Abstract
Genetic variants in the opioid receptor mu 1 (OPRM1) and dopamine receptor d2 (DRD2) genes are implicated in behavioral phenotypes related to substance use disorders (SUD). Despite associations among OPRM1 (rs179971) and DRD2 (rs6277) genes and structural alterations in neural reward pathways implicated in SUDs, little is known about the contribution of risk-related gene variants to structural neurodevelopment. In a 3-year longitudinal study of initially SU- naïve adolescents (N = 129; 70 females; 11 – 14 years old), participants underwent an MRI structural scan at baseline and provided genetic assays for OPRM1 and DRD2 with SU behavior assessed during follow-up visits. Baseline differences in key reward-related brain regions (i.e., bilateral caudate and cingulate cortex) were detected in those with genetic liability for SU in OPRM1 who went onto engage in SU at subsequent waves of data collection. In addition, main effects of OPRM1, DRD2, and SU were related to variability in structure of the putamen, anterior cingulate, and nucleus accumbens, respectively. These data provide preliminary evidence that genetic risk factors interact with future SU to confer structural variability prior to SU in regions commonly implicated in risk for SU and the development of SUDs.
Introduction
Despite an overall decline in rates of adolescent risky substance use (SU; e.g., binge drinking has declined approximately 13% in the past decade), adolescent drug and alcohol use remains relatively prevalent (Johnston et al., 2020). Approximately 35% of adolescents report lifetime use of illicit substances (e.g., marijuana, cocaine, methamphetamines, hallucinogens) and 42% report lifetime alcohol use (Johnston et al., 2020). These rates are concerning given that SU during adolescence is likely to impact neurodevelopmental trajectories in ways that increase the propensity for a variety of adverse outcomes (Scott et al., 2017). For instance, early onset SU (i.e., prior to 16 years of age) is an established predictor of subsequent SU disorders (SUDs) (Moss et al., 2014). Moreover, adolescent SU has been associated with functional and structural alterations of reward-processing regions that are implicated in the development of SUD (Cheetham et al., 2014; Heitzeg et al., 2015) and portends the entrenchment and persistence of SUDs (Kravitz et al., 2015). Implementing strategies that identify adolescents at heightened risk for SU, prior to SU initiation, could offer a potential point of entry for preventative intervention strategies aimed at recalibrating reward circuitry and, in turn, sensitivity to drug-related reinforcers. One approach to ascertaining risk is the identification of biomarkers that are predictive of SU outcomes, such as genetic variants that influence brain structure in regions that underlie the SUD phenotype. By identifying those genetic variants that forecast structural variability and phenotypic characteristics of SUDs, prevention efforts could be more effectively targeted to prevent changes in brain structure that ultimately confer greater risk for SU/SUD.
There are many genetic variants that have been implicated in the physiological and behavioral responses to commonly misused substances and which predict the likelihood of SUD (Trucco et al., 2019). Further, a number of those genes whose variants appear to confer risk of SU/SUD have well-established roles in the structural attributes of brain regions that are consistently implicated in SU and dependence phenotypes. Of particular importance may be those genetic variants that contribute to the structure and function of brain regions that have been shown to mediate different stages of substance use trajectories, from initiation to sustained use to SUD (e.g., mesocorticolimbic dopamine pathway regions). Critical variants include the dopamine transporter (DAT1), dopamine receptor D2 (DRD2), catechol-O-methyltransferase (COMT) and opioid receptor mu 1 (OPRM1) genes (Batalla et al., 2018; Nemmi et al., 2018; Roussotte et al., 2015). Variability in these same genes has also been linked to the likelihood of SU behaviors in developmental populations (Kleinjan et al., 2013; Miranda et al., 2010).
For example, the DRD2 gene is implicated in dopamine synthesis and regulation and has been associated with striatal response to reward as well as susceptibility to SU and SUD in adolescent samples (Brody et al., 2013; Macare et al., 2018). The rs6277 single nucleotide polymorphism (SNP) variant of the DRD2 gene also appears to influence structural pathways in the brain that subserve reward and motivation processes (i.e., ventral tegmental area, caudate, putamen, nucleus accumbens (NAcc), medial prefrontal cortex, and anterior cingulate). Importantly, there are mixed reports regarding which rs6277 allele confers greater risk for SUD (Hill et al., 2013; Swagell et al., 2012). Specifically, in adult samples, the minor ‘G’ allele of this SNP appears to predict low availability of striatal dopamine (Hirvonen et al., 2009) and an increased risk of substance dependence across an array of commonly misused substances (e.g., nicotine, alcohol, heroin (Gao et al., 2017; Swagell et al., 2012; Voisey et al., 2012)). In contrast, this same allele may be protective against risk for SUD such that it predicts later onset of alcohol use during adolescence (Brody et al., 2013), a behavioral phenotype that is associated with a reduced long-term risk of SUD (Behrendt et al., 2009). This suggests that this variant may be protective against subsequent SUD. In adolescence and emerging adults, carrying the ‘G’ allele of DRD2 rs6277 has also been associated with larger caudate and putamen volumes and better executive functioning performance compared to those who are homozygous for the major ‘A’ allele (Hill et al., 2013; Markett et al., 2013), which is implicated in SUD. The mixed findings may, in part, be due to some studies incorporating adolescents and emerging adults (Brody et al., 2013; Hill et al., 2013; Macare et al., 2018), while others focus on adult samples with established substance misuse or SUDs (Gao et al., 2017; Swagell et al., 2012; Voisey et al., 2012).
The OPRM1 A118G SNP (rs1799971) also seems to contribute to heterogeneity in reward pathway regions, particularly with respect to function; an effect that may influence individuals’ risk of SU/SUD. The OPRM1 gene plays a primary role in the regulation of opioid signaling in reward pathways in the brain and in the reinforcing effects of a variety of commonly misused substances (Burns et al., 2019). Relatedly, in individuals with SUDs, endogenous opioids are downregulated in the striatum, possibly increasing the reward value of abusable substances (for review, Burns et al., 2019). The OPRM1 A118G SNP has been shown to regulate the opioid signaling and subsequent release of dopamine in reward-related regions differentially among individuals who are homozygous for the A allele compared to G-allele carriers (Popova et al., 2019) and is thought to influence responsivity to drugs. Adults who carry the high-risk ‘G’ allele (i.e., AG/GG) experience more positive acute effects of alcohol (e.g., intoxication, positive mood) compared to those who are homozygous for the A allele (Ray & Hutchison, 2004). This differential influence of the AG/GG vs. AA allele status of OPRM1 A118G has also been noted in adolescents who engage in alcohol use (Miranda et al., 2010). Specifically, adolescent G allele carriers are 3 times more likely to develop an alcohol use disorder (AUD) during adolescence compared to AA homozygotes (Miranda et al., 2010). Thus, being a G-allele carrier for A118G may represent a significant SUD risk factor.
While there is a dearth of evidence regarding an impact of the OPRM1 A118G variant on structural variability, functional magnetic resonance imaging (fMRI) findings suggest there is OPRM1-dependent functional modulation in reward-related brain regions in those with a SUD. Notably, there is a stark lack of work examining this SNP in developmental populations, as existing literature has almost exclusively focused on adult samples with established SUDs. For example, the A118G SNP moderates neural responses during cue-induced craving paradigms in adults with SUDs. Compared to AA homozygous individuals, G allele carriers demonstrate greater neural cue-reactivity to SU-related cues in striatum, insula, and medial/inferior frontal gyrus (Bach et al., 2015; Ray et al., 2014). Prior studies also suggest that this SNP moderates the relationship between fronto-striatal connectivity and SU severity in young adults, such that having at least one copy of the G allele predicts stronger associations between cue-related connectivity and SU severity. This implies that, in those who carry this risk allele, the extent to which the functional response to SU-related cues predicts sensitivity to the reinforcing effects of misused substances is more pronounced (Courtney et al., 2013; Korucuoglu et al., 2017). However, while functional connectivity studies consistently implicate fronto-striatal connectivity in the functional impacts of A118G, they are mixed in terms of the directionality of effects (i.e., weaker vs. stronger connectivity in G allele carriers (Courtney et al., 2013; Korucuoglu et al., 2017; Ray et al., 2014)).
Collectively, these studies suggest that the putative role of these two variants in conferring risk for SU and SUD may occur via their influence on the function and, in the case of DRD2 rs6277, structure of brain regions that mediate reward processes and which have been consistently implicated in key cognitive and behavioral phenotypes that are related to SU and SUD outcomes. However, it remains to be determined whether the association between these variants and brain structure occurs prior to the onset of SU. Studying such associations in an SU-naïve sample who go onto engage in SU is a crucial step in delineating predictive factors of SU that exist prior to onset of SU. Most studies of adolescent SU do not include an SU-naïve baseline sample, which limits conclusions given the profound neurocognitive effects that actual substances may have on the developing brain (Fishbein et al., 2016). Thus, the present study aimed to isolate neurodevelopmental factors (i.e., brain structure in key reward regions and genetic risk factors) that are thought to relate to the onset of SU behaviors. In the current study, we considered how OPRM1 and DRD2 genetic variants corresponded with structural variability in SU-naïve youth and how these associations might vary in participants who eventually initiated SU (i.e., across a 3-year period). Examining the interactions between genetic variants that predict risk for SU/SUDs and structural variability in brain regions implicated reward processing and in addiction (Koob & Volkow, 2016), prior to the onset of SU, may shed light on mechanisms that underlie heightened risk for SUDs and provide novel targets for SU/SUD prevention. Specifically, based upon prior meta-analyses of SUD risk (Pando-Naude et al., 2021), current theoretical models of addiction (Koob & Volkow, 2016), and empirical work reviewed here, we examined structural variability in the bilateral caudate, putamen, globus pallidus, NAcc, amygdala, thalamus, hippocampus, anterior (ACC) and middle cingulate cortices.
Materials & Methods
Participants
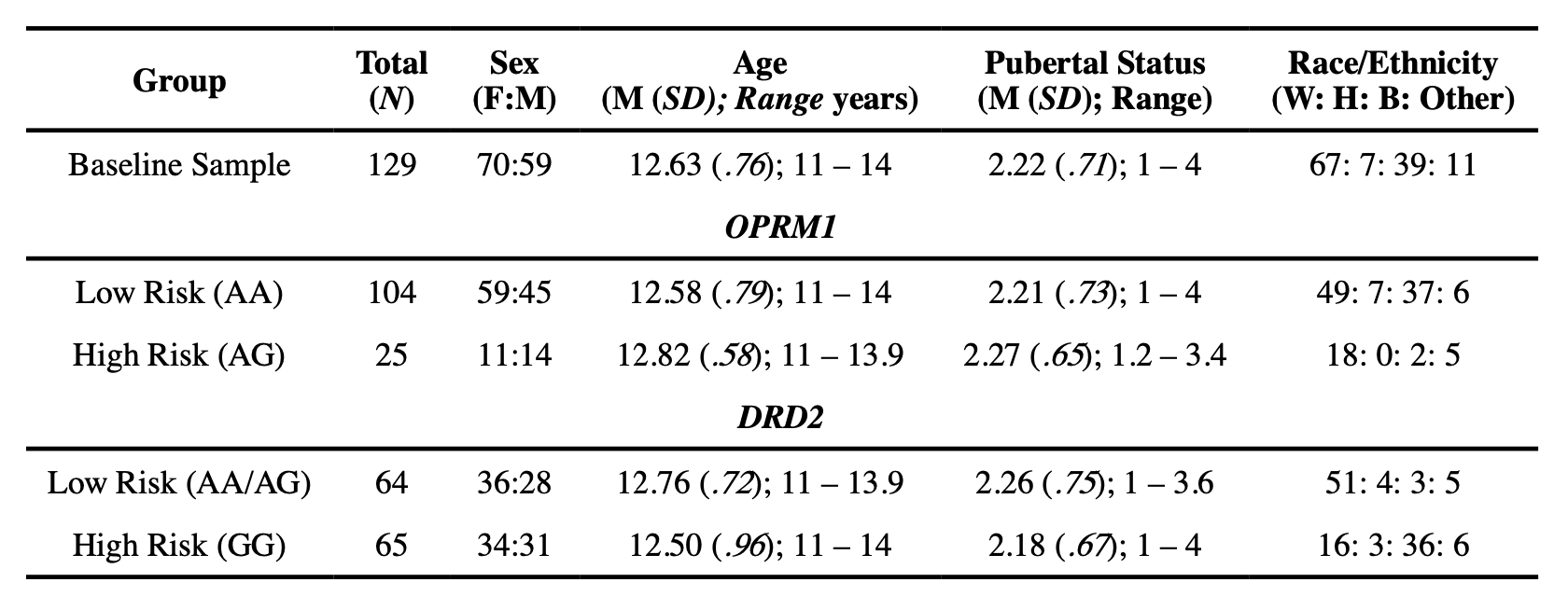
SU naïve adolescents (N=147; age = 11 – 13 years old) were recruited to participate in the Adolescent Development Study (ADS), a prospective, longitudinal study of the neural, cognitive, and behavioral precursors and consequences of adolescent SU in the greater Washington D.C. area. A community-based sample was recruited by targeting households based upon rates of neighborhood crime, income, and rates of SU, with even the lower-risk county in the catchment area having adolescent SU rates that tended to increase with age (see Fishbein et al., 2016 for further details). Participants were excluded on the basis of SU prior to enrollment, previous head injury, left-handedness, neurodevelopmental disorders (e.g., epilepsy, autism spectrum disorders, and motor disorders), and other contraindications for magnetic resonance (MR) imaging (e.g., MR incompatible metal foreign bodies). RTI International and the Georgetown University Institutional Review Boards approved the study. All caregivers and participants provided consent or assent, respectively, prior to assessments.
In order to capture measurable traits that likely play significant roles in SU initiation and escalation, a range of cognitive, behavioral, brain imaging, and demographic measures were acquired at an initial assessment (i.e., baseline), and at 18- and 36-month follow-up visits (i.e., Waves 2 and 3) (for a complete description of methods, see Fishbein et al., 2016). The current analyses considered relationships between brain structure and genetic variants known to be associated with risk for SU and/or SUD in a subsample of adolescents for whom the following data types were available: genetics data, imaging data at baseline, and behavioral data at baseline and subsequent waves of data collection (see Table 1 for demographic information at baseline for the entire sample and genetic high and low risk allele groups).
Procedures
Substance Use.
Adolescent participants completed two self-report surveys to assess their drug use status at baseline, wave 2, and wave 3 visits: Drug Use Screening Inventory-Revised (DUSI-R; Tater, 1990) and the Tobacco, Alcohol and Drug (TAD) questionnaire (Bucholz et al., 1994). The DUSI-R consists of 20 questions regarding a variety of abusable substances (e.g., alcohol, marijuana, tobacco products, stimulants, over the counter medications). The TAD assesses use of tobacco, alcohol, and illicit substances (i.e., cocaine, methamphetamine, opiates, marijuana, salvia, synthetic marijuana, ecstasy, inhalants, prescription drugs not used as prescribed). Participants who confirmed the use of any drugs (e.g., alcohol, marijuana) on either questionnaire in the period since their last study visit at either wave 2 or 3 were aggregated into a single “user” group. This was done, rather than considering each wave separately, as there were not sufficient numbers of users reporting first use at wave 2 or 3 to sufficiently power the inclusion of users by wave. Given the age of our sample, any SU was considered problematic given that early SU (prior to age 16) is a critical risk factor in the development of subsequent SUDs (Jordan & Andersen, 2017). In other words, we did not seek to distinguish between genetic risk for SU and SUD since, at this early stage, any SU is predictive of poor outcomes. It should be noted that, as expected, Wave 2 users were significantly younger than those reporting use for the first time at Wave 3 (t(33) = 5.23, p < .001; Wave 2 SU M = 14.77 years, SE = .11; Wave 3 SU M = 15.76 years, SE = .14), but both groups were within the age range of what is considered ‘early’ use in prior literature (i.e., <16 years old) (Moss et al., 2014). Wave-related age differences in use were anticipated since those who initiated SU at Wave 2 were expected to be younger than those who initiated SU at Wave 3 given the longitudinal design of the study. Importantly, the age of participants at the time of use was not related to variability in brain structure at baseline.
MRI Data Acquisition.
Participants underwent training in a mock scanner to prepare for the scanning environment and tasks. Participants were scanned using a 3T Siemens Tim Trio MRI machine with a 12-channel head coil. Foam padding was placed around participants’ heads to minimize head motion. A high-resolution T1-weighted anatomical scan was acquired using a magnetization prepared rapid acquisition gradient echo (MPRAGE) protocol with the following parameters: TR/TE = 1900 ms/TE = 2.52 ms, TI = 900 ms, flip angle = 9°, FOV = 250 mm, 176 slices with a thickness of 1.0 mm, effective resolution = 1mm3, scan time = 4 min, 18 s).
MRI Data Quality Control.
Prior to the FreeSurfer analysis, MPRAGE images were visually inspected for quality by at least 3 trained raters. These structural images were scored on a scale ranging from 1 (high quality) to 5 (low quality) for ghosting and ringing. Clipping and wrapping were scored with either a 1 (low quality) or 0 (high quality). A single quality control (QC) metric was calculated using the following computation (QC = 4*wrap + 10*clip + 2*ring + 1*ghost). Images with a QC metric >11 were deemed to be of poor quality and excluded from further analysis (n=18).
MRI Data Processing.
To extract cortical thickness and subcortical volume estimates, cortical reconstruction and volumetric segmentation were performed using the FreeSurfer image analysis suite (v6.0). The complete technical details of these procedures are described in prior publications (Fischl et al., 2002; Reuter et al., 2010). Briefly, image processing included motion correction and averaging (Reuter et al., 2010) of volumetric T1-weighted images, removal of non-brain tissue (Ségonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and gray matter structures (Fischl et al., 2002) intensity normalization (Sled et al., 1998), tessellation of the gray matter/white matter boundary, automated topology correction (Ségonne et al., 2007), and surface deformation following intensity gradients. To address reconstruction errors and defects that can occur in the automated FreeSurfer pipeline, 5 trained editors (supervised by an expert FreeSurfer editor) manually edited T1s. Specifically, skull-stripped images were inspected and cleaned by the trained editors. For example, any dura or skull included in the pial surface by FreeSurfer were manually deleted. Voxels labeled by FreeSurfer as white matter in grey matter surfaces were manually deleted. Control points were added to fix intensity normalization errors in white matter. All edits were documented and FreeSurfer was re-run to incorporate all edits.
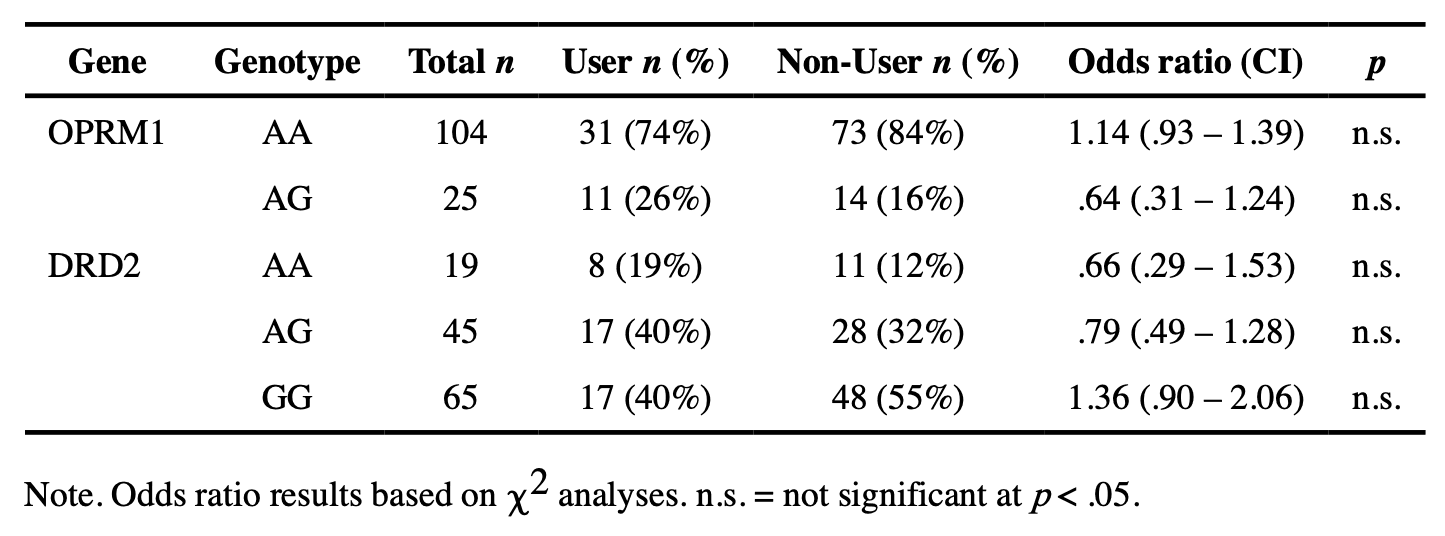
Genotyping Protocol.
DNA from saliva samples was extracted using a modified version of a previously described method (Freeman et al., 2003). Taqman SNP Genotyping Assays were performed using an allelic discrimination assay protocol (Life Technologies, Carlsbad, CA, USA). Genotyping analyses considered in the current analysis included determination of SNPs for OPRM1 (rs1799971) and DRD2 (rs6277) genes. These SNPs were selected using multiple criteria. While prior candidate genes studies suggest that they play a role in the development of substance use disorder via an impact on neurodevelopmental pathways, this was not the only criterion for selection. Indeed, we focused on those genes/variants that have also been identified in genome-wide association studies as being significantly associated with substance use and/or substance use disorders (Lopez-Leon et al., 2021). The OPRM1 SNP considered here met this criteria and while the Taq1A DRD2 SNP is more commonly identified in genome-wide studies of substance use disorders, this variant is in strong linkage disequilibrium with the C957T/rs6277 variant that we considered and, importantly, prior studies suggest that it may be this latter variant that is predictive of dopamine D2 receptor availability (Smith et al., 2017). Genotype frequencies, along with odds ratios for SU risk, are reported in Table 2. As a consequence of the relatively low minor G allele frequency for A118G, no individuals were GG homozygotes for OPRM1 A118G in our sample. Therefore, analysis of OPRM1 focused on homozygous non-risk individuals (AA) vs. carriers of the risk ‘G’ allele (AG/GG). Prior population-based studies of this gene have reported minor allele frequencies between 19 – 21%, which is comparable to what we report here (Rouvinen-Lagerström et al., 2013). Similarly, given the small number of participants with an AA genotype for DRD2 rs6277, analyses focused on the comparison of GG vs. AA/AG genotypes, which is consistent with prior literature. Based upon the 1000 Genomes project, the minor allele frequency was expected to be around 33% for this gene (Auton et al., 2015).
Data Analysis.
Data models were designed to determine the relative and interactive associations between genetic risk (i.e., carrying a single copy of the ‘G’ allele for OPRM1 and AA/AG for DRD2) and post-baseline SU (i.e., SU at either Wave 2 or Wave 3) with brain structure at baseline. We conducted ANCOVAs (covariates discussed below) to determine relationships between genetic risk status and SU status (i.e., user vs. non-user) at follow-up that relate to thickness or volume of ROIs at baseline selected a priori. Analytical models focused only on these specific regions and the impact of the two noted variants. Models were run separately for DRD2 and OPRM1 to examine their relative associations with brain structure. ROIs selected were those that are known to be highly relevant to SU (i.e., regions that show SU/SUD-related functional and/or structural variability) and which have been shown to be impacted by the genes under study, including bilateral caudate, putamen, globus pallidus, NAcc, amygdala, thalamus, hippocampus, anterior (ACC) and middle cingulate cortices. For all models, significant interactions were subjected to independent samples t-tests. The threshold for significance was FDR corrected for multiple comparisons in the ANCOVA models interhemispherically for each gene, and separately by volume and thickness.
Age, sex, ethnicity/race, and pubertal status were included in all ANCOVA models as nuisance variables. Puberty was measured with the Pubertal Development Scale (PDS; (Carskadon & Acebo, 1993)), which sums self-report questions regarding gonadarche and adrenarche in boys and girls on a 4-point scale (1=not yet started; 2=barely started; 3=definitely started; 4=seems complete). There were not any significant associations between genetic risk status for either gene and age, sex, or pubertal status. However, the frequency of risk carriers for OPRM1 (F(3,120) = 4.88, p = .003) and DRD2 (F(3, 119) = 3.83, p = .01) was associated with race. Specifically, for OPRM1 Bonferroni-corrected post hoc tests revealed the risk variant was more common in Caucasian/White and “Other race” participants than Black/African American participants (i.e., p = .04 and p = .02, respectively). Conversely, for DRD2, Black/African American participants were more likely to be risk variant carriers than Caucasian/White participants (p = .01), which is consistent with prior literature (Müller et al., 2012; Villalba et al., 2015). Therefore, it was deemed appropriate to include race in all ANCOVA models as a covariate of no interest.
Results
The final analyses included 129 participants (M age = 12.63, SD = .76; 54% female). Of those participants, 42 participants reported SU at Waves 2 or 3 (M baseline age = 12.86 years, SD = .10; 61% female). Here, we report significant findings surviving FDR p-value adjustment.
Main Effects of Genotype and SU
There were several significant main effects of gene status in OPRM1 and DRD2 and SU on volumetric and thickness measures that were not superseded by significant interactions (Table 4). Specifically, there was a significant main effect of OPRM1 risk status on both left and right putamen volumes whereby carriers of the risk “G” allele had significantly larger putamen volumes compared to the non-risk homozygous participants (left putamen: AG M = 5877.03 mm3, SE = 124.64, AA M = 5559.11 mm3, SE = 66.32; right putamen: AG M = 5929.38 mm3, SE = 122.97, AA M = 5636.37 mm3, SE = 65.43).
In addition, there was a main effect of DRD2 risk status on left anterior cingulate thickness such that participants homozygous for the high-risk “G” allele had significantly thinner left anterior cingulate cortex (GG M = 3.06 mm, SE = .02) compared to those carrying the non-risk “A” allele (AA/AG M = 3.13 mm, SE = .02) (Table 3).
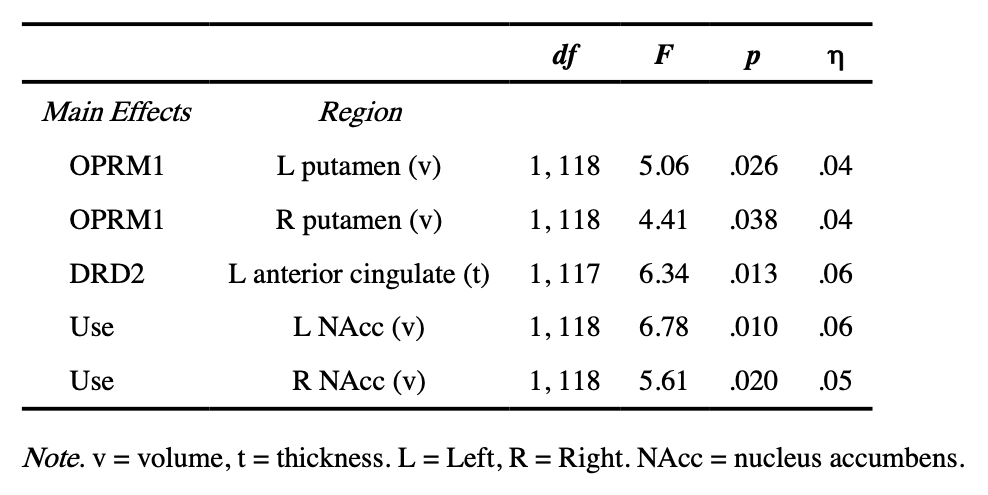
Lastly, there were two significant main effects of subsequent SU status on left and right NAcc volumes whereby adolescents who initiated SU had larger left and right NAcc volumes compared to adolescents who did not initiate SU (left NAcc: users M = 531.73 mm3, SE = 19.25, non-users M = 470.17 mm3, SE = 13.23; right NAcc users M = 692.73 mm3, SE = 16.91, non-users M = 643.54 mm3, SE = 11.62) (Table 3).
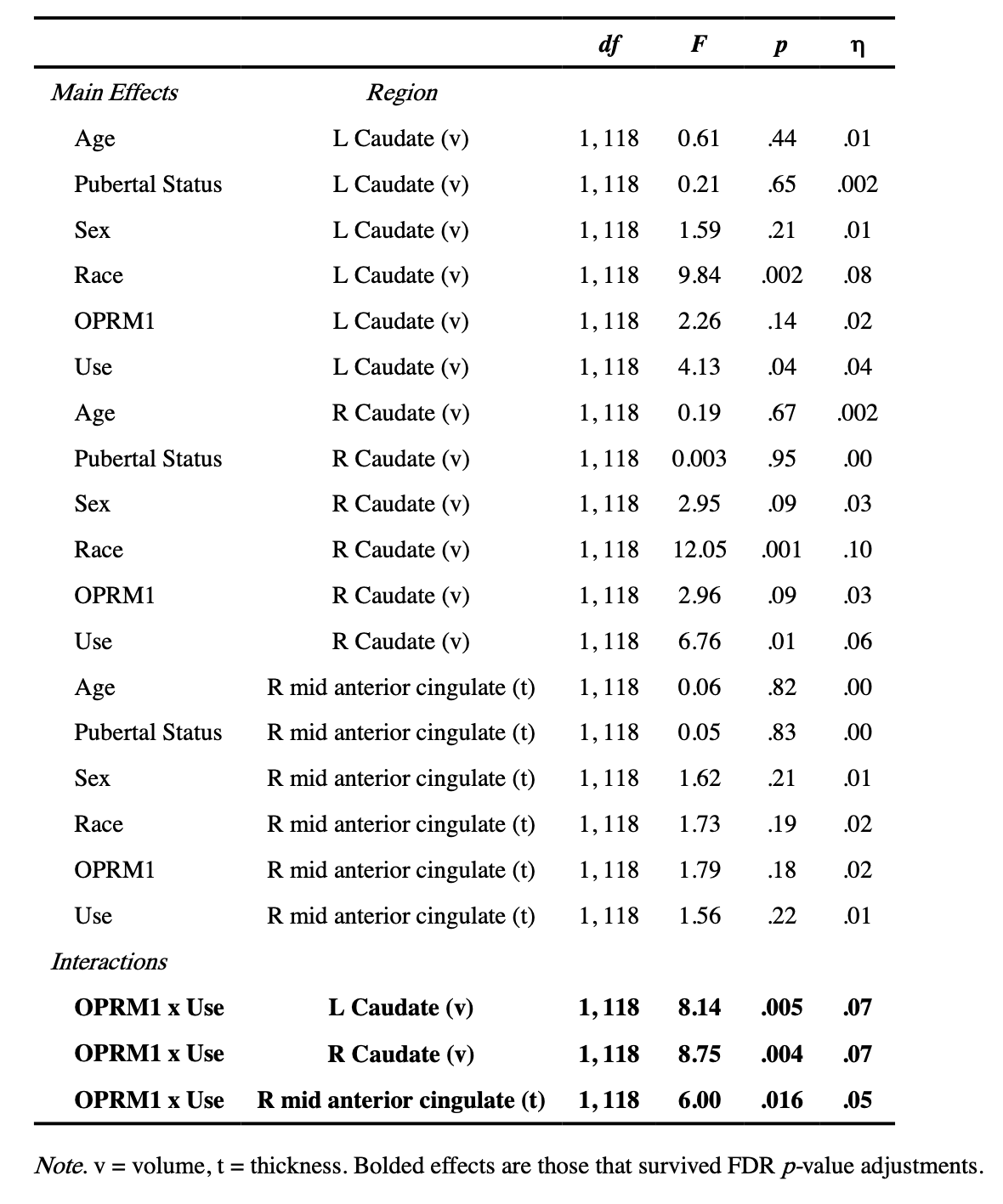
Genotype x SU Interactions
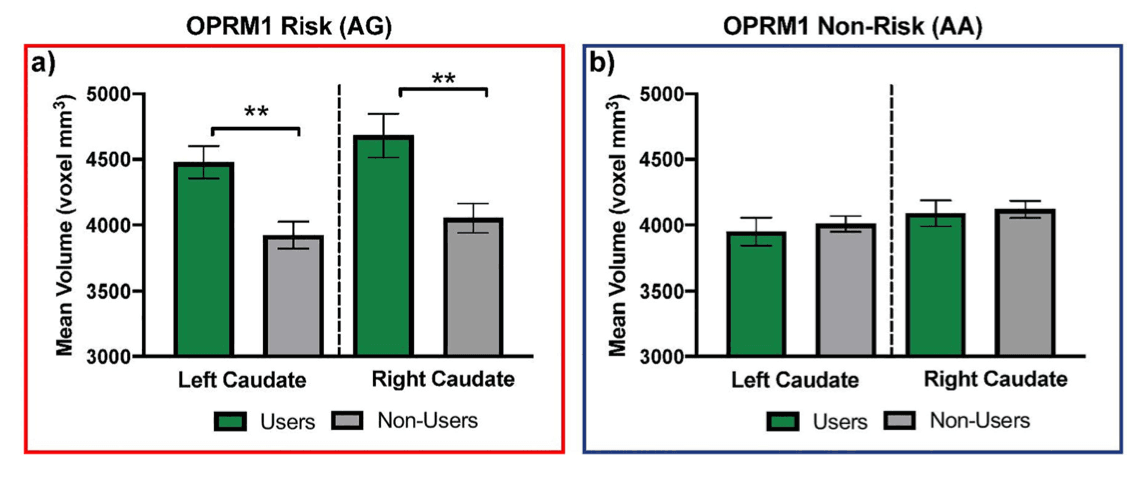
After controlling for effects of participant age, pubertal status, race, and sex, we uncovered interactions between OPRM1 and subsequent SU on left and right caudate volume (Table 4). In both the left and right caudate, follow-up independent samples t-test analyses revealed that in those carrying the risk G allele (AG), adolescents who went on to initiate SU had larger caudate volumes compared to those who did not initiate (left caudate: t(23) = 3.38, p = .003, users M = 4477.81 mm3, SE = 124.24, non-users M = 3932.71 mm3, SE = 102.53; right caudate: t(23) = 3.29, p = .003, users M = 4681.67 mm3, SE = 165.20, non-users M = 4052.02 mm3, SE = 111.36). Conversely, in homozygous non-risk individuals (AA), whether they did or did not initiate SU did not relate to caudate volumes at baseline (left caudate: t(102) = 0.51, p= .61, users M = 3948.98 mm3, SE = 106.71, non-users M = 4008.96 mm3, SE = 60.45; right caudate: t(102) = 0.25, p = .81, users M = 4089.17 mm3, SE = 97.54, non-users M = 4118.58 mm3, SE = 64.80). Results are shown in Figure 1.
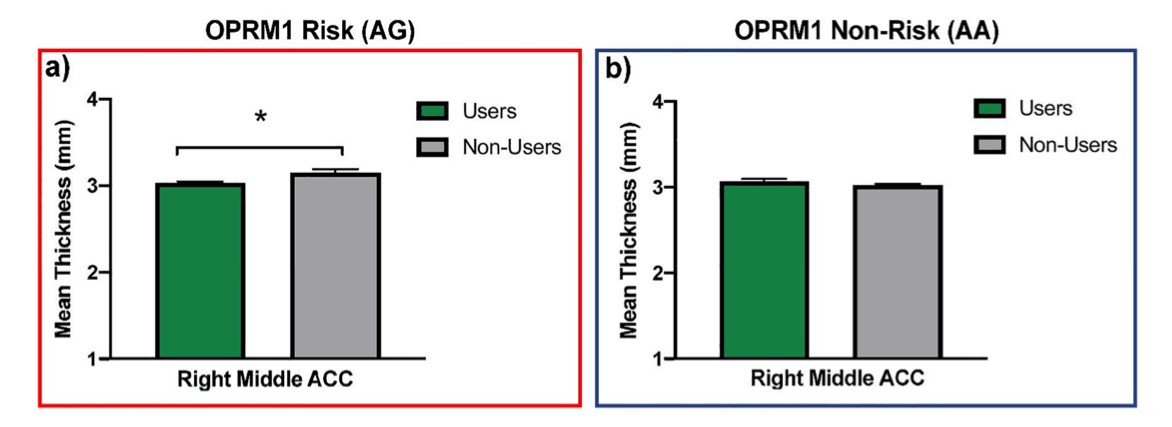
In addition, a significant interaction between OPRM1 and use on right middle anterior cingulate cortex thickness was found (Table 4). Follow-up independent samples t-test analyses revealed that in those carrying the risk G allele (AG), adolescents who initiated SU had thinner right middle anterior cingulate cortices than those who did not initiate SU (t(23) = 2.64, p = .02, users M = 3.03 mm, SE = .02, non-users M = 3.15 mm, SE= .04). In contrast, no significant differences in cortical thickness were found between those who were homozygous non-risk individuals (AA) (t(102) = 1.43, p = .16; users M= 3.07 mm, SE = .03, non-users M = 3.02 mm, SE = .02). Results are shown in Figure 2. No interactions between DRD2 and use were uncovered.
Discussion
This study considered the association between genetic variants known to confer genetic liability for SU and SUD (i.e., OPRM1 A118G and DRD2 rs6277), subsequent SU behavior, and brain structure in regions previously implicated in the development of SU and SUD. These associations were considered at baseline in a sample of initially SU-naïve adolescents who did or did not subsequently initiate SU. This is one of the first studies to establish relationships between these variants, subsequent SU during adolescence, and brain structure in SU-naïve adolescents. Prior work has largely focused on correspondence between genetic liability for SU and brain function in reward pathway regions predominantly in adults with heavy SU or SUD (Bach et al., 2015; Courtney et al., 2013; Ray & Hutchison, 2004), with only one study focusing on adolescent users (Korucuoglu et al., 2017). Of note, this is the first study of its kind to include an SU-naïve baseline sample, as the only other adolescent study we could locate included participants with 3–4 years of prior SU experience (Korucuoglu et al., 2017). Thus, the present study contributes to the literature by establishing relative and interactive associations between genes and subsequent adolescent SU that relate to preceding alterations in reward-related brain structures, above and beyond developmental effects of age and pubertal status.
Two sets of key findings reported herein include: 1) interactive effects of OPRM1 and subsequent SU relating to the structure of brain regions key in reward processing prior to SU onset and 2) associations between OPRM1 and DRD2 genotype, and later SU associated with patterns of cortical thickness and volume in reward-related regions prior to the onset of SU. In the first set of findings, we found a consistent pattern of effects of OPRM1 such that in those carrying the A118G risk G allele (i.e., AG individuals), subsequent users had greater bilateral caudate volumes and thinner middle ACC at baseline compared to adolescents who did not initiate SU. Conversely, in those who were homozygous for the non-risk A allele, there were no differences in brain structure at baseline between users and non-users. As part of the dorsal striatum, the caudate has consistently been implicated in responsiveness to rewards, craving substances, and is key in the shift from voluntary to compulsive SU (Vollstädt-Klein et al., 2010). Similarly, the ACC is involved in craving or anticipation of rewards. As noted previously, there is a paucity of literature examining whether ACC and/or caudate structure is associated with OPRM1 status. In so far as functional and structural findings can inform one another, functional findings have shown increased negative functional connectivity between the caudate and frontal circuitry in dependent individuals carrying the risky ‘G’ allele of OPRM1 when receiving alcohol taste cues compared to water (Ray et al., 2014). In an alcohol-taste cue paradigm in adolescent drinkers, OPRM1 risky ‘G’ allele carriers’ decreased connectivity between the caudate and frontal regions correlated with greater pleasantness and urge ratings compared to those who were homozygous for the ‘A’ allele (Korucuoglu et al., 2017). The present study adds to this by elucidating that even prior to SU, there are structural alterations to the caudate and ACC that interact with OPRM1 status. In this way, our findings suggest that these structural modifications may precede alterations induced by actual SU, as those found by Ray and colleagues (2014). However, this hypothesis will need to be explicitly evaluated in future work.
In the second set of findings, we report associations between indices of brain structure at baseline and OPRM1 and DRD2, and subsequent SU. Findings revealed that carriers of the OPRM1 risky ‘G’ allele had increased bilateral putamen volume compared to those who were homozygous for the non-risk ‘A’ allele. The putamen plays an important role in the initiation of addictive behaviors through a shift from voluntary to habitual responses (Everitt & Robbins, 2013). OPRM1 A118G-related structural variability in this region prior to the onset of SU may be an important aspect of developmental pathways that lead to SU. Though evidence is scant regarding how variability in the volume of the putamen contributes specifically to SU, a study of biological siblings found that those with SUD had larger putamen volumes, supporting the notion that greater putamen volume may serve as an endophenotypic biomarker for risk of SU and SUD (Ersche et al., 2012). While structural variability cannot be simply interpreted as a mapping directly to brain function (Batista-García-Ramó & Fernández-Verdecia, 2018), our results are consistent with prior work examining functional responsivity in the putamen and SU. For example, elevated responsivity to monetary rewards in the putamen is predictive of subsequent onset of SU in adolescence (Stice et al., 2013). Moreover, risk ‘G’ allele carriers show disrupted processing (i.e., greater neural response to SU-related taste cues) in reward regions, including the putamen (Bach et al., 2015; Ray & Hutchison, 2004). Thus, the existing literature suggests that carrying the ‘G’ allele of OPRM1 A118G confers neurocognitive risk for SU/SUD via functional modulation of reward-related brain regions. Our results expand upon this to imply that such endophenotypic effects extend to brain structure in these same regions. That these effects occur prior to SU suggests that OPRM1 A118G genotype may be an important biomarker to be considered in the development of preventative interventions.
For DRD2, compared to those carrying the non-risk allele (i.e., AA/AG), those who were homozygous for the risk ‘G’ allele for DRD2 evinced decreased thickness in the left ACC. These results are consistent with a prior meta-analysis that identified consistently decreased gray matter in the right ACC in those with SUD (Ersche et al., 2013). Moreover, previous work showing a similar reduction in left ACC as reported here noted that this structural variability was predictive of later alcohol misuse in adolescents (Cheetham et al., 2014). Though not specific to cingulate thickness, our results support other work showing patterns of brain structure differences in G homozygotes specific to reward-related regions, which may put them at disproportionate risk of SU and SUD. Reductions in ACC and posterior cingulate thickness may point to potential alterations in affective and cognitive processes that are highly relevant to the risk of SU and the development of the SUD phenotype (e.g., response inhibition, working memory (Cheetham et al., 2014)). Indeed, our findings support prior research suggesting that being homozygous for the G allele of DRD2 may coincide with structural alterations in the cingulate, which may be an identifiable precursor to adolescent SU and SUD.
Finally, subsequent SU was associated with baseline bilateral NAcc volumes. Extant literature largely supports the notion that alterations in the NAcc (both functionally and structurally) are related to and may precede adolescent SU (Cope et al., 2019; Morales et al., 2019; Weissman et al., 2015). Of relevance to the current study, previous work has noted that larger NAcc volume relates to future SU, particularly in adolescent females (Morales et al., 2019). In those with a positive family history of SUDs, females tend to have larger left NAcc volume (Cservenka et al., 2015). In addition, college binge drinkers have greater NAcc volume compared to abstinent controls (Sousa et al., 2020), suggesting that alterations to the NAcc are evident prior to and following SU initiation. In contrast to the current findings, prior work has found that adolescents with smaller left NAcc were more likely to have initiated SU at follow-up (Urosevic et al., 2014). This finding is not consistent with other studies, including the present study, which may be due to methodological differences. For example, the current study evaluated a younger (11 – 13 years old) group of adolescents across 3 time points, while Urosevic and colleagues’ (2014) sample included older adolescents (15 – 18 years old) who were evaluated across 2 time points. It may be that the age of the current sample provided opportunity to observe a higher risk group, as SU initiation before 15 is typically referred to as early use (Moss et al., 2014). Taken together, our findings add to a growing literature demonstrating that there are both functional and, in the present study, structural alterations in key reward-related regions that relate to subsequent SU and predate SU intitation. This indicates that regions implicated in the intitation and instantiation of SU and SUDs (e.g., craving, reinforcing effects of substances) are likely vulnerable even before adolescents initiate SU.
Limitations
There are limitations of the current study that temper definitive conclusions. First, although the study is adequately powered to investigate neural effects and larger effects of genetic risk variants on brain structure, it is likely not sufficiently powered to examine more nuanced genetic effects. In other words, results reported here should be interpreted with caution until larger, adequately powered cohort studies are conducted and replicate the current findings. However, while many other studies have considered the contribution of genetic risk variants to variability in brain structure and function in substance-dependent adults, there are few other studies that have investigated genetic liability in conjunction with indices of neural structure in SU-naïve adolescents. Thus, despite potential concerns regarding overall power, this study presents novel findings in regions that are highly relevant to the development of SU and SUD and, as such, represents a promising avenue for future research examining biological risk factors of adolescent SU and SUD. Moreover, it is notable that prior imaging genetics studies investigating similar topics have comparable (Hill et al., 2013) or even smaller sample sizes compared to the current study (Markett et al., 2017).
The potential power issue in our study is further compounded by the relatively low frequency of the minor allele for both the genes. Indeed, we were restricted to comparisons of AA and AG individuals for the OPRM1 A118G SNP since we did not have any GG individuals. For the DRD2 SNP, we had fewer AA individuals (i.e., 19), and thus decided to combine AA and AG individuals, which is consistent with the prior literature and allowed us to maximize our power. More adequately powered studies able to recruit a greater number of individuals who are homozygous for the minor allele for these types of variants will be better positioned to disentangle the relative contributions of carrying one or more copies of the risk or non-risk allele. It may be the case that having two copies of a risk allele confers greater associations between SU severity and variability in SU-relevant brain structures compared to having a single copy by exerting additive or synergistic effects.
Relatedly, although we were able to uncover gene x SU interactions that predicted variability in brain structure, we did not detect significantly increased odds of subsequent SU based upon genotype alone. It is also notable that only initiation of SU was considered and participants did not develop SUD during the study period. In other words, subsequent work investigating similar questions within a longer follow-up period may yield different patterns than those observed here. Future work will need to examine longitudinal interactions between SU and genetic liability on patterns of brain structure development and how these patterns relate to the development of SUD.
Finally, it is possible that complementary measures of SU that were not examined here may aid in uncovering genetic impacts on the likelihood of use as well as the influence of genetic variability on brain structure in SU/SUD-relevant regions. Here, we used a multi-measure approach which effectively captured use of myriad substances across an 18-month period, including establishing the SU-naïve cohort at baseline. However, future studies may benefit from evaluating different dimensions of adolescent SU (e.g., SU severity, different SU patterns, polysubstance use) or SU measured over a different time scale (e.g., past week or past month use rather than use over longer periods, as was the case here) that may allow for more nuanced predictive ability of variability in brain structure.
Implications and Conclusions
The current study provides preliminary evidence for interactions between OPRM1 and subsequent SU related to bilateral caudate volume and right middle ACC thickness in a sample of SU-naïve adolescents. Moreover, specific effects of OPRM1 and DRD2 risk status, as well as subsequent SU were associated with the structure of key reward-related regions (e.g., putamen, NAcc, ACC). These are brain regions whose structure and function have been consistently implicated in reward-related processes and that subserve cognitive and behavioral processes that typify various stages in the cycle of addiction (Koob & Volkow, 2016). Our data provide initial evidence that gene-related structural variability relates to subsequent SU, even prior to SU in regions implicated in SU risk and the development of SUDs. In other words, in our substance-use naïve sample, consistent patterns of alterations in brain structure (i.e., greater subcortical volumes) were apparent in those carrying genetic risk variants who subsequently went on to engage in SU.
More work is needed to disentangle associations between these genetic variants and their role in the developmental course of these brain structures pertinent to SUD, as well as their concomitant effects on SU-relevant behaviors. Our results constitute a critical step in delineating correspondence between genetic and neural risk factors of adolescent SU. The SU-naïve baseline sample is a crucially informative aspect of the current study, as it helps to illuminate precursors for potential substance misuse, apart from effects that SU are known to have on the developing brain. Identifying those developing brain structures that may serve as endophenotypes (i.e., intermediate phenotypes) for genetic discovery in the context of adolescent SU is important to the premise that there are brain-mediated factors influencing SU initiation and SUD risk. Moreover, the identification of endophenotypic biomarkers that may mechanistically contribute to an elevated risk for SU and eventual SUD has significant implications for the development of preventative intervention strategies for adolescent SU aimed at recalibrating reward circuitry and sensitivity in at-risk adolescents. Identifying such endophenotypes provides an opportunity to develop targeted interventions designed to bolster neurocognitive skills supported by brain regions highlighted in the current study; such interventions could be optimized to enable acquisition of specific neurocognitive skills (e.g., behavioral and emotional regulation) that may help to delay SU initiation and diminish long-term consequences of adolescent SU.