Abstract
Substance use disorder (SUD) is a chronic relapsing disorder with long-lasting changes in brain intrinsic networks. While most research to date has focused on static functional connectivity, less is known about the effect of chronic drug use on dynamics of brain networks. Here we investigated brain state dynamics in individuals with opioid use (OUD) and alcohol use disorder (AUD) and assessed how concomitant nicotine use, which is frequent among individuals with OUD and AUD, affects brain dynamics. Resting-state functional magnetic resonance imaging data of 27 OUD, 107 AUD, and 137 healthy participants were included in the analyses. To identify recurrent brain states and their dynamics, we applied a data-driven clustering approach that determines brain states at a single time frame. We found that OUD and AUD non-smokers displayed similar changes in brain state dynamics including decreased fractional occupancy or dwell time in default mode network (DMN)-dominated brain states and increased appearance rate in visual network (VIS)-dominated brain states, which were also reflected in transition probabilities of related brain states. Interestingly, co-use of nicotine affected brain states in an opposite manner by lowering VIS-dominated and enhancing DMN-dominated brain states in both OUD and AUD participants. Our finding revealed a similar pattern of brain state dynamics in OUD and AUD participants that differed from controls, with an opposite effect for nicotine use suggesting distinct effects of various drugs on brain state dynamics. Different strategies for treating SUD may need to be implemented based on patterns of co-morbid drug use.
Introduction
Substance use disorder (SUD) is a chronic relapsing disorder and a main contributor to global disease burden. In the United States, around 80,816 deaths occurred from opioid overdoses in 2021 and each year more than 140,000 people die from alcohol-related causes. Therefore, a better understanding of the neurobiological mechanisms is relevant for addressing consequences from SUD.
Resting-state fMRI (rfMRI) has advanced our fundamental understanding of functions of large-scale brain networks in a task-free condition. A growing body of evidence suggests that chronic drug use can have long-lasting effects on intrinsic brain networks, thereby compromising cognitive and affective functions, which are crucial in the development and maintenance of SUD. So far, most studies on SUD have focused on quantifying differences in static functional connectivity, which assumes that connectivity between regions is static within the time period of the scan. Connectivity differences between individuals with SUD and matched controls are often observed in the default mode network (DMN), which is typically involved in self-referential thinking, in the salience network, which plays an important role in directing attention to internal and external stimuli and in the executive control network, which is relevant for cognitive control and goal-directed behaviors.
While static functional connectivity reveals spatial properties of intrinsic brain networks and emphasizes connectivity strength between different regions averaged over time, dynamic functional connectivity provides additional insights in their temporal profiles, which may underlie essential aspects of cognition, emotions and behavior. Although there has been growing interest, few studies have examined brain dynamics in SUD. In chronic smokers, altered dynamic functional connectivity density was observed in the visual network (VIS), the DMN, and in reward circuitry. Importantly, in the latter study, dynamic analysis significantly outperformed the static analysis and showed greater sensitivity to detect subtle differences between chronic smokers and controls. In another study, cocaine users showed higher DMN state occurrence rate and higher probability of transitioning from the salience state to the DMN state. Additionally, different classes of drugs and their co-use might distinctly affect brain dynamics when compared to healthy controls. In this study, we examined brain states in patients with opioid use disorder (OUD) and with alcohol use disorder (AUD) and explored how co-use of opioid, alcohol, and nicotine affected brain state dynamics. We hypothesized that compared to healthy controls (HC), OUD and AUD participants would show imbalanced brain state dynamics. As both opioid and alcohol are sedating drugs, we expected similar effects on brain state dynamics and an exacerbation with their co-use; whereas we predicted that nicotine, which has stimulant effects, would have opposite effects. Various analytical approaches have been proposed to capture temporal features of brain networks. In the current study, we applied a data-driven clustering approach to identify recurrent co-activation patterns of brain networks i.e., brain states and their dynamics. This approach was chosen because it uses the maximum temporal resolution offered by fMRI and determines brain states at a single time frame. It differs from the dynamic functional connectivity by identifying network co-activations rather than calculating the connectivity between networks within a defined time window.
Materials and methods
Participants
We used data from two cohorts to investigate brain states in OUD (Cohort 1) and AUD (Cohort 2), respectively. In Cohort 1, data from 27 OUD participants, who were recruited from treatment programs, or the community and 38 HC were included for analyses. OUD participants met the criteria for a DSM-5 OUD diagnosis in their lifetime and had a minimum 5-year history of opiate misuse. Twenty-one (out of 27) OUD participants were being treated with medications for OUD (buprenorphine or methadone). In Cohort 2, data from 107 AUD participants and 99 HC were included for analyses. Among AUD participants, 22 were non-treatment seeking and 85 were treatment seeking. Treatment-seeking participants were enrolled in a short-term inpatient detoxification program at the NIH clinical center and the average number of detoxification days prior to the scan day was 19.41 ± 7.52 days. A standardized clinical interview for DSM-IV or DSM-5 was used for AUD diagnosis. The Timeline Follow-back was used to assess daily alcohol consumption in the 90 days prior to the study. For the AUD cohort, we excluded participants with other SUDs apart from AUD or nicotine dependence, whereas for the OUD cohort, we did not exclude them if they had an additional SUD. For both cohorts, participants were given a breathalyzer and a urine drug screen (cocaine, tetrahydrocannabinol, opiates, amphetamine, methamphetamine, and oxycontin) on each day of testing. Participants who failed the drug screen were excluded, except for the presence of opiates in OUD. HC had no history of SUD or other psychiatric disorders nor current use of prescribed or over-the-counter psychoactive medications. The Alcohol Use Disorders Identification Test and the Fagerstrom Test for Nicotine Dependence (FTND) were used to identify harmful drinking behavior and nicotine dependence, respectively. All participants were asked not to smoke in the 2 h prior to their MRI scan and/or to remove their nicotine patch if they had one. There were no smokers among HC. Written informed consent approved by the Institutional Review Board at the NIH was obtained from all participants.
MRI acquisition
In Cohort 1, participants were scanned on a 3 T Magnetom Prisma scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) with a 32-chaneel head coil. For rfMRI, data were collected for 8 min; a multi-echo, multiband echo-planar imaging (EPI) sequence was used (Multiband factor = 3, TR = 891 ms, TE = 16, 33 and 48 ms, FA = 57°, 45 slices with 2.9 × 2.9 × 3.0 mm voxels, 520 time points). Multi-echo images for each time point were combined using an echo-time weighted average of TEs. A fixation cross was presented on a black background and participants were instructed to keep their eyes open, using an LCD monitor. T1-weighted 3D MPRAGE (TR/TE = 2400/2.24 ms, FA = 8°) and T2-weighted variable flip angle turbo spin-echo (Siemens SPACE; TR/TE = 3200/564 ms) pulse sequences were used to acquire high-resolution anatomical brain images with 0.8 mm isotropic voxels FOV = 240 × 256 mm.
In Cohort 2, participants were scanned on a Siemens 3T Magnetom Skyra scanner with a 20-channel head coil. The rfMRI data were collected for 10 min with eyes open and an EPI sequence was used (TR = 2000 ms, TE = 30 ms, FA = 90°, 3.8 mm isotropic voxels, multi-slice mode: interleaved, 300 time points). High resolution structural images were collected using a T1-weighted MPRAGE sequence (TR/TE = 1900/3.09 ms, FA = 10°, FOV: 240 × 240 mm, 1 mm isotropic voxels).
Due to the differences in MRI acquisition, all analyses were conducted for Cohort 1 and Cohort 2 separately.
Replicate brain states with an independent dataset with a single-echo multiband EPI (Cohort 3)
An independent dataset with 55 healthy individuals (Age 42.0 ± 13.2; 30 Females) was used for validating identified brain states. A single-echo multiband EPI (multiband factor = 8, TR/TE = 720/37 ms, FA = 52° and 72 slices with 2 mm isotropic voxels, 1238 time points) were used to record resting-state BOLD responses for 15 min.
MRI preprocessing
The data were preprocessed using CONN toolbox 21a including rigid body realignment, spatial normalization to MNI space, smoothing (FWHM = 6 mm), band-pass filtering (0.01–0.08 Hz), linear detrending, head motion regression (three rotational, three translational and their derivatives), removal of signals within the CSF and the WM using aCompcor, a method for identifying principal components associated with segmented WM and CSF. Using custom MATLAB code, we further scrubbed volumes with a FD threshold of 0.25 mm and DVARS threshold of 150%. Participants who had a mean FD < 0.6 mm before scrubbing and the number of time frames greater than 180 after scrubbing were included in the analyses.
Analysis of brain states and dynamics
To identify brain states, i.e., brain co-activation patterns, denoised voxel-level data were first parcellated into 400-node Schaefer atlas [21] and demeaned. We then concatenated ROI timeseries from all participants combining HC and patients (Matrix row: NSubjects*Ntime points; column: NROIs) and applied k-means clustering. This approach allowed us to investigate brain dynamics with the maximal temporal resolution of 1 TR. We performed k-means clustering for k = 2–22 in Cohort 1 and k = 2–17 in Cohort 2, where k was the number of clusters (k2 must be less than the number of TRs to capture all transitions) using Pearson correlation as the distance metric and repeated 50 times with the random initializations before choosing the solution with the best data separation. The optimal number of clusters (k) was determined based on incremental variance explained by the lowest error solution at each value of k. In Cohort 1, k = 6 was chosen because the additional variance explained by increasing k beyond k = 6 was less than 1%. In Cohort 2, additional variance explained by increasing k beyond k = 5 was less than 1%. However, to keep consistency between the two cohorts, we chose k = 6 for the second cohort as well (Fig. S1). Another reason we chose k = 6 rather than k = 5 is that with k = 6 we were able to identify three anticorrelated pairs of brain states in both cohorts. To further ensure the reliability of our partitions, we independently repeated the process ten times and computed adjusted mutual information between each of the ten resulting partitions. We then selected the partition that shared the greatest adjusted mutual information with all other partitions for further analysis. Clusters were defined as brain states and labeled by assessing the cosine similarity of the positive and negative activations of their centroid with a binary presentation of seven a priori-defined brain functional networks. Positive values of cluster centroid reflect activations above mean (high amplitude), while negative values reflect activations below mean (low amplitude).
Following this, we then analyzed the dynamic characteristics of the six identified brain states. The fractional occupancy was defined as proportion of TRs assigned to each brain state. Dwell time was calculated by averaging the length of time (number of contiguous TRs *TR) spent in a brain state. Appearance rate was determined by the total number of times a state appeared per minute. Additionally, transition probability between states i and j was defined as the probability that state j occurs at the TR after state i, given that state i is occurring.
Analysis of static functional connectivity
Since instantaneous network co-activation and static functional connectivity provide complementary information on brain networks at rest, we additionally analyzed resting state functional connectivity between intrinsic networks. Denoised voxel-level data were first parcellated into seven networks using Yeo atlas. Pearson’s correlation coefficients between the network time courses were computed. Correlation coefficients were then converted to normally distributed Z-scores using the Fisher transformation for the second-level group analysis.
Statistics
For group comparisons (OUD/AUD vs HC), two sample t test were used. In OUD and AUD participants, we also assessed how co-morbid nicotine dependence affected brain state dynamics by calculating the correlations with the FTND scores. Additionally, we calculated the correlations between brain state dynamics with the AUDIT scores to examine the effect of co-morbid alcohol use in OUD and the effect of alcohol use severity in AUD. As the sample size in Cohort 2 was large enough, we also performed one-way ANOVA to investigate differences between AUD smokers (n = 57), AUD non-smokers (n = 50) and HC. Fisher’s LSD tests were used for ANOVA posthoc tests. SPSS 22 (IBM, Armonk, NY), MATLAB (R2022b) and RStudio were used for analyzes. Benjamini-Hochberg procedure (BH) was applied for corrections for multiple comparisons. Both uncorrected and BH-corrected p values as well as effect sizes were reported. Findings with puncorr < 0.05 were discussed.
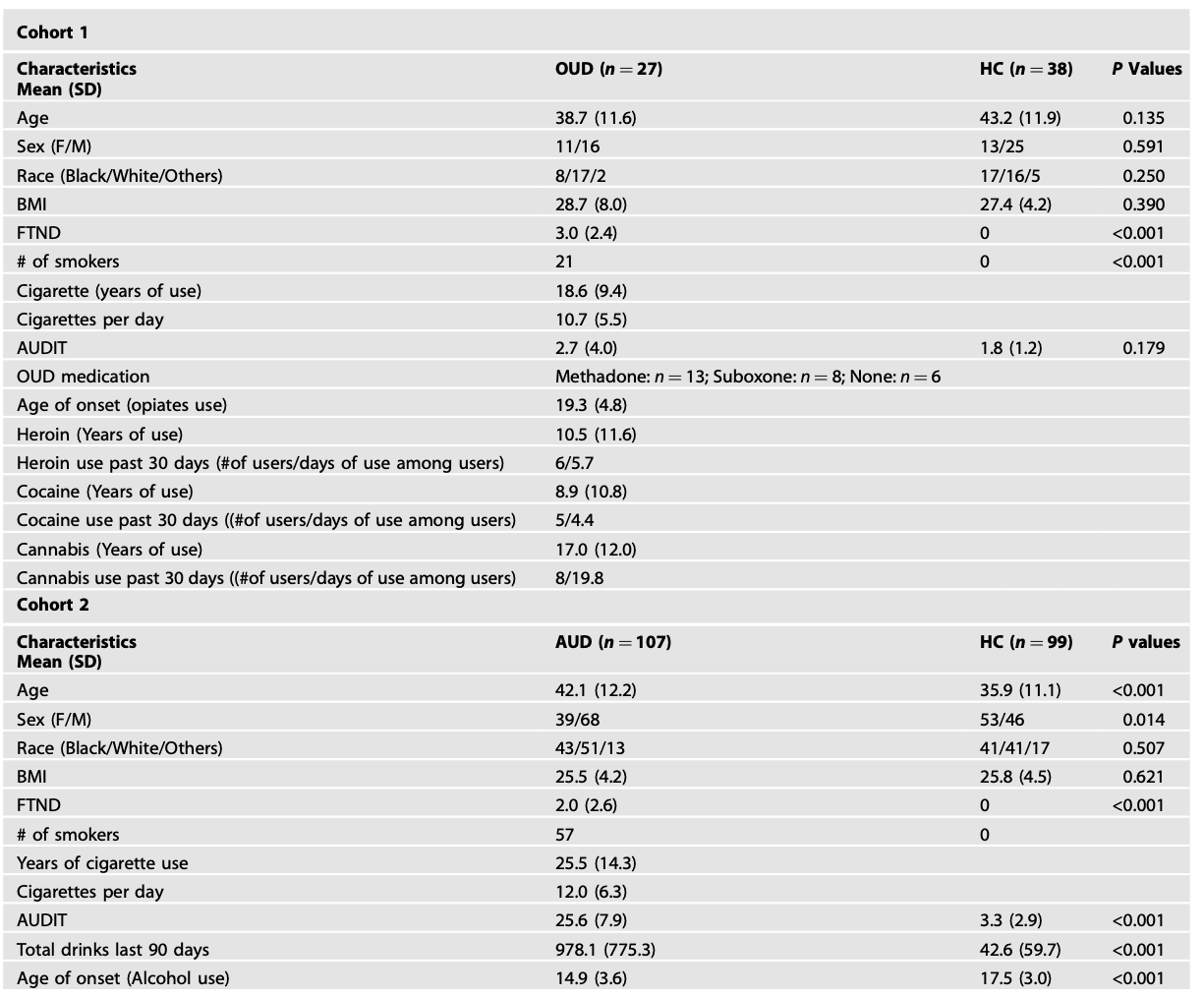
Recurrent brain states
Clustering algorithm identified six co-activity patterns i.e., brain states in both cohorts (Fig. 1). The six brain states remained when identifying brain states by clustering groups (OUD/AUD vs HC) separately (Figs. S2 and S3). We further replicated our results with an independent dataset indicating the robustness of the identified brain states (Fig. S4). Based on cosine similarity to Yeo’s 7 networks, brain states were labeled as SOM+, SOM-, VIS+, VIS-, DMN+, DMN-/LIM-. In both cohorts, we also observed the hierarchical relationship among the six identified brain states, which could be grouped into three anti-correlated pairs (Fig. S5). Similar findings of anticorrelated sub-states have been previously reported by others using this method.
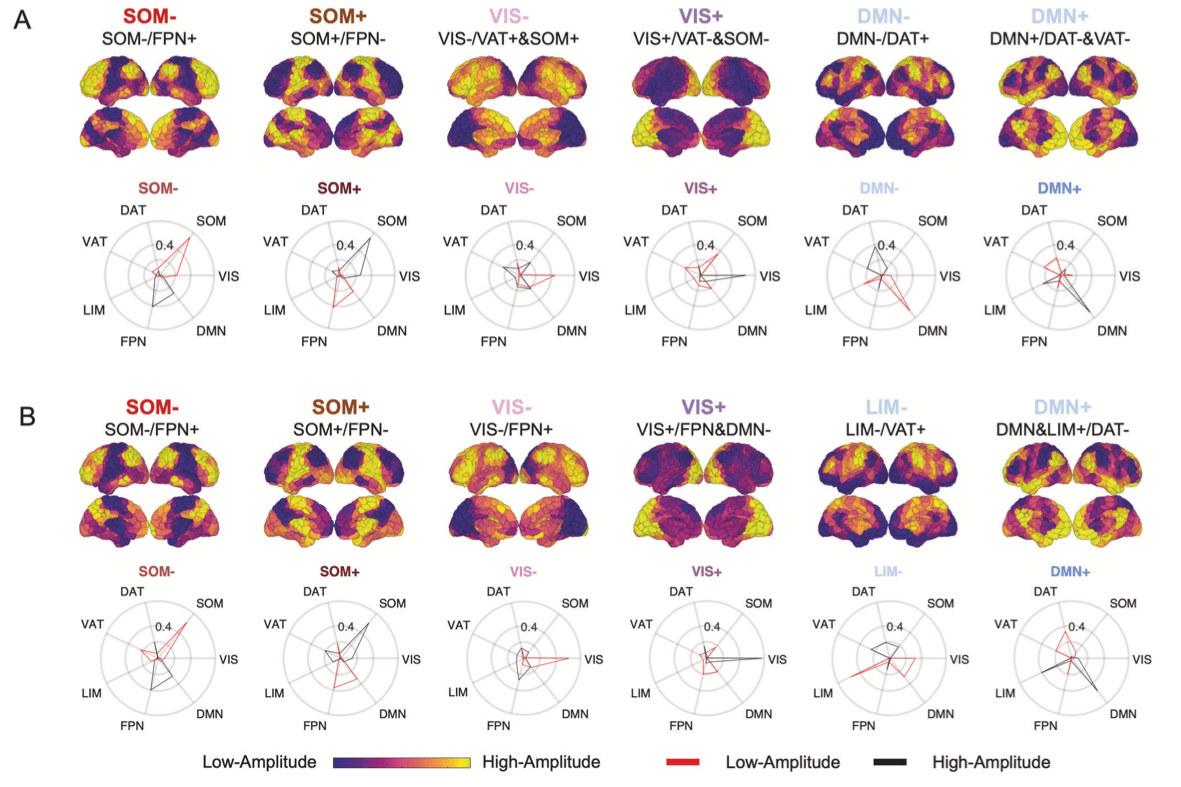
Temporal dynamics of the recurrent brain states
To capture dynamic characteristics of the identified brain states, we calculated fractional occupancy (probability of occurrence), dwell time (duration of persistence) and appearance rates (frequency of appearance per minute). OUD participants showed lower fractional occupancy in DMN+ (t63 = −2.69, puncorr = 0.009, Cohen’s d = 0.68, pBH = 0.05 for six comparisons), shorter dwell time in DMN+ (t63 = −2.19, puncorr = 0.032, Cohen’s d = 0.55, pBH = 0.09) and DMN- (t63 = −2.28, puncorr = 0.026, Cohen’s d = 0.57, pBH = 0.09), and higher appearance rate in VIS+ (t63 = 2.22, puncorr = 0.030, Cohen’s d = 0.53, pBH = 0.10) (Fig. 2). There were no significant differences between AUD and HC when combining AUD smokers and non-smokers. Since age and sex differed between AUD and HC (Table 1), we further controlled them as covariates and did not find group differences.
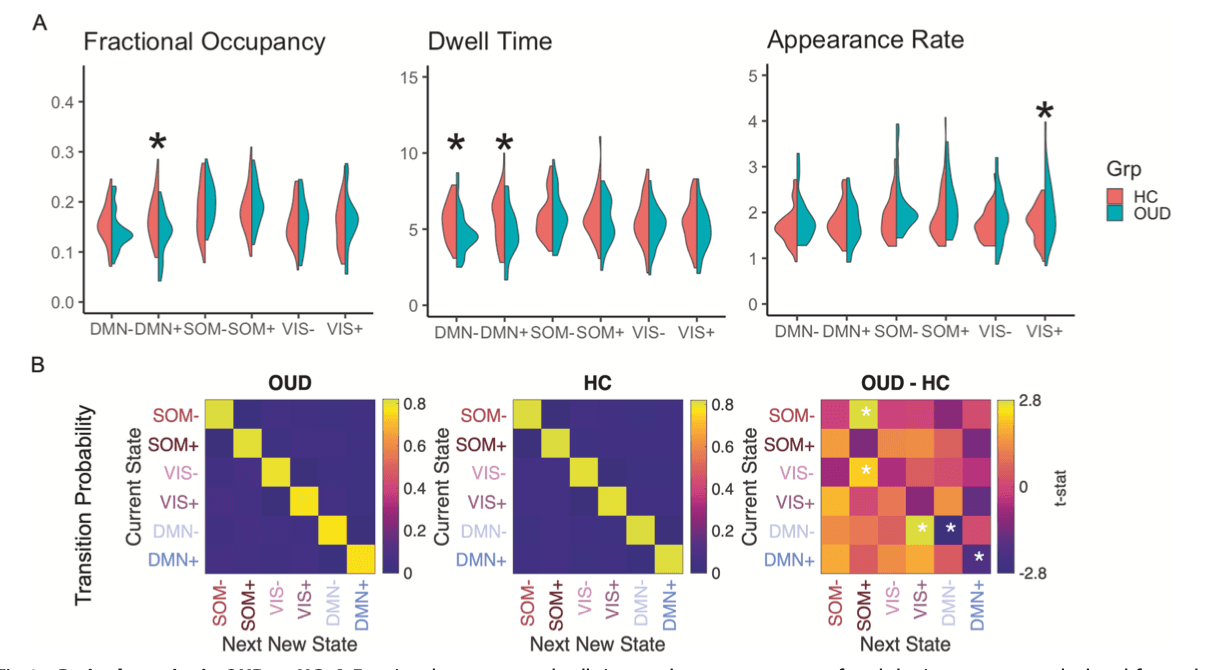
Transition probabilities between brain states were calculated for each participant. The probability of persistence in DMN+ and DMN- was 5% and 4% lower in OUD than HC, respectively (DMN + : t63 = −2.10, puncorr = 0.039, Cohen’s d = 0.51, pBH = 0.28 for 36 comparisons; DMN-: t63 = −2.80, puncorr = 0.007, Cohen’s d = 0.68, pBH = 0.08), consistent with shorter dwell time in DMN+ and DMN-. Compared to HC, higher probability of transitioning from states DMN- to VIS+ (2%, t63 = 2.80, puncorr = 0.007, Cohen’s d = 0.69, pBH = 0.08), from VIS- to SOM+ (2%, t63 = 2.24, puncorr = 0.029, Cohen’s d = 0.56, pBH = 0.26), and from SOM- to SOM+ (1%, t63 = 2.77, puncorr = 0.007, Cohen’s d = 0.65, pBH = 0.08) was observed in OUD (Fig. 2). Transition probabilities did not differ between AUD and HC when combining AUD smokers and non-smokers.
Static functional connectivity between networks
In Cohort 1, we found significant anticorrelations between SOM and FPN, and between DMN and DAT, which were consistent with their contra-activation in dynamic brain states i.e., SOM-/FPN+, SOM+/FPN-, DMN-/DAT+ and DMN+/DAT- (Figs. 1 and S6). Similarly, in Cohort 2, we found significant anticorrelations between SOM and FPN, and between DMN and DAT, as well as anticorrelations between VIS and all other networks that contributed to VIS+ and VIS- brain states. Strong functional connectivities between SOM and VAT and between LIM and DMN were also consistent with co-activation pattern in the SOM-, SOM+ and DMN+ brain states (Figs. 1 and S7). In sum, there were consistencies between resting functional connectivities and co-activation patterns of brain networks i.e., brain states.
OUD participants showed weaker anticorrelation between DMN and DAT (t63 = 2.33, puncorr = 0.023, Cohen’s d = 0.58) and greater functional connectivity between DMN and FPN than HC (t63 = 2.01, puncorr = 0.040, Cohen’s d = 0.51) (Fig. S6). Since findings from both brain states and resting-state functional connectivity indicated changes in DMN, we further explored the relationship between DMN functional connectivity and brain dynamics in OUD participants. Weaker anticorrelation between DMN and DAT but not DMN-FPN connectivity was correlated with shorter DMN- dwell time (r65 = −0.487, p = 0.010) and low probability of persisting in DMN- state (r65 = −0.416, p = 0.031). AUD participants had higher functional connectivity between SOM and VAT than HC (t63 = 2.69, puncorr = 0.008, Cohen’s d = 0.38) (Fig. S6). Controlling for age and sex did not change the results.
Effect of drug co-use
Among OUD participants, co-morbid nicotine dependence was associated with lowered fractional occupancy in VIS- (r27 = −0.487, puncorr = 0.010, pBH = 0.06 for six comparisons), dwell time in VIS+ (r27 = −0.396, puncorr = 0.041, pBH = 0.12), and VIS- (r27 = −0.453, puncorr = 0.018, pBH = 0.10), and reduced persistence in VIS- (r27 = −0.416, puncorr = 0.006, pBH = 0.22 for 36 comparisons) or VIS+ states (r27 = −0.427, puncorr = 0.004, pBH = 0.22). Also, nicotine dependence enhanced the transition probability from VIS- to DMN- (r27 = 0.567, puncorr = 0.002, pBH = 0.07), from VIS+/DMN- to DMN+ states (r27 = 0.418, puncorr = 0.030, pBH = 0.22/r27 = 0.533, puncorr = 0.004, pBH = 0.07) (Fig. 3A). Similarly in AUD participants greater nicotine dependence was associated with lower fractional occupancy (r107 = −0.268, puncorr = 0.005, pBH = 0.03) and appearance rate in VIS+ (r107 = −0.266, puncorr = 0.006, pBH = 0.03) and higher fractional occupancy (r107 = 0.232, puncorr = 0.016, pBH = 0.04) and longer dwell time in DMN+ state (r107 = 0.215, puncorr = 0.026, pBH = 0.16). In terms of transition probability, in AUD, higher FTND scores were associated with lower probabilities of transitioning from SOM-/VIS-/DMN+ to VIS+/VIS- state (all r107 < −0.190, puncorr < 0.050, all pBH > 0.05), and higher probabilities from VIS- to LIM-, from VIS- to SOM+, or of persisting in DMN+ state (all r107 > 0.200, all puncorr < 0.039, all pBH > 0.05 Fig. 3B). Consistently, we found different brain dynamics for AUD smokers and non-smokers. AUD smokers had longer dwell time in DMN+, higher fractional occupancy in SOM+, lower fractional occupancy and lower appearance rate in VIS+ than AUD non-smokers. Compared to HC, AUD non-smokers showed higher VIS+ fractional occupancy, while AUD smokers had higher SOM+ fractional occupancy (Fractional occupancy: VIS+: F(2,203) = 5.649, puncorr = 0.004, partial η2 = 0.053, pBH = 0.02; SOM+: F(2,203) = 4.519, puncorr = 0.012, partial η2 = 0.043, pBH = 0.03; Appearance rate: VIS+: F(2,203) = 3.277, puncorr = 0.040, partial η2 = 0.031, pBH = 0.24; Dwell time: DMN+: F(2,203) = 3.259, puncorr = 0.040, partial η2 = 0.031, pBH = 0.24; all post-hoc tests p < 0.05) (Fig. 4A). Regarding transition probabilities, compared to HC, there was higher transition to the VIS+ or VIS- states in AUD non-smokers (1–2%, all t147 > 2.08, puncorr < 0.039, Cohen’s d > 0.33, all pBH > 0.05), while lower transitions to VIS+ in AUD smokers (2%, t154 = 2.07, puncorr = 0.040, Cohen’s d = 0.36, pBH = 0.56). AUD smokers had higher transitions to SOM+ (2–3%), lower transition to VIS+ or VIS- brain states (1–3%) and higher persistence in DMN+ state (4%) than AUD non-smokers (all t105 > 2.15, puncorr < 0.034, Cohen’s d >0.42, all pBH > 0.05) (Fig. 4B). Differences in brain state dynamics were also reflected in static functional connectivity. Greater dwell time in DMN+ state and higher possibility of persistence in DMN+ state in AUD smokers was associated with stronger anticorrelation between DMN and VAT (Fig. S7) (all r206 < −0.349, all p < 0.001). In contrast, lower fractional occupancy and lower persistence in SOM+ in AUD non-smokers were associated with weaker anticorrelation between SOM-FPN (Fig. S7) (all r206 < −0.473, p < 0.001).
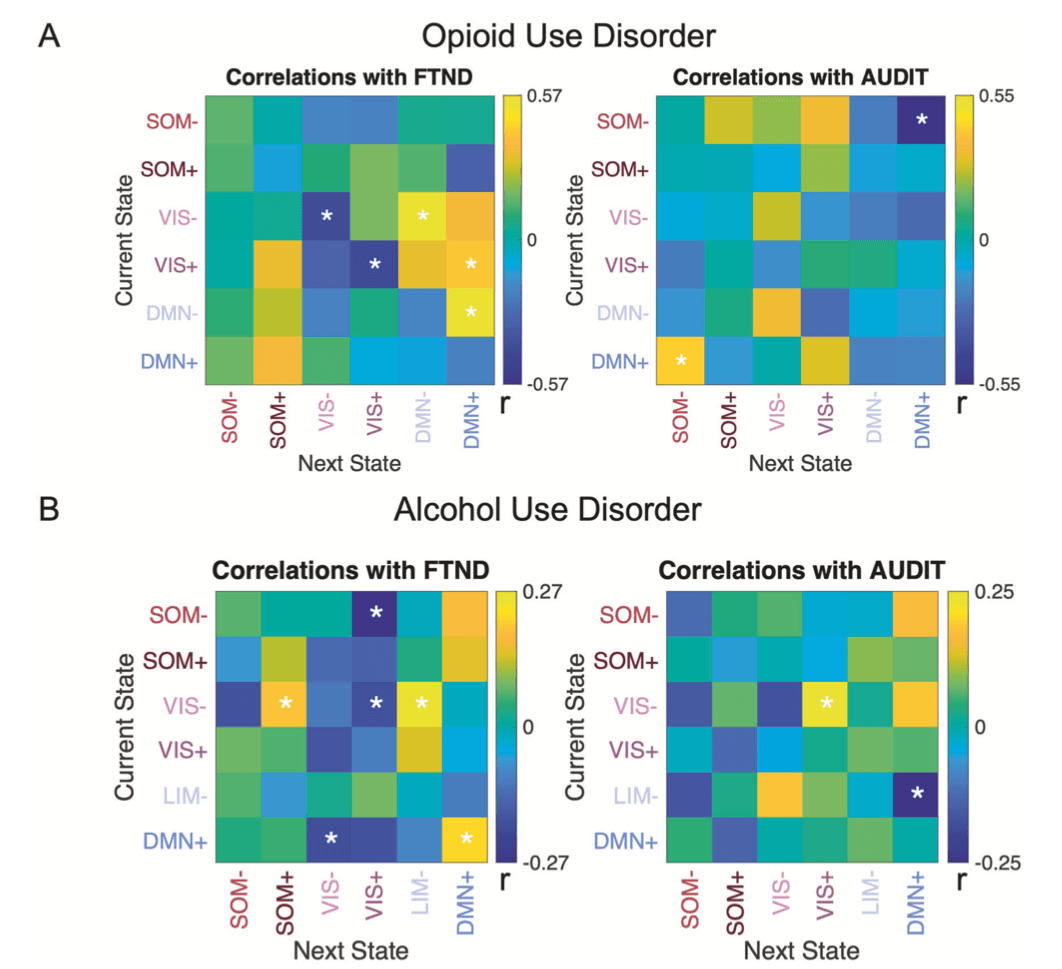
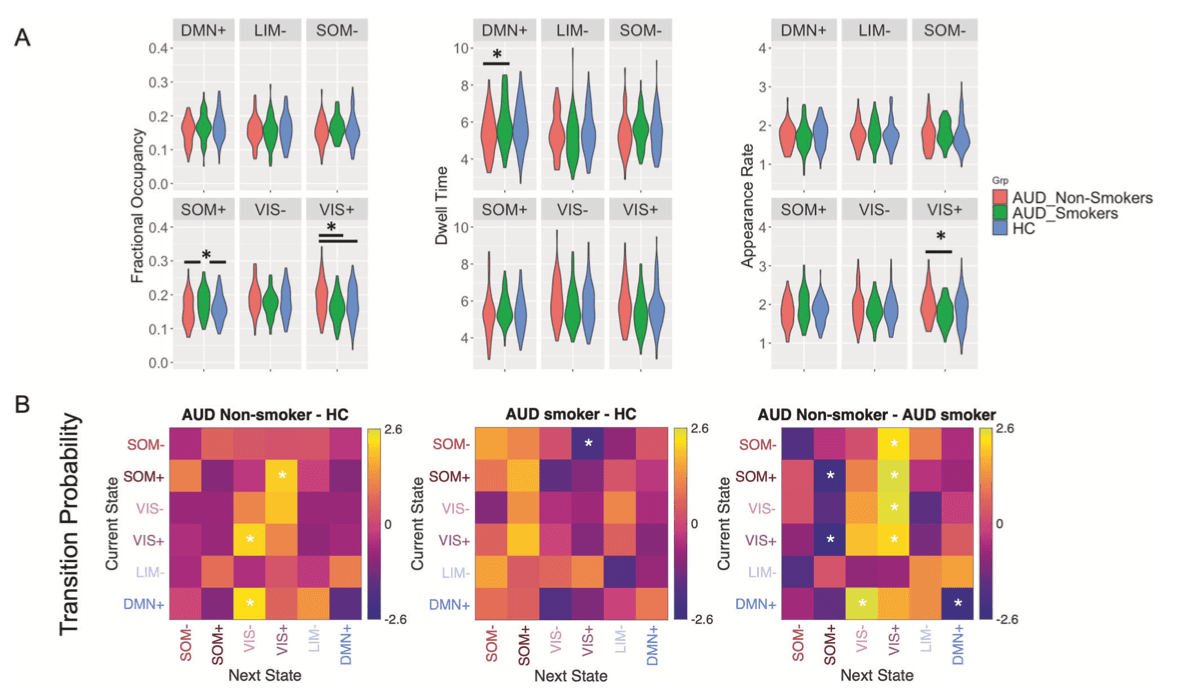
In OUD, co-morbid alcohol use was negatively associated with fractional occupancy (r27 = −0.501, puncorr = 0.008, pBH = 0.048 for six comparisons) and appearance rate in DMN+ (r27 = −0.500, puncorr = 0.008, pBH = 0.048). In line with this, severity of alcohol use problems decreased probability of transitioning from SOM- to DMN+ (r27 = −0.549, puncorr = 0.003, pBH = 0.11 for 36 comparisons), while it increased the transition probability from DMN+ to SOM- (r27 = 0.427, puncorr = 0.026, pBH = 0.47) in OUD participants (Fig. 3A). Higher AUDIT scores in AUD participants were associated with higher transition from VIS- to VIS+ (r99 = 0.235, p = 0.019, pBH = 0.34) and lower transition from LIM- to DMN+ (r99 = −0.246, puncorr = 0.014, pBH = 0.34) (Fig. 3B).
Effects of OUD medications and AUD detoxification
To assess if medications for OUD affected dynamic brain states and static functional connectivity in OUD participants, we compared those treated with Methadone, Buprenorphine, or without medications. Analyses showed no differences between OUD groups in brain state dynamics (one-way ANOVA, all F(2,24) < 3.405, all puncorr > 0.05) or static functional connectivity (one-way ANOVA, all F(2,24) < 1.188, all puncorr > 0.323).
In the AUD cohort who underwent inpatient detoxification, we investigated how days of detoxification affected brain state dynamics. Analyses showed that longer withdrawal days prior to the scan were associated with greater fractional occupancy in DMN+ (r61 = 0.27, puncorr = 0.038, pBH = 0.22), higher transition probabilities from VIS- to DMN+/DMN+ (all r61 > 0.28, all puncorr < 0.029, all pBH = 0.21), and lower transition probabilities from SOM+ to SOM- (r61 = −0.28, puncorr = 0.028, pBH = 0.21), from VIS- to VIS+ (r61 = −0.30, puncorr = 0.019, pBH = 0.21), from VIS+ to DMN- (r61 = −0.25, puncorr = 0.048, pBH = 0.27) and from DMN- to SOM+ (r61 = −0.35, puncorr = 0.006, pBH = 0.21).
Discussion
Aberrant brain functional connectivity has been reported in individuals suffering from various SUDs. Here, we revealed altered temporal dynamics in recurrent brain states in OUD and AUD participants. OUD and AUD non-smokers displayed similarities in brain dynamic changes including decreased fractional occupancy or dwell time in DMN-dominated brain states and increased fractional occupancy or appearance rate in VIS+ brain states, which were also reflected in transition probabilities of brain states. The observed alterations in brain state dynamics were greater in OUD participants who had more severe alcohol use and in the AUD participants with the more severe disorder. Interestingly, co-morbid nicotine dependence mitigated the disrupted brain states in individuals with OUD and AUD. Specifically, greater nicotine dependence was associated with higher DMN-dominated brain states and lower VIS-dominated brain states. Overall, the observed effect sizes of changes in brain state dynamics were greater in OUD than in AUD participants. Of note, OUD and AUD participants were from two different cohorts whose data were analyzed separately. Therefore, a direct comparison was not possible.
We identified six brain states comprised of combinations of active and inactive brain networks in both cohorts and validated the brain states in an independent sample scanned with different sample rate, scan lengths and EPI sequences (Figs. 1 and S4). The identified brain states were very robust and not affected by different scanning protocols. Compared to HC, OUD participants spent less time in brain states characterized by contraposition of DMN with DAT while they showed increased appearance rate in the brain state with high VIS activation. The findings of brain state dynamics and brain state transition probabilities were in agreement with each other. While DMN states were less persistent, the transition to states with high-amplitude activity in SOM and VIS was more likely in OUD than in HC. For AUD participants, we did not find significant differences from healthy controls when combining smokers and non-smokers, and differences only emerged when we compared HC with AUD who were non-smokers. Specifically, AUD non-smokers displayed very similar changes in brain dynamics and transitions as OUD participants i.e., decreased dwell time in DMN-dominated brain state, increased occurrence in VIS-dominated brain state accordant with higher probabilities of transitioning to a VIS-dominated state and lower probability of persisting in a DMN+ state. An additive effect on brain state differences in the participants with OUD and co-morbid alcohol misuse further supported the similar effects of chronic opioid and alcohol use on brain state dynamics. In both AUD and OUD participants, greater nicotine dependence was associated with lower occurrence and transition to a VIS-dominated state, whereas higher occurrence and transition to a DMN-dominated state. These results were also confirmed by an alternative analysis that compared AUD participants who were smokers versus non-smokers. As there were only six non-smokers among OUD participants, we were not able to compare OUD non-smokers with OUD smokers. We expect that changes in brain dynamics might be greater in OUD non-smokers than OUD smokers, but this will need to be confirmed in a larger sample size.
Our nicotine findings are consistent with previous reports of decreased dynamic functional connectivity density in the visual cortex and increased in the orbitofrontal cortex, anterior key node of the DMN, in chronic smokers compared to non-smokers. Similar findings on brain dynamics were reported in cocaine users who showed higher occurrence rate in the DMN state than HC. The counteracting effects of nicotine on the changes in brain state dynamics in AUD that we observed, are consistent with findings in two large datasets showing that participants with comorbid alcohol and nicotine misuse had smaller changes in static functional connectivity than participants who were only drinkers or smokers. Alcohol consumption increased ratings of desire to smoke and a similar effect was reported for opioid consumption and craving for cocaine. Combined use of drugs may serve to modulate the effects of one drug over the other e.g., stimulants to overcome mental state changes induced by sedatives and vice versa. The dynamics in DMN and VIS states might be important for shifting neural activity between internally and externally directed processes.
We interpret our findings as the result of neuroadaptations from chronic drug exposures. However, we cannot distinguish between temporal neuroadaptations that drive withdrawal (physical dependence) versus persistent neuroadaptations that sustain addiction. For AUD participants alcohol use was discontinued prior to the testing day. For the subgroup of AUD participants who were enrolled in an inpatient detoxification program, days in detoxification prior to the scan were associated with normalization of brain state dynamics as reflected by greater fractional occupancy and transition probability to the DMN+ state. For the OUD cohort, brain state dynamics did not significantly differ between participants based on medication status (Methadone vs Buprenorphine vs None). However, the lack of a significant effect of opioid medication could be attributed to the small sample size i.e., only six OUD participants were not under medication treatment (Table 1). Additionally, none of these six subjects used illicit opioids in the past 30 days indicating that these six OUD participants were in recovery even without OUD medication treatment. Thus, a larger cohort is needed to rule out the contribution of medication for OUD and the effects of long-term abstinence and recovery. As for nicotine, participants from both cohorts were asked to discontinue nicotine use 2 h before the scan. Based on the relatively low FTND scores and daily cigarette consumption of less than a pack per day (<20) in both AUD and OUD (Table 1), most participants were light smokers [11]. Thus, it is unlikely that they would have experienced severe nicotine withdrawal after 2 h of abstinence. However, since we did not record the time since last cigarette use nor did we assess withdrawal symptoms at the time of scanning, we could not control for these variables in the analyses. Therefore, our findings may reflect a mixture of short and long-term neuroadaptations associated with physical dependence and withdrawal and more persistent ones associated with addiction. Further, because we did not measure nicotine in plasma at the time of scanning, our findings could also be confounded by the presence of nicotine in plasma from the last cigarette, though the 2-hour nonsmoking period would have minimized this. Interestingly, a previous study that allowed chronic smokers (light and heavy smokers) to smoke before the scan to prevent withdrawal symptom, showed similar results of brain dynamics in chronic nicotine users indicating that effects cannot be attributed to nicotine withdrawal. In non-smokers, nicotine biases resting-state brain function away from the DMN+ and toward the salience network-dominated state, which is opposite to the effect observed in our study in participants with OUD or AUD who were also nicotine dependent. Therefore, the reported findings in the current study are more likely to relate to nicotine addiction than to withdrawal or to acute nicotine effects.
There are at least two kinds of neural underpinnings that might lead to altered brain state dynamics. First are structural changes. White matter damage has been associated with reduced DMN brain states occupancy in patients with cerebral small vessel disease. Damage of structural white matter connectivity has been reported in individuals with OUD and with AUD, which could impact brain states and transitions. Structural changes in gray matter such as cortical thinning in AUD and OUD could also affect brain state dynamics. OUD and AUD displayed common patterns of cortical thinning that were not present in patients with stimulant use disorder comorbid with AUD. Second, brain network dynamics are likely modulated by neurotransmitters including dopamine and serotonin. Since chronic opioid or alcohol use impacts dopaminergic and serotonergic signaling, altered neurotransmission in these systems might have contributed to the brain state dynamics imbalances seen in OUD and AUD participants. Moreover, alcohol stimulates the opioid system and opioid agonist medications (buprenorphine and methadone) as well as antagonists (naltrexone) reduce alcohol consumption in OUD suggesting that altered opioid signaling could also account for brain dynamics changes in OUD and AUD participants and might explain the greater effects observed in OUD than AUD. The counteracting effects of nicotine could be due to its interaction with opioid system. When used in combination, nicotine enhances opioid-induced antinociception. Furthermore, modulation of nicotinic acetylcholine receptors affects alcohol intake as well. Together, our finding suggests complex interactions among various neurotransmitter systems. Understanding the neural origins of altered brain state dynamics and transitions in SUD deserves further investigation.
The relationship between recurrent brain states and static functional connectivity is complex. Though static functional connectivity is associated with dynamic brain states, it cannot fully explain instantaneous coactivation of functional networks. In the current study, we found that anticorrelations between SOM and FPN and between DMN and DAT were reflected by brain states displaying contra-activations of these anti-correlated networks. Further, weaker/stronger DMN-DAT anticorrelation was associated with less/more time spent in DMN states. The neurocircuitry involved in addiction has been characterized by preclinical and clinical studies, however the dynamics underlying the stages of the addiction cycle (intoxication, withdrawal, and rumination/craving) and the transitions to remission and recovery are much less well understood. Our findings of a disruption in the dynamic mental states patterns in OUD and AUD participants, most of whom were studied during short-term detoxification (withdrawal) or while under OUD medication, suggests that addiction disrupts the stability of mental state functional networks towards externally dominated states over internal ones. Further work is needed to understand how brain state dynamics influence the risk for relapse including studies on how therapeutic interventions such as neuromodulation affect brain state dynamics and their relationship to symptom control.
The current study provides evidence for changes in brain state dynamics in OUD and AUD participants. We revealed similar effects of chronic opioid and alcohol use on brain dynamics and transitions at rest and a counteracting effect of nicotine dependence in these participants. Our findings highlight the importance of strategizing the treatment for drug co-use. For instance, one may consider nicotine replacement therapies as an adjunct intervention for treating AUD and OUD. Also, while discontinuing nicotine use in AUD and SUD, additional monitoring/intervention such as mindfulness training that prolongs the time spent in the DMN state, might be necessary to prevent worsening of symptoms. Future interventions that modulate brain network dynamics based on participants’ drug co-use might provide additional benefits to recovery.