Abstract
Globally, millions of people suffer from various substance use disorders (SUD), including mono-and polydrug use of opioids and methamphetamine. Brain regions such as the cingulate cortex, infralimbic cortex, dorsal striatum, nucleus accumbens, basolateral and central amygdala have been shown to play important roles in addiction-related behavioral changes. Clinical and pre-clinical studies have characterized these brain regions and their corresponding neurochemical changes in numerous phases of drug dependence such as acute drug use, intoxication, craving, withdrawal, and relapse. At present, many studies have reported the individual effects of opioids and methamphetamine. However, little is known about their combined effects. Co-use of these drugs produces effects greater than either drug alone, where one decreases the side effects of the other, and the combination produces a prolonged intoxication period or a more desirable intoxication effect. An increasing number of studies have associated polydrug abuse with poorer treatment outcomes, drug-related deaths, and more severe psychopathologies. To date, the pharmacological treatment efficacy for polydrug abuse is vague, and still at the experimental stage. This present review discusses the human and animal behavioral, neuroanatomical, and neurochemical changes underlying both morphine and methamphetamine dependence separately, as well as its combination. This narrative review also delineates the recent advances in the pharmacotherapy of mono- and poly drug-use of opioids and methamphetamine at clinical and preclinical stages.
Introduction
Dependence on drugs and alcohol is a serious worldwide problem from social, economic, and health perspectives (Pakri Mohamed et al., 2018; Das and Horton, 2019; Sontate et al., 2021). Globally, the number of methamphetamine and opiate users has continued to grow at an alarming rate despite numerous stringent drug abuse laws (Bach et al., 2020; Dezman et al., 2020). A recent report indicates a nearly four-fold increase in methamphetamine-related hospitalizations and a more than 10-fold increase in stimulant-related deaths (Winkelman et al., 2018; Ruhm, 2019) surpassing the overdose death rate of prescription opioids (Hedegaard et al., 2020). Likewise, the prevalence of opioid overdose and overdose-related deaths were also escalated in the past years (Stevens et al., 2017; Sivaraman et al., 2021). What’s more concerning is, almost half of psychostimulant use-related deaths involve opioids, and an increase in trend is also observed in opioid use related-deaths involving methamphetamine (Ihongbe and Masho, 2016; Lancet, 2018; Gladden et al., 2019; Kariisa et al., 2019), indicating a spike in polysubstance use (Palamar et al., 2018; Zuckermann et al., 2019; Compton et al., 2021). It is estimated that the global cost for the treatment of 4.5 million drug users is about $35 billion annually (INCB, 2013), which is accounted for only one in six drug users. If all of the dependent drug users were to seek treatment, it would cost an estimated 0.3–0.4% of the global gross domestic product ($200 billion) (INCB, 2013). The cost of untreated and continuing use is significantly higher than investment in treatment alone, research finds. Reports from the United States National Drug Intelligence Center (NDIC) indicate the drug-related healthcare cost includes both direct and indirect costs related to inpatient drug treatment, medical intervention such as emergency services, and research for prevention and treatment (NDIC, 2011).
Currently, one of the most well-researched treatment options for substance dependence is opioid dependence. Methadone maintenance therapy (MMT) has been employed as one of the harm reduction approaches to manage opiate addiction (Ali et al., 2018), with some reporting its efficacy in reducing high-risk behaviors (Zhang et al., 2019), and whereas some have argued that for long term treatment, MMT may not significantly improve the quality of life among patients (Teoh Bing Fei et al., 2016). MMT also requires lifelong commitments from drug users. Other drugs such as buprenorphine or buprenorphine-naloxone are mainly used in private settings due to the high cost, as a maintenance therapy (Vijay et al., 2015). Buprenorphine is an opioid agonist like methadone, whereas naloxone is a short-acting opioid antagonist commonly given by injection to reverse opioid overdoses (Webster et al., 2016). In several countries, buprenorphine or buprenorphine-naloxone combinations were injected illicitly by the majority of opioid users, increasing the incidences of opioid dependence (Yokell et al., 2011). Oral treatment of naltrexone for opioid dependence is ineffective due to poor treatment adherence (Minozzi et al., 2011). Naltrexone implant, on the other hand, has produced some positive results in the treatment of opioid or polydrug abuse (Kelty et al., 2019; Krupitsky et al., 2019). Nevertheless, the clinical efficacy of the implant in the long-term has not been reported and the potential opioid overdose associated with naltrexone implant has not been sufficiently explored (Saucier et al., 2018).
To date, there are no significantly convincing treatment outcomes in the pharmacotherapy of methamphetamine use disorder (MUD) (Morley KC. et al., 2017; Ballester et al., 2017). Systematic analysis of existing literature revealed some positive outcomes with dexamphetamine, methylphenidate, naltrexone, and topiramate, whereas anti-depressants, such as selective serotonin reuptake inhibitors, and tricyclic antidepressants were being the least effective in the management of MUD (Siefried et al., 2020). Individual clinical studies have reported efficacy in the use of buprenorphine (Ahmadi and Razeghian Jahromi, 2017; Ahmadi et al., 2019), N-acetylcysteine (Salehi, 2015), and methylphenidate (Rezaei et al., 2015) in reducing the craving score of methamphetamines, whereas some have reported lack of efficacy among drugs such as bupropion (Anderson et al., 2015), modafinil (Heinzerling et al., 2010; Anderson et al., 2012), varenicline (Briones et al., 2018) in methamphetamine dependence treatment.
Therefore, this present review discusses the human and animal behavioral and neurochemical changes underlying both morphine and methamphetamine dependence separately, as well as its combination. This review also delineates the recent advances in the pharmacotherapy of mono and poly drug-use of opioids and methamphetamine at clinical and preclinical stages.
Opioid Use Disorder
Opioid abuse originates from over prescription for the patients’ pain relief, while the increasing availability of low-cost opioids also has exacerbated its potential for abuse (Darcq and Keiffer, 2018). Patients develop tolerance to the opioid’s analgesic effect after treatment over an extended period. Administration of opioids at a higher dose is used to overcome this tolerance, however, patients will then be vulnerable to severe side effects such as withdrawal symptoms, and the threat of respiratory depression (Hayhurst and Durieux, 2016). Worldwide, the prevalence of opioid use was the highest in North America (UN World Drug Report, 2021). Analysis of individual data from the United Kingdom, United States, Australia, Germany, and France revealed that almost 1 in 5 reported abuse and 1 in 4 individuals reported misuse of opioid analgesics obtained through a prescription (Morley KI. et al., 2017). Heroin, fentanyl and morphine were the most commonly used opioids amongst others which include methadone, buprenorphine, codeine, tramadol, oxycodone, and hydrocodone (UN World Drug Report, 2019). According to WHO estimates, there were approximately 115,000 casualties from opioid overdoses globally, and COVID-19 has further exacerbated the fatality rate (Centers for Disease Control and Prevention, 2020; UN World Drug Report, 2021).
Morphine abuse negatively affects the users once the addiction cycle is engaged due to the tolerance developed following prolonged use of morphine, which is defined as the need to increase the dose to achieve the same initial effect due to decreased analgesic efficacy (Dai et al., 2018). The Food and Drug Administration (FDA) defines a person is opioid-tolerant if the person has been receiving oral morphine 60 mg/day for 1 week, where different types of opioids have different durations such as transdermal fentanyl, oral oxycodone, oral hydromorphone, oral oxymorphone with 25 mg/h, 30 mg/day, 8 mg/day, 25 mg/day, respectively (Rabin et al., 2017). The users potentially succumb to dependence due to the severity of the withdrawal symptoms including abdominal pain, nausea, diarrhea, lacrimation, and generalized piloerection. In contrast to the drug pain-relieving effects, drug cessation in the morphine-dependent state results in the genesis of negative effects such as anxiety, agitation, and dysphoria (Verster et al., 2021). Psychological dependence on the other hand refers to the state of the patient where they are craving for the drug, to relieve its withdrawal symptoms, or for its gratifying effects (Jacobs, 1986). The withdrawal symptoms that are brought forth from abstinence lead to craving with disinhibition, leaving the user vulnerable to relapse (Kalant, 2010; Campbell et al., 2013). Moreover, there was heightened impulsivity and impaired strategic planning in opioid-dependent patients (Tolomeo et al., 2016), along with increased anhedonia (Kras et al., 2018; Kiluk et al., 2019). Withdrawal symptoms are a key driver behind continued abuse, and a barrier to opioid discontinuation (Pergolizzi et al., 2020).
Behavior parameters established using various models of abuse under controlled environmental and drug administration regimens mimic the psychological status of humans in the presence or absence of substances depending on the animal models (Kumar et al., 2013; Kumar et al., 2016; Iman et al., 2021). Likewise, in opioid dependence animal models, depressive-like behaviors are significant at 1 week after prolonged withdrawal where experiments showed that there was a decreasing level of social interaction and elevation in immobility time which reflects a state of lowered mood or depression-like behavior (Anraku et al., 2001). The social avoidance symptoms and emotional despair mirrored by these mice reflect depression (Jia et al., 2013). Anxiety is another prominent affective symptom that manifests during abstinence from chronic morphine administration. Animal studies have shown that there is a significant increase in anxiety-like behaviors in the elevated plus maze and light/dark box paradigms (Zhang et al., 2008; Buckman et al., 2009; Miladi-Gorji et al., 2012). Apart from that, another prominent withdrawal symptom that accompanies abstinence is impulsive behavior, which encapsulate poor inhibitory response control (impulsive action) and impulsive decision making (impulsive choice) where observations suggest that the opioid system plays a significant role in decision making (Pattij et al., 2009). Morphine exposure also increases motor impulsivity in animal models (Kieres et al., 2004; Colin et al., 2012; Moazen et al., 2018), as well as deficits in learning and memory (Iman et al., 2021).
In Europe, fentanyl abuse was related to over 250 fatalities, while in 2017 alone there have been 25 fatalities associated with fentanyl and its synthetics analogs such as carfentanil, butyryl fentanyl, fluorobutyrylfentanyl, furanylfentanyl, and alfentanil (European Monitoring Centre for Drug and Drug Addiction, 2018; Hikin et al., 2018). According to the National Survey on Drug Use and Health in the United States, fentanyl use appears to be on the rise although the most commonly misused prescription opioids are hydrocodone, oxycodone, codeine, and tramadol (UN World Drug Report, 2018). Fentanyl and fentanyl analogs are full agonists at the MOR with varying degrees of potencies, where acetylfentanyl is 5–15 times more potent than heroin (Yonemitsu et al., 2016), butyfentanyl is 7 times more potent than morphine (Steuer et al., 2017) and ocfentanil is almost 90 times more potent than morphine (Fletcher et al., 1991). There is a high demand for opioids that are popularly derived from fentanyl, which are available at a cheaper cost compared to heroin (Marchei et al., 2018; Rothberg and Stith, 2018). Fentanyl is 50 times more potent than heroin (Rothberg and Stith, 2018), it is often found in heroin samples as a cutting agent that is meant to give heroin a much higher potency, which is more favorable to drug abusers (Marchei et al., 2018). Fentanyl also causes drowsiness, sedation, euphoria (lesser than heroin and morphine), respiratory depression, anxiety, hallucinations and have associated with withdrawal symptoms such as diarrhea, abdominal cramps, anxiety, sweating, bone pain, and shivers (Stanley, 2014; Suzuki and El-Haddad, 2017; Kuczynska et al., 2018).
Rats that undergo short-term withdrawal from fentanyl self-administration (0.0032 mg/kg/infusion followed by 24 h abstinence) were found to have a disrupted brain immune response where there was an increase of inflammatory responses in the NAc simultaneously resulting in immunosuppression in the hippocampus (Ezeomah et al., 2020). It was suggested that the changes in immune outcomes in the central nervous system contribute to the relapse in OUD, however, the authors interestingly noted that the inflammation levels did not correlate with the opioid receptor expression (Grace et al., 2015; Liang et al., 2016). Cisneros and colleagues proposed that the fentanyl-associated change in the immune response contributes to neuroimmune adaptations that might drive the development of OUD, and increase the onset and severity of neurocognitive disorders (Cisneros and Cunningham, 2021). Chronic self-administration of fentanyl in rats (2.57 μg/kg per i.v. infusion, 30 days), significantly decreased ultrasonic vocalization, suggesting an aversive response to repeated fentanyl use, thus indicating negative reinforcement (Dao et al., 2021). Cessation of fentanyl administration (1.2 mg/kg/day for 14 days) also resulted in a time-dependent elevation in brain reward thresholds and somatic withdrawal signs, displaying severe deficits in brain reward function (Brujinzeel et al., 2006). In addition, chronic administration of high dose fentanyl (0.3 mg/kg/i.p. for 28 days) reduced anxiety-like behavior in rats in the open field and elevated plus maze tests (Colasanti et al., 2011; Fujii et al., 2019), reduced muscle strength and locomotion (Fujii et al., 2019) On the contrary, during withdrawal, increase in anxiety-like behavior and hyperalgesia was noticed in mice, and neither high nor low doses of fentanyl had any negative effects on the animals’ cognition (Fujii et al., 2019). In a separate study, 25 μg/kg of fentanyl reduced the grimace scale in mice and rats inflicted with injury of the infraorbital nerve (Akintola et al., 2017). In a more recent study, extended access to self-administration of a vaporized fentanyl to rats altered their behavioral economic metrics consistent with the development of an addiction-like state (McConnell et al., 2021).
The Centre for Disease Control and Prevention reported that there were a cumulative 14000 heroin users died from an overdose in the United States (Centers for Disease Control and Prevention, 2020). In addition, there is a 97.5% increase in heroin use among non-medical users of other prescription drugs suggesting increased polydrug abuse (Jones et al., 2015). Key factors behind the polydrug use of heroin and other substances are the high cost and low availability of heroin, making drug abusers seek cheaper and more lasting highs (Siegal et al., 2003; Lankenau et al., 2012; Mateu-Gelabert et al., 2015). Symptoms of heroin withdrawal include restlessness, insomnia, diarrhea, muscle and bone pain and cold flashes, depression, and nausea, peaking around 48–72 h after the last dose and may last 5–10 days (National Highway Traffic Safety Administration, 2004). Chronic administration of heroin (5 mg/kg at 12 h intervals for 34 days) impaired spatial learning and memory along with increased expression of proapoptotic proteins, relating the cognitive detriment to neural apoptotic damage (Garcia-Fuster et al., 2003; Astals et al., 2008). Withdrawal from intravenous self-administration of heroin (5 daily sessions, limited to 25 number of infusions 0.04 mg/infusion after 7 days increased to 75 number of infusions maximum), results in motivational deficit shown by a significant increase in latency to collect earned food, which was hypothesized as a consequence of the diminished perceived value of the food reward (Goldberg and Schuster, 1967; Harris and Aston-Jones, 2003; Dalley et al., 2005).
Methamphetamine Use Disorder
Methamphetamine is a powerful psychostimulant that has been abused as a recreational drug instead of its intended use as a second-line treatment for attention deficit hyperactivity and obesity (Kish et al., 2001). Methamphetamine remains a significant public health concern over its abuse, especially in its crystalline form, where its use is rapidly increasing in East and South-East Asia (UN World Drug Report 2021). According to the UN World Drug Report 2021, the highest prevalence of Amphetamine Type Stimulant (ATS) abuse was reported in North America and the lowest in Africa. But the prevalence of non-medical use of pharmaceutical stimulants and methamphetamine was the highest in North America as well as South East Asia. Malaysia, however, reported 65.2% ATS use among its drug and substance abusers according to the National Anti-Drug Agency Report (National Anti-Drug Agency, 2019).
Clinical findings have associated chronic use of methamphetamine with manifestations of withdrawal symptoms including fatigue, sleep disturbance, dysphoria, agitation or psychomotor retardation, increased appetite, depression, and anxiety (American Psychiatric Association, 2013; Zhao et al., 2021). Anxiety and depression appear to be prominent and severe especially during the early withdrawal period (Zhang et al., 2015; Ren et al., 2017; Luan et al., 2018; Luan et al., 2018), where longer duration of methamphetamine use was associated with a higher odds ratio of depression, and co-occurring anxiety and psychotic symptoms (Ma et al., 2018). Whereas, symptoms such as craving and sleep disturbance were reported to persist as long as 4 weeks of post-abstinence (Zorick et al., 2010; Mancino and Gentry, 2011). Increased impulsivity also was reported among methamphetamine users during abstinence which is suggested to be a negative reinforcer to maintain drug use (Jones et al., 2016). In a study using the Iowa gambling task, methamphetamine dependence significantly affected inhibitory control and decision making, suggesting abnormal reward processing and inhibitory control (Fitzpatrick et al., 2020). Methamphetamine dependence also affects the cognitive ability of dependent users such as visual memory (Moon et al., 2007), attention/processing speed learning/memory, working memory, timed and executive function (Kalechstein et al., 2003), and decision making (Mizoguchi and Yamada, 2019). Moreover, it was also reported that a month of abstinence did not improve the impaired cognition of methamphetamine-dependent subjects (Simon et al., 2010).
Studies employing mice, induced methamphetamine withdrawal through various dosage regimens, where some researchers achieved this by administering the substance through varied durations such as 8 weeks (5 mg/kg, i.p, once a day, 5 days per week; Ru et al., 2019), 2 weeks (2 mg/kg, 12-h intervals; Hosseini et al., 2021), and 10 days (5 mg/kg, i.p, once a day; Georgiou et al., 2016; Jacobskind et al., 2019). Whereas, some tested escalating dose regimen for 10 days (D1: 2 mg/kg, D2: 4 mg/kg, D3-10: 6 mg/kg). Researchers using rats opted for 10 days of methamphetamine exposure (2 mg/kg, intramuscular; Li et al., 2021), some for 14 days (2 mg/kg, 12 h interval; Damghani et al., 2016), 21 days (10 mg/kg; Yasuj et al., 2019), 14 days (inhalation of methamphetamine, 1W: 5 mg/kg, 2W: 10 mg/kg; Rezaeian et al., 2020), 7 days (2 mg/kg once per day, i.p; Etaee et al., 2019), and 4 days (2.5, 5 or 7.5 mg/kg every 3 h, 3 times per day, i.p; García-Cabrerizo and García-Fuster, 2019). Withdrawal from chronic methamphetamine (various doses of methamphetamine given for 8 weeks, 21, 14, and 4 days) resulted in changes in behaviors such as anxiety and depressive symptoms when tested in the open field, sucrose preference test, forced swim test, and splash test (Damghani et al., 2016; Shabani et al., 2018; Ru et al., 2019; Yasuj et al., 2019; Rezaeian et al., 2020; Hosseini et al., 2021). In addition to this, some reported no changes in the locomotion of animals during the withdrawal period (Hosseini et al., 2021; mice; 2 mg/kg, 12-h intervals), whereas some reported increase in locomotion in methamphetamine treated animals (Rezaeian et al., 2020; rats; inhalation of methamphetamine, 1W: 5 mg/kg, 2W: 10 mg/kg). These discrepancies could be due to the differences in strains of animals tested, mode of methamphetamine intake, dose, and duration of intake as well.
Co-Abuse of Opioid and Methamphetamine
Polysubstance use is a serious public health concern across the globe (Morley KC. et al., 2017; Lyons et al., 2019; Zuckermann et al., 2019), especially among young adults and adolescents (Tomczyk et al., 2016; Silveira et al., 2019; Willis et al., 2019; Zuckermann et al., 2019). Among the adolescents, the common polysubstance use comprised cigarettes/E-cigarettes/tobacco, alcohol, and marijuana (Tomczyk et al., 2016; Zuckermann et al., 2019, 2020; Tan et al., 2020). Systematic analysis of data from the US, United Kingdom, France, Germany, and Australia associated benzodiazepine with four-fold greater odds of misuse and six-fold greater odds of abuse with prescription opioid analgesics (Morley KI. et al., 2017). Data from the National Survey on Drug Use and Health (2015–2018; 18–64 years old) revealed that the prevalence of opioid and methamphetamine use was higher among those from the age group 18–49 (Shearer et al., 2020). In the US, methamphetamine use was significantly increased among treatment-seeking opioid users, from 18.8% in 2011 to 34.2% in 2017 (Ellis et al., 2018). In line with this, an increase in methamphetamine use was reported among primary treatment admissions, from 1 in 50 in 2008 to 1 in 8 in 2017 (Jones et al., 2020). In Malaysia, a steep increase in polydrug abuse was reported, with 8,841 polydrug abusers in 2018, by 2019 it has increased to 15,166 polydrug abusers which is around a 71.5% increase in a year’s time (National Anti-Drugs Agency, 2019). Moreover, in the US, almost half of the psychostimulant use-related deaths involve opioids, and likewise, opioid use related-deaths involve methamphetamine co-use (Ihongbe and Masho, 2016; Lancet, 2018; Gladden et al., 2019; Kariisa et al., 2019; Compton et al., 2021).
Polydrug abuse refers to the fairly common activity where drug users combine the desired effects of multiple different drugs in one administration or on separate occasions. The combination that is most popular among polydrug users is the co-use of stimulants and opioids, which is known as “speedball: combination of opioids and cocaine” (Trujillo et al., 2011) or “goofball: combination of opioids and methamphetamine” (Glick et al., 2021). Individuals with OUD often co-use methamphetamine through separate use or co-injection (Al-Tayyib et al., 2017), to balance the two drugs’ relative effects, attaining a synergistic high or mitigate the risk of overdose or withdrawal (Ellis et al., 2018; Palmer et al., 2020; Baker et al., 2021). Patients taking medications for OUD, use methamphetamine to attain an alternative high to opioids and/mitigate the sedative effects of the medications (McNeil et al., 2020; Palmer et al., 2020). Polydrug abuse also refers to the sequential use of drugs, which is the consumption of a substance after the peak effect of another substance, reportedly to alleviate withdrawal symptoms or to prolong a state of euphoria (Preston et al., 2016). Combinations most popularly included stimulant and depressant substances (Rigg and Ibañez, 2010; Silva et al., 2013) with the main motivation behind this sequential combination being the alleviation of withdrawal symptoms. However, the sequential polydrug combination does not exclude substances of the same class which aim to ease the effects of the drug (Lankenau et al., 2012; Kecojevic et al., 2015). Prolonging a high also was a motivation behind the sequential use of stimulants and opioids, which manages the opposing psychotropic effects (Valente et al., 2020). Moreover, it was reported that methamphetamine users with a history of polysubstance use (such as heroin, ketamine, and ecstasy) are more prone to develop anxiety symptoms during the early period of abstinence (Su et al., 2017). In line with this, polydrug use was associated with anxiety and depression by a 10-years prospective study (Burdzovic Andreas et al., 2015). A study involving psychostimulant-dependent patients with a history of polydrug use revealed that the severity of negative symptoms in psychostimulant-associated psychosis is not related to the psychostimulant use, but rather due to the use of opioids (Willi et al., 2016). Furthermore, it was also reported that co-use of methamphetamine and morphine results in differential physical symptoms compared to the use of morphine or methamphetamine alone. For instance, co-used patients reported increased catecholaminergic hyperstimulation of respiratory, cardiovascular, and peripheral nervous systems, and more severe neuropsychiatric symptomatology (Liu et al., 2015).
Although there have been a number of preclinical studies that attempt to characterize the polydrug abuse phenomenon, there is still insufficient evidence to completely understand the behavioral and neurochemical consequences that come with it. Chronic administration of morphine and methamphetamine increased the incidence of jumping behavior (Kaka et al., 2014), where morphine assigned rats were given cumulative doses of 5, 10, 20, 30, and 40 mg/kg per day within 5 days while methamphetamine was assigned rats were given cumulative doses of 1, 2, 4, 6 and 8 mg/kg per day for 5 days, and lastly, on day 6, a combination of 8 mg/kg methamphetamine and 40 mg/kg morphine was injected. It is indicative of an attempt to escape the test chamber due to withdrawal-induced anxiety and stress (Liu et al., 1999). Manifestation of withdrawal symptoms in the methamphetamine or morphine alone administered animals were dissimilar to the animals exposed to both methamphetamine and morphine. None of the methamphetamine-administered animals displayed escape behaviors and other behaviors such as ptosis and chewing were more pronounced in the morphine-treated animals (Kaka et al., 2014). The co-use of drugs often masks the unwanted effects of the other drug. In line with this, it was reported that the co-use of morphine and low dose methamphetamine (7.5 mg/kg and 1.0 mg/kg respectively) caused sensitization of the opioid receptor system with the psychostimulant masking the sedative effects of morphine (Ridzwan et al., 2018). The effects of methamphetamine and morphine co-use depend on the drugs’ dose and behaviors assessed, and very often synergistic effects of the drugs have been reported with concurrent co-use. An acute combination of morphine (5 mg/kg) and methamphetamine (1 mg/kg) injected subcutaneously resulted in more than twice of ambulation and more than 50% of rearing than the animals administered with each drug alone, indicating synergistic effects from the co-use (Trujillo et al., 2011). Furthermore, it was also reported that methamphetamine (0.032 mg/kg/infusion) had no reinforcing effects on rats withdrawn from morphine or morphine-dependent rats, whereas fentanyl produced high reinforcing effects on morphine withdrawn animals, and reduced effects on morphine-dependent animals. These results suggest that the reinforcing effects of methamphetamine are independent of the withdrawal or dependence state of opioid use (Seaman et al., 2021). Likewise, morphine (0.75 mg/kg i.p.) also produced synergistic effects on methamphetamine-induced (5 mg/kg i.p.) conditioned place preference and sensitization of stereotyped behaviors along with methamphetamine (Lan et al., 2009).
Neurological Changes in Drug Dependent Brains
Addiction experts at the World Health Organization proposed in 1950 that drug addiction is primarily characterized by psychological dependence, regardless of the type of drug (Eddy and Isbell, 1959). Due to this, early psychological hypotheses linked addiction to symptoms like psychic tolerance (which was thought to be the source of increasing drug consumption) and abstinence agony (also known as withdrawal syndrome) (the presumed main obstacle to abstinence) (Solomon and Corbid, 1973). For many years, researchers speculated that the mesotelencephalic dopamine system was responsible for the rewarding effects of both opiates (such as heroin and morphine) and psychostimulants, building on the discovery that electrical stimulation of certain brain areas may produce reward (for example, cocaine, amphetamine, and methamphetamine) (Wise, 1978; Di Chiara and Imperato, 1988). Motivational effects of drug-associated signals and psychomotor sensitization to addictive substances were both linked to this system (Stewart et al., 1984). Using these neuropharmacological findings, the 1987 psychomotor stimulant theory of addiction and later theories highlighted shared psychobiological foundations for addiction, spanning drug classes, were based on these neuropharmacological breakthroughs (Wise and Bozarth, 1987; Badiani et al., 2011).
Opioid Dependence
Acute administration of morphine to healthy volunteers (not on any type of opioids) results in positive signal changes in reward-associated regions, including the amygdala, nucleus accumbens, hippocampus, and orbitofrontal cortex (Becerra et al., 2006). Similarly, acute opioid withdrawal (naloxone-precipitated) in healthy male subjects (21–34 years old) increased neural activity in rewards-prediction and reward-association regions, including the pregenual cingulate, caudate, middle orbital gyrus, orbitofrontal gyrus, and putamen. Whereas, reduced neural activity was seen in the areas involved in the sensorimotor integration, network dysregulation, and body attentional monitoring such as the bilateral precentral and postcentral gyri, posterior insula, left anterior precuneus, and bilateral temporal lobe (Chu et al., 2015). Chronic opioid-dependent patients undergoing abstinence also recorded reductions in the midbrain-thalamic grey matter connectivity (Tolomeo et al., 2016). In a separate study on opiate-dependent patients (18–59 years old; 18 males, 11 females), baseline drug use severity and opioid withdrawal symptoms were positively correlated with the neural response to drug cues in the orbitofrontal cortex, nucleus accumbens, and amygdala. Craving, however, did not mediate such changes (Shi et al., 2021). The neurological and behavioral changes seen in opioid abstinent patients are time-dependent as well. For instance, recently withdrawn opioid-dependent patients showed reduced hedonic response to natural rewards, increased drug-related cues, increased cortisol levels compared to opioid-dependent patients that have been abstinent for 2–3 months. Furthermore, the recently withdrawn patients also had stronger dorsolateral prefrontal cortex responses to drug cues and higher cortisol levels (Bunce et al., 2015), indicating neuroplasticity in reward- and stress-associated brain regions over the abstinence period.
Heroin is greatly implicated with impulsive and poor decision-making due to its deteriorating effects in regions associated with cognitive functions (Kirby and Petry, 2004; Pirastu et al., 2006). Past fMRI findings indicate that heroin-dependent individual (HDI) groups had significant functional changes in the left prefrontal cortex, bilateral orbital frontal cortices, and left anterior cingulate gyrus as compared with control groups, where the HDI groups exhibited a disruption in the white matter structural networks (Zhang et al., 2016). Chronic heroin use is associated with white matter structural connectivity impairment in bilateral frontal lobe sub-gyrus, cingulate gyrus, medial frontal gyrus, posterior thalamic radiation, left temporal lobe sub-gyrus, and right superior frontal gyrus (Li et al., 2011) resulting in different activation patterns in the networks of reward, motivation, memory/learning and control that are heavily involved in drug abuse and addiction (Zhang et al., 2011). Functional connectivity is also compromised in chronic heroin users due to the dysregulation of brain regions (prefrontal cortex, anterior cingulate cortex, supplementary motor area, ventral striatum, insula, amygdala, and hippocampus) that lead to the decrease of the monitoring function, impairing inhibitory control and inducing deficits in stress regulation (Liu et al., 2009). The aforementioned neurological changes weakened the executive control, which manifests as increased impulsivity, based on findings from the Iowa Gambling Task (IGT) and the Barrett Impulsiveness Scale (BIS), where a positive correlation was found between poor performance in the IGT (indicating impaired decision making) with heroin use (Qiu et al., 2011; Ma et al., 2015). As for the BIS, studies investigating impulsivity in heroin-dependent individuals showed that weakened executive control is positively correlated with the BIS score (Qiu et al., 2013; Wang et al., 2016).
Acute fentanyl treatment (50 ug/kg/i.p) to rats decreased [123I]b-CIT binding to dopamine transporter in the striatum by 30%. Similarly, in a human subject, reduced [123I] b-CIT binding was noticed in the basal ganglia by 37% in the presence of fentanyl. Whereas, subacute (10 ug/kg, twice a day, i.p) in animals and following 2 weeks of drug-free period (human) recorded no significant alterations in the dopamine transporter activity. The findings indicate the differential effects of fentanyl on the reuptake of dopamine sensitive to the time frame of administrations as well (Bergström et al., 1998). In a more recent study, in nonhuman primates, intravenous self-administration of fentanyl (1 ug/kg) recorded reduced functional connectivity in the brain regions associated with the effects of opioid agonists such as the striatum, cingulate cortex, and midbrain, indicating a reduced function in motoric, cognition, and sensory-related faculties. Whereas, functional connectivity of nucleus accumbens with other regions were increased, suggesting escalation in the activities of regions associated with reward processing, drawing similarity with other types of opioid that promote addiction (Withey et al., 2022). Furthermore, chronic intake of fentanyl also has caused cognitive detriments such as opioid-related acute amnestic syndrome with MRI findings of a patient revealing restricted diffusion of the hippocampi, and 10% loss of volume in the cornu amnomis, subiculum hippocampal subfields, and dentate regions (Butler et al., 2019).
Methamphetamine Dependence
One of the most frequently methamphetamine-associated changes in the brain is cognitive deterioration, which was shown to affect brain regions such as the prefrontal cortex and anterior cingulate cortex that involved in cognitive control, and prefrontal cortex, anterior cingulate cortex, and striatum in decision making (Sabrini et al., 2019). Chronic methamphetamine intake also caused severe gray matter deficits in the limbic, cingulate, and paralimbic cortex, reduced the hippocampal size, and the neurological findings correlated with cognitive impairment (Thompson et al., 2004). Some researchers reported significant improvement in cognitive function after withdrawal from methamphetamine use over 6 months (Proebstl et al., 2019), especially abstinence as long as 1 year was shown to normalize the cognitive function (Ludicello et al., 2010). Some reported slight improvement just after 1 month of abstinence (not significant) (Simon et al., 2010), whereas some findings indicate that even with an average abstinence period of 46 days, both abstinent and dependent patients still perform worse than the control group in cognitive assessments (Farhadian et al., 2017), suggesting a longer duration of abstinence needed to reverse the chronic methamphetamine-induced cognitive deficits. In line with this, a separate study reported that prolonged abstinence from methamphetamine use improved the grey matter volume of cognition-associated regions (Zhang et al., 2018). Such findings also imply the important roles of these brain regions in the development of methamphetamine dependence (London et al., 2015). Compared to adults, adolescent brains are more vulnerable to methamphetamine-induced alterations, even with a shorter duration of use and smaller doses, particularly affecting the frontostriatal system (Lyoo et al., 2015), which is also been reported in adults (London et al., 2015). Using an animal model, it was reported that withdrawal from chronic psychostimulant use remodels the functional architecture of the brain, causing a shift from cortical (sensory/motor) regions to the more subcortical network (Kimbrough et al., 2021). Another common symptom associated with methamphetamine dependence, that is psychosis was reported due to decreased activity in the left precentral gyrus and the left inferior frontal gyrus, and increased activity in the putamen and pallidum (Vuletic et al., 2018).
Neurochemical Changes in Drug Dependent Brains
Dopamine
The dopaminergic neurotransmitter system innervates brain regions associated with addiction, including the striatum, hippocampus, prefrontal cortex, amygdala, and others (Ogawa and Watabe-Uchida, 2017; Menegas et al., 2018). Variations in the inhibitory and excitatory outputs from D1 and D2 receptors of the dopamine system (Kravitz et al., 2012) somehow produces differential responses to rewards, aversive stimuli, and prediction of rewards and punishment (Ljungberg et al., 1992; Mileykovskiy & Morales 2011). D1 receptors are relatively denser in the striatum, nucleus accumbens, olfactory bulb, amygdala, hippocampus, substantia nigra, hypothalamus, and frontal cortex, while D2 receptor and its subtypes are expressed mainly in the cortex, substantia nigra, and hypothalamus (Mishra, et al., 2018).
Acute intake of opioids by opioid naïve subjects was shown to increase the dopamine release in the striatum in preclinical (Spanagel et al., 1992) and clinical (Spagnolo et al., 2019) studies, mediating the reinforcing effects of the drug. In contrast, prolonged exposure to opioids dampens the striatal dopamine release (Jia et al., 2005; Shi et al., 2008; Yeh et al., 2012) due to drug-induced adaptations in the dopamine neurotransmitter system. Such hypodopaminergic state was associated with reward deficiency syndrome, which behaviorally manifests as insufficiency in the feeling of satisfaction (Blum et al., 2015). Nevertheless, there have been conflicting findings in the dopamine levels of chronic opioid users, consistent with a human postmortem study that reported no difference in striatal dopamine transporters between opioid users and healthy deceased subjects (Kish et al., 2001; Cosgrove, 2010). However, reduced dopamine levels may induce feedback mechanisms to increase dopamine receptor expressions, which has been reported in a postmortem study of opioid users, where both D1 and D2 receptors were upregulated in the ventral tegmental area, nucleus accumbens and the amygdala (Sadat-Shirazi et al., 2018).
Psychostimulants invoke higher dopamine release in the ventral striatum compared to opioids, upon acute intake (Martinez et al., 2003; Spagnolo et al., 2019), which could be due to the direct actions of the stimulants on the dopamine transporters (Tsukada et al., 1999). Studies indicate that there is a general downregulation of dopamine receptors with stimulant use, where the action of methamphetamine is dose-dependent with methamphetamine acting primarily as a dopamine transporter blocker at low concentrations and reversing dopamine transport at high concentrations (Calipari et al., 2013; Ashok et al., 2017). Such changes cause deficits in functions of the dopamine receptors-enriched brain areas, which could be the reason why drug-dependent users crave or even relapse because the endogenous dopamine is no longer sufficient for stimulation (Wang et al., 2012; Härtel -Petri et al., 2017). The reductions in both pre-and postsynaptic dopamine receptors possibly due to the loss of dopamine neurons or damage to the dopaminergic terminals, mediated by methamphetamine-induced apoptosis through activation of caspases and formation of free radicals (De Vito and Wagner, 1989; Tata and Yamamoto, 2007; Cunha-Oliveira et al., 2008). Chronic administration of methamphetamine reduces the levels of dopamine transporters in the striatum, orbitofrontal and dorsolateral prefrontal cortex, and amygdala (McCann et al., 1998; Sekine et al., 2001; Volkow et al., 2001; Sekine et al., 2003). Animals that self-administered methamphetamine exhibited dose-dependent decreases in striatal dopamine and striatal dopamine transporter levels, as well as significant reductions in dopamine and dopamine transporter levels in the cortex (Krasnova et al., 2010). Exposure to methamphetamine reduced the levels of dopamine transporter availability which is suggested to be the mechanism behind deficits in inhibitory control that emerge in dependent individuals (Groman et al., 2012). However, there have been studies that reported no effects of methamphetamine treatment in the striatum and nucleus accumbens (Melega et al., 2008).
Opioid Receptors
Opioid receptor subtypes are mu (MORs), kappa (KORs), and delta (DORs) (For review on opioids alone, kindly refer to Darcq and Keiffer, 2018). The MORs mediate behavioral changes such as motivational aspects (Laurent et al., 2015), impulsivity (Olmstead et al., 2009), aversion processing (Boulos, 2016), and despair-like behavior (Lutz et al., 2014). The MORs bind readily to endorphins and are mainly found in the mesocorticolimbic networks (Le Merrer et al., 2009). The KORs bind to dynorphins and act as an “anti-reward” system (Koob and Le Moal, 2008; Koob et al., 2014), mediating negative affective states such as depression, stress, dysphoria, and aversion (Crowley and Kash, 2015), that are more pronounced during the abstinence period of opioid dependence (Chavkin and Koob, 2016). The KORs are present in the striatum, hypothalamus, and periaqueductal gray (Wang, 2019). The MORs potentiates dopamine release in the nucleus accumbens, whereas the KORs inhibit dopamine release terminals in the nucleus accumbens and prefrontal cortex, hence causing dysphoria (Spanagel et al., 1992; Bals-Kubik et al., 1993). The initial positive, and negative reinforcing effects in the later stage of addiction allow the transition from recreational drug use to dependence (Gerrits et al., 2003). In rats, exposure to morphine significantly elevated the levels of accumbal MORs, but decreased levels in the ventral tegmental area (Vassoler et al., 2016). Withdrawal from chronic morphine, however, enhanced the MOR activity in the ventral tegmental area suggesting it may be an adaptive response to the elevation of cAMP levels during morphine withdrawal (Meye et al., 2012). Furthermore, withdrawal from morphine also increased MOR mRNA levels in the other reward-associated regions including the lateral hypothalamus, nucleus accumbens core, and caudate-putamen (Zhou et al., 2006). Nevertheless, the chronic opioid or withdrawal-induced mRNA changes have been inconsistent where some reported a decrease (Duttaroy & Yoburn, 2000), an increase (Sehba et al., 1997) while another reported no changes (Castelli et al., 1997), which could be due to the differences in the brain regions examined, exposure time, dose and route of opioid agonist administration.
Prolonged administration of opioids also decreased endogenous endorphin production, where administration of Fentanyl slowed down the endorphin production in patients under general anesthesia (Ballantyne, 2017). Furthermore, there is also downregulation of MORs along with the uncoupling of MORs from their ligand-gated voltage channels (Sprouse-Blum et al., 2010), causing the users to be dependent on the exogenous opioids to replicate the endogenous opioids that are unresponsive, mediating the risk for drug tolerance and addiction (Toubia and Khalife, 2019). Contrary to MORs and KORs, the DORs are not associated with the drug reward system, but more towards learning and memory (Klenowski et al., 2015; Pellissier et al., 2016), and also attenuates negative mood (Lutz et al., 2014). The DORs bind to enkephalin and are expressed in the basal ganglia (Wang, 2019), mood, motivation, and learning-related regions (Erbs et al., 2015). Chronic intake of morphine decreased the density of DOR-expressing neurons in the mice hippocampus, which persisted even after 4 weeks of abstinence (Erbs et al., 2015). This contributes to reduced inhibition of the firing activity of the hippocampus, resulting in disturbances in memory processes (Erbs et al., 2015), which is commonly reported as cognitive deficits in opioid-dependent patients, especially during the early period of abstinence (Rapeli et al., 2006).
Neuroadaptation occurs with the persistent increase in striatal MOR following methamphetamine treatment, which occurred concurrently with the emergence of anxiety-related symptoms during withdrawal (Georgiou et al., 2016). Opioid receptors do not respond similarly to methamphetamine, where a study using a 7-days regimen revealed that binding of MOR was not changed on day 2 and 5 but downregulated on day 8 then gradually returned to normal on day 11—while there were no changes in KORs and sigma opioid receptors on any given day examined (Chiu et al., 2006). Activation of the dopamine receptor is required for the increased expression of MOR mRNA, at least in the nucleus accumbens (Azaryan et al., 1996), in the presence of stimulants such as cocaine, indicating a substantial interaction between the dopaminergic and opioid system mediating the rewarding effects of stimulants. Moreover, it was also reported that the MOR is important in modulating the development of methamphetamine-induced behavioral sensitization through the dopaminergic neurotransmission (Tien and Ho, 2011). Further corroborating this were findings by Park and colleagues, who reported a decrease in the dopamine 1 receptor-ligand binding in the striatum of methamphetamine-treated MOR knockout mice (Park et al., 2011).
In a tail withdrawal test, methamphetamine (5 mg/kg) was as analgesic as 10 mg/kg morphine. However, the lower doses of methamphetamine (1 and 2 mg/kg) were not. The analgesic effects of methamphetamine were reversed by administration of naltrexone (1 mg/kg; non-selective opioid receptor antagonist), indicating the interaction between MORs and methamphetamine at higher doses. The analgesic effects of 5 mg/kg of methamphetamine were equipotent to the morphine (10 mg/kg) (Ridzwan et al., 2018). It was previously reported that daily intake of methamphetamine (2.5 mg/kg) significantly reduced the expression of MORs (Chiu et al., 2006). The researchers reported a profound decrease in the expression of MORs on day 8, not day 2 or 5, whereas Ridzwan et al. (2018) conducted the tail withdrawal test minutes upon drug administrations. At present, it is unclear whether methamphetamine able to reduce the threshold for analgesic activity by downregulating the MORs within a shorter time frame.
Polydrug Use
Co-use of methamphetamine and morphine may alter the brain and behavior differently compared to the use of either drug alone. Previous studies have reported greater rewarding effects from the co-use of morphine and methamphetamine compared to the individual doses of the drugs (Negus et al., 1998; Ranaldi and Wise, 2000). A combined administration of methamphetamine (0.75 mg/kg) and morphine (5 mg/kg) produced higher conditioned place preference (CPP) and slower decline of CPP than equivalent individual doses of the drugs (Lan et al., 2009). Such drug-induced reinstatement has also been reported in other preclinical studies testing low doses of morphine (2 mg/kg) and methamphetamine (0.5 mg/kg) (Manzanedo et al., 2005; Tatsuta et al., 2007), which coincides with human findings where low doses of morphine and methamphetamine were reported to mediate the reinforcing effects (Lamb et al., 1991; Melega et al., 2007). Similarly, repeated administration of combined low doses of methamphetamine (0.75 and 2.5 mg/kg/day) and morphine (5 mg/kg/day) for 5 days was reported to elevate dopamine level in the nucleus accumbens compared to either drug alone (Zhu et al., 2015), indicating the higher reinforcing effects of the drugs when taken together.
Challenge administration of morphine (5 mg/kg) and methamphetamine (0.75 mg/kg) on day 40 (post chronic drugs administration) significantly increased striatal dopamine levels compared to either drug alone, but decreased dopamine turnover in the striatum (Lan et al., 2009). Whereas, challenge administration of methamphetamine alone (0.75 mg/kg) significantly decreased dopamine turnover, but morphine (5 mg/kg) produced no profound changes. The reduction in combined drug-induced dopamine turnover was lesser than methamphetamine-induced (Lan et al., 2009), indicating differential effects of the combination of drugs than either drug alone on striatal dopaminergic neurotransmission in the development of behavioral sensitization. Similar findings were also reported in a previous study, however on individual doses of methamphetamine (2 mg/kg) and morphine (10 mg/kg), where methamphetamine significantly increased dopamine release and reduced dopamine turnover in the striatum. Whereas, morphine slightly increased the dopamine levels in the striatum, and had no effects on dopamine turnover. Furthermore, the effects of methamphetamine on dopamine release and turnover were greater in the striatum than nucleus accumbens, whereas for morphine, a significant increase in the release and turnover of dopamine was seen in the nucleus accumbens than striatum (Mori et al., 2016). The findings indicate the differences in the effects of psychostimulants and opioids in the mesolimbic and nigrostriatal dopamine systems.
Effects of low doses of cocaine are enhanced in an additive manner by the addition of low dose heroin, where the drug combination significantly increased the extracellular levels of nucleus accumbens dopamine (Smith et al., 2006). The author suggests that the neurochemical effects are likely through MORs and DORs in the nucleus accumbens. However, a study showed that only the MORs in the nucleus accumbens is involved in the reinforcing effects of combined administration of heroin and cocaine where the author suggested that the DOR had no effect on speedball self-administrations because doses might have been too low and DORs is regionally specific to the shell area of the nucleus accumbens (Cornish et al., 2005). Dopamine receptors such as the D1 and D2 receptors play different roles in the combined administration of heroin and cocaine, particularly D1 receptors enhance the individual self-administration of heroin or cocaine, whereas stimulation of D2 receptors inhibits the reinforcing effects of heroin when administered together (Rowlett et al., 2007).
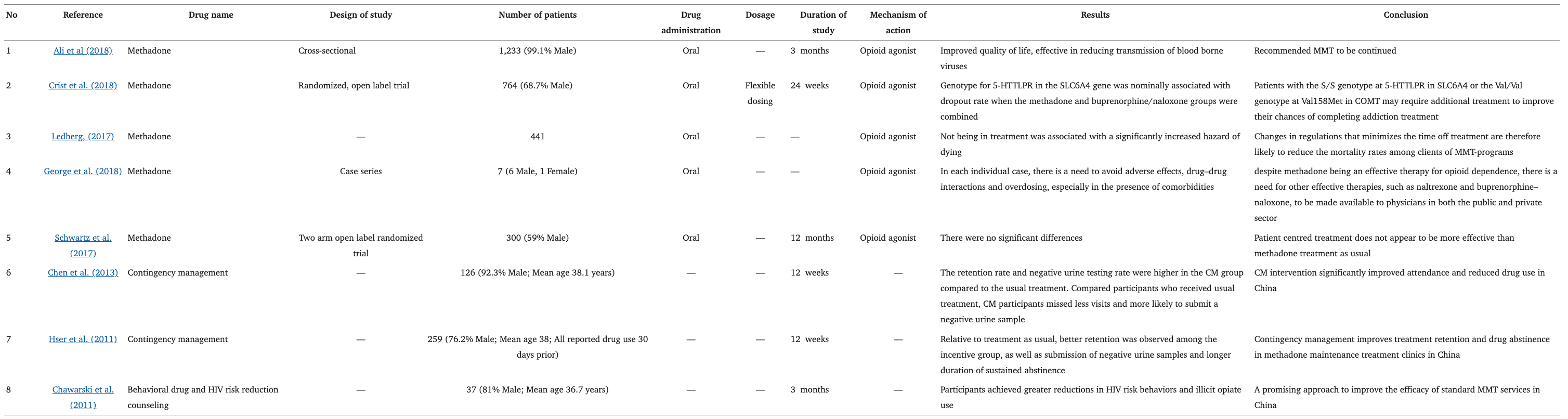
Pharmacotherapy for Opioid Use Disorder and Methamphetamine Use Disorder
Opioid Use Disorder
Methadone doses that are considered low, intermediate, and high are <50, 50–100, and >100 mg/day, respectively whereas the dose for methadone maintenance treatment varies between 30 and 125 mg/day (Robles et al., 2002). Oral intake of methadone for over 3 months improved the quality of life and reduced transmission of blood-borne diseases among opioid-dependent patients (Ali et al., 2018). Despite the efficacy of MMT in harm reduction, there are still other clinical concerns regarding the safety of the therapy. The patients undergoing MMT often have co-morbidities, where they are already on prescription drugs, therefore when added with methadone it might lead to unwanted drug-drug interactions, such as the development of “opioid withdrawal-like symptoms” in the case of efavirenz and zidovudine which are common treatments for HIV patients who are highly prevalent under the MMT program (George et al., 2018). Patients with opioid dependence also tend to have higher rates of mood disorders and other illicit substance abuse, where a combination of these factors may lead to the possibility of central nervous system effects and worsening behavioral symptoms (George et al., 2018). Other potential adverse effects of methadone include nephrotoxicity (Atici et al., 2005; Lentine et al., 2015) and cardiotoxicity (Kumar, 2010).
Buprenorphine is an opioid partial agonist that sustains abstinence, delays the time of resumption to opioid use, and retains patients in treatment (Schottenfeld et al., 2005). Buprenorphine has a long half-life of 24–60 h and typical dosages for maintenance treatment are 8–16 mg/day (Walsh et al., 1994; Kampman and Jarvis, 2015). Due to problems associated with diversion and abuse with buprenorphine treatment, the buprenorphine/naloxone combination tablet was introduced (Vicknasingam et al., 2010). Other combinations of buprenorphine exist as well; however, they were injected illicitly, which instead increased opioid dependence (Yokell et al., 2011).
Methamphetamine use was reported among treatment-seeking OUD patients with a prevalence of 85% in the United States (Ellis et al., 2018), where most of these patients recorded a significantly higher percentage of positive results in the urine morphine test which indicated relapse of opioid use (Liu et al., 2018). In addition, methamphetamine use was also associated with a higher risk of buprenorphine non-retention (Tsui et al., 2020). Whereas, another study found no such associations between methamphetamine use and opioid abstinence in OUD pharmacological management (methadone) (Smyth et al., 2018). The overall impact of methamphetamine use on OUD treatment outcomes are still unclear, but patients have described a balancing effect of the drugs (increases functionality of the drug that is associated with a lower perceived need for medications for OUD) that lead towards non-retention of treatment (Mcneil et al., 2020) (Table 1).
A retrospective study in Rhode Island was the first to investigate the efficacy of MMT in fentanyl abuse, which reported that the majority of patients that underwent 6 months of methadone maintenance achieved abstinence (89%), but the relapse rate was still high (59%) (Stone et al., 2018). Silverstein and colleagues analyzed qualitative data from 63 interviews, to investigate the presence of illicit non-pharmaceutical fentanyl in the current environment and how it has affected practices of non-prescribed use of buprenorphine. The participants consisted of OUD patients on non-prescribed buprenorphine, where they used illicit opioids such as buprenorphine not in seek of euphoria, instead as a form of self-treatment. However, some reported that non-pharmaceutical fentanyl defeated the harm reduction brought by buprenorphine as there were unanticipated experiences of withdrawals (Silverstein et al., 2019). However, the Zurich or Bernese method has been considered a valuable modification to buprenorphine induction for the treatment of fentanyl abuse where it utilizes micro-dosing of buprenorphine. Micro-dosing or micro-induction of buprenorphine is a method of administering buprenorphine in small incremental doses during initiation of treatment that slowly builds buprenorphine at opioid receptors without precipitating withdrawal (Ahmed et al., 2021). Overlapping induction of buprenorphine while being on full mu agonists such as methadone is feasible, where patients experienced very mild opioid withdrawal and craving (Hämmig et al., 2016).
Psychosocial interventions in conjunction with medications for the treatment of opioid addiction are approved as a part of comprehensive treatment for opioid addiction such as contingency management (CM) and cognitive behavioral therapy (CBT), with the majority focusing on methadone treatment (Dugosh et al., 2016). Studies showed that CM participants attended more days of treatment and had longer durations of continued abstinence (Hser et al., 2011; Chen et al., 2013) while CBT participants displayed significant improvements in their positive appraisal at the 6-months assessment and lower emotional discharge at the 12-months assessment compared to control group MMT alone (Kouimtsidis et al., 2012). Other psychosocial interventions include behavioral drug and HIV risk-reduction counseling, motivational interviewing, acceptance and commitment therapy, general supportive counseling, and web-based behavioral interventions (Chawarski et al., 2011; Stotts et al., 2012; Gu et al., 2013; Marsch et al., 2014).
Table 1. Current treatment in opioid dependence.
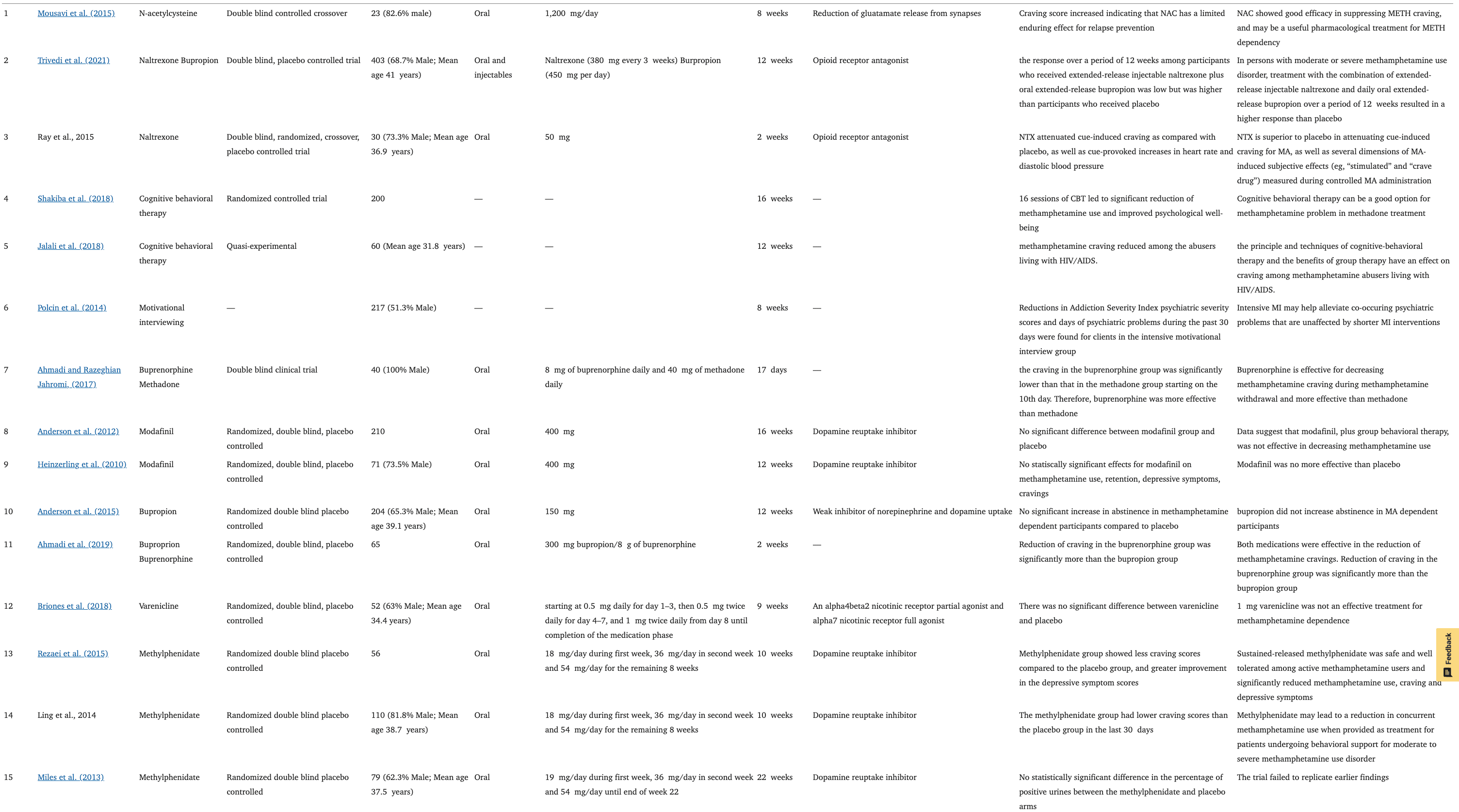
Methamphetamine Use Disorder
In addition to OUD, MMT is also prescribed to chronic methamphetamine users as treatment (Singh et al., 2020). Comparison between the methadone (a full agonist of the MORs) and buprenorphine (a partial agonist of the MORs) in the reduction of methamphetamine craving revealed more significant craving-attenuating effects of buprenorphine during methamphetamine withdrawal (Ahmadi and Razeghian Jahromi, 2017). In another study, buprenorphine significantly reduced methamphetamine cravings compared to bupropion (weak inhibitor of dopamine and norepinephrine reuptake) for 14 days (Ahmadi et al., 2019). Bupropion also did not significantly increase abstinence duration in methamphetamine-dependent patients compared to placebo (Anderson et al., 2015).
N-acetyl cysteine (NAC) reduces the synaptic release of glutamate (Dean et al., 2012). Preclinical studies and early pilot clinical investigations suggested that NAC may be useful in the treatment of methamphetamine dependence, showing good efficacy in suppressing methamphetamine craving however, there was no report made on methamphetamine use outcomes (Ebrahimi et al., 2015). A combination of NAC and naltrexone was found to be no more superior than a placebo in reducing methamphetamine craving (Grant et al., 2010). Modafinil (dopamine reuptake inhibitor), was not effective in decreasing methamphetamine consumption compared to the placebo (Heinzerling et al., 2010). Whereas, another study reported that those who were compliant in taking the modafinil drug were more likely to reduce drug use (Anderson et al., 2012). Both controlled trials were comparing modafinil daily doses ranging from 200 to 400 mg.
Varenicline at 1 mg (an α4β2 nicotinic receptor partial agonist and α7 nicotinic receptor full agonist) taken twice daily for 9 weeks had no significant effects on end-of-treatment-abstinence and treatment effectiveness score compared to placebo in methamphetamine dependence (Briones et al., 2018). Sustained release of methylphenidate (daily dosing regimen of 18 mg at week 1, 36 mg at week 2, and 54 mg for the remaining weeks) on the other hand was safe and well-tolerated among active methamphetamine users and significantly reduced methamphetamine use, craving, and depressive symptoms (Tiihonen et al., 2012; Miles et al., 2013; Rezaei et al., 2015). Methylphenidate is a dopamine reuptake inhibitor (Karila et al., 2010).
Sixteen weeks of CBT reduced methamphetamine dependence and improved the psychological well-being of patients undergoing methadone therapy. The 30 participants in the treatment group became abstinent at post-test and remained abstinent at the 3-months follow-up (Shakiba et al., 2018). The CBT also reduced craving among methamphetamine abusers living with HIV/AIDS (Jalali et al., 2018). Significant reductions in methamphetamine use and psychiatric symptoms were seen following the psychosocial interventions (Polcin et al., 2014; Rawson et al., 2021). The matrix model, which is a multi-component treatment adopting elements of CBT, MI, family, and group therapy, was found to be more effective in increasing methamphetamine abstinence compared to treatment as usual (CBT only) (Rawson et al., 2021). It is reported that sessions of both MI and CBT significantly increased abstinence as well (Baker et al., 2004, 2005). The treatment combining MI and CBT was found to be effective in improving abstinence where participants reported fewer negative consequences of methamphetamine use at follow-up and intensive matrix program produced a higher abstinence rate compared to CBT alone (Smout et al., 2010) (Table 2).
Table 2. Current treatment in methamphetamine dependence.
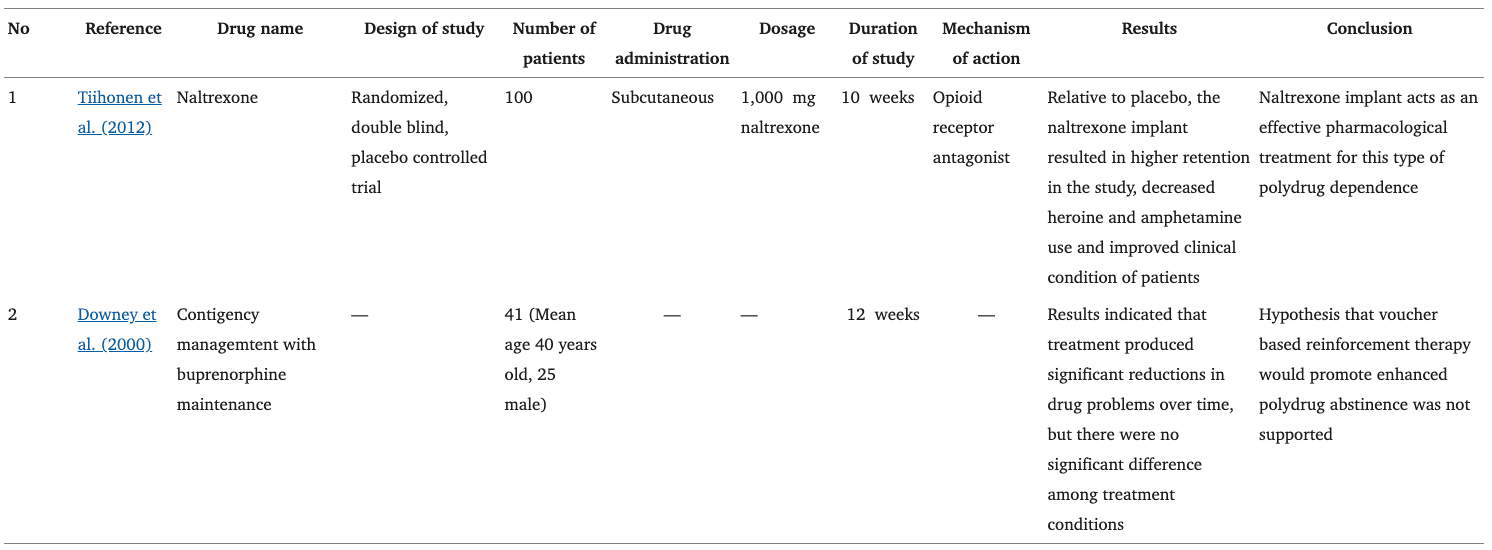
Polysubstance Abuse
Naltrexone subcutaneous implants (1,000 mg) for 12 weeks showed higher retention of patients with decreased use of heroin and methamphetamine, providing some of the earliest evidence for effective pharmacological treatment (Tiihonen et al., 2012). Furthermore, a combination of 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone treatment in an 18-days experiment was reported to reduce relapse in the cocaine and morphine co-administration (McCann, 2008; Cordery et al., 2014). Apart from that, based on heroin-dependent polydrug abusers with contingency management and buprenorphine maintenance (2 mg for 5 weeks), it was suggested that for patients who have already achieved polydrug abstinence, contingency management may enhance treatment outcomes. However, participants generally did not produce any significant treatment outcomes which could possibly be due to the population sample where buprenorphine-maintained polydrug abusers continued to use illicit opiates at fairly high levels (Downey et al., 2000). The use of 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone treatment was studied in morphine and methamphetamine polydrug dependent mice and results show that the combination successfully attenuated polydrug-reinstatement (Suhaimi, 2017).
Methadone maintenance at a relatively high dose of 30 mg/kg a day in 3 h, attenuated heroin and cocaine-seeking behavior, possibly by reducing the incentive value of drug-related cues (Leri et al., 2004). In a separate study, hypothermia was observed after 360 min of coadministration of methamphetamine and morphine. The cooling was beneficial after 30 min (golden hour) of co-administration. During this early stage, methamphetamine plus morphine-induced significant hyperthermia (Namiki et al., 2005). Another study also proved that the lethal effect induced by co-administration of methamphetamine and morphine was significantly and almost completely diminished by cooling from 30 to 90 min afterward, with normal behaviors such as grooming, sniffing, and rearing returned with the normalization of colonic temperature (Mori et al., 2007). Both studies indicated a “golden hour” of between 30 and 90 min for cooling in the treatment of subacute toxicity and lethality produced by the co-administration (Table 3).
Table 3. Current treatment in polydrug dependence.
Conclusion
The prevalence and risk associated with polydrug use are threats to the current public health resources worldwide. Thus, improving and pooling the understanding of mechanisms behind individual drugs and their combined use is essential to accurately reflect their effects on the neurochemical systems. However, this knowledge is still limited, especially in its polydrug combinations that can contribute to unique addiction potential and the development of addictive behaviors. It is important to appreciate novel preclinical experiments that investigate the pathophysiology and pharmacotherapy targeting the mono and polydrug abuse of morphine and methamphetamine. Given how complex addiction as a disease is with many powerful elements playing their roles, we need to understand the mechanisms behind the relationship between polydrug abuse and addiction to determine better and more effective treatments for this ongoing public health crisis.