Abstract
The past 15 years has seen a rapid expansion in the number of studies using neuroimaging techniques to investigate maturational changes in the human brain. In this paper, I review MRI studies on structural changes in the developing brain, and fMRI studies on functional changes in the social brain during adoles- cence. Both MRI and fMRI studies point to adolescence as a period of continued neural development. In the final section, I discuss a number of areas of research that are just beginning and may be the subject of devel- opmental neuroimaging in the next twenty years. Future studies might focus on complex questions including the development of functional connectivity; how gender and puberty influence adolescent brain develop- ment; the effects of genes, environment and culture on the adolescent brain; development of the atypical adolescent brain; and implications for policy of the study of the adolescent brain.
Introduction
Half a century ago, very little was known about how the human brain develops and it is unlikely scientists expected that at the turn of the millennium it would be possible to look inside the brains of living humans of all ages and track changes in brain structure and function across development. In the second half of the twentieth century, interest in brain development rapidly increased. Most research in this field relied on non-human animal brains. Data on human brain development were rare because of the scarcity of post-mortem human brains of different ages. It is only in the past 15 years or so, because of advances in imaging techniques, that research has revealed a great deal about the development of the living human brain across the lifespan. Technical advances in neuroimaging methods, in particular, magnetic resonance imaging (MRI) and functional MRI (fMRI), have revolutionized what we know about how the human brain develops and have facilitated the rapid expansion of this young research field.
In this paper, I first review early histological studies on the development of the brain. In the next section, I outline recent advances that have made it possible to study the development of the living human brain. In the third section, I review recent MRI studies on structural development in the living human brain. Next, I briefly discuss fMRI developmental imaging studies on the social brain during adolescence. Finally, I look ahead and speculate on some of the key questions for the next 20 years of developmental neuroimaging.
Early studies
Ground-breaking experiments on animals, starting in the 1950s, showed that, soon after birth, sensory regions of the brain go through sensitive periods during which environmental stimulation appears to be crucial for normal brain development and normal perceptual development to occur (Hubel and Wiesel, 1962; Wiesel and Hubel, 1965). Early in postnatal development, the brain begins to form new synapses, so that at some point in early development the synaptic density greatly exceeds adult levels. This process of synaptogenesis lasts up to several months or years, depending on the species of animal, and is followed by a period of synaptic pruning (Cragg, 1975). Which synapses survive and which are selectively eliminated is partly experience-dependent (Changeux and Danchin, 1976; Low and Cheng, 2006). Much of what we know about how the brain develops comes from animal research. For example, research carried out in rhesus monkeys demonstrated that synaptic densities in visual cortex reach maximal levels two to four months after birth, after which time pruning begins (Bourgeois et al., 1994; Rakic, 1995; Rakic et al., 1986; Woo et al., 1997; Zecevic and Rakic, 2001). Synaptic densities gradually decline to adult levels at around three years, around the time rhesus monkeys reach sexual maturity.
In the late 1960s and 1970s research on post-mortem human brains revealed that some brain areas, in particular the prefrontal cortex, continue to develop well beyond early childhood (Huttenlocher, 1979; Huttenlocher et al., 1982; Yakovlev and Lecours, 1967). First, it was found that the myelination of axons follows a chronologic sequence, and that the last cortical areas to be myelinated are the association areas, the prefrontal cortex (PFC) among them, where the process of myelination continues for years, well into adolescence (Yakovlev and Lecours, 1967). Second, post-mortem human brain data suggested that synaptic reorganisation continues throughout childhood and adolescence in certain brain regions (Webb et al., 2001). Histological studies of human prefrontal cortex have shown that there is a prolif- eration of synapses in the subgranular layers of the prefrontal cortex during early and mid-childhood, followed by a plateau phase and a subsequent elimination and reorganisation of prefrontal synaptic connections during adolescence (Huttenlocher, 1979). This finding has recently been supported and expanded by a larger scale study of prefrontal synaptic spine development in 32 post-mortem human brains of different ages across the lifespan (aged one week to 91 years; Petanjek et al., 2011). This study demonstrated that pre- frontal dendritic spine density increases in childhood, resulting in numbers that exceed adult levels two- or three-fold by puberty, and then decreases gradually after puberty (Fig. 1). The elimination of synaptic spines continued beyond adolescence throughout the third decade of life, providing evidence for astonishingly protracted dendritic reorganisation in the human prefrontal cortex.
Figure 1
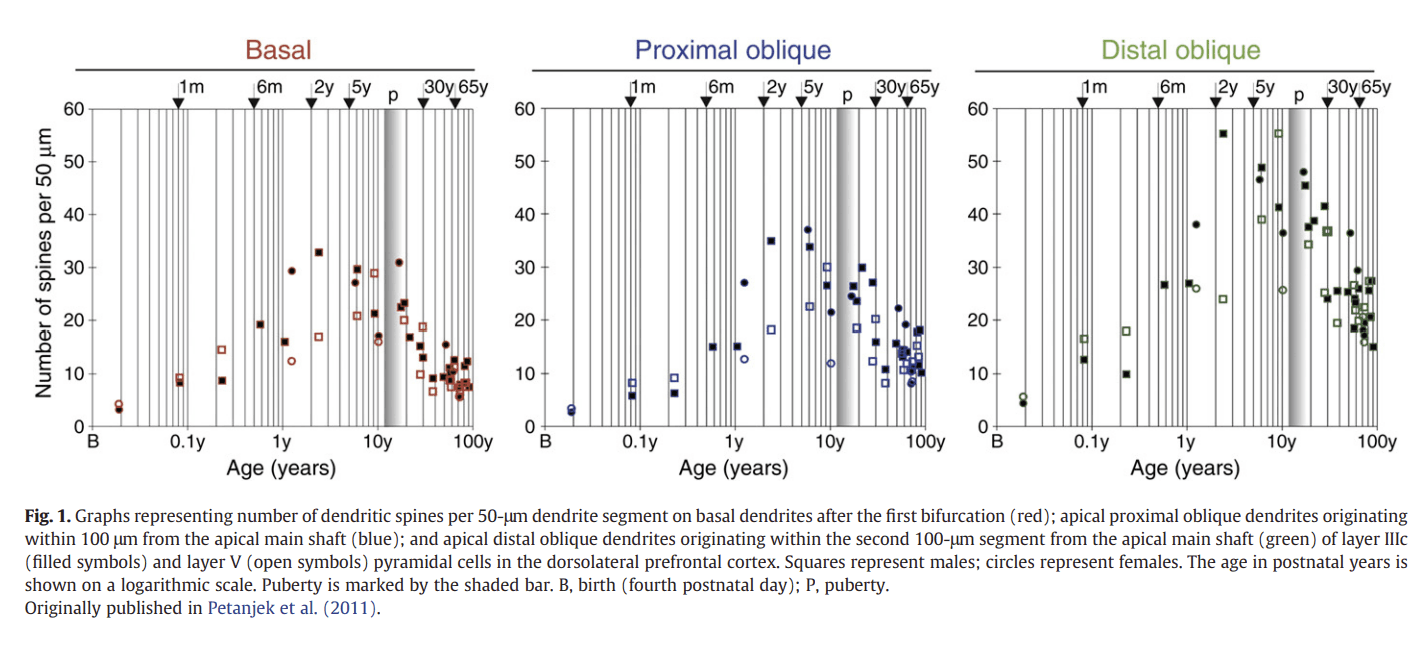
Figure 2
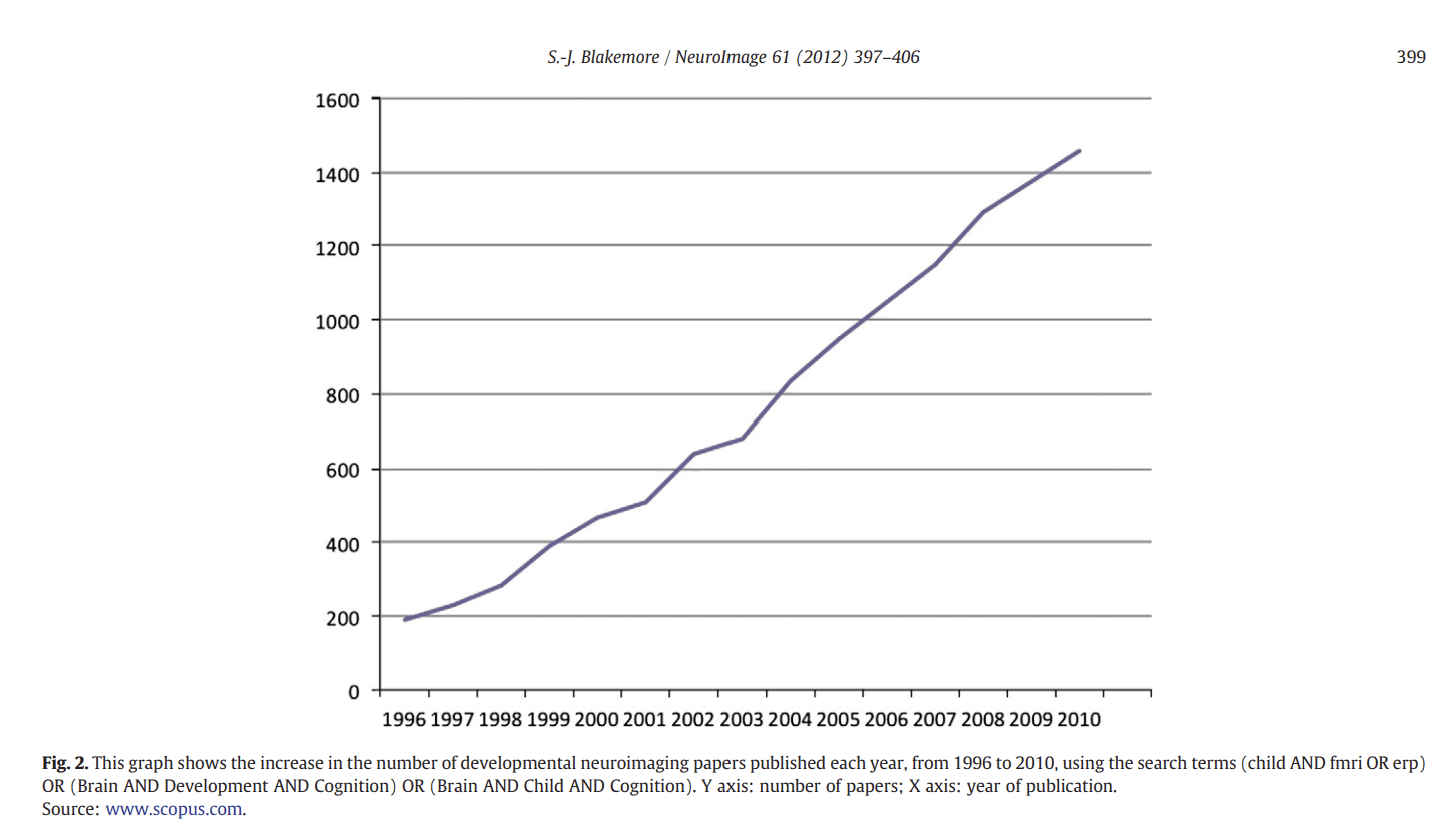
Recent advances using MRI
In the past decade or so, the field of developmental cognitive neuroscience has undergone unprecedented expansion, mostly due to technological advances in neuroimaging techniques. There has been a year-on-year increase in the number of papers reporting studies using paediatric neuroimaging published since 1996, as shown in Fig. 2. There have been high profile books (e.g. Johnson, 2004), special issues of several scientific journals and conferences, as well as a new journal (Developmental Cognitive Neuroscience), dedicated to this growing field (see Blakemore et al., 2010a).
Several different neuroimaging techniques have advanced to the point where they can be used reliably to study human brain development across age. EEG and event-related potentials (ERP) have long been regarded as the neuroimaging methods of choice with babies and young children. They have obvious appeal because of their safety, ease of use, and good temporal resolution. The development of func- tional near-infrared spectroscopy (fNIRS) is providing a new means to look at cortical activation in infants, since it is non-invasive, rela- tively low cost and portable (Gervain et al., 2011). However, more than any other advance, the increased use of MRI and fMRI in devel- opmental populations has created new opportunities to track structural and functional changes in the developing human brain. This work has advanced our knowledge of how the human brain de- velops, and the data from developmental neuroimaging studies have in turn triggered new interest in the changing structure and function of the brain over the lifespan.
Structural development
Research using MRI to acquire structural images from participants across the lifespan has revealed that the human brain continues to develop for many decades (e.g. Shaw et al., 2008). Age-associated region-specific, linear and non-linear changes in white matter tracts (Giedd et al., 1999; Lebel and Beaulieu, 2011; Ostby et al., 2009; Paus et al., 1999) and cortical grey matter (volume, density, and thickness; Ostby et al., 2009; Paus, 2005; Shaw et al., 2008; Tamnes et al., 2010) have been described in structural MRI studies.
White matter development
One of the most consistent findings from MRI studies is that there is a steady increase in white matter volume in several brain regions during childhood and adolescence. An early developmental MRI study revealed differences in the density of white and grey matter be- tween the brains of a group of children (average age 9 years) and a group of adolescents (average age 14 years; Sowell et al., 1999). The results showed adolescents had a higher volume of white matter and a lower volume of grey matter in the frontal cortex and parietal cortex compared with the younger group. Increased white matter and decreased grey matter density in the frontal and parietal cortices during adolescence is a finding that has been corroborated by several studies carried out by a number of different research groups with increasingly large numbers of subjects (Barnea-Goraly et al., 2005; Giedd et al., 1996, 1999; Paus et al., 1999; Pfefferbaum et al., 1994; Reiss et al., 1996; Sowell et al., 1999). In addition, increases in white matter volume are accompanied by progressive changes in MRI mea- sures of white matter integrity, such as the magnetisation-transfer ratio (MTR) in MRI, and fractional anisotropy (FA) in diffusion- tensor MRI (Fornari et al., 2007; Giorgio et al., 2010; Paus et al., 2008). The MTR indexes the efficiency of magnetization exchange be- tween different tissue compartments, and is strongly influenced by the integrity of myelin membranes. FA is the extent to which the diffusion of water molecules in the brain is anisotropic (not equal in all directions), and higher FA values are thought to reflect increasing organization of white matter tracts (due to processes including myelination and axon density), since water molecules will tend to diffuse in parallel with the tracts. Generally, there is evidence for in- creasing FA during adolescence (see Schmithorst and Yuan, 2010, for review). The increase in white matter seen with age (Fig. 3) has been interpreted as reflecting continued axonal myelination (and/or axonal calibre; Paus et al., 2008) during childhood and adolescence.
Figure 3
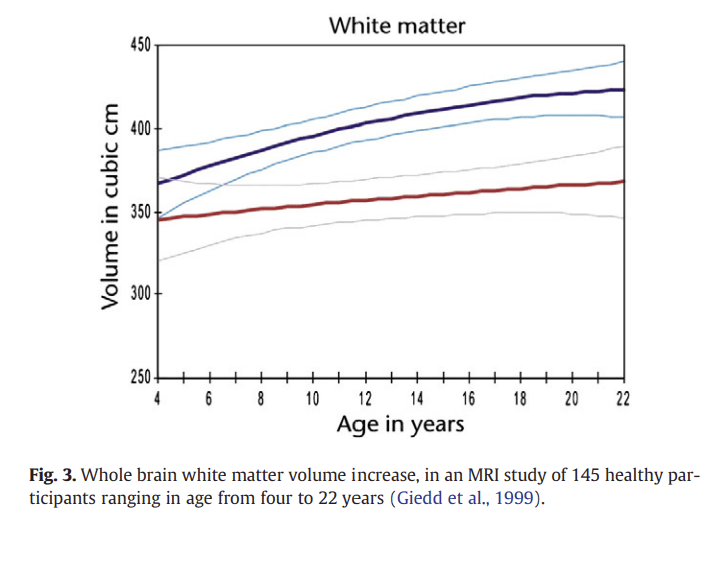
Recent DTI evidence for non-linear changes in white matter development has been reported in a longitudinal study of 103 participants from 5 to 32 years (Lebel and Beaulieu, 2011). 10 major white matter tracts were assessed for FA and mean diffusivity (MD; which also corresponds to white matter tract strength). In contrast with earlier studies showing linear increases in white matter volume, this study showed nonlinear development trajectories for FA and MD. FA and MD showed more rapid changes at early ages (increases for FA, decreases for MD), and slower changes or levelling off during young adulthood.
Grey matter development
In another pioneering developmental MRI study, emanating from the National Institute of Mental Health paediatric neuroimaging project, Giedd et al. (1999) performed longitudinal MRI scans on 145 healthy participants ranging in age from about four to 22 years. Scans were obtained from each participant at two-year intervals. The volume of grey matter in the frontal lobe increased during late childhood and early adolescence with a peak occurring at around 12 years. This was followed by a decline during adolescence (Fig. 4). Similarly, parietal-lobe grey matter volume increased during child- hood to a peak at around 12, followed by decline during adolescence. Grey matter development in the temporal lobes was also non-linear, but the peak was reached later at about 17 years. In another longitu- dinal study by the same group, participants aged between 4 and 21 were scanned every two years for 8 to 10 years (Gogtay et al., 2004). In terms of cortical grey matter density, sensory and motor brain regions matured earliest, followed by the remainder of the cor- tex, which matured (in terms of grey matter loss) from posterior to anterior regions. This loss of grey matter occurred last in the superior temporal cortex. A later study analysed cortical thickness and investi- gated the age of at which peak cortical thickness was reached, and again showed earlier maturation in sensory and motor regions and later maturation in parts of the frontal and temporal lobes (Shaw et al., 2008).
Figure 4
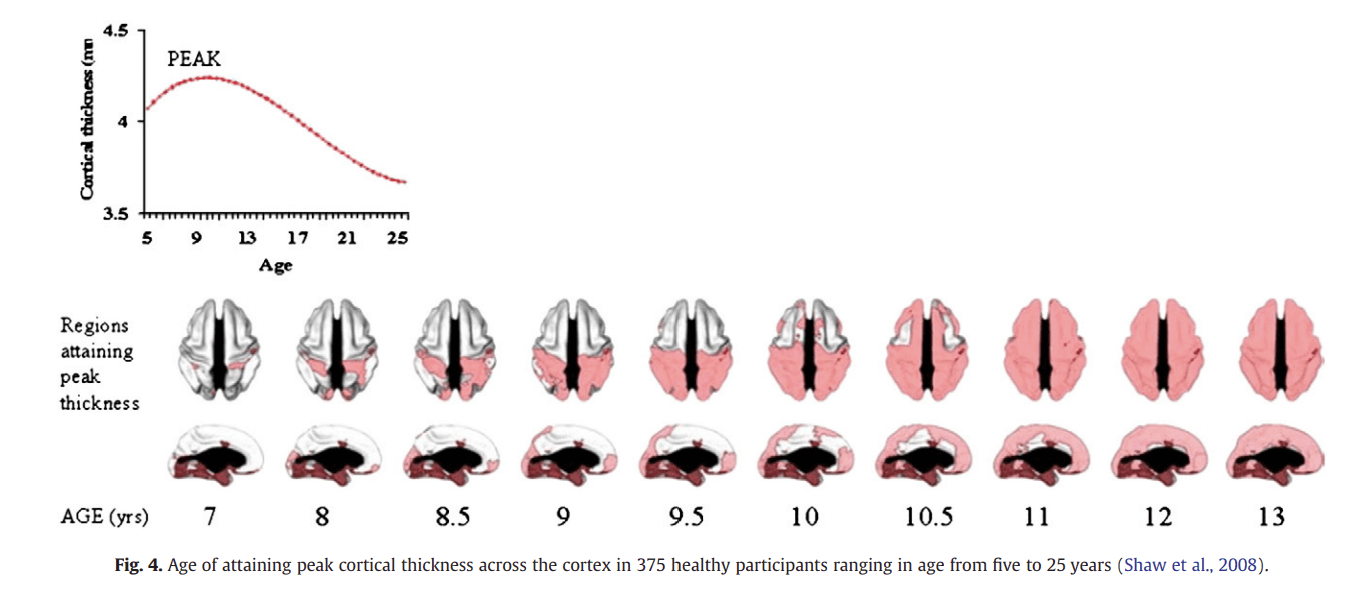
An early MRI study by a different group demonstrated a sharp acceleration in grey matter loss between childhood and adolescence in the dorsal prefrontal cortex and the parietal cortex (Sowell et al., 2001). The regions exhibiting the most robust decrease in grey matter density (e.g. the dorsal prefrontal cortex) also exhibited the most robust increase in white matter density. This study revealed that the loss of grey matter in the frontal cortex continued up to the age of 30. A further MRI study of participants ages 7 to 87 revealed a reduction in grey matter density in the dorsal prefrontal, parietal and temporal cortices, accompanied by an increase in white matter, which continued up to the age of 60 (Sowell et al., 2003).
The MRI results demonstrating non-linear developmental changes in grey matter in various brain regions throughout adolescence have been interpreted in several ways. First, age-related decreases in grey matter volume shown in MRI studies have been proposed to be pre- dominantly due to intracortical myelination and increased axonal calibre (Giorgio et al., 2010; Paus et al., 2008; Perrin et al., 2008). This would result in an increase in the volume of tissue that is classified as white matter (and a net reduction in grey matter) in MRI scans. A second explanation is that the grey matter changes reflect the synaptic reorganisation that occurs during puberty and adolescence (Huttenlocher, 1979; Petanjek et al., 2011). Thus, specu- latively, the increase in grey matter apparent at around the age of pu- berty onset (Giedd et al., 1999) might reflect a wave of synaptic proliferation at this time, while the gradual decrease in grey matter density that occurs in certain brain regions during adolescence has been attributed to synaptic pruning (Giedd et al., 1999; Gogtay et al., 2004; Sowell et al., 2001). Although changes in synaptic density are likely to be accompanied by changes in glia and other cellular components (Theodosis et al., 2008), whether such changes would be visible as volumetric changes in MRI scans is debated (see Paus et al., 2008).
Developmental functional neuroimaging studies of the social brain in adolescence
Developmental functional imaging, using fMRI, has rapidly expanded in the past decade. In this section I focus on development of the social brain in adolescence as an example of research in this burgeoning field.
The social brain is defined as the network of brain regions in- volved in understanding other people. It includes the network that is involved in theory of mind, or mentalising, the process that enables us to understand other people's actions in terms of the underlying mental states that drive them (Frith and Frith, 2007). For example, we interpret another person reaching towards a coffee pot in terms of a desire for coffee, rather than the mechanical forces used in such an action. Over the past 20 years, a large number of neuroimaging studies in adults have shown remarkable consistency in identifying the brain regions that are involved in mentalising. These studies have employed a wide range of stimuli including stories, sentences, words, cartoons and animations, each designed to elicit the attribution of mental states (see Lieberman, 2012-this issue; Amodio and Frith, 2006; Gilbert et al., 2010). In each case, the mentalising task resulted in the activation of a network of regions including the poste- rior superior temporal sulcus (pSTS)/temporo-parietal junction (TPJ), the anterior temporal cortex (ATC) including the temporal poles and the medial prefrontal cortex (MPFC; see Burnett and Blakemore, 2009, for evidence that these regions function as a network).
Recent meta-analyses of MPFC activation by different mentalising tasks indicate that the peak activation lies within the anterior dorsal MPFC (dMPFC; Amodio and Frith, 2006; Gilbert et al., 2006). This region is activated when one thinks about psychological states, regardless of whether these psychological states are applied to oneself (Johnson et al., 2002; Lieberman, 2012-this issue; Lou et al., 2004; Ochsner et al., 2004; van Overwalle, 2009; Vogeley et al., 2001), one's mother (Ruby and Decety, 2004), imagined people (Goel et al., 1995) or animals (Mitchell et al., 2005). Frith has proposed that the dMPFC is involved in the necessary decoupling of mental states from physical reality, whereas the pSTS/TPJ is involved in predicting what movement a conspecific is about to make (Frith, 2007; although see Saxe, 2006, for alternative viewpoint).
fMRI studies of mentalising during adolescence
While many studies over the past 30 years have investigated the development of mentalising in infancy and childhood, pointing to step-wise changes in social cognitive abilities during the first five years of life (Frith and Frith, 2007), recently experimental studies have focused on the development of the social brain beyond child- hood. Recent cognitive neuroscience studies have focused on adolescence as a period of profound social cognitive change. Adolescence is defined as the period of life between puberty and the attainment of a stable, independent role in society (Steinberg, 2010). Adolescence is characterised by psychological changes in terms of identity, self- consciousness and relationships with others (Steinberg, 2010). Compared with children, adolescents are more sociable, form more com- plex and hierarchical peer relationships and are more sensitive to acceptance and rejection by peers (Steinberg and Morris, 2001). While the causes of these social changes in adolescence are likely to be multi-factorial, development of the social brain might play a signif- icant role.
A growing number of fMRI studies have investigated the development of the functional correlates of mentalising during adolescence. These studies have generally compared brain activity during a mentalising task in adolescents and adults. Despite having used a wide variety of mentalising tasks, the results are remarkably consistent: in each of these studies dorso-medial prefrontal cortex (dMPFC) activity was greater in an adolescent group than in an adult group during a mentalising task compared to a control task (see Fig. 5 for meta-analysis).
Figure 5
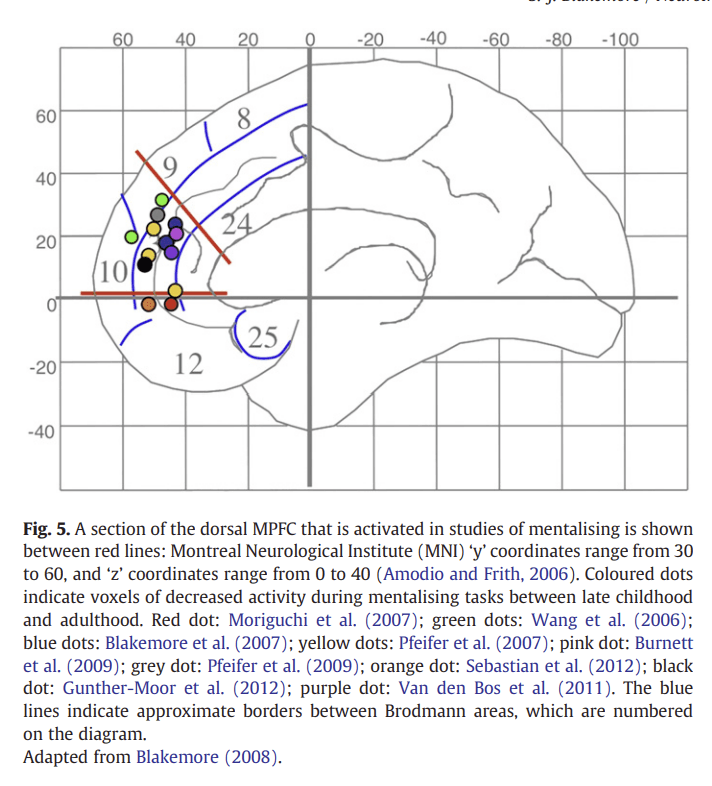
An early developmental fMRI study of mentalising investigated the development of communicative intent, using a task in which participants had to decipher a speaker's intention (whether they were being sincere or ironic; Wang et al., 2006). In young adolescents (aged 9–14), the dMPFC and left inferior frontal gyrus were more active during this task than in adults (aged 23–33) (see Fig. 5 green dots). A similar region of the dMPFC was found to be more active in adolescents than in adults in an fMRI study that involved thinking about one's own intentions (Blakemore et al., 2007). Adolescents (aged 12–18) and adults (aged 22–38) were presented with scenarios about intentional causality (involving intentions and consequential actions) or physical causality (involving natural events and their consequences). The dMPFC was more active in adolescents than in adults during intentional causality relative to physical causality (Fig. 5 blue dots). Conversely, a region in the right pSTS was more ac- tive in adults than in adolescents when they were thinking about intentional causality compared with physical causality. These results suggest that the neural strategy for thinking about intentions changes from adolescence to adulthood. Although the same neural network is active, the relative roles of the different areas change with age, with activity moving from anterior (dMPFC) regions to posterior (pSTS) regions.
In another developmental study that focused on the processing of self-related sentences (Pfeifer et al., 2007), children (aged 9.5–10.8) and adults (aged 23–31.7) were asked to indicate whether character traits accurately described themselves (self) or someone else (other). The dMPFC was more active in children than in adults during self-knowledge retrieval (see Fig. 5 yellow dots; a similar finding was reported in Pfeifer et al., 2009; grey dot). In another study of mentalising, participants aged 9 to 16 were scanned during an animation-based mentalising task (Moriguchi et al., 2007). Here the picture was more complex: there was a positive correlation between age and activity in the dMPFC, and a negative correlation between age and activity in the ventral MPFC (Fig. 5 red dots). The researchers suggest that this might reflect a change in strategy, from simulation in childhood (based on the self, which involves the ventral MPFC) to a more objective strategy in adults (involving the dMPFC). Note that the oldest participants in this study were 16, and it is unknown how activity within the dMPFC during the animations task changes after this age; it cannot be ruled out that activity in this region de- creases between 16 years of age and adulthood.
Recent studies have focused on emotional aspects of mentalising, and have revealed similar developmental patterns of activity changes. In one study, adolescents (aged 10–18) and adults (aged 22–32) read scenarios that pertained to social emotions such as guilt and embarrassment (Burnett et al., 2009). Unlike basic emotions such as fear and anger, social emotions require the representation of another per- son's mental states (for example, feeling guilty involves imagining how someone else would feel as a consequence of your action). Thinking about social relative to basic emotions activated regions of the social brain, and the dMPFC was more highly activated in adoles- cents than in adults (Burnett et al., 2009; Fig. 5 pink dot). The left ATC showed the opposite pattern of activity: this region was more highly activated by social compared with basic emotions in adults relative to adolescents. These results again suggest that the relative roles of the different areas within the mentalising network change with age, with activity moving from anterior (dMPFC) to posterior (ATC) regions.
Another recent study investigated distinct and overlapping neural substrates of cognitive mentalising (understanding thoughts and intentions) and affective mentalising (understanding emotions), using a theoretical framework proposed by Shamay-Tsoory et al. (2010). A group of adolescents (aged 11–16) and adults (aged 24–40) were scanned while looking at cognitive and affective mentalising cartoons (Sebastian et al., 2012). Both types of cartoon activated the social brain network, while the affective mentalising cartoons activated ventral mPFC to a greater extent than did cognitive mentalising car- toons. Affective mentalising was associated with increased ventral mPFC activity in the adolescents relative to the adults (Fig. 5 orange dot).
Gunther Moor et al. (2012) compared brain activity in young adolescents (aged 10–12), mid-adolescents (14–16) and young adults (19–23) while participants carried out the mind in the eyes paradigm (Baron-Cohen et al., 1997). This task involves making judgements about the mental states and emotions a person is feeling based only on photographs of their eyes. At all ages, greater activity was found in the pSTS during the reading the mind in the eyes task, relative to a control condition that involved making age and gender judgments about the same facial stimuli. Only early adolescents showed addi- tional involvement of the dMPFC (Gunther Moor et al., 2012; Fig. 5 black dot).
A recent developmental fMRI study investigated the Trust Game, in which participants were second players in an investment game. They were given trust by the first player with an amount of money that they could either divide equally between themselves and the first player (reciprocate) or distrust the first player and keep most of the money for themselves (defect) (Van den Bos et al., 2011). A certain degree of mentalising is involved when deciding whether to defect or reciprocate the first player who gave trust in the first place. Three groups of participants, early adolescents (aged 12–14), mid-adolescents (15–17) and young adults (18–22), took part. This rather different type of mentalising-related study demonstrated an age-related decrease in dMPFC activity for reciprocal choices. Specifically, all participants showed activity in this region for defect choices, but only in early adolescence was this region also engaged in reciprocal choices. The activation for reciprocal choices decreased between early and late adolescence and remained stable into early adulthood (Fig. 5 purple dot).
Note that, in these latter two studies, the groups labelled adults were very young; it is unknown how brain activation on these tasks changes after the early twenties. An interesting point here is that much of what we know about adult human behaviour and cognition, and associated neural responses, is based largely on undergraduate students who are over-represented in subject pools. It could be argued that the brain within the typical undergraduate age range (18–22) is still very much developing.
To summarise, a number of developmental neuroimaging studies of mentalising show striking consistency with respect to the direction of change in MPFC activity. Despite the variety of mentalising tasks used, fMRI studies of mental state attribution have consistently shown that MPFC activity during mentalising tasks decreases between adolescence and adulthood. It is not yet understood why this should be, and at least two non-mutually exclusive explanations have been put forward (see Blakemore, 2008, for detailed discussion). One possibility is that the cognitive strategy for mentalising changes between adolescence and adulthood. For example, mentalising in adults may be more automatic than in adolescents, who instead might base their judgement on novel computations performed in the MPFC. This possibility may be related to the skill learning hypothesis (Johnson, 2011), whereby one region first supports a certain function, but another brain region may take over later in development. This would fit with the findings from some of the developmental fMRI studies of mentalising showing that temporal regions of the social brain increase in activation, while dMPFC decrease, during adoles- cence (Blakemore et al., 2007; Burnett et al., 2009). According to this idea, the PFC may be particularly involved during the learning of new abilities and might decrease in activity once a skill has become more automatic (Johnson, 2011).
A second possibility is that the functional change with age is due to neuroanatomical changes that occur during this period. Decreases in activity are frequently interpreted as being due to developmental reductions in grey matter volume, presumably related to synaptic pruning. However, the relationship between structure and function is likely to be more complex than this, and there is currently no direct way to test the relationship between number of synapses, synaptic activity and BOLD signal in humans (see Blakemore, 2008; Harris et al., 2011; and Relationship between structural and functional development).
Developmental cognitive neuroscience — the next twenty years
The field of developmental neuroimaging is still young, and there are still many questions that remain to be tackled. In this section, I look at a few key areas that are predicted to be the focus of research in the next few decades.
Gender differences and puberty
As described in Grey matter development, MRI studies have shown that cortical grey matter changes during childhood and adolescence in a region-specific and predominantly non-linear manner (Giedd et al., 1999; Shaw et al., 2008; Sowell et al., 1999; Tamnes et al., 2010). An early paper by Giedd et al. (1999) showed that the frontal and parietal lobes attain peak grey matter volume at around age 11 in girls and 12 in boys. The ages at which these peaks occur approximately correspond to the sexually dimorphic ages of gonadarche onset, which suggests possible interactions between puberty hormones and grey matter development. Other MRI studies have shown the gradual emergence of sexual dimorphisms across puberty, with increases in amygdala volume during puberty in males only, and increases in hippocampus volume in females only (Lenroot et al., 2007; Neufang et al., 2009).
Relatively little is known about the relationship between gender, puberty and neural development in humans. Evidence from animal studies indicates that the hormonal events of puberty exert profound effects on brain maturation and behaviour (Cahill, 2006; Sisk and Foster, 2004; Spear, 2000). It has been suggested that the hormonal events of puberty trigger a second period of structural reorganisation in the human brain (Sisk and Foster, 2004; Petanjek et al., 2011). However, there is little understanding of the specific relationships be- tween puberty and adolescent brain development (Blakemore et al., 2010b; Dorn, 2006). In recent years, a number of MRI studies have investigated the relationships between structural brain development, gender and puberty (e.g. Raznahan et al., 2010). Neufang et al. (2009) investigated relationships between grey matter volume, gender and puberty measures in participants aged 8–15. Irrespective of gender, there was a positive relationship between pubertal measures and grey matter volume in the amygdala, and a negative relationship be- tween pubertal measures and hippocampal volume. In addition, there were gender-specific effects: females showed a positive relationship between oestrogen levels and limbic grey matter, and males showed a negative relationship between testosterone and parietal cortex grey matter. Furthermore, an adolescent structural MRI study by Peper et al. (2009) showed evidence for a positive association between testosterone levels and global grey matter density in males (and not in females), while females showed a negative association between oestradiol levels and both global and regional grey matter density.
A recent MRI study investigated the effect of sex differences on brain structure in 80 adolescent boys and girls matched on sexual maturity, rather than age (Bramen et al., 2011). The authors investigated measured physical pubertal maturity and testosterone and found significant interactions between sex and puberty in regions with high sex steroid hormone receptor densities: sex differences in the right hippocampus, bilateral amygdala, and cortical grey matter were greater in more sexually mature adolescents. Larger grey matter vol- umes were found in MTL structures in more sexually mature boys, whereas smaller volumes were observed in more sexually mature girls.
These findings are preliminary and require replication, but they represent an important first step in this new area of research. Further work is needed to investigate mechanisms underlying region- specificity and sexual dimorphism in the relationship between puberty hormones and structural brain development.
A number of developmental fMRI studies show gender differences in neural activity in a range of cognitive paradigms (a full review of these findings is beyond the scope of this paper). Generally, the findings of gender differences in activity patterns of the developing brain are not particularly consistent, and there is a need to discover whether gender differences can be replicated, under what conditions and at what ages. Even if robust gender differences are discovered, their aetiology is difficult to decipher. Gender differences may be due to a wide range of innate or environmental factors, including: pre-natal sex hormone effects; gender differences in neurovasculature and gyrification; puberty-independent effects of genes encoded on the sex chromosomes; changes in hormones at puberty; and/or social expectations in the environment. Further studies are needed to eluci- date these complex relationships.
Functional and effective connectivity
Until recently there had been little research on the development of structural, functional and effective connectivity, even though this has potential to elucidate differences in behaviour, cognition and mental state. Functional connectivity analysis examines the statistical dependencies between different brain areas. A baseline measure of this, in the absence of any task, is referred to as resting state function- al connectivity (rsfMRI). Recent studies have employed rsfMRI to describe changes in functional networks across different developmental periods (Fair et al., 2007, 2008, 2009; Supekar et al., 2009; see Power et al., 2010 for a review). These studies have shown differences in organization within and between functional networks between childhood and adulthood, with significant changes occurring during adolescence. Changes within specific networks, such as the default mode network (the network of brain regions that are active during baseline), have been reported during adolescence across several stud- ies employing different analytic approaches (Fair et al., 2008; Jolles et al., 2011; Lopez-Larson et al., 2011; Supekar et al., 2010). An emerging theme within rsfMRI studies is that interactions between different networks reduce with age and that this might reflect enhanced within-network connectivity and more “efficient” (and thus reduced) between-network influences (Fair et al., 2007; Stevens et al., 2009).
There are principally two different ways of analysing task- dependent connectivity: functional and effective connectivity. A classic example of functional connectivity is psycho-physiological interaction (PPI) analysis. PPI analysis is a statistical technique based on linear regression and provides insights that are independent and fundamentally different from those gained by conventional analysis. PPI analysis is based on the principle that if activity in one region (area A) predicts the activity in another region (area B), then the strength of the prediction reflects the influence area A could be exerting on area B. If the strength of the prediction varies with the psychological context in which the physiological activity is measured (i.e. experimental condition) then this is evidence for a psychophysiological interaction (Friston et al., 1997). In PPI analysis, a brain region of interest is defined as the physiological source. Only one previous social brain study has investigated functional connectivity development over adolescence (Burnett and Blakemore, 2009). This study showed that functional connectivity within the mentalising system was higher during social versus basic emotions in adults and adolescents, and that there was a developmental reduction in functional connectivity within the mentalising network, possibly due to increased specialisation and increasingly “efficient” between-network influences (cf Stevens et al., 2009).
In contrast to functional connectivity as assessed by PPIs, effective connectivity methods such as dynamic causal modelling (DCM) (Friston et al., 2003) provide additional information about the directionality of the influence of one area over another. There are surprisingly few developmental neuroimaging studies that have employed effective connectivity methods. Two recent studies using effective connectivity methods have found that, compared to adults, both children (Bitan et al., 2006) and adolescents (Hwang et al., 2010) show weaker top–down modulatory influences from frontal areas during different tasks.
Relationship between structural and functional development
The cellular changes that underlie the change in activity shown by particular brain regions during development are as yet unknown. One hypothesis is that changes at the synapses contribute to developmental changes in BOLD signal. For example, a possible consequence of excess synapses in the human PFC and other cortical regions in early adolescence is that it renders information processing in the relevant brain regions less “efficient”. The excess, “untuned” synapses are thought to result in a low signal-to-noise ratio. Input- dependent synaptic pruning eliminates those excess synapses, thereby effectively fine-tuning the remaining connections into specialised functional networks. Excess synaptogenesis during child- hood might result in increasing levels of activity in the relevant brain region due to a low signal-to-noise ratio of the relevant neuronal networks. After pruning, it is possible that it takes fewer synapses to do same amount of work, because the remaining synapses are more efficient. This would engender a system with a higher signal- to-noise ratio, which might result in more efficient cognitive proces- sing, and possibly lower BOLD signal and improved performance with age. This might account for decreases in BOLD signal between late childhood/early adolescence and adulthood observed in certain cognitive tasks.
However, this is a purely speculative idea and in reality the relationship between structure and function is probably more complex. First, different regions develop at different rates and structural and functional changes within a region are not always parallel. Second, some regions show increases in BOLD signal with age during certain tasks, and decreases in other tasks (see Church et al., 2010). Third, the suggested relationship between synapses and BOLD signal makes several assumptions that are yet to be tested (see Harris et al., 2011 for review). It assumes that a larger number of synapses in a given unit of brain tissue results in an increased BOLD signal if those synapses are active. The notion that the magnitude of the BOLD response is associated with synaptic density assumes a more or less linear relationship between synaptic density and the BOLD sig- nal. Exactly how linear the coupling is between synaptic density and the BOLD response remains to be determined. It is likely that it is dif- ferent across brain regions, and that there is at least some degree of nonlinearity (Lauritzen, 2005). It also makes the assumption that vas- cular changes correlate with synaptic changes; whether this is the case is as yet unknown (Harris et al., 2011). Furthermore, it would be useful to know whether there are correlations between structural changes (in grey matter volume) and functional (BOLD signal) changes in the same individuals. This is surprisingly rarely studied in developmental neuroimaging studies, although recent neuroimag- ing studies on executive control (Konrad et al., 2005), stimulus- independent thought (Dumontheil et al., 2010a), relational reasoning (Dumontheil et al., 2010b), and reading (Lu et al., 2009) have attempted to correlate grey matter changes and functional development. Other studies have combined white matter changes and functional development (for example, in a working memory task; Olesen et al., 2003). These studies have generally found that structural changes account for some, but not all, of the developmental pattern of BOLD signal. Future research should attempt to disentangle possible causal contributions to functional brain development as viewed by fMRI.
Individual differences in brain development
There are large individual differences in adolescent social and behavioural development. Understanding the mechanisms that contribute to these individual differences may shed light on why some adolescents negotiate the social pressures of this developmen- tal period well, while others do not. In addition, vulnerability to many psychiatric disorders, including depression, anxiety disorders and eating disorders, increases steeply during adolescence (Paus et al., 2008). Twin studies suggest that this is in part due to the emer- gence of new genetic effects during puberty and adolescence (Scourfield et al., 2003). Genetic factors are likely to play an impor- tant role in individual differences. It is well established that genetic polymorphisms (inter-individual variation) affect cognition (Green et al., 2008). Highly heritable individual differences in cognitive ability are associated with structural differences in specific regions of the cortex (Toga and Thompson, 2005). How genes interact with environment in the context of brain development is already the focus of a growing number of developmental neuroimaging studies.
A distinctively under-researched area in cognitive neuroscience is how context and culture affect brain development. Research from cross-cultural psychology has shown that the experience of adoles- cence is variable and contingent upon culture (Choudhury, 2010). The lives of teenagers from different cultures can be dramatically different and it is unknown how this shapes their brains. It has been suggested, for example, that characteristics that are common in teenagers in the West, such as intergenerational conflict, stem from cultural values of individualism in Western societies, which are less prominent in pre-industrial societies (Choudhury, 2010). There are cultural variations in puberty onset, which tends to be earlier in in- dustrialized countries than in pre-industrial societies, possibly due to differences in dietary intake (Berkey et al., 2000). In addition, the end of adolescence, being socially defined, is vastly different between different cultures. A critical issue for future research is to disentangle genetically pre-programmed developmental changes and changes that are due to culture and the environment. An interesting specula- tion is that adolescence represents a period of synaptic reorganisation and, as a consequence, some areas of the brain might be particularly sensitive to experiential input at this period of life.
Translational outcomes of developmental neuroimaging
Much of what we have learned from developmental neuroimaging is focused on the typically developing brain. While the basic science is of course necessary to pave the foundations for translational research, in recent years studies have started to focus on the health and educa- tion consequence of the developing adolescence brain. Here, I mention three areas: mental health, education and the law.
A disproportionate number of psychiatric conditions typically have their onset in adolescence (Paus et al., 2008). These include anxiety disorders, depression, eating disorders, addiction and schizophrenia (usually at the end of adolescence). This suggests that adolescence may represent a sensitive period in terms of the neurobiological events that underlie these conditions. There is already a large and rapidly expanding literature on brain development in developmental disorders and psychiatric conditions, and some of these are large-scale and longitudinal (e.g. Giedd and Rapoport, 2010; Shaw et al., 2010). A full review of this large and growing literature is beyond the scope of this paper. Current and future research should include longitudinal aspects to track brain development in adolescents who are at high risk from developing a certain condition, and compare development in those who do, versus those who do not, develop the condition.
Research into the cognitive implications of continued brain maturation beyond childhood may be relevant to the social development and educational attainment of adolescents. Further studies are necessary to reach a consensus about how axonal myelination, and synaptic pruning and proliferation impact on social, emotional, linguistic, mathematical and creative development. In other words, which skills undergo perturbation, which undergo sensitive periods for enhancement and how does the quality of the environment interact with brain changes in the development of cognition is unknown whether greater emphasis on social and emotional cognitive development would be beneficial during adolescence is unknown, but research will provide insights into potential intervention schemes in secondary schools, for example, remediation pro- grammes or schemes for tackling anti-social behaviour.
Research in developmental neuroimaging can also contribute to the debate about juvenile crime, for instance the age of criminal responsibility, which in the UK is 10 years. In the USA, some minors receive life sentences without the possibility of parole. A dialogue between psychologists, neuroscientists, the legal profession and policy makers has already begun in the context of juvenile crime and the age of criminal responsibility. Continued input from neuroscience would be useful to shape future legislative procedures concerning adolescents. The plentiful data that consistently paint a picture of the adolescent brain as relatively immature might speak against the relatively young age of criminal responsibility and harsh sentences for adolescents. Of course, these debates are profoundly complex and neuroscience data cannot provide the answers alone. Developmental neuroimaging data also suggest that the brain is still developing during adolescence, and that it is not too late for rehabilitation.
Conclusion
In this paper, I have reviewed MRI and fMRI studies on structural and functional changes in the adolescent brain. The ability to see inside the developing human brain and to track developmental changes, both in terms of structure and function, is relatively recent. The past 15 years has seen an explosion in the number of studies using neuroimaging techniques to investigate maturational changes in the human brain. These studies point to adolescence as a period of continued neural development. The next twenty years of developmental neuroimaging might focus on more complex questions including the development of functional connectivity; how gender and puberty influence adolescent brain development; the effects of genes, environment and culture on the adolescent brain; development of the atypical adolescent brain; and implications for policy of study of the adolescent brain. This is an exciting time for developmental cognitive neuroscience, a young field that is set to continue to expand and mature over the next two decades.