Abstract
Mu-Opioid Receptors (MORs) are well-known for participating in analgesia, sedation, drug addiction, and other physiological functions. Although MORs have been related to neuroinflammation their biological mechanism remains unclear. It is suggested that MORs work alongside Toll-Like Receptors to enhance the release of pro-inflammatory mediators and cytokines during pathological conditions. Some cytokines, including TNF-α, IL-1β and IL-6, have been postulated to regulate MORs levels by both avoiding MOR recycling and enhancing its production. In addition, Neurokinin‐1 Receptor, also affected during neuroinflammation, could be regulating MOR trafficking. Therefore, inflammation in the central nervous system seems to be associated with altered/increased MORs expression, which might regulate harmful processes, such as drug addiction and pain. Here, we provide a critical evaluation on MORs’ role during neuroinflammation and its implication for these conditions. Understanding MORs’ functioning, their regulation and implications on drug addiction and pain may help elucidate their potential therapeutic use against these pathological conditions and associated disorders.
1. Mu-Opioid receptors
Mu-Opioid Receptors (MORs) belong to the family of Opioid Receptors (ORs), which are metabotropic receptors well-known for binding opioidergic drugs such as morphine and heroin. Other members of the ORs family include Delta (DOR), Kappa (KOR) and opioid receptor like-1 (ORL1). All ORs can bind with differential affinity distinct endogenous (own produced by the organisms) opioids, including endorphins (recognized by MORs), enkephalins (DORs), dynorphins (KORs) and nociceptins (ORL1) (Bodnar, 2022). ORs also differ in their distribution within the nervous system. MORs, DORs and ORL1 are widespread in the central nervous system (CNS), whereas KORs are more restricted to the midline and ventral structures (Allen Institute for Brain Science, 2011). ORs participate in many processes including analgesia, neuroprotection, respiratory control, ionic homeostasis, peristalsis, mood regulation, cardioprotection and sedation, among others (reviewed elsewhere: Shenoy and Lui, 2018).
The effects of endogenous opioids also depend on where in the nervous system they bind their receptors, the cell type they have their effects on, and the specific receptor(s) to which they bind. In particular, they can produce analgesia, euphoria, reinforcement, sedation, dysphoria, miosis, addiction, truncal rigidity, hedonia, aversion, nausea, and reduce the rate of respiration and cough reflex in the CNS (McNicol et al., 2003, Hyman et al., 2006, Le Merrer et al., 2009, Al-Hasani and Bruchas, 2011, Castro and Berridge, 2014a). Interestingly, activation of the different ORs might result in opposite outcomes. Indeed, MORs activation within the mesocorticolimbic system (MCLS) induces euphoria, whereas KORs and DORs activation leads to dysphoric behaviors(Anand et al., 2010). ORL1 participates in pain modulation, anxiety, mood, and memory formation (Thompson et al., 2012). Although these are the main effects of the ORs activation, readers should be aware that ORs might behave differently even when activated with the same agonist and within the same brain area, creating what it is known as ‘hotspots’ and ‘coldspots’ (Hipólito et al., 2008, Castro and Berridge, 2014b, Castro and Berridge, 2017, Al-Hasani et al., 2015, Campos-Jurado et al., 2017).
MORs are widely distributed throughout the brain (see Fig. 1) and participate in many mechanisms regulating CNS functions, including pain processing, reinforcement, and related processes. Due to MORs pivotal role in the reward and pain systems (Cuitavi et al., 2021a), changes in their regulation at either gene, mRNA or protein level might result in disruption of these physiological processes and thus, predispose to pathological state or disorders such as addiction, opioid-induced hyperalgesia, and pain comorbidities. Interestingly, these affections are related to dysregulation of the intracellular signalingcascades triggered by MORs after their activation through G proteins (see Fig. 2). Among the different G protein types, MORs are known to couple with Gi1, Gi2, Gi3, Go1, Go2, Gz and G16, although with different frequency or potency (Connor and Christie, 1999).
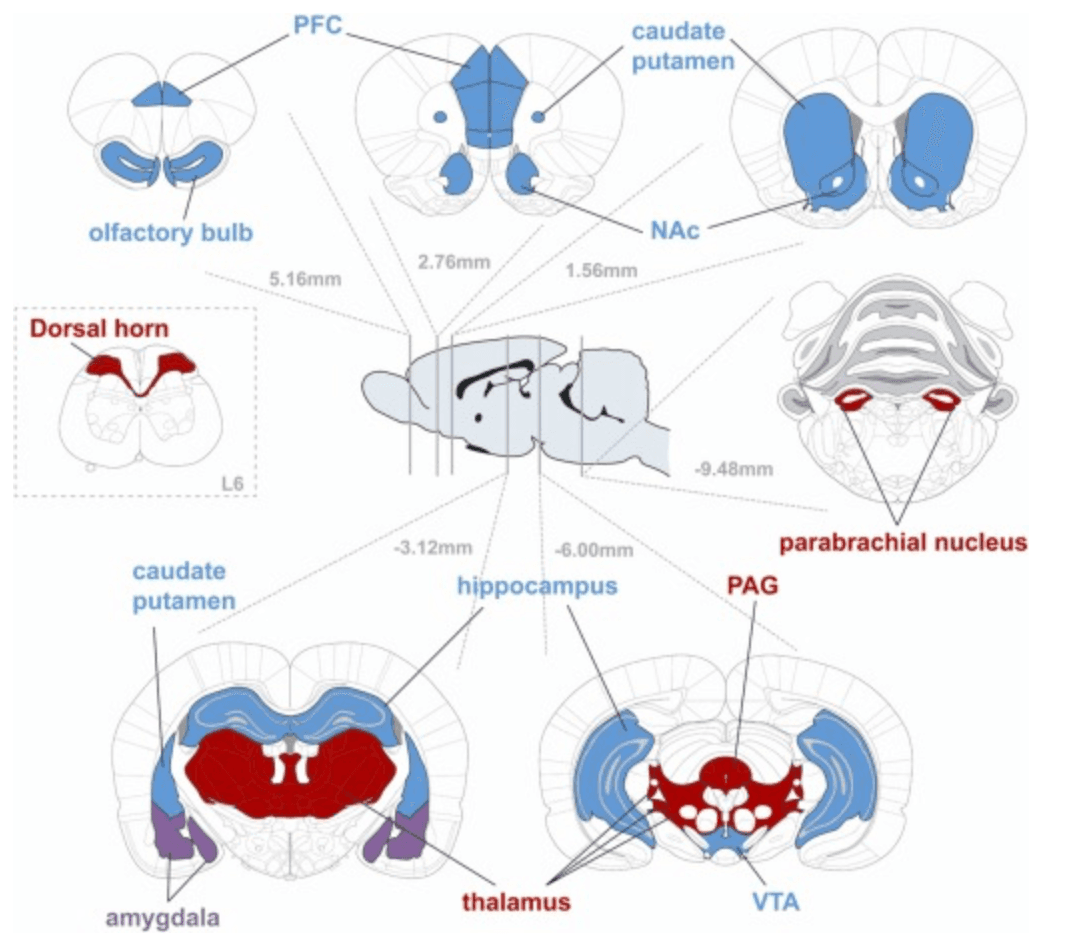
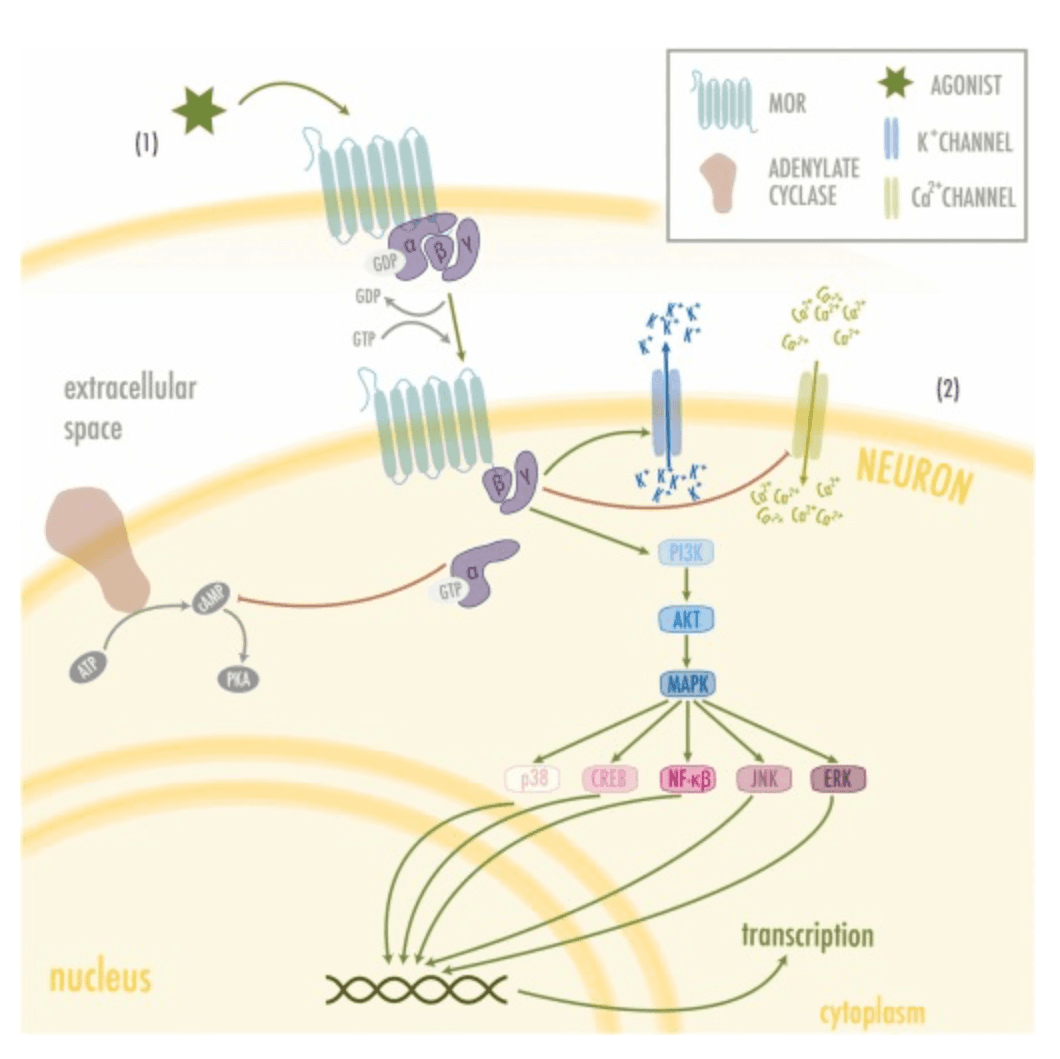
In the last decade, an increasing amount of studies have demonstrated that MORs are implicated in neuroinflammatory regulation (Merighi et al., 2013, Gessi et al., 2016, Cahill and Taylor, 2017, Shrivastava et al., 2017, Reiss et al., 2022). A critical evaluation of the crosstalk between MORs and neuroinflammation, and its implication for drug addiction and pain processing, might help clinical and pre-clinical research to uncover novel therapeutic opportunities for neurological disorders where the endogenous opioid system plays a key role.
2. Neuroimmunity: A new way of understanding MORs
Up until very recently, the brain was conceived as an immune privileged organ. The blood-brain barrier, composed by endothelial cells, astrocytes and pericytes, protects the CNS from pathogens and other chemical substances (Kadry et al., 2020). Nonetheless, the discovery of immune cells and processes within the brain changed this misconception. On the one hand, microglia, resident macrophages in the CNS, stand as the main protectors of the brain. Under non-pathological conditions, microglial cells are responsible for a myriad of functions such as neuronal survival and death, synaptogenesis, and protection against infection (Li and Barres, 2018). On the other hand, astrocytes also play a pivotal role in neuroinflammation. Interestingly, based on the stimuli that activates astrocytes, their response can be protective or detrimental (Colombo and Farina, 2016). MORs seem to be active players in inducing immune responses through glial cells in the CNS. However, in a systemic level, MOR agonistshave traditionally shown immunosuppression and hypothesis about the effect of opioid therapies increasing vulnerability to infection or uncontrolled tumor growth are nowadays under careful evaluation (Plein and Rittner, 2018, Sekandarzad et al., 2017). Thereby, although we focus on the neuroinflammatory processes induced by MOR activation in the CNS in this section we also give a brief update on MOR-mediated systemic immunosuppression.
2.1. MOR and systemic immunosuppression
Opioids have traditionally linked to immunosuppression in a systemic level (Zhang et al., 2020). In fact, there is evidence that they, directly or indirectly, can reduce the activity of a myriad of immune cells such as natural killers, T and B cells, neutrophils, mast cells and dendritic cells, which can be reversed by treating with MORs antagonists. In fact, MOR mRNA and protein has been identified in immune cells at peripheral but also at central level (Machelska and Celik, 2020). This information has been recently reviewed by Plein and Rittner (2018) and Eisenstein (2019), however we present a summary in this section highlighting the most recent data.
Natural killer cells are lymphocytes belonging to the innate immune system, which play a role in limiting the spread of microbial infections and tumors (Vivier et al., 2008). Several reports reviewed at Einsenstein 2019 have shown the suppressive effect of MOR agonists on NK cells activity at peripheral but also when administered directly in the periaqueductal grey nucleus (weber and Pert Science 1989). Interestingly, recent data of Maher et al., 2019, Maher et al., 2020 has increased the knowledge in this specific matter. In the first experiment Maher et al. (2019) showed that opioids such as morphine, methadone and buprenorphine reduced natural killer cells cytotoxicity on K562 tumor cell line in vitro through MORs. Here, authors extracted cells from human plasma and treated them with opioids for 2 h. It is important to notice that when cells were pretreated with naloxone the decrease in the activity was not observed. This very same phenomenon was observed again in a later experiment in which they treated cells with morphine as their opioid of choice (Maher et al., 2020). Paradoxically, authors also observed a MORs-mediated increase in the levels of granzymes A and B, secreted serine proteases involved in apoptotic processes, which were significantly reduced when naloxone was used as pretreatment. Interestingly, IL6, a proinflammatory cytokine, show a tendency to increase in the medium after treating NK cells with morphine in the presence of K562 cells that was completely impaired by the pretreatment with naloxone. Altogether these data may indicate a dynamic response to morphine of the NK cells from initial activation to final inhibition of its cytotoxic properties that deserved to be further investigated. It is also important to remark that Maher et al., 2019, Maher et al., 2020 only used NK isolated from male humans’ plasma and it would be interesting to study is the same changes can be found in women. Moreover, some opioids seem to be more immunosuppressive than others. In fact, oxycodone, hydromorphone, and buprenorphine do no alter natural killer cells activity (Meserve et al., 2014) and tramadol even increases it (Santamaria et al., 2010).
B lymphocytes belong to the adaptative immune system and oversee antibody production and immune memory through their different phenotypes (Sharonov et al., 2020). D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO), a synthetic MOR agonist, reduces antibody production, which is reversed by the addition of naloxone (Reviewed at Eisenstein, 2019). Moreover, morphine decreases the expression of major histocompatibility complex class II (MHC-II), essential to recognize pathogens, in B cells (Plein and Rittner, 2018). However, a gap in the literature aiming the specific pathways by opioids alter lymphocytes B function makes it difficult to understand the effect of opioids, chronic or acutely given in antibody production. Together with this reported action in B lymphocytes, opioids have also shown to alter T lymphocytes function. T lymphocytes, which are adaptive immune system pathogen presenter cells, are also compromised when an opioid is present. In fact, morphine inhibits T lymphocyte-mediated apoptosis and alters T cells differentiation and function (Roy et al., 2011). Other opioids such as methadone decreased the expression of the CD8 + T markers of activation CD69 and CD25 in human patients (Mazahery et al., 2020). Finally, sufentanil, an opioid used in postoperative analgesia, has been shown to reduce the levels of different phenotypes of T cells in rats, including T helpers and regulatory T cells (Peng et al., 2020).
Regarding the data presented in this section, it is important to pinpoint that, although there is evidence of MOR-induced systemic immunosuppression, this subject is very complex and holds very variables. On top of the aforementioned differences found in natural killer cells activity when different opioids are used, there are further data suggesting that opioid could act systemically without promoting immunosuppression. Some studies using breast cancer mouse models observed that morphine did not promote tumor growth or angiogenesis (Doornebal et al., 2013, Doornebal et al., 2015). In fact, there is a lack of evidence regarding opioid-induced metastasis (Hooijmans et al., 2015; Sekandarzad et al., 2017) or opioid increased incidence of infection (Häuser et al., 2015). Taking all of this into account, and as Plein and Rittner (2018) pointed out in their review “there is not strong enough data to establish a clinical relevance of opioid-induced immunosuppression”. Further research is required to shed light on this specific topic by investigating which opioids, which doses and the temporal dynamics that may lead to immunosuppressive effect of these medications. This information can help to design the best therapeutical approach.
2.2. MORs and neuroinflammation
Many of the functions carried out by microglia and astrocytes involve neuroinflammatory-mediated signaling cascades which promote oxidative stress and regulate inflammatory cytokines, chemokines, and mediators through the action of the toll-like receptors (TLRs) (Kumar, 2019). Although opioids have been classically posed as immunosuppressors at systemic but also in the nervous system (central and peripheral), data available are contradictory. Curiously, recent research places microglial MORs as neuroinflammation promoters in the CNS as summarized in Table 1(Merighi et al., 2013, Gessi et al., 2016, Cahill and Taylor, 2017, Shrivastava et al., 2017, Zhang et al., 2020, Reiss et al., 2022). However, the mechanisms by which they function remain unclear, since the relation that connects the TLR and the MOR pathway, although hinted, is yet to be scientifically proven (see Fig. 3) (Zhang et al., 2020). Nonetheless, the PKCe–Akt–ERK1/2 pathway stands out as the best candidate to connect both receptors. In an in vitro experiment where microglia cell cultures were treated with both lipopolysaccharides (LPS, a model of inflammation) and morphine, it was reported that this pathway, which leads to the release of inflammatory mediators, reached its maximal activation 10 min after the treatment (Limiroli et al., 2002). Furthermore, this morphine-induced activation was higher than that of LPS alone (Merighi et al., 2013). Similarly, Gessi et al. (2016) found that morphine increases NF-κB translocation to the nucleus in microglia cell cultures activated with LPS. Moreover, IKappaB Kinases (IKKs) activation was higher when morphine or DAMGO were added alongside LPS. It is important to notice that, in all these cases, morphine and DAMGOseem to increase an existing neuroinflammatory response and fail to induce one on their own. In this sense, both Merighi et al. (2013) and Gessi et al. (2016) suggest that MORs activation might be enhancing TLR-promoted response by an upregulation of proinflammatory cytokines. Nonetheless, there is evidence that these responses were pharmacologically blocked with Naloxone, a MOR antagonist, and by silencing MOR by using siRNAµ.
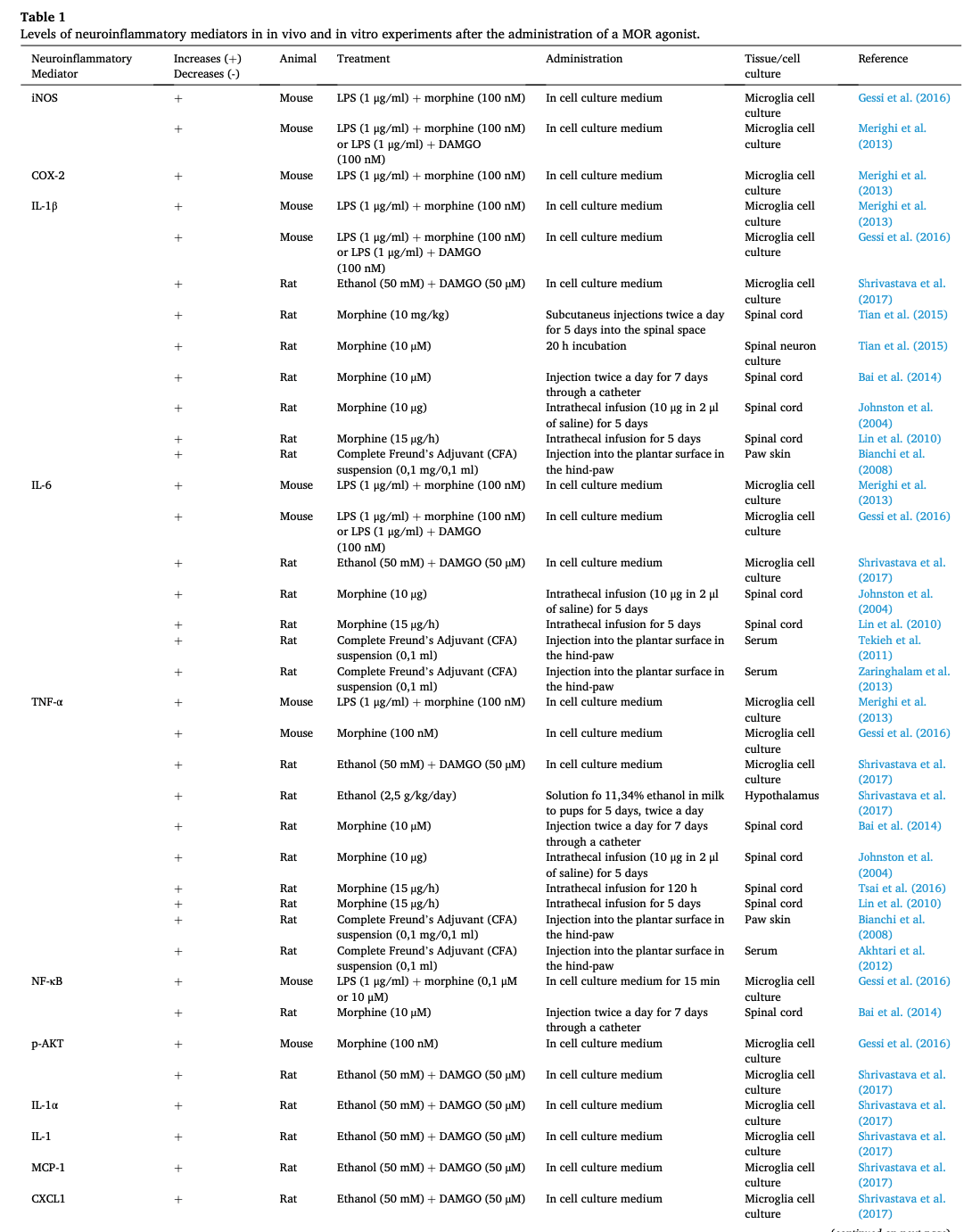
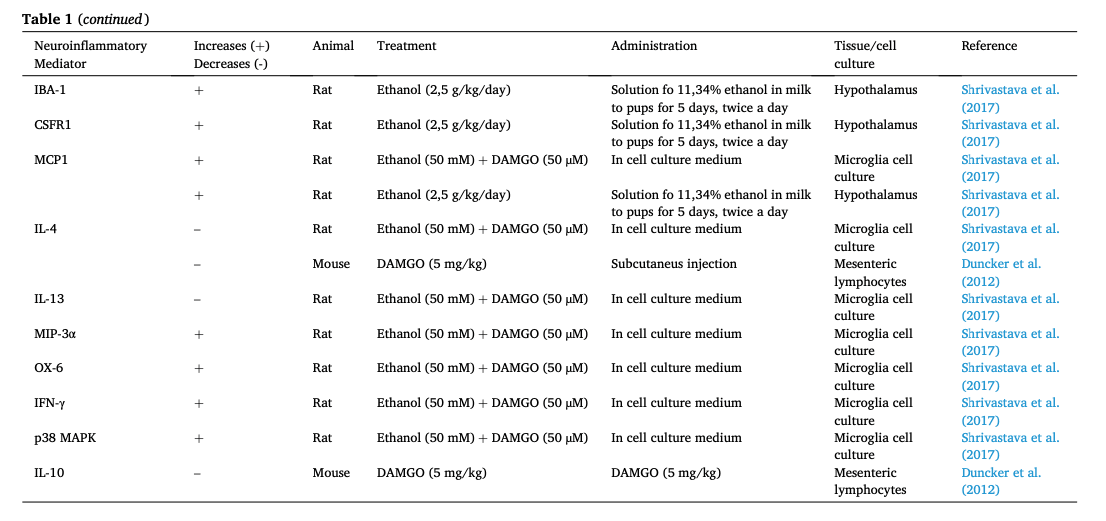
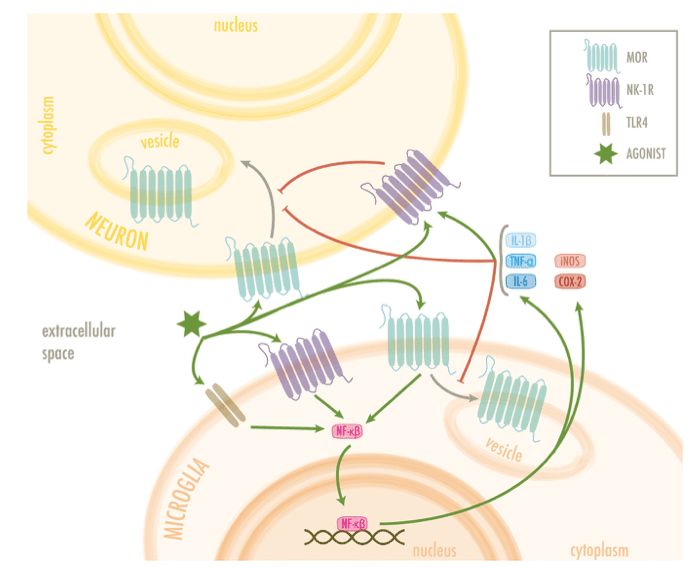
Further research points at pro- and anti-inflammatory cytokines as markers confirming the relationship between MORs and neuroinflammatory events. Shrivastava et al. (2017)found that some proinflammatory cytokines such as TNF-α, IL-1β and IL-6, known to promote inflammation, were upregulated, whereas the anti-inflammatory cytokines IL-4 and IL-13 were downregulated in primary microglia cell cultures from rats treated with DAMGO. These findings were further validated in vivo in the hypothalamus of rat pups. This very same study also used ethanol, which stimulates immune responses (Cantacorps et al., 2017) and promotes MOR activation through several mechanisms (Reviewed by Peana et al., 2017), obtaining a similar outcome. Other studies carried out in different immune cell types show the downregulation of anti-inflammatory interleukins when MORs are activated. Concretely, Duncker et al. (2012) described how mesenteric lymphocytes obtained from mice subcutaneously treated with DAMGO showed lower levels of IL-4 and IL-10. Another study observed how acute dosage/administration of morphine in vivo in a mice model inhibited the production of IL-10 in thioglycolate-elicited peritoneal macrophages when treated with LPS (Limiroli et al., 2002), although this study only shows how morphine is acting again as a potentiator of LPS. MOR activation also seems to be related to astrocytosis. Sil et al. (2018) demonstrated both in vitro and in vivo that morphine induces astrocyte activation and neuroinflammation, which are reverted after a treatment with naltrexone. Moreover, this mechanism has been proposed to promote morphine tolerance via extracellular vesicles signaling (Ma et al., 2021).
Notwithstanding this information, there are different studies that suggests that an acute or chronic treatment with morphine induces glial immunosuppression by different mechanisms. In vitro studies showed that morphine acute treatment impaired chemotaxis and release of pro-inflammatory cytokines or chemokines in microglia and astrocytes cultured cells (Chao et al., 1997, Hu et al., 2000, Mahajan et al., 2002, Mahajan et al., 2005). Very recently Peng and colleagues showed that morphine chronic treatment impaired lysosomal activity and reducing mitophagy both in vitro and in vivo (Peng et al., 2022).
2.3. Role of neuroinflammation as MORs expression and trafficking controller
The aforementioned neuroimmune mechanisms seem to correlate with MORs expression. Although cytokines’ role regulating the gene expression of MORs was previously described in different cell types (Ruzicka et al., 1996, Vidal et al., 1998, Kraus et al., 2001, Kraus et al., 2003, Börner et al., 2004), it was not until the last decade that this was further confirmed. Langsdorf et al. (2011) stimulated cultured macrophages (line TPA-HL-60) with LPS and, as expected, cytokines and Reactive Oxygen Species(ROS) were upregulated in the cell medium. Afterwards, they treated SH-SY5Y cells, which are a neuroblastoma cell line, with that medium and found an increase in the MOR messenger levels. Finally, Byrne et al. (2012) treated U97 MG human astrocytomacells with IL-1β and observed an upregulation of both mRNA and protein levels for MOR. This study also showed that morphine downregulates the levels of MOR in the membrane of the astrocytoma cells. However, those levels were restored when IL-1β was administered. Although no mechanism has been proposed for this event, it is known that the presence of Il-1β reduces the amount of the G Protein-Coupled Receptor Kinase 2 (GRK2) when it is released, producing allodynia (Kleibeuker et al., 2008, Willemen et al., 2010). Therefore, since GRK2 is a kinase phosphorylating MORs and that is needed for MORs’ internalization (Møller et al., 2020), this suggests that the release of IL-1β prevents MOR from being endocytosed.
Another possible mechanism for neuroinflammation-induced MOR alterations involves Neurokinin Receptors (NKRs). These receptors, which are G protein coupled receptors, spread all along the nervous system and are involved in the regulation of stress, pain, muscle contraction and inflammation responses (Almeida et al., 2004). NK1Rs, a type of Neurokinin receptor, are closely related to MORs. In fact, MOR activation has been suggested to inhibit the release of Substance P, an agonist of NK1R (Xiao et al., 2016). Furthermore, in some instances, the activation of NK1R seems to result in the release of pro-inflammatory mediators (Zieglgänsberger, 2019). For example, treating RAW 264.7 cells (murine macrophages) with Substance P at nanomolar concentrations activates intracellular pathways (ERK1/2 and p38 MAPK) leading to a release in pro-inflammatory mediators (Sun et al., 2008). Additionally, NK1Rs can regulate MORs’ availability stage by endosomal encapsulation when activated (Schmidlin et al., 2002, Bowman et al., 2015). Similarly, NK1Rs can be upregulated by the action of IL-1β (Guo et al., 2004). Nonetheless, the stated MOR-NK1R relationship remains a hypothesis that needs further confirmation.
3. Neuroimmunity as a driver of drug addiction
Drug addiction is a neuropsychiatric disorder characterized by a recurring desire to continue taking the drug despite harmful consequences (Liu and Li, 2018). There are many substances that can lead to addiction including both legal and illegal drugs, such as cannabis, opioids, alcohol, cocaine, tobacco, caffeine, etc. (American Psychiatric Association, 2013). All of them activate a common neurochemical pathway in the mesocorticolimbic system (MCLS) due to their properties as reinforcers (Liu and Li, 2018). This system, which processes reward and aversion, is one of the most affected neuronal circuits in disorders associated with use of drugs. Natural reinforcers activate this system to facilitate behaviors that increase survival (Fields et al., 2007). In order to do so, the MCLS comprises brain areas that play a crucial role in reward management. Concretely, Nucleus Accumbens (NAc), Prefrontal Cortex (PFC), extended amygdala, Ventral Tegmental Area (VTA), hippocampus and hypothalamus are the main brain structures that shape the neural reward circuitry (Koob and Volkow, 2016).
Opioid receptors in the MCLS play an important role in modulating reward responses when activated by both endogenous and exogenous opioids (Darcq and Kieffer, 2018). Interestingly, exogenous opioids can disrupt MCLS’s normal activity. Opioids such as morphine or heroin can bind to MORs at GABAergic neurons of the VTA (Fields and Margolis, 2015). MORs’ activation inhibits GABAergic neurons, which in turn disinhibits VTA dopaminergic neurons. Thus, opioid receptors activation increases the release of dopamine within the NAc, which is the main target of VTA dopaminergic neurons (Le Merrer et al., 2009). In addition, MORs agonists have also shown to modulate DA extracellular levels within the NAc, thus regulating DA release at terminal level (Hipólito et al., 2008). Depending on the subarea studied within the NAc, the administration of DAMGO differentially regulated DA extracellular levels, suggesting that MORs might be located in a different neuron type or location in the shell and in the core of the NAc. The increase in the dopaminergic activity accounts for the reinforcing effect, which is essential in opioid and other drugs reinforcing events that ultimately are crucial for the development of addiction (Becker and Chartoff, 2019).
3.1. The role of cytokines in the MCLS for drug addiction
Multiple drugs can promote the release of neuroinflammatory mediators within different areas from the MCLS (Cahill and Taylor, 2017, Cantacorps et al., 2017). Similarly, the expression of different cytokines can also interfere with the process of drug addiction. IL-2 participates through modulating the sensitivity of the dopaminergic circuitry within this system (Zalcman, 2001). IL-6 is essential in promoting methamphetamine-induced dopamine neurotoxicity (Ladenheim et al., 2000). Another cytokine that interferes in the MCLS is TNF-α (Lewitus et al., 2016, Norlin et al., 2021). Pre-treatment with an intraperitoneal (i.p.) injection of TNF-α in mice was able to reduce the extracellular striatal dopamine release evoked by methamphetamine (Nakajima et al., 2004). Likewise, markers of neuroinflammation are related to stress and depression (Maydych, 2019). Krasnova et al. (2016) demonstrated that methamphetamine self-administration in rats triggered neuroinflammation in the striatum. They also suggest that this event could be related to the classical neurocognitive dysfunctions found in human methamphetamine users including depression. Moreover, Cahill and Taylor (2017) suggested that events associated with neuroinflammation within the MCLS may trigger depression.
Multiple studies have demonstrated that predisposition to addiction by drugs of abuse has a significant genetic component (Marcos et al., 2008, Bevilacqua and Goldman, 2009, Liu et al., 2009). Accordingly, some studies have found genetic associations between neuroinflammation and drug addiction. On the one hand, a study comparing the IL-1β gene from 60 opioid addicts versus 60 healthy people showed a significant difference in two different single nucleotide polymorphisms (SNPs), both associated with an increased production of IL-1β. Those SNPs were found at a higher frequency in opioid addicts, thus suggesting neuroinflammation plays a role in addiction (Liu et al., 2009). On the other hand, another SNP at the IL-10 gene promoter seems associated with a decrease in this anti-inflammatory interleukin. One study quantified the amount of people with this polymorphism in both alcoholic patients and healthy controls. Interestingly, this SNP was more present in alcoholics than in healthy people (Marcos et al., 2008).
3.2. Neuroinflammation: The key to understanding drug relapse and tolerance?
Recent studies have provided a direct connection between neuroinflammation and relapse (Brown et al., 2018, Berríos-Cárcamo et al., 2020, Cuitavi et al., 2021b, Fernández-Rodríguez et al., 2022). Glial cells are involved in synaptic plasticity; they modulate the effects of drugs of abuse and vice versa (Jiménez-González et al., 2021). Moreover, microglial activation has been related to addiction and to an increased expression of cytokines (Schwarz and Bilbo, 2013, Loftis and Janowsky, 2014, Taylor et al., 2016, Cantacorps et al., 2017). Therefore, 3-isobutyryl-2-isopropyl pyrazolo-[1,5-a] pyridine (ibudilast), which is an anti-inflammatory drug that decreases glial activation, has been used in the past to prevent relapse into drug use disorders (Linker et al., 2019). Hutchinson et al. (2009) also used ibudilast in Sprague–Dawley rats and discovered that it attenuated the pro-inflammatory cytokine release induced by morphine and, therefore, it reduced opioid withdrawal. Additionally, studies in humans showed that heroin craving was significantly reduced when patients were treated with ibudilast(Metz et al., 2017). In another investigation, a traumatic brain injury in the frontal cortex in Long-Evans rats led to less IL-10 when compared to controls. Interestingly, animals were more prone to relapse into cocaine consumption as assessed by the operant conditioning chambers paradigm (Vonder Haar et al., 2018).
Curiously, there is an increase of pro-inflammatory cytokines in morphine tolerance rats (Hutchinson et al., 2008), and the inhibition of the expression of pro-inflammatory mediators restores the effect of opioids (Raghavendra et al., 2004) thus suggesting they participate in the development of opioid tolerance. Furthermore, the PKCɛ pathway alongside Akt and ERK phosphorylation are involved in an increase in the release of pro-inflammatory mediators in microglia due to opioid administration (Zheng et al., 2011, Merighi et al., 2013), since PKCɛ inhibition can reduce opioid tolerance (Smith et al., 2007). Moreover, a chronic morphine treatment also induces the activation of the PI3k/Akt signaling pathway by interacting with MORs, which produces an increase in IL-1β levels and the development of morphine tolerance (Xu et al., 2014, Tian et al., 2015).
All things considered, morphine tolerance is enhanced when combined with glial activation since the levels of pro-inflammatory cytokines are higher than with just glial activation. These pro-inflammatory cytokines are produced via NF-kβ through different pathways that are activated by morphine and nociceptive stimulation, and alter MOR transcriptional levels (Zhou et al., 2021).
4. Pain-derived neuroinflammation: role of MORs
Pain, defined as an “unpleasant sensory and emotional experience associated with actual or potential tissue damage” by the International Association for the Study of Pain (Raja et al., 2020), is a very complex function of the nervous system. Physiological pain is needed to promote survival, and it must be well regulated since both down- and up-regulation can be detrimental (Breivik, 2008). When pain persists beyond the healing process (3 months or more) and is no longer physiologically relevant, it can be considered chronic (Nicholas et al., 2019).
Chronic pain is one of the most prevalent illnesses in developed countries, with an increasing number of patients over the years (Reid et al., 2011, Breivik et al., 2013, Souza et al., 2017, Steingrímsdóttir et al., 2017). Moreover, chronic pain shares comorbidities with other illnesses, such as depression, anxiety, sleep disturbances, drug use disorders, and lack of energy, all of which interfere with people’s quality of life (Banks and Kerns, 1996, Anisman and Hayley, 2012, Tajerian et al., 2014, Hipólito et al., 2015, Lorente et al., 2022a). Interestingly, the number of sufferers increases with age, and it is more prevalent in women. Thus, chronic pain represents a disruption in the normal life for many individuals and a significant economic burden for our societies (Kurita et al., 2012, Landmark et al., 2013, Sá et al., 2019).
Despite the recent number of reviews on pain, its mechanism, its comorbidities, and potential therapeutics (Garcia-Larrea and Quesada, 2022; Haanpää and Treede, 2022; Hu et al., 2022; Lorente et al., 2022b; Song et al., 2022), the crosstalk between neuroinflammation, opioids and pain remains poorly understood. Nonetheless, numerous in vivo experiments in rats have shown that morphine administration provokes the release of inflammatory mediators in the dorsal horn of the spinal cord, which is a key area in pain management (Johnston, 2004; Bai et al., 2014; Tsai et al., 2016; Eidson and Murphy, 2019). Specifically, Bai et al. (2014) injected 10 µg of morphine twice a day for 7 days to Sprague Dawley rats through a catheter located into the dorsal horn of the spinal cord. After that period rats were sacrificed, and the levels of some proteins were measured by western blotting. Results showed that morphine administration triggered the inflammatory pathway; there was an increased translocation of NF-κB to the nucleus and higher levels of TNF-α and IL-1β. The authors suggested that this effect is not only promoted by MOR, but also by TLRs, since its blockage diminished the effect of morphine administration.
4.1. Neuroinflammation to understand MOR-based Opioid-induced hyperalgesia and tolerance
Nowadays, opioids are the best analgesic treatment for chronic pain. However, prolonged opioid administration can induce different adverse effects including tolerance, opioid-induced hyperalgesia (OIH) and addiction (Furlan and Murphy, 2022). It has been reported that the development of opioid tolerance leads to higher levels of opioid consumption, which in turn enhances OIH in a process that seems to be modulated by neuroinflammatory mechanisms. Raghavendra et al. (2002) demonstrated a positive synergistic effect by which the neuroinflammation induced by neuropathic pain and morphine treatment was higher than that produced just by neuropathic pain. Therefore, this suggests that opioid treatment can aggravate the neuroinflammation produced by certain pathologies.
As previously mentioned, opioid treatments can produce neuroinflammation without the presence of pain. Furthermore, spinal glial activation in neuropathic pain and opioid treatment accounts for the release of pro-inflammatory cytokines, which leads to a reduction in the antinociceptive effect of morphine or morphine tolerance (Song and Zhao, 2001, Watkins et al., 2005, Tsai et al., 2008). In fact, IL-1β inhibition allows morphine to have its antinociceptive effect, showing the role of pro-inflammatory cytokines in opioid tolerance and in neuropathic pain (Johnston et al., 2004, Shavit et al., 2005, Sung et al., 2005, Watkins et al., 2005). Moreover, naloxone, a MOR antagonist, reduced morphine tolerance and the levels of spinal TNF-α, IL-1β and IL-6 in animals that followed an opioid treatment (Lin et al., 2010). Additionally, IL-10 in spinal cord induces nociception since it inhibits spinal glial activation, decreases the production of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), and attenuates morphine tolerance (Milligan et al., 2006, Watkins et al., 2007, Lin et al., 2010). Altogether, these results suggest that MORs activation produces an increase in pro-inflammatory mediators, which leads to a major sensitivity towards a noxious stimulus.
One of the main causes of OIH is cytokine signaling to neurons of the CNS (Vanderwall and Milligan, 2019). Cytokines can cross the blood brain barrier, attributable to an increased permeability produced by neuroinflammation (Matsuda et al., 2019), and then emit signals through brain endothelial cells or through glossopharyngeal and vagus sensory nerves (Watkins and Maier, 2005). In an animal model of arthritis produced by a Complete Freund’s Adjuvant (CFA) injection in the knee of the animal, three cytokines, TNF-α, IL-1β and IL-6, were upregulated, and this increase was associated with hyperalgesia via MORs regulation (Bianchi et al., 2008, Tekieh et al., 2011, Akhtari et al., 2012, Zaringhalam et al., 2013). Moreover, blocking these cytokines reduced hyperalgesia (Delery and Edwards, 2020). Additionally, at least one part of its anti-hyperalgesic effects is mediated by an increased MORs spinal expression (Tekieh et al., 2011, Akhtari et al., 2012, Zaringhalam et al., 2013). By blocking IL-6 the anti-hyperalgesic effects are only present in the seven first days of treatment. In the second and third weeks of treatment the anti-IL-6 has a hyperalgesic effect which indicates that IL-6 regulates hyperalgesia in a time dependent manner (Tekieh et al., 2011). In agreement, it has previously been shown that certain analgesic drugs (i.e. Bisphosphonate ibandronate) can lead to a reduction in hyperalgesia and release of pro-inflammatory cytokines produced by CFA (Bianchi et al., 2008).
The release of pro-inflammatory cytokines seems to result in reduced MOR expression. Conversely, the activation of MORs produces an upregulation of pro-inflammatory cytokines. An in vitro study has demonstrated that a c-Jun NH2-terminal kinase (JNK) inhibition increases MORs gene expression, and it can be reversed by inhibitors of P38 MAPK and NF-kβ (Wagley et al., 2013). An in vivo study has shown that JNK activation regulates G proteins coupled to opioid receptors, which can interfere with the tolerance produced by DAMGO (Melief et al., 2010). Furthermore, it has been observed in spinal astrocytes that the activation of the JNK pathway by an acute ultra-low dose of morphine leads to thermal hyperalgesia (Sanna et al., 2015). Additionally, inhibition of either NF-kβ or MEK1/2, when in combination with morphine administration, attenuates neuropathic pain development and thermal and mechanical hyperalgesia in another murine model (Popiolek-Barczyk et al., 2014). Therefore, this suggests that the relationship between hyperalgesia and neuroinflammation is bidirectional.
5. Conclusion, limitations and way forward
This review presents an updated summary of current discoveries on MORs and their role in neuroinflammatory processes. In just the last 10 years, this field of research has seen an exponential growth, which indicates that elucidating the relationship between MORs and neuroinflammation poses a significant interest to basic and translational researchers as well as clinicians. Elucidating MOR-neuroinflammation relationship will serve as a promising venue for better personalized and accurate treatments for pain, drug addiction, and other MOR-related conditions. Importantly, neuroinflammatory mediators seem to regulate MORs presence on the cell membrane through positive feedback. This relationship is of special relevance to better assess and treat associated phenomena such as drug addiction, OIH, and opioid tolerance.
New treatments targeting the MOR-neuroinflammation relationship to address addiction and tolerance find a great limitation. In this reviewer, we have emphasized the dualistic nature of MOR activation, which can result in immunostimulation and/or immunosuppression. The outcome of how an opioid has an effect in the immune system might be different in and out of the CNS and have different consequences for patients treated with opioids, which is a huge limitation in the existing literature. In particular, this issue is a matter of debate among anesthesiologist and surgeons since the use of perisurgical opioids for tumor removal, could result in immunodepression. The same applies when alleviating a cancer-induced pain condition or a non-malignant pain treated with opioids. In this sense, finding new ways of safely administering opioids including the idea of targeting specific regions will be of key interest. Be that as it may, further research is warranted to verify the clinical relevance of the crosstalk between opioids and immune system observed in vitro and in vivo using animal models.
Moreover, in order to further understand this relationship, the first and most important aspect that must be elucidated is the specific link(s) connecting MORs and the TLR pathways. As suggested by Zhang et al. (2020), the PKCe–Akt–ERK1/2 is the main candidate, although further research is required to find out which molecule, or composition of several molecules are playing a key linker role. Additionally, it is of crucial importance to ascertain possible sex-dependent factors which could have an impact on the above-mentioned aspects. Traditionally, there has been a huge gap in many scientific fields regarding potential sex differences and this one is not an exception, with only a few recent research papers accounting for this factor (Shrivastava et al., 2017, Doyle and Murphy, 2018, Cuitavi et al., 2021b). Moreover, as shown in this review, nearly all the articles presented work with either cell cultures or animal models (see Table 1). Nonetheless, results seem promising and establish the basis of the crosstalk between MORs and neuroinflammation, which positively impacts its translational value. Therefore, the next step should be confirming this crosstalk in humans and assess its impact on addiction and pain conditions.