEN-lCEN connectivity, both AP and NAP sub-groups were not significantly different from controls (figure 3A, supplementary material S8).
Given the effect of SUD on rCEN-CON connectivity seen in figure 2, we investigated whether the stronger negative connectivity between rCEN-CON observed in patients with current SUD co-morbidity (current+; n = 32) would be different in AP and NAP patients.
Stronger negative connectivity between rCEN-CON was observed in NAP patients with SUD compared to those without current SUD (−1.177, t = −2.43, P = .017, 95% CI = −2.140 to −0.215). However, connectivity between rCEN-CON did not differ between AP patients with or without current SUD (−0.279, t = −0.74, P = .464, 95% CI = −1.033 to 0.474) (figure 3B).
Between-Network Connectivity, SUD, and Symptom Severity
Given the effect of current SUD on rCEN-CON, an exploratory correlation analysis was performed in patients to investigate if rCEN-CON connectivity was associated with symptom severity (3 PANSS sub-scales). rCEN-CON connectivity was significantly correlated with PANSS positive symptom severity (r = −0.2069, P = .0286).
3 structural equation models were compared to model the relationships among current+, positive symptoms, and Rcen-CON connectivity: (1) Independent model in which positive symptoms and current+ independently affect rCEN-CON connectivity; (2) Mediation model with positive symptoms as the mediator; (3) Mediation model with SUD as the mediator (figure 4).
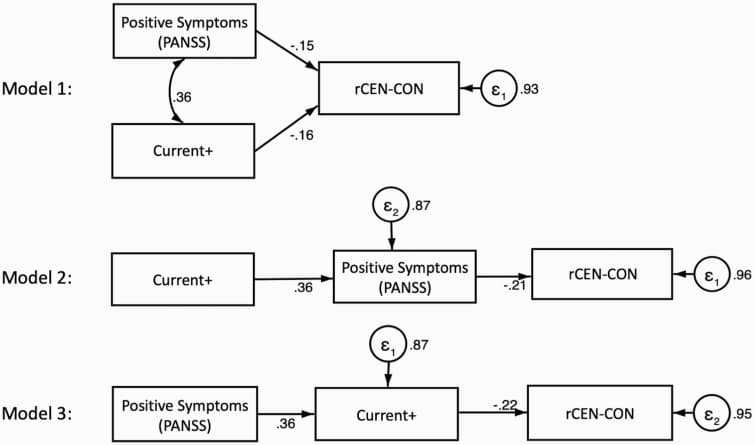
Goodness-of-fit tests indicated that model 3 fit the data best (supplementary material S9), where positive symptom severity positively influenced SUD status (0.36, P < .001), which in turn negatively affected rCEN-CON connectivity (−0.22, P = .016).
Discussion
In this study, we found that SUD co-morbidity altered between-network connectivity across DMN, CEN, and SN subsystems in ESP patients. We observed that past and current SUD had specific effects on connectivity between different network-pairs. We also observed that NAP patients showed greater alterations in between-network connectivity relative to AP patients. Furthermore, current SUD co-morbidity had a greater impact on rCEN-CON in NAP patients compared with AP. Finally, we compared three alternative models to assess whether psychotic symptoms may mediate the association between SUD and connectivity, or whether SUD mediates the association between symptoms and connectivity and found that it is the latter − SUD partially explains the association between psychosis symptoms and rCEN-CON connectivity.
Triple Network Framework
In our study, we observed stronger negative FC between sub-networks of the CEN and DMN (rCEN-tempDMN) in controls, with only weak negative FC in ESP patients. This finding is in line with the literature where Manoliu et al. also reported alterations in connectivity between the DMN and CEN in schizophrenia patients. We have now shown that abnormalities in between-network connectivity are already present at the early stage of psychosis, and occur to a lesser degree in AP patients relative to NAP patients. ESP-control differences were observed in NAP patients for two network-pairs (rCEN-tempDMN, SAL-CON). This result follows a general pattern in the psychosis literature, where abnormalities in various domains (eg cognition, functional connectivity, morphometric measures) are more severe in NAP patients. Our findings support a framework where psychosis symptoms (eg hallucinations) manifest as a result of an inappropriate shift of salience from external events to internal processes.
While previous research has mostly focused on the DMN, SN, and CEN as a whole, we investigated sub-networks of these systems. Our findings were largely specific to the temporal lobe sub-network of the DMN, the right lateralized CEN, and the CON sub-network of the SN. Therefore, it appears that the abnormalities in between-network connectivity in the Triple Network framework are specific to distinct sub-networks in the SN and DMN that are closely linked to CEN functions. Our results also suggest lateralization of CEN functions, as we only observed abnormalities for network-pairs involving the right CEN. The right CEN has been linked to multiple cognitive processes including reasoning, attention, inhibition, and memory; whereas the left CEN has been strongly mapped to language processing, including semantic, phonologic, and orthographic tasks.
Effect of SUD
We showed that patients with past and current SUD had alterations in between-network connectivity, resulting in two observed patterns. rCEN-CON FC (figure 2B 2nd panel) had stronger negative connectivity in patients with current SUD co-morbidity relative to patients with past SUD, suggesting a direct acute effect of SUD. This finding is consistent with previous studies showing that SUD affects CEN-SN connectivity. The addiction literature hypothesizes an inappropriate shift of salience attributed to substance-related cues, with decreased sensitivity to non-substance reinforcers. Reduced CEN-SN connectivity has also been associated with impairments in cognitive control and higher impulsivity. Thus, altered rCEN-CON FC could be a mechanism underlying an overlapping feature in both SUD and psychosis.
In addition, our study provides some evidence that past SUD has persistent effects on brain connectivity. While we did not specifically test the hypotheses that past+ would be significantly different from sub- to minimize multiple comparisons, the past+ group had the greatest differences relative to controls for rCEN-tempDMN and Sal-CON connectivity. This observation suggests that alterations in connectivity between these network-pairs may be linked to abstinence or withdrawal symptoms. SN has been linked to acute nicotine abstinence. Thus, even if substance use decreases after FEP, abnormalities in between-network FC may persist.
Our study also showed that SUD had diagnosis-specific effects on between-network connectivity. rCEN-CON was significantly more negative in NAP patients with SUD, but not in AP patients, suggesting that NAP patients are particularly vulnerable to substance use. Literature indicates that AP patients have higher cognitive reserve and better premorbid IQ than NAP patients, suggesting that cognitive reserve, premorbid IQ, and functioning (proxy for greater brain weight and increased efficiency of connectivity) might play a role in mediating higher vulnerability in NAP patients. AP patients in our sample had higher education and lower PANSS negative score (associated with cognitive performance), which indirectly supports greater cognitive reserve and differential vulnerability. Our results suggest between-network connectivity abnormalities as a potential mechanism underlying the worsened clinical outcomes in NAP patients, but not AP patients, with SUD.
Relationship With Positive Symptoms
Our finding of a negative correlation between rCEN-CON and positive symptom severity is consistent with other published findings and suggests that engagement and disengagement of the rCEN is associated with the manifestation of positive symptoms. Notably, the rCEN contains the right temporoparietal junction, and disruption of activity in this region has been linked to hallucinatory misperceptions. Regions in both the rCEN and CON are thought to be the key components of a “reorienting system”, whose improper functioning can result in misperceptions of environmental stimuli and self.
Given the observation that alterations in rCEN-CON connectivity were associated with positive symptom severity and with SUD in ESP, it is important to parse out whether each risk factor occurs independently from underlying vulnerabilities; whether SUD contributes to exacerbated psychosis symptoms, resulting in greater connectivity abnormalities; or whether patients develop SUD to cope with increased symptoms (“self-medication model”). Our study is the first to model these relationships and our results suggest that patients use substances as a coping mechanism for positive symptoms. Reports, where patients described using substances to alleviate positive symptoms, and relieve non-psychotic effects such as general dysphoria and anxiety, support the self-medication hypothesis. Nevertheless, the temporal relationship between SUD and psychosis is difficult to determine due to the difficulties in establishing when illness begins. In our study, the age of onset of substance use is lower than the age of onset of psychosis. Model 3 (SUD as mediator) only performed slightly better than model 2 (positive symptoms as mediator). Therefore, it is likely both scenarios (“self-medication” and “substance use precipitates psychosis”) co-exist in our ESP population. All in all, our data suggests a mechanism where ESP patients use substances for short-term relief of illness-related discomfort, resulting in greater brain abnormalities, and future exacerbation of symptoms.
Limitations
Some limitations have to be considered. First, the sample sizes within each sub-group, especially current+, was small. Therefore, we chose to limit our analyses to specific hypotheses, and looked at SUD as a whole. Second, we only investigated a diagnosis of SUD via SCID, rather than levels of drug use. The categorical effects of SCID-derived SUD may be different than the dimensional effects using different drug assessments. Furthermore, we did not collect detailed information on remission for patients with past SUD. Information on past or current nicotine use disorder was also not available. However, few patients and none of the controls were smokers in our study. Finally, motion effects remain a potential confounder in neuroimaging studies. While we have corrected for motion in our processing pipeline, we also included a motion parameter as a co-variate in our models, and main findings were unchanged (supplementary material S10). Future work could include additional cohorts of substance induced psychosis or SUD patients without psychosis to further explore the associations between SUD and psychosis. It would also be interesting to study if different types of substances (eg alcohol, cannabis, stimulants) have different mechanistic effects on brain connectivity.
Conclusion
Our study provides evidence that SUD affects connectivity between SN and CEN in ESP patients, which could underlie clinical outcomes. In particular, rCEN-CON showed the greatest abnormalities in patients with current SUD co-morbidity, and was associated with positive symptom severity and modulated by diagnosis. In addition, we found persistent effects of SUD on brain connectivity. More research is warranted to elucidate the complex interactions between diagnosis, SUD, functional connectivity and clinical outcomes.