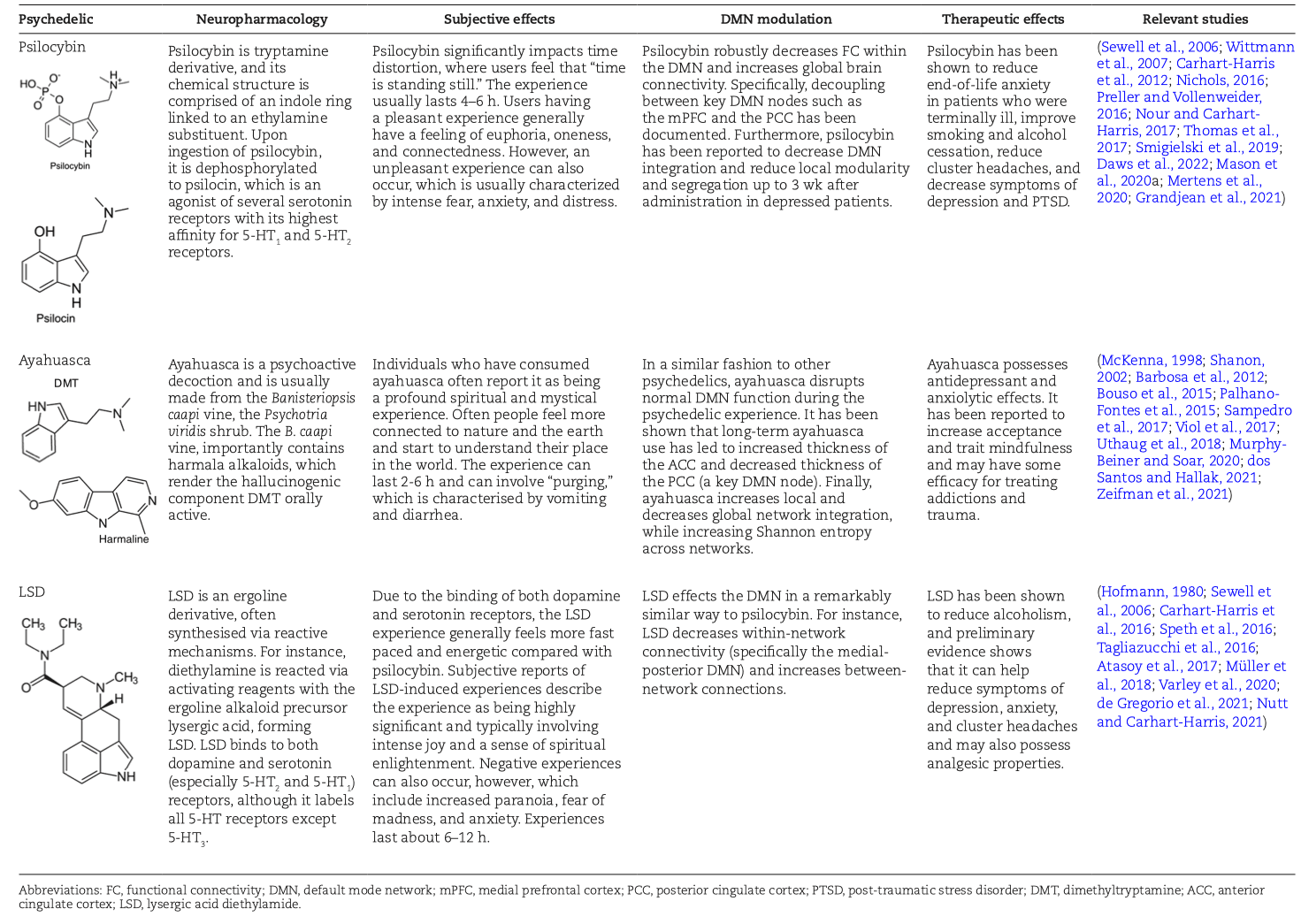
Abstract
Psychedelics are a unique class of drug that commonly produce vivid hallucinations as well as profound psychological and mystical experiences. A grouping of interconnected brain regions characterized by increased temporal coherence at rest have been termed the Default Mode Network (DMN). The DMN has been the focus of numerous studies assessing its role in self-referencing, mind wandering, and autobiographical memories. Altered connectivity in the DMN has been associated with a range of neuropsychiatric conditions such as depression, anxiety, post-traumatic stress disorder, attention deficit hyperactive disorder, schizophrenia, and obsessive-compulsive disorder. To date, several studies have investigated how psychedelics modulate this network, but no comprehensive review, to our knowledge, has critically evaluated how major classical psychedelic agents—lysergic acid diethylamide, psilocybin, and ayahuasca—modulate the DMN. Here we present a systematic review of the knowledge base. Across psychedelics there is consistent acute disruption in resting state connectivity within the DMN and increased functional connectivity between canonical resting-state networks. Various models have been proposed to explain the cognitive mechanisms of psychedelics, and in one model DMN modulation is a central axiom. Although the DMN is consistently implicated in psychedelic studies, it is unclear how central the DMN is to the therapeutic potential of classical psychedelic agents. This article aims to provide the field with a comprehensive overview that can propel future research in such a way as to elucidate the neurocognitive mechanisms of psychedelics.
INTRODUCTION
Psychedelics are a class of hallucinogenic agents that have cultural, spiritual, and scientific implications (McKenna, 1998; Nichols, 2016). The etymology of the term psychedelic is from the Greek words ψυχή (psyche, “soul, mind”) and δηλοῦν (deloun, “to manifest”). Therefore, psychedelics are “mind/soul manifesting” and are suggested to illuminate hidden terrains of the human psyche (Weil and Winifred, 2004). “Classical psychedelics” is a broad term describing a variety of substances whose primary mechanism of action resides in serotonin (5-HT2A) receptors in the brain and, in turn, produce profound alterations of consciousness, including modulations in the sense of self, sensory perception, and emotions (Table 1) (Morris, 2008; Nichols, 2016; Speth et al., 2016).
Neuroimaging studies have shown that when an individual is at rest, multiple interconnected brain regions are activated above baseline, and, conversely, there is decreased activity in these regions during task-dependent attention (Raichle, 2015) (Figure 1). These regions amalgamate to create 4 functional hubs: the medial prefrontal cortex (mPFC), the posterior cingulate cortex (PCC), precuneus, and the angular gyrus, together referred to as the Default Mode Network (DMN) (Andrews-Hanna et al., 2014). The DMN is one of many resting-state networks (RSNs). RSNs are derived from the cognitive origin hypothesis of resting-state connectivity, where resting-state connectivity is defined as the synchronous fluctuation of low-frequency signals between functionally related brain areas (Biswal et al., 1997).
![Figure 1.Reprinted by permission from [Springer Nature]: Nature [Nature Reviews Neuroscience]. The default mode network in cognition: a topographical perspective, Smallwood et al. (2021).](/_next/image?url=https%3A%2F%2Fimages.ctfassets.net%2F9ge8ptsf9tdf%2F7xufcU86FLZenCZWMQ2r0M%2F30f7ea81e2bf31a32a5194753148bfef%2FScreenshot_2025-07-30_at_2.00.15%C3%A2__PM.png&w=1920&q=75)
Functional connectivity (FC) is defined as the temporal coactivation patterns of neural activity between anatomically distinct brain regions, it is an important and well-established variable of interest when understanding DMN connectivity (van den Heuvel and Pol, 2010). RSNs, such as the DMN, are imaged prior to the experimental stimulus (i.e., engaging in a memory task), and DMN FC has been found to be anti-correlated and orthogonal to task-dependent brain networks such as the salience network (SLN) (Raichle, 2015; Smallwood et al., 2021). Furthermore, altered FC within the DMN has been correlated with a variety of psychometric components in clinical questionnaires, which may have downstream therapeutic effects. For instance, reduced FC with brain regions that comprise the DMN has been associated with positive states of ego dissolution such as Oceanic Self-Boundlessness (measured by the 5-Dimensional Altered States of Consciousness Rating scale; see Table 2), which may facilitate the cognitive reappraisal that can occur during the psychedelic experience (Smigielski et al., 2019; Yaden and Griffiths, 2020).
Alterations in the FC signatures of the DMN, consisting of intra-connectivity changes (FC alterations within the DMN brain regions) and inter-connectivity changes (changes in FC parameters between brain regions within the DMN and brain regions within other cognitive networks), have been implicated in a variety of complex cognitive functions such as theory of mind, self-referencing, memory, and rumination (Andrews-Hanna, 2012; Raichle, 2015). However, the underlying mechanisms are not fully understood (Smallwood et al., 2021). Additionally, altered DMN function has been implicated in a range of neuropsychiatric and neurodegenerative conditions such as depression, attention deficit hyperactivity disorder, schizophrenia, anxiety, and post-traumatic stress disorder, Alzheimer’s disease, and aging in general (Mohan et al., 2016; Zhang and Volkow, 2019). As such, altered DMN connectivity in specific clinical populations or groups consuming therapies that impact functional brain activity (e.g., psychedelics) appears to offer a window into the variability of complex human functioning that can also be modulated by psychedelic treatment.
The “mind-manifesting” properties of psychedelics illuminated by figures like Aldous Huxley, Timothy Leary, and Albert Hofmann created a surge of trials during the 1960s, which highlighted psychedelics as potential therapeutic agents for a range of mental health and substance use disorders (Carhart-Harris, 2019). In particular, lysergic acid diethylamide (LSD) was on the market as Delysid/Sandoz in the 1950s and 1960s, prescribed for the treatment of “psychoneuroses, psychoses” as well as other neuropsychiatric disorders, always to be administered in a controlled setting (psychiatric clinic or hospital) by properly trained health professionals. The use of LSD and all other psychedelics in clinical or preclinical research was severely constrained for political reasons in the 1970s, although LSD continued to be prescribed by psychiatrists outside the United States well into the 1990s. Based on the promising yet preliminary findings with LSD and other drugs such as psilocybin, N,N-dimethyltryptamine (DMT), ayahuasca, or mescaline, but also 3,4-methylenedioxymethamphetamine (MDMA) and ketamine, psychedelics are regaining scientific attention, especially in the field of clinical neuroscience. As a result, a number of more recent studies have begun to elucidate how psychedelics modulate the DMN (Carhart-Harris et al., 2012, 2016; Bouso et al., 2015; Palhano-Fontes et al., 2015; Thomas et al., 2017; Millière et al., 2018; Müller et al., 2018; Kelly et al., 2019; Smigielski et al., 2019; Mason et al., 2020; Mertens et al., 2020; Daws et al., 2022; de Gregorio et al., 2021; Grandjean et al., 2021).
Psychedelics often induce meaningful and mystical experiences that have been associated with increased measures of brain entropy (Carhart-Harris, 2018). Entropy is a measure of the uncertainty of the system, and regarding the brain, it is associated with functional disorder, unpredictability, and flexibility, which may lead to an enhanced array of dynamic brain states (Tagliazucchi et al., 2014; Atasoy et al., 2017; Carhart-Harris, 2018). Yaden and Griffiths (2020) argue that a considerable variance in the therapeutic outcome associated with psychedelics can be explained by the altered state of consciousness that psychedelics produce. Furthermore, one of the most ubiquitous and transformative components of the psychedelic experience is the feeling of ego dissolution, a relaxation of subject-object distinctions during which the borders and constraints of the self seem to dissolve (Letheby and Gerrans, 2017; Mason et al., 2020). Feelings of ego dissolution also appear to be a central component of the unitive experience (a sub-component of the mystical states), which is an experience of perceived union with nature or a higher power or an all-pervading sense of oneness (Nour et al., 2016). It has been hypothesized that ego dissolution can be therapeutic because an individual’s cognitive attributions and affect are viewed with a greater distance and objectivity (Letheby and Gerrans, 2017; Mason et al., 2020). Psychedelic-induced ego dissolution may be precipitated via Bayesian belief updating wherein reduced precision of previously held beliefs lends to revision of the self and world, as hypothesized by Stolicker and colleagues (2022). This has parallels with the practice of meditation, which is also associated with decreased activity of the DMN and alterations to precision-weighting of beliefs and attention (Brewer et al., 2011; Palhano-Fontes et al., 2015; Letheby and Gerrans, 2017; Millière et al., 2018). Functional magnetic resonance imaging (fMRI) studies involving psychedelics show that the modulation of the DMN acutely decreases connectivity and blood flow within nodes of this network (Table 2). These changes are paralleled by magneto/electroencephalography studies showing neuronal desynchronization of alpha power (itself previously shown to be highly correlated with DMN connectivity), at times specifically located in the PCC (one of the main hubs of the DMN), which has been tentatively hypothesized to result in a mind that is less constrained, more flexible, and less self-referential and egoic (Muthukumaraswamy et al., 2013; Letheby and Gerrans, 2017; Nour and Carhart-Harris, 2017; Carhart-Harris, 2018; Mason et al., 2020).
Although the DMN is a RSN that is featured in a vast array of psychedelic trials, there is still some ambiguity as to what changes/modulation to this network mean and what motifs and differences are seen across the literature regarding this network. Therefore, this systematic review seeks to explore the following aims: (1) to critically evaluate the impact of classic psychedelics on the DMN; (2) to evaluate the evidence regarding DMN modulation and resultant psychotherapeutic effects; (3) to identify challenges and limitations associated with the current literature surrounding DMN modulation by psychedelics; and (4) to provide a discussion surrounding future research and the potential future utility of DMN modulation–focused psychedelic therapies. This review focuses on the classical psychedelics: LSD, psilocybin, DMT, mescaline, and ayahuasca (which contains DMT and harmala alkaloids), complementing previous reviews of these agents (Carhart-Harris and Friston, 2019; Aday et al., 2020; Inserra et al., 2021). To this end we conducted a literature review via Scopus and PubMed databases on August 28, 2021, up to November 14, 2021.
METHODS
This systematic review followed the PRISMA statement (Moher et al., 2015) for transparent and comprehensive reporting. The review was not registered, and a review protocol was not prepared.
Search Strategy
We conducted an electronic database search of PubMed and Scopus from inception to April 2022. We structured our search according to the PICO framework (Schardt et al., 2007) using search terms related to the DMN, along with the names of the classical psychedelics, to return all potentially eligible studies. The search string was: default mode network OR DMN OR neuro circuitry AND ayahuasca OR DMT OR dimethyltryptamine OR psilocybin OR psilocin OR LSD OR Lysergic acid diethylamide OR mescaline OR peyote OR psychedelic. A search of Google Scholar was conducted to identify any additional relevant articles.
Eligibility Criteria
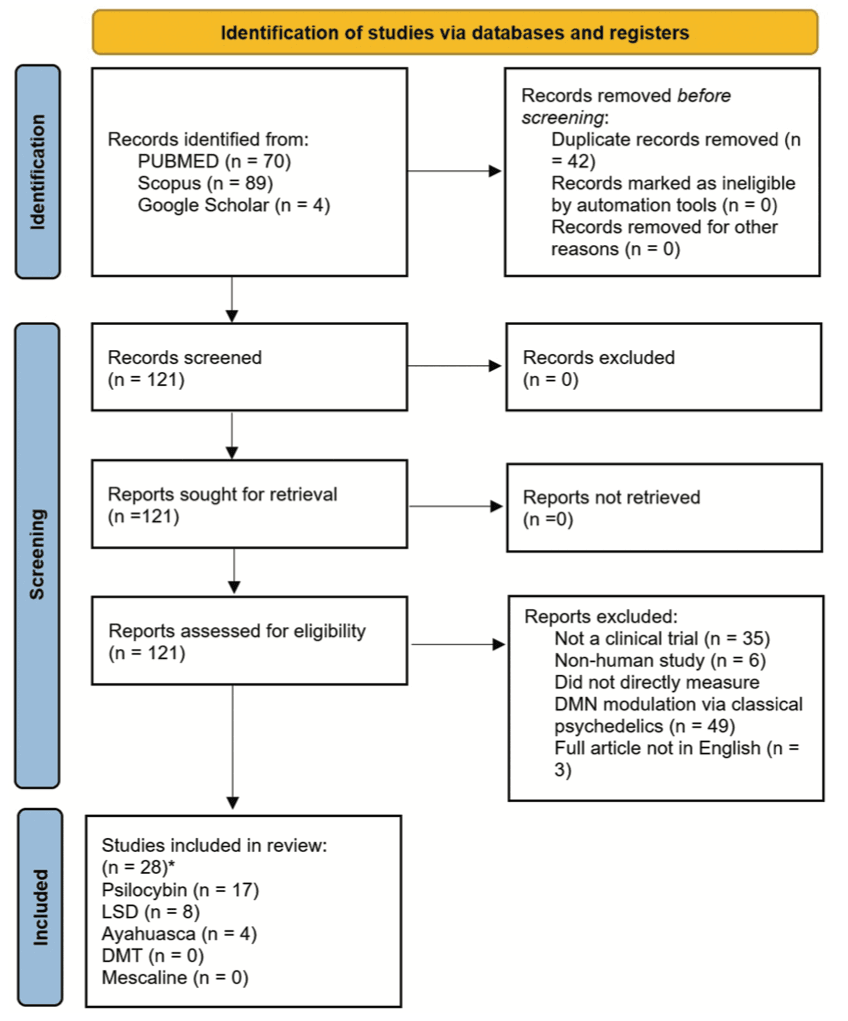
Articles were screened by 2 reviewers (J.G., S.R.). Disagreements were resolved through discussion until consensus was reached (with J.S. arbitrating if required). We included only human clinical trials (rodent studies were excluded) assessing DMN modulation by the classical psychedelics. Papers that performed novel analyses on previously published datasets were also included [i.e., Carhart-Harris et al., (2013) and Varley et al., (2020)]. Only English language papers were eligible, and there was no psychedelic dose requirement and no requirement on the length of treatment or on the statistical methods used. All clinical diagnoses and healthy controls were included. The full article screening and selection process is detailed in Figure 2.
RESULTS
Results Overview
The initial database search was performed on 28th of August 2021 and a second database search was performed on the 20th of April 2022. The search returned 163 results which were reduced to 119 after the duplicates were removed. A further 95 articles were removed due to ineligibility. This left 28 articles that were included in the study (Figure 2).
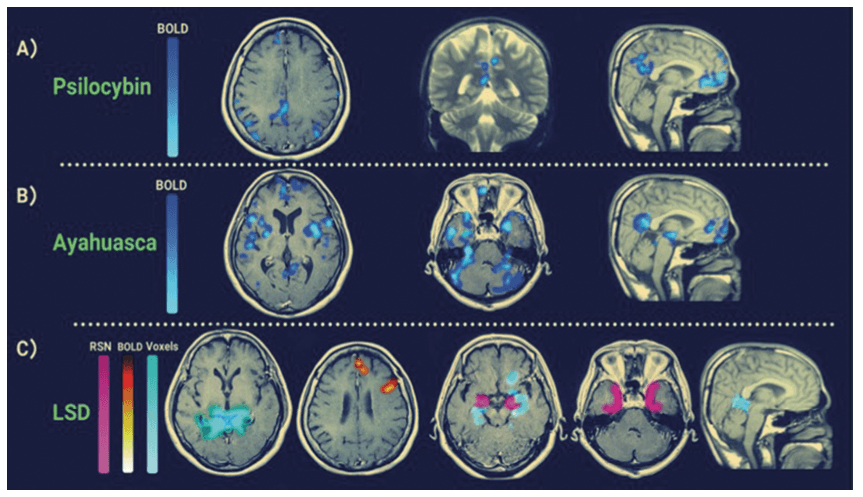
A total of 17 psilocybin studies were assessed, twelve using fMRI (2 of 12 using 7 Tesla fMRI, remaining using 3 Tesla) and two using Electroencephalogram (EEG) or Magnetoencephalogram (MEG). The average age of subjects across these studies was 34.6 years with a standard deviation (SD) of 8.8 years. The average number of participants across the studies was 26 with a SD of 19. Out of the 17 studies, 13 had a placebo control group. Four Ayahuasca studies were assessed, with all four using fMRI (two using 1.5 Tesla and two, 3 Tesla). The average age of subjects across these studies was 35 years with a SD of 17.8 years, and the average number of participants was 28 with a SD of 5.9 years. Only one of these studies had a placebo control group. Eight LSD studies were assessed, with seven using (3 Tesla) fMRI, one using EEG and one using MEG. The average age across these studies was 29.6 years with a SD of 2.8, and the average number of participants was 20, and the SD was 6.4 participants. All studies had a placebo control group. No published research papers assessing how mescaline and DMT modulates the DMN in humans were found. Table 2 is a summary of the findings and study characteristics for the research articles included in this systematic review. Figure 3 shows the specific DMN brain regions detected via fMRI that are modulated by psychedelics.
DISCUSSION
Synthesis of Data
Based on the evidence (see Table 2), there are clear associations between a psychedelic’s ability to reduce the functional connectivity within the DMN (and increase its connectivity to other networks), altered states of consciousness, and therapeutic outcomes (Letheby and Gerrans, 2017; Müller et al., 2018; Smigielski et al., 2019; dos Santos and Hallak, 2021; Mason et al., 2021; Daws et al., 2022). At this stage, however, it is difficult to ascertain the direction of causality.
A recent review from Aleksandrova and Phillips (Aleksandrova and Phillips, 2021) also revealed that the classical psychedelics can induce marked neuronal structural and functional changes via neurotrophic signaling and neuroplasticity. These psychoplastic changes are most likely mediated by brain-derived neurotrophic factor and mammalian target of rapamycin (Inserra et al., 2021). Furthermore, Martin and Nichols (2017) showed that psychedelics have the potential to alter gene expression and immunomodulatory mechanisms. These effects are proposed to rewire pathological cortical networks (possibly by reducing neuronal inflammation, a burgeoning hypothesis of addiction, depression and neurodegeneration), thereby explaining how psychedelics may neurobiologically induce positive long-term health outcomes. Thus, it is challenging to delineate how much of the benefit in psychedelic therapy is derived from neuroplastic changes on the cellular level or on the network level (DMN modulation) and the degree to which the two are linked. This is further confounded by the fact that studies use varying dosages of psychedelics are not all placebo controlled and often investigate participants who are not drug naïve.
Depression is often characterized by excessive activity in the mPFC, and there appears to be an inverse correlation between depressive symptomatology and the degree of 5-HT2Areceptor stimulation in this region (Morris, 2008; Nichols, 2016; Carhart-Harris, 2019). Psilocybin can acutely increase anxiety, which may be mediated by glutamate hyperfrontality; however, long-term reductions in anxiety could occur due to 5-HT2Areceptor downregulation in this area (Mason et al., 2020). Another hypothesis is that psilocybin, and the classical psychedelics more broadly, have a significant affinity for and agonize the 5-HT1A receptors. These are the primary inhibitory serotonergic receptors and may be responsible for the decreased blood oxygen level–dependent (BOLD) coactivation often seen in the DMN and other brain regions on the administration of psychedelics. On the macro-scale, connectivity between the parahippocampal cortex and prefrontal regions was a reliable predictor of depressive symptoms 5 weeks after psilocybin treatment (Carhart-Harris et al., 2018).
A review by Thomas et al. (2017) concluded that based on the totality of evidence at that time (7 clinical trials), psilocybin, by disrupting the hyperconnectivity in the DMN of psychiatric conditions, may be a novel treatment for a range of neuropsychiatric conditions. A recent analysis by Daws et al. (2022) found that psilocybin therapy reduced depressive symptoms for up to 6 weeks post intervention and that this effect was likely dependent on increased brain network integration. Interestingly, this group also found that increased within-DMN connectivity and reduced between-network DMN connectivity (specifically with the executive and salience network) were correlated with baseline depressive severity, which aligns with the previous findings of other researchers (Hamilton et al., 2011; Lydon-Staley et al., 2019; Feurer et al., 2021).
The literature reviewed clearly indicates that classical psychedelics acutely decrease functional connectivity within the DMN and increase between-network connectivity (see Table 2) (Roseman et al., 2014; Lebedev et al., 2015; Palhano-Fontes et al., 2015; Carhart-Harris et al., 2016; Nichols, 2016; Tagliazucchi et al., 2016; Atasoy et al., 2017; Müller et al., 2018; Mason et al., 2020; Mertens et al., 2020; de Gregorio et al., 2021; Grandjean et al., 2021; Daws et al., 2022; Madsen et al., 2021). Thus, during the psychedelic experience, there seems to be a unique shift in neural connectivity, reflecting a shift from a more modular, segregated brain to a more interconnected global network (Palhano-Fontes et al., 2015; Smigielski et al., 2019). This decrease in between-network segregation seems to be somewhat specific to the classical psychedelics, although this decreased segregation is also seen in ketamine (Bonhomme et al., 2016) and salvinorin A (Doss et al., 2020). It is not seen with selective serotonin reuptake inhibitor antidepressants (Klaassens et al., 2015; Daws et al., 2022), stimulants (Konova et al., 2015), sedatives (Stamatakis et al., 2010), or MDMA (Roseman et al., 2014). This increase in inter-network FC can be interpreted via a dynamical systems theory approach [see Roseman et al., (2014) for more details]. For instance, RSNs are canonical networks of brain activity and when reflected graphically, the valleys in this 2D plane are indications of the metastable “sub-states” of these RSNs. Thus, deeper valleys imply a prolonged sub-state with greater rigidity, whereas shallower valleys indicate less stable and more flexible sub-states. The increase in FC between the DMN and other RSNs seems to align with the latter graphical representation (i.e., shallow troughs), which may indicate that the brain/mind can access a greater dynamic repertoire of metastable sub-states. This more flexible brain state seems to persist in the recovered state of patients with major depressive disorder (MDD) treated with psilocybin (Daws et al., 2022).
Psychedelics may in part disrupt the DMN because they promote a cognitively intensive experience where one is dealing with the “task” of one’s subconscious and grappling with the deep metaphysical and personal questions that generally arise during the experience (Shanon, 2002). This aligns with the definition of the DMN, which is anticorrelated with task-based activities. Although mind-wandering is associated with increased DMN activity, awareness of mind-wandering—as seen in meditation—decreases the resting FC of the DMN (Brewer et al., 2011; Palhano-Fontes et al., 2015). This is a plausible mechanism by which ayahuasca, as well as psilocybin and LSD, also decrease activity within the DMN because the experience is characterized by a hyper-awareness of one’s internal dispositions and attributions (Brewer et al., 2011; Preller and Vollenweider, 2016; Millière et al., 2018). Furthermore, it could be argued that hyper-awareness of one’s mind and internal cognition is a feature of a brain that has increasing entropy and is approaching criticality (Carhart-Harris et al., 2012, 2016; Carhart-Harris, 2018; Millière et al., 2018), although this needs to be further examined. It is also clear that various individuals will respond differently to distinct treatments because their baseline levels of awareness will differ. Another plausible mechanism of reduced coactivation within the DMN is that psychedelics produce less mentation to the past, as seen with LSD, but whether this effect is unique to psychedelics is unclear (Speth et al., 2016).
Models of Psychedelic Action
It is important to note that the “relaxed beliefs under psychedelics” (REBUS) model is 1 of 3 prominent models of the biological mechanisms underpinning psychedelics’ mode of action, and each model exhibits unique strengths and weaknesses. The REBUS model has been the primary focus of this article (refer to Table 2, where 24 of the 28 papers were most closely aligned with the REBUS model) because it included DMN activity and modulation as a central axiom (REBUS is based on the brain’s hierarchical organization with DMN at the top of this hierarchy). The two other models suggest mechanisms that are relatively independent of DMN modulation (Doss et al., 2022).
Relaxed Beliefs Under Psychedelics and Brain Entropy
It has been hypothesized that the underlying neurobiology of DMN perturbation and its downstream effects is caused by entropy (Carhart-Harris, 2018). Psychedelics exert their hallucinogenic effects primarily by activating the 5-HT2A receptor, resulting in increased 5-HT release, enhancing the excitability of layer V pyramidal cells in the cortex and hence leading to increased glutamate in the neocortex (Nichols, 2016; Carhart-Harris, 2019; Mason et al., 2020). In the cortex 5-HT2A receptor activation can lead to asynchronous glutamate release resulting in desynchronized ensembles of neurons (Nichols, 2016; Riba et al., 2002; Tagliazucchi et al., 2014). Subsequently, the brain becomes more desynchronized resulting in a loss of oscillatory power, aligning with the brain entropy hypothesis (Aghajanian and Marek, 1999; Tagliazucchi et al., 2014; Carhart-Harris, 2018).
Brain entropy is characterized by increased randomness, unpredictability, and disorderliness (with respect to neuronal firing), which in turn disrupts top-down, goal-oriented cognitive processing (Carhart-Harris, 2018, 2019; Kelly et al., 2019; Varley et al., 2020). It is proposed that this disruption in top-down processing may facilitate increased neural and cognitive flexibility (Doss et al., 2021), which is a viable explanation for the mechanistic underpinnings of the therapeutic effect of psychedelics (Gallimore, 2015; Mason et al., 2021; Daws et al., 2022). The REBUS model postulates that psychedelics serve to relax the precision-weighting of previous beliefs while freeing up and increasing the information flow from bottom-up information processing (Carhart-Harris and Friston, 2019). This process can enable the revision of pathologically overweighted priors, which affects the functional systems of the brain such as those related to the self (Ho et al., 2020).
Evidence from Barrett et al. (2020a) and Daws et al. (2022) may support this model and the notion of a “resetting mechanism” because the acute disruption in top-down cognitive control showed enduring benefits (up to 1 month) in top-down control of emotion, leading to less negative affect and increased positive affect after psilocybin administration. Anatomical connectivity can be conceived as a Bayesian prior on FC, and under anesthetic, external information is minimally processed and integrated, which means that there is a strong association between the FC and structurally encoded priors. However, under classical psychedelics, the brain is less constrained by pre-existing structural priors and therefore these pre-existing priors have less of an effect on cognition, aligning with the REBUS model.
Interestingly, the decreased weight of structural priors frees up the ability for the brain to create a greater array of FC patterns and networks, supposedly enabling the bizarre and ineffable experiences associated with psychedelics (Luppi et al., 2021) [see Table 1 and Preller and Vollenweider, (2016) for the phenomenology of psychedelics]. Specifically, a reduction in FC of the mPFC (a key DMN hub) has been observed following the administration of LSD, and this area is known to be implicated in reality monitoring (Taylor et al., 2007; Subramaniam et al., 2020). Thus, a disruption in this brain region’s function may underpin and support the attenuated top-down processing, impairing an individual to accurately differentiate endogenously or exogenously generated percepts. Further evidence for the REBUS model is seen by the findings that entropy and mystical experiences involving ego dissolution (MacLean et al., 2011) have been correlated with increases in trait openness (Lebedev et al., 2016), which itself is associated with creativity, intelligence, and even increased grey matter in the inferior parietal lobule (Schretlen et al., 2010; Taki et al., 2013). Long-term meditators have decreased DMN FC (Brewer et al., 2011; Brewer and Garrison, 2014; Millière et al., 2018), with psychedelic and meditative states sharing some key characteristics such as heightened levels of perceptual sensitivity, ego dissolution, and decreased negative rumination (Brewer et al., 2011; Millière et al., 2018). Increased awareness of thoughts and feelings also seems to be a key characteristic of the ayahuasca experience and may explain the long-term thinning of the PCC in experienced users (Bouso et al., 2015). Furthermore, thinning of the PCC and thickening of the ACC, supporting DMN-TPN orthogonality, has been associated with greater levels of attention, emotional regulation, and feelings of self-transcendence, which is positively correlated with well-being and negatively associated with depression and end-of-life anxiety (Reed, 2008; Bouso et al., 2015).
Cortico-Striatal-Thalamo-Cortical Model (CSTC)
The CSTC postulates that 5-HT2A receptor activation leads to alterations in the CSTC circuitry, resulting in disinhibition of the thalamus and reduced sensory gating, thereby increasing the amount of sensory information reaching the cortex (Vollenweider et al., 1997). Evidence for this model comes from Preller et al. (2019), who found that LSD increased excitatory connections from the thalamus to the PCC and reduced effective connectivity from the ventral striatum to the thalamus. These connectivity patterns suggest that LSD increases bottom-up informational flow by reducing thalamic-sensory gating, lending credence to the CSTC model.
Cortico-Claustro-Cortical (CCC) Model
The third model is known as the cortico-claustro-cortical model. The claustrum is situated between the insula and putamen and is a thin, curved sheet of neurons embedded in white mater. It contains a large density of 5-HT2A receptors and bidirectional glutamatergic connections to most of the cortex. The CCC model proposes psychedelic effects are a function of activating receptors in the claustrum [the claustrum also contains k-opioid receptors, the primary target of Salvia A (Stiefel et al., 2014)], which leads to a disruption in higher cortical networks through CCC circuits that may underpin the neural and subjective effects that have been associated with the psychedelic experience. Evidence for this model is supported by the findings from Barrett et al. (2020b) (Table 2). Barret and colleagues observed that psilocybin reduced the BOLD signal of the claustrum, which was also predictive of the participant’s subjective experience.
Limitations of REBUS, CTSC, and CCC Models
The REBUS model needs to be clearer as to what constitutes higher and lower brain regions and would benefit from dividing key brain regions (for example the hippocampus) into its constituent nuclei. Furthermore, functional outcomes of increased entropy (and region-specific entropy) are not entirely clear. For example, a recent study showed that individuals with Major Depressive Disorder (MDD) had increased entropy in bilateral hippocampi (Xue et al., 2019) and it does not seem that MDD patients have a ‘richer subjective experience’ and tend to have a more rigid rather than dynamic cognition (Carhart-Harris et al., 2017). Finally, the majority of studies supporting the REBUS model employ a seed-based approach, which assumes dysconnectivity across homogeneous brain structures, regions and networks. Thus, this presupposition has an a priori connectivity bias which limits the measurement of pharmacologically induced connectivity alterations in unaccounted brain regions and networks (Preller et al., 2018). However, it is promising that Preller et al., (2018)who used the data-driven Global Brain Connectivity (GBC) approach, recapitulated connectivity patterns from previous seed-based analyses.
The CSTC model is also incomplete because it does not account for the “efference copies” that also interact with sensory and predictive cortices (Pynn and DeSouza, 2013). Additionally, like the REBUS model, the CSTC model would be strengthened by a revision that includes more specificity regarding the models’ neuroanatomical constituents (e.g., difference in efferent and afferent output of various thalamic nuclei). Finally, the CCC model is the most recent and thereby has the least amount of support, and as newer technologies emerge this will facilitate research investigation into claustrum connectivity (because the claustrum is naturally hard to measure) in response to psychedelic drugs, which will enable this model’s validity and reliability to be further evaluated.
It is important to discuss that these models are not necessarily distinct from one another and that they are all likely involved in mediating psychedelic therapeutic effects. For instance, the feedforward thalamocortical loop that occurs due to increased serotonergic activity (5-HT2A receptor agonism in the CSTC circuit), which leads to “sensory overload” and ego-dissolution, is compatible with the increased bottom-up information flow, relaxed priors, and entropy findings that support the REBUS model. Additionally, it is also possible that 5-HT2A receptor activation within the claustrum leads to CSTC alteration and DMN modulation, increasing entropy and brain plasticity (Barrett et al., 2020b). Hence, it may be appropriate for psychedelics researchers to strive for a unifying psychedelic theory where various theoretical and empirical models are simultaneously tested and juxtaposed with one another. This will enable a more integrated mechanistic understanding of psychedelics’ mode of action; we can potentially understand the similarities and differences of these models and how they relate to the complex phenomenology and clinical outcomes associated with psychedelics.
Current Challenges and Limitations of the DMN
This article rests on the fundamental premise that the DMN is an inherently valuable and meaningful network; however, this presupposition is not without challenge (Fair et al., 2008). It has been argued that the DMN is plagued by the methodological confounder that certain brain regions show intrinsic structural connectivity through vascular coupling rather than functional connectivity (Andrews-Hanna, 2012). However, the assessment of the DMN is not limited to measurements of BOLD fMRI; it has also been assessed through positron emission tomography, which measures glucose metabolism via radioactive tracers (Raichle, 2015). In addition, the neuronal activity of the DMN can be estimated by the electrical activity and magnetic fields associated with this electrophysiological activity via EEG (Foster and Parvizi, 2012) and MEG (Brookes et al., 2011), respectively.
Additionally, Morcom and Fletcher (2007) question the assumption that the DMN reveals substantial information about cognition; however, these early criticisms were based on the simple subtraction design utilized by Raichle et al. (2001) and the scarcity of literature at the time. Furthermore, Morcom and Fletcher concede that controlled experimental manipulations are necessary to further explore the DMN, and the unique experimental conditions that meditation and psychedelics offer have addressed exactly this. Over a decade later, there is a surge in publications suggesting the DMN can offer insight into the variability of human cognition (Brewer et al., 2011; Palhano-Fontes et al., 2015; Raichle, 2015; Mohan et al., 2016; Speth et al., 2016; Smigielski et al., 2019; Zhang and Volkow, 2019; Marstrand-Joergensen et al., 2021; Smallwood et al., 2021; Yeshurun et al., 2021). Furthermore, the DMN has continuously been correlated with the sense of self, ego dissolution, top-down cognitive processes (executive function), cognitive flexibility, awareness, and a number of neuropsychiatric conditions (Andrews-Hanna et al., 2014; Lebedev et al., 2015; Palhano-Fontes et al., 2015; Mohan et al., 2016; Carhart-Harris et al., 2018; Smigielski et al., 2019). For instance, a review by Barrett and Griffiths (2017)hypothesized that decreased activity and FC within DMN hubs such as the PCC and mPFC on administration of classical psychedelics are key facets that mediate the mystical experience via decreased self-referencing and disintegration in the feeling of self. These authors further posit that decreased activity and FC in the inferior parietal lobule (a lateral node of the DMN) is responsible for the feeling of timelessness and spacelessness that often governs the psychedelic experience. Therefore, although Morcom and Fletcher provided valid critiques at the time (2007), current evidence suggests the DMN is a revealing and insightful network that should remain in the purview of cognitive neuroscience and psychedelic research.
Psychedelics are not the only class of drug to alter the DMN. Alcohol also reduces FC within the DMN (Fang et al., 2021). Indeed, this decrease in resting state FC can explain 33% of the variance in alcohol craving in individuals with alcohol use disorder, thereby showing that DMN connectivity profiles/signatures may act as a possible biomarker for this condition (Fede et al., 2019). Fang et al. (2021) found that moderate alcohol consumption acutely and significantly decreased FC within the right hippocampus and right medial temporal gyrus. Unlike psychedelics, alcohol did not significantly affect the inter-network connectivity of the DMN with other cortical networks. Therefore, the acute disruption in intra-network connectivity of the DMN induced by both alcohol and psychedelics may in part explain the euphoric experience characteristic of intoxication with both drugs. The ability of psychedelics to increase global integration and connectivity (via increased internetwork connectivity) juxtaposed with its neuroplastic properties may explain its unique capacity to produce positive therapeutic health outcomes. Interestingly, it does seem that ketamine has the capacity to decrease FC within the DMN (at doses that alter consciousness) and alter the FC between the DMN and other networks (Bonhomme et al., 2016), and it has been argued that neuroplasticity is a convergent mode of action between psychedelics and ketamine (via mechanisms described above) (Aleksandrova and Phillips, 2021). Additionally, findings from Doss et al. (2020) showed that the k-opioid receptor agonist and atypical dissociative Salvinorin A decreased within network FC and increased between network FC. Static and entropic functional connectivity were best predicted by the DMN, questioning the specificity of the entropic brain hypothesis to the classical psychedelics. It is unclear if Salvinorin A elicits the same neuroplastic properties as ketamine and the classical psychedelics and whether this relates to their intra- and internetwork modulation capacity.
Table 2 outlines and summarizes the limitations of each study included in the systematic review, so a brief overview of the common limitations will be discussed. The majority of included studies were, for ethical reasons, conducted on people with prior psychedelic experience and were of small sample sizes. Therefore, it is encouraged that future researchers report effect sizes, as a power analysis by McCulloch et al., (2022) found that sample sizes greater than 60 are needed for adequately powered studies. Strikingly, only 2 of 28 papers had 60 or more participants (Mason et al., 2020, 2021). Furthermore, a number of studies included new analysis on two previously small sample datasets (Carhart-Harris et al., 2012, 2016), which is problematic for a number of reasons. The primary limitation from this sort of analysis is that one would expect that in the absence of any serious fundamental methodological flaw in the dataset that different analytical methods should support the same hypothesis. Thus, we emphasize that these findings are not necessarily independent evidence, though the fact that different analytical methodologies generated similar conclusions is reassuring. Future research should aim to carry out similar analysis on novel datasets, which would provide more robust and reliable evidence for the REBUS and entropic brain hypothesis.
The problem of inferring psychological processes or cognition from patterns of activation via neuroimaging technologies is known as reverse inference. It is not entirely clear as to whether alterations in DMN activity and connectivity are merely a by-product or epiphenomenon of psychedelics or if they play a mediating role in psychedelics’ specific psychological effects and therapeutic benefit. Thus, researchers should be tentative in drawing causal conclusions from correlative evidence.
With any field of research there is a risk of publication bias. In the context of the papers reviewed in this article, when initial articles linking the DMN to various cognitive outcomes were established in prestigious journals (refer Figure 3, Carhart-Harris et al., 2012, 2016, and Palhano-Fontes et al., 2015), it directs the field of psychedelic research and expected findings and hypotheses generated by researchers. Thus, we urge the scientific community to remain wary of overly enthusiastic claims and consider theories as parsimonious models rather than established facts. Pre-print journals such as bioRxiv and PsyArXiv can also be useful in sharing studies that may have difficulty getting published elsewhere due to negative results but can still contribute to the corpus of scientific knowledge. However, it is important to consider that due to the renewed interest in psychedelic medicine, publications with negative findings are still likely to be published and the currently published data have all been attached to registered trials.
Potential Future of DMN Modulation–Focused Psychedelic Therapies
Studies have highlighted the potential application of the intrinsic FC of the DMN as a biomarker. The DMN has been utilized as a biomarker for attention deficit hyperactivity disorder, early antidepressant response, chemotherapy-related brain injury, depression, epilepsy, Parkinson’s disease (Yanbing et al., 2020), bipolar affective disorder, and schizophrenia (Meda et al., 2014). Using resting and task-influenced DMN activity as a cognitive biomarker is aligned with the research domain criteria of the National Institute of Mental Health. Research domain criteria is a research framework for investigating mental disorders and places an emphasis on using different biological and cognitive markers as transdiagnostic tools. Therefore, using the DMN as a biomarker of neuropsychiatric conditions can help overcome many of the limitations often associated with diagnosing these pathologies via symptomatology. These include heterogeneity of symptoms for the same condition and less reliance on the subjective judgment of a clinician.
The REBUS and predictive-coding models may offer explanatory power in terms of psychedelics transdiagnostic potential. Neuropsychiatric conditions are characterized by distinct DMN signature abnormalities, which psychedelics may therapeutically normalize. Little is known regarding how psychedelics influence top-down and bottom-up information-processing streams and whether these pathways converge upon the DMN. Future research might take Bayesian approaches to determine if psychedelics alter hierarchical sensory processing and if this may contribute to updating models of the self and world as proposed in the REBUS model. Moreover, belief system updating may alter top-down processing, with greater cognitive flexibility modulating distinctions of exogenous and interoceptive information. These hypotheses can be directly assessed in future research by examining how nodes of the DMN may modulate thalamic activity via effective connectivity analyses, and vice versa, to gain a deeper understanding about information transfer as demonstrated by Preller et al. (2019).
Micro-dosing of psychedelics (particularly LSD and psilocybin), which generally involves taking sub-hallucinogenic doses, has been reported to lead to decreased mind-wandering (Polito and Stevenson, 2019), which at the neural level is reflected by reduced DMN activity. For instance, a double-blinded placebo-controlled study by Murray et al., (2022)administered low doses of LSD (13 or 26 μg sublingual). Using EEG, scalp electrodes were placed on the midline of the brain to infer source localization for key DMN regions and showed a reduction in broadband oscillatory power, consistent with findings using larger doses. Across all EEG frequencies (delta, theta, alpha, beta, and gamma), the 26-μg dose saw greater reductions in oscillatory power compared with the 13-μg dose. There is, however, currently no direct evidence or clinical trials that show how micro-dosing modulates the DMN, and this should be explored in subsequent research. This can illuminate how much DMN modulation (and its potential downstream benefits) is dependent on the rich subjective experience associated with larger doses of psychedelics. Furthermore, studies could aim at determining a “minimum effective dose,” which would likely cause fewer challenging experiences that may result from administration of high-dose psychedelics. The same rationale also applies to non-hallucinogenic psychedelic analogues, which are currently being explored and have shown anti-depressant effects, at least in rodent models (Cao et al., 2022). However, it is important to note that challenging experiences may also be conducive of positive therapeutic outcomes (Yaden and Griffiths, 2020).
A worthwhile future direction would be the comparison of DMN modulation for experienced psychedelic users and psychedelic naïve individuals because it would help decipher the interaction between the acute and enduring effects of these substances on this network. Alternatively, well-designed longitudinal research involving several scanning sessions could clarify how modulations of DMN FC may reflect the dynamics of lasting effects of psychedelics in brain activity as demonstrated by McCulloch et al. (2022). Interestingly, in this recent study within and between DMN FC differences were not significantly (with low effect size, d = 0.2) modulated at 1-week and 3-months post injection. This study design could be replicated with a greater sample size and additional acute time points to further delineate the acute and enduring connectivity patterns of psychedelic drugs.
Clinically, psychedelics could be used in conjunction with psychotherapeutic techniques such as mindfulness meditation. Mindfulness and psilocybin retreats have shown promising outcomes for people with depression (Carhart-Harris et al., 2017). It seems that mindfulness and psilocybin therapy modulate the DMN in similar ways (a reduction in FC and activity within the DMN) and may act synergistically. This may be because mindfulness, which acts to increase and cultivate non-judgmental awareness of one’s thoughts and feelings, may help facilitate a positive psychedelic experience and vice versa.
Additionally, a legitimate research question in the context of depression is whether infrequent psilocybin treatment (i.e., quarterly) juxtaposed with standard frontline treatments such as cognitive behavioral therapy and selective serotonin reuptake inhibitors such as escitalopram may yield a superior continued reduction in depressive symptomatology. This type of study design could be within the purview of future research scientists and, if effective, could be translated to a variety of other neuropsychiatric conditions. Furthermore, it is important that clinical (and pre-clinical) research promotes a “bench-to-bedside” approach; with scheduled drugs such as psychedelics, there will be a need for government lobbying and policy reform [see Marks and Cohen, (2021) for a roadmap for psychedelic therapy].
CONCLUSIONS
This systematic review provides evidence to support the notion that classical psychedelics are capable of modulating the DMN, which is correlated with ego dissolution, increased brain entropy, and improved mental health and well-being. Our review of the data shows psychedelics are a valuable tool for investigating this network and considers the potential therapeutic implications of this effect. Psychedelics are showing promise as tools to aid our understanding of the brain and mind in greater detail. Finally, this understanding can be harnessed to treat a variety of neuropsychiatric conditions and potentially increase healthy individuals’ psychological well-being.