Abstract
Adolescence is a time of considerable development at the level of behaviour, cognition and the brain. This article reviews histological and brain imaging studies that have demonstrated specific changes in neural architecture during puberty and adolescence, outlining trajectories of grey and white matter development. The implications of brain development for executive functions and social cognition during puberty and adolescence are discussed. Changes at the level of the brain and cognition may map onto behaviours commonly associated with adolescence. Finally, possible applications for education and social policy are briefly considered.
Introduction
Adolescence is a time characterised by immense hormonal and physical changes (Coleman & Hendry, 1990; Feldman & Elliott, 1990). This transition from childhood to adulthood is also characterised by dramatic changes in identity, self-consciousness and cognitive flexibility (Rutter & Rutter, 1993). There seems to be a qualitative shift in the nature of thinking such that adolescents are more self-aware and self-reflective than prepubescent children. Adolescents develop a capacity to hold in mind more multidimensional concepts and are thus able to think in a more strategic manner. Empirical research on cognitive and neural development during puberty and adolescence is in its initial stages. In the past few years, several pioneering experiments have investigated the development of brain and cognitive processes during this period of life.
This review begins by describing the cellular studies that first demonstrated anatomical brain developments during adolescence. It then describes how recent brain imaging techniques have supported these findings and have shed some light on the trajectories of structural brain maturation during adolescence. The following sections discuss investigations of cognitive development, in particular studies of executive functions and social cognition using behavioural and functional imaging techniques. Finally, applications for education and social policy are briefly suggested.
The first experiments on adolescent brains
Until recently, very little was known about brain development during adolescence. The notion that the brain continues to develop after childhood is relatively new. Experiments on animals, starting in the 1950s, showed that sensory regions of the brain go through sensitive periods soon after birth, during which time environmental stimulation appears to be crucial for normal brain development and for normal perceptual development to occur (Hubel & Wiesel, 1962). These experiments suggested the human brain might be susceptible to the same sensitive periods in early development. Indeed, later experiments demonstrated sensitive periods in the first year of life for sensory capacities such as sound categorisation (Kuhl, Williams, Lacerda, & Stevens, 1992). Based on these experiments, the idea that the human brain may continue to undergo substantial change after early sensitive periods seemed unlikely.
It was not until the late 1960s and 1970s that research on post-mortem human brains revealed that some brain areas, in particular the prefrontal cortex, continue to develop well beyond early childhood. Studies carried out in the 1970s and 1980s demonstrated that the structure of the prefrontal cortex undergoes significant changes during puberty and adolescence (Huttenlocher, 1979; Huttenlocher, De Courten, Garey, & Van Der Loos, 1983; Yakovlev & Lecours, 1967).
Two main changes were revealed in the brain before and after puberty. As neurons develop, a layer of myelin is formed around their extension, or axon, from supporting glial cells. Myelin acts as an insulator and massively increases the speed of transmission (up to 100 fold) of electrical impulses from neuron to neuron. Whereas sensory and motor brain regions become fully myelinated in the first few years of life, although the volume of brain tissue remains stable, axons in the frontal cortex continue to be myelinated well into adolescence (Yakovlev & Lecours, 1967). The implication of this research is that the transmission speed of neural information in the frontal cortex should increase throughout childhood and adolescence.
The second difference in the brains of pre-pubescent children and adolescents pertains to changes in synaptic density in the prefrontal cortex. An adult brain has about 100 billion neurons; at birth the brain has only slightly fewer neurons (Pakkenberg & Gundersen, 1997). However, during development many changes take place in the brain. Neurons grow, which accounts for some of the change, but the wiring, the intricate network of connections – or synapses – between neurons, sees the most significant change. Early in postnatal development, the brain begins to form new synapses, so that the synaptic density (the number of synapses per unit volume of brain tissue) greatly exceeds adult levels. This process of synaptic proliferation, called synaptogenesis, lasts up to several months, depending on the species of animal and brain region. At this point, synaptic densities in most brain regions are at their maximum. These early peaks in synaptic density are followed by a period of synaptic elimination (or pruning) in which frequently used connections are strengthened and infrequently used connections are eliminated. This experience-dependent process, which occurs over a period of years, reduces the overall synaptic density to adult levels.
These data came mainly from studies of sensory regions of animal brains. The first demonstration of synaptogenesis was in 1975, when it was found that in the cat visual system the number of synapses per neuron first increases rapidly and then gradually decreases to mature levels (Cragg, 1975). Further research carried out in rhesus monkeys (Rakic, 1995) demonstrated that synaptic densities reach maximal levels two to four months after birth, after which time pruning begins. Synaptic densities gradually decline to adult levels at around 3 years, around the time monkeys reach sexual maturity.
However, synaptogenesis and synaptic pruning in the prefrontal cortex have a rather different time course. Histological studies of monkey and human prefrontal cortex have shown that there is a proliferation of synapses in the subgranular layers of the prefrontal cortex during childhood and again at puberty, followed by a plateau phase and a subsequent elimination and reorganisation of prefrontal synaptic connections after puberty (Huttenlocher, 1979; Bourgeois, Goldman-Rakic, & Rakic, 1994; Woo, Pucak, Kye, Matus, & Lewis, 1997; Zecevic & Rakic, 2001). According to these data, synaptic pruning occurs throughout adolescence and results in a net decrease in synaptic density in the frontal lobes during this time. The focus of this review will be on cognitive implications of this second wave of synaptogenesis in the frontal cortex at the onset of puberty and the process of synaptic pruning that follows it after puberty.
Synaptic pruning is believed to be essential for the fine-tuning of functional networks of brain tissue, rendering the remaining synaptic circuits more efficient. Synaptic pruning is thought to underlie sound categorisation, for example. Learning one's own language initially requires categorising the sounds that make up language. New-born babies are able to distinguish between all speech sounds. Sound organisation is determined by the sounds in a baby's environment in the first 12 months of life – by the end of their first year babies lose the ability to distinguish between sounds to which they are not exposed (see Kuhl, 2004 for review). For example, the ability to distinguish certain speech sounds depends on being exposed to those distinct sounds in early development. Before about 12 months of age babies brought up in the USA can detect the difference between certain sounds common in the Hindi language, which after 12 months they cannot distinguish (Werker, Gilbert, Humphrey, & Tees, 1981). In contrast babies brought up hearing the Hindi language at the same age become even better at hearing this distinction because they are exposed to these sounds in their language. This fine-tuning of sound categorisation is thought to rely on the pruning of synapses in sensory areas involved in processing sound. The studies on post-mortem brain development suggest that development of cognitive processes associated with the frontal lobes may well continue throughout adolescence.
Viewing the adolescent brain with MRI
Until recently, the structure of the human brain could be studied only after death. The scarcity of post-mortem child and adolescent brains meant that knowledge of the adolescent brain was extremely scanty. Nowadays, non-invasive brain imaging techniques, particularly Magnetic Resonance Imaging (MRI), can produce detailed three-dimensional images of the living human brain (see Figure 1). Since the advent of MRI, a number of brain imaging studies have provided further evidence of the ongoing maturation of the frontal cortex into adolescence and even into adulthood.
Figure 1: Magnetic Resonance Imaging (MRI)
Linear increases in white matter during adolescence
In the past few years, several MRI studies have been performed to investigate the development of the structure of the brain during childhood and adolescence in humans (cf. Paus, 2005; Casey, Tottenham, Liston, & Durston, 2005). One of the most consistent findings from these MRI studies is that there is a steady increase in white matter in certain brain regions during childhood and adolescence. In one MRI study, a group of children whose average age was 9 years, and a group of adolescents whose average age was 14, were scanned (Sowell et al., 1999). This study revealed differences in the density of white and grey matter between the brains at the two age groups. The results showed a higher volume of white matter in the frontal cortex and parietal cortex in the older children than in the younger group. The younger group, by contrast, had a higher volume of grey matter in the same regions. Myelin appears white in MRI scans, and therefore the increase in white matter and decrease in grey matter with age was interpreted as reflecting increased axonal myelination in the frontal and parietal cortices during this time period.
The increased white matter and decreased grey matter density in the frontal and parietal cortices throughout adolescence has now been demonstrated by several studies carried out by a number of different research groups with increasingly large groups of subjects (Barnea-Goraly et al., 2005;Giedd et al., 1996, 1999a; Pfefferbaum et al., 1994; Reiss, Abrams, Singer, Ross, & Denckla, 1996; Sowell, Thompson, Tessner, Toga, 2001; Sowell et al., 2003). Different studies point to developmental changes in white matter density in different brain regions. Paus et al. (1999a) analysed the brain images of 111 children and adolescents aged between 4 and 17 years, and noted an increase in white matter specifically in the right internal capsule and left arcuate fasciculus. The left arcuate fasciculus contains white matter tracts that connect anterior speech regions (Broca's area) and posterior language regions (Wernicke's area). Thus the increase in white matter in this region was interpreted as reflecting increased connections between the speech regions. The corpus callosum, the dense mass of fibres that connects the two hemispheres of the brain, has also been found to undergo region-specific growth during adolescence and up until the mid-twenties (Barnea-Goraly et al., 2005; Giedd et al., 1999b; Pujol, Vendrell, Junque, Marti, & Josep, 1993).
While structural neuroimaging studies diverge in terms of the precise brain regions in which white matter density increases have been found, they generally agree on the pattern of white matter change. Most studies point to a steady, more-or-less linear increase in white matter with age (see Figure 2) (Barnea-Goraly et al., 2005; Giedd et al., 1999a; Paus et al., 1999a; Paus, Evans, & Rapoport, 1999b; Reiss et al., 1996; Pfefferbaum et al., 1994) and in light of histological studies, this has consistently been interpreted as reflecting continued axonal myelination during childhood and adolescence.
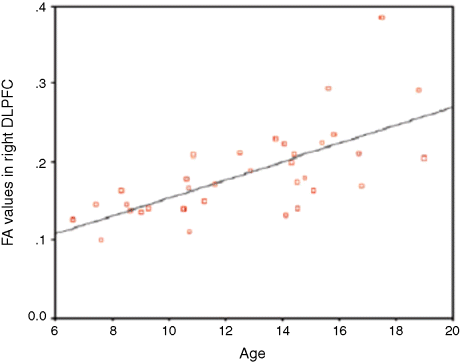
Figure 2: Linear development of white matter with increasing age in the right DLPFC. The graph shows the correlation between age and amount of white matter (as indicated by fractional anistotropy value (FA) determined by structural features of tissue such as fibre density and diameter) (Barnea-Goraly et al., 2005).
Non-linear decreases in grey matter during adolescence
While the increase in white matter in certain brain regions seems to be linear across all brain areas, the changes in grey matter density appear to follow a region-specific, non-linear pattern. In other words, while white matter development follows a steady, progressive course, grey matter development is at certain stages progressive and at other times regressive. As the following studies have shown, its pattern of development in certain brain regions follows an inverted-U shape.
Giedd et al. (1999a) performed a longitudinal MRI study on 145 healthy boys and girls ranging in age from about 4 to 22 years. At least one scan was obtained from each of 145 subjects (89 were male). Scans were acquired at two-year intervals for 65 of these subjects who had at least two scans, 30 who had at least three scans, two who had at least four scans and one who had five scans. Individual growth patterns revealed heterochronous grey matter development during adolescence. Changes in the frontal and parietal regions were similarly pronounced. The volume of grey matter in the frontal lobe increased during pre-adolescence with a peak occurring at around 12 years for males and 11 years for females. This was followed by a decline during post-adolescence. Similarly, parietal-lobe grey matter volume increased during the pre-adolescent stage to a peak at around 12 years for males and 10 years for females, and this was followed by a decline during post-adolescence. Grey matter development in the temporal lobes was also non-linear, but the peak was reached later at about 17 years. In the occipital lobes, grey matter development had a linear course (Giedd et al., 1999a).
A further MRI study of 35 normally developing children (7–11 years), adolescents (12–16 years) and young adults (23–30 years) demonstrated a sharp acceleration in the loss of grey matter between childhood and adolescence in the dorsal prefrontal cortex and the parietal cortex (Sowell et al., 2001). In the frontal lobes, the decrease in grey matter density was even more pronounced between adolescence and adulthood. In addition, an inverse relationship between dorsal prefrontal cortex growth and grey matter density was found to exist between childhood and adolescence. The regions exhibiting the most robust decrease in grey matter density also exhibited the most robust post-pubescent increase in white matter density in the dorsal prefrontal cortex.
In a longitudinal study of participants aged between 4 and 21, Gogtay et al. (2004) scanned 13 children every two years for 8 to 10 years. In terms of grey matter density, they found that sensory and motor brain regions matured first. This was followed by the remainder of the cortex maturing from the back to the front (parietal cortex to frontal cortex). The loss of grey matter occurred last in the superior temporal cortex. The authors noted that phylogenetically older brain areas matured earlier than newer ones.
A similar pattern of development was found in a longitudinal study of children aged from 3 to 15 years (Thompson et al., 2000). In this experiment, high spatial resolution maps of the brain's growth patterns were obtained using tensor mapping, with the same subject being scanned across time spans of up to four years. In the older group (11 to 15 years) a localised grey matter decrease in the frontal cortex was observed. This study provided further evidence of a sharp acceleration of grey matter density loss between childhood and adolescence in dorsal frontal cortex.
Thus, rather than a simple linear change in grey matter with age, studies suggest perturbation in grey matter density development that more or less coincides with the onset of puberty. At puberty, grey matter volume in the frontal lobe reaches a peak, followed by a plateau after puberty and then a decline throughout adolescence continuing until early adulthood. The MRI results demonstrating a non-linear decrease in grey matter in various brain regions throughout adolescence have been interpreted in two ways. First, it is likely that axonal myelination results in an increase in white matter and a simultaneous decrease in grey matter as viewed by MRI. A second, additional explanation is that the grey matter changes reflect the synaptic reorganisation that occurs at the onset of and after puberty (Huttenlocher, 1979; Bourgeois et al., 1994). Thus, the increase in grey matter apparent at the onset of puberty (Giedd et al., 1999a) might reflect a wave of synapse proliferation at this time.
The gradual decrease in grey matter density that occurs after puberty in certain brain regions has been attributed to post-pubescent synaptic pruning (Giedd et al., 1999a; Sowell et al., 2001; Gogtay et al., 2004). In other words, the increase in grey matter at puberty reflects a sudden increase in the number of synapses. At some point after puberty, there is a process of refinement such that these excess synapses are eliminated (Huttenlocher, 1979), resulting in a steady decline in grey matter density.
Gender differences in development of brain structure
A cross-sectional MRI study of 46 children and adults (mean age 11) revealed significant differences in grey and white matter between girls and boys, particularly in the inferior frontal gyrus (IFG) (Blanton et al., 2004). A significant age-related increase in white matter volume in the left IFG was found in boys but no significant volumetric changes were found in girls in any frontal regions (see Figure 3). Furthermore, even after correcting for total cerebral volume, boys had significantly greater grey matter volume in the IFG relative to girls. The authors speculated that these structural differences may arise from the difference in steroid levels between girls and boys during pubertal maturation. It was suggested that the effect of inhibition of synaptic pruning from testosterone may account for greater volume in boys than in girls. On the other hand, it was proposed that greater hemispheric specialisation (cf. Shaywitz et al., 1995) among boys may account for the gender differences in structure. So far, structural studies are not in full agreement with regard to gender differences. For example, in a longitudinal study, it was found that boys had 10% greater total cortical grey matter volume than girls, but that developmental trajectories of grey and white matter volume were not significantly different (Giedd et al., 1999a). Larger longitudinal studies will help to overcome variability in frontal cortex anatomy, to discern gender differences more clearly.
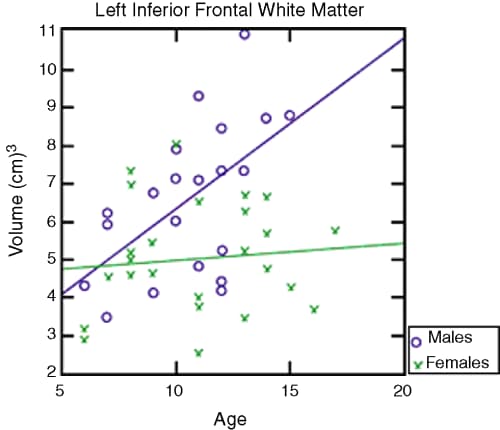
Figure 3: Relationship between age and volume of white matter in the IFG for males and females. A significant linear relationship was found between age and IFG white matter for males (Blanton et al., 2004).
Brain changes continue after adolescence
Recent MRI studies indicate that the time at which the brain reaches maturity may be much later than the end of adolescence. One such study of participants aged between 7 and 30 revealed that the loss of grey matter in the frontal cortex accelerated during adulthood between the early 20s and up to the age of 30 (Sowell et al., 2001).
A further MRI study by the same group involved scanning 176 individuals between 7 and 87 years (Sowell et al., 2003). The results revealed a reduction in grey matter density in the dorsal prefrontal, parietal and temporal cortices, which was accompanied by an increase in white matter. The pattern of grey matter changes was non-linear during the period of adolescence. Although the decrease in grey matter was most dramatic from childhood to young adulthood, the data revealed that white matter volume continued to increase well beyond this stage and even up to the age of 60. The non-linear decrease in grey matter was concomitant with a linear increase in white matter, consistent with earlier MRI data and with post-mortem studies.
To summarise, while early cellular studies in animals suggested that morphological changes in the brain more or less cease early on in infancy, in vivo MRI studies coupled with post-mortem cellular studies of human brains have revealed an extended period of development, in particular in the frontal and parietal cortices. Taken together, the studies provide consistent evidence for the dynamic nature of the adolescent brain with respect to maturational changes of grey and white matter. The main changes that have been observed are a non-linear reduction in frontal grey matter density and a simultaneous linear increase in white matter.
Changes in behaviour and cognition after puberty
Two of the brain regions that have consistently been shown to undergo continued development during adolescence are the prefrontal cortex and the parietal cortex. Given the continued structural changes in these brain regions during adolescence, it might be expected that cognitive abilities that rely on the functioning of these regions and their complex interconnectivity with other regions should also change during this time period. Most studies to date have investigated cognitive abilities subserved by the frontal lobes, in particular executive function.
Development of executive function
The term executive function is used to describe the capacity that allows us to control and coordinate our thoughts and behaviour (Luria, 1966; Shallice, 1982). These skills include selective attention, decision-making, voluntary response inhibition and working memory. Each of these executive functions has a role in cognitive control, for example filtering out unimportant information, holding in mind a plan to carry out in the future and inhibiting impulses. Much work on human and monkey brain and behaviour has associated these strategic behaviour skills with the frontal lobes. Lesion studies (Goldman-Rakic, 1987; Rakic, Bourgeois, & Goldman-Rakic, 1994; Shallice, 1982) and functional imaging experiments (e.g. Casey et al., 1997; Rubia et al., 2001; Rubia, Smith, Brammer, & Taylor, 2003) suggest that such skills rely heavily on the frontal lobes. Since MRI studies have demonstrated changes in frontal cortex during adolescence, executive function abilities might be expected to improve during this time. For example, selective attention, decision-making and response inhibition skills, along with the ability to carry out multiple tasks at once, might improve during adolescence. While many studies have investigated the development of executive function skills in early and late childhood (e.g. Paus, 1989; Paus, Babenko, & Radil, 1990; Casey et al., 1997; Brocki & Bohlin, 2004; Klenberg, Korkman, & Lahti-Nuuttila, 2001), only a handful of studies have investigated the changes in executive function skills during adolescence.
Behavioural studies show that performance of adolescents on tasks including inhibitory control (Leon-Carrion, Garcia-Orza, & Perez-Santamaria, 2004; Luna, Garver, Urban, Lazar, & Sweeney, 2004a), processing speed (Luna et al., 2004a), working memory and decision-making (Luciana, Conklin, Cooper, & Yarger, 2005; Hooper, Luciana, Conklin, & Yarger, 2004) continues to develop during adolescence. Luna et al., for example, showed that performance on an oculomotor task undergoes a large improvement from childhood to adolescence, followed by a plateau between adolescence and early adulthood (Luna et al., 2004a). Another study investigating performance on a variety of executive function tasks between the ages of 11 and 17 demonstrated a linear improvement in performance on some tasks but not others (Anderson, Anderson, Northam, Jacobs, & Catroppa, 2001). Improvement during adolescence was observed on tasks of selective attention, working memory and problem solving, whereas strategic behaviour, as tested by the Tower of London task (Shallice, 1982), seemed to have been formed earlier in childhood. Different aspects of executive function, therefore, may have different developmental trajectories. These studies used a systems neuroscience approach to cognition to speculate that the developments in performance are linked to the pruning and myelination processes occurring during adolescence in the frontal cortex.
Prospective memory is the ability to hold in mind an intention to carry out an action at a future time (Ellis, 1996), for example remembering to make a phone call at specific future time. Prospective memory is associated with frontal lobe activity (Burgess, Veitch, Costello, & Shallice, 2000) and has been shown to develop through childhood as we develop our future-oriented thought and action (Ellis & Kvavilashvili, 2000). Multitasking is believed to be a test of prospective memory as it requires participants to remember to perform a number of different tasks, mirroring everyday life. In a recent study of the development of prospective memory from childhood to adulthood, a multitask paradigm was used to test children aged between 6 and 14 and adults (Mackinlay, Charman, & Karmiloff-Smith, 2003). Participants were scored for both efficiency and the strategies used to carry out the task effectively. A significant improvement in both the efficiency and quality of strategies was found between the ages of 6 and 10. However, between the ages of 10 and 14, there was no significant change in performance. The adult group (mean age 25), on the other hand, significantly outperformed the children. The authors therefore suggested that prospective memory continues to develop during adolescence, in line with the notion of frontal maturation in the brain. It is possible that the lack of improvement in performance between the 10- and 14-year-olds was related to their pubertal status.
A non-linear pattern of development was found in a recent behavioural study that used a match-to-sample task (McGivern, Andersen, Byrd, Mutter, & Reilly, 2002). In this task, volunteers were shown pictures of faces showing particular emotional expressions (happy, sad, angry), or words describing those emotions (‘Happy,’‘Sad,’ Angry’), and were asked to specify, as quickly as possible, the emotion presented in the face or word. In a third condition, volunteers were shown both a face and a word, and had to decide whether the facial expression matched the emotional word. The rationale behind the design of the task was that the face/word condition places high demands on frontal lobe circuitry, since it requires working memory and decision-making. The task was given to a large group of children aged 10 to 17 years and a group of young adults aged 18 to 22 years.
The results revealed that at the age of puberty onset, at 11–12 years, there was a decline in performance in the matching face and word condition compared with the younger group of children. A 10–20% increase in reaction time on the match-to-sample task occurred at the onset of puberty in the 10–11-year-old group of girls and in the 11–12-year-old group of boys, compared to the previous year group of each sex (age 9–10 and 10–11 in girls and boys, respectively) (see Figure 4). The results suggest that there is a dip in performance on this kind of task at the onset of puberty. After puberty, from age 13–14, performance improved until it returned to the pre-pubescent level by the age of about 16–17 years.
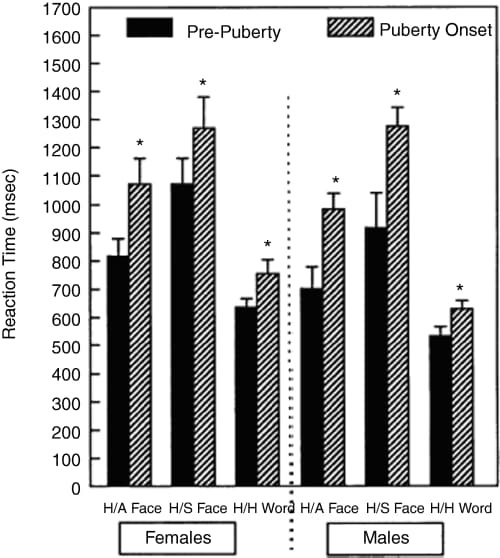
Figure 4: Significant difference in reaction times for emotional match-to-sample task between the year preceding and the average age of puberty onset (McGivern et al., 2002).
The researchers linked this pubertal dip in performance to the proliferation of synapses that occurs at the onset of puberty. Based on these psychophysical results and in the context of structural MRI studies discussed above, it was suggested that until pruning occurs after puberty, synaptic connections in the frontal cortex generate a low signal to noise ratio due to an excess of synapses, which renders the cognitive performance less efficient. Therefore, the sudden proliferation of synapses that occurs at puberty results in a perturbation of cognitive performance. Only later, after puberty, are the excess synapses pruned into specialised, efficient networks which may explain the post-pubescent improvement on this table (McGivern et al., 2002).
Development of social cognition
In addition to executive functions, there is evidence that the prefrontal cortex is involved in several other high-level cognitive capacities, including self-awareness (Ochsner, 2004) and theory of mind (Frith & Frith, 2003), that is the ability to understand other minds by attributing mental states such as beliefs, desires and intentions to other people (Frith, 2001). In addition to neural development, there are major changes in hormones at puberty. While it is impossible to tease apart all of the important influences on the social and emotional behaviour of adolescents, significant neural development and hormonal changes are likely to influence social cognition. Social cognition, then, may also be expected to change during this time period. In addition, the interaction may be two-way. During this time, what is perceived as important in the social world around us also changes and leaves its imprint on the pruning process. Accumulating new social experiences, for example, when entering a new school, may influence the development of social cognitive processes. So far, very few studies have addressed the effect of puberty and adolescence on social cognitive abilities.
Perspective taking
Perspective taking is the ability to take on the viewpoint of another person. The ability to take another's perspective is crucial for successful social communication. In order to reason about others, and understand what they think, feel or believe, it is necessary to step into their ‘mental shoes’ and take their perspective. Perspective taking is related to first-order theory of mind in that it involves surmising what another person is thinking or feeling. Perspective taking includes awareness of one's own subjective mental states (‘first-person perspective’, or 1PP) and the ability to ascribe mental states to another person (‘third-person perspective’ or 3PP). Despite much theoretical debate, there is little consensus about the mechanisms underlying perspective taking. One prevalent view is that we understand others by mentally simulating their actions and thoughts (Harris, 1995; Gallese & Goldman, 1998). In support of this ‘simulation theory’, a growing body of evidence from neurophysiological studies has demonstrated that common brain areas are activated both when we execute an action and when we observe another person perform the same action (Rizzolatti, Fadiga, Gallese, & Fogassi, 1996a; Rizzolatti et al., 1996b; Grafton, Arbib, Fadiga, & Rizzolatti, 1996; Decety et al., 1997; Buccino et al., 2001). Common brain areas are also activated when subjects perceive a visual scene or answer a conceptual question from their own, first-person, perspective and from another person's perspective. Functional neuroimaging studies have revealed that the parietal and frontal cortices are associated with making the distinction between 1PP and 3PP at the motor (Ruby & Decety, 2001), visuo-spatial, (Vogeley et al., 2004), conceptual (Ruby & Decety, 2003) and emotional (Ruby & Decety, 2004) level. These studies suggest that the organisation of motor, social and affective knowledge is not distinct, but that they are intertwined with one another. It is proposed that ‘mirror neurons’ that fire when an agent both performs an action or observes another person performing the action provide a basis for integrating perceptual, motor and social functions (see Rizzolatti, Fogassi, & Gallese, 2001 for review). In each of these contexts, superior frontal and right inferior parietal cortex are activated to a greater extent during 3PP than during 1PP. Several neuroimaging studies have implicated the inferior parietal cortex in the distinction between the self and others at the sensorimotor level (Blakemore, Wolpert, & Frith, 1998; Farrer & Frith, 2002; Ruby & Decety, 2001).
So far there has been a lack of attention to the development of social cognition during adolescence. In a recent study, the development of perspective taking was investigated before, during and after puberty (Choudhury, Blakemore, & Charman, 2005). These data suggest that development of social perspective taking undergoes a perturbation during puberty in parallel with the discontinuous processes of brain maturation. As described earlier, histological studies of human prefrontal cortex have shown that there is a proliferation of synapses in the prefrontal cortex during childhood, followed by a plateau phase and a subsequent elimination and reorganisation of prefrontal synaptic connections after puberty (Huttenlocher, 1979). Cognitive processes that depend on the prefrontal cortex might undergo a perturbation at puberty due to the synaptic reorganisation that occurs at this time.
To our knowledge, this is the first study that has investigated the development of perspective taking during adolescence. Further experiments on the development of other social cognitive processes are being carried out in our laboratory and other research centres and may shed more light on the effect of puberty and adolescence on social cognition.
With regard to face processing, differential developmental trajectories for recognition of the six Ekman emotion faces were reported from a behavioural study of 484 children and adolescents aged between 6 and 16 years (Lawrence et al., submitted). The computerised task required participants to match an emotional label (happy, sad, angry, fearful, disgusted and surprised) to images of facial expressions, and responses were recorded. Accuracy data indicated that emotion recognition abilities develop during adolescence. In particular, recognition of fear and disgust showed the greatest linear improvements with age, while there was no improvement in the ability to recognise sad and angry expressions in this age range. In addition, pubertal status, independent of age, affected emotion recognition. Recognition of fear, disgust and anger improved with pubertal development, as determined by the Pubertal Development Scale (Peterson et al., 1988). Given that the processing of facial expressions is associated with prefrontal activity (Sprengelmeyer, Rausch, Eysel, & Przuntek, 1998), it was proposed that the structural developments in the adolescent prefrontal cortex and the concomitant change in the hormonal environment differentially affect neural circuits involved in particular aspects of emotion recognition.
These recent findings are in line with previous studies that also showed an interruption at puberty in the developmental course of face recognition (Carey, Diamond, & Woods, 1980; Diamond, Carey, & Back, 1983; Flin, 1983). In one study, for example, the percentage of correct responses in a behavioural face recognition task improved by over 20% between the ages of 6 and 10 (Carey et al., 1980). However, this was followed by a decline around the age of puberty. Between ages 10 and 12, participants showed a drop in accuracy of over 10%. Performance on the task recovered again from the age of approximately 14 up to 16. Similarly, in another study, face encoding was found to be worse in pubescent girls compared with pre- and post-pubescent girls matched for age (Diamond et al., 1983). The authors suggested that the decline in performance was a result of hormonal changes at puberty that may impact directly on cognitive performance or alternatively that adolescents’ new form of self-awareness and awareness of other people in response to their bodily changes at puberty may lead to a reorganisation of face representation.
Further investigation of the influence of puberty on brain development and cognition would, however, benefit from objective measures of pubertal development. Currently, if they measure puberty at all, researchers tend to rely on self-ratings, parent reports or the judgements of teachers in schools. Investigators consistently report the difficulties met in ascertaining the level of pubertal development in adolescence, even using these methods. Not only do parents and teachers often consider them to be inappropriate to administer in schools, where much cognitive testing for such studies takes place, but they are also crude indices of pubertal status. It may be worth working with endocrinologists to investigate alternative reliable methods, such as using saliva swabs to test hormone levels.
Viewing the adolescent brain in action with fMRI
Functional MRI (fMRI) provides us with a safe, non-invasive tool to study interactions between brain and behaviour (see Figure 5). fMRI has been used only in a handful of studies investigating the neural bases of cognitive development using tasks designed to tap specifically into prefrontal cortex function, in particular executive function tasks.
Figure 5: Functional MRI (fMRI)
For example, through fMRI studies, the development of response inhibition, and the neural structures supporting it, have been well studied. A popular paradigm for studying inhibition is the Go/No-Go task, which involves inhibiting a response when a certain stimulus is shown. In one fMRI study that employed a version of this task, a group of children (7–12 years old) and young adults (21–24 years old) were presented with a series of alphabetic letters and were required to press a button upon seeing each one, except when the letter X appeared (Casey et al., 1997). Volunteers were instructed to refrain from pressing any buttons if they saw the letter ‘X’– the No-Go stimulus. This task requires executive action: the command to inhibit a habitual response.
The results showed that in both children and adults, several regions in the frontal cortex, including the anterior cingulate, orbitofrontal cortex and inferior and middle frontal gyri, were activated during the task that required inhibiting the normal response. While the location of activation was essentially the same for both age groups, there was a significantly higher volume of prefrontal activation in children than in adults, specifically in the dorsolateral prefrontal cortex and extending into the cingulate. By contrast, adults showed more activity in the ventral region of the prefrontal cortex. Thus the activation in the dorsolateral prefrontal cortex could be negatively correlated with behavioural performance (as interpreted from error rates), as distinct from the orbitofrontal cortex whose activation increased with improvement in behavioural performance. In line with this pattern, those subjects who performed best (that is, those who had lowest error rates) and had the greatest orbitofrontal activation also had the least dorsolateral prefrontal activation.
The greater and more diffuse activity in the dorsal region of the prefrontal cortex in children suggests that there is a heavier dependence on this region in children compared with in adults. The researchers suggested that during adolescence, the network recruited for this task is modified until adulthood, at which stage activation of a smaller, more focal region of the prefrontal cortex is used to perform the same task.
To substantiate speculations about brain activation during inhibition during the transition between childhood and adulthood, the study was replicated with a wider age range of participants (Tamm, Menon, & Reiss, 2002). In the second study, the same Go/No-Go task was used but participants included children as well as adolescents – this time, subjects ranged in age from 8 to 20 years old. While there was no difference in accuracy on the task with age, reaction times to inhibit responses successfully significantly decreased with age. fMRI data revealed age-related increases in activation in the left inferior frontal gyrus extending to the orbitofrontal cortex and, consistent with Casey et al.'s results, age-related decreases in activation in both the left superior and middle frontal gyrus extending to the cingulate. These results demonstrated a dissociation between prefrontal areas in the development of inhibitory control and a negative correlation between age and brain activation.
This pattern of age-dependent activation has been corroborated by fMRI studies of generativity. Word generation tasks, for example, have been extensively used in experimental and clinical studies and are consistently linked to prefrontal cortex activation and are therefore useful to study development of generativity in adolescence (Brown et al., 2005; Gaillard et al., 2000). A study using a verbal fluency task required children (average age 11 years) and adults (average age 29 years) to generate different words starting with the same letter as quickly as possible in the scanner (Gaillard et al., 2000). The results of this study revealed that children performed worse on the task and had on average 60% greater activation in the left inferior frontal cortex and the dorsolateral prefrontal cortex than did adults.
Thus, reaction times and imaging data together suggest that in children, an immature stage of the brain where excess synapses, possibly as a result of a burst of proliferation, accounts for the poorer performance and extensive and less efficient frontal activation. A pruned and more myelinated adult brain could explain the faster reaction times and focal activation of the frontal cortex, the area associated with generativity (Frith, Friston, Liddle, & Frackowiak, 1991) and the inhibitory response (Konishi et al., 1999). The Go/No-Go task requires multiple executive functions including working memory and inhibition albeit at a relatively low level. One possibility is that extensive activation in children is a compensatory strategy used while the brain is less efficient in integrating executive functions.
Adolescents are renowned for engaging in risky behaviour. A recent neuroimaging study suggests that differences in brain activation in mesolimbic circuitry during incentive-driven behaviour between adolescents and adults might account for this (Bjork et al., 2004). A group of 12 adolescents and 12 young adults were scanned while they carried out a task that involved anticipating the opportunity for both monetary gains and losses and the notification of their outcomes. Compared to adults, adolescents showed reduced recruitment of the right ventral striatum and right amygdala while anticipating responses for gains. Activation patterns during monetary gain notification did not differ between groups. This suggested lower activation for motivational but not consummatory components of reward-directed behaviour. To explain risky behaviour commonly associated with adolescence, the authors postulated that adolescents are driven to seek more extreme incentives to compensate for low recruitment of motivational brain circuitry.
Anecdotally, adolescents are known to be poor at decision-making, especially when risk is involved. In an fMRI study that investigated the neural mechanisms that might account for differences between adolescents and adults in decision-making, participants were presented with one-line scenarios (e.g. ‘Swimming with sharks’) and were asked to indicate via a button press whether they thought this was a ‘good idea’ or a ‘not good idea’ (Baird, Fugelsang, & Bennett, 2005). There was a significant group by stimulus interaction, such that adolescents took significantly longer than adults on the ‘not good idea’ scenarios relative to the ‘good idea’ scenarios. Furthermore, adults showed greater activation in the insula and right fusiform face area compared to adolescents, during the ‘not good’ ideas. On the other hand, adolescents showed greater activation in the dorsolateral prefrontal cortex (DLPFC) during the ‘not good’ ideas and there was a significant correlation between DLPFC activation and reaction time. It was proposed that when confronted with a risky scenario, adults’ relatively efficient responses were driven by mental images of possible outcomes and the visceral response to those images, in line with the somatic marker hypothesis (Damasio, 1996). However, adolescents relied more on reasoning capacities and therefore activated the DLPFC, hence the relatively effortful responses compared to adults.
Neural plasticity of the developing brain may underpin different propensities for learning new skills, such as problem solving, at different stages of the life cycle. For example, sensitive periods for learning phonemes of one's mother tongue occur in the first six months of life (Kuhl et al., 1992) and the ability to learn a second language declines with age (Hakuta, Bialystok, & Wiley, 2003). Logical reasoning required to solve mathematical problems activates both parietal and frontal cortex in both adolescents and adults. An fMRI study that required subjects to solve algebraic equations before and after a practice period demonstrated differential activation patterns after four days of learning in adolescents and adults (Luna, 2004b; Qin et al., 2004). Both adolescents and adults showed an increase in prefrontal, parietal and motor activation while solving the equations. Both groups also demonstrated a reduction in prefrontal areas after practice. However, adolescents, as distinct from adults, additionally demonstrated a reduction in parietal regions after the practice period. The authors proposed that the parietal cortex represents an ‘imaginal’ component necessary for this sort of abstract reasoning task. They proposed that after the learning period, the adolescents are less reliant on this area than the adults. However, the directions of cause and effect remain ambiguous. It is unclear whether the adolescents’ decrease in parietal activation with practice was a result of an immature parietal cortex and hence a higher relative dependence on the prefrontal cortex. Alternatively, more localised parietal activation in adolescents might indicate an advantage among adolescents for this sort of task; that is, adolescents depend less heavily on more specialised parietal circuitry, compared to adults. Further functional imaging studies combined with behavioural analysis will clarify the significance of focal versus diffuse brain activation patterns for the propensity to learn.
Although several developmental studies emphasise the decrease in frontal activity with age, in others, activity in this and other regions has been found to increase with age. Using a visuo-spatial working memory task, Kwon, Reiss, and Menon (2002) found that performance gradually improved with age between 7 and 22 years. Age-related changes were found in several brain regions including the dorsolateral prefrontal cortex and the posterior parietal cortex bilaterally (see Figure 6). Similarly, Rubia et al. (2001) reported increased activation in various frontal and parietal regions on a task of response inhibition. Using the Stroop colour–word interference paradigm, which involves inhibition of inappropriate responses, Adleman et al. (2002) observed age-related increases in activity in a left frontal-parietal network of areas. They found no evidence of decreased activity with age.
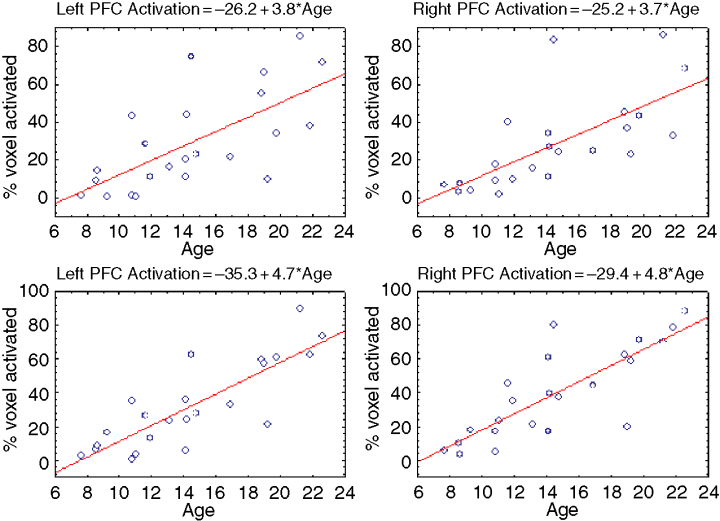
Figure 6: Increasing activation in left and right prefrontal cortex and posterior parietal cortex during working memory task (Kwon et al., 2002).
Confounding effects of task performance
One remaining issue is the confounding effect of task performance differences in fMRI studies. If one group's task performance is worse than that of the other group, then any difference in brain activity between the two groups is difficult to interpret. It might cause the difference in task performance, or it might be an effect of these differences. Future studies should attempt to match task performance between groups to avoid this interpretation problem.
Development of social cognition in the brain
Recognition of facial expressions of emotion is one area of social cognition that has been investigated during adolescence (see Herba & Phillips, 2004). Much of the literature has focused on early development of facial recognition (Nelson, 1987). Relatively little is known about the continued development of emotion processing during adolescence. The amygdala has consistently been associated with emotion processing (Dolan, 2002; Phillips, Drevets, Rauch, & Lane, 2003). An fMRI study of the role of the amygdala in normal adolescents involved scanning 12 adolescent participants (Baird et al., 1999). They found significant amygdalar activation in response to the perception of fearful facial expressions. A similar fMRI study investigated the neural processing of other facial expressions (happiness and sadness) in a group of 12 adolescent subjects (aged 13–17 years). The perception of happy faces compared with neutral faces was associated with significant bilateral amygdalar activation in adolescents (Yang, Menon, Reid, Gotlib, & Reiss, 2003). Neither of these studies contained an adult or a younger child group, so comparisons before and after puberty of the neural processing of facial emotion could not be made. Furthermore, there was no exploration of how age affects emotion expression processing. Thomas et al. (2001) addressed some of these issues by studying amygdala activation to fearful facial expressions in two groups: a group of children (mean age 11 years) and adults. Adults demonstrated greater amygdala activation to fearful facial expressions, whereas children showed greater amygdala activation to neutral faces. The authors argued that the children might have detected the neutral faces as more ambiguous than the fearful facial expressions, with resulting increases in amygdala activation in response to the neutral faces.
Killgore, Oki, and Yurgelun-Todd (2001) studied developmental changes in neural responses to fearful faces in children and adolescents. Results indicated sex-differences in amygdala development: although the left amygdala responded to fearful facial expressions in all children, left amygdala activity decreased over the adolescent period in females but not in males. Females also demonstrated greater activation of the dorsolateral prefrontal cortex over this period, whereas males demonstrated the opposite pattern. The authors interpreted these findings as evidence for an association between cerebral maturation and increased regulation of emotional behaviour; the latter mediated by prefrontal cortical systems. It is possible that the pattern of decreased amygdala and increased dorsolateral prefrontal activation in girls with increasing age reflects an increased ability to contextualise and regulate emotional experiences per se.
In a recent study a group of adolescents (aged 7 to 17) and a group of adults (aged 25–36) viewed faces showing certain emotional expressions. While viewing faces with fearful emotional expressions, adolescents exhibited greater activation than adults of the amygdala, orbitofrontal cortex and anterior cingulate (see Figure 7) (Monk et al., 2003). When subjects were asked to switch their attention between a salient emotional property of the face, like thinking about how afraid it makes them feel, and a non-emotional property, such as how wide the nose is, adults, but not adolescents, selectively engaged and disengaged the orbitofrontal cortex. These fMRI results suggest that both the brain's emotion processing and cognitive appraisal systems develop during adolescence. The authors interpret these results in the context of their Social Information Processing Network model (cf. Nelson, Leibenluft, McClure, & Pine, 2005).
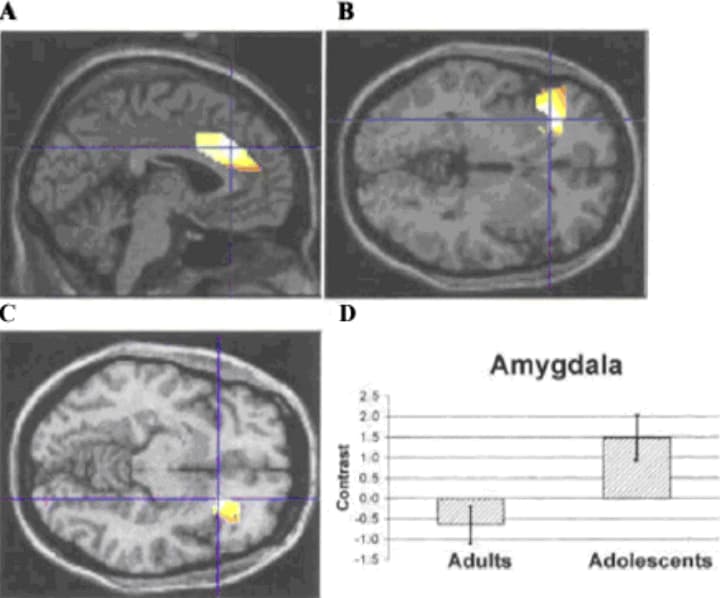
Figure 7: Activation patterns in adolescents relative to adults when passively viewing fearful impact to neutral faces. (A–D) Activation in the ACC, left OFC, right OFC, and amygdala, respectively. Crosshairs indicate cluster maxima (Monk et al., 2003)
Implications for teenagers
An interesting speculation based on the results of the studies discussed in this review is that puberty represents a period of synaptic reorganisation and as a consequence the brain might be more sensitive to experiential input at this period of time in the realm of executive function and social cognition. This sensitive period might be akin to the sensitive periods of brain development that are evident in the early sensory system. Much like sound categorisation during language acquisition (see above), experience with executive functions and certain social cognitive skills might be much more difficult to incorporate into brain networks once they are established after puberty. This notion is purely speculative and further research, preferably with input by multiple disciplines including educational researchers, cognitive scientists and neuroscientists, may shed light on this.
Research into the cognitive implications of continued brain maturation beyond childhood may be relevant to the social development and educational attainment of adolescents. Further studies are necessary to reach a consensus about how axonal myelination and synaptic proliferation and pruning impact on social, emotional, linguistic, mathematical and creative development. In other words, which skills undergo perturbation, which undergo sensitive periods for enhancement and how does the quality of the environment interact with brain changes in the development of cognition? Longitudinal studies of the effect of early deprivation on the cognitive development of Romanian adoptees in the UK have begun to investigate this question (O'Connor & Rutter, 2000). Whether greater emphasis on social and emotional cognitive development would be beneficial during adolescence is unknown but research will provide insights into potential intervention schemes in secondary schools, for example for remediation programmes or anti-social behaviour.
Research in psychology and cognitive neuroscience can also contribute to the debate about juvenile crime, for instance on the current use of Anti-Social Behaviour Orders (ASBOs) in the UK. ASBOs are civil orders which can be imposed against anyone aged 10 or over who is deemed to have acted in a manner which ‘causes harassment, alarm or distress’ to anyone, and which, if breached, become criminal offences. A dialogue between psychologists and parliamentarians would be useful to shape future legislative procedures concerning adolescent social behaviour. Current theoretical and philosophical underpinnings of criminal law are grounded in the principle of autonomy: individuals are regarded as rational autonomous human beings who can ‘choose’ their actions and are therefore held responsible by criminal law. This framework of the law is borrowed from philosophy rather than psychology. Drawing on recent experimental evidence from cellular, behavioural and brain imaging studies, neuroscientists and psychologists can evaluate the efficacy of ASBOs. Firstly, they can investigate the role of brain development in causing problem behaviour among adolescents. Secondly, given that the brain is still developing, psychologists can explore the long-term psychological effects of receiving an ASBO on the adolescent. Finally, neuroscience may offer insights into alternatives to current punitive methods. It may, for example, be worth allocating more resources to educational and rehabilitation programmes designed to take into account the natural developmental changes in adolescent psychology.
Conclusion
The study of the development of executive function and social cognition beyond childhood is a new but rapidly evolving field with applications for medical diagnosis, education and social policy. The finding that changes in brain structure continue into adolescence and early adulthood challenged accepted views and has given rise to a recent spate of investigations into the way cognition might change as a consequence. In this paper, we have focused on research in developmental cognitive neuroscience, but a richer account of changes in adolescent learning, and strategic and social behaviour requires a multidisciplinary approach that recognises the complex interactions between genetics, brain structure, physiology and chemistry and the environment. Studying the development of adolescent cognition using complementary in vivo methods that exploit the advantages of each – such as combining fMRI with electroencephalography (EEG) or diffusion tensor imaging (DTI) – within a theoretical framework that regards motor, affective, social and perceptual functions as intertwined promises to further inform our understanding of typical and atypical adolescent behaviour.