Abstract
Drug-related cues hijack attention away from alternative reinforcers in drug addiction, inducing craving and motivating drug-seeking. However, the neural correlates underlying this biased processing, its expression in the real-world, and its relationship to cue-induced craving are not fully established, especially in opioid addiction. Here we tracked inter-brain synchronization in the Nucleus Accumbens (NAc), a hub of motivational salience, while heroin-addicted individuals and healthy control subjects watched the same engaging heroin-related movie. Strikingly, the left NAc was synchronized during drug scenes in the addicted individuals and non-drug scenes in controls, predicting scene- and movie-induced heroin craving in the former. Our results open a window into the neurobiology underlying shared drug-biased processing of naturalistic stimuli and cue-induced craving in opiate addiction as they unfold in the real world.
Opioid-related overdose deaths in the United States have increased by 109% between 2015 and 2020, including a 38% increase in 2020 alone. Heroin use in particular increased by approximately 40% in response to the COVID-19 pandemic. Despite the magnitude of this drug use and overdose epidemic, however, and compared to other drugs of abuse, the neurobiological substrates of human opioid use disorder (OUD) have been largely understudied. In addiction to other drug classes (e.g., stimulants), drug cues have been shown to divert attention away from alternative reinforcers (e.g., social, food, sex), a core imbalance in motivational salience suggested to drive drug use and perpetuate the addiction cycle. Whether a similar hijacking occurs in opioid addiction, however, remains an open question. Here we were interested in the extent (i.e., its expression as a phenotype shared by groups of individuals) of such drug-biased neural processing in real-world contexts and its relationship to cue-induced craving in heroin addiction.
To explore the brain substrates of cue reactivity and craving in human drug addiction, neuroimaging studies commonly employ static images (e.g., pictures of drugs). Instead, here we used an engaging heroin-related movie, a dynamic, narrative-based, and context-rich natural stimulus, to better approximate actual real-world experiences in individuals with OUD (iOUD). Specifically, we used inter-subject synchronization to analyze fMRI BOLD responses to the movie, hypothesizing a shared brain signature in iOUD. Our primary region of interest was the Nucleus Accumbens (NAc), a major processing hub for motivational salience attribution, reward anticipation, and craving, where we expected to document unique synchronized tracking of drug-related content as predictive of cue-induced craving in the iOUD.
Functional MRI BOLD activity was recorded in 29 treatment-seeking medically stabilized iOUD (40.4±10.3 years, 23 Male, 19 White) and 16 age, sex, and race-matched healthy controls (HC) (43.8±10.3 years, 10 M, 11 W), while subjects watched the first 17 minutes of the movie “Trainspotting”, which contains scenes of explicit heroin use, as well as food and social scenes that are highly salient but not directly related to heroin use. Importantly, the overarching narrative structure of the movie is centered around drug-addiction, including complex contextual elements relevant to OUD (e.g., social, emotional, and economic challenges of addiction). To infer the movie features that led to synchronized NAc activity, we used an approach developed by Hasson et al., which was inspired by reverse correlation analyses of single unit recordings. We were interested in whether the iOUD would show differential synchrony in the NAc during the drug-related content at the expense of other typically motivating stimuli.
Following Hasson et al., we decomposed the BOLD signal in each voxel during movie watching into two components. The first component, which we call the global component, relies on the observation that engaging narratives evoke a wave of global synchronized activity across much of the brain, driven by surprising and emotionally engaging moments of a movie. Here, the global component was derived by averaging the BOLD signal across all grey matter voxels and then z-scoring the resulting time series within each subject. The second component, which we call a selective component, was derived by regressing the global component out of the time series at each voxel, yielding a residual signal that is not explained by the global activation wave throughout the brain and is specific to each voxel. Hasson et al. has shown that a reverse correlation analysis of this selective component can recover canonical response properties from traditional GLM-based fMRI tasks [e.g., fusiform face area responds to scenes with faces]. Here, for the first time, we applied this method for the purpose of tracking the neural signature of drug related motivation/incentive salience in subcortical regions in heroin-addicted individuals.
Applying this reverse correlation method to the mean selective component separately in the left and right NAc (LNAc and RNAc, respectively), we found a set of significant time points during the movie that elicited a synchronized NAc response in each subject group (see Identifying synchronized TR’s and region-specific reactivity in supplemental methods). Accounting for the hemodynamic lag, we then identified clips of the movie that contributed to this synchronization (5-10 sec before each significant time point). Stitching these clips together yielded separate NAc-specific “movies” for each group. Remarkably, the LNAc-specific movie contained almost exclusively clips of drug use in the OUD group and no scenes of drug use in the HC group (Fig. 1; see supplemental videos).
For statistical confirmation purposes, and based on previous methods, we quantified the content of the LNAc-specific movies by first breaking the entire movie into 24 distinct scenes. Each scene was a priori classified as drug or non-drug, based on whether drugs or drug use were explicit in the scene (see supplemental methods section Labeling of region-specific movies for detailed criteria). Each TR from the LNAc-specific movie could then be matched to a scene (multiple TRs could be matched to the same scene) and thereby labeled as drug or non-drug, yielding a count of scene types to be used for statistical comparisons. Using this count as the dependent measure, chi-square tests revealed a significantly different scene type distribution between the iOUD and HC for the LNAc-specific movie, such that for iOUD it fell almost exclusively within the drug scenes, while for HC it was close to what could be expected if scenes were selected at random (iOUD: 40/8, HC: 25/40 drug/non-drug; χ 2 (1)=20.95, p=0.0000047). Indeed, nonparametric tests also indicated that the number of drug scenes was significantly different (𝛼=0.05) from random phase shuffled signals for iOUD, but not HC (Fig. S1). We further compared the magnitude of the LNAc-selective component directly between the two groups and found that it was significantly different during each of the LNAc-specific movies (Fig. S2).
Given the skewed responsiveness of the LNAc to drug stimuli in iOUD and this region’s role in craving, we inspected whether the magnitude of the LNAc-selective component predicts cue-induced craving for drugs. In-scanner subjective heroin craving ratings were collected immediately before and after subjects watched the movie (for calculation of movie-induced craving). After leaving the scanner (within 45 minutes after watching the movie), subjects also completed a custom questionnaire intended to probe scene-specific cravings. The LNAc-specific component averaged over significant time points for each subject predicted both sceneand movie-induced cravings (Fig. 2A,B). Interestingly, the correlation with baseline craving (measured with the heroin craving questionnaire, HCQ) (12) was not significant (Fig. 2C), suggesting that the LNAc synchronous reactivity scales with the dynamic experience of craving induced by watching the movie.
We next tested whether the LNAc reactivity was related to other potential explanatory variables including the demographic measures that differed between the groups and, in iOUD, measures of recent and lifetime drug use, withdrawal symptoms and severity of dependence (Table S2). The LNAc reactivity signal was not significantly correlated (𝛼=0.05) with any of these measures, further suggesting that it was not driven by the demographic factors that commonly differ between individuals with and without addiction, and that it may specifically be predictive of craving induced by movie-watching. To better examine the specificity of the above results to the LNAc, we repeated the above analyses (including statistics and correlations) for the global component, several other regions of interest involved in drug cue reactivity [ventromedial prefrontal cortex (13), dorsolateral prefrontal cortex, anterior cingulate cortex (15), insula (16), putamen], and a control region [fusiform cortex (18)]. Other than the LNAc, only the left anterior insula showed significant drug-biased reactivity in OUD (Table S3). However, only the LNAc showed a complete reversal of scene-type preferences between the groups (i.e., drug scenes for iOUD and non-drug scenes for HC) and a correlation with the cue-induced craving measures, suggesting specificity of these results to the LNAc.
Supporting the impaired response inhibition and salience attribution model, and other prominent neurobiological theories of addiction, which posit an aberrant incentive salience attribution to drug-related stimuli at the expense of other typically motivating stimuli, we revealed a bias toward naturalistic depictions of drug-use in the LNAc of iOUD. Documenting this imbalance during exposure to a movie, a narrative-based and context-rich dynamic stimulus, suggests that such neural bias may dominate during real-life daily ongoing experience. Here we documented such a shared narrowing of responses in addiction towards drug-related stimuli even in currently abstinent, treatment-seeking and medication-maintained individuals. Predicting cue-induced craving, this interpretable brain signal may also be a powerful predictor of later drug use and relapse, as remains to be ascertained with longitudinal follow-up studies in these subjects.
Whether the dynamic bias of the NAc to a drug-related narrative is generalizable to other substance use disorders, or to individuals at risk for developing drug addiction, remains an open question. Future efforts may also benefit from more sex-balanced samples to interrogate potential sex-differences related to mapping drug cue reactivity and craving in a naturalistic context. Such efforts may also warrant larger samples, although the inter-subject correlation based statistics yield stable results with comparable samples in traditional block designs, and even smaller samples in naturalistic designs. Furthermore, future efforts could employ a whole-brain search for inter-brain synchronization during movie watching, complementing our regional focus (that accounted for the global component and corrected for multiple comparisons). A real-time measure of engagement during movie watching (e.g., with eye-tracking or online scene-specific ratings) may have provided more information about participants’ experiences during the movie, but we decided in this first effort not to disrupt the continuous narrative structure and engagement during movie viewing, instead obtaining a comprehensive post-movie survey.
To the best of our knowledge, this is the first use of dynamic naturalistic stimuli for the demonstration of drug-biased processing as a predictor of cue-induced craving in the addicted human brain. The interbrain synchronization and scene classification methods introduced here for the first time in the context of heroin addiction allow us to highlight specific scenes that drive the shared striatal reactivity; as compared to typical block- or event-related picture-based studies, these methods can better decode the actual features/identity of the craving-inducing stimuli even in this treatment-seeking group. Crucially, this approach effectively operationalizes daily-life drug-related experiences, opening a window into the neurobiology underlying drug-biased processing and cue-induced craving as they unfold in the real world. As one iOUD referred to the movie stimulus “it gives me that feeling like even though I'm not getting high, I just, I feel like I'm doing it with them.”
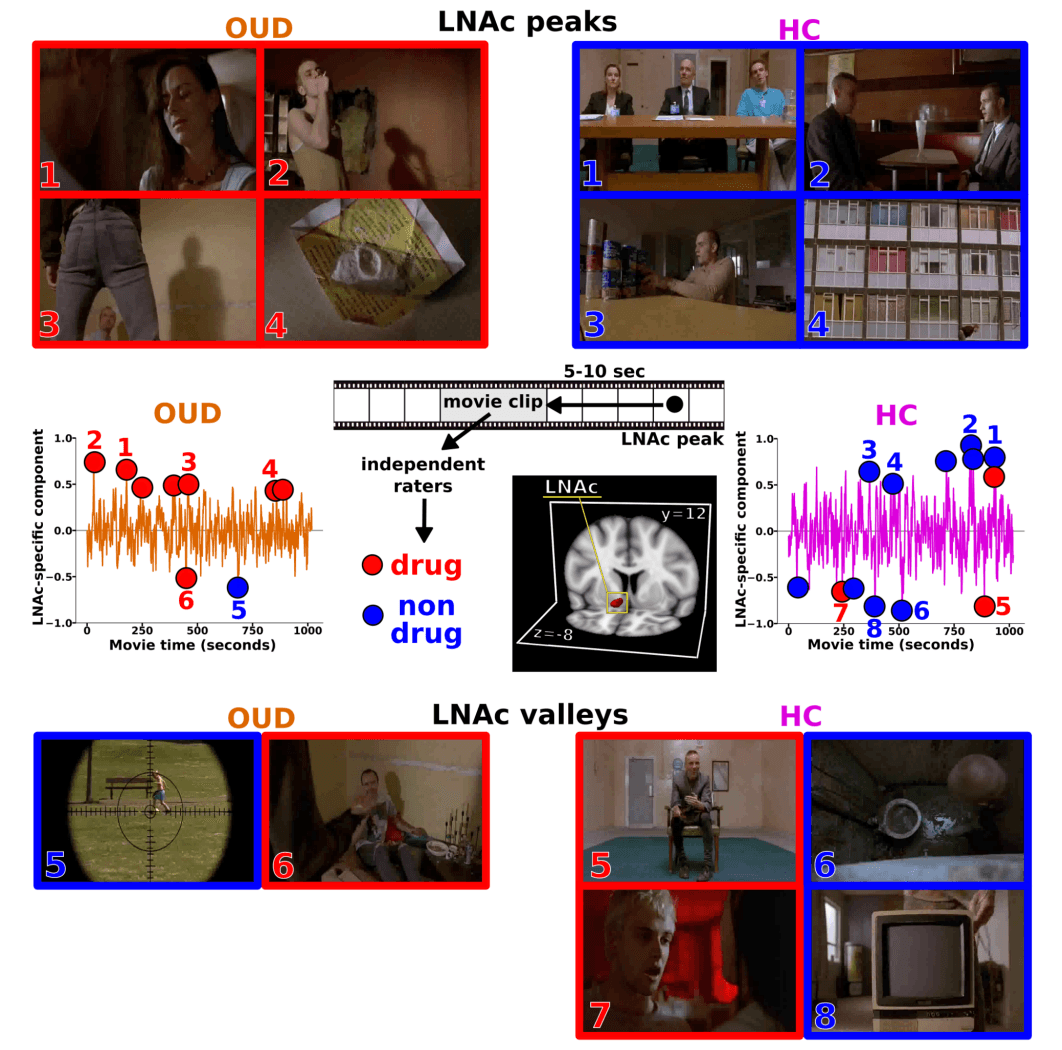
Fig. 1. LNAc responds almost exclusively to drug use in OUD and non-drug scenes in HC
The mean LNAc-specific component for each group is displayed with filled circles indicating significantly synchronized time points for each group (left: OUD; right: HC). Movie clips 5-10 s prior to each significant time point were labeled by independent raters as drug (red) or non-drug (blue). Representative still images are displayed for the four most significant peaks (top) and valleys (bottom) of the LNAc-specific component. While OUD responded almost exclusively to drug use, HC peaks corresponded to other typically motivating stimuli (e.g., food or social cues) and HC valleys to typically aversive stimuli (e.g., pain, embarrassment, feces). A 3 dimensional rendering of the LNAc mask (Harvard-Oxford Atlas) is displayed in MNI space.
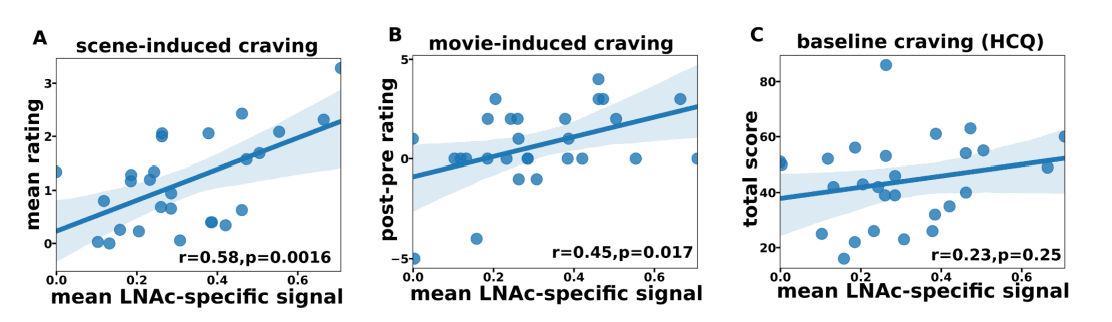
Fig. 2. LNAc reactivity correlates with cue-induced heroin craving in OUD
A. LNAc reactivity correlated with mean scene-induced craving ratings. These craving ratings were collected outside the scanner in response to 3 sec video clips from the movie (averaged over all clips). B. LNAc reactivity correlated with immediately post-minus-pre movie subjective ratings for heroin craving. C. LNac reactivity did not correlate with baseline craving as measured by the heroin craving questionnaire (HCQ). For all plots, outliers with a ±3 SD from the mean threshold criteria were removed. Line and shaded area represent least squares linear fit with 95% CI. All reported r and p values are based on Pearson correlation.