Abstract
Alcohol is one of the most widely used substances. Alcohol use accounts for 5.1% of the global disease burden, contributes substantially to societal and economic costs, and leads to approximately 3 million global deaths yearly. Alcohol use disorder (AUD) includes various drinking behavior patterns that lead to short-term or long-lasting effects on health. Ethanol, the main psychoactive molecule acting in alcoholic beverages, directly impacts the GABAergic system, contributing to GABAergic dysregulations that vary depending on the intensity and duration of alcohol consumption. A small number of interventions have been developed that target the GABAergic system, but there are promising future therapeutic avenues to explore. This review provides an overview of the impact of alcohol on the GABAergic system, the current interventions available for AUD that target the GABAergic system, and the novel interventions being explored that in the future could be included among first-line therapies for the treatment of AUD.
1. Introduction
Alcoholic beverages have been consumed for recreational purposes in most parts of the world since before recorded history began. According to the latest World Health Organization (WHO) global estimates (WHO, 2021), about 5.1% of the global adult population is living with alcohol use disorders (AUD). Another study by the global burden of disease (GBD) collaborative network reported a 1.5% global AUD prevalence in 2019, highlighting variabilities between countries (Castaldelli-Maia and Bhugra, 2022). Ethanol, the main active component of alcoholic beverages, is currently one of the most used psychoactive drugs on the market. Ethanol produces a state of anxiolysis and disinhibition, which is commonly sought after in social situations or in individuals with AUD (Gilman et al., 2008). Alcohol consumption is also causally related to the development of approximately 230 diseases or disorders, including infectious diseases, malignant neoplasms, cardiovascular system due to ethanol’s effect on blood pressure and inflammation (Chiva-Blanch and Badimon, 2019), mental and behavioral disorders, neurological diseases, digestive diseases, and injuries (Rehm et al., 2017). While consumption patterns vary, the impact of ethanol at low doses on overall health remains unclear (Larsson et al., 2020; Zhao et al., 2023). A recent systematic meta-analysis of cohort studies showed no statistically significant protective effect of alcohol on all-cause mortality at low ethanol intakes (Zhao et al., 2023). Studies have highlighted that abstinence from alcohol has many health benefits, including improved sleep. On the contrary, the risk of certain types of cancer, heart disease, and stroke increases with increased alcohol consumption (Savin et al., 2018; Paradis et al., 2022), and chronic consumption of ethanol in high doses is also linked to feelings of dysphoria, cognitive deficits, and an increased risk of developing AUD (Trantham-Davidson and Chandler, 2015).
Two major diagnostic classification systems are used to define AUD. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), developed by the American Psychiatric Association, defines AUD as a cluster of behavioral and physical symptoms, including withdrawal, tolerance, and craving (American Psychological Association, 2013). The International Classification of Diseases 11th Revision (ICD-11), developed by the World Health Organization, divides AUD into a harmful pattern of alcohol use and alcohol dependence. Alcohol dependence is characterized by “a strong internal drive to use alcohol, which is manifested by an impaired ability to control use, increasing priority given to use over other activities, and persistence of use despite harm or negative consequences” (WHO-ICD11, 2022). According to the ICD-10 definition of AUD, it was estimated that in 2016, approximately 8.6% of adult men and 1.7% of adult women suffered from AUD globally (Carvalho et al., 2019).
AUD may be characterized by the development of tolerance due to homeostatic adaptation in the brain compulsive seeking and withdrawal upon cessation of consumption (Liang and Olsen, 2014). AUD symptomatology includes a wide range of behaviors such as poor control over drinking and impulsivity (a failure to inhibit excessive drive), reward deficiency (a reduced response to natural rewards), maladaptive learning (the growing incentive salience of a drug’s predictive cues with chronic use), the emergence of opponent processes (the power of negative motivational states underlying withdrawal), faulty decision making (inaccurate computation in preparation for action) or automaticity of responses (inflexibility of stimulus–response habits) (Volkow et al., 2013). Due to neuronal dependency on alcohol for regular activity in individuals with AUD, cessation of alcohol consumption often leads to withdrawal (Littleton, 1998). Sudden cessation might result in acute withdrawal symptoms, including delirium, seizures, and cognitive dysfunctions (Jesse et al., 2017; Laniepce et al., 2020). However, the symptoms seen in alcohol withdrawal range in severity depending on the volume and duration of ethanol consumption and inter-individual variability (Newman et al., 2023). Withdrawal symptoms are often related to hyperexcitability, such as insomnia, anxiety, palpitations, agitation, and even seizures (Saunders et al., 2019), likely related to alteration in the functioning of the brain inhibition system.
Due to its hydrophilic nature, ethanol readily penetrates all biological membranes and crosses the blood–brain barrier. Once in the organism, ethanol metabolism happens in the liver but also in the brain due to the presence of alcohol dehydrogenase (ADH), catalase, and P450 (CYP2E1) in both organs. Such metabolism routes produce mainly three metabolites: acetaldehyde, salsolinol, and acetate (Gil-Mohapel et al., 2019; Wilson and Matschinsky, 2020). After reaching the brain, ethanol and its metabolites induce diverse disturbances such as reduced glucose uptake, increased monocarboxylate uptake, dopaminergic, GABAergic, and glutamatergic alterations (Peana et al., 2017).
Since, ethanol and its metabolites act on multiple biological pathways of the central nervous system (CNS), therapeutic interventions relying on various approaches have been developed with variable degrees of efficacy. However, there is still a significant need to understand better the underlying mechanism leading to AUD and associated symptoms and develop more efficient intervention strategies. While impacting many CNS pathways, one of the main pathways altered by alcohol is the inhibitory pathway utilizing gamma-aminobutyric acid (GABA).
This review provides an overview of the impact of ethanol on brain functions related to GABA, describes existing therapeutic interventions, lists their shortcomings, and summarizes the existing knowledge around GABAergic functions in AUD involved in the expression of symptoms and outcomes before providing insight into the development of future therapeutic interventions acting on the GABAergic system.
2. Impact of ethanol on brain
Ethanol produces a wide variety of behavioral and physiological effects in the body, but exactly how it acts to produce these effects is still poorly understood. Like most dependence-producing substances, ethanol binds and acts on multiple proteins, receptors, and signaling pathways throughout the brain (Figure 1A), including amino acids, opioids, enzymes, and ion channels (Heinz et al., 2009; Koob and Volkow, 2016). The primary targets behind ethanol-induced behavioral phenotypes (disinhibition, hyperlocomotion, and anxiolysis) are GABAA receptors. Besides modulating GABAA receptor activity, ethanol can directly bind and modulate the activity of several proteins, including ionotropic glutamatergic (NMDA) receptors, alcohol dehydrogenase (ADH), and glycine receptors (Grant and Lovinger, 2018). Further, it has been observed that ethanol is capable of indirect modulation of other neurotransmitters (dopamine, serotonin, opioid, and cholinergic), particularly in brain regions involved in the mesolimbic reward system [i.e., amygdala, hippocampus, striatum, and ventral tegmental area (VTA)] via GABAergic/glutamatergic neurons or their respective receptors present on other types of neurons (Abrahao et al., 2017). Therefore, chronic ethanol consumption in large volumes drives a chemical imbalance in the brain and forces a homeostatic response to maintain neurochemical equilibrium and functionality (De Witte, 2004). As the brain chemically adapts to excess ethanol, it forms a new equilibrium in which ethanol becomes integral in neuronal function (Figure 1B; Valenzuela, 1997; Pérez-Ramírez et al., 2022). In individuals with AUD, this is manifested through increased tolerance to the effects of ethanol, which can lead to the consumption of alcohol near toxicity levels to experience the effects of alcohol, such as relaxation, anxiolysis, or disinhibition. Consistent with this notion, magnetic resonance spectroscopy (MRS) studies generally demonstrate lower cortical GABA levels in individuals with AUD, specifically during withdrawal, than in control participants (Prisciandaro et al., 2019; Kirkland et al., 2022; Shyu et al., 2022).
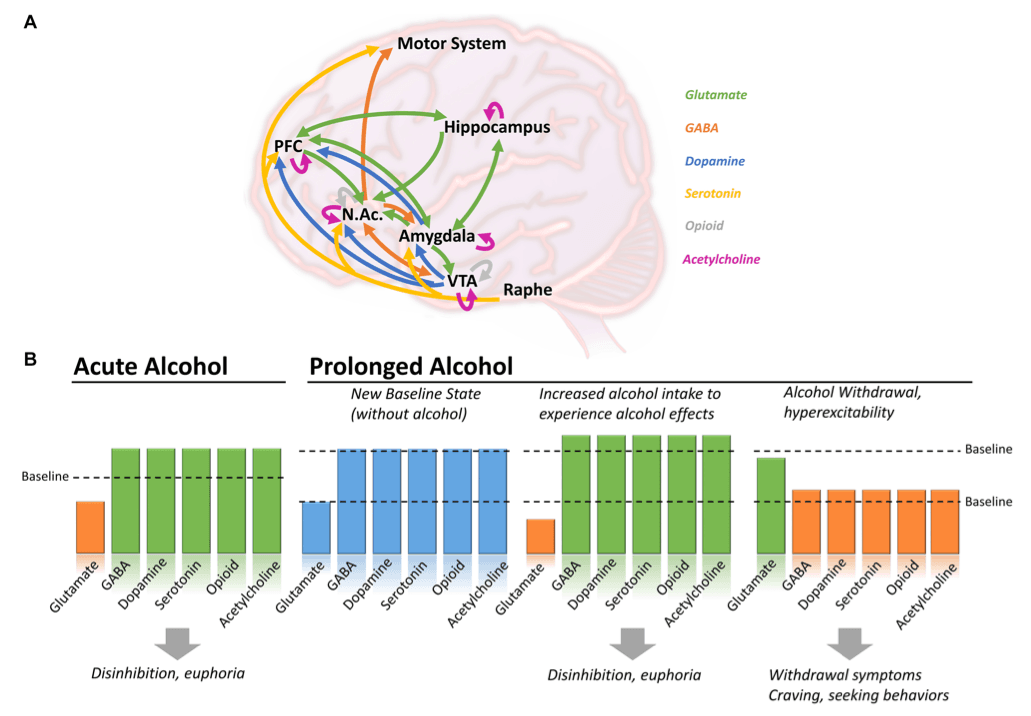
Therefore, the main activity of ethanol is thought to be on glutamatergic and GABAergic signaling pathways, with an increase or decrease of function depending on the state (acute consumption, chronic consumption, or withdrawal), inducing a cascade of events acting on dopamine, serotonin, and endogenous opioid release (Ferraguti et al., 2015).
2.1. Impact of ethanol on glutamate and GABA
Preclinical and clinical studies showed that ethanol binds to and inhibits the functions of the glutamatergic receptors (NMDA, AMPA, Kainate, and mGluR5) (Möykkynen and Korpi, 2012; Ferraguti et al., 2015). It also binds to and facilitates the functions of the GABAA and GABAB receptors (Valenzuela and Jotty, 2015; Olsen and Liang, 2017), which, combined with the effect of glutamatergic receptors, causes an overall imbalance in neuronal activity, thought to be responsible for “blackout” moments after acute heavy drinking (Wetherill and Fromme, 2016; Yang et al., 2022) and contributing to excitotoxicity and loss of synaptic plasticity (Chandrasekar, 2013). Data from studies using human transcranial magnetic stimulation (TMS), a non-invasive neuromodulation approach that probes GABA-receptor-mediated cortical inhibition, confirmed that alcohol intake increases GABA-inhibitory neurotransmission and decreases NMDA-receptor-activated excitatory neurotransmission (Ziemann et al., 2015). Interestingly, the activity of ethanol metabolites on glutamatergic and GABAergic targets seems different, which could explain the dynamic changes happening during drinking episodes (see Section 2.6 below).
Preclinical studies in rats have also confirmed the critical impact of ethanol on the regulation of ethanol-maintained responses through GABAA receptor-dependent signaling in the central nucleus of the amygdala (Avegno et al., 2018; Barchiesi et al., 2021; Kisby et al., 2021). Preclinical studies have also confirmed the impact of alcohol on behavioral outcomes [compulsive behavior (Giuliano et al., 2018), withdrawal-induced hyperalgesia (Avegno et al., 2018), increased anxiety (Barchiesi et al., 2021), altered cognitive functions], and biological pathways [GABA and glutamine (McCunn et al., 2022), glutamate (Vengeliene et al., 2005; Mira et al., 2019), dopamine (Ma and Zhu, 2014; Solanki et al., 2020)] as well as provided insights onto therapeutic interventions (Foo et al., 2019).
2.2. Impact of ethanol on acetylcholine
Ethanol intake in rats was also shown to bind to the nicotinic-subtype receptor of acetylcholine (Davis and de Fiebre, 2006) and to increase acetylcholine levels in the VTA (Larsson et al., 2005), facilitating the influx of dopamine onto the nucleus accumbens (NAc). Such activity in the VTA and NAc is thought to contribute to positive reinforcement of alcohol. In contrast, modulation of the nicotinic receptors of the hippocampus and amygdala is thought to be involved in negative effects (Tarren et al., 2016). Ethanol’s binding and activity at nicotinic receptors are also thought to interfere with nicotine-induced desensitization, which could explain the high prevalence of co-use of alcohol and tobacco (Davis and de Fiebre, 2006; Addolorato et al., 2012).
2.3. Impact of ethanol on dopamine
As a downstream effect of alcohol consumption, ethanol induces an indirect increase in dopamine release and acetylcholine activity from the VTA to the NAc, a brain region strongly associated with reward and motivation (Boehm II et al., 2004). Preclinical Studies have also shown that dopamine is released in the ventral striatum and NAc, contributing to drug reward, which could be further increased by nicotine co-administration (Tizabi et al., 2007). The activation of central GABAergic neurotransmission, particularly through GABAB receptors, is also linked to the mesolimbic dopaminergic neurotransmission during rewarding processes, altogether contributing to the addictive properties of ethanol (Addolorato et al., 2012).
2.4. Impact of ethanol on serotonin
Acute alcohol consumption increases serotonin release, contributing to the rewarding aspect of consuming alcohol (Banerjee, 2014). Previous studies showed that acute ethanol augments the firing rate of the serotoninergic 5-HT3 receptors, and longer consumption can affect the expression and function of various other subtypes, including 5-HT2, without a clear understanding of whether it is a direct effect or mediated by a cascade of events or adaptation (Lovinger, 1997).
2.5. Impact of ethanol on opioids
Consumption of alcoholic beverages has also been shown to increase the levels of endogenous opioids (Mitchell et al., 2012), which are subsequently drastically reduced during withdrawal, leading to craving and increasing the risk of opioid-seeking behaviors (Turton et al., 2020). The activity of ethanol at GABAA receptors in the VTA and NAc facilitates endogenous opioid release in the VTA, contributing to the alcohol-induced feeling of euphoria (Colasanti et al., 2012). Opioid-targeting treatments such as naltrexone or nalmefene diminish these effects of alcohol (Turton et al., 2020), providing further evidence of the impact of alcohol on the opioid system.
2.6. Impact of ethanol metabolism on various neurotransmitters
Acetaldehyde, salsolinol, and acetate, metabolites of ethanol, seem to participate in the effect of alcohol, but their contribution is less understood. Acetaldehyde in the brain causes euphoria at low doses and plays a vital role in ethanol’s reinforcing properties, thereby facilitating alcohol addiction (Quertemont et al., 2005; Peana et al., 2017). One of the primary studies reported that acetaldehyde increased GABA uptake but did not affect both its release and synthesis (Bobrova and Covaltchuk, 1980). Acetaldehyde has been shown to stimulate dopaminergic neurons (Melis et al., 2007) and μ opioid receptors (Sanchez-Catalan et al., 2014). Acetaldehyde is a highly reactive and short-lived metabolite of ethanol that reacts with biogenic amines like dopamine and forms condensation products like Salsolinol.
Studies reported that salsolinol may exert some of the effects of ethanol by activating μ opioid receptors on GABAergic neurons signaling onto dopaminergic neurons in the mesolimbic system. However, the mechanisms are complex, and it seems like salsolinol would reduce GABAergic activity while ethanol increases it, suggesting opposite responses on GABAergic receptor activity from ethanol and one of its metabolites, also causing a downstream opposite effect on dopamine release (Peana et al., 2017).
Finally, the direct role of acetate on GABAergic regulation has not been reported. However, acetate was reported to contribute to increased cerebral blood flow (Tanabe et al., 2019), increased neuronal excitability, and enhanced glutamatergic activity (Chapp et al., 2021), whereas ethanol boosts GABA-mediated inhibition. Accordingly, existing literature indicates that concrete experimental evidence is required to confirm the effects of ethanol’s metabolites on the GABAergic system.
3. GABAergic mechanisms involved in AUD
GABA is the main inhibitory neurotransmitter in the brain. It exerts its function by binding to two types of receptors: GABAA and GABAB. GABAA receptors are ionotropic chloride channels (Enna, 2007), while GABAB are metabotropic G-coupled protein receptors (GPCR) (Pinard et al., 2010). GABAB receptors mediate slow inhibitory transmission, while GABAA mediates fast inhibition. GABAA and GABABhave been extensively reviewed for their potential in pharmacotherapies (Sarasa et al., 2020) and link to AUD (Ghit et al., 2021; Holtyn and Weerts, 2022).
GABAA receptors are heteropentamers composed of various subunits such as α, β, γ, δ, ε, θ, π, and ρ (Figures 2A,B), which are found throughout the brain (Fritschy and Mohler, 1995), including regions involved in alcohol-related use such as the prefrontal cortex, thalamus, cerebellum, or the amygdala (Bowery et al., 1987). Ethanol acts as a positive allosteric modulator (PAM) of GABAA receptors, binding to several subunits, mostly α-subunits, thus explaining its sedative and neuromodulating properties (Ghit et al., 2021). Other PAMs include benzodiazepines and Z-drugs that promote sedation, anxiolysis, muscle relaxation, and anti-seizure properties.
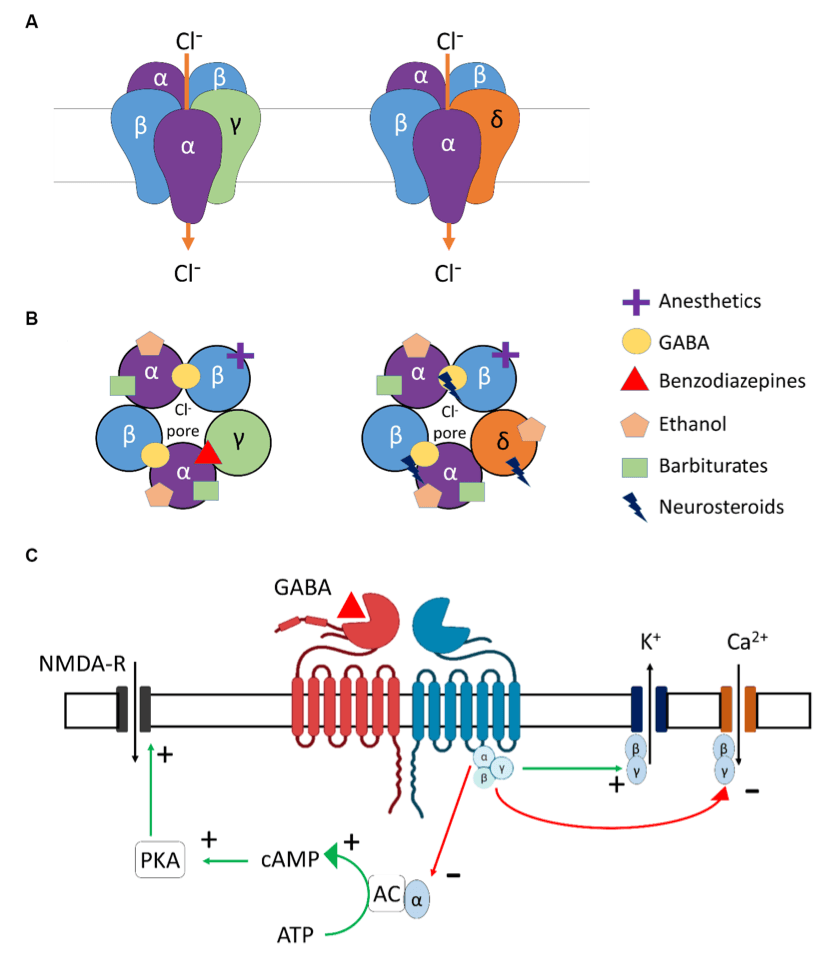
GABAB receptors are the only metabotropic G protein-coupled receptors for GABA (Figure 2C) and can be found in presynaptic (auto-inhibitory) and postsynaptic membranes and distributed throughout the CNS and PNS. The two main subunits of the GABAB receptor are GABABR1 and GABABR2. For the GABAB receptors to be active and functional, these subunits need to interact to form a stable heterodimer. Importantly, orthosteric agonists and antagonists bind to GABABR1, while PAMs bind to the GABABR2 subunit. GABAB receptors are primarily found in the cerebellum, prefrontal cortex, and thalamus, in addition to the interpeduncular nucleus and the olfactory nucleus (Bowery et al., 1987). Alcohol is known to interact with the GABABreceptors in the brain, but the exact binding site and mechanism of action are not completely understood. GABAB receptor-binding drugs have anti-convulsant and analgesic properties (Terunuma, 2018) and are also found to reduce craving and withdrawal symptoms in dependent individuals [for example, Baclofen (Logge et al., 2022)].
4. From alcohol use to alcohol use disorders – the GABAergic system
DSM-5 classifies substance-related disorders into substance-use disorders (SUD) and substance-induced disorders (intoxication, withdrawal, and other substance/medication-induced mental ailments). Clinically, SUDs occur in a range of severity based on a number of symptom criteria endorsed. Mild (2–3 symptoms), moderate (4–5), and severe (>5). The DSM-5 diagnostic criteria do not describe levels or types of alcohol use or alcohol use harms (American Psychological Association, 2013); however, for this review, we chose to include some of the most commonly used categories of this kind (e.g., binge alcohol use) for a better illustration of the AUD pathophysiology and the involvement of GABAergic system to align with clinical presentations of AUD and alcohol withdrawal. AUD encompasses various disorders characterized by different consumption patterns, impacting the brain and the GABAergic system. Alcohol consumption, including alcohol use not meeting the criteria for AUD, also impacts the GABAergic system. For example, minimal alcohol intake will enter the brain and target GABAA receptors, causing a cascade of regulatory events, potentially leading to behavioral changes. When consumption becomes chronic, or during binge drinking episodes, the impact of alcohol on the brain is even more profound, triggering activation/inhibition of other biological pathways (as described earlier in Figure 1). Table 1 below summarizes alcohol use at different levels, explains the different considerations given for men and women, and highlights the impact on the GABAergic system and symptoms related to the use of alcohol.
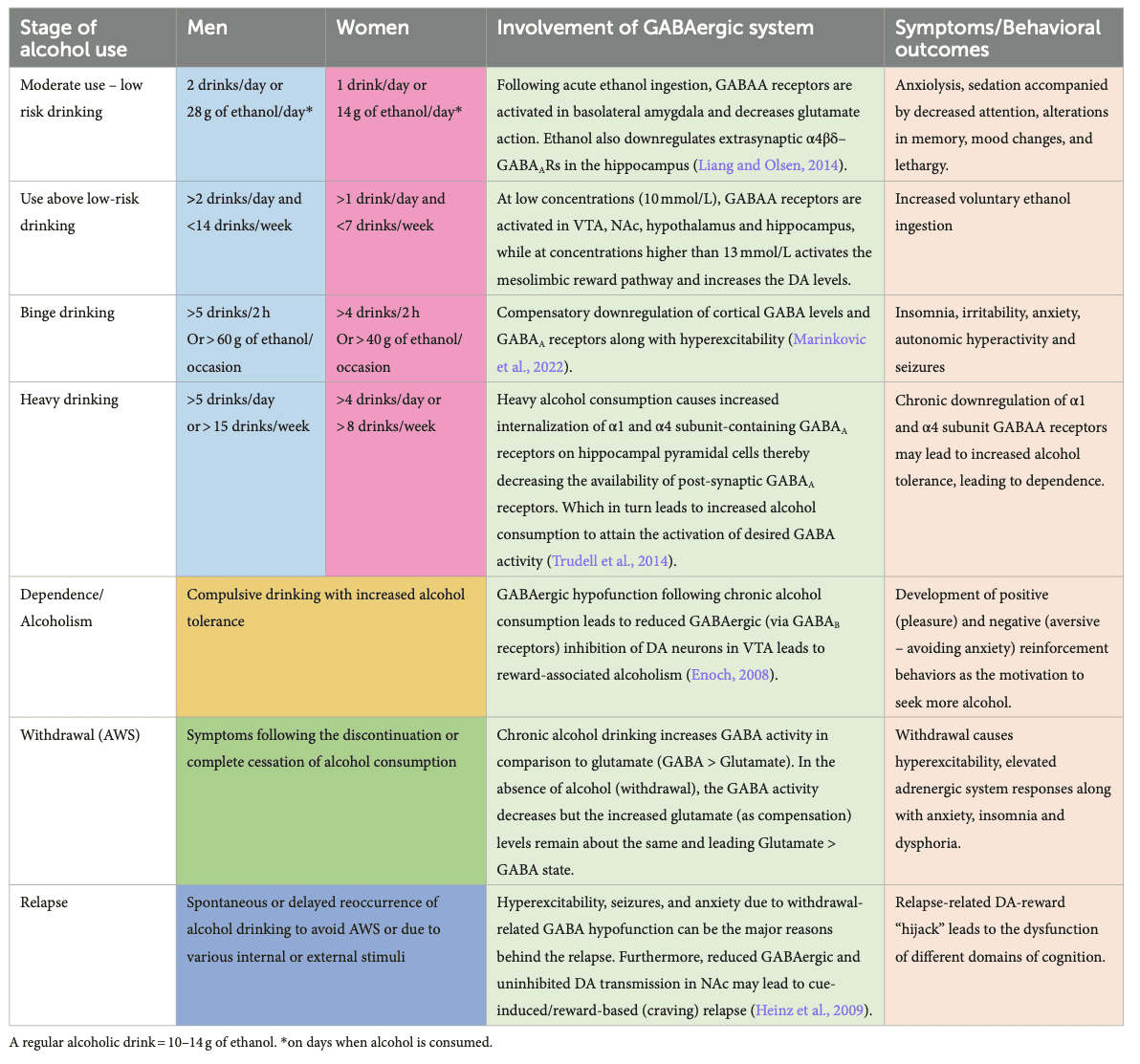
4.1. Occasional, moderate, and safe use of alcohol with low risk for AUD
The safe or moderate use of alcohol is considered with less than 2 drinks per day for men and 1 for women (0.02–0.04 g/dL blood alcohol concentration), where the risk for developing AUD remains low. Even with such use, the acute or low level of ethanol present in the system is enough to potentiate the action of GABA at GABAAreceptors, inducing relaxation. Even in rats, acute ethanol administration induces a state of anxiolysis driven by the potentiation of the GABAA receptor in the basolateral amygdala, acting on multiple cell populations (Herman and Roberto, 2016). Low levels of ethanol already play a role in the expression and trafficking of GABAAreceptors in the brain by rapidly downregulating α4β3δ-GABAA receptors in the hippocampus (Chandler et al., 2017). Expression of the α1β3γ2-GABAA receptors is also downregulated after several hours of consumption, followed by an upregulation of α4β3γ2 and α2β3γ1 after a couple of days. This demonstrates the broad and long-lasting kinetics of an acute consumption of ethanol, which is reversible, but the recovery timeline is dose-dependent (Holford, 1987).
During medium-risk drinking, i.e., drinking episodes of alcohol when the volume of alcohol is consumed in a short period but not binge drinking (not more than 5 drinks in 2 h for men, 4 in 2 h for women, and < 0.08 g/dL) (World Health Organization, 2000), ethanol levels can range from 5 to 30 mmol/L. This potentiates the GABAAreceptors in the brain, decreasing excitatory glutamatergic neurotransmission and causing slight sedation, a feeling of relief, slight alteration of short-term memory, decreased attention, and potential mood changes (Liang and Olsen, 2014). Studies in rats have demonstrated that this dose level increases GABAergic firing rate and afferent-evoked synaptic response in the VTA, a central hub for dopaminergic projections in the brain, regulating motivation, cognition, reward valuation, and addiction. This impact on the VTA, potentially driven by changes in firing rates from the GABAergic system, contributes to increased alcohol intake (Tateno and Robinson, 2011).
Interestingly, preclinical studies using rats also demonstrated that reducing α4-subunit expression via a viral-mediated RNA interfering with the α4-protein synthesis in the NAc allowed for a reduction of self-administered ethanol. Similar results were observed when pharmacologically blocking the GABAA receptors in the paraventricular nucleus of the hypothalamus, further confirming the role of GABAergic potentiation in increasing alcohol intake and seeking behaviors (Li et al., 2011).
4.2. At-risk drinking patterns
Greater than the threshold set for safe and moderate use described above, consumption of alcohol is considered at risk (NIAAA, 2018). In this case, ethanol induces GABAA receptor activation in the VTA, NAc, hypothalamus, and hippocampus, causing an overall imbalance in excitation/inhibition, leaning toward increased inhibition. At a certain point, thought to be above 13 mmol/L (Liang and Olsen, 2014), the reward pathways of the mesolimbic system are directly and indirectly activated (as described in the previous section on Impact of Ethanol on the Brain), allowing dopamine release, which fosters the development of addictive properties of alcohol consumption.
4.3. Alcohol use disorder
AUD is considered when the drinking pattern is above established standards, either due to volume or frequency of intake. One tool used worldwide to identify AUD is the Alcohol Use Disorder Identification Tool (AUDIT), developed by WHO (Higgins-Biddle and Babor, 2018). While the classification of AUD has changed over the years and is country-dependent, most medical and addiction professionals frequently break AUD into two categories: binge and heavy drinking (Kranzler and Soyka, 2018).
Binge drinking is the acute consumption of large amounts of alcohol (for example, five or more drinks in less than 2 h for men and four or more for women, leading to >0.08 g/dL of blood alcohol concentration). Binge drinking leads to cognitive deficits, reduced inhibition, and reduced ability to control alcohol intake voluntarily, thereby increasing the chances of developing more frequent AUD in the future (Chmielewski et al., 2020). Risk factors for binge drinking include age, male sex, alcohol consumption at a young age, a patient’s state of mental health, and genetic susceptibility (NIAAA, 2020).
Preclinical studies of psychological changes and alcohol consumption have determined that in young rats (postnatal days 28–42), binge drinking induces a state of anxiety-like behavior and leads to alcohol dependence in adulthood (Pandey et al., 2015). Stress and withdrawal-induced anxiety are correlated to increased voluntary ethanol drinking in alcohol-preferring rats (Meyer et al., 2013), and chronic psychosocial stressed male mice showed increased voluntary ethanol drinking (Bahi, 2013). Human magnetic resonance spectroscopy studies have shown that cortical GABA levels are reduced in young adult binge drinkers (Marinkovic et al., 2022). Following acute high-dose ethanol administration in rats, thalamic α4-GABAAreceptor levels were regulated temporally, as a decrease was observed at 2 h followed by a delayed transient increase (Werner et al., 2016). Other studies using a transgenic dopaminergic D3 receptor knockout mouse model combined with an α6-GABAA receptor ligand (RO 15–4,513) also showed that increased GABAergic inhibition in the NAc contributes to reducing binge drinking, confirming the critical role of GABAergic neurotransmission in reducing alcohol intake (Leggio et al., 2019).
Heavy drinking is defined as drinking more than recommended during a week, leading to 0.1–0.2 g/dL of blood alcohol concentration, depending on the number of drinks. For a man, having more than 15 standard alcoholic drinks weekly is considered heavy drinking. For women, having more than 8 drinks a week meets the criteria for heavy drinking (NIAAA, 2018). Heavy drinking leads to increased neuronal atrophy and reduces white matter fiber integrity (Daviet et al., 2022), associated with increased risk for dependence, anxiety, depression, cognitive deficits, altered control over drinking habits, cardiovascular diseases, and other health risks.
Studies have shown that the behavioral changes are primarily due to the plastic changes of GABAA receptors that occur after chronic ethanol exposure, which include significantly reduced post-synaptic α1 and increased α4-containing GABAA receptors. The subunit composition of GABAA receptor subtypes is expected to determine their physiological properties and pharmacological profiles. An in-depth study of GABAA-subunits using genetically engineered mice has shown that the α1 subunit involves sedation, anti-convulsant activity, anterograde amnesia functions, etc., while the α4subunit is involved in changes in mood and anxiety. Thus, these GABAA receptor subunit composition changes are a mechanism underlying the behavioral changes after chronic ethanol exposure, which leads to additional risks of developing dependence. Heavy drinking triggered by chronic stress and any induced anxiety is an additional risk factor for developing alcohol dependence, observed in animal models and humans (McCaul et al., 2017). Conversely, stopping or reducing alcohol consumption, in turn, aggravates stress or anxiety due to an overall imbalance in brain homeostasis (Schmidt et al., 2016).
4.4. Chronic/daily alcohol use leading to dependence
With chronic alcohol consumption comes an increased risk for reward-associated habitual alcohol abuse, pronounced craving behavior for alcohol, and inability to stop seeking alcohol. This is usually highly linked to the development of dependence, a severe form of AUD that occurs when a person develops tolerance to the effect of alcohol and, therefore, seeks further alcohol consumption to prevent experiencing withdrawal symptoms. Alcohol dependence is a serious condition that requires comprehensive treatment to address the physical, emotional, and behavioral aspects of AUD.
Postmortem studies found a loss of GABAergic markers in the human brains of adults with alcohol dependence, particularly in men (Behar et al., 1999; Dodd et al., 2006). Transcranial magnetic stimulation (TMS) studies also demonstrated that chronic alcohol dependence has some level of impact on GABAA and GABAB receptor function, which seems to vary from study to study (Mohammadi et al., 2006; Ziemann et al., 2015). Several studies found no effects on short-interval cortical inhibition or TMS-evoked N45 potential (Conte et al., 2008; Nardone et al., 2010; Mon et al., 2012; Naim-Feil et al., 2016), thought to index GABAA receptor function. However, other studies found a general decrease in GABA levels (Prisciandaro et al., 2019; Shyu et al., 2022), including in youth with alcohol dependence (Kaarre et al., 2018). Given the dynamic nature of alcohol’s effects on GABA, the GABA levels depend on several states (e.g., recently detoxified or more prolonged abstinence) and traits (e.g., age). One report on long-interval cortical inhibition thought to index GABAB showed decreases in alcohol-dependent patients (Naim-Feil et al., 2016).
Multiple preclinical studies demonstrated that chronic ethanol consumption alters GABAA receptor plasticity, leading to ethanol dependence (Olsen and Liang, 2017). Other preclinical studies established that general GABAA receptor expression and function changes in cases of alcohol dependence, both synaptically and extra-synaptically, in brain regions highly involved in establishing dependence and symptom emergence (i.e., the cortex, hippocampus, and central amygdala). This translates into a general loss of phasic and tonic GABAergic inhibition, tolerance to ethanol, and cross-tolerance to benzodiazepines and other sedative-hypnotics acting on GABA receptors (Kumar et al., 2009; Olsen and Liang, 2017; Bohnsack et al., 2018).
With such alteration in overall GABAergic functioning, a drastic imbalance in excitation/inhibition develops across multiple brain regions [medial prefrontal cortex (Pleil et al., 2015)], amygdala circuit (Herman et al., 2016; Herman and Roberto, 2016; Hughes et al., 2019), intrahippocampal circuits (Liang et al., 2004), and VTA circuits (Arora et al., 2013) causing a decrease in inhibitory control in multiple neurotransmitter firing activity, leading to the emergence of various behavioral changes including cognitive deficits, seeking behavior, humor changes, and others (Morrow et al., 2020).
Chronic alcohol consumption in heavy drinking, dependence, and associated GABAAplasticity changes also lead to DA release changes in the reward neurocircuitry. During acute alcohol withdrawal, changes occur, such as upregulation of α4-containing GABAA receptors and downregulation of α1- and α3-containing GABAAreceptors (Liang and Olsen, 2014). GABAA receptor downregulation may contribute to anxiety and seizures of withdrawal. During withdrawal periods, rats show a significant decrease in DA and serotonin levels in the reward neurocircuitry commonly associated with dysphoria, depression, and anxiety disorders. These psychological changes may also contribute to ethanol-seeking behavior, again demonstrating the complexity of changes induced by chronic alcohol consumption.
5. Existing interventions
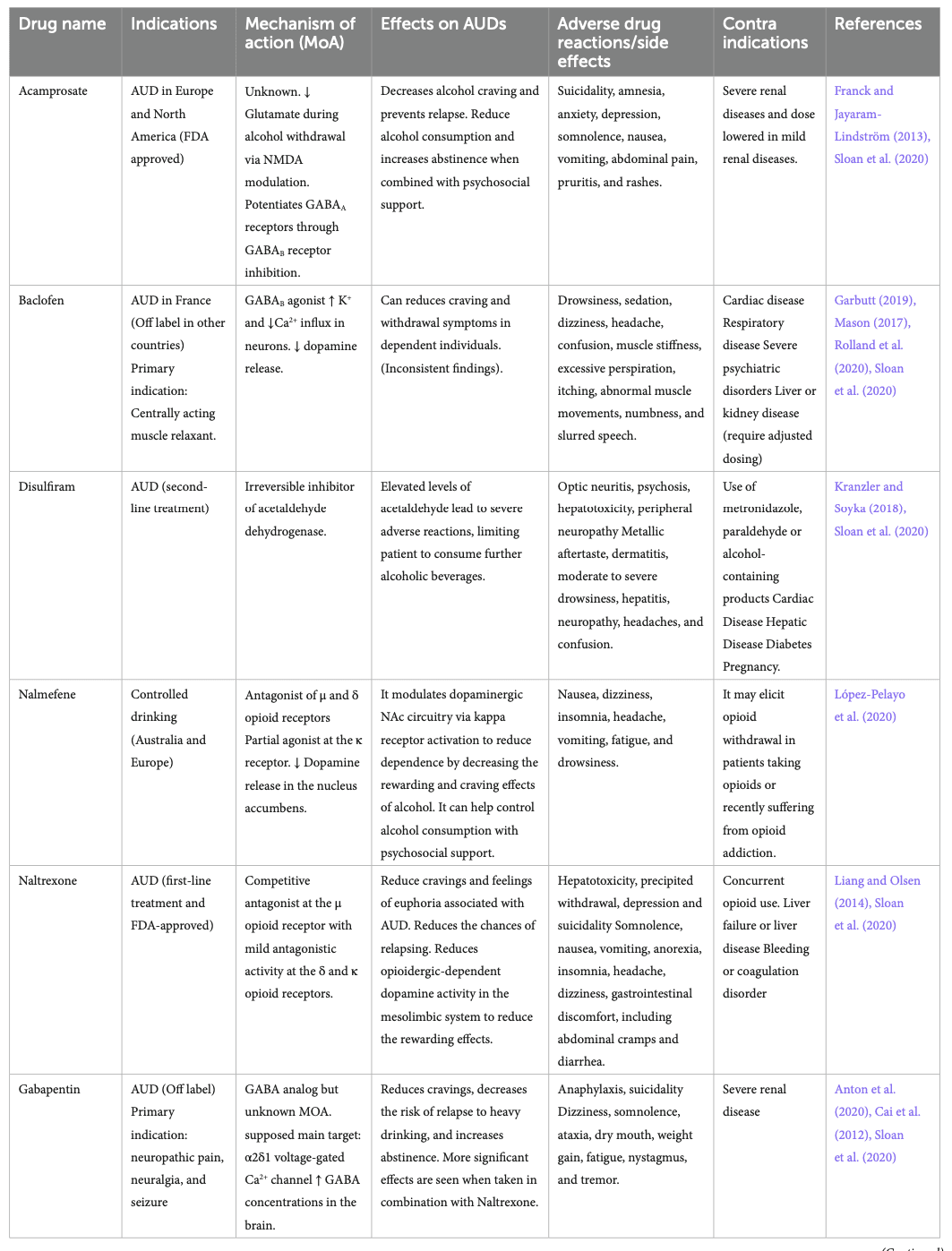
Existing therapeutic interventions for AUD and alcohol withdrawal have attempted to harness the various CNS systems on which alcohol acts to limit the harms associated with alcohol consumption. The existing therapeutic interventions have diverse efficacy levels, various side effects, and contraindications (Table 2). Several clinical trials have shown the efficacy of certain pharmacotherapies that are approved by regulatory agencies for treating AUD or withdrawal and that are used off-label (Carpenter et al., 2018; Sloan et al., 2020).
5.1. Non-GABAergic pharmacologic interventions
Disulfiram has been an FDA-approved drug used to treat AUD since 1951. It inhibits the acetaldehyde dehydrogenase enzyme involved in ethanol metabolism, leading to higher plasma concentrations of acetaldehyde, which induces unpleasant side effects if a patient consumes alcohol while taking this medication, preventing further drinking. Disulfiram-induced reactions can include hepatotoxicity and death, which is why disulfiram needs to be used with caution (Kranzler and Soyka, 2018; Stokes and Abdijadid, 2022). Nowadays, the most used pharmacotherapy is naltrexone (commercialized under the brand name Revia®), a competitive μ opioid receptor antagonist and a partial antagonist of the δ and κ opioid receptors (Liang and Olsen, 2014; Sloan et al., 2020; Singh and Saadabadi, 2023). It decreases craving by reducing the rewarding and euphoric effects of alcohol and is one of the few AUD pharmacotherapies approved by the FDA. It is generally well tolerated but has minor side effects (Singh and Saadabadi, 2023).
Acamprosate is an FDA-approved drug used in Europe and North America for alcohol craving and relapse prevention (Franck and Jayaram-Lindström, 2013; Kalk and Lingford-Hughes, 2014). Although its exact mechanisms are unknown, it decreases glutamate during alcohol withdrawal through NMDA receptor modulation and indirectly potentiates GABAA receptors. Acamprosate is generally well tolerated (Kalk and Lingford-Hughes, 2014).
Nalmefene is another antagonist of the μ and δ opioid receptors but is a partial agonist at the κ receptor. It is currently approved for AUD indication in Australia and Europe. Nalmefene decreases dopamine release in the NAc and reduces alcohol dependence and consumption by decreasing the rewarding and craving effects of alcohol (Paille and Martini, 2014). It can help control alcohol intake and has shown better results in those benefiting from psychosocial support. It has mild side effects, which generally disappear with time (Paille and Martini, 2014; López-Pelayo et al., 2020).
5.2. GABAergic pharmacologic interventions
Baclofen is only approved for the treatment of alcohol withdrawal in France (Garbutt, 2019). Despite multiple trials supporting its efficacy in reducing the risk of relapse and increasing abstinence days (Agabio et al., 2023), its efficacy remains controversial, and systematic reviews consider the evidence of its efficacy insufficient (Jonas et al., 2014). It acts as an agonist at the GABAB receptor and decreases dopamine release in the mesolimbic system, which reduces craving and withdrawal symptoms in dependent individuals. Baclofen has multiple side effects, limiting its use (Romito et al., 2021).
Gabapentin is a GABA analog used as an anti-epileptic medication for over 30 years. Clinical trials have shown dose-dependent efficacy in reducing craving, reducing anxiety, and facilitating abstinence (Anton et al., 2020). However, some studies also raise concerns due to its sedating properties and documentation of extra-medical use of this medication (Modesto-Lowe et al., 2019; Weresch et al., 2021). It was also found that Gabapentin causes respiratory depression when used alone and increases the risk of opioid-related deaths when combined with opioids (Gomes et al., 2017). Despite being a GABA analog, its mechanism of action is still unclear and seems unrelated to GABAergic modulation. Its main target seems to be the α2δ-subunit of the voltage-gated calcium channel. It also increases GABA concentrations in the brain (Cai et al., 2012).
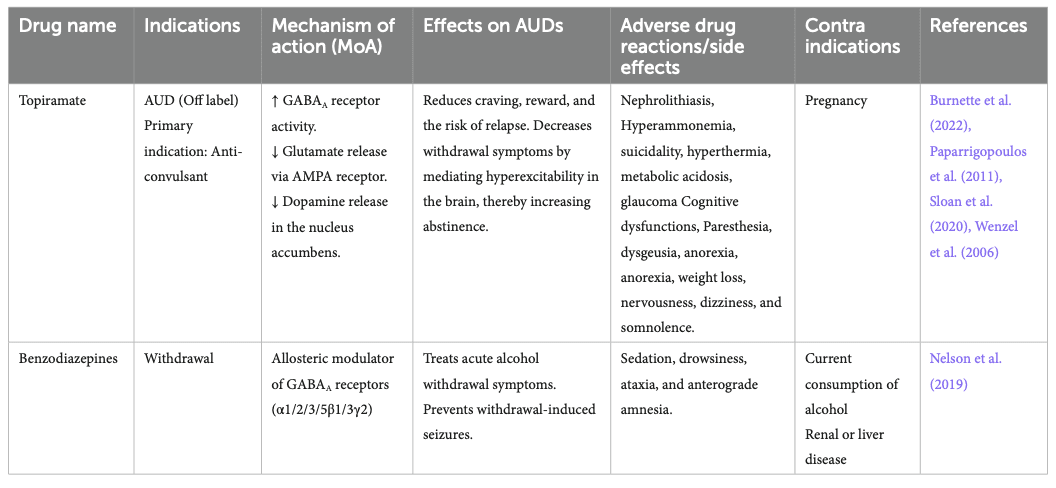
Topiramate is not yet approved by the FDA for the treatment of AUD. Still, clinical trials have demonstrated reductions in craving and risk of relapse and increasing abstinence (Kranzler et al., 2014; Manhapra et al., 2019; Wetherill et al., 2021). It is an approved anti-convulsant for treating epilepsy and seems to act through GABAAreceptor modulation (Fariba and Saadabadi, 2022). It also binds the AMPA receptor to decrease glutamate release and decreases dopamine release in the NAc. It has some side effects, including paresthesia, dysgeusia, anorexia, and cognitive impacts such as slowing mental and physical activity and trouble concentrating or attention (Wenzel et al., 2006).
Benzodiazepines (BZ) are allosteric modulators of the GABAA receptor that bind to the α1, 2, 3, 5, and γ subunits. They enhance the activity of GABA when binding at its receptor and are recommended in managing acute alcohol withdrawal (Nelson et al., 2019), but not for the treatment of AUD itself. They can lead to sedation, ataxia, anterograde amnesia, and have abuse potential (Engin, 2022). Alcohol delays the metabolism of BZ (Hoyumpa, 1984), prolonging its bioavailability, causing psychomotor impairment, and increasing the risk of overdosing of BZ. Studies showed that BZ also modulates part of ethanol’s reinforcing and/or aversive properties. BZ and ethanol co-consumption is also known to amplify the effect of alcohol.
5.3. Psychotherapeutic interventions
In contrast to pharmacological interventions, Cognitive Behavioral Therapy (CBT) is a form of psychotherapy that involves challenging automatic thoughts, cognitive distortions, existing beliefs, and problematic behaviors (Chand et al., 2023). It is one of the most studied forms of treatment for SUD and has the most support from evidence-based studies. Adults with problematic drinking who received CBT showed decreased alcohol consumption, and newer variants of CBT, such as virtual reality-assisted CBT (Thaysen-Petersen et al., 2023), appear to be more successful than traditional methods (Carroll and Kiluk, 2017).
Motivational Enhancement Therapy (MET) is another psychosocial treatment that applies principles from motivational psychology. MET is often the foundation of brief interventions for risky alcohol use, and indeed, protocols can be very short, requiring only a few sessions of client-centered interventions (Ceci et al., 2022). MET focuses on identifying a reason for a change in alcohol consumption, but outcomes vary substantially with commitment and readiness to change to have an impact (Hodgins et al., 2009).
However, existing therapeutic options have shown limitations. Some drugs, repurposed from other indications, show direct or indirect activity in the GABAergic system (Gabapentin, topiramate, and baclofen). The GABAergic system is a key player in the pathophysiology of AUD and alcohol withdrawal and is a desirable target for drug development (Liang and Olsen, 2014; Mirijello et al., 2015). Indeed, the previous sections showed how intertwined central pathways are in the context of ethanol consumption and how instrumental the GABAergic system is in modulating most of the effects, directly or indirectly. However, AUD is broad and can vary in expression in multiple ways (volume consumed, acute or chronic consumption, etc.). Therefore, the impact of ethanol on the GABAergic system may vary depending on the manifestation of AUD, and different interventions acting on different aspects of the GABAergic system may be required to elicit optimal outcomes in treating AUD or alcohol withdrawal. The following sections will present novel GABAergic interventions currently being investigated.
6. GABAergic interventions in preclinical models and their impact on alcohol-related symptoms: reconciling risk and benefits
6.1. GABAA: involvement in AUD and therapeutic potential
Since ethanol facilitates the activity of GABA and has such a large effect on GABAergic receptor expression and function, it can be difficult to anticipate what impact a GABAergic drug would have on individuals with AUDs. Benzodiazepines (BZ), binding at the interface between α1-2-3-5 and γ subunits of the GABAA receptors, are known enhancers of phasic GABAergic inhibition across brain regions and induce internalization of synaptic GABAA receptors (Gallager et al., 1984; Tehrani and Barnes, 1997). Therefore, BZs promote the mechanisms leading to some ethanol-induced deficits in GABAergic inhibition. However, BZs have beneficial effects in the context of acute withdrawal symptoms as they act as a substitute for ethanol and can help individuals in withdrawal re-establish a new excitation/inhibition balance without alcohol (refer to Figure 1B).
In recent years, BZ-derivatives acting preferentially at selected α-subunits were developed and tested in preclinical models for their activity on ethanol self-administration and craving behaviors (Table 3). Activation of α2/α3-GABAA receptors by the HZ-166, XHe-II-053, YT-III-31, or YT-III-271 PAMs in ethanol discrimination studies augmented the reinforcing effects of ethanol via increasing the self-administration in rhesus monkeys (Berro et al., 2019). These findings are aligned with clinical evidence that demonstrated a positive association of both the GABRA2 and GABRA3 gene expression with an increased risk for developing alcoholism (Covault et al., 2004; Enoch, 2008; Soyka et al., 2008; Mallard et al., 2018). Similarly, potentiation of the α5-GABAA receptor via QH-ii-066 administration was also shown to enhance the reinforcing effects of alcohol in non-human primates, while using an inverse agonist at the α5-GABAA receptor (Xli-093) inhibited such reinforcement effects (Rüedi-Bettschen et al., 2013). Consistently, intra-hippocampal infusions of an α5-GABAA receptor inverse agonist RY023 reduced ethanol-maintained responses in a dose-dependent manner, suggesting that the α5-GABAAreceptors in the hippocampus play an important role in regulating ethanol-seeking behaviors (June et al., 2001). This was further supported by studies using the partial α5-GABAA receptor inverse agonist Ro 15–4,513, by the selective α5−GABAA inverse agonist (α5IA-II) (Stephens et al., 2005) and by the use of the α5-GABAA receptor knockout mice model showing reduced ethanol preference (Boehm II et al., 2004; Stephens et al., 2005).
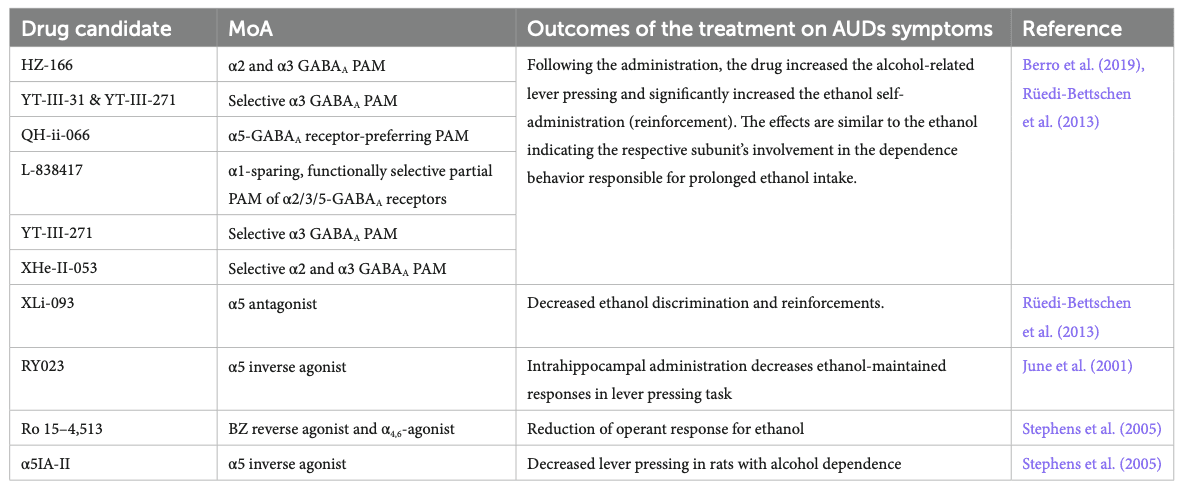
However, the studies mentioned above all evaluated the impact of positive modulation of the αx-GABAA receptor in the context of alcohol consumption or alcohol discrimination when the system is already sensitized to further GABAergic activity (Figure 1B – central panel). However, it remains unclear how such modulation would play in the context of withdrawal when the system is deficient in GABAergic regulation, which is, in turn, causing craving behaviors. Knowing the anti-craving effect of BZ (Nelson et al., 2019), one could expect that the α2-, α3- or α5-PAMs can contribute to the anti-craving effect of BZ in a brain system during a withdrawal state and could further elicit beneficial effects without the side effects observed with benzodiazepines.
While BZ and derivatives bind and act at the interface between α1-2-3-5 and γ subunits, neurosteroids bind between α and β subunits of the GABAA receptors. Furthermore, such binding is greatly facilitated by the presence of the δ subunit in the pentamer (Gatta et al., 2022; Figure 2B). Neurosteroids are potent and effective neuromodulators synthesized from cholesterol in glial and neuronal cells of the central (CNS) and peripheral nervous systems (PNS). They act at extrasynaptic receptors, facilitating tonic inhibition (Chen et al., 2019; Belelli et al., 2022). With acute alcohol intake, the cerebral levels of allopregnanolone were found to be increased, whereas its levels were reduced during chronic alcohol consumption and withdrawal (Romeo et al., 1996). In addition, stimulation of neurosteroidogenesis by metyrapone was found to reduce cocaine intake in rats (Goeders and Guerin, 2008), and one could suppose a similar effect for alcohol intake.
Recent studies found that allopregnanolone has antidepressant properties for women with postpartum depression (Pinna et al., 2022), a disorder with reduced GABAergic function (Prevot and Sibille, 2021). Therefore, with their action of the GABAergic system, and their involvement in arousal, cognition, emotion, and motivation, neurosteroids may hold therapeutic potential in treating AUD (Zorumski et al., 2013; Gatta et al., 2022), and such effects are being investigated (Morrow et al., 2020; Mounier et al., 2021).
6.2. GABAB: involvement in AUD and therapeutic potential
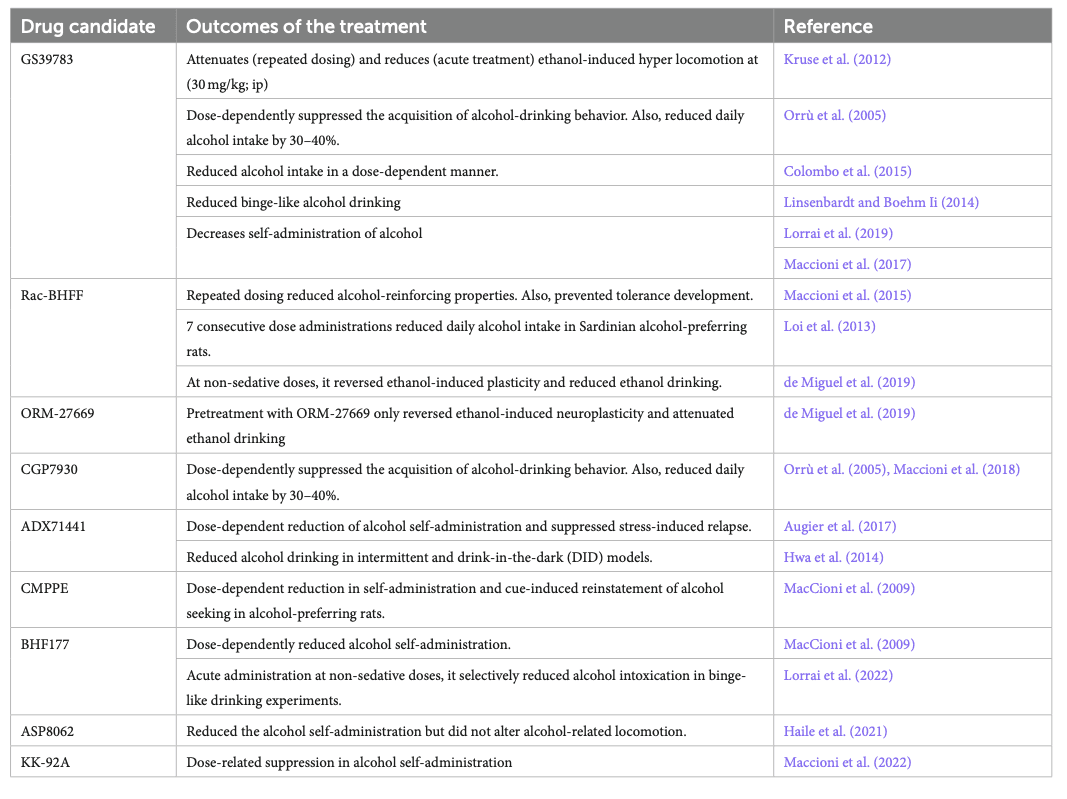
The involvement of GABAB receptors in the development of AUD is still unclear. However, studies in clinical populations (using Baclofen) and animals [experimental candidates listed in Table 4 (Maccioni and Colombo, 2019)] showed that GABABreceptor modulation was beneficial in AUD management. For instance, rats receiving baclofen showed reduced hyper-locomotion caused by acute alcohol administration (Besheer et al., 2004), and reduced anxiety-like behavior and tremors following chronic alcohol withdrawal (Knapp et al., 2007). Table 4 includes a list of GABABPAMs such as CGP7930, GS39783, BHF177, Rac-BHFF, ADX71441, CMPPE, COR659, and ORM-27669 that were primarily studied in rodent models and were found to be beneficial in AUDs.
7. Novel therapeutic agents targeting the GABAergic system in clinical trials
7.1. Pharmacological interventions
With the increased characterization of the impact of alcohol on the GABAergic system and the increasing characterization of the link between GABAergic functions, receptor subtype, and symptom relief in the context of AUD, more clinical trials are being initiated to investigate how GABAergic modulation can contribute to better treatment of AUDs and alcohol withdrawal (Table 5). Interventions acting on GABAA receptors are investigated in multiple clinical trials. For example, DZ is already the standard of care for reducing withdrawal symptoms. Midazolam, another benzodiazepine, and propofol, a GABAA receptor agonist, were withdrawn from Phase 4 studies in 2016 due to logistical reasons. They were studied for their potential effect on stress response and immune functions in mechanically ventilated patients with AUDs.
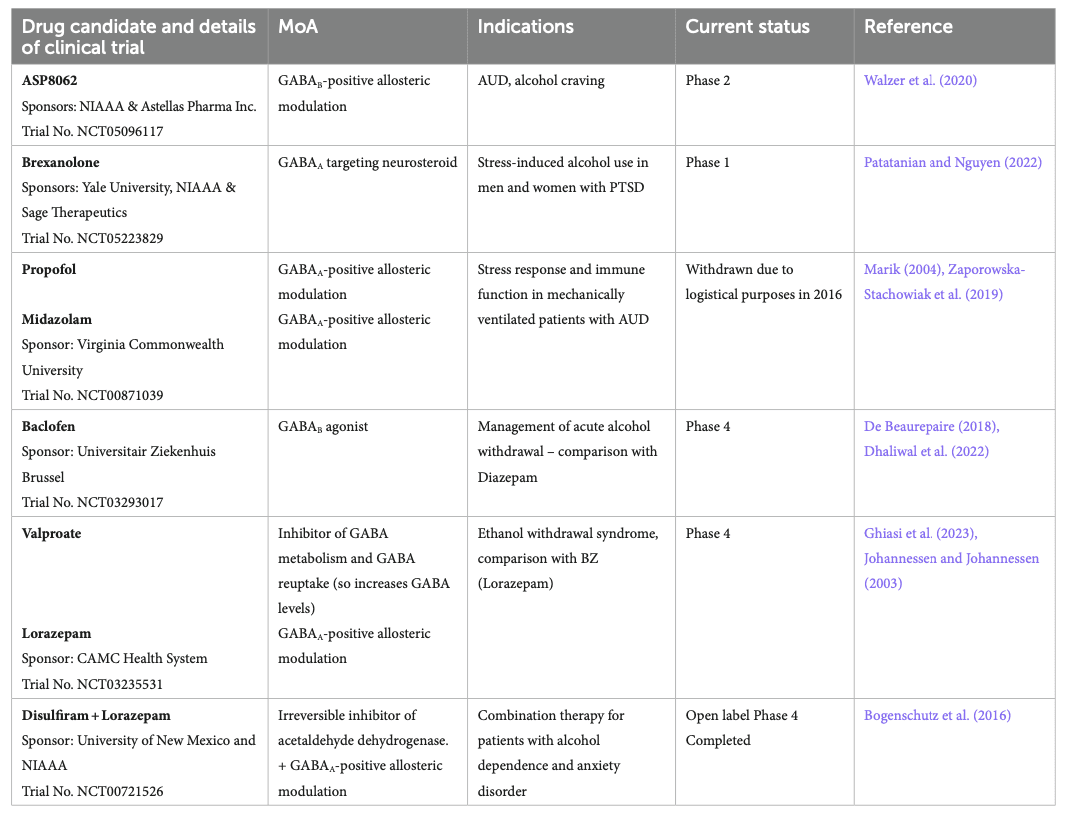
Brexanolone, a GABAA-targeting neurosteroid, is about to start recruiting for a Phase 1 study to demonstrate safety before assessing efficacy in participants with AUD and PTSD. Brexanolone is already approved for treating postpartum depression (Morrow et al., 2020).
Baclofen, a GABAB agonist, is currently under Phase 4 to assess its efficacy in managing acute alcohol withdrawal. As mentioned in Table 1, baclofen is already approved in France for reducing craving and withdrawal syndrome, but some literature suggests its efficacy for this indication is limited (Cooney et al., 2019). ASP8062, a GABAB-PAM, is currently investigating the efficacy of 2 weeks of treatment in a Phase 2 study in participants with moderate AUD at reducing alcohol cravings. Preclinical studies in rats showed promising effects in reducing alcohol self-administration without side effects observed with baclofen (Haile et al., 2021), and phase 1 studies in humans confirmed the safety of ASP8062 (Ito et al., 2022).
The antiepileptic valproate also acts indirectly on the GABAergic system by blocking the metabolism of GABA and by blocking GABA reuptake, increasing GABA levels in the brain (Janmohamed et al., 2020). Clinical trials are ongoing to determine the efficacy of valproate treatment at reducing ethanol withdrawal, compared to benzodiazepines, here lorazepam. Lorazepam was also used in another open-label clinical trial completed in 2013 to assess the efficacy of a combination with disulfiram. Reports showed a significant reduction in anxiety, depression, and craving; such effects were observed 24 weeks after intervention (Bogenschutz et al., 2016).
7.2. Non-pharmacological interventions – rTMS
Repetitive transcranial magnetic stimulation, i.e., rTMS, is a noninvasive neurostimulation modality delivering focused magnetic field pulses to the cortex that modulate cortical activity. Treatment sessions are generally delivered daily over several weeks, which results in the induction of long-term changes in cortical excitability through neuroplasticity. This includes modulation of the implicated neurocircuits underlying alcohol use disorder and is under investigation as a potential treatment. Enduring changes in cortical activity (namely inhibition and excitation) resulting from rTMS have implications for enduring changes in GABA activity (Daskalakis et al., 2006). Over a decade ago, the first published clinical trial demonstrated efficacy in reducing cravings in adults with AUD over a sham-control condition (Mishra et al., 2010). Since then, the majority of trials have delivered rTMS over the left or right dorsolateral prefrontal cortex, with a recent meta-analysis showing a signal for reduced alcohol craving with rTMS treatment (Sorkhou et al., 2022), potentially driven by the impact of rTMS on GABAergic signaling. However, most RCTs have been small single-center trials, and given the substantial heterogeneity in parameters utilized across studies, the optimal protocol has not yet been determined.
Additionally, there is growing interest in using deep rTMS™ using coils (H Coils) that can induce a broader electrical field within the cortex. For example, a recent RCT using rTMS with an H7 Coil stimulating the bilateral medial prefrontal cortex and anterior cingulate cortex showed positive results in reducing craving and alcohol consumption in treatment-seeking patients with AUD (Harel et al., 2022). Moreover, another trial that utilized a coil that stimulates the bilateral lateral PFC and insula showed efficacy for nicotine dependence in a large, definitive, multi-site RCT that subsequently paved the way for FDA clearance for this indication (Zangen et al., 2021), demonstrating the first time FDA cleared indication for any substance use disorder. Taken together, further exploration of the therapeutic potential of rTMS for AUD is warranted. Given the well-described link between GABA dysfunction in AUD and rTMS effects on the GABAergic system, it will be important to explore whether biomarkers of GABAergic functions can serve as mediators or moderators of rTMS efficacy.
8. Conclusion
Alcohol use-related disorders are significant risk factors for other high mortality-causing diseases. Although the mechanisms are elusive, the GABAergic system’s involvement seems critical in AUD development. Currently, GABAergic drugs are used in the second or third line of treatment of AUD and mitigation of alcohol withdrawal. Studies indicate that pharmacological modulation of GABA receptors may be a promising therapeutic option in achieving long-term abstinence by decreasing the daily alcohol intake and withdrawal effects. However, extensive research is needed in this line to uncover the pharmacological potential of the GABAergic system in managing alcohol use-related disorders.