Abstract
Adolescent risk-taking is a public health issue that increases the odds of poor lifetime outcomes. One factor thought to influence adolescents' propensity for risk-taking is an enhanced sensitivity to appetitive cues, relative to an immature capacity to exert sufficient cognitive control. We tested this hypothesis by characterizing interactions among ventral striatal, dorsal striatal and prefrontal cortical regions with varying appetitive load using functional magnetic resonance imaging (fMRI) scanning. Child, teen, and adult participants performed a go nogo task with appetitive (happy faces) and neutral cues (calm faces). Impulse control to neutral cues showed linear improvement with age, whereas teens showed a nonlinear reduction in impulse control to appetitive cues. This performance decrement in teens was paralleled by enhanced activity in the ventral striatum. Prefrontal cortical recruitment correlated with overall accuracy and showed a linear response with age for nogo versus go trials. Connectivity analyses identified a ventral frontostriatal circuit including the inferior frontal gyrus and dorsal striatum during nogo versus go trials. Examining recruitment developmentally showed that teens had greater between-subjects ventral-dorsal striatal coactivation relative to children and adults for happy nogo versus go trials. These findings implicate exaggerated ventral striatal representation of appetitive cues in adolescents relative to an intermediary cognitive control response. Connectivity and coactivity data suggest these systems communicate at the level of the dorsal striatum differentially across development. Biased responding in this system is one possible mechanism underlying heightened risk-taking during adolescence.
Adolescent behavior is qualitatively different from that seen in children and adults in numerous ways. These differences are particularly evident when considering US health statistics on the prevalence and causes of mortality in teenagers and the heightened risk-taking behavior related to these outcomes. Epidemiological studies report enhanced risk-taking behavior during the adolescent years, as evidenced by substantial influx in drug and alcohol experimentation, accidental death, and unprotected sex (Eaton, et al., 2008). A better understanding of the cognitive and biological mechanisms that underlie this behavioral shift may improve targeted interventions aimed to prevent these risky behaviors.
We have developed a theoretical framework characterizing aspects of neurobiological maturation that may bias adolescent behavior toward the approach of expected rewards (Casey, Getz, & Galvan, 2008; Casey, Jones, & Hare, 2008; Somerville & Casey, 2010). This model, consistent with others (Ernst, Pine, & Hardin, 2006; Steinberg, 2008) and grounded in empirical work in the animal and human, proposes that interactions between brain circuitry representing motivational load and cognitive control vary dynamically across development, with adolescence characterized by an imbalance between the relative influence of motivational and control systems on behavior. Specifically, dopamine-rich brain regions representing the appetitive value of potential rewards such as the ventral striatum (Carlezon & Wise, 1996; Pontieri, Tanda, Orzi, & DiChiara, 1996; Wise, 2004; Galvan, et al., 2005; Haber & Knutson, 2009; Spicer, et al., 2007) show strong signaling during adolescence which may be indicative of earlier maturation (Galvan, et al., 2006; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Van Leijenhorst, et al., 2009). In contrast, brain circuitry important for integrating motivational and cognitive control processes including ventrolateral frontostriatal networks (Balleine, Delgado, & Hikosaka, 2007; Delgado, Stenger, & Fiez, 2004; Rubia, et al., 2006) remain less structurally and functionally mature during the adolescent years (Giedd, et al., 1999; Luna, et al., 2001). When these systems interact, signaling of the ventral striatum with less downregulation by control systems exerts a stronger influence on subsequent behavior, effectively signaling enhanced approach motivation left unchecked by control systems.
Though recent neurobiological research has largely supported this conceptualization, the majority of evidence informing these theoretical models has separately targeted either reward processing or cognitive control systems. A notable exception is recent work demonstrating how incentive can upregulate cognitive control abilities (Geier, et al., 2010; Hardin, et al., 2009), in which participants were rewarded for correctly suppressing an otherwise neutral behavior. Here we address the capacity of adolescents to regulate the approach to appetitive cues themselves, by requiring participants to withhold a prepotent response toward faces that are neutral or positive. This design is arguably a relevant experimental model with which to inform adolescents' reduced ability to resist temptation in everyday life.
In the present study, we utilized a go nogo paradigm (e.g., Durston, Davidson, et al., 2003; Hare, Tottenham, Davidson, Glover, & Casey, 2005) with happy faces representing appetitive cues and nonthreatening calm faces representing a control condition of lower appetitive value. The assertion that happy faces represent an appetitive stimulus is based on data showing that response latencies to approach happy stimuli (via button press) are speeded relative to less emotional calm expressions (Hare et al., 2005, see Results). This paradigm contains trials in which the participant is instructed to respond to a stimulus and others in which the participant should suppress this response. Child, teen, and adult participants from a sample partially overlapping with a prior report (Hare et al., 2008) completed the task during functional magnetic resonance imaging (fMRI) scanning. Behavioral responses to each stimulus type were identified and fMRI analyses focused on circuitry previously implicated in cognitive control across development (frontostriatal circuitry) and areas of the brain sensitive to reward (ventral striatum). Specifically, we focused on how interactions between these systems predicted cognitive control failures to salient, appetitive cues across a broad range of ages, including during the transition into and out of adolescence.
Methods
Participants
Eighty-three participants between the ages of 6 and 29 were scanned for this experiment. Data from 7 participants were excluded for insufficient correct trials to analyze in one or more conditions (not completing all runs of the experiment, poor overall accuracy, and/or lack of responding). Data from 12 participants were excluded based on excessive head motion (as defined by >2mm translational or 2 degrees rotational motion within a run). Two additional participants were excluded due to technical problems, leaving a total of 62 usable subjects (30 female) in all reported analyses. Portions of the data acquired in this task have been published in a separate report (Hare et al., 2008) focused on an experimental condition not reported on here (see Experimental Task). Relative to the Hare et al. (2008) sample, the present sample consists of n=57 of the same participants and also includes n=5 additional child participants.
For demographic information about the developmental sample, see Table 1. Participants reported no neurological or psychiatric illnesses and no use of psychotropic medications in a brief screening module assessing scanning risks, self-reported health problems, medication usage, and past diagnoses and treatment of psychiatric illnesses. Before participation, all subjects provided informed written consent (parental consent and subject assent for children and adolescents) approved by the Institutional Review Board of Weill Cornell Medical College.
Table 1
Age and gender demographics by age group.
N | Age Range | Mean Age (SD) | Percent female | |
Children | 18 | 6–12 | 9.5 (1.64) | 50 |
Teens | 19 | 13–17 | 15.9 (1.4) | 42 |
Adults | 25 | 18–29 | 23.7 (3.18) | 56 |
Experimental Task
Participants completed a go-nogo task (Hare, et al., 2005; Hare, et al., 2008) with fearful, happy, and calm facial expressions serving as stimuli. The current report focuses on the happy and calm conditions and omits the fear condition from group analyses, which was the focus of a prior report (Hare et al., 2008). Within a single fMRI run, two expression types were presented, one as a `go' (i.e., target) stimulus to which participants were instructed to press a button, and the other expression serving as a `nogo' (i.e., nontarget) stimulus for which participants should withhold a button press. All combinations of expressions were used as both targets and nontargets resulting in a 2 (response: go, nogo) by 3 (emotion: fear, calm, happy) factorial design. Prior to the onset of each run, a screen appeared indicating which expression served as the target stimulus, instructing participants to respond to that expression and no other expression. Participants were also instructed to respond as fast as possible but to try to avoid making errors.
Stimuli and apparatus
Stimuli consisted of happy, fearful and calm faces of unique identities from the NimStim set of facial expressions (Tottenham, et al., 2009). Calm faces (mildly pleasant versions of neutral faces) were used because prior work has indicated that neutral faces can be construed as negative in developmental populations (Gross & Ballif, 1991; Herba & Phillips, 2004; Thomas, et al., 2001). The task was presented using EPrime software, viewable by subjects on an overhead liquid crystal display (LCD) panel integrated with the IFIS-SA system (fMRI Devices Corporation, Waukesha, WI). EPrime software, integrated with the IFIS system, logged button responses and reaction times.
Task parameters
Data were acquired in six functional runs representing each combination of emotion (happy, calm, fear) and response (go, nogo; Figure 1) using a rapid event-related design. For each trial, a face appeared for 500 milliseconds followed by a jittered intertrial interval ranging from 2 to 14.5 seconds in duration (mean 5.2 seconds) during which participants rested while viewing a fixation crosshair. A total of 48 trials were presented per run in pseudorandomized order (36 go, 12 nogo). In total, 24 nogo trials and 72 go trials were acquired for each expression type.
Figure 1
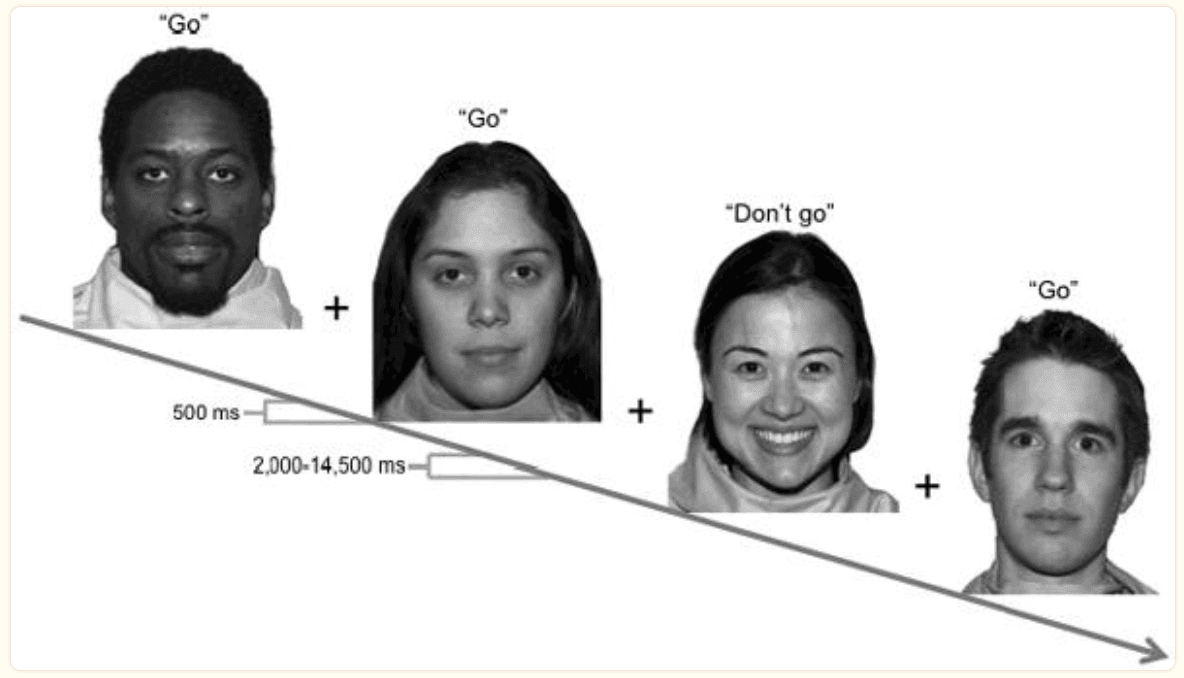
Schematic of four trials within an fMRI run. In this example, calm faces are the target stimuli, for which participants should `go' by pressing a button. Happy faces are the nontarget (`nogo') stimulus, to which participants should withhold a button press. Each face was displayed for 500 ms followed by a variable intertrial interval. Words above faces in quotes are not displayed during the experiment.
Image Acquisition
Participants were scanned with a General Electric Signa 3.0T fMRI scanner (General Electric Medical Systems, Milwaukee, WI) with a quadrature head coil. A high-resolution, T1 weighted anatomical scan spoiled gradient sequence ([SPGR] 256 × 256 in-plane resolution, 240-mm field of view [FOV], 124 × 1.5-mm axial slices), or a 3D magnetization prepared rapid acquisition gradient echo sequence ([MPRAGE] 256 × 256 in-plane resolution, 240-mm FOV; 124 × 1.5-mm sagittal slices) was acquired for each subject for transformation and localization of data to Talairach grid space. A spiral in and out sequence (Glover & Thomason, 2004) was used to acquire functional data (repetition time = 2500ms, echo time = 30, FOV = 200 mm, Flip angle = 90, skip 0, 64 × 64 matrix). Thirty-four 4-mm-thick coronal slices were acquired per TR a resolution of 3.125 × 3.125 mm covering the entire brain except for the posterior portion of the occipital lobe.
Analysis of behavioral data
Behavioral data were analyzed for accuracy by calculating hit (correct response), miss (incorrect lack of response), correct rejection (correct withholding of response), and false alarm (incorrect response) rates for happy and calm conditions. For analysis purposes, participants were grouped into child (aged 6–12), teen (aged 13–17) and adult (18 years or older) subgroups.
Analysis of fMRI data
FMRI data analysis was performed within Analysis of Functional Neuroimages (AFNI) software (Cox, 1996). Functional data were slice-time corrected, realigned within and across runs to correct for head movement, coregistered with each participant's high resolution anatomical scan, scaled to percent signal change units, and smoothed with a 6 mm full-width at half-maximum (FWHM) Gaussian kernel.
For each participant, a general linear model analysis was performed to characterize task effects by incorporating task regressors of interest (calm-go, calm-nogo, happy-go, happy-nogo, fear-go, fear-nogo, errors) convolved with a gamma-variate hemodynamic response function, and covariates of non-interest (motion parameters, linear and quadratic trend for each run). For completeness, fear trials were modeled as task regressors (convolved with a canonical gamma-variate hemodynamic response function) but were not analyzed further. Parameter estimate (β) maps representing task effects were then transformed into the standard coordinate space of Talairach and Tournoux (1988) by applying the warping parameters obtained from the transformation of each subject's high resolution anatomical scan. Talairach transformed parameter estimate maps were resampled to a resolution of 3 × 3 × 3mm.
Random effects group analyses were performed to identify functional regions of interest (ROIs) for subsequent analysis. Specifically, the conditions happy-go, happy-nogo, calm-go, and calm-nogo were carried to a 2 × 2 × 3 group linear mixed effects model with factors of emotion (within-subjects: happy, calm), response (within-subjects: go, nogo), and age (between-subjects: child, teen, adult). The main effect of response map identified candidate regions differentially engaged as a function of cognitive control demands including the right inferior frontal gyrus (x = 32, y = 23, z = 3). Responses modulated by development were identified in the main effect of age map, including a cluster in the ventral striatum (x = −4, y = 11, z = −9).
Imaging findings considered statistically significant exceeded whole-brain correction for multiple comparisons to preserve an alpha < 0.05 by using a p-value/cluster size combination stipulated by Monte Carlo simulations run in the Alphasim program within AFNI. The single exception to whole-brain thresholding was in the analysis of age effects. Given the role of the striatum in the development of impulse control (Vaidya et al., 1998; Casey et al., 2000; Luna et al., 2001; Durston, Thomas, Yang, et al., 2002, Galvan et al., 2006; Somerville & Casey, 2010) it was treated as an a priori region of interest for voxelwise analysis of age effects. Specifically, age effects were queried for within an inclusive anatomical mask containing voxels in the dorsal and ventral striatum, with p < 0.05, corrected statistical thresholding applied based on the striatum search volume (1,060 voxels). For clarity, we refer to thresholding of the age effect data as p < 0.05 small volume corrected (svc) throughout the manuscript.
Regions of interest were created as spheres with a 4mm radius centered about the peaks listed above, each containing ten 3×3×3 voxels. Parameter estimates were extracted for the 4 conditions (happy-go, happy-nogo, calm-go, calm-nogo) for each participant and ROI and were submitted to offline analyses to determine the directionality of effects. Response, emotion, and developmental effects (independent of the voxelwise contrast with which the ROI was defined) were evaluated using 2 (emotion: calm, happy) × 2 (task: go, nogo) × 3 (age: child, teen, adult) ANOVAs. Offline analyses were conducted in SPSS Statistics 17.0 software (SPSS, Chicago, IL).
Significant effects were tested for performance modulation by submitting parameter estimates to bivariate correlations against subjects' mean false alarm rates. Significant performance effects were followed up with partial correlation analyses to test whether performance effects remained significant when controlling for age. Conversely, significant age effects were followed up with partial correlation analyses to identify whether age effects remained significant when controlling for performance.
Prior work with the go-nogo paradigm has established a role for frontostriatal circuitry in supporting successful behavioral inhibition (Casey et al., 2000; Durston, Thomas, Yang, et al., 2002; Hare et al., 2005). To identify this circuitry in the current dataset, a psychophysiological interaction analysis (PPI) was employed that was sensitive to differential task-based functional connectivity with a seed region in the right inferior frontal gyrus, for which regional activity predicted performance differences across ages. Specifically, this analysis was sensitive to brain regions showing greater functional coupling with the right IFG for correct nogo trials relative to go trials. The PPI analysis was carried out using standard processing steps (Friston, et al., 1997) by extracting the functional timecourse within the seed region (right IFG ROI described above x = 32, y = 23, z = 3), removing sources of noise and artifact, deconvolving the neural signal, and convolving the timecourse data with no go versus go task timings and the canonical hemodynamic response function (as specified in Gitelman, Penny, Ashburner, & Friston, 2003). Group results including all participants, thresholded at p < 0.05, corrected for multiple comparisons at the whole-brain level, identified a single cluster showing significantly greater functional connectivity with the right IFG during nogo than to go trials. This cluster extended medial and posterior from the right IFG to the dorsal striatum specifically to the caudate. A dorsal striatum region of interest was generated based on the connectivity map by centering a 4mm sphere about the cluster sub-peak within the anatomical boundaries of the dorsal striatum (x = 9, y = 13, z = 6).
Signal change values were extracted from this ROI and tested for between-subject coactivation with the ventral striatum and right IFG. Specifically, ventral striatal, dorsal striatal and right IFG signal change values from the ROIs previously described were extracted for the happy-nogo versus happy-go contrast. These values were then submitted to between subjects bivariate correlations within child, teen and adult participant groups. These analyses identify the degree of coactivation across subjects for nogo relative to go trials between these regions within each age group. Identified coactivation values represent the extent to which the tendency to activate one region predicts activation in another region across participants.
Control analyses
Additional analyses were conducted to verify that reported developmental effects were not due to lower-level aspects of the data. As task performance was significantly different across age groups, the number of correct trials varied during first-level GLM analyses. Therefore, a second set of first-level GLMs were estimated in which number of correct trials were equated across conditions (happy-go, happy-nogo, calm-go, calm-nogo) and participants to match the lowest mean number of correct trials across all age groups (calm nogo trials in children; mean = 17). To do so, new regressors were generated by randomly selecting n=17 trials per condition for inclusion. All other trials were modeled, but as separate regressors that were not examined further. Findings from the 17-trial regressors were extracted from previously defined ROIs, tested for replication, and reported in Results.
In addition, overall data quality was evaluated across age groups by calculating mean signal-to-noise ratio (SNR) in each of the ventral striatum, dorsal striatum, right IFG ROIs and in the whole brain. SNR values were computed as the ratio between the mean baseline estimate from first-level general linear modeling and the standard deviation of the residual timeseries, as described by Murphy and colleagues (Murphy et al., 2007) and used in our previous neuroimaging work (Johnstone et al., 2005). SNR values did not systematically differ across age groups in any of these regions or in the whole brain (one way ANOVA (age: child, teen, adult), ROIs all p's > 0.2; whole brain p > 0.3). Whole-brain SNR values were also included as covariates in the coactivation analyses to verify that between-subject differences could not simply be attributed to differences in data sensitivity within each age group (see Results).
Results
Behavioral performance
Here we focus on the two types of possible errors in this task: misses (failure to press during go trial) and false alarms (erroneously pressing during nogo trial). For miss rates, results of a 2 (emotion: happy, calm) by 3 (age: child, teen, adult) mixed ANOVA yielded a main effect of emotion (F(1,59) = 15.44, p < 0.001), with greater overall miss rates for calm (5.0% +/− 0.6) relative to happy faces (2.6% +/− 0.4). However, tests for a main effect of age (F(2,59) = .24, p > 0.7) and an age by emotion interaction (F(2,59) = .13, p > 0.8) were not significant, suggesting that miss rates were not differentially modulated by age for either emotion condition (Figure 2, gray line plots hit rates [inverse of miss rates]). This was further supported by nonsignificant results in independent samples t-tests evaluating differential miss rates for happy relative to calm trials in children versus teens, teens versus adults, and children versus adults (all p's > 0.5).
Figure 2
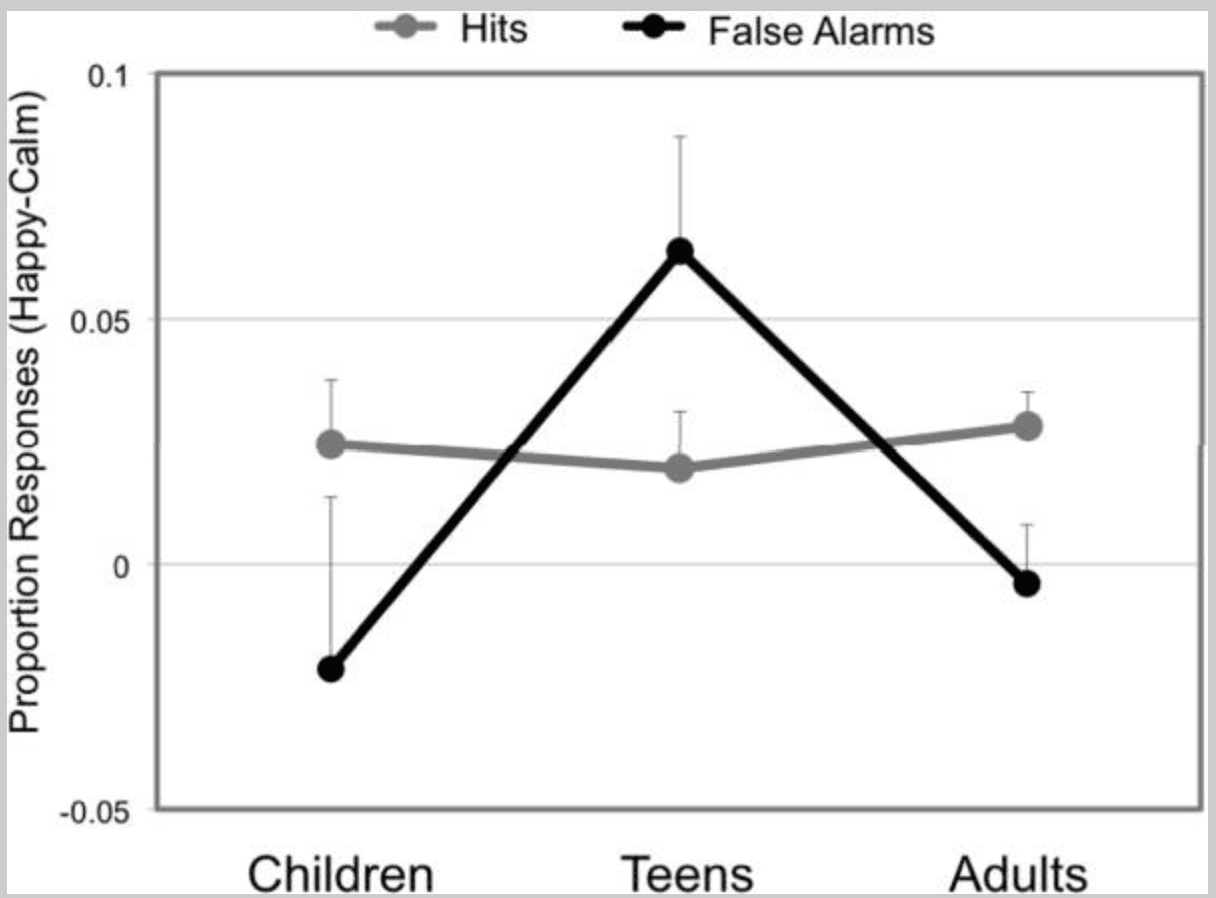
Behavioral performance by emotion and development. Gray line represents proportion of correct hits out of total go trials; black line represents proportion of false alarms out of total no-go trials. The y axis represents the proportion of responses for happy trials adjusted for proportion of responses for calm trials.
For false alarm rates, we observed a main effect of age (F(2,59) = 12.57, p < 0.001) and an age by emotion interaction (F(2,59) = 3.59, p = 0.034; children: calm 28.85% +/− 4.4, happy 26.71 +/− 4.2; teens: calm 22.1, +/− 3.4, happy 28.4 +/− 4.3, adults: calm 9.3% +/− 1.5, happy 8.9 +/− 1.7) and no main effect of emotion (F(1,59) = 1.18, p > 0.2; Figure 2, black line). To explore the directionality of the interaction, we conducted a series of independent samples t-tests comparing false alarm rates for happy relative to calm trials across age groups. Teens generated significantly more false alarms for happy relative to calm trials compared to children (t(35) = 2.04, p = 0.049) and adults (t(42) = 2.62, p = 0.012). Demonstrated another way, the false alarms committed by adolescents were significantly loaded in the happy condition (happy versus calm t(18) = 2.87, p = 0.01), whereas the false alarms committed by children and adults were equally distributed across happy and calm expression types (happy versus calm; children p > 0.5, adults p > 0.9). Finally, for calm trials, false alarms demonstrated a linear pattern of improvement with increasing age (linear term F(1,59) = 22.3, p < 0.001; quadratic term p > 0.4), whereas for the happy trials, quadratic (inverted U) and linear contrasts explained a significant portion of the variance in responding (quadratic term F(1,59) = 6.52, p = 0.013; linear F(1,59) = 14.31, p < 0.001).
Reaction time data suggest that happy faces facilitate speeded responses relative to calm faces (mean speeding to happy relative to calm +/− standard deviation: 53.5 ms +/− 68 ms; F(1,59) = 36.09, p < 0.001). This effect was evident in all three age groups when tested separately (p's =/< 0.01). Descriptive reaction time data are as follows: children (mean reaction time +/− standard deviation, in milliseconds; calm: 767.7 +/− 194; happy: 710.0 +/− 186), teens (calm: 549 +/− 91; happy: 518.9 +/− 86), adults (calm: 626.4 +/− 100; happy: 558.0 +/− 66).
To test whether differential error rates across age groups could be explained by a general speed-accuracy tradeoff, we analyzed reaction time data for correct `go' trials. A speed-accuracy tradeoff account could explain the differential accuracy findings across age if the conditions of poorest accuracy were also the fastest. We found no evidence of speed-accuracy tradeoff effects because unlike the accuracy findings, the test for an interaction between age and emotion in reaction times was not significant (F(2,59) = 1.78, p > 0.15). In other words, all three groups demonstrated equivalently speeded responses to happy faces that did not mirror the accuracy findings.
fMRI results
Responses modulated by development were identified in the main effect of age map, including a cluster in the ventral striatum (x = −4, y = 11, z = −9; p < 0.05 svc; Figure 3A). Post-hoc analysis of the age main effect showed that adolescents engaged the ventral striatum significantly more than children and adults to happy faces (p's =/< 0.01; Figure 3B) and to a lesser extent, to calm faces (p's =/< 0.06; means +/− standard deviation of percent signal change for calm versus rest: children: −0.095 +/− 0.21; teens: 0.046 +/− 0.16; adults: −0.051 +/− 0.17). Analysis of the best-fitting function that represents responding across ages to happy faces showed that a quadratic (inverted U) function explained a significant portion of variance in response to happy faces (F(1,59) = 10.05, p < 0.003) whereas a linear function did not (F(1,59) = 0.54, p > 0.4). The nonlinear enhancement in recruitment in teens remained significant when controlling for differences in task performance (false alarm rate; F(2,59) = 6.77, p < 0.002) and in the control analysis with matched numbers of trials (F(2,59) = 7.80, p = 0.007). The magnitude of activity to happy trials, calm trials, and no-go versus go trials was not associated with task performance (p's > 0.2).
Figure 3
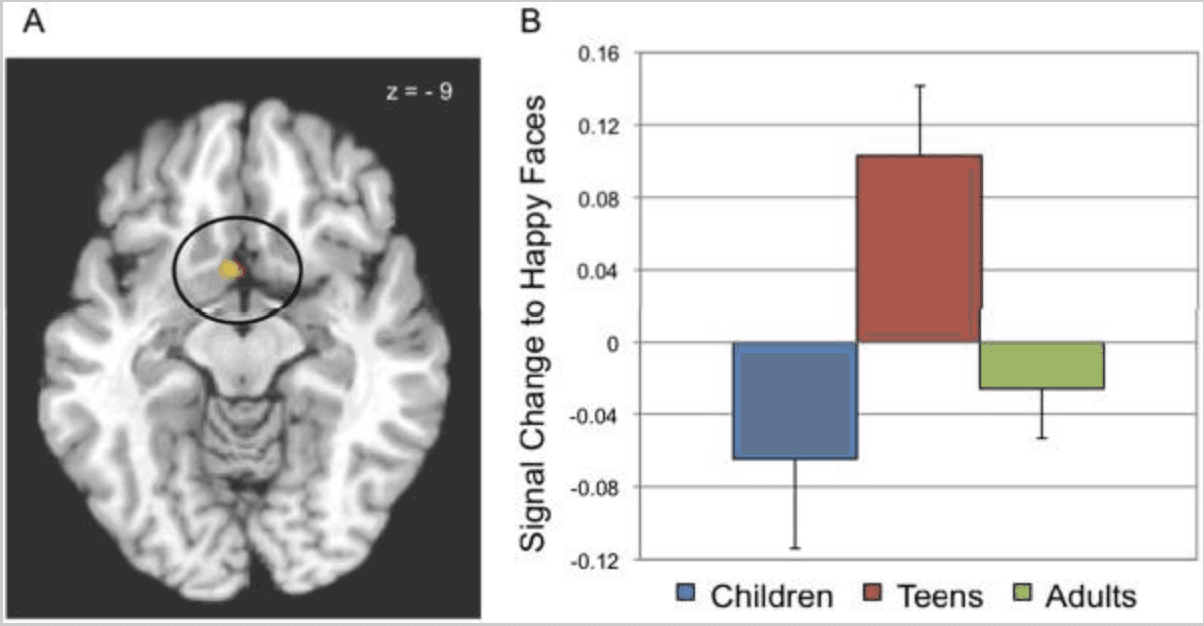
A) Brain regions showing differential activity as a function of age. Activations, threshold p < 0.05, svc are rendered on a representative high resolution anatomical scan.
B) Plot of activity in the ventral striatum (circled in A) response to happy faces (nogo and go conditions collapsed) relative to rest as a function of age. Adolescents show a significantly larger magnitude of activation relative to both children and adults. The left side of image corresponds to the left side of the brain.
The main effect of response map (nogo versus go) identified regions differentially engaged as a function of cognitive control demands including the right inferior frontal gyrus (IFG; x = 32, y = 23, z = 3), showing significantly greater responses to nogo relative to go trials (p's < 0.05, whole brain corrected; Figure 4A). Post-hoc analyses testing for the best fitting function indicated the right IFG response was significantly explained by a linear function (F(1,59) = 4.53, p = 0.037) and not a quadratic function (F(1,59) = .17, p > 0.6). Posthoc analyses indicated that the right IFG also showed greater activity to calm relative to happy faces (F(2,59) = 8.95, p < 0.005). Further, the right IFG ROI showed a linear decrease in response magnitude with increasing age to nogo trials relative to go trials (r(61) = −0.28, p = 0.026; Figure 4B).
Figure 4
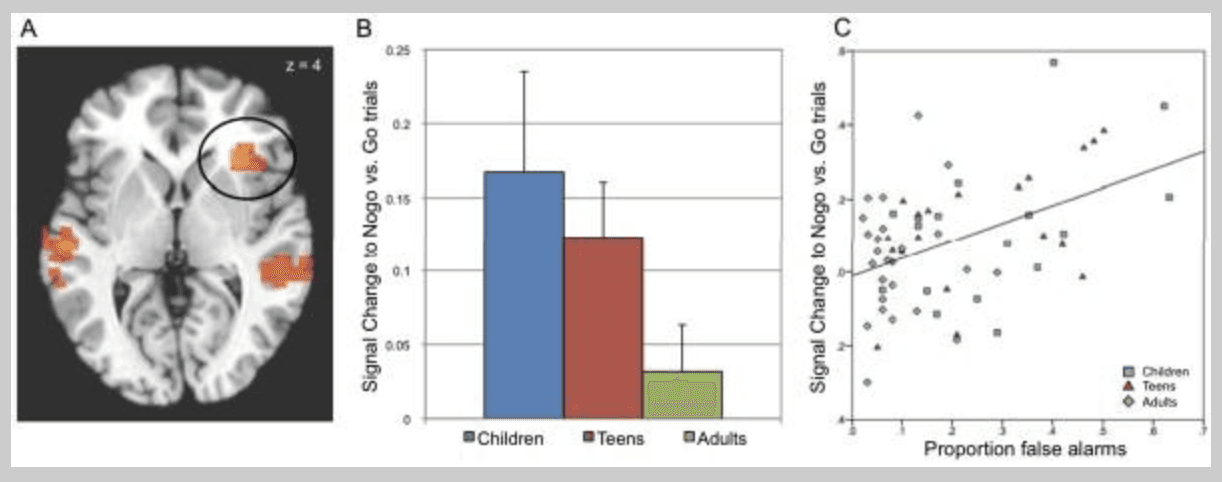
A) Brain regions showing differential activity as a function of task (nogo > go). Activations, thresholded p < 0.05, whole brain corrected are rendered on a representative high resolution anatomical scan.
B) Plot of activity in the right inferior frontal gyrus (circled in A) to nogo relative to go trials (happy and calm conditions collapsed) as a function of age. Increasing age predicts a linear decrease in recruitment.
C) Plot of activity in A) as a function of performance. Generally worse performance (greater false alarm rate on x-axis) predicted greater recruitment for successful suppression trials (correct nogo trials collapsed across emotion) relative to go trials (collapsed across emotion). The left side of image corresponds to the left side of the brain.
When controlling for performance effects, the task x age interaction in the right IFG was no longer significant (p > 0.4), indicating performance was a more robust predictor of activity in the right IFG than age. This relationship was demonstrated by a significant correlation between response magnitude to correct nogo vs. go trials and overall performance (as measured by false alarm rate; r(61) = 0.39, p = 0.002; see Figure 4C), which was replicated in the control analysis with a matched number of trials (r(61) = 0.28, p = 0.026). Figure 4C depicts this relationship with one participant excluded who was found to be an extreme outlier (defined as more than three interquartile ranges above the third or below the first quartile value). Although the correlation is significant including this individual, excluding this individual renders the resulting correlation even more reliable (r(60) = 0.45, p < 0.001). All reported analyses represent responses to correct trials. Thus, individuals more susceptible to false alarms tend to recruit the right IFG more to the nogo trials for which they successfully suppressed a behavioral response.
Connectivity analyses
The PPI analysis yielded a single cluster of voxels showing significantly greater functional connectivity with the right IFG for correct nogo trials relative to go trials. This cluster extends from near the right IFG seed region medially and posteriorly into the right dorsal striatum (x = 9, y = 13, z = 6, see Figure 5). These findings implicate a functional frontostriatal circuit showing significantly greater coordinated activity during trials in which response suppression was correctly engaged relative to trials in which response suppression was not required.
Figure 5
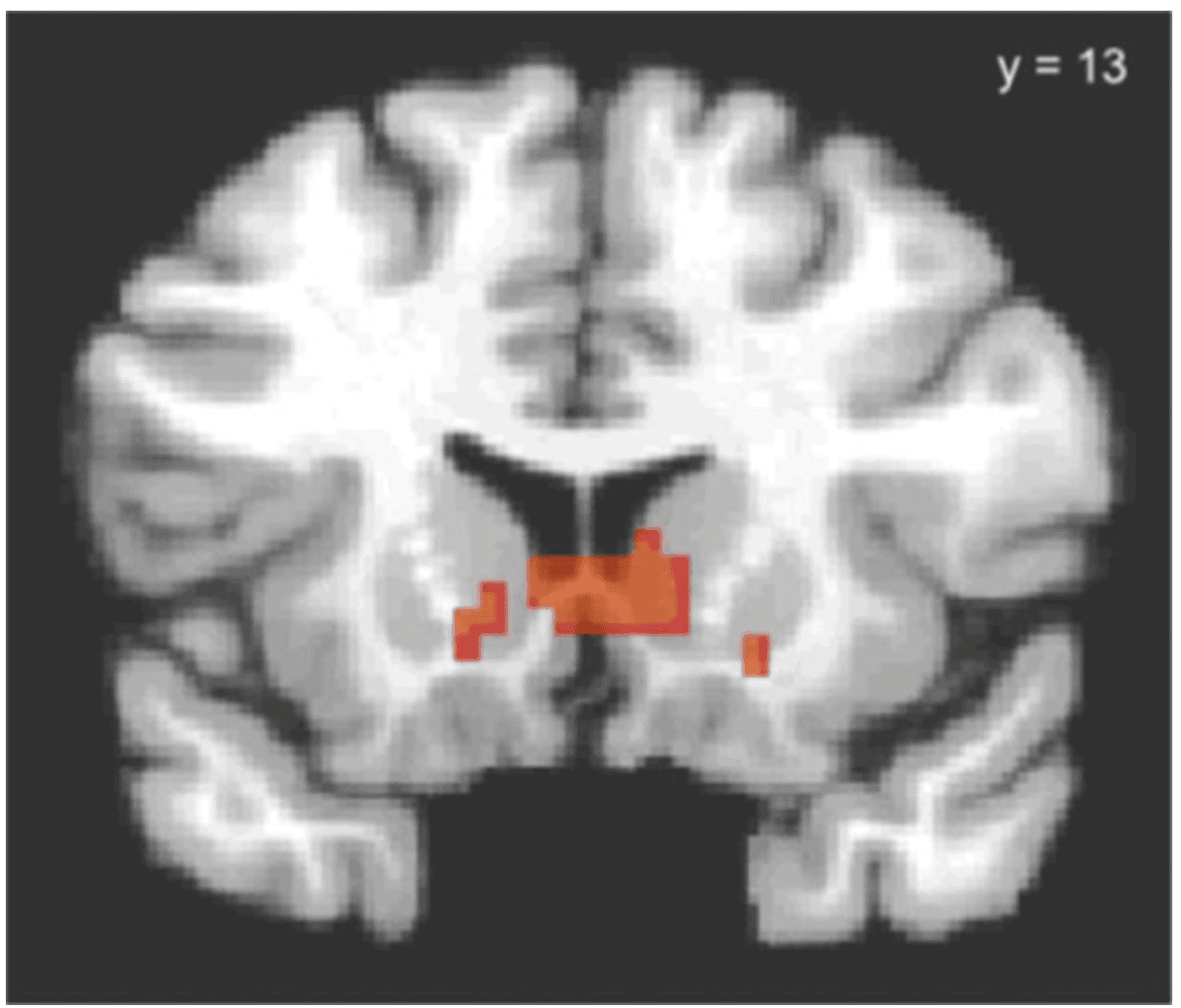
Psychophysiological interaction results based on seed region in right inferior frontal gyrus (IFG; circled in Figure 4A). The right dorsal striatum (caudate) demonstrates significantly greater functional coupling with the right IFG during nogo relative to go trials (threshold p < 0.05, whole brain corrected and rendered on a representative high resolution anatomical scan). The left side of image corresponds to the left side of the brain.
Follow-up analyses tested whether frontostriatal circuitry showed differential degrees of coactivity across ages for nogo relative to go trials. A series of between-subjects correlations tested the degree of coactivation between ROI signal values (nogo versus go contrast) from the ventral striatum (shown in Figure 3), the right IFG (shown in Figure 4) and the dorsal striatum (shown in Figure 5) within each age group. Data for the happy condition summarized in Figure 6 and below. We focus on the happy condition because happy-nogo relative to happy-go trials encompass the psychological construct of suppressing approach responses toward potential rewards. Children showed marginal coactivation between the ventral and dorsal striatum during happy nogo versus go trials (r(17) = 0.41, p = 0.09) whereas coactivation between the dorsal striatum and right IFG was less reliable (p > 0.12). Conversely, adults showed significant coactivation between the dorsal striatum and right IFG (r(24) = 0.49, p = 0.013) but not between the ventral and dorsal striatum (p > 0.8). Teens showed significant coactivation between the ventral and dorsal striatum (r(18) = 0.57, p = 0.012), as well as the dorsal striatum and right IFG (r(18) = 0.54, p = 0.016). All correlations remained significant in partial correlation analyses controlling for differences in whole-brain signal to noise ratio across participants with the exception of the dorsal striatum-right IFG correlation in adults, which becomes a nonsignificant positive trend.
Figure 6
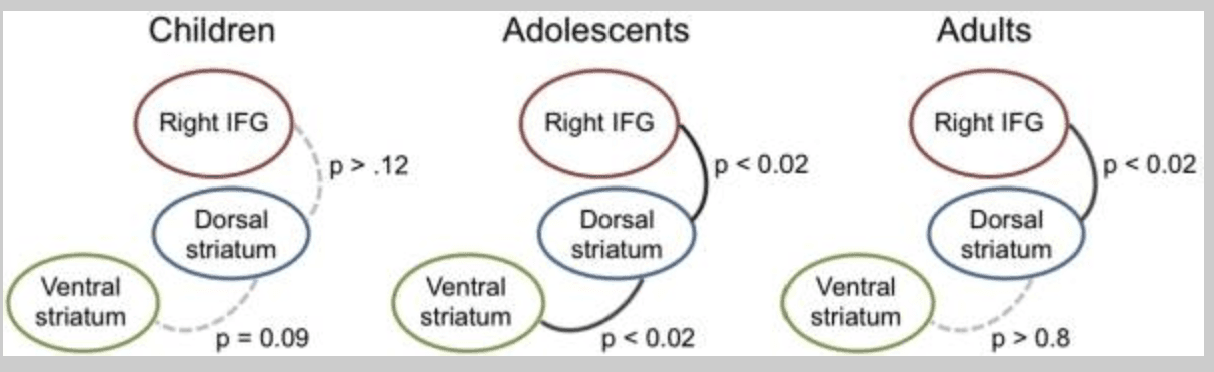
Between-subjects functional coactivation results for happy nogo trials relative to happy go trials in child, adolescent, and adult participants. Labeled bubbles represent regions depicted in Figure 3 (ventral striatum), Figure 4 (right IFG) and Figure 5 (dorsal striatum). P-values represent level of between-region co-activation across participants. Dotted line: coactivation not significant; gray line: significant at p < 0.05. All correlations are positive. IFG = inferior frontal gyrus.
Discussion
The capacity to exert control over one's actions is especially challenged when confronted with salient, appetitive cues. In this study, we sought to provide empirical evidence for reduced of impulse control in adolescents when faced with cues signaling appetitive value. Using a task that contains salient, appetitive stimuli (e.g., happy faces) that facilitated approach responses, we tested the developmental trajectory of subjects' ability to flexibly approach or avoid positive or neutral stimuli in a context-dependent manner. We found that teens demonstrated a unique pattern of errors relative to both children and adults, characterized by a reduction in the capacity to suppress approach behavior toward a salient, appetitive cue.
These behavioral findings suggest that although adolescents can engage behavioral suppression in neutral contexts at a proficiency intermediate to children and adults, they demonstrate a specific failure to override approach motivation toward appetitive cues. These findings cannot simply be explained by speed-accuracy tradeoff effects, because each of the three age groups demonstrated faster performance to happy than neutral cues, which did not predict poorer performance. This behavioral profile is consistent with theoretical accounts of adolescents as biased to engage in risky behavior at the service of approaching potential rewards (Steinberg, 2004) and converges with animal models of development showing enhanced reward seeking during developmental periods comparable to adolescence (Spear, 2000). Recently, Cauffman and colleagues (2010) used a series of decision making tasks with varying reward load and demonstrated that reward sensitivity shows an inverse U shaped function, rising to peak from 14–16 years of age and then declining. Laboratory demonstrations of biased approach motivation in adolescents (see also Figner, Mackinlay, Wilkening, & Weber, 2009) bolster the conclusion that adolescent risk-taking behavior is not simply a function of changes in independence or societal treatment (e.g., Epstein, 2007, see Dahl, 2004 for further discussion). It is also not solely attributable to immature cognitive regulation abilities (Yurgelun-Todd, 2007), as motivational aspects of the environment influence the ability to regulate behavior in a given context. Rather, this work suggests that the maturation trajectories of both cognitive and affective processes interact to influence the influx in risk-taking during adolescence (Casey, Getz, et al., 2008; Steinberg, 2008). The current behavioral findings suggest that when required to suppress behavioral approach to salient appetitive cues, adolescents' performance shows impairment not observed in other age groups.
Behavioral findings lead to neurobiological hypotheses regarding differential maturation of cognitive control and motivational systems. Based on nonhuman and human work to date, we specifically targeted frontostriatal and ventral striatal circuitry as candidate regions whose dynamic interactions across development are thought to mediate adolescents' reduced ability to resist approaching potential rewards (Somerville & Casey, 2010). We observed a region of the ventral striatum showing a nonlinear pattern of engagement with maximal activity in teens to happy faces. This finding converges with prior work demonstrating exaggerated representation of reward properties of stimuli in adolescents. For example, receipt of a monetary incentive led to exaggerated responses in the ventral striatum of adolescents compared to adults (Ernst, et al., 2005) and children (Galvan, et al., 2006; Van Leijenhorst, et al., 2009). Relative to adults, adolescents show enhanced ventral striatal activity while preparing for a trial for which reward is at stake (Geier, et al., 2010), suggesting upregulation of motivated behavior at the level of ventral striatum in adolescents. In addition, we observed a marginally greater response to neutral facial expressions in adolescents in the ventral striatum, though to a lesser extent than happy faces. This pattern suggests that although appetitive stimuli recruit ventral striatal responses more prominently, engagement of the ventral striatum in adolescents may also be marked by reduced specificity relative to children and adults.
Comparing nogo to go trials enabled the isolation of responses to trials in which suppression was correctly engaged (nogo trials) relative to trials in which cognitive control demands were low. It should be noted that as in past work (Durston, Davidson, et al., 2003; Hare, et al., 2005; Hare, et al., 2008), error trials were modeled separately, and thus activity differences here represent those to which correct suppression was accomplished. During nogo trials, we observed greater prefrontal recruitment in individuals with younger age. Prefrontal activity also predicted performance, such that individuals who were overall less successful at suppressing approach responses showed more right IFG activity for successful suppression trials. This pattern is consistent with prior work using the go nogo paradigm (Durston, Davidson, et al., 2003; Durston, Thomas, Yang, et al., 2002; Luna & Sweeney, 2004), reporting engagement of the inferior frontal gyrus for trials in which suppression was correctly invoked. The relationship between activity and performance suggests that prefrontal control resources were engaged to a greater degree in individuals who had the most difficulty accomplishing response suppression (i.e., younger participants).
More generally, there is less agreement in the literature about the nature of developmental shifts in recruitment of lateral prefrontal regions in contexts of cognitive demand. In the current study, we relied on differences in behavioral performance to interpret age-related changes in activation magnitude. Some studies, consistent with what is presented here, have demonstrated progressively lesser recruitment of prefrontal cortical regions with increasing age (Hardin, et al., 2009; Velanova, Wheeler, & Luna, 2008). This pattern could be interpreted as a relatively less specialization in younger populations resulting in more diffuse engagement (Durston, et al., 2006). Greater recruitment in younger ages may also be a result of increasing cognitive demands required of younger individuals in order to successfully complete the same task as older individuals, as suggested by Velanova and colleagues (2008) based on similar findings using an antisaccade task. Using performance variability, our observation that greater recruitment was found in the participants who had the greatest number of false alarm errors supports this interpretation. However, it should be noted that there is still debate as to whether stronger or weaker activation is a marker of `maturity' (Bunge & Wright, 2007; Luna, Padmanabhan, & O'Hearn, 2010) as other work has suggested larger-magnitude activity as an indicator of functional maturation (Klingberg, Forssberg, & Westerberg, 2002; Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Rubia, et al., 2006; Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006). Future developmental work will be required to more fully inform this issue.
Connectivity analyses identified frontostriatal circuitry, specifically the right dorsal caudate and inferior frontal gyrus that demonstrated significantly stronger functional coupling during correct suppression trials relative to trials not requiring suppression. Striatocortical interactions have been shown across tasks and species to be central to accomplishing goal-directed behavioral regulation (Delgado, et al., 2004; Durston, Thomas, Yang, et al., 2002; Schultz, Tremblay, & Hollerman, 2000), and more specifically in the suppression of impulses (Miller & Cohen, 2001). Interactions between the dorsal striatum and prefrontal cortex have been shown in primates to be critical to integrating reward associations with behavioral output (Pasupathy & Miller, 2005), a finding paralleled by adult human imaging literature (Galvan, et al., 2005; Poldrack, Prabhakaran, Seger, & Gabrieli, 1999). Developmentally, engagement of right frontostriatal circuits support suppression of a compelling response in children and adults (Casey, et al., 1997; Durston, Thomas, Worden, Yang, & Casey, 2002; Durston, Thomas, Yang, et al., 2002) and are hypo-responsive in impulse control disorders such as ADHD (Casey, et al., 2007; Durston, Tottenham, et al., 2003; Epstein, et al., 2007; Vaidya, et al., 1998). These findings support a general role for this circuitry in the shaping of goal-oriented actions.
After defining this circuitry, we tested for differential coactivation patterns among child, adolescent and adult participants. Adult and teen participants showed significant between-subjects coupling of dorsal striatal-prefrontal responses. In other words, adult and teen participants who tended to engage the dorsal striatum also tended to engage the inferior frontal cortex when correctly suppressing approach responses to happy faces. Though indirect, these findings support the notion that striatocortical responses show a relatively greater degree of functional organization in teens and adults relative to children. In adolescent participants, this frontostriatal response was also accompanied by a significant ventral-dorsal striatal coupling. Based on what is known about this circuitry (Haber, Kim, Mailly, & Calzavara, 2006), we speculate that teens who tended to activate the ventral striatum more strongly, also required greater dorsal striatal-prefrontal engagement in order to correctly suppress approach to positive cues.
Interactions between the ventral striatum, dorsal striatum, and prefrontal cortex are critical to the learning, expression, and regulation of motivated behavior. Indeed, individuals with Parkinson's disease who suffer from focal disruption of striatal activity demonstrate selective deficits in identifying and selecting motivationally relevant information in the environment (Cools, Ivry, & D'Espostio, 2006). By tracking anatomical projection fields, work by Haber and colleagues (Haber, et al., 2006) has implicated the dorsal striatum as a key convergence point for valuation-relevant signaling from the ventral striatum, and signals from regions of the brain important for cognitive control, including the prefrontal cortex (see also Haber & Knutson, 2009). Moreover, “parallel” striatocortical loops involved in different forms of goal directed behavior (motor, oculomotor, stimulus-driven, response-driven or motivational) have long been suggested to communicate at the level of the basal ganglia (Alexander & Crutcher, 1990; Casey, 2000; Casey, Durston, & Fossella, 2001; Casey, Tottenham, & Fossella, 2002). Our findings are consistent with differential biasing of these loops at the level of the striatum, when subcortical systems appear to be reaching functional maturity and suggest that while signaling of subcortical regions develops relatively early, top down signaling from these control regions may be more protracted.
Limitations
The findings presented here should be considered in light of their limitations. First, it should be explicitly acknowledged that a third emotional category, fearful faces, was present during the experimental task and the focus of a previous report (Hare et al., 2008). The calm face condition served as a control condition in both reports. Though behavioral findings suggest the presence of fearful faces in a functional scan did not modulate behavioral accuracy differently than the other two emotion categories, it is possible that the presence of fearful faces influenced the findings in ways to which the available measures were not sensitive. In addition, happy faces differ from calm faces in valence and salience, both of which could have contributed to the observed effects of appetitive value. A second methodological limitation is in the use of calm faces as a control condition. Though normative data suggest that calm faces are less positive and arousing than happy faces (Tottenham et al., 2009), we did not explicitly collect these ratings and it is possible that the calm faces were interpreted as mildly positive in their own right. In terms of results, the modest nature of the coactivation findings should also be acknowledged. Finally, measures of pubertal status and endogenous hormones were not acquired. Seminal research has demonstrated ways in which circulating gonadal hormones affect both organizational and activational mechanisms to influence brain function across development (Romeo & Sisk, 2001; Sisk & Foster, 2004; Steinberg, 2008) and shown a predictive relationship between pubertal status and such appetitive behaviors as sensation seeking and drug abuse (Martin et al., 2002; see Forbes & Dahl, 2010). Future research including measures of hormones may inform the relationship between striatocortical development, hormonal maturation, and behavioral outcomes (Blakemore, Burnett, & Dahl, 2010).
Conclusion
Adolescence has been described as a period of social reorientation (Nelson, Leibenluft, McClure, & Pine, 2005), with less time spent with parents and more time spent with peers, relatively unmonitored. With this relative influx in freedom comes an increasing need to regulate one's own behavior, which contrasts with childhood when behavior tends to be constrained by parents and other caregivers. Although immature cognitive control capacity has often been considered a sufficient explanation for adolescents' influx in risky behavior, there is a growing body of evidence including the current findings implicating biased motivational drives in adolescence, both at the behavioral and neurobiological level. Indeed, the relatively greater freedom experienced during this time may support stronger motivational drives, as independence also facilitates opportunity to seek out potentially rewarding experiences. This approach motivation may be supported by strong subcortical signaling of the ventral striatum. When placed in contexts in which one must regulate their own behavior, control failures – some resulting in risky behavior – may be a product of a prefrontal regulatory system that is relatively inexperienced and thus not functionally mature. Over time, experience shapes the capacity to regulate these approach behaviors, which shifts toward a state of greater balance between dynamic approach and regulatory signaling circuitries and strengthening of the ability to resist temptation.