Abstract
Adolescence is a period of dramatic neural reorganization creating a period of vulnerability and the possibility for the development of psychopathology. The maturation of various neural circuits during adolescence depends, to a large degree, on one's experiences both physical and psychosocial. This occurs through a process of plasticity which is the structural and functional adaptation of the nervous system in response to environmental demands, physiological changes and experiences. During adolescence, this adaptation proceeds upon a backdrop of structural and functional alterations imparted by genetic and epigenetic factors and experiences both prior to birth and during the postnatal period. Plasticity entails an altering of connections between neurons through long-term potentiation (LTP) (which alters synaptic efficiency), synaptogenesis, axonal sprouting, dendritic remodeling, neurogenesis and recruitment (Skaper et al., 2017). Although most empirical evidence for plasticity derives from studies of the sensory systems, recent studies have suggested that during adolescence, social, emotional, and cognitive experiences alter the structure and function of the networks subserving these domains of behavior. Each of these neural networks exhibits heightened vulnerability to experience-dependent plasticity during the sensitive periods which occur in different circuits and different brain regions at specific periods of development. This report will summarize some examples of adaptation which occur during adolescence and some evidence that the adolescent brain responds differently to stimuli compared to adults and children.
This symposium, “Experience during adolescence shapes brain development: from synapses and networks to normal and pathological behavior” occurred during the Developmental Neurotoxicology Society/Teratology Society Annual Meeting in Clearwater Florida, June 2018. The sections will describe the maturation of the brain during adolescence as studied using imaging technologies, illustrate how plasticity shapes the structure of the brain using examples of pathological conditions such as Tourette's' syndrome and attention deficit hyperactivity disorder, and a review of the key molecular systems involved in this plasticity and how some commonly abused substances alter brain development. The role of stimulants used in the treatment of attention deficit hyperactivity disorder (ADHD) in the plasticity of the reward circuit is then described. Lastly, clinical data promoting an understanding of peer-influences on risky behavior in adolescents provides evidence for the complexity of the roles that peers play in decision making, a phenomenon different from that in the adult. Imaging studies have revealed that activation of the social network by the presence of peers at times of decision making is unique in the adolescent. Since normal brain development relies on experiences which alter the functional and structural connections between cells within circuits and networks to ultimately alter behavior, readers can be made aware of the myriad of ways normal developmental processes can be hijacked. The vulnerability of developing adolescent brain places the adolescent at risk for the development of a life time of abnormal behaviors and mental disorders.
Introduction
“Plasticity is an intrinsic property of the human brain and represents evolution's invention to enable the nervous system to escape the restrictions of its own genome and thus adapt to environmental pressures, physiologic changes, and experiences” (Pascual-Leone et al., 2005) p 377). Plasticity entails an altering of connections between neurons through long-term potentiation (LTP) (which alters synaptic efficiency), synaptogenesis, axonal sprouting, dendritic remodeling, neurogenesis and recruitment (Skaper et al., 2017). While the focus of this report is on the adolescent brain, experience and neuronal plasticity, brain maturation throughout adolescence occurs on a backdrop of genetic and environmental interactions beginning prior to conception and continuing throughout gestation and postnatal life. Experience during early childhood has been shown to set a trajectory toward a lifetime of health or disease, both mental and physical, as documented in the Adverse Childhood Experiences (ACE) study (Felitti et al., 1998) We now know that experience during childhood and adolescence can modify the trajectory of the structural and functional changes induced by environment and experience that occur during the rest of life (Abraham and Bear, 1996). During childhood and adolescence, the brain exhibits sensitive periods when experience can greatly alter its functional and structural characteristics. Many studies have demonstrated that various types of experiences (e.g., enriched environment or foot shock) can alter the appearance, types and longevity of dendritic spines, which are almost exclusively associated with excitatory synapses, throughout adolescence and into adulthood (Trachtenberg et al., 2002; Jung and Herms, 2014) and that these effects can be sex-specific (Baratta et al., 2019). These sensitive periods occur in different brain regions at different times of development and may even occur in different layers of the same cortical region at different times (Petanjek et al., 2011). For the purpose of this manuscript, the term “Critical period” will refer to a restricted sensitive period wherein changes can only occur within this restricted period of time. Beyond the critical period, further modification is limited to molecular tuning of synaptic strength (functional plasticity) as the major mechanism of adapting to the environment. Typically, the sensory systems, such as the visual system, exhibit critical periods during early postnatal development and have been widely studied (e.g. (Hubel and Wiesel, 1970). The prefrontal cortex (PFC), which is the seat of the highest order executive function in the human, exhibits the largest overproduction of synapses and the slowest rate of elimination of all regions examined (Petanjek et al., 2011). In fact, these authors propose that the PFC exhibits anatomical and functional changes which are consistent with a dynamic reorganization of synaptic circuitry beyond a relatively simple activity-driven molecular tuning of stable synapses (Petanjek et al., 2011). This “molecular tuning of stable synapses” or functional plasticity which can occur in any brain region at almost any time in development or adulthood encompasses a range of adaptations of synapses including alterations in neurotransmitter content, receptors, transporters as well as second messenger systems. Functional plasticity is more difficult to study in the living human shifting the focus of investigators toward structural plasticity as visualized in postmortem material and various imaging modalities. The development of 2 photon in vivo imaging has facilitated the examination of dendritic spines across the lifespan and demonstrated that different types of microcircuitry within the neocortex appear to have different rates of synaptic formation and elimination e.g., (Holtmaat et al., 2006). Interestingly, the age when learning and acquisition of knowledge is at a peak coincides with an overall decrease in number of synapses rather than an increase (Petanjek et al., 2011). Generally, as the brain matures, the cortex thins due to pruning of the less activated synapses, increased efficiency and maturation of white matter structure (Giedd and Rapoport, 2010).
According to Hensch (2004), there are 8 properties which govern the response of the nervous system during sensitive periods: Initially, there must be a genetically-determined structure allowing functional competition between inputs. Secondly, electrical activity (action potentials) mediates plasticity, the change in neural excitation/depression. Thirdly, selected pathways responsive to experience undergo consolidation from the level of the dendritic spine up to the level of the network or circuit. Fourth, the regulation of the sensitive period is determined by experience (appropriate neural activation) not simply age. If a circuit experiences little activity, it will remain “sensitive’ for a longer amount of time. Fifth, each brain region has a unique timing and duration of sensitive periods. Each level of processing lays a foundation for more complex processes within a given network to ultimately shape brain function. Sixth, the molecular mechanisms which are responsible for these changes are diverse and vary for individual connections. Seventh is the importance of inhibition in plasticity in that inhibitory interneurons arbitrate which synapses are eliminated and which are retained and strengthened. Eighth, is the importance of attention and motivation under which the experiences can lead to learning. This aspect is particularly salient for the adolescent since the alert state of the infant/child diminishes with age –with the possible exception of stimuli related to social and emotional spheres (Hensch, 2004).
The topic of this report, experience-dependent plasticity during adolescence, encompasses a wide range of complex phenomena on a background of early developmental influences For example, during the postnatal period, the ocular dominance columns develop in visual cortex following normal binocular visual input; once the critical period has passed, visual deprivation will not disrupt the major features of the columns while visual deprivation prior to closure of the critical period will (Wiesel and Hubel, 1965). This is experience-dependent plasticity within the visual system. During adolescence, hypothetically, the networks responsive to social stimuli, emotional stimuli and higher order cognition (and have been shown to be responsive using fMRI imaging) are available to undergo plastic changes such as pruning and potentiation, as stimuli are experienced. The resulting structural and functional changes in the responsive networks are then the underpinnings of ensuing behavioral changes. While there is ample evidence that the adolescent brain responds differently to many stimuli compared to the adult, empirical evidence that experience modifies specific connections which in turn modify specific behaviors is lacking (Paus, 2013). However, identical twin studies have demonstrated that the experience of each twin leads to alterations in the epigenetic profile of each which is suggestive of the importance of experience in modifying the genetic expression and therefore behavior (Fraga et al., 2005). A great deal of correlational data exists and some will be presented in this report. On the other hand, animal studies have provided strong empirical evidence that experiences alter structure. For example, housing rats in enriched environments produces alterations in dendritic fields and synapses and these changes in structure are associated with reliable behavioral changes. In an elegant study by the Kolb group, increased dendritic arborization and changes in dendritic length within the medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) which differ depending on the type of play experience in rats between 21 and 60 days of age were documented (Bell et al., 2010). Behavioral alterations were also described. Therefore, experience-dependent plasticity involves both selective regression or elimination of synapses (which have not been cohesively activated) and selective growth of new or altered connections (following coincident stimulation of pre and post-synaptic elements).
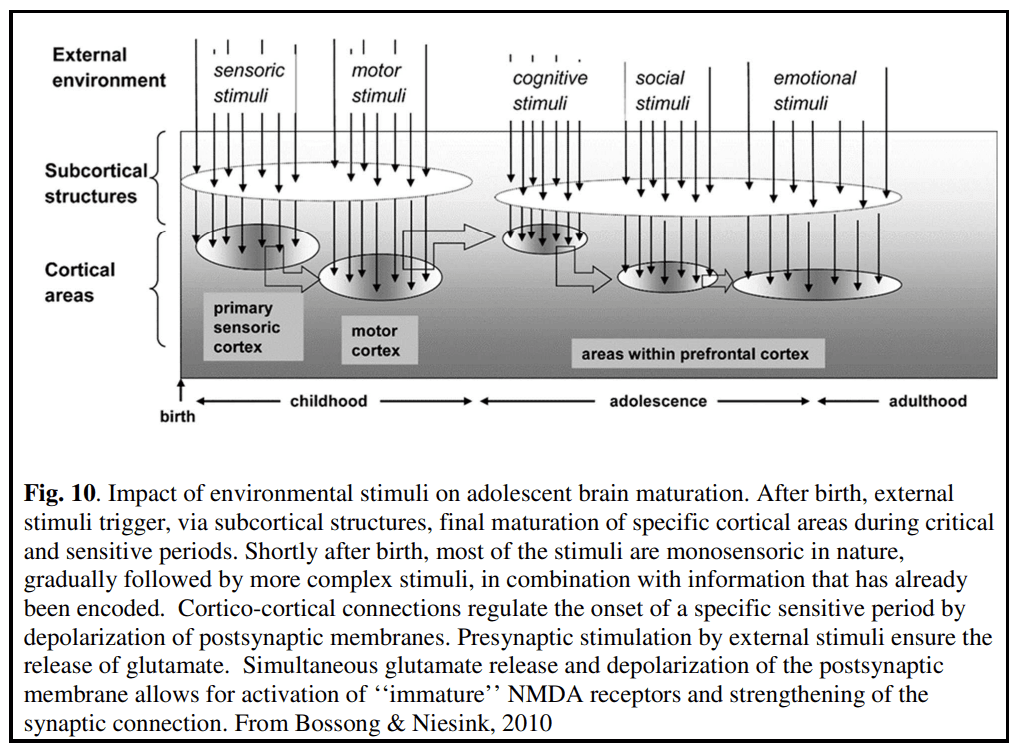
In summary, while childhood includes the sensitive periods for the development of the sensory and motor systems, adolescence includes sensitive periods for social, emotional and cognitive development in that the networks subserving these domains are undergoing plasticity based on the experiences of the individual (Bossong and Niesink, 2010). Adolescence is characterized by a continued maturation of functions mediated by the PFC including working memory, planning, concept formation, inhibitory control and others, a thinning of the cortex within the PFC (and many other brain regions) and an increase in white matter density and volume (see review by Wahlstrom et al. (2010)). With the maturation of these complex behaviors comes a vulnerability to exogenous influence and the increased possibility that functional and structural maturation can become abnormal and psychopathology can ensue.
This report contains summaries of presentations made at the Developmental Neurotoxicology Society Annual Meeting with the Teratology Society in June 2018 at Clearwater Florida. The first section by MacMaster discusses the various methods which have been used to assess brain structure, function and metabolism and how these indicators change over time. The next section by Peterson reviews several human conditions wherein the exertion of control over undesirable behaviors appears to alter brain structure. Thirdly, the basic physiology of synaptic plasticity is discussed and then a summary of effects of commonly-abused substances in adolescence is presented (Niesink). Then, the effects of methylphenidate on brain and behavior in the rodent and humans illustrate the plastic changes induced by a common therapeutic drug (Andersen). Lastly, the complex influences of peers in adolescent decision making illustrate the unique way social stimuli alter brain function in the adolescent (Braams). Together, the reader will become aware of the complexity of the interactions between experiences, brain structure and behavior as they shape the brain of the young adult.
Building the adolescent brain
Introduction
For decades, understanding the biologic underpinnings of brain development during adolescence has been elusive. However, with the arrival of new methods to evaluate brain structure, chemistry, and function, researchers are beginning to better understand brain development.
How can we learn about brain development during adolescence?
Until recently, our understanding of brain development was limited to post-mortem studies, animal models, and studies of performance during specific neuropsychological tasks (i.e., working memory). This was not sufficient to develop a fulsome understanding of the neurobiology of development during adolescence. The advent of brain imaging techniques overcame this impediment.
Brain structure
Studies of regional brain structures, volume, and shape typically use magnetic resonance imaging (MRI) (see Fig. 1). Present-day conceptualizations of cortical thickness and other basic morphological studies using MRI are enhanced using diffusion tensor imaging (DTI). DTI allows for the investigation of the integrity and organization of white matter tracts (see Fig. 2). Combinatorial approaches such as these are currently being used to help disentangle the overlapping neurobiology of commonly comorbid disorders (i.e., ADHD and motor disorders (Langevin et al., 2014)). A landmark study has also advanced the understanding of white matter development in children through adulthood (Lebel et al., 2008)
Brain chemistry
The physiological functioning of the brain in adolescence is not well understood. However, the study of metabolites in the brain can yield many clues as to brain function, development, disease onset, progression, and also informs potential targets for intervention. There are three commonly used tools for understanding brain chemistry in youth: (1) Single Photon Emission Computed Tomography (SPECT), (2) Positron Emission Tomography (PET), and (3) Magnetic Resonance Spectroscopy (MRS). Each provide specific inherent advantages and disadvantages.
SPECT and PET have limited use in adolescents as they require the injection of radioactive isotopes. Despite impressive advantages (i.e., it can measure cerebral blood flow, glucose metabolism, and neurochemistry; including key neurotransmitters, transporters, and receptors), PET has not been utilized in longitudinal studies in young people, as benefits must be weighed heavily against the potential for harm. Controlled studies are also challenging to conduct due to the inherent ethical issues of exposing typically developing control participants with minimal potential benefit to the individual.
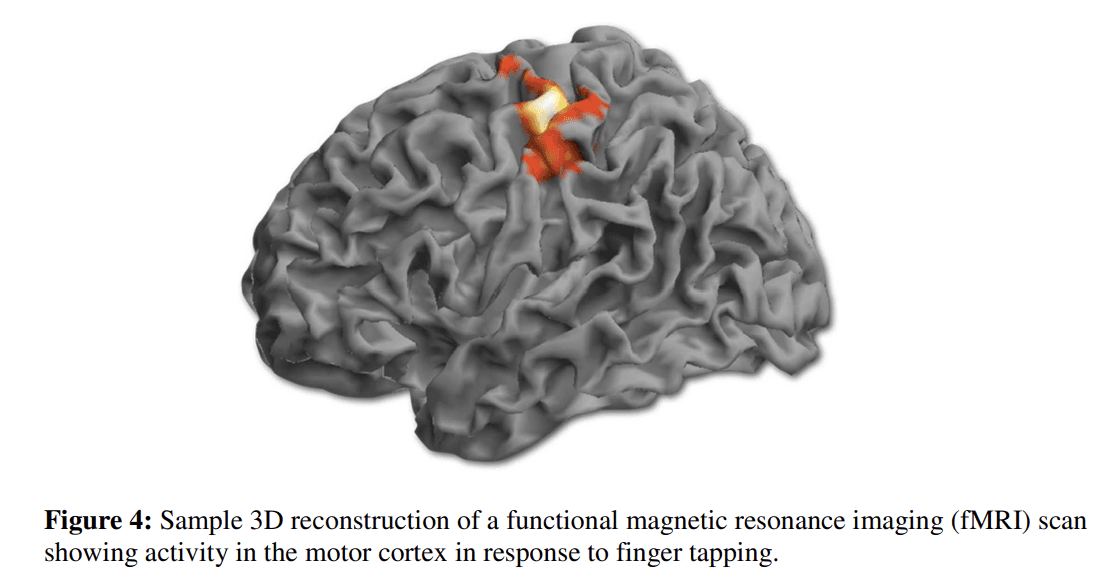
MRS methods are similar to MRI in that no ionizing radiation is used. This bypasses the safety limitations of PET and SPECT. Though, the capacity to probe various biological compounds is limited by comparison. Phosphorous (31P) and proton (1H) MRS are the two most commonly used in studies involving children and adolescents (i.e., MacMaster et al., 2008; Stanley et al., 2008). 1H-MRS permits the study of a variety of metabolites such as N-acetyl-aspartate (Birken and Oldendorf, 1989), a neuronal marker; creatine/phosphocreatine (Rackayova et al., 2017), involved in energy metabolism; choline compounds (Lin and Grant, 2013), membrane components; myo-inositol, a secondary messenger involved in signal transduction; and glutamate (Shen, 2013), glutamine, and γ-aminobutyric acid (GABA) (Best et al., 2013) –all of which occur in relatively high concentrations in brain (see Fig. 3). 31P-MRS permits the analysis of membrane synthesis compounds (phosphomonoesters (PMEs) like phosphoethanolamine and phosphocholine) and breakdown compounds (phosphodiesters (PDEs) like glycerophosphoethanolamine and glycerophosphocholine), along with substrates of energy metabolism.
Brain function
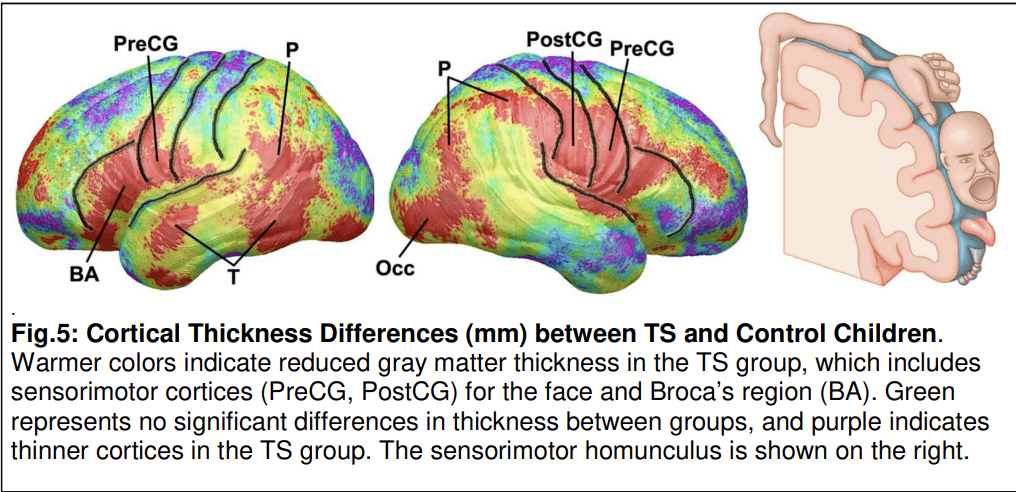
Functional MRI, or fMRI, is the prominent tool in the study of functional development in adolescents. Basically, fMRI detects changes magnetic properties associated with blood flow. This is typically used to determine activity during tasks (see Fig. 4) or the degree of functional connectivity between brain regions during the so-called resting state. The study of regions of the brain displaying synchronous activity while not engaged in higher-order tasks, the default network, allows an examination of basal brain function and offers an innovative method for determining differences in brain function between groups and conditions, including age-related changes in connectivity within specific networks (Vinette and Bray, 2015). Connectivity between regions during task engagement would reflect an activated state which is expected to be different from resting state connectivity.
Considerations
There are two pragmatic considerations regarding the execution of MRI based studies in adolescent participants. First, the obligation to remain still can be challenging; even for healthy youth. Second, anxiety can be induced by being in a limited space, can reduce participant comfort and impede the capacity to acquire accurate images. While this response occurs in a minority of patients, the effect of motion artifacts is a major impediment to the utility of imaging data. Sedation is an option for clinical scans, but due to its inherent risks it is not typically applied in research studies involving typically developing adolescents. Training participants in a “mock scanner” prior to the actual scan reduces subjective distress and heart rate during the actual procedure. This facilitates the successful completion of most research studies. Other technical considerations in brain imaging include the tremendous variation in brain structure, function, and chemistry over the course of childhood into adolescence. Also, cognitive task-based imaging data is obviously influenced by performance on tasks and functions under scrutiny. Children may participate poorly on a task in the MRI if they consider it too hard or too tedious. The resulting brain scans can reflect a wide range of emotional states in addition to the activation of networks engaged in the cognitive task.
Typical brain development
To begin to understand brain development during adolescence, one can focus on four core principles (see Table 1). First, brain development is not linear and uniform. This means different parts of the brain grow at different rates at different times, and not just simply larger. Second, as the brain develops, it becomes more connected and specialized; function matures. Third, the changing dynamic between frontal/executive regions and limbic/arousal/reward regions influences behavior strongly. Finally, the malleability of the developing brain represents a double edge sword, on the one hand, of plasticity/learning and, on the other hand, vulnerability to disease, disorder, and exposures.
Table 1. Four principles of brain development during adolescence.
1. Brain development is not linear during adolescence |
2. The brain gets more connected and specialized during adolescence |
3. The changing dynamic between frontal/executive and limbic/arousal/reward regions influences behavior strongly. |
4. The malleability of the developing brain represents a double edge sword of plasticity/learning and vulnerability to disease, disorder, and exposures. |
Overall brain size increases significantly from in utero through the first two years of life. During this time, synaptogenesis is most active. In the time leading to early adolescence, two major dynamic processes are ongoing in the maturing brain: (1) synaptic pruning (Huttenlocher, 1979) and (2) myelination. Synaptic pruning is the process of synapse elimination, associated with both regulation of required synaptic connections, and terminal pruning associated with learning and memory. Myelination is formation of the myelin sheath around a nerve, allowing for improved conduction of impulses. It is thought that increased myelination, connectivity, and changing functional activation patterns indicate that increasingly efficient connections between brain regions are being made from childhood into adulthood.
Gray matter volume (i.e., neuronal bodies, neuropil, glia, synapses and capillaries) peaks in mid-childhood into early adolescence, then temporarily plateaus and then decreases following puberty with each region following a unique trajectory (Courchesne et al., 2000). Gray matter volume maturation approximately parallels changes in local cerebral metabolic rates for glucose as a function of age (Chugani, 1998). These changes are the consequence of synaptic pruning, i.e., an increase in the efficiency of neural processing. It is essential to note that brain development is heterochronous; meaning the timing, degree, and rate of cortical and subcortical gray matter change are regionally specific. White matter volume increases relatively linearly through childhood into adolescence (Courchesne et al., 2000), with connections being formed during maturation. The corpus callosum, which connects the right and left hemispheres of the brain and topographically maps onto the cortex, undergoes sex-specific synaptogenesis in associated cortical regions and appears to parallel the development of associative reasoning skills (Thompson et al., 2000).
Biological sex also plays an important role in brain development. Parietal and frontal gray matter volume was found to peak earlier in females and then decline after puberty, while temporal lobe gray matter volume reached its peak in both sexes at approximately the same time (Giedd et al., 1999). Subcortically, basal ganglia, hippocampus, and amygdala development also appear to be influenced by sex (Durston et al., 2001). Gray matter loss in the basal ganglia is dramatic in boys between the ages 6 of 12 years. In girls, hippocampus volume increases more than in boys with age, while the reverse is true for the amygdala. These maturational alterations coincide with sex-specific differences in the timing of puberty and suggest a role for gonadal hormones in brain development. Differential sex trajectories of specific brain regions may be reflected in preponderance of specific disorders within each sex that are associated with those regions (i.e., basal ganglia – obsessive compulsive disorder (OCD) and attentional deficit hyperactivity disorder (ADHD) in males, and major depressive disorder (MDD) in females). Finally, the pituitary gland demonstrates a striking gender difference (MacMaster et al., 2007), with females developing a larger pituitary gland than males, particularly during adolescence. Again, this may be associated with the differential timing of puberty in males and females.
Specialization is critical for proper brain function. Previously using 1H-MRS, we found that reading (phonological processing speed) was associated with increases frontal lobe glutamate, creatine, and inositol in small children (Lebel et al., 2016). Reading and language are skills that develop early and require early specialization. Aspects of executive function (i.e., attention, working memory, planning) develop much later and so does the function and structure of the brain region that subserves it, the dorsolateral prefrontal cortex (Fuster, 2008). Connectivity matters as well. For example, in youth, better vocabulary skills are associated with greater anatomical coupling in several linguistically relevant regions of cortex, including the left inferior parietal (temporal-parietal junction), inferior temporal, middle frontal, and superior frontal gyri and the right inferior frontal and precentral gyri (Lee et al., 2014). Indeed, longitudinal work by Raznahan et al. (2011) using a seed within their resting state fMRI found that regions that fired together tended to wire together (coupling). This plasticity is greatest during development. For example, it has been shown that music lessons can improve connectivity from the left primary motor cortical area to the right cerebellum in youth (Alves-Pinto et al., 2015).
The differential trajectories in regional brain volumes are also responsible for changes in behavior over time. Indeed, the shifting dynamic between slower developing frontal/executive regions and earlier developing limbic/arousal/reward regions influences behavior strongly (Casey et al., 2011). This is responsible for the oft-observed ‘risk taking’ behavior seen in adolescents. They are wired, at that time, to be especially responsive to reward, especially social reward. Indeed, the extended period of frontal lobe development represents a time of special plasticity and vulnerability. It has been suggested that delays in frontal specialization and coupling may be reflected in superior intelligence (Shaw et al., 2006). The plasticity mentioned above (Alves-Pinto et al., 2015; Raznahan et al., 2011) is on the positive side of learning. The biological processes that permit this plasticity also render an adolescent especially vulnerable to psychiatric illness, to the effects of exposure to adverse events or compounds, and the like. This is the double-edged sword discussed earlier.
Future directions
Conducting brain-imaging studies of development in adolescents poses special challenges. However, this important work is positioned to provide a critical neurobiological understanding of the mechanisms underlying specific neuropathologies and the effect of interventions. Practically, MRI methods are particularly useful in adolescents, as they pose no threat to safety with radiation and hence lend themselves naturally to longitudinal studies. As the technology continues to evolve, resolution (spatial and temporal) will improve, novel methods will emerge, and consensuses in application will develop. This will allow for even greater progress into understanding brain development across adolescence in typical and atypical populations.
Experience-dependent plasticity in adolescent brain development (interaction between experience and symptoms in brain diseases)
Neural plasticity is the structural reorganization of neurons in response to developmental and experiential demands. It is a dynamic process superimposed on other major maturational changes during childhood, adolescence, and adulthood, and it is generally mediated by the strengthening of existing synapses or the creation of new ones. In persons with a neuropsychiatric illness, neural plasticity supports compensatory responses that modulate the severity of symptoms. Our understanding of these compensatory responses also requires an understanding of what is being compensated for in each illness.
Context
Neural plasticity represents a relative refinement of organization in brain structure and neural circuit development that has occurred largely during fetal and early postnatal life. It is superimposed on the progressive cascade of maturational events culminating in the exuberant production of neural elements typically reaching >150% of adult levels during childhood. Thereafter, a reduction in overall numbers through the processes of apoptosis for neurons and pruning for dendrites and synapses occurs. Activity is the dominant determinant for which elements survive and which die off during development; the surviving fine-grained architecture is defined by the rule, “use it or lose it”. Despite the decline in the overall number and density of dendritic arbors and synapses in postnatal life, dendritic trees and the synapses upon them are constantly being formed, pruned, and refined in a highly dynamic reshaping of their architectures, and therefore of their information processing functions, according to experience (Tau and Peterson, 2010).
Less appreciated historically is the importance of glial cells in brain development and plasticity. Glial cell proliferation occurs primarily in the second half of human gestation and is primarily responsible for the dramatic increase in brain size and weight during that time. Glial cells are important for axon guidance during neural migration, as well as regulation of the extracellular environment, recycling of excitatory and inhibitory neurotransmitters, and modulation of synaptic connections. They also support the process of myelination in postnatal life, which is particularly important for long axons that require fine calibration of conduction speeds for coordination of long-distance signal transmission. The majority of new myelin in adolescence and adulthood is formed through an activity-dependent, plastic remodeling of the number, thickness, and length of existing myelin sheaths (Gibson et al., 2014; Yeung et al., 2014; Young et al., 2013). Neuronal activity can also trigger new myelin production in adolescence and adulthood through the differentiation of oligodentrocyte precursor cells into new oligodendrocytes (Rivers et al., 2008). Thus plasticity involves structural reorganization of not only dendrites and synapses in gray matter, but also of myelin in white matter. We have recently provided compelling evidence for the neuroplastic reorganization of white matter tracts, particularly in fiber bundles interconnecting frontal cortex with the basal ganglia and thalamus, that mediates the increasing capacity for self-regulatory control in youth 9 through 12 years of age, during the transition from childhood to adolescence (Nelson et al., in press).
Evidence for neural plasticity from studies of human illnesses
The evidence for neural plasticity in humans is largely inferred, as the dynamic processes of plasticity can be captured in vivo only through brain imaging techniques that are unable to visualize the changes in synaptic architecture at the cellular level that defines plasticity, but instead image brain tissue at a spatial resolution of >1 mm3, a volume that can contain hundreds of thousands of synapses. Most of the evidence for neural plasticity in humans comes indirectly, from studies of psychopathology. Here we present three examples of human disease which have their origins in childhood and adolescence and describe how symptom severity can relate to structural alterations in brain.
Tourette syndrome
Many imaging studies collectively suggest that the core brain pathology that produces the tic symptoms of Tourette syndrome (TS) is a hypoplasia and hyperexcitability of motor pathways within cortico-striatal-thalamo-cortical (CSTC) circuits that loop from the cortex, to the basal ganglia, thalamus, and back to the cortex. Cortical thickness in TS compared with control children is significantly reduced bilaterally in inferior portions of the sensorimotor strip and Broca's region – regions that control oro-facial-laryngeal musculature and vocalization, which tics most commonly affect – as well as parietal and posterior temporal cortices (Fig. 5) (Sowell et al., 2008). More severe tic symptoms were associated with more extensive cortical thinning in the sensorimotor strip. Similarly, basal ganglia volumes, particularly of the caudate nucleus, were significantly smaller in 154 TS children and adults (6–63 years old) compared with 130 age-matched healthy controls (Peterson et al., 2003). Caudate volumes in children from the same cohort correlated significantly and inversely with the severity of tic symptoms 8 years later, in late adolescence or early adulthood (Bloch et al., 2005).
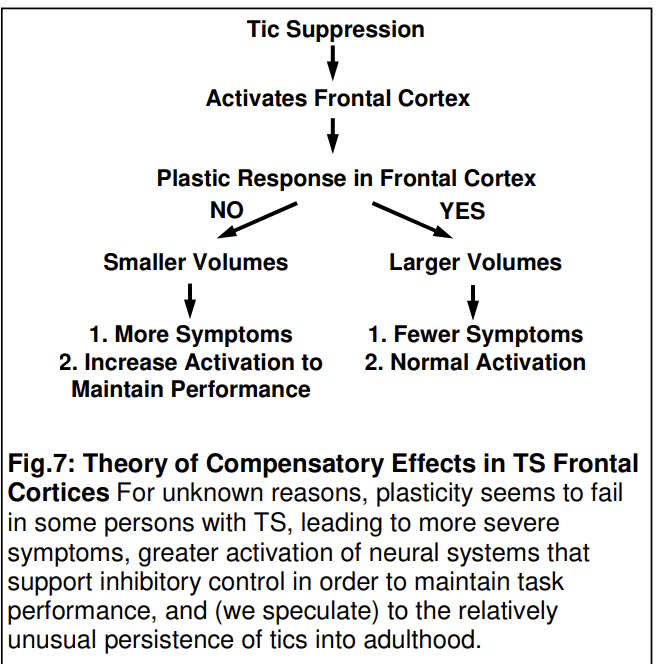
In contrast to these reduced volumes in motor circuits, volumes of dorsal prefrontal regions were significantly larger in this same large sample of TS child and adult participants compared with controls (Peterson et al., 2001). This effect, however, derived primarily from larger volumes in TS children, and in fact TS adults had significantly smaller volumes in these regions, producing a significant age-by-diagnosis interaction that derived from an inverse correlation of dorsal PFC volumes with age in the TS group that was absent in the controls. Frontal and parietal volumes correlated significantly and inversely with the severity of tic symptoms in both age groups at the time of MRI scan, suggesting that the larger volumes in children represented a compensatory response to help attenuate symptoms, presumably in response to the frequent, powerful activation of dorsal prefrontal and parietal regions when suppressing tics (Peterson et al., 1998), which TS children often do throughout their day, particularly in social settings. We speculate that children who exhibit this neuroplastic, compensatory response in prefrontal and parietal cortices are the subset of children whose tics attenuate progressively during adolescence; in contrast, those who for unknown reasons do not generate this neuroplastic response (and who therefore have smaller than average prefrontal and parietal volumes) have reduced inhibitory cortical reserve and are those whose tics either do not remit or even increase during adolescence. This latter group, whose tic symptoms do not attenuate, are more likely to compose clinical samples of adults with TS, such as the adults in our sample who had severe tic symptoms, thereby accounting for the significantly larger volumes in the TS children in our sample and the significantly smaller volumes in TS adults.
In this same large sample of children and adults, the overall volumes of the hippocampus and amygdala were significantly larger (3% and 6%, respectively) in the TS group (p < 0.006). Surface analyses showed that overall hippocampal enlargement in the TS group derived from larger local volumes of the head and medial surface of the hippocampus, over the length of the dentate gyrus (Peterson et al., 2007). Local volumes in these subregions correlated inversely with the severity of tic, OCD, and ADHD symptoms (Fig. 6), even though these symptoms correlated minimally with one another. These findings suggest that local enlargement of the dentate gyrus has a compensatory, neuromodulatory influence over tic-related symptoms, similar to enlargement of the dorsal prefrontal cortex, a structure with which the hippocampus is connected and works in concert. This interpretation of experience-dependent hypertrophy is consistent with the known plasticity of the dentate gyrus.
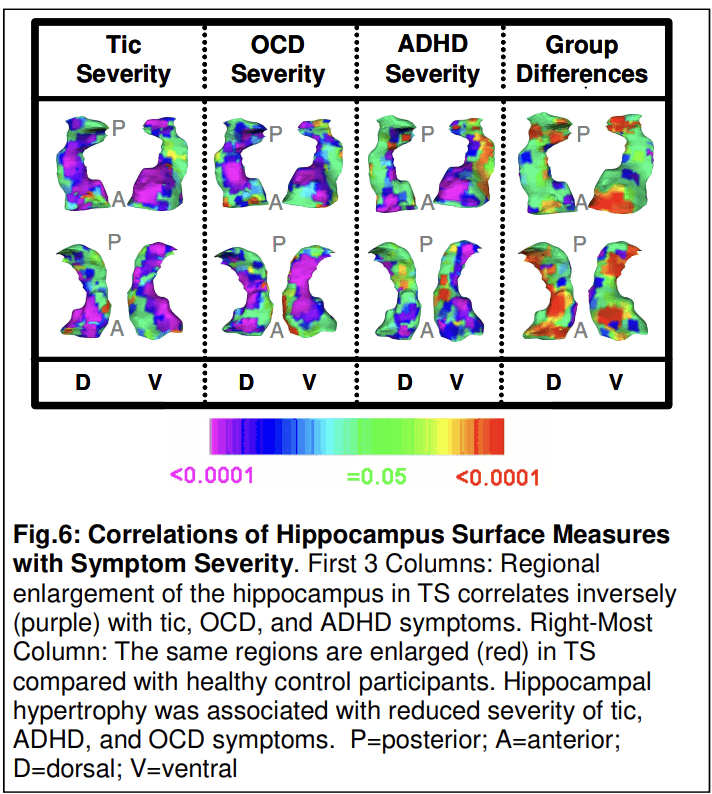
Studies such as these have yielded a theory of TS pathogenesis in which the severity and persistence of symptoms is determined by a developmental interplay between a genetically determined biological vulnerability (hypoplasia in motor portions of CSTC circuits) and experience-dependent plasticity in portions of CSTC circuits (particularly frontal cortex and hippocampus) that exert top-down control over activity in those motor loops. The capacity to generate a plastic hypertrophy contributes to better inhibitory control and less severe tic symptoms, whereas failure to generate a plastic response produces more severe symptoms and the persistence of tic symptoms into adulthood (Fig. 7). TS is thus an illness in which the expression of a specific genetic burden is modified by compensatory and neuromodulatory responses in distributed neural systems which determine whether and to what degree an individual child develops these behaviors, and whether those symptoms will remit or attenuate during adolescence (Plessen et al., 2009).
Attention-deficit/hyperactivity disorder
A similar balance between a maturational vulnerability and neuroplastic compensation seems to operate in determining the severity of ADHD symptoms. Cross-sectional imaging studies of individuals with ADHD have suggested the presence of reduced gray matter in frontal and anterior temporal cortices (Sowell et al., 2003). Those studies suggest the absence of an interaction of diagnosis with age, indicating that the magnitude of the differences from healthy controls in gray matter is reasonably constant in these regions from childhood through adolescence. A large, longitudinal study of 223 ADHD youth and 223 healthy controls, however, demonstrated that these group differences detected in prior cross-sectional studies were not static, but instead represented a substantial delay (approximately 3 years) in maturation of frontal and temporal gray matter in the ADHD group relative to controls, finally catching up to control values by early adulthood (Shaw et al., 2007). This delayed maturation and eventual catch-up may explain the general improvement of impulsivity and hyperactivity commonly noted during adolescence in prior longitudinal phenotypic studies of ADHD.
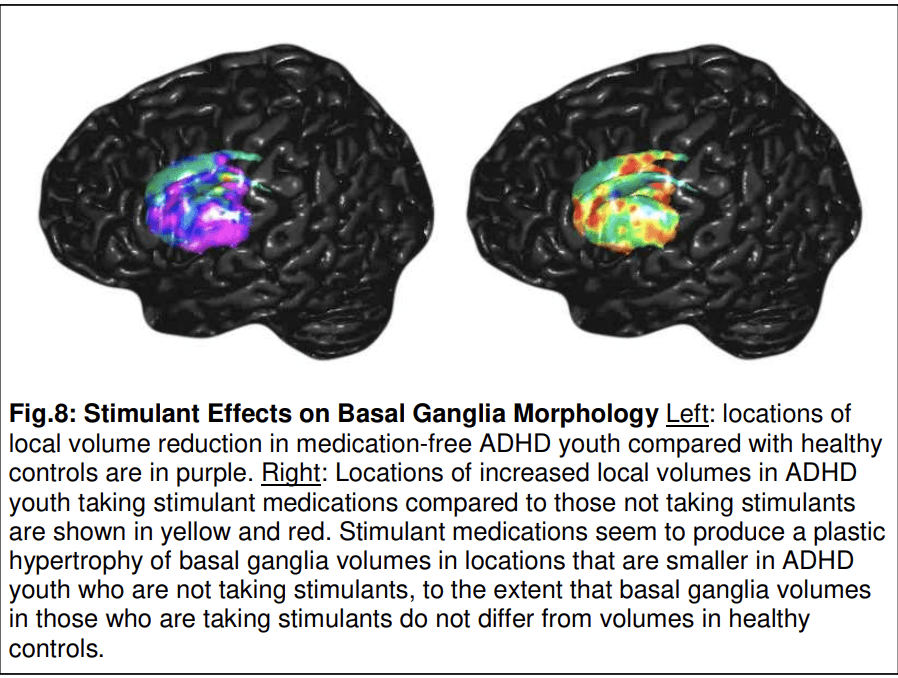
Another maturational vulnerability noted consistently in prior studies of ADHD is hypoplasia of the basal ganglia (Norman et al., 2016; Sobel et al., 2010) and the pulvinar nucleus in the thalamus (Ivanov et al., 2010), which together with the frontal and temporal dysmaturation suggests that CSTC circuits are hypoplastic, as in Tourette syndrome, though in cognitive more so than in motor portions of those circuits. Like the cortical portions of those circuits, the hypoplasia of the basal ganglia may not be immutable, however, as the use of stimulants compared with non-use in youth with ADHD has been associated with enlargement of the same portions of the basal ganglia (Fig. 8) and thalamus that are hypoplastic in those not taking stimulants (Ivanov et al., 2010; Sobel et al., 2010). In other words, stimulants may normalize localized volume abnormalities in the basal ganglia and thalamus portions of CSTC loops, presumably representing plasticity in these subcortical nuclei, but conditioned and made possible by the stimulation of dopamine receptors in the basal ganglia that stimulant medications provide.
Evidence for plasticity has also been detected in the hippocampus, with effects nearly identical to those observed in TS. Overall volumes of the hippocampus were nearly 7% larger in the ADHD compared with healthy control groups, with enlargements localized primarily to the head of the hippocampus. In those same locations, local volumes correlated inversely with the severity of symptoms, such that the larger the deviation from control values, the less severe were the symptoms (Plessen et al., 2006). These findings suggest that the local hypertrophy in the head of the hippocampus is a response to help compensate for the presence of functional disturbances in the hypoplastic circuits subserving cognitive and executive functions in ADHD.
Depressive illness
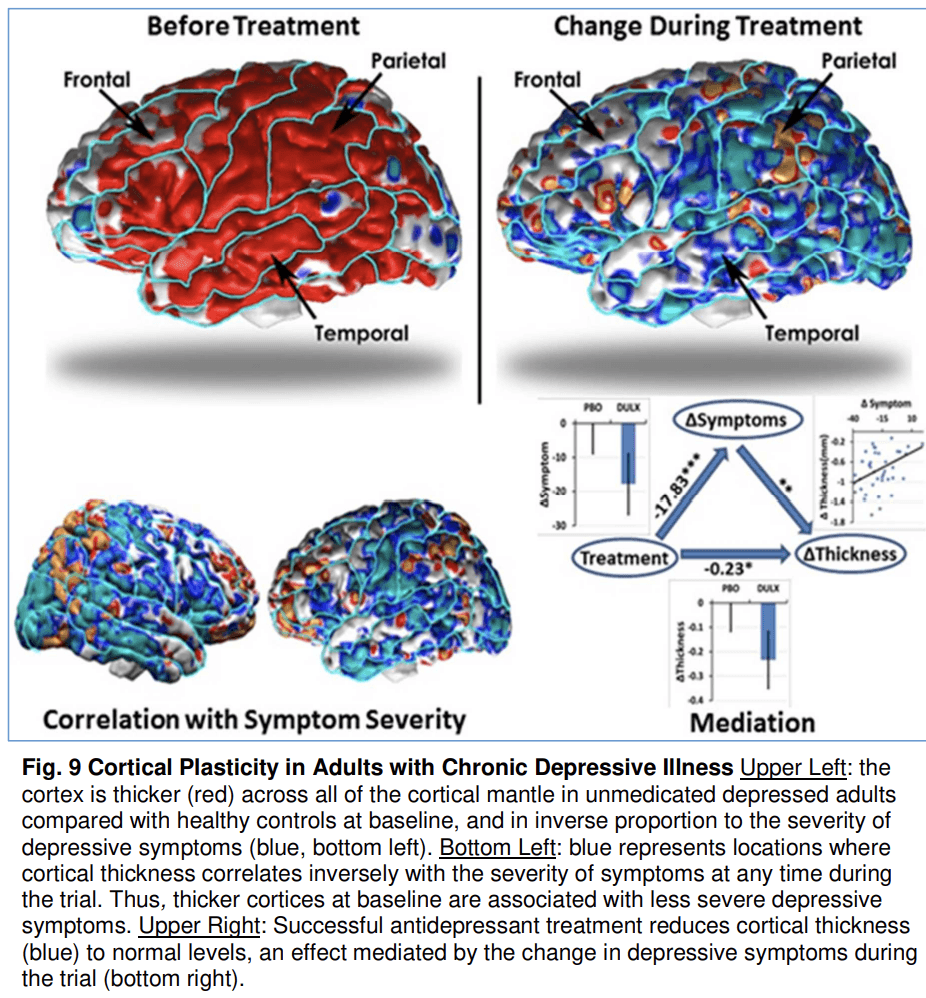
Most of the evidence for activity-dependent plasticity in psychiatric illnesses comes from naturalistic observational studies as described above. The yoking of imaging studies to randomized controlled trials (RCTs), however, affords the opportunity of studying brain changes associated with true control of an experimental variable, in this case treatment assignment. This experimental control has given us probably the strongest evidence to date for the importance of plasticity in modulating illness severity. MRI acquisition was yoked to a 10-week randomized, placebo-controlled RCT of the antidepressant medication duloxetine in treating previously unmedicated adults with dysthymia (Bansal et al., 2018). At study baseline, the depressed group had significantly thicker cortices across all portions of the neocortex compared with a matched healthy control group, and in direct inverse proportion to the severity of their depressive symptoms (Fig. 9), suggesting that the cortical hypertrophy was adaptive and helped to attenuate symptoms. Medication reduced symptoms compared with placebo, and it also normalized cortical thickness measures, whereas placebo had no effect. Mediation analyses showed that medication reduced cortical thickness through the reduction of symptoms (rather than reducing symptoms by way of normalizing cortical thickness), suggesting that adaptive cortical plasticity was no longer needed in those who responded to medication, and therefore thickness values returned to normal. Although this study does not directly assess the role of coritcal plasticity in adolescent depression, depression most commonly begins in adolescence or young adulthood, suggesting that neural plasticity during adolescence (or its failure in some youth) may play an important role in pathogenesis. It also illustrates that substantial remodeling of brain structure occurs beyond childhood and that cortical hypertrophy may occur when an individual attempts to control symptoms.
Conclusions
These examples reveal that in humans, ample evidence suggests that plasticity plays a role in the structural brain changes observed in TS, ADHD and depression, in pathogenesis and natural history of these disorders, and in treatment-induced symptom attenuation.
Cortical development in adolescence: the roles of intrinsic and environmental factors
Introduction
Initially, brain development is genetically guided, but after birth, the brain is shaped by a wide range of experiences including sensory stimuli, social relationships, intellectual stimuli and stress (Ordaz et al., 2010; Yang et al., 2012). During adolescence, external stimuli are implicated in anatomical and functional changes in the brain. Apart from the structural changes, brain neurotransmitter systems also undergo dramatic changes. This section highlights some of the neurophysiological processes that underlie these changes in a nutshell. Whereas specific stimuli are important during critical periods in specific areas such as the visual system, more general stimuli such as social, emotional and cognitive stimuli are the triggers for changes in brain areas undergoing maturation during adolescence. The changes in neurocircuitries start with long-lasting, activity-dependent changes in synaptic efficacy, embodied in long-term potentiation (LTP) and long-term depression (LTD) (Section 4.2). Glutamate and glutamate-receptors are critically involved in the delimitation of the “critical”, or more correctly “sensitive”, periods for these changes (Section 4.3). Further, the endocannabinoid system is critically involved in the regulation of the glutamate system (Section 4.4). Structural changes resulting from changes at the level of neural networks and some resulting changes in neurotransmitter systems are the subject of 4.5 Brain maturation and neuroanatomical development in adolescence, 4.6 Development of neurotransmitter systems. Adolescence is associated with an increase in risk-taking behaviors. The initiation of drinking alcohol, tobacco smoking, and cannabis use typically begins in the early teen years (Johnston et al., 2018). How these behaviors might interfere with normal brain development is the subject of Section 4.7. Finally, some promising ongoing studies that will help to further unravel the influence of external factors on the development of the adolescent brain are described (Section 4.8).
Experience-induced cortical maturation during sensitive periods
An individual's ‘experience’ helps shape his or her neural circuitry and is therefore crucial for shaping the adult behavior repertoire. Animal and human studies have revealed that the reorganization of neural circuits in the adolescent brain involves many of the same developmental processes that are used during the initial construction of the nervous system: dendritic pruning, the loss of synaptic contacts and the strengthening of often-used synapses (Koss et al., 2014; Andersen et al., 2000; Huttenlocher, 1979; Lenroot and Giedd, 2006; Sowell et al., 2001).
A plethora of deprivation studies have shown that an intact nervous system fails to develop normally when submitted to decreased sensory or motor input (for a comprehensive review, see Kolb (2018)). The activity-dependent pruning of synaptic contacts plays a critical role in the shaping of neuronal circuitries in response to environmental influences (Henson et al., 2017) and there are critical periods for these events, with the most thoroughly studied being the visual system. Although during development, the brain is influenced by multiple experiences, most experiments are performed in laboratory animals where manipulation of a single experiential domain permits the examination of selective activity-dependent pruning.
Long-term potentiation (LTP) and long-term depression (LTD) are neural responses that are critically involved in synaptic pruning and strengthening (Tessier and Broadie, 2009). Afferent sensory inputs to the cortex are relayed by thalamocortical neurons and provide stimuli that result in either LTP or LTD (Bear, 2003; Selemon, 2013; Selemon and Zecevic, 2015). If the input to the cortex provides repeated synchronized stimuli, the result will be a LTP of the postsynaptic neuron and the subsequent strengthening of the synaptic connection. When the activity is asynchronous, it may result in LTD and the subsequent weakening and eventually loss of the synapse. The time window or the critical period when synaptic modification occurs depends on the brain region and the specific features of the sensory stimuli (Levelt and Hubener, 2012). The absence of appropriate stimuli during the critical period can lead to the improper development of cortical neural circuits and the development of inappropriate capabilities and behaviors that persist into adulthood.
It has been suggested that there are parallels between the processes involved during adolescence and early sensory/critical periods (Crews et al., 2007; Bossong and Niesink, 2010). It is assumed that the higher-order neural circuits that mature during adolescence also possess critical periods or more likely sensitive periods since modification of networks can occur outside of the “critical period”. Experiences that occur before or after the sensitive period will have less effect on the maturation of the circuit but some plasticity can still occur. The experience-dependent shaping of “higher-level circuits” cannot occur until the “lower-level circuits” have become competent (Knudsen, 2004; Tau and Peterson, 2010). Higher-order association cortices mature only after lower-order somatosensory and visual cortices, the functions of which they integrate, have developed. Phylogenetically older brain areas mature earlier than newer ones (Gogtay et al., 2004). Regions associated with more primary functions (e.g., primary motor cortex) develop earlier than regions involved in more complex and integrative tasks (Fig. 10).
Although higher-order inputs might shape specific neural networks within the PFC in a fixed order, brain development is a dynamic process. The development of complex functions involves cascades of sensitive periods that affect different levels of processing at different ages (Knudsen, 2004).
Glutamate, the NMDA receptor and its role during sensitive periods
Glutamate, the most important excitatory neurotransmitter, activates two different groups of receptors: ionotropic and metabotropic. Ionotropic glutamate receptors are ligand-gated ion channels that are divided into three main sub-groups: 2-amino-3-3-hydroxy-5-methyl-isoxazol-4-yl propanoic acid (AMPA), kainate and N-Methyl-d-aspartic acid (NMDA) receptors (for a review, see Traynelis et al. (2010)). Glutamate and the NMDA receptor have been shown to play key functions in the fine-tuning of neural circuitry during development (Colonnese and Constantine-Paton, 2006; Hofer and Constantine-Paton, 1994; Citri and Malenka, 2008).
The majority of NMDA receptors are tetrameric assemblies of two GluN1 and two GluN2 subunits (Vyklicky et al., 2014). The two GluN2 subunits, GluN2B and GluN2A, play different roles in the modulation of synaptic elimination (Ohno et al., 2010). GluN2B is predominant at the start of a sensitive period, while the number of GluN2A subunits grows until they eventually outnumber GluN2B subunits (Carmignoto and Vicini, 1992; Erisir and Harris, 2003; Barria and Malinow, 2005). The greater the ratio of GluN2B to GluN2A subunits, the longer the NMDA receptor will remain open. The loss of GluN2B subunits is thought to be instrumental in terminating the sensitive period of plasticity (Erisir and Harris, 2003).
The endocannabinoid system
The endocannabinoid system (ECS) also plays a crucial role in neurodevelopment. The ECS consists of two G-protein-coupled cannabinoid receptors (CB1 and CB2), endocannabinoid ligands (endocannabinoids; primarily anandamide (AEA) and 2-arachidonoylglycerol (2-AG)), and the enzymes responsible for the synthesis and degradation of these ligands (De Petrocellis and Di Marzo, 2009). The CB1 receptor is thought to mediate most of the CNS effects of cannabinoid drugs, while the CB2 receptor is more abundant in peripheral tissues, particularly immune tissues (Svizenska et al., 2008). CB1 receptors are predominantly located presynaptically and activation by endocannabinoids can inhibit transmission in both glutamatergic and GABA-ergic synapses (Freund et al., 2003).
The CB1 receptor is expressed at high levels throughout the cerebral cortex, hippocampus, basal ganglia, and cerebellum (Herkenham et al., 1990; Herkenham et al., 1991). The endocannabinoid system undergoes functional and structural changes during adolescence (Rodriguez de Fonseca et al., 1993; Mato et al., 2003; Ellgren et al., 2008; Dalton and Zavitsanou, 2010; Eggan et al., 2010). In the rat PFC, the expression of CB1R gradually decreases from mid- to late adolescence (Ellgren et al., 2008; Heng et al., 2011). In humans, the expression of CB1R increases from infancy to young adulthood in the frontal cortex, striatum and hippocampus (Mato et al., 2003). Although the role of the ECS in adolescent neurodevelopment is not yet fully understood, emerging evidence indicates a correlation between CB1 density in certain regions and the sensitive period for that region (see review by Bossong and Niesink (2010)). It might be expected that more CB1 receptors are present in specific areas rich in glutamatergic synapses at the beginning of a sensitive period and are present in lower levels at the end of the sensitive period. The activation of CB1 receptors on the terminals of principal neurons controls excitatory synaptic responses in the forebrain. This suggests that excitatory synaptic transmission in forebrain is directly modulated by the CB1 receptors expressed on presynaptic axon terminals that originate from glutamatergic neurons (Domenici et al., 2006).
Rubino et al. (2015) showed that THC exposure during adolescence resulted in impaired endocannabinoid signaling and impaired endocannabinoid-mediated LTD in the adult PFC. Furthermore, THC altered the maturational fluctuations of NMDA subunits and was associated with larger amounts of gluN2B in adulthood. Since gluN2B subunits of the NMDA receptor are the immature form of the receptor, THC may extend the sensitive period for plasticity in the PFC. Finally, adolescent THC exposure can alter a range of behaviors including cognition in adulthood (Rubino and Parolaro, 2016). All these effects seem to be triggered by the disruption of the physiological role played by the ECS during adolescence. Meanwhile, evidence for a role of adolescent endocannabinoid signaling in regulating the hypothalamic pituitary adrenal (HPA) axis stress responsivity and the development of emotional behavior is accumulating (Dow-Edwards and Silva, 2017; Lee and Gorzalka, 2015).
Brain maturation and neuroanatomical development in adolescence
Different areas of the cerebral cortex develop at different speeds. The initially large production of neurons and synaptic connections is followed by a decrease in production (Nie et al., 2013). This decrease in gray matter is accompanied by an improvement in function. Tamnes et al. (2017) recently conducted a multisample MRI study to investigate the relationship between cortical volume and cortical thickness from late childhood to early adulthood (Tamnes et al., 2017). Their results showed that although most of the individual variation in adult cortical volume is due to variation in surface area and not thickness (Im et al., 2008), the greatest contributor to the decrease in volume that occurs between the ages of 7 and 29 years is thinning of the cortex (Tamnes et al., 2017). This is due to pruning. Pruning is the retraction of axons and dendrites which occurs with experience and coincides with an improvement of efficiency of remaining neural elements. During adolescence, pruning occurs predominantly at asymmetric synapses, which are primarily excitatory glutamatergic synapses (Brenhouse and Andersen, 2011). Therefore, pruning mainly impacts glutamatergic neurotransmission, especially in the PFC (Bourgeois and Rakic, 1993). Within cortical networks, GABAergic interneurons are the primary neurons in charge of the dynamic adjustment of excitation levels. The role of GABAergic interneurons in the maintenance of the dynamic balance between excitation and inhibition is essential to cortical function (Shu et al., 2003; Meunier et al., 2017). Functional maturation of GABAergic interneurons in the PFC lasts until late adolescence and involves NMDA-receptors (Li et al., 2011; Li et al., 2016). Synaptic pruning at excitatory synapses and the sparing of inhibitory synapses changes the balance of excitation/inhibition both in individual neurons and within networks. Many of the functional processes involved in circuit maturation and the fine-scale refinement of network activity depend on proper interactions between GABAergic and glutamatergic synapses.
Development of neurotransmitter systems
Adolescents are more impulsive, exhibit more risk-taking, are more responsive to incentives and have poorer inhibitory control than adults (Casey and Jones, 2010; Lydon et al., 2014). These behavioral differences are accompanied by the incomplete maturation of frontostriatal connections and neurotransmitter systems (Somerville and Casey, 2010). During adolescence, different neurotransmitter systems develop at different rates. Differences in maturation of the mesolimbic and mesocortical dopamine (DA) systems, and a slower development of the descending glutamatergic cortical innervation of the nucleus accumbens are often cited as the reason for the increase in risky behavior and substance abuse in adolescence (O'Dell, 2009).
O'Dell (2009) also suggested that adolescents have a relatively active glutamate system and an underdeveloped GABA system, both of which contribute to more robust reinforcement effects and weaker withdrawal effects than are observed in adults. This increase in reinforcement and weaker withdrawal symptoms compared to adults may make adolescents more vulnerable to substance abuse than adults (O'Dell, 2009).
Changes in neurotransmitter levels have been most thoroughly studied in animals. In rats, NMDA receptors peak in cortex during early adolescence, followed by a loss of approximately 1/3 by postnatal day 60 (P60) (Insel et al., 1990). Weanling rats exhibit only approximately 70% of adult levels of the DA transporter in the PFC and nucleus accumbens (Coulter et al., 1997). However, by early-mid adolescence (P28–35), binding to DA and 5HT transporters occurs at levels not significantly different from those found in adults (Tanner, 1991). In rats, DA inputs to the superficial layers of the PFC increase until P35, while increases in other layers continue through to P60 (Kalsbeek et al., 1988). Neurochemical assessments of DA concentrations in the frontal pole of the rat brain also showed that there is a marked increase in DA activity between postnatal 4 and 6 weeks of age (Leslie et al., 1991). In nonhuman primates, DA inputs to the PFC also increase during adolescence and peak at levels notably higher than those seen either earlier or later in life (Rosenberg and Lewis, 1995; Lewis, 1997). All these changes in neurotransmitter systems suggest that the PFC undergoes a protracted period of development lasting well into adulthood.
Environmental factors that influence cortical brain development: social behavior, alcohol, tobacco and cannabis use
The initiation of drinking alcohol, tobacco smoking and cannabis use typically begins in the early teen years (Johnston et al., 2018). Exposure to exogenous substances, such as alcohol, nicotine, and cannabis can interfere with normal brain development during adolescence and produce structural and behavioral disruption. During adolescence, there is also an increasing influence of the social environment, especially since the activities of peers become increasingly important. The social environment of an adolescent has a profound, but complex, influence on drug use, and drug exposure during adolescence markedly alters later social interactions, both in animals and in humans (Trezza et al., 2014).
Social behavior
Social play behavior in animals is the result of coordinated activity in a network of corticolimbic structures (the social brain), and its monoamine, opioid and endocannabinoid innervation (Vanderschuren et al., 2016). Engaging in social play behavior has been shown to be important for proper maturation of brain and behavior.
Bell and colleagues found that play behavior promotes pruning in the medial PFC and nucleus accumbens (Trezza et al., 2008; Trezza et al., 2014; Bell et al., 2010). Social isolation in adolescence decreases the number of dendritic dopamine receptors in the PFC. This effect may be tempered by local CB1-mediated retrograde signaling, suggesting an interaction between the ECS and social experience-induced maturation of the dopaminergic cortical system (Fitzgerald et al., 2013). The permanent behavioral deficits observed in rats isolated during adolescence are thought to result from disturbances in pruning of the glutamatergic neurons in limbic brain regions and dopaminergic neurons in the ventral tegmental area that project to the PFC (Fitzgerald et al., 2013).
Alcohol
Ethanol's detrimental effects are mediated by the modulation of the expression of NMDA receptor subunits, which are highly dependent on the phase of development, the brain area and the substructure levels. Alcohol regulates the GluN2A to GluN2B expression ratio. As stated above, this NMDA receptor subunit ratio ultimately defines the sensitive period for synaptic plasticity (Naassila and Pierrefiche, 2019) and may be a mechanism whereby alcohol disrupts neuroplasticity during adolescence.
A cross-sectional study of a large adolescent cohort found that frontal and temporal volumes were smaller and the frontal, temporal and cingulate cortices thinner in a high alcohol exposure group (Pfefferbaum et al., 2016). Data from longitudinal studies suggest that cortical thickness declines in the PFC and temporal regions and white matter growth is attenuated in individuals who initiated alcohol consumption during adolescence (Luciana et al., 2013; Squeglia et al., 2015; Squeglia et al., 2014). However, in a review of human imaging studies of adolescent alcohol and drug users, Silveri et al. concluded that the available structural imaging data indicate that frontal lobe volume abnormalities are both a pre-existing risk factor and a consequence of adolescent alcohol use (Silveri et al., 2016).
Nicotine
Nicotine can both activate and desensitize neuronal nicotinic acetylcholine receptors (nAChRs) (Dani, 2001). nAChRs regulate catecholamine development during the adolescent period. Exposure to nicotine during adolescence is thought to interfere with limbic circuitry and to produce enhanced vulnerability to (nicotine) addiction, increased impulsivity, and mood disorders (Dwyer et al., 2009).
Prospective and longitudinal human studies have indicated that adolescent exposure to nicotine has permanent effects on behavior such as an increased risk of developing substance use disorder and mental health problems, including anxiety and depression (Counotte et al., 2011). Therefore, while the effects of nicotine on behavior are established, imaging studies would be helpful to understand the structural basis for these behavioral changes.
Cannabis
Cannabis exposure during adolescence may disrupt the last steps of brain development, thereby altering the maturation of higher cognitive function (Bossong and Niesink, 2010). Exposure to cannabis during adolescence can affect neurobehavioral functions, especially cognition, emotional functioning, the risk of psychosis, and addiction. Exposure to exogenous cannabis is thought to interfere with the normal trajectories and mechanisms underlying brain maturation (Rubino and Parolaro, 2014; Bossong and Niesink, 2010; Renard et al., 2016).
A strong association has been found between early, frequent, and heavy adolescent cannabis exposure and poor cognitive and psychiatric outcomes in adulthood; these impairments have been related to the duration, frequency, dose and age at onset of cannabis use. Cognitive deficits may wane with abstinence, but abnormalities may be long-lasting in subjects who began smoking cannabis before age 16 (Schubart et al., 2011; Galvez-Buccollini et al., 2012). Several prospective longitudinal studies have shown that cannabis use is associated with almost a 4-fold increase in psychotic disorders, particularly schizophrenia, than is observed in controls (Marconi et al., 2016). In short, consumption of cannabis during adolescence is detrimental to the normal development of the brain.
Future Developments
In adolescence, the activity-dependent pruning of synaptic contacts plays a critical role in shaping neuronal circuitry (Bossong and Niesink, 2010; Henson et al., 2017). The large-scale loss of synapses is one way in which neuronal circuits are refined by experience. In recent years, the results of both animal and human studies have provided further support for the notion that disrupting the glutamatergic system during adolescence (either directly or indirectly through a myriad of exogenous substances) plays an important role because it results in aberrant structures and behaviors later on.
Some progress has been made in the understanding of individual brain development by the examination of the brain connectome which is unique to each individual (Barch et al., 2013). By using connectivity fMRI, Finn and colleagues were able to follow phenotypic changes in the cerebral cortex in individual subjects (Finn et al., 2015). The authors noted that analogous to the exposome, functional connectivity profiles act as a ‘fingerprint’ that can be used to accurately identify subjects in a large group. The use of this technique in prospective epidemiological studies in which individuals can be followed from before they have begun using alcohol, nicotine or cannabis into adulthood would allow conclusions to be drawn about the effects of exposure to these substances on brain development. Using such a personalized approach would represent a substantial step forward in the study of the consequences of substance use on (individual) brain development.
Many questions remain regarding the impact of changes in the social environment and the role of substance use in the developing brain. To more comprehensively explore the impacts of substance use and other experience on adolescent brain development, longitudinal studies including large cohorts of children ranging in age from childhood through adolescence using neuroimaging and other sophisticated tools are needed (Volkow et al., 2018). Prospective longitudinal studies, such as The Adolescent Brain Cognitive Development (ABCD) Study (Feldstein Ewing et al., 2018) in the US, and the Generation R Study in The Netherlands (Jaddoe et al., 2012) will help us to understand the effects of alcohol, tobacco and marijuana use on adolescent brain, cognitive, social, and emotional development.
Exposure to stimulants during development: Translational studies and implications
Introduction
Illicit drug use that is initiated before 14 years of age increases the risk for lifelong addiction by 4–11 fold compared to drug use initiated at 16 years or older (Grant et al., 2011). Teenagers with untreated attention deficit hyperactivity disorder (ADHD) initiate illicit drug use earlier than the general population, which makes them more vulnerable to develop addiction (Dunne et al., 2014; Kaye et al., 2014). However, treatment before adolescence reduces risky behaviors and substance use according to most (Mannuzza et al., 2003; Mannuzza et al., 2008; McCabe et al., 2016); Wilens et al., 2003), but not all (Hinshaw and Arnold, 2015), studies.
Treatment for ADHD symptoms typically targets cortical functions such as attention, working memory, and impulsivity. As discussed in Section 3, the development of cortical thickness in ADHD is delayed three years relative to age-matched controls and is delayed even more when localized to the medial prefrontal cortex (Shaw et al., 2007). This delay is due to a slower cortical growth rate in children with untreated ADHD compared with the rate for control subjects or those children whose ADHD was treated (Shaw et al., 2009). The abnormal trajectory of ADHD normalizes by 25 years of age in a subset of patients (Shaw et al., 2013), although it is not clear if this is due to stimulant treatment, including methylphenidate (MPH) (Pretus et al., 2017; Schweren et al., 2013). As clinical studies can only establish a relationship between ADHD treatment and behavioral and anatomical changes, preclinical studies are well poised to determine the causative effects of stimulant treatment on brain and behavior development.
The goal of treatment should move beyond intervention and focus on prevention. There are three components that need to be taken into account for the prevention of most psychiatric conditions: 1) the age of exposure; 2) age of assessment; and 3) gender. The timing of treatment needs to coincide with a sensitive period to have lifelong, beneficial effects (Andersen, 2005). A sensitive period occurs when the maturing brain is most influenced by external stimuli such as medication or experience. However, the drugs must interact with developing brain tissue before they can initiate enduring changes. The third factor, gender, is under-studied. We proposed a model that demonstrates how the first two factors interact with early drug exposure resulting in lasting—and opposite–changes compared to the same drug exposure in adulthood (Andersen, 2005).
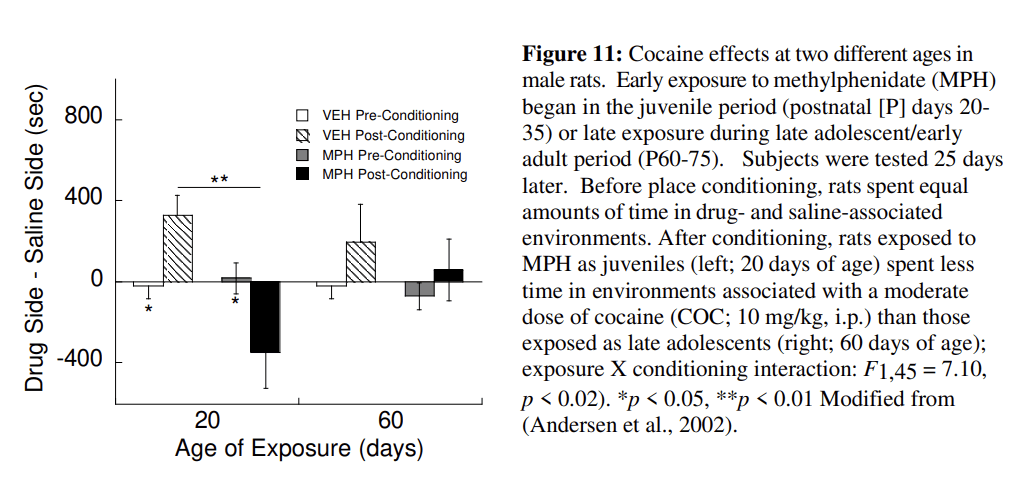
Behavior and neuronal changes in ADHD exemplify how these three factors work in a preventative approach to reduce substance use. Paradoxically, the treatments of choice for ADHD are psychostimulants such as MPH and amphetamine. The administration of psychostimulants during a sensitive period of reward development leads to a permanent reprogramming of the underlying neural circuitry. For example, pre-pubertal treatment of ADHD with stimulants decreases risk for substance use in a sub-population of teens with ADHD (Wilens et al., 2003). Stimulant exposure prior to the maturation of the reward system in animals produces delayed effects in adolescence (see Fig. 11). Treatment later in adolescence and adulthood, that is, after the sensitive period, results in different effects than in younger subjects. In addition to psychostimulants, Section 5.5 discusses additional mechanisms either underlying ADHD itself or its treatment that can reduce addiction-related behaviors and alter brain function during development.
A caveat to a number of experiments that have examined early drug exposure is the use of normal animals. Proposed animal models for ADHD include the dopamine transporter knock-out mouse, viral-mediated overexpression of the transporter (Adriani et al., 2009), the Spontaneous Hypertensive rat (Sagvolden and Johansen, 2012), the Naples High Excitability rat (Gonzalez-Lima and Sadile, 2000), and the use of 6-hydroxydopamine to deplete dopamine levels (Shaywitz et al., 1976); all models reviewed by Russell (2011). These models display a number of different behaviors that are found in ADHD with no one model capturing the full clinical spectrum. Reductions in behaviors are observed in response to acute administration of stimulants (Leo et al., 2018; Oades et al., 2005; Jordan et al., 2014; Lukkes et al., 2016), but few of these models have been used to examine the enduring effects of developmental treatment.
Age of exposure
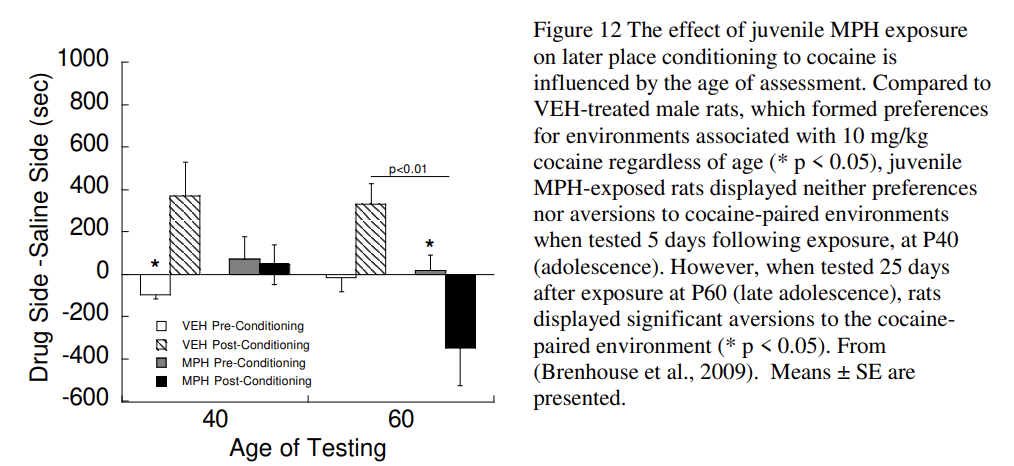
Clinical studies have found enduring reductions in substance use in adolescents with ADHD who were treated in childhood with psychostimulants (Mannuzza et al., 2003; Mannuzza et al., 2008; McCabe et al., 2016; Wilens et al., 2003). These changes are more strongly associated with children with comorbid conduct disorder (Mannuzza et al., 2008) whom have higher rates of substance abuse than individuals with a diagnosis of ADHD alone (Disney et al., 1999). Animal studies are uniquely suited to clarify the effects of timing on vulnerability to use drugs of abuse. Juvenile exposure to MPH in rats (Postnatal day, P20–35) produces an aversion to cocaine-associated environments. Similarly, cocaine self-administration is reduced following MPH treatment from P30–240, which includes exposure during the periadolescent period (Thanos et al., 2007). In contrast, post-pubertal exposure alone (P50–65) produces a place preference for cocaine (Andersen et al., 2002) (Fig. 12) that is consistent with increased cocaine self-administration in animals exposed to MPH during P35–60; (Brandon et al., 2001). The observation that MPH exposure at an earlier age increases cocaine self-administration (Crawford et al., 2011) suggests a possible second sensitive period occurs prior to the one identified at P20–35. MPH treatment at P7–35 (juvenile to early adolescence) increases cortical dopamine innervation in adulthood by 55% compared to controls while adult-exposed animals showed no change in innervation (Gray et al., 2007). These data support a sensitive period for the effects of MPH that varies depending on the measure being examined (cocaine preference, self-administration, DA innervation) and is quite different from the classic sensitization that occurs following repeated administration of psychostimulants to adults.
Age of assessment
The enduring effects of early drug treatment are often not observed immediately after exposure. Drug experimentation and use during adolescence is typically elevated and then decreases with maturation into adulthood (SAMHSA, 2014). Early drug exposure interacts with a sensitive period to sculpt the brain with the effects manifesting when regional brain development is complete. For example, the expression of the behavioral effects of MPH in rats is delayed until late adolescence or later (Andersen, 2005; Brenhouse et al., 2009). Also, Fig. 12 shows that juvenile MPH blocks preference for a cocaine-associated environment at P40 and produces an aversion for the environment at P60. Thus the expression of the effects of MPH varies with the age of assessment. In individuals with treated and remitted ADHD, the rate of gray matter changes is closer to control values than the rate in individuals with untreated ADHD (Shaw et al., 2013). Taken together, the changes induced by stimulant medication emerge in late adolescence or adulthood.
Knowing that treatment effects are delayed has two implications for intervention. First, unmasking of changes is an important prevention for later, and more serious, behaviors that are associated with ADHD. For example, early treatment is associated with reduced levels of substance abuse that occur in untreated ADHD (Wilens et al., 2003). Second, the early intervention can result in improved functioning later in life that will increase self-sufficiency, self-esteem, and lead to a greater quality of life.
Gender/sex differences
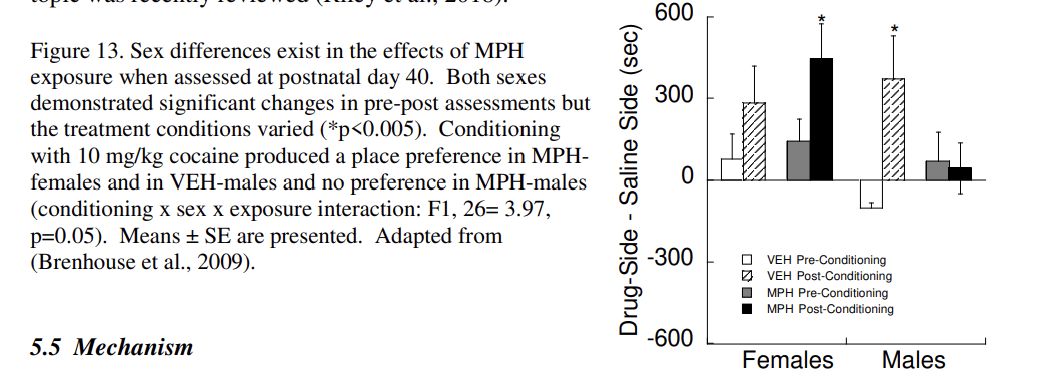
The prevalence of ADHD is higher in boys than girls, and ADHD boys are at higher risk for substance use whereas rates in girls do not differ from controls (Hinshaw et al., 2012). However, a number of diagnostic issues in girls may underestimate both the prevalence of ADHD and drug use. Rodent studies show that sex differences in sensitivity to stimulants later in life are influenced by the age of drug administration. Prenatal exposure to cocaine has no effect on cocaine-induced male locomotion in adulthood, but sensitizes female locomotion (Torres-Reveron and Dow-Edwards, 2006). Juvenile exposure (P20–35) to MPH (or cocaine) produces an aversion in males (Andersen et al., 2002; Brenhouse et al., 2009; Carlezon Jr. et al., 2003), but increases preferences for cocaine-associated environments in females (Fig. 13; (Brenhouse et al., 2009). In all, sex differences in stimulant responses are apparent in behavior, neurochemistry, and the neuroendocrine system; the topic was recently reviewed (Riley et al., 2018).
Mechanism
Magnetic resonance imaging (MRI) provides a point of translation between humans and animals. Several clinical studies of MPH effects have revealed lasting alterations in blood flow using functional MRI. For example, MPH treatment for ADHD increases blood flow in the dorsal anterior cingulate cortex region in adults (Bush et al., 2008). Children (10–12 years) with ADHD who were treated with MPH for 16 weeks show increased cerebral blood flow in the striatum and thalamus in response to a MPH challenge one week after treatment was stopped (Schrantee et al., 2016). No significant changes are observed in blood flow in adults or in the placebo group. In parallel with the clinical findings, juvenile rats exposed to MPH have increased blood flow following a MPH challenge in the medial prefrontal cortex and striatothalamic regions (Andersen et al., 2008).
A different approach to understanding blood flow changes is pharmaco-MRI (phMRI). PhMRI detects changes in blood flow following a challenge with an agonist or antagonist to probe an underlying mechanism (Choi et al., 2006). Amphetamine-induced changes in blood flow are reduced with the D3 antagonist in normal, adult animals (Chen et al., 2005), suggesting D3 involvement. PhMRI is also sensitive to age-related changes. For example, acute MPH decreases blood flow in young animals and increases blood flow in older animals (Chen et al., 2010). When MPH is given chronically during the juvenile period, however, a challenge with MPH in adulthood increases blood flow in animals. These data are consistent with the hypothesis that early exposure to drugs programs the circuitry in a manner that is opposite to its acute effects.
Increases in the D3 receptor occur following extended drug psychostimulants use in adults of many species (Keck et al., 2015). D3 agonists have rewarding properties that are similar to those of cocaine (Nader and Mach, 1996). Activation of the D3 receptor is involved with the motivation to take drugs under high requirements, reactivity to drug-associated cues, and reinstatement (Sokoloff and Le Foll, 2017). In adults, treatment with a D3 antagonist reduces cocaine use (Le Foll et al., 2002). Juvenile male rats exposed to the D3 agonist ±7-OH-DPAT have reduced preferences for cocaine-associated environments, reduced locomotor activity, and reduced D3 receptors in the prefrontal cortex in adolescence (Andersen et al., 2008). D3 mRNA is decreased by MPH exposure in female rats (Lukkes et al., 2016). Therefore, within an equal, but opposite framework of developmental drug exposure, increased D3 stimulation by juvenile MPH or ±7-OH-DPAT reduces D3 expression later in life. Drug addiction in adulthood is treated with D3 antagonists (Heidbreder et al., 2005; Le Foll et al., 2002) and thus, reduced D3 resulting from early intervention could contribute to reduction of later drug addiction.
MPH treatment during a sensitive period modulates the developmental trajectory by fluctuations in the level of brain-derived growth factor (BDNF). Low levels of BDNF partly define a sensitive period of high plasticity (Morishita et al., 2015; Takesian and Hensch, 2013). Juvenile treatment with MPH increases BDNF in male rats in most studies (Andersen et al., 2008; Chase et al., 2007; Fumagalli et al., 2010) and in females (p = 0.07; (Lukkes et al., 2016). Similarly, juvenile exposure to ±7-OH-DPAT increases BDNF to approximately the same levels as MPH. The increase in BDNF reduces plasticity, and in this instance, closes the sensitive period for other drug effects.
A number of alternative targets for treatment exist that are known to interact with BDNF, and thus could modulate a sensitive period. Serotonin has been implicated in ADHD (Comings, 2001; Hall et al., 2014) and treatment in animals decreases activity and impulsivity, and increases cognitive flexibility (Adriani et al., 2006; Borycz et al., 2008). An additional potential target is the trace amine-associated receptor 1 (TAAR1) that is associated with ADHD (Leo et al., 2018; Lindemann et al., 2008; Sotnikova et al., 2009). TAAR1 modulates juvenile MPH effects (Leo et al., 2018), D3-associated activities (Siemian et al., 2017), and increases dopamine levels by modifying the DAT. While TAAR1 has the characteristics of a novel drug target, findings suggest that more research is needed (Leo et al., 2018).
Independent of known effects on sensitive period-related mechanisms, changes in noradrenergic activity are associated with ADHD and its treatment (Arnsten, 2006). Clinically, the alpha2a agonist guanfacine is typically used in conjunction with other stimulants in ~5% of children with ADHD (Sikirica et al., 2012). Both attention and impulsivity are risk factors associated with childhood/teen risk for addiction (Khurana et al., 2013). Guanfacine reduces these behaviors (Arnsten, 2009) underscoring the need to target behaviors antecedent to substance use. Noradrenergic activity modulates impulsivity in adolescent (Sun et al., 2012) and adult rats (Abela and Chudasama, 2014) and drug self-administration in adult rats (Schmidt et al., 2017). Juvenile exposure to guanfacine in rats reduced impulsivity, but also delayed the initiation of cocaine self-administration in males, while accelerating initiation of use in females (Freund et al., 2019).
Conclusions
Parallels can be drawn across humans and animals for the beneficial effects of early drug treatment in ADHD. Treatment in juveniles produces persistent changes in behavior (i.e., stimulant responses and drug taking) and neural development. The data presented demonstrate how a properly timed intervention can change the course of development.
Peer influence on adolescent decision making: dissection of relevant constructs
Adolescence is a time of life associated with increased risk taking behavior. Risk taking behaviors are those behaviors that can pose adverse consequences for health including increased traffic related injuries, increased unintentional injury and high prevalence of substance use such as binge drinking. The prevalence of these consequences differs across age and type of risky behavior. High risk taking behavior is especially common for 18- to 21-year-olds and, depending on the comparison groups, this age category can be defined as late adolescence or young adulthood. Here this age category is referred to as adolescence. It should be noted that some amount of risk taking behavior is normative and important for adolescents to explore and learn about their environment (Crone and Dahl, 2012). However, excessive amounts of risk taking behavior can pose serious and long term adverse consequences for health (Eaton et al., 2010). The first step to development of effective interventions is understanding of the mechanisms that lead to increased risky decision making in adolescence. Our current understanding of these mechanisms is limited and, for instance, efforts to delay the onset of substance use and minimize its use in adolescence have been mildly effective at best (West and O'Neal, 2004).
Adolescents are highly influenced by the social context and peers influence real world risk taking behavior. Adolescents are, for instance, 2.5 times as likely to engage in risky driving when one teen passenger accompanies them in the car and over 3 times more likely to engage in risky driving when accompanied by two or more teen passengers (Tefft et al., 2013). Similar effects are shown for criminal behavior (McCord and Conway, 2005) and alcohol use (Grüne et al., 2017; Mayer et al., 1998). These real life observations show that social context is an important factor for risky decision making. However, it is unclear which aspects within the social context lead to increases in risky decision making. To increase our mechanistic understanding of risk taking behavior, laboratory studies can be used to decompose risky decision making and investigate which aspects of risky decision making are elevated in adolescence and under which conditions. The studies discussed below focus on the influence of the social context on adolescent risky decision making and aim to dissect what aspects of the social context contribute to increased risky decision making in adolescence.
Social influence on risky decision making
In one of the earliest studies that tested peer influence, participants performed a driving game (Gardner and Steinberg, 2005). This study used an ecologically valid paradigm, i.e. driving, and peer manipulation, i.e. presence of real life friends, and showed that increases in risky decision making can be recreated in the lab. This experiment demonstrated that risk taking behavior during adolescence can be manipulated and is not just an effect of increased access to risk. To further probe why and under which conditions adolescents show increased risky decision making in the social context, follow up studies have systematically dissected social context effects. Peer influence is a broad construct and can be dissected into several different operationalizations of the social context. At the most minimal level a peer can be present, but not observing choices. Increasing levels of peer engagement are operationalized by peer observation and finally peer encouragement. A study testing how peer presence and peer observation differentially influence risky decision making in children, adolescents and adults showed that peer observation increased risky decision making in 16- to 18-year-olds while the mere presence of a peer did not (Somerville et al., 2018). Another study provided participants with information about previous choices of others (Braams et al., 2018). Results showed that participants follow choices of others and showed increased risky decision making when others had previously selected a risky option, and the inverse when others had previously selected the safe option. Developmental analyses showed that adolescents were most likely to follow safe choices of their peers and least likely to follow risky choices. These results are in contrast to the increased risky decision making under peer influence in the real world. Together, the results of these studies show that, even though adolescents show increased risky decision making in real life, the effects of social context on adolescent risky decision making are more complex than previously assumed. The risk increasing effects of peers are specific to certain types of decisions and peer manipulations.
One possible explanation for the increase in risky decision making under peer observation and peer encouragement is reputation management. Peer acceptance and status within the peer group are important to adolescents (Brown et al., 1986; Brown, 2004; Crone and Dahl, 2012) and popularity is positively related to risky behavior (Mayeux et al., 2008). Under conditions in which peers can observe decisions, these decisions have consequences for how the adolescent is perceived by peers. Under conditions in which adolescents are merely presented with information about others' choices, there are no reputational costs to making a safe decision and indeed results showed that adolescents are not prone to more risky choices in this social context.
Structural and functional changes underlying social influence on risk taking in adolescence
The brain undergoes major changes in both structure and function during adolescence. Two areas important for risk taking behavior, the ventral striatum and the prefrontal cortex, show differential developmental patterns in adolescence. An influential model on adolescent risk taking states that the ventral striatum (VS) shows hyperreactivity to rewards during adolescence and the prefrontal cortex (PFC), the seat of executive function, is not yet fully developed (Somerville et al., 2010). The ventral striatum is a region associated with receipt of rewards (Delgado, 2007). Two recent studies showed that the nucleus accumbens, a subpart of the ventral striatum involved in reward processing, showed decreases in volume over development (Herting et al., 2018; Wierenga et al., 2018). Volumetric decreases in the nucleus accumbens were related to greater behavioral reward sensitivity (Urošević et al., 2012). The PFC is related to cognitive control and shows protracted development up to at least 30 years of age (Petanjek et al., 2011). The developmental mismatch in VS and PFC has been tested both structurally and functionally. In a longitudinal study of structural changes over development, almost all participants, spanning 7–30 years, showed a developmental mismatch between subcortical structures, including the nucleus accumbens, and the PFC (Mills et al., 2014). A large, longitudinal study spanning the ages 8–27 investigating functional recruitment of the nucleus accumbens showed that the nucleus accumbens response to rewards is elevated in adolescence compared to childhood and adulthood (Braams et al., 2015). Nucleus accumbens responses to reward correlated with self-reported alcohol use in a subset of this sample aged 12–27 (Braams et al., 2016). These studies together give insight in to possible neural mechanisms underlying increased risk taking in adolescence.
Social influence
Increases in risk taking behavior under social influence can be attained in at least two different ways. There could be less activation in the PFC or increased activation in the nucleus accumbens. The effects of the social context have been tested by using a virtual driving game in which participants are instructed to drive as fast as possible from A to B (Gardner and Steinberg, 2005). On the way they encounter several crossroads. At each of the crossroads they see a stoplight which just turns yellow as they approach. They can decide to either stop and make a safe decision, or they can decide to go which may result in a faster time or a crash. When participants do this task in the absence of a peer, there were no differences in number of risky decisions or number of crashes between a group of adolescents, young adults and adults (Chein et al., 2011). Participants performed the task inside the MRI scanner and no differences were observed in recruitment of the VS. However, when participants were observed by a peer, the adolescent group showed increases in risky decisions and number of crashes and the VS showed increased recruitment. Brain areas associated with cognitive control were less strongly recruited by adolescents than by adults and did not vary with social context (Chein et al., 2011). That is, the study showed that the presence of peers observing the subject in a simulated driving task sensitized the adolescent brain to the reward value of the risky choices. In a similar setup, it was tested how adolescent participants made decisions in this task while their mother was observing their choices. In this social context, adolescents made fewer risky decisions and increased ventral lateral PFC and medial PFC activation was observed when adolescents made decisions to stop (Telzer et al., 2015). Together, these studies show that function in both the VS and the PFC is amenable to the social context.
Although the focus so far has been on the role of the social context in risk taking behavior, which is often seen as a negative effect of social influence, social influence can also increase prosocial decision making. In a public goods game in which participants can choose to invest part of their endowment in a public goods pot or keep their money to themselves, adolescents show increased prosocial, i.e. investing, behavior when they receive positive feedback for investments in the public goods pot. Increased investments in the public goods pot was associated with increased activation in a network of brain regions associated with social processes (Van Hoorn et al., 2016). Prosocial social influence can be an avenue for future interventions in risk taking behavior.
Practical implications for intervention
As stated above, peer influence on decision making is a broad construct and can be dissected into peer presence, peer observation, peer encouragement and other forms of information about peers such as information about their choices. These different types of peer influence have different effects on behavior. Intervention in substance use in adolescence is possible if we better understand under which conditions adolescents are prone to increase risky decision making. One successful example is the introduction of graduated driving laws in which adolescents are not allowed to drive with peer passengers until 18 years of age. This type of graduated driving law has been implemented in several states across the US and in Europe. These laws decrease accidents and risky driving in adolescents by as much as 30 to 40%. A better understanding of the social context effects on substance use could lead to similar implementation of either legislation or development of new intervention programs. One possibility would be to develop interventions targeted at peer norms around substance use, which would decrease the social motivation and incentives for substance use. One study showed successful changes in willingness to drink by exposing adolescents to anti-alcohol peer norms. Adolescents who are heavy drinkers at baseline developed a more negative view of adolescent drinkers and they expressed less willingness to drink after being exposed to peer anti-alcohol norms (Teunissen et al., 2014). This study shows that peer norms can be a powerful way of changing adolescent risk taking behavior.
Summary
In summary, to understand risk taking behavior in adolescence, it is critical to take the social context into account. The influence of peers on risky behavior by adolescents is complex yet amenable to investigation in the laboratory. The findings discussed here show that different types of peer influence, i.e. peer presence vs peer advice, result in differential peer effects. Potentially these differences are due to differential importance of reputation management, peer acceptance within the group and popularity for adolescents compared to adults. We have made strides in dissecting the influence of peers on certain types of risk-taking behavior such as driving and these results have successfully been implemented in legislation to reduce accidents. Future studies should focus on the role of peers in other risky behaviors such as substance use. Furthermore, we know that the neural architecture underlying these mechanisms is developing during adolescence. Demonstration of the various patterns of activation under different social contexts provides a better understanding of the physiologic underpinning of the effects of social context on decision making during adolescence.
Summary and conclusions
This report illustrates many of the ways that the structure and function of the adolescent brain are vulnerable to influence by the environment. Experiences encompassing all domains of behavior affect neuronal activity, synapses, dendritic spines, circuits and networks which ultimately determine how the individual responds to subsequent experiences. First, MacMaster presented a discussion of the various imaging modalities available today and how they have been used to show brain structural and functional development during adolescence. He makes the point that while the extended period of plasticity in frontal brain regions creates the opportunity for extensive cognitive development, it also makes the brain vulnerable to disruption potentially resulting in neuropathologies; the “double-edged sword”. Peterson illustrated how effort to control abnormal behaviors can result in hypertrophy or pruning of the brain areas which mediate the behaviors and their control. That is, “neural plasticity supports compensatory responses that modulate the severity of symptoms” in TS, ADHD and depression thus providing evidence that activation of circuits can influence the structure of those circuits. Niesink reviewed the molecular mechanisms involved in synaptic plasticity and how glutamate and the endocannabinoids are critical to these processes. He also discussed drug use during adolescence and the fact that early drug exposure increases future drug taking behavior. The reward circuit is highly plastic during adolescence and exposure to a variety of drugs dysregulates this system. This point is also made in the following section by Andersen who described the effects of MPH, the psychostimulant used to treat ADHD, on brain structure and drug taking. In this case, clinically relevant doses of MPH given to young rats reduced drug liking in adulthood, an effect that was opposite that seen when MPH is administered to adults. All of these sections address the unique vulnerability of the adolescent brain to exogenous influences, experience and activation. Lastly, Braams discussed the complexities of peer influence on adolescent decision making and described imaging studies showing that the presence of peers can increase recruitment of the ventral striatum in risky situations. This study illustrates the unique response of the adolescent brain to social settings.
It is widely known that adolescents undergo dramatic changes in socialization, including heightened self-consciousness, increased importance and complexity of peer relationships and an improvement in the relationships with peers and others (Steinberg and Morris, 2001) and reviewed by Lamblin et al. (2017). We propose that the social brain, being widely activated during adolescence, is subject to robust plasticity during this sensitive period. The social brain is the network of brain regions that allows us to recognize others and evaluate their mental states, feelings, dispositions and actions. It includes the medial prefrontal cortex (mPFC), anterior cingulate cortex, inferior frontal gyrus, superior temporal sulcus (STS), the amygdala and anterior insula (Blakemore, 2008). Social behavior matures during adolescence due primarily to engagement of this network and the plasticity within its components. The roles of the different areas change with age and experience with the biggest changes, including synaptic reorganization, seen in the mPFC and superior temporal sulcus. During a mentalizing exercise involving intention, there is an age-related shift in activation from the mPFC to the STS (Blakemore, 2008). This shift is due to natural maturation and experiences. During this process, the underlying networks are vulnerable to outside influences. Repeated activation of a circuit during a sensitive period such as that which occurs in the social network will ultimately result in apparently permanent changes in that circuit (see Section 3). It is interesting to speculate that the presence of peers in situations where drugs are present (a risky situation) would increase the reward value of the drug (recall the presence of peers increases activation of the ventral striatum), potentially increasing drug use, perhaps permanently altering the reward circuits and increasing drug use throughout life.
Clearly, the adolescent brain is not mature. While most authors consider 24 years the age when brain maturation stops, Somerville makes the case that brain maturity is an elusive concept since the age of the developmental asymptote (when changes are at asymptote) is different in different brain regions (Somerville, 2016). For example, changes in fractional anisotropy in frontal cortex continue until 32 years of age while this measure reaches asymptote at 25 years of age in occipital cortex. Does this suggest that the behaviors these regions subserve do not mature until these ages? Most investigators would agree that brain maturation is indeed protracted particularly in the late developing PFC. In fact, a detailed analysis of the synaptic spines within the PFC provides evidence that the PFC exhibits overproduction of spines during childhood and that the remodeling and elimination of spines continues beyond adolescence and throughout the third decade of life (Petanjek et al., 2011). This suggests that the PFC, the seat of executive function, remains vulnerable to experiences through the 20's and into the 30's.
It has been almost 20 years since the Neuroscience community has begun to examine adolescent development and the development of non-invasive imaging techniques such as MRI and fMRI has resulted in an explosion of studies on brain/behavior relationships in the adolescent (see Luna et al. (2010) for revi). Our understanding of the relationship between regional brain function and behavior has facilitated an understanding of what happens during normal brain maturation and thus what happens when things go wrong. The development of the individual “fingerprint” of brain connectivity and how this changes with age may help scientists follow the effects of therapeutic interventions so important in psychopathology and substance abuse, two morbidities with roots in adolescence. Perhaps during the next ten years, we will see a change in the development of the brain circuitry due to the pervasive use of social media. In addition, the increased sensitivity of adolescents to acceptance and rejection through social media may make them more reactive to emotion-arousing media (Crone and Konijn, 2018). Hopefully, creative engagement of social media will facilitate adolescents gaining resilience and improved self-image necessary to prepare them for their futures.