Abstract
Early life stress (ELS) has been established as a major risk factor for a multitude of psychiatric and medical disorders. ELS is highly prevalent in the general population and constitutes a major public health concern. The current review will focus on the clinical literature that suggests a link between adverse early life experiences and vulnerability for adolescent and adult substance use disorders. It will investigate the characteristics of ELS that appear to increase risk for disorder onset and a more severe disease course, characterized by earlier onset, greater risk of relapse, and treatment resistance. The authors explore how ELS may increase risk for adverse substance use outcomes through long-lasting changes in the HPA axis and development of stress, reward, and executive control brain systems. The review will also discuss potential pathways to substance use disorder following ELS, with a focus on the role of comorbid mood and anxiety disorders and other modifiable traits. Finally, the authors will discuss how the current body of work presents the potential for prevention and intervention strategies to reduce the psychosocial consequences following early life stress and minimize adverse substance use outcomes.
Introduction
Early life stress (ELS) is a major public health concern. ELS—defined as exposure to adverse childhood experiences including childhood maltreatment (sexual, physical, and emotional abuse in addition to physical and emotional neglect) and household dysfunction (i.e., parental maladjustment)—is common in the general population, with the National Comorbidity Survey Replication reporting that 53% of adults have experienced ELS before the age of 18 (Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky, et al., 2010). ELS has been found to account for a large proportion of population attributable risk for substance use disorders (SUDs), mood and anxiety disorders, post-traumatic stress disorder (PTSD), and antisocial and borderline personality disorder, as well as various medical illnesses including cardiovascular disease and diabetes (Nemeroff, 2016; Lippard & Nemeroff, 2020). Overall, ELS contributes to a significant reduction in life span. As it is no longer a question of if the environment shapes gene-directed development, but rather a question of how it shapes development, studies investigating the exact biological and neurobiological mechanisms by which ELS increases risk for psychiatric illness are emerging. An intimate understanding of these mechanisms is crucial for the development of interventions combatting ELS-related consequences. This review focuses on the relations between ELS and development of, and recovery from, SUDs, which includes alcohol and other illicit drugs, and discusses the neurobiology and pathways that may mediate these relations.
Early Life Stress and Substance Use Disorders
Drug addiction is a chronic relapsing disorder characterized by compulsive drug seeking and use, loss of control over limiting intake, and emergence of negative emotional states reflecting motivational withdrawal (Koob & Volkow, 2010). ELS impacts each stage of the addiction cycle and is associated with a more pernicious disease course. The relation between ELS and increased risk for SUDs has been observed across a broad range of substances of abuse, including alcohol, opioids, nicotine, marijuana, and cocaine (Le Moal & Koob, 2007; Sinha, 2008; Pilowsky, Keyes, & Hasin, 2009; Enoch, 2011; Garami, Valikhani, Parkes, Haber, Mahlberg, Misiak, et al., 2019). One study found that 56% of young adults who experienced court-documented cases of physical abuse or neglect or sexual abuse between ages 0 and 11 have met the criteria for alcohol or drug abuse or dependence later in life (DuMont, Widom, & Czaja, 2007). Studies have also uncovered a graded relationship between ELS and risk for SUDs. A landmark study demonstrated that individuals having experienced four or more categories of ELS, including childhood maltreatment, possess a 7.2-fold increase in risk for developing alcohol use disorders (Anda, Croft, Felitti, Nordenberg, Giles, Williamson, et al. 2006). Similarly, another large-scale study found that individuals having experienced five or more adverse childhood experiences were seven- to tenfold more likely to report illicit drug use problems and addiction to illicit drugs (Skeer, McCormick, Normand, Buka, & Gilman, 2009). While the majority of largescale studies substantiating these relationships to date have been retrospective, several prospective, longitudinal studies have emerged and confirmed a positive association between ELS and SUDs, thereby strengthening inferences about causality (Shin, Edwards, & Heeren, 2009; Skeer et al., 2009).
ELS is associated with an earlier age of substance use initiation (Dube, Felitti, Dong, Chapman, Giles, & Anda, 2003; Ompad, Ikeda, Shah, Fuller, Bailey, Morse, et al., 2005; Enoch, 2011). In a nationally representative sample of 3,592 young adults, Rothman and colleagues found a positive association between adverse childhood experiences and initiation of drinking prior to the age of 15, while drinking initiation was found to occur after the age of 20 in those without adverse childhood experiences (Rothman, Edwards, Heeren, & Hingson, 2008). Dube, Miller, Brown, Giles, Felitti, Dong, et al., (2006) reported that for every adverse childhood experience, the likelihood of having initiated alcohol use by the age of 14 was increased by two- to threefold (N = 8417) and the likelihood of having initiated illicit drug use by age 14 was increased by two- to fourfold (N = 8613) (Dube et al., 2003, 2006). Notably, these studies all demonstrate inverse dose-response relationships between total number of adverse childhood experiences and age of substance use initiation. In another large-scale study of 122,824 Minnesota public school students (grades 6, 9, and 12), Harrison, Fulkerson, and Beebe (1997) also found that individuals with a history of physical and sexual abuse were almost twice as likely to have initiated alcohol use and three times as likely to have initiated drug use by age 12, compared to individuals without a history of abuse (Harrison et al., 1997). ELS is also associated with adolescent problem substance use behaviors, e.g., risky alcohol use patterns (Enoch, 2011). The US National Longitudinal Study of Adolescent Health (AddHealth) (N = 12,478 adolescents) showed that childhood maltreatment prior to the age of 11 increased risk for adolescent (ages 12–18) binge drinking (Shin et al., 2009). Hamburger, Leeb, and Swahn (2008) also found that childhood sexual abuse was associated with heavy episodic drinking in males (Hamburger et al., 2008). Adolescent substance use has lasting influences on lifelong substance use behaviors, and these behaviors constitute well-established risk factors for development of SUDs (DeWit, Adlaf, Offord, & Ogborne, 2000; Englund, Egeland, Oliva, & Collins, 2008). Earlier drug use initiation and problem adolescent substance use (i.e., binging) may, at least in part, mediate the relationship between ELS and SUDs. In fact, childhood maltreatment has been associated with a faster transmission from substance use to SUDs (Larance, Gisev, Cama, Nelson, Darke, Larney, et al., 2018), and childhood trauma appears to be more prevalent in early-onset alcoholism (before the age of 25) than in late-onset alcoholism (after the age of 25) (Dom, De Wilde, Hulstijn, & Sabbe, 2007).
ELS has been associated with increased risk of relapse and a poor treatment response in individuals with SUDs. Patients with a SUD and a history of ELS remain abstinent for shorter periods of time (Greenfield, Kolodziej, Sugarman, Muenz, Vagge, He, et al., 2002), relapse more often (Heffner, Blom, & Anthenelli, 2011; Van Dam, Rando, Potenza, Tuit, & Sinha, 2014), and are less adherent to treatment (Jaycox, Ebener, Damesek, & Becker, 2004), compared to patients with a SUD who do not have a history of ELS. For example, a prospective study investigating the relationship between childhood abuse and response to inpatient alcohol treatment found sexual abuse was associated with fewer abstinent days, higher prevalence of relapse (defined as three or more standard drinks per drinking day for women, and five or more for men), more heavy drinking days, and a shorter time period to first drink and relapse during the first year following discharge from inpatient treatment (Greenfield et al., 2002). Greater substance use during and following outpatient treatment is also reported in patients with a SUD and history of ELS (Shane, Diamond, Mensinger, Shera, & Wintersteen, 2006; Sacks, McKendrick, & Banks, 2008; Williams, Smith, An, & Hall, 2008). Increased treatment resistance and relapse may stem from greater craving and more severe withdrawal symptoms (Schumacher, Coffey, & Stasiewicz, 2006; Sacks et al., 2008; Francke, Viola, Tractenberg, & Grassi-Oliveira, 2013), as these factors appear to be heightened in individuals with a SUD who experience ELS. In a 3-week follow-up study of early abstinence in crack cocaine-dependent women, Francke et al. (2013) found that women with a history of childhood physical neglect experienced higher levels of withdrawal symptoms, as measured by the Cocaine Selective Severity Assessment, during detoxification than did women without a history of physical neglect (Francke et al., 2013). Notably, this study also found that craving levels remained higher and lasted longer in women with a history of physical neglect. It has been hypothesized that individuals with a history of ELS represent a clinically and biologically distinct subtype, highlighting the possibility that unique molecular underpinnings following ELS contribute to increased craving, withdrawal symptoms, and ultimately relapse (Teicher & Samson 2013; Teicher, Samson, Anderson, & Ohashi, 2016). For example, ELS has been associated with a unique inflammatory status (e.g., lower adiponectin and resistin plasma levels, higher tumor nercrosis factor (TNF)-alpha, TNF-related weak inducer of apoptosis, interleukin-6 and interleukin-4 levels, and a T-helper 1/T-helper 2 immune imbalance) during early abstinence in detoxified women with crack cocaine addictions (Levandowski, Viola, Tractenberg, Teixeira, Brietzke, Bauer, et al., 2013, 2014, 2016). Some studies have also suggested that neural differences distinguishing clinically healthy controls from individuals with psychiatric illness are restricted to, or more robust in, the subset of patients with a history of ELS. Hippocampal morphology is an example of this (Opel, Redlich, Zwanzger, Grotegerd, Arolt, Heindel, et al., 2014; Teicher et al., 2016); hippocampal gray matter volume deficits observed in individuals with SUDs appear to be uniquely associated with childhood maltreatment (Van Dam et al., 2014). Therefore, treatment specifically addressing ELS and its neurobiological impact may be instrumental in improving treatment response in this population.
ELS Timing, Duration, and Type
Stressful life events occurring at all stages of development, from infancy to early adulthood, have profound effects on the developing brain and on increasing risk for psychopathology (Dunn, Nishimi, Powers, & Bradley, 2017). However, as demonstrated in Fig. 1, brain regions mature at different stages in development, with each region possessing a unique and relatively brief exposure-sensitive period during which these regions are thought to be maximally susceptible to the effects of ELS (Andersen, Tomada, Vincow, Valente, Polcari, & Teicher, 2008; Baker, Williams, Korgaonkar, Cohen, Heaps, & Paul, 2013; Pechtel, Lyons-Ruth, Anderson, & Teicher, 2014). Thus, age at which ELS occurs may differentially affect brain development and eventual risk for psychopathology as previously reviewed (Teicher et al., 2016). Specifically, the hippocampus appears to be maximally susceptible to ELS between ages 3 and 5 years. Amygdala peak sensitivity appears to occur between ages 10 and 11 years, and the prefrontal cortex (PFC), a relatively late developing region, is suggested to be most sensitive to stress between the ages of 14 and 16 years. Despite these windows of heightened brain region vulnerability, there is evidence to suggest that the earlier ELS is experienced, the more extensive its impact is on development and psychiatric outcomes. Longitudinal clinical studies have shown that childhood maltreatment occurring prior to the age of 5, as compared to individuals with maltreatment occurring later in life, is related to higher levels of psychopathology, including more internalizing and externalizing behaviors, greater emotional dysregulation, decreased neurocognitive functioning (i.e., inhibitory control, working memory), and increased suicidal ideation (Keiley, Howe, Dodge, Bates, & Petti, 2001; Kaplow & Widom, 2007; Lansford, Miller-Johnson, Berlin, Dodge, Bates, & Pettit, 2007; Kim & Cicchetti, 2010; Cowell, Cicchetti, Rogosch, & Toth, 2015; Gould, Dunlop, Rosenthal, Iosifescu, Mathew, Neylan, et al., 2019). This is in line with developmental psychopathology models that posit that because rapid development across various domains occurs during childhood, the earlier the stress occurs, the greater the likelihood of adverse outcomes in childhood, which then further interferes with development during adolescence (Cicchetti & Toth, 1995).
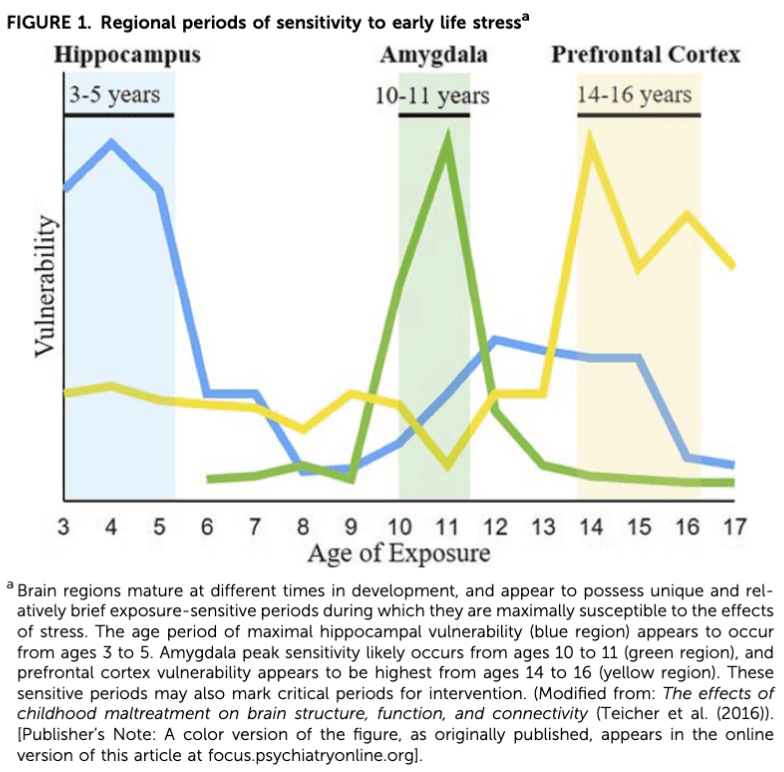
Literature documenting the contribution of ELS timing on SUD-related outcomes is relatively sparse and inconsistent. Several studies have reported that maltreatment occurring during adolescence confers heightened risk for illicit drug use during late adolescence and early adulthood than does maltreatment occurring in either early (ages 0 to 5 years) or late (ages 6 to 11 years) childhood (Thornberry, Ireland, & Smith, 2001; Smith, Ireland, & Thornberry, 2005). A recent study of 300 young adults found that individuals with a history of early life physical abuse, particularly occurring during adolescence, compared to individuals with no history of physical abuse, were at increased risk for greater alcohol use and associated behaviors during adulthood (i.e., frequent monthly drinking and alcohol related problems) (Shin, Chung, & Rosenberg, 2016). These studies converge to suggest that stress during adolescence may have an especially robust impact on substance use outcomes. Because greater autonomy and exposure to illicit substances is characteristic of adolescence, it is possible that maltreated adolescents are more likely to engage in the unhealthy coping strategies that may including abusing alcohol and other drugs in the face of stress (Tarter, 2002; Brown, McGue, Maggs, Schulenberg, Hingson, Swartzwelder, et al., 2008). Indeed, large-scale studies have reported that individuals with a history of adverse childhood experiences are more likely to use substances to cope with problems than individuals without such a history (Harrison et al., 1997; Rothman et al., 2008). Drinking and drug use as a coping strategy has been associated with compulsive substance use and dependence symptoms (Laurent, Catanzaro, & Callan, 1997). Drinking to cope with negative emotions has also been shown to partially mediate the relationship between childhood sexual abuse and alcohol problems (Grayson & Nolen-Hoeksema, 2005). However, the aforementioned large-scale studies did not report investigating the age at which adverse experiences occurred, so it is unclear whether stress occurring in later childhood and adolescence independently increases risk for greater substance use and associated behaviors or if stress occurring very early in life adversely sculpts the stress response in a way that increases risk for substance-related coping later in life. ELS timing may not only affect initiation and risk for developing a SUD but may also affect the likelihood of relapse. For example, Schumacher et al. (2006) showed that individuals who experienced trauma prior to the age of 13, compared to individuals who experienced trauma after the age of 13, reported greater alcohol craving following presentation of a personalized trauma-related cue; however, there was no relation between age of first trauma and age of first episode of drunkenness (Schumacher et al., 2006).
Studies have also demonstrated that the longer duration, greater severity, and type of ELS experienced may be associated with worse and distinct outcomes (Arnow, 2004; Dunn, Nishimi, Gomez, Powers, & Bradley, 2018). It is hypothesized that there is an additive effect of ELS (Anda et al., 2006), as cumulative effects of childhood adversity have been shown to increase risk for mood, anxiety, and SUDs and their comorbidity (Green et al., 2010; Putnam, Harris, & Putnam, 2013). Chronicity of ELS may be a greater risk factor for SUDs than is severity of any single stress event. One study reported that having multiple incidents of childhood sexual abuse more strongly predicted heavier alcohol use and binge drinking in adulthood than did having a single event of childhood sexual abuse, regardless of how severe the event was (Jasinski, Williams, & Siegel, 2000). This work supports the cumulative stress hypothesis that the magnitude and chronicity of ELS is a robust risk factor for later psychopathology (Turner & Lloyd, 1995, 2003, 2004; Pilowsky et al., 2009). Studies suggest that the type of ELS also matters (Carr, Martins, Stingel, Lemgruber, & Juruena, 2013). These results, however, should be interpreted with caution as the type of ELS may be inherently confounded with developmental stage at which it occurs (i.e., emotional neglect may appear to have the largest impact in the first year of life because it is the most common form of maltreatment during this period (Gunnar & Donzella, 2002)). Because types of ELS do not occur in isolation, and additive effects have been reported, it has been difficult to uniquely investigate a single type of ELS (Anda et al., 2006).
Parental Substance Use and Peer Context
The role of parental substance use on both ELS and offspring substance use should be considered. Not only do parental SUDs increase risk for offspring substance use problems by increasing genetic vulnerability and access to substances (Chassin, Curran, Hussong, & Colder, 1996), but parental SUDs, themselves, are considered a form of ELS and are highly associated with childhood maltreatment (Dube et al., 2001). The adverse childhood experience study found that individuals raised by one or more alcoholic parents were twice as likely to have experienced childhood maltreatment (Dube et al., 2001), and prospective work has further supported that a parental SUD increases risk for childhood maltreatment (Cicchetti & Handley, 2019). Thus, future work should consider the role of parental SUDs, specifically in terms of how genetic risk, increased access to substances, and ELS may interact to contribute to the development of offspring SUDs. It is also important to consider the salient role that peer contexts play in adolescence and the social motives/pressures that accompany them (Spear, 2000). It is possible that adolescents who have experienced family-related stress place an especially strong emphasis on peer relationships. Such relationships may be characterized by minimal adult supervision and high social pressure to engage in illicit substance use. Indeed, affiliation with substance using peers is a predictor of adolescent substance use (Chuang, Ennett, Bauman, & Foshee, 2005).
Neurobiological Underpinnings That May Contribute to SUDs Following Early Life Stress
ELS may sculpt the developing brain in a way that increases risk for subsequent development of SUDs. Specifically, ELS has been demonstrated to interfere with typical development of the hypothalamic-pituitary-adrenal (HPA) axis and corticotropin-releasing factor (CRF) brain systems that underlie the stress response, subcortical brain systems associated with reward processing and sensitivity (Teicher et al., 2016), and prefrontal cortical regions that underlie executive function (Lupien, McEwen, Gunnar, & Heim, 2009). These systems all play a major role in addiction-related behaviors and likely interact to contribute to risk for SUDs. The brain regions involved in these systems are highly sensitive to ELS (Arnsten, 2009; Hart & Rubia, 2012), with even brief periods of stress exposure resulting in significant structural remodeling (Holmes & Wellman, 2009). Preclinical studies have suggested a causal relationship between ELS and neural alterations, elucidating potential stress-induced remodeling processes (i.e., reduced dendritic length and branching, decreased spine density, and suppressed neurogenesis) (Arnsten, 2009; Holmes & Wellman, 2009; Lupien et al., 2009). To date, the literature has largely focused on how alterations in stress systems may mediate the relationship between ELS and SUDs; thus, the majority of this section will discuss effects of ELS on the neurobiological underpinnings of stress response, and subsequently how alterations in stress systems may interact with reward systems, as well as the potential role of altered executive functioning.
HPA Axis and CRF Brain Systems
ELS results in dysregulation of the HPA axis, altering homeostasis and reactivity to stressors (Lupien et al., 2009). Studies have also consistently observed HPA axis dysregulation in SUDs (Wand & Dobs, 1991; Lovallo, 2006), and work investigating addiction-related processes suggests that cortisol levels influence drug self-administration, drug reinforcement (i.e., subjective “high”), withdrawal symptoms, and stress and drug-induced craving and relapse (Sinha, 2001; Cleck & Blendy, 2008). The prevailing view is that a blunted HPA stress response in individuals with a history of ELS confers addiction vulnerability (Sinha, 2008). Specifically, suppressed cortisol secretion in the face of stress has been associated with enhanced drug motivation and self-administration in individuals with and without a SUD (Blaine & Sinha, 2017). Human studies have shown that blunted stress-induced cortisol responses predict subsequent increases in both frequency and quantity of substance use (Moss, Vanyukov, Yao, & Kirillova, 1999). For example, Blaine, Nautiyal, Hart, Guarnaccia, & Sinha, (2019) found that lower cortisol responses to stress and drug cues in heavy and moderate social adult drinkers predicted greater alcohol consumption during an alcohol taste test (Blaine et al., 2019). Blunted HPA activity following stress has also been observed in individuals with SUDs (Sinha, 2009) and appears to be predictive of relapse (Higley, Crane, Spadoni, Quello, Goodell, & Mason, 2011). These results are supported by preclinical models of stress-induced reinstatement (Sinha, 2001). Additionally, individuals with a family history of alcohol use disorders exhibit a blunted HPA axis response to stress (Dai, Thavundayil, Santella, & Gianoulakis, 2007).
However, conflicting findings from studies investigating alterations in the HPA axis and risk for SUDs have been reported. Specifically, HPA axis hyperactivity has also been associated with greater substance use (de Wit, Vicini, Childs, Sayla, & Terner, 2007; Huizink, Greaves-Lord, Oldehinkel, Ormel, & Verhulst, 2009). This association has primarily been substantiated by preclinical work (Piazza & Le Moal, 1996; Fahlke, Lorenz, Long, Champoux, Suomi, & Higley, 2000). Rodent studies have shown that elimination of the corticosterone response lowers stress-induced reinstatement of substances (Goeders & Guerin, 1996; Mantsch & Goeders, 1999). Fahlke et al., (2000) found that non-human primates stressed in early life (i.e., maternal deprivation) exhibited elevated cortisol levels in response to stressors, which was associated with increased alcohol self-administration (Fahlke et al., 2000). A hyperactive stress response is also linked to increased drug sensitivity and greater withdrawal symptoms in preclinical models (Varghese, Montalvo-Ortiz, Csernansky, Eiger, Herrold, Koola, et al., 2015). These studies converge to suggest that dysregulation of the HPA axis (both blunting and hyperactivity), in general, may serve as a biomarker for risky substance use behaviors, as well as a pre-morbid risk factor for developing SUDs. More research, particularly in human populations, is needed to understand the heterogeneity of findings and should include study of SUD risk in vulnerable populations (e.g., those with ELS and comorbid psychopathology) and other clinical and environmental factors that may contribute to heterogeneous findings (e.g., timing, type, and duration of ELS and familial factors).
ELS also results in long-lasting neural alterations in CRF brain systems that underlie the stress response and bidirectionally interact with the HPA axis. A recent study investigating the relationship between lifetime trauma exposure, HPA axis activity, and neural responses to acute stress in healthy adults found that greater trauma was associated with lower morning cortisol levels, and that greater trauma and lower cortisol are related to increased amygdala and hippocampal responses to stress (Seo, Rabinowitz, Douglas, & Sinha, 2019). The study reported that increased amygdala and hippocampal neural activity mediated the relationship between life trauma and decreased cortisol levels, suggesting that the effects of trauma on HPA activity may occur indirectly through a sensitized stress response in the limbic regions involved in positive and negative feedback of the HPA axis. Another imaging study, in healthy adults, reported that ELS is associated with stress-induced hyperactivity of limbic-striatal regions (including amygdala, hippocampus, insula, and striatum) and hypoactivity of orbitofrontal cortex (OFC) (Seo, Tsou, Ansell, Potenza, & Sinha, 2014). Lower volume and activity of the prefrontal cortex (PFC), including the OFC, has been consistently observed following ELS (Teicher et al., 2016). The PFC has a high density of stress-susceptible glucocorticoid receptors, receptors that cortisol binds to, rendering it highly sensitive to the effects of HPA dysregulation. For example, both elevated and suppressed levels of glucocorticoids have been found to impair prefrontal brain development through apoptosis, delays in myelination, inappropriate pruning, and decreased brain growth factor (McEwen, 2007; De Bellis & Zisk, 2014). Lower PFC volume may contribute to a sensitized limbic-striatal stress response as the PFC, and OFC, in particular, has many anatomical connections with limbic-striatal regions and regulates limbic-striatal responses to stressful and emotional stimuli. In fact, sustained PFC hypoactivation to stress has been related to substance use-related coping and binge drinking in adults without alcohol use disorders (Sinha, Lacadie, Constable, & Seo, 2016) and is predictive of craving and time to relapse in individuals with alcohol use disorders (Blaine, Seo, & Sinha, 2017). Because stress is a primary cause of relapse, stress sensitization following ELS likely contributes to the higher rates of relapse observed in these individuals. These data converge to suggest that altered neural stress systems following ELS may underlie stress sensitivity and contribute to increased stress-induced alcohol and drug intake across different stages of the addiction cycle.
Most of the studies investigating ELS and associated changes in stress-related brain systems have been conducted in healthy developing adults and patients with depression or PTSD. However, several studies investigating how ELS affects the neurobiology of stress systems in individuals with SUDs are emerging. Bachi, Parvaz, Moeller, Gan, Zilverstand, Goldstein, et al. (2018) identified lower OFC gray matter volume (GMV) in individuals with cocaine use disorders and histories of ELS, compared to individuals with cocaine use disorders and no ELS history (Bachi et al., 2018). Another imaging study found that lower GMV in subfields of the hippocampus were uniquely associated with childhood maltreatment and predictive of severity of relapse (Van Dam et al., 2014). Altered functional responses of corticolimbic stress systems (including the amygdala, OFC, and insula) have also been reported, when utilizing functional magnetic resonance imaging (fMRI), in a sample of alcohol-dependent individuals (Yang, Spence, Briggs, Rao, North, Devous, et al., 2015). It is important to note that changes following development of an alcohol use disorder may also contribute to changes in the HPA axis and CRF-containing circuits; studies have shown that initially drugs activate HPA activity and facilitate acquisition of drug use, but following chronic use, drug-induced HPA activity become blunted and dysregulated (Koob, 2008). Therefore, both pre-morbid stressful experiences and alcohol use may contribute to subsequent stress reactivity and most likely interact to contribute to detrimental outcomes. However, longitudinal study of individuals beginning prior to development of SUDs is needed to clarify neural alterations that occur following ELS (in the absence of drug abuse) in order to disentangle neurophysiological mechanisms that confer risk for initiation of, and transitioning to, a SUD, and mechanisms that may contribute to relapse and worse outcomes following development of a SUD. Such large-scale longitudinal studies have begun to emerge. For example, the Adolescent Brain Cognitive Development (ABCD) study is an ongoing landmark NIH-funded study following 10,000 healthy children from ages 9–10 into early adulthood, collecting neuroimaging data to track brain growth and allowing researchers to investigate environmental effects on brain development and social outcomes, including development of SUDs.
Subcortical Brain Systems Associated With Reward Processing and Reward Sensitivity
Changes in subcortical brain systems associated with reward processing and reward sensitivity are also suggested to contribute to increased risk for SUDs following ELS. Altered brain activity during reward anticipation is consistently reported in SUDs (Balodis & Potenza, 2015). ELS may confer pre-morbid risk for addiction vulnerability through its long-lasting neurobiological impact on reward processing and associated brain regions (Edmiston, Wang, Mazure, Guiney, Sinha, Mayes, et al., 2011; Enoch, 2011; Hart & Rubia, 2012). Most notably, fMRI studies have converged to suggest reduced sensitivity to rewards, as diminished basal ganglia activity to anticipated rewards is commonly observed in individuals with a history of ELS (Teicher & Samson, 2016). For example, during a monetary incentive delay task, childhood maltreatment was related to decreased positive subjective responses to reward-predicating cues as well as lower activation in the globus pallidus (Dillon, Holmes, Birk, Brooks, Lyons-Ruth, & Pizzagalli, 2009). Hanson, Albert, Iselin, Carre, Dodge, & Hariri (2016) demonstrated that higher cumulative ELS was associated with lower monetary reward-related activity in the ventral striatum (Hanson et al., 2016). Also probing reward anticipation, Boecker, Holz, Buchmann, Blomeyer, Plichta, Wolf, et al., (2014) observed that higher ELS relates to lower activation of both the ventral striatum and putamen (Boecker et al., 2014). While abnormalities in basal ganglia structure are reported (Edmiston et al., 2011), there have been few structural positive findings, suggesting that striatal structural changes may not necessarily underlie functional changes (Brake, Zhang, Diorio, Meaney, & Gratton, 2004). It has also been hypothesized that depressed striatal activity may result from alterations in other brain regions involved in stress systems (Teicher & Samson, 2016), as discussed below.
Interactions Between Stress and Reward Systems
Drug and stress sensitivity appear to be linked, with neurobiological models proposing that neuroadaptations in stress pathways interact with reward systems to alter sensitivity to the rewarding properties of alcohol and other drugs of abuse (Koob, 2008). ELS is associated with altered sensitivity to the subjective effects of substances (i.e., levels of drug-induced euphoria), a known risk factor for SUDs (Wand, Oswald, McCaul, Wong, Johnson, Zhou, et al., 2007). Cortisol may influence reward sensitivity and drug self-administration through its interaction with the mesocorticolimbic reward system. As many dopamine (DA) neurons in mesocorticolimbic regions express glucocorticoid receptors, cortisol released in response to stress heavily influences DA functioning in these regions. Suppression of corticosterone release lowers levels of DA under basal conditions and in response to stress and administration of drugs of abuse (Piazza & Le Moal, 1996; Barrot, Marinelli, Abrous, Rouge-Pont, Le Moal, & Piazza, 2000). Studies have largely demonstrated a positive association between ELS and mesocorticolimbic DA release following stress (Meaney, Brake, & Gratton, 2002), with increased HPA activity suggested to mediate the relationship. Pruessner, Champagne, Meaney, and Dagher (2004) found that healthy college students with a history of low parental care exhibited increased cortisol responses to a stress task, which positively related to DA levels in the ventral striatum (Pruessner et al., 2004). Work has also shown that drug administration itself induces a greater DA response in the ventral striatum in those with a history of childhood adversity than in those without a history of such adversity (Oswald, Wand, Kuwabara, Wong, Zhu, & Brasic, 2014). This may also be related to higher levels of stress- and drug-induced cortisol, as another study found that amphetamine-induced cortisol response was associated with dopamine binding in ventral striatum and self-reported amphetamine-induced euphoria (Wand et al., 2007). Taken together, data converge to suggest that following stress and drug administration, excessive stimulation of the DA reward system—due to increased cortisol release—enhances the reinforcing properties of substances of abuse (Piazza & Le Moal, 1996). However, the literature is mixed. A study by Besheer, Fisher, Grondin, Cannady, and Hodge (2012) showed that chronic cortisol treatment in rats results in decreased sensitivity to the interceptive effects of alcohol, as measured using drug discrimination methods (Besheer et al., 2012). Also, rodent models bred for high sensitivity to alcohol tend to exhibit blunted corticosterone responses to stress and alcohol (Raatesalmi, Virtanen, Sarviharju, Pelto, & Korpi, 2002). Considering that reward system functioning evolves throughout the course of the addiction cycle, it is possible that acute and chronic changes in cortisol levels and response differentially affect DA functioning.
Prefrontal Cortex and Executive Function
In addition to regulating limbic-striatal stress and reward systems, the PFC contributes to many higher-order executive functions (Girotti, Adler, Bulin, Fucich, Paredes, & Morilak, 2018). Among other functions, the PFC plays an essential role in attentional and cognitive processes, behavioral control, impulsivity, working memory, and decision-making (Goldstein & Volkow, 2011). Both the PFC and the aforementioned executive functions are thought to be critical to the development of addiction. Alterations in the PFC and executive functions are commonly observed in individuals with SUDs and have been linked to a more severe clinical course characterized by increased craving, greater drug use, denial of illness, and increased likelihood of relapse (Goldstein & Volkow, 2011; Blaine & Sinha, 2017). Neuroimaging studies have consistently demonstrated altered PFC function during tasks assessing executive functioning in individuals with SUDs (Goldstein & Volkow, 2011). For example, using a Stroop task to assess inhibitory control, Salo, Ursu, Buonocore, Leamon, and Carter (2009) found that methamphetamine-dependent subjects showed lower dorsolateral PFC and anterior cingulate activity, compared to non-substance abusers (Salo et al., 2009). While it is widely accepted that drug exposure directly alters PFC structure and function, and hence drug exposure may contribute to deficits in executive function, it is also important to note that recent work has indicated that PFC dysfunction may be a pre-morbid risk factor for SUDs (Goldstein & Volkow, 2011). Notably, a prospective study by Cheetham, Allen, Whittle, Simmons, Yücel, & Lubman (2014) found that lower prefrontal, particularly anterior cingulate, cortical volume in substance-naive adolescents predicts later alcohol-related problems (Cheetham et al., 2014). This group also found lower orbitofrontal gray matter volume to prospectively predict the initiation of cannabis use (Cheetham, Allen, Whittle, Simmons, Yucel, & Lubman, 2012).
As decreased PFC volume has been consistently observed following ELS (Teicher et al., 2016), it is possible that ELS alters PFC development in a way that impairs executive functions and ultimately increases risk for addiction. In addition to the wealth of literature linking ELS with altered PFC structure, impaired executive functioning, including decreased working memory, attention, and inhibitory control, has also been consistently observed following ELS (Holmes & Wellman, 2009; Hart & Rubia, 2012). Further support for PFC dysfunction following ELS comes from functional neuroimaging studies examining PFC-related performance during fMRI tasks investigating executive functions. One such study found that individuals with a history of childhood maltreatment, compared to those without, exhibited medial PFC hypoactivation during the encoding and recognition of emotional and neutral words, suggesting diminished recruitment of prefrontal engagement during higher order processing (van Harmelen, van Tol, Dalgleish, van der Wee, Veltman, Aleman, et al., 2014). Preclinical studies have provided insight into the molecular changes (i.e., reduced dendritic length, branching, and spine density) that might underlie altered PFC functioning following stress exposure and have linked such dendritic changes with impaired attentional and working memory processes (Arnsten, 2009). Following ELS, the PFC may be taken “offline,” allowing subcortical brain regions to exert greater bottom-up influence on behavior.
It is possible that the neural and executive functional consequences of ELS may confound or interact with those observed in SUDs (Teicher & Khan, 2019). While very few studies have considered the potential role of ELS on neural and executive functioning outcomes in SUDs, one study found that individuals with an alcohol use disorder and a history of maltreatment performed significantly worse on a task measuring sustained attention than did diagnostic controls without maltreatment (De Bellis, Morey, Nooner, Woolley, Haswell, & Hooper, 2019). These results highlight the need for more work aimed at examining whether ELS independently or additively contributes to prefrontal and executive deficits observed in SUDs.
Additional Pathways to SUDs Following Early Life Stress
Comorbid Psychopathology
ELS is also a well-established risk factor for mood and anxiety disorders and for their comorbidity with SUDs (Kessler, McLaughlin, Green, Gruber, Sampson, Zaslavsky, et al., 2010). Rates of childhood maltreatment appear to be especially high in individuals with these comorbidities, with one study finding that 95% of individuals with comorbid SUDs and mental health problems have experienced a traumatic event during childhood (Wu, Schairer, Dellor, & Grella, 2010). While it is possible that the effects of ELS are non-specific (i.e., a shared risk factor that indiscriminately increases risk for a range of psychiatric outcomes), it has also been postulated that mood and anxiety disorders mediate the increased risk for SUDs following ELS (i.e., primary mood/anxiety disorder, secondary SUD) (Brady & Sinha, 2005). In fact, adult psychological distress, which commonly occurs in mood and anxiety disorders, has been identified to mediate large proportions of alcohol use disorders in individuals with childhood abuse and neglect (Strine, Dube, Edwards, Prehn, Rasmussen, Wagenfeld, et al., 2012). One recent retrospective study of 690 adults from the general population found that the effect of childhood maltreatment on subsequent alcohol problems was mediated by depression (Salokangas, From, Luutonen, Salokangas, & Hietala, 2018). While not investigating ELS, a 10-year prospective study of 5001 participants identified through the National Comorbidity Survey found that baseline mental health disorders contributed significant prospective risk for the onset of nicotine, alcohol, and illicit drug dependence over the follow-up period (Swendsen, Conway, Degenhardt, Glantz, Jin, Merikangas, et al., 2010). Comorbid psychiatric problems have also been suggested to contribute to poor treatment outcomes following ELS. For example, assessment of over 20,000 veterans with SUDs in treatment found that psychiatric problems at baseline mediated the association between lifetime sexual or physical abuse and poorer treatment outcomes (Rosen, Ouimette, Sheikh, Gregg, & Moos, 2002). Another study observed that the positive association between sexual abuse and worse drinking outcomes following inpatient treatment was no longer significant when considering baseline psychosocial factors and psychiatric illness, including depression (Greenfield et al., 2002). Francke et al. (2013) conducted a 3-week follow-up study, with results suggesting that mood symptoms intensify the severity of withdrawal symptoms during SUD treatment; however, causality cannot be inferred. In a sample of crack cocaine-dependent women in treatment, another study found that a history of childhood physical neglect was associated with more prolonged and severe withdrawal symptoms during detoxification and that depression severity was positively correlated with the intensity of these symptoms (Francke et al., 2013). A self-medication hypothesis has also been proposed and documented; however, studies have also reported a relationship between childhood maltreatment and greater stress-related drinking even after controlling for mood-related symptoms (Young-Wolff, Kendler, & Prescott, 2012). These results suggest mechanisms beyond self-medication contribute to higher rates of SUDs in mood and anxiety disorders. For example, stress sensitization is well documented in mood disorders (Dienes, Hammen, Henry, Cohen, & Daley, 2006) and may be one mechanism by which ELS increases risk for comorbid mood and SUDs. Another possibility is that brain changes associated with mood/anxiety disorders increase vulnerability for problematic substance use (i.e., increasing impulsivity, altering the rewarding effects of drugs). For example, ADHD has been associated with neurobiological changes in brain circuits that are also associated with drug craving. Obsessive-compulsive disorder related behaviors have been suggested to engage ventral striatal circuits involved in drug reward (Fontenelle, Oostermeijer, Harrison, Pantelis, & Yucel, 2011).
There is also evidence to suggest that SUDs are an intermediate risk factor for mood/anxiety disorders (i.e., primary SUD, secondary mood/anxiety disorder) following ELS (Strakowski & DelBello, 2000). Risky behaviors, including smoking and alcohol and drug use, are prevalent in individuals with ELS and likely contribute to adverse psychiatric and medical outcomes (Anda, Felitti, Bremner, Walker, Whitfield, Perry, et al. 1999). Addiction has been shown to worsen clinical course, prognosis, and treatment outcomes in mood/anxiety disorders (Balanzá-Martínez, Crespo-Facorro, González-Pinto, & Vieta, 2015). It is possible that substance use initiates mood and anxiety disorders in vulnerable individuals who might not have otherwise developed the disorder (Winokur, Coryell, Akiskal, Maser, Keller, Endicott, et al., 1995; Strakowski & DelBello, 2000). For example, mood episodes may be more likely to occur due to changes in neurotransmitter signaling systems caused by excessive alcohol use (Sonne & Brady, 2002). Alcohol intoxication has also been found to exert glutamatergic effects and generate reactive oxidative species that may diminish cortical control over limbic systems that subserve emotional regulation (Stanfield, Moorhead, Job, McKirdy, Sussmann, Hall, et al., 2009; Rakofsky & Dunlop, 2013). It has been suggested that with every withdrawal episode, neurons are more likely to depolarize autonomously, resulting in mood episodes with no apparent stressors (Post & Kalivas, 2013). Binge drinking and withdrawal states are known to induce epigenetic changes in the brain, including histone methylation, phosphorylation, acetylation, and DNA methylation. Such changes result in altered gene expression, ultimately leading to more and longer mood episodes over time (Rakofsky & Dunlop, 2013). In a neuroimaging study of methamphetamine-dependent adults, Dean, Kohno, Hellemann, and London (2014) found that childhood maltreatment was positively associated with limbic connectivity, with amygdala and hippocampal connectivity correlated with current levels of depression, anxiety, and emotional dysregulation (Dean et al., 2014). While directionality cannot be inferred, these results support altered brain activity, including connectivity; following childhood maltreatment in individuals with SUDs may contribute to negative emotional states.
As illustrated in Fig. 2, the relations between SUDs and comorbid psychopathology are undoubtedly complex. A greater understanding of these relations could increase efficacy of treatment and improve outcomes. Research has suggested that having a primary mood disorder and secondary SUDs (i.e., mood disorder onset first) is clinically and etiologically distinct from primary SUDs and secondary mood disorders (Winokur, Cook, Liskow, & Fowler, 1993; Feinman & Dunner, 1996; Strakowski, DelBello, Fleck, Adler, Anthenelli, Keck, et al., 2005). Determining the primary versus secondary disorder may be crucial in guiding effective treatment, as studies have shown that treatment is most successful when targeting the primary disorder (Fontenelle et al., 2011). For example, a double-blind placebo-controlled study of lithium treatment for adolescents with primary bipolar disorder and secondary SUD found that lithium not only improved psychiatric outcomes, but it also reduced positive urine tests for substances of abuse (Geller, Cooper, Sun, Zimerman, Frazier, Williams, et al., 1998). A recent study by Liu, Oshri, and Duprey (2018) took a developmental approach to understanding the direction of the relationship between depressive symptoms and alcohol use following childhood maltreatment (Liu et al., 2018). The group confirmed their hypothesis that the relationship between depression and alcohol use is bidirectional and influenced by developmental timing. These findings further support tailoring treatment and intervention to target the primary disorder. More longitudinal work disentangling the temporal relationship between substance use and mood disorders following ELS is critically needed.
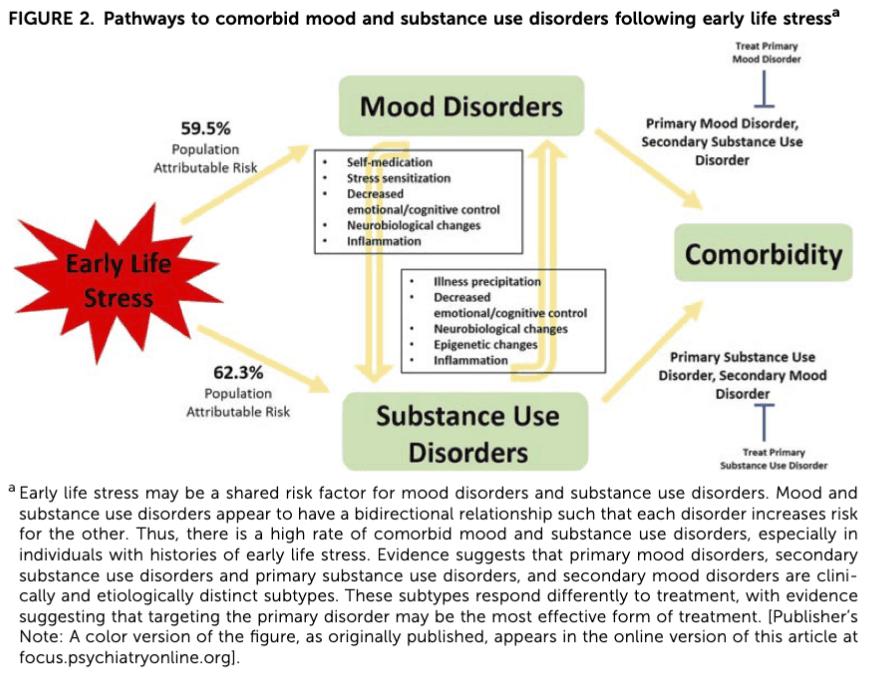
Another treatment approach may be to identify and target processes implicated in the etiology of both mood and SUDs. For example, ELS is associated with systemic inflammation (Nemeroff, 2016), which is thought to be a key risk factor for the development and progression of mood and SUDs. While patients affected by mood and SUDs exhibit elevated levels of inflammatory markers (Lippard & Nemeroff, 2020 accepted), it is unclear to what degree ELS-related to inflammation may contribute to this finding, especially in light of work showing that substances of abuse themselves also increase inflammation. Recently, work has suggested that inflammation may contribute to HPA axis dysregulation (Chrousos, 2000) and precipitate changes in neural structure and function (Tsai, Gildengers, Hsu, Chung, Chen, & Huang, 2019). The observed HPA axis and neural abnormalities appear to overlap with those reported following ELS. Interestingly, it is also suggested that HPA axis dysregulation may contribute to inflammation (Miller, Chen, Fok, Walker, Lim, Nicholls, et al., 2009), suggesting bidirectional communication between the HPA axis and immune functioning. Thus, anti-inflammatory treatment may provide a promising therapeutic strategy in mood and SUDs, especially in individuals with ELS; future work investigating the role of inflammation in the course of mood and SUDs, and their comorbidity, is warranted.
Impulsivity
Considering that impulsivity is a major risk factor for addiction (Dawes, Targer, & Kirisci, 1997), and that individuals with a history of ELS exhibit elevated levels of impulsivity (Elam, Wang, Bountress, Chassin, Pandika, & Lemery-Chalfant, 2016), it has been hypothesized that impulsivity contributes to risk for SUDs following ELS. In fact, studies have demonstrated that impulsivity partially mediates the relationships between ELS and adverse substance use outcomes (Shin, Lee, Jeon, & Wills, 2015; Proctor, Lewis, Roesch, Thompson, Litrownik, English, et al., 2017; Kim, Hwang, Kim, Hwang, Cho, Kang, et al., 2018). Negative urgency, or impaired self-control in the face of negative emotionality, is one dimension of impulsivity that has been consistently identified as elevated following ELS (Shin, McDonald, & Conley, 2018). This facet of impulsivity is also a known risk factor for problematic substance use (Stautz & Cooper, 2014). Shin et al., (2015) showed that negative urgency mediated the relationship between childhood emotional abuse and frequency of alcohol use, binge drinking, and alcohol use disorders in a community sample of young adults (Shin et al., 2015). Wardell, Strang, and Hendershot (2016) found that negative urgency was the only facet of impulsivity to mediate the relationship between childhood maltreatment and alcohol and cannabis use problems (Wardell et al., 2016). However, other studies have identified additional dimensions of impulsivity that also mediate the relationship between ELS and substance use. For instance, Oshri, Kogan, Kwon, Wickrama, Vanderbroek, Palmer, et al., (2018) found that negative urgency, positive urgency (the tendency to be impulsive in the face of positive emotions), and sensation seeking all mediated the relationship between childhood maltreatment and alcohol and cannabis use (Oshri et al., 2018). Ramakrishnan, McPhee, Sosnowski, Rajasingaam, and Erb (2019) found positive urgency to partially mediate the relationship between ELS and substance-related problems (Ramakrishnan et al., 2019). Kim et al. (2018) showed that response impulsivity, including sensation seeking, reflection impulsivity, and aggression, partially mediated the association between ELS and alcohol use disorder severity (Kim et al., 2018). Indeed, another study demonstrated that externalizing behaviors, including impulsivity, mediated the effect of childhood maltreatment on age of alcohol and marijuana use initiation (Proctor et al., 2017). A prospective study following 1421 adolescents from ages 10 through 22 found that impulsivity-related externalizing (i.e., aggression, delinquency), but not internalizing (i.e., social withdrawal, somatic complaints, and anxious/depressed behaviors), problems partially mediated the risk for SUDs following early life familial conflict (Skeer et al., 2009). While discordant results are reported, perhaps because different facets of impulsivity may not be generalizable across substances, they converge to suggest impulsivity as a risk factor for SUDs. Indeed, there is some evidence that distinct impulsive traits may be differentially related to type and/or frequency of substance use (Sunny Hyucksun Shin, Chung, & Jeon, 2013; Ramakrishnan et al., 2019). Additionally, brain imaging studies suggest that ELS results in changes in brain regions thought to regulate impulsive behaviors (Teicher & Samson, 2016). For example, a study by Teicher, Anderson, Ohashi, and Polcari (2014) uncovered functional connectivity differences in brain networks that regulate emotions and urges in young adults with a history of maltreatment, compared to young adults without a history of maltreatment (Teicher et al., 2014).
Summary and Future Directions
ELS increases risk for SUDs and is associated with a more severe disease course characterized by earlier onset, greater craving and relapse, treatment resistance, and greater comorbidity and suicidal behavior. Examining these relationships in human studies is difficult; there is often limited control over confounding factors (i.e., disease-related confounds), retrospective cross-sectional studies may be clouded by recall bias, and study design often limits causal inferences. Preclinical animal models do not face these limitations and have been especially informative in studying the relations between ELS and SUDs. Not only does the wealth of data from these preclinical studies support these relations (Sinha, 2001; Moffett, Vicentic, Kozel, Plotsky, Francis, & Kuhar, 2007), but they have also advanced our knowledge because they allow for exclusion of potential confounds, direct manipulation of ELS, and close examination of neurobiological mechanisms (see Box 1 for a summary of preclinical models). These preclinical models also have considerable ecological validity. Continued preclinical study, in conjunction with clinical research, is necessary to ascertain a more complete understanding of the pathways to SUDs following ELS, as well as to test possible intervention techniques to mitigate adverse outcomes. With that said, caution must be taken in interpreting the results of these preclinical studies. Animal models may not have the capacity to capture and integrate the complexities underlying the relationship between ELS and SUDs, and greater attention to the generalizability and translational validity of these models is critical. For example, animal models might not be capable of capturing the complex interaction between comorbid anxiety/mood disorders and SUDs. Additionally, as timing and duration of ELS are factors that heavily influence neural and behavioral outcomes, another limitation in terms of translational relevance stems from the incomparable rates of development between animals, particularly rodents and mice, and humans.
Despite the complexity of studying these processes in humans, clinical studies have consistently demonstrated that ELS increases risk for SUDs through altering development of the HPA axis, CRF circuits, and mesocorticolimbic reward systems and their interactions. Comorbid mood/anxiety disorders mark one factor that likely has a mutually detrimental bidirectional relationship with SUDs following ELS. More research is needed in investigating comorbid psychopathology following ELS, especially in light of the significance this may have on treatment efficacy. Considering the high rates of suicide in the United States, greater investigation of the relationship between ELS, SUDs, and suicide is also warranted. While relatively little is known about this relationship, studies have documented a positive association between ELS and rates of suicidal behavior in individuals with SUDs (Roy, 2003; Maloney, Degenhardt, Darke, Mattick, & Nelson, 2007; Huang, Schwandt, Ramchandani, George, & Heilig, 2012). Additionally, how genes interact with the environment to influence substance use outcomes marks another avenue in need of further exploration. Although genetic and environmental factors correlate highly within families, genes have emerged as a mechanism that influence how ELS manifests in adverse outcomes, including SUDs. Studies in this area have predominantly focused on how specific candidate genes, commonly those involved in the HPA axis and CRF circuits, interact with the environment to increase risk for subsequent substance use and SUDs (Heath & Nelson, 2002; Enoch, 2006). However, candidate genes studies are limited by methodological and conceptual limitations (Dick, Agrawal, Keller, Adkins, Aliev, Monroe, et al., 2015). Future work should extend beyond genetic polymorphisms of candidate genes, including investigating epigenetic changes and polygenetic risk, to garner a better understanding of these complex gene by environment interactions underlying associations between ELS and development/course of SUDs. It is clear that additional factors play a role in the development of SUDs following ELS. For example, sex differences, both following ELS and in pathways to addiction (Lippard, Mazure, Johnston, Spencer, Weathers, Pittman, et al., 2017), are suggested. One prospective study found that severity of childhood trauma predicted cocaine relapse and escalation of drug use after initial relapse in women but not men (Hyman, Paliwal, Chaplin, Mazure, Rounsaville, & Sinha, 2008), with similar sex differences also reported in relapse in individuals with alcohol use disorders (Heffner et al., 2011). Future studies should consider sex differences when investigating pathways to SUDs following ELS.
It goes without saying that serious efforts should be made toward the prevention of early life maltreatment. Additionally, an intimate understanding of the mechanisms that translate ELS into risk for SUD is crucial for the development of interventions combatting ELS-related consequences. Childhood and adolescence mark periods of rapid development, and while high brain plasticity during this time increases susceptibility to the effects of stress, it also makes it a critical window for intervention (Romeo & McEwen, 2006). Indeed, recent work has found that psychosocial factors (i.e., living situation, parenting, environmental enrichment, social support) impact risk for SUDs, in that these factors are associated with decreased disease vulnerability and improved disease course following ELS (Lansford, Malone, Stevens, Dodge, Bates, & Pettit, 2006; DuMont et al., 2007; Braithwaite, O'Connor, Degli-Esposti, Luke, & Bowes, 2017; Cheong, Sinnott, Dahly, & Kearney, 2017). For example, supportive relationships with family members and peers may constitute strong protective factors, because these relationships appear to teach healthy stress-coping strategies and foster healthy stress response systems (Bellis, Hardcastle, Ford, Hughes, Ashton, Quigg, et al., 2017). Bellis et al. (2017) (N = 7047) found that the increased adult prevalence of daily smoking and heavy alcohol consumption following adverse childhood experiences was decreased in those with support from a trusted and always available adult during childhood (Bellis et al., 2017). Psychosocial treatment interventions have also been successful in improving outcomes following ELS. Trauma-focused, cognitive behavioral therapy, for example, has garnered empirical support (Cohen, Mannarino, Murray, & Igelman, 2006) and marks another avenue for further development. Future research should investigate if certain psychosocial factors/interventions are more effective at different stages in life as brain regions thought to contribute to risk following ELS mature at different period in development (Fig. 1). Additionally, identifying neurobiological differences between individuals susceptible and resilient to the effects of ELS could inform treatment strategies (Teicher et al. 2016). Studies have shown that ELS-induced modifications may be reversible. For example, lower cortisol release following ELS appears to be transient, as improved childcare and alleviation of chronic stress reverses the state (Fries, Hesse, Hellhammer, & Hellhammer, 2005; Gunnar & Quevedo, 2008). Stress-induced morphological remodeling (i.e., dendritic atrophy and branching) of the PFC and hippocampus is also suggested to be reversible following alleviation of chronic stress (Vyas et al., 2004; Radley, Rocher, Janssen, Hof, McEwen, & Morrison, 2005). A controlled study of Romanian orphans found that children randomly assigned to a newly established system of higher-level foster care, as opposed to institutional care, not only exhibited lower levels of adverse clinical outcomes (Smyke, Zeanah Jr., Fox, & Nelson 3rd, 2009) but also a reversal of previously observed decreased white matter volume (Sheridan, Fox, Zeanah, McLaughlin, & Nelson 3rd, 2012). It is also important to consider that brain changes in some individuals following ELS may be adaptive (Callaghan & Richardson, 2011). For example, accelerated brain development following ELS has been observed in both rodents and humans and has been suggested to reflect an adaptation to adversity (Callaghan & Richardson, 2011). Specifically, Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer, et al. (2013) found that following maternal deprivation, some children exhibit accelerated development of PFC-amygdala connectivity (Gee et al., 2013). Longitudinal study is critically needed to disentangle what ELS-related differences contribute to risk or resilience to aid development of future interventions. Despite the need for more study, there appears to be great opportunity to improve outcomes following ELS and decrease development of SUDs and related outcomes. Data suggesting the effects of ELS may be reversible require serious attention, with future study aimed at developing psychosocial and/or psychopharmacological interventions and environmental enrichment programs. These interventions/programs may improve outcomes, strengthen resiliency, and decrease societal and individual burden.