Abstract
Comorbidity rates in Bipolar disorder rank highest among major mental disorders, especially comorbid substance use. Besides cannabis, alcohol is the most frequent substance of abuse as it is societally accepted and can be purchased and consumed legally. Estimates for lifetime comorbidity of bipolar disorder and alcohol use disorder are substantial and in the range of 40–70%, both for Bipolar I and II disorder, and with male preponderance. Alcohol use disorder and bipolarity significantly influence each other's severity and prognosis with a more complicated course of both disorders. Modern treatment concepts acknowledge the interplay between these disorders using an integrated therapy approach where both disorders are tackled in the same setting by a multi-professional team. Motivational interviewing, cognitive behavioral and socio- therapies incorporating the family and social environment are cornerstones in psychotherapy whereas the accompanying pharmacological treatment aims to reduce craving and to optimize mood stability. Adding valproate to lithium may reduce alcohol consumption whereas studies with antipsychotics or naltrexone and acamprosate did not affect mood fluctuations or drinking patterns. In summary, there is a continuous need for more research in order to develop evidence-based approaches for integrated treatment of this frequent comorbidity.
Epidemiology of Bipolar Disorder and Substance Use Disorder
Both bipolar affective disorder (BD) and substance use disorder (SUD) are wide-spread in the general population. Most epidemiological and treatment studies were conducted according to DSM-IV or ICD-10 criteria that distinguishes between substance abuse and dependence as diagnostic entities on its own. Depending on the diagnostic system (ICD or DSM) used and subject sample studied, bipolar affective disorder (BD) in the general population has a lifetime prevalence between 1.3 and 4.5% (1). The World Health Organization World Mental Health Survey Initiative (2) conducted across eleven countries reported a 4.8% lifetime prevalence of all manifestations of bipolarity, including subthreshold and spectrum disorder.
Looking at specific countries, a representative survey applying the Composite International Diagnostic Interview [CIDI (3)] for ICD 10 and DSM-IV criteria reports a 1-year prevalence rate of 1% for BD -I and 0.6% for BD-II disorder for Germany (4). The same study reports on a 1-year prevalence of 5.7% for substance abuse (except nicotine) according to DSM-IV criteria. Three percent fulfilled criteria for alcohol dependence and 1.8% for abuse (4). In a prior survey, looking at lifetime prevalence rate, the same group reports on similar numbers for BD, and 9.9 and 8.5% for alcohol abuse and dependence, respectively (5). These numbers are in a similar range as in other European countries; while prevalence rates from the US are much higher, both for BD and substance abuse/dependence (6). Whereas numbers for legal substances, e.g., alcohol, are considered as relatively robust and reproducible, many cases of illicit drug use remain undetected in patients with BD. Cannabis is likely to be second after alcohol as substance of abuse in BD patients, affecting approximately one quarter of bipolar patients (7).
Whereas, the incidence of BD across countries and cultures is within a similar range, reported rates for AUD differ considerably due to cultural and religious diversity. For example, a representative household survey in Iran found a 12-month prevalence of alcohol use disorders of 1% according to DSM-IV criteria and 1.3% according to DSM-5, with higher prevalence rates in urban vs. rural areas (8). For comparison, a recent US household survey reports a 12-month prevalence of DSM-5 AUD of 13.9% (9).
SUD comorbidity is not exclusive to adult bipolar patients but starts early in life. Pediatric onset BD rarely occurs in the absence of comorbid conditions, and the co-occurrence of additional disorders complicates both the accurate diagnosis of BD and its treatment. Manifestation of BD in children and adolescents is not as infrequent as previously assumed, with rates of bipolar spectrum disorder reaching an estimated 4%, especially in US samples (10).
In the meantime, DSM-5 (11) abolished the distinction between substance use, abuse and dependency by defining threshold numbers of criteria for different grades of severity of substance use. Of the 11 criteria, 2–3 should be fulfilled to diagnose mild alcohol use disorder (AUD) (12). Also, BD criteria experienced some adaptions with yet speculative consequences for epidemiological figures. Whereas, criteria for a manic episode were tightened (13, 14) preceding substance use per se is no more an exclusion criterion for a genuine BD diagnosis as long as the mental alterations exceed well the physiological effect of the substance. This may change figures of future epidemiological studies on SUD and BD comorbidity to some degree.
Both disorders follow a chronic course and considerably impair social functioning and quality of life (15–17), general health and ultimately life expectancy (18–20). Antecedent SUD has been associated with earlier age of onset of BD (21) and a greater need of hospitalization at onset of BD (22). In addition, both disorders have a significantly increased rate of suicides and suicide attempts with an added risk in case of coexistence of both disorders (23–25).
Comorbidity of Bipolar Affective Disorders in Alcohol Use Disorder Patients
Among mental health disorders, BD has probably the highest risk of having a second, comorbid DSM -IV axis I disorder (26). Epidemiological data from the US report life-time prevalence rates of up to 90% for comorbidities in BD (6), with 62.3% for AUD (39.1% for DSM-IV alcohol abuse and 23.2% for alcohol dependency) followed by cannabis (46%), cocaine (24%) and opioids (8.5%) (27). The already cited WHO census across 11 countries showed a mean SUD life time comorbidity with BD of 36.6% with a large variation between countries (2). A meta-analysis including nine national surveys conducted between 1990 and 2015 revealed a mean prevalence of 24% for AUD and of 33% for any SUD except nicotine (28). Analyzing SUD and bipolar comorbidity in clinical settings, the same group reports the highest prevalence for AUD (42%) followed by cannabis use (20%) and any other illicit drug use (17%) (21). Cannabis ranking second after AUD has also been confirmed in other studies (7, 27, 29). Similar rates of SUD were also reported in the Systematic Treatment Enhancement Program Bipolar Disorders (STEP BD) study including 3,750 Bipolar I or II patients (30).
Our own study in BD patients recruited from the Stanley Foundation Bipolar Network (SFBN) outpatient clinic with more comprehensive care found life-time SUD in 42% and AUD in 33% of BD patients (31). Secondary analysis of this data examined the gender-specific relationships between AUD and BD. In line with epidemiological catchment area studies (26), absolute numbers of AUD in males with BD were higher (49 vs. 29%), however, the relative risk of suffering from AUD was significantly greater for women with BD [odds ratio (OR) = 7.35] than for men (OR = 2.77) in relation to the general population (32). Among other factors such has higher rates of depression, previous trauma might play a role for the higher OR in female BD patients; additional analysis in a larger sample of SFBN patients revealed that bipolar women with AUD had also a significantly higher rate of post-traumatic stress disorder (PTSD) than those without AUD (33).
The risk of developing comorbid SUD is obviously much higher in BD than in Major Depressive Disorder (MDD) suggesting that depressive mood is not the main driver of comorbidity in BD, but other factors imminent to BD, such as personality, early onset and impaired functionality, might play a decisive role. The OR for developing a SUD has been estimated 1.8 in patients with a lifetime MDD and 6.9 for those with a lifetime BD-I, compared with the general population (34), and prevalence rates for SUD are ~25–50% higher in BD-I than BD-II patients (26, 35). The latter appears to be mainly driven by illicit drugs (OR 7.46 in BD-I and 3.30 in BD-II) (28). For AUD, however, a recent meta-analysis of 22 studies showed no difference between BD-I (OR 3.78) and BD-II (OR 3.81) (28). A recent catchment area study in Northeast England found a 40% lifetime comorbidity between BD II and AUD, surprisingly with little difference between female (38%) and male (43%) subjects (36).
As mentioned, there is a wide variation of prevalence rates for BD-SUD comorbidity across countries (2) with higher rates in the US than in other industrialized countries. Analyzing the SFBN sample of the two German centers revealed a life-time prevalence of 17.8% for AUD only—compared to 33% in the whole SFBN which included four US and three European centers (two in Germany, one in the Netherlands). The transatlantic difference for illicit drug use might be even higher, as SUD other than AUD was only present in 8.5% of the German SFBN sample (37). The higher SUD comorbidity rates in the US might directly relate to the poorer prognosis and higher treatment resistance in the SFBN US compared to the European sample (38).
Less data has been generated for the rate of BD in samples of AUD patients. Not only that BD pre-disposes for SUD, also the opposite is true: According to the already cited meta-analysis by Hunt and colleagues, people with an AUD were 4.1 times on greater risk of having a BD compared to those without an AUD (21). The US- National Comorbidity Survey (39) found that 6.5% of males and 10.6% of females with alcohol dependency had also suffered from at least one manic episode. As a limitation, this survey did not differentiate between manic episodes which preceded SUD, those which followed SUD and those which were possibly induced by substance use. In addition, it is fair to assume that there is a substantial dark figure as symptoms of BD are often masked by SUD. Recognizing an underlying BD in SUD patients, however, is essential to tailor adequate treatment plans.
Is There a Shared Etiology Between BD and Aud?
Family studies indicate that AUD and affective disorders, especially BD have a shared genetic pre-disposition. Examining the family history of bipolar patients participating in the SFBN we observed that, among others, AUD not only in parents (40), but also grandparents of a bipolar patient is associated with a more severe course of BD and poor prognosis (41). Temperament traits, prevalent in BD and AUD and genetically determined such as sensation seeking behavior may play a decisive role across illness boundaries (42, 43). Candidate genes include a shared polymorphism of the aldehyde hydrogenase and alcohol dehydrogenase (44), a Ser23Cys (rs6318) polymorphism of the 5HT2C gene (in female bipolar patients) (45) and a VAL-158-MET polymorphism of the cathechol-o-methyltransferase (COMT) (46) impacting on the monoamine metabolism. However, recent GWAS finding pointed out that the correlation between genetic alterations and psychiatric disorders is not simple; the genetic connection of substance use and psychiatric disorders is rather highly pleiotropic and involves shared neurodevelopmental path, neurotransmission, and intracellular trafficking (47).
Diagnosis of AUD in Bipolar Patients
Symptoms of AUD and SUD may often obscure an underlying diagnosis of BD, and frequently result in a long delay before a BD diagnosis has been established by careful clinical observation. Brown et al. reported rates of SUDs in patients with BD ranging from 14 to 65% in treatment settings (48) but only a minority has received a correct diagnosis so far. Given the high incidence of psychiatric comorbidities in AUD, the German S3 Guideline recommend in every patient with AUD to carefully screen for psychiatric comorbidities after completing treatment of acute intoxication or withdrawal (49).
Uncovering AUD in people with BD appears less problematic. A recent systematic review on comorbidity of BD and AUD (50) recommends the use of the Alcohol use disorder Identification Test [AUDIT, (51, 52)] to detect heavy and frequent alcohol use, and AUDIT appears to be also sensitive in patients with comorbid mental health disorders. The AUDIT is also recommended to screen comorbid individuals by several evidence- based guidelines, e.g., the German S3-Guidelines on AUD (49, 53).
Consequences of Comorbidity
The detrimental impact of substance use and BD has been well-established, both for the individual and for society (54, 55). Numerous investigations demonstrated that comorbid AUD influences the clinical course of BDs unfavorably [for a review, see (50)]. Especially in younger people BD as well as SUD results in severe and lasting impairment and a loss of healthy years lived (56, 57). BD and SUD are afflicted with high rates of suicide attempts and suicide that are even topped in case of coexistence of both disorders (24). A Brazilian study reports of at least one suicide attempt in 68% of BD patients with AUD compared to 35% in BD without AUD, with virtually no difference between BD patients with DSM-IV alcohol abuse and dependence (23).
Gender differences have a significant influence on treatment outcomes in BD (58) but not as much on outcomes in alcohol dependence (59). AUD also facilitates additional comorbidities in BD patients such as anxiety disorders in female patients (33) and has a detrimental effect on the course of BD in general with an earlier onset (28), delayed recovery from episodes, more frequent mood switches, rapid cycling, mixed states, more severe depression and suicidal ideation (30, 32, 60) and lower adherence to treatment (61). Especially a history of verbal abuse and rates of social phobia and depression are higher in female than male BD patients with AUD (32). Whereas, AUD in female BD patients fosters rather self-destructive consequences, males appear more likely to externalize anger and impulsivity, and stand out by a history of criminal actions (62). Specific numbers for AUD and BD are not available, but for affective disorders (AD) in general and SUD, criminal behavior has been observed twice as frequent in AD with SUD compared to AD without (63).
The relationship between BD and AUD is bidirectional. In younger patients, it appears that alcohol use and bipolar symptoms are more likely to increase or decrease in unison (64). Depression increases alcohol craving in BD patients with AUD. An exploratory sub-analysis (65) examined the impact of depressive symptoms on craving and drinking behavior in 30 comorbid patients participating in a 8-week, placebo-controlled relapse prevention study (acamprosate vs. placebo). The analyzed subgroup of bipolar patients was well-stabilized on different mood stabilizers (antipsychotics, antiepileptics, or lithium). Severity of depression correlated significantly with craving and drinking behavior 1 week later.
However, also the reverse is true (66), the pattern and frequency of AUD can foster new episodes of BD, both mania and depression (67, 68); increasing severity of AUD predicts occurrence of a new major depressive episode (MDE) (69). Co-occuring BD has a detrimental impact on subjects with AUD. The Collaborative Study on the Genetics of Alcoholism is a family pedigree investigation that enrolled treatment-seeking alcohol-dependent probands who met the DSM-IV criteria for alcohol dependence (70). Of the 228 Bipolar probands, 75.4% (74% in bipolar I patients and 77% in bipolar II patients) fulfilled criteria for DSM-IV life time alcohol dependence. Comparing 5-year prospective data of BP-I and -II probands with and without alcohol dependence confirmed (71), in line with previous retrospective studies (60), a more severe course of BD in comorbid bipolar I individuals, whereas bipolar II individuals were less severely impaired by comorbid alcohol use.
The sequence of onset of each respective disorder might be of importance for early detection and possibly treatment of persons on risk. In a study by Frank et al., substance use preceded in 60% but succeeded in 7% the first manic episode which favors SUD and AUD as a trigger for BD. In one third of cases the temporal sequence remained unclear (72). Analysis of data from the National Comorbidity Survey Replication study revealed that SUD starting in adolescence leads to an ~3-fold increased odds of subsequent mood disorders, especially BD (73). The fact that juvenile-onset BD is a risk factor for SUD was also replicated in other studies (74, 75). Other studies, however, are in support of BD as the primary disorder followed by SUD and/or AUD. Preisig et al. (76) concluded from their study on familial relationship between mood disorders and alcoholism that BD tends to precede AUD. Comparing retrospectively three samples of bipolar patients (group 1: BD without AUD and SUD, group 2: onset of BD precedes AUD and SUD, and group 3: onset of a SUD precedes BD), Feinman and colleagues found that subjects in the second group showed a significantly earlier onset of affective symptoms than those in the other two groups, also suggesting that BD is a breeding ground for AUD or SUD (77). Whether SUD or AUD onset prior to BD results in a more or less severe course of BD is still a matter of discussion. The study of Feinman and Dunner found higher rates of suicide attempts in their group 3 (SUD prior to BD), whereas Winokur et al. report on a milder course of BD in those with prior onset of SUD (78). The relationship between SUD or AUD and BD is probably not just bidirectional but more complex with several confounding variables. McElroy et al. (79), for example, retrospectively showed an association between early onset BD, mixed symptoms, psychiatric comorbidity and SUD. Thus, early detection of both BD and being on risk for SUD is essential to avoid disastrous outcomes (10), but further prospective research of the complex relationship in larger samples is still needed.
Treatment Strategies in Comorbid BD and AUD—General Principles of Treatment
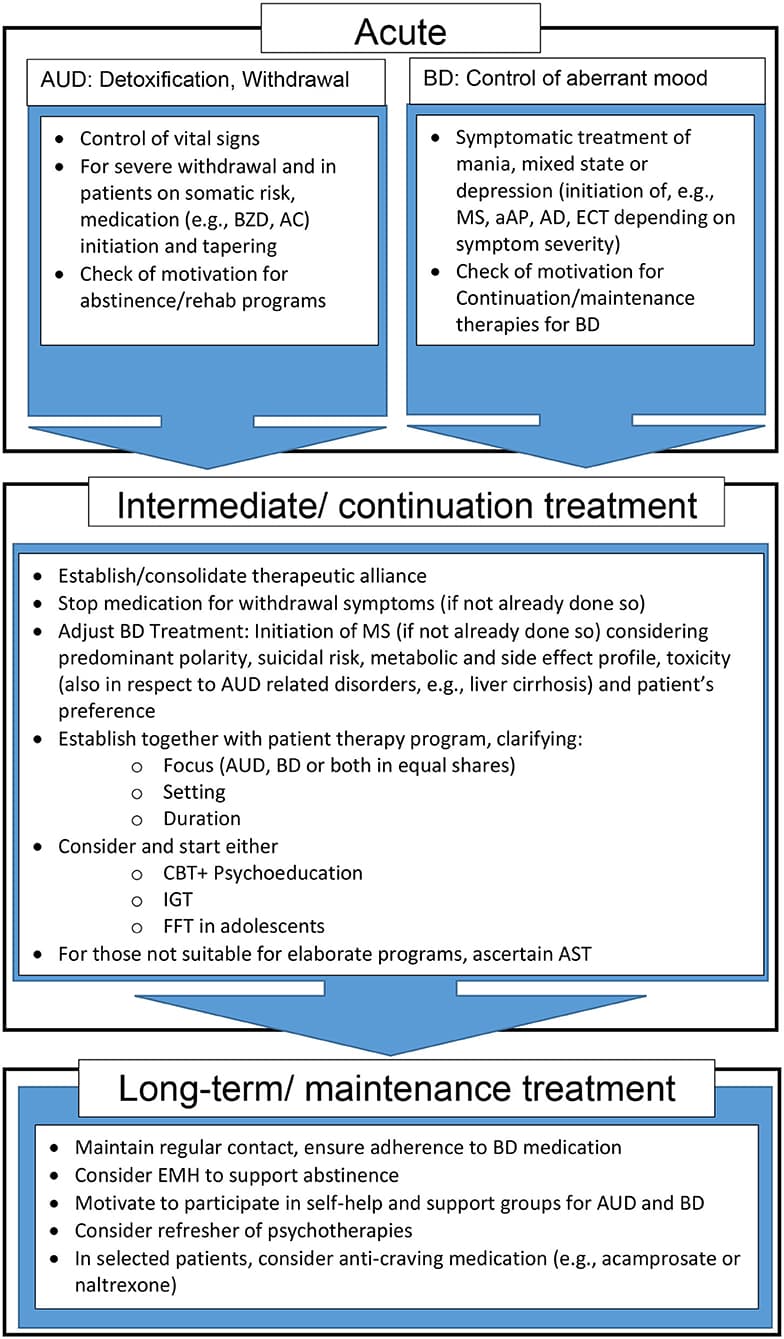
This chapter deals with the intermediate and long-term treatment of comorbid BD and AUD. We do not recap acute treatments for detoxification or delirium on one side, and mania or severe depression on the other side. These acute treatments are symptom-orientated, rarely different in comorbid vs. non-comorbid patients and depend on the predominant symptomatology (affective vs. addictive) that needs attention first. For intermediate and long-term treatment, the dogma persisted for a long time that AUD needs to be treated first and sufficiently before attention should be paid to the mental health disorder. Today, strategies that promote concomitant therapy of dual disorders are the established treatment of choice (80) and recommended in major guidelines (81). However, treatment adherence and compliance remain a challenge in this special group, since medications are often not taken as prescribed (61) and psychotherapy appointments are often missed. Studies support that the most important predictor of non-adherence in BD is comorbid alcohol and/or drug abuse (82, 83). Thus, effective psychosocial (84), psychoeducational (85, 86) or psychotherapeutic (87, 88) intervention for AUD and BD can also positively impact on medication adherence and, by this, ameliorate the course especially of BD (84).
Successful treatment of comorbid BD and AUD is a time-consuming process. Except from few specialized long-term inpatient settings for comorbid patients (89) the emphasis of all treatment concepts is on outpatient settings as behavioral changes and building up resilience is a long process in both disorders. As relapses and recurrences are rather the rule than the exception, regular outpatient contacts, emergency numbers to call in case of an imminent relapse and a timely and easy access to inpatient treatment for either one of the disorders are crucial. The German S3 Guidelines for AUD recommend that both disorders, BD and AUD, should be treated in one setting and by the same therapeutic team (49, 81). If not feasible, a close coordination of therapies, e.g., by means of a case manager, should be established.
Psychotherapy
The evidence base for suitable psychotherapies in comorbid BD and AUD remains poor. The German S3 Guidelines for AUD (49) recommends cognitive behavioral therapy (CBT) as the best evidenced modality whereas there is no recommendation for other psychotherapies due to insufficient data.
This recommendation is, by large, based on the CBT studies conducted by Farren et al. In a prospective cohort study, 232 comorbid patients with alcohol dependence and an affective disorder (among whom 102 were individuals with BDs), received inpatient treatment with cognitive behavioral therapy for 4 weeks (90). The program also included psychoeducation on both disorders. At 6-month follow-up both groups (depressive and bipolar patients) showed a significant reduction of alcohol consumption, but no difference was found between patients with unipolar and bipolar disorder. At 5-year follow-up, there was still a significant long-term benefit, particularly in those who engaged in post-discharge supportive therapy. Early abstinence predicted later abstinence, and a significant number of those who reduced their drinking by 6 months also achieved complete abstinence after 5 years (91).
Other guidelines, e.g., the Canadian Network for Mood and Anxiety Treatments (CANMAT) do not recommend CBT but rather the integrated group therapy (IGT) developed by Weiss and colleagues which includes CBT and psychoeducation components. IGT has been studied in a pilot study (92) and 2 separate RCTs (93, 94) comparing it with either group drug counseling or no treatment. This manualized program with 20 weekly group sessions demonstrated effectiveness both for the prevention of alcohol and bipolar relapses (93) even at 8-month follow-up. IGT topics are identification of triggers preceding substance use, refusing drugs and alcohol, coping with BD without abusing substances, medication adherence, relationships with friends and family members, self-help group participation, weighing the pros and cons of recovery, and recognizing the warning signs of relapse, among other topics (95). A slimmed version with twelve sessions, developed by the same group, also demonstrated effectiveness (94).
The evidence for Assertive community treatment (AST) that has been examined in two RCTs is inconclusive, with one study showing a reduction of alcohol use, the other not when compared to standard clinical case management. Both studies included also patients with other major mental health disorders, such as MDD and schizophrenia; thus, both do not supply information exclusively about changes in the course of BD (96, 97). Only a follow-up evaluation of the first study after 3 years specifically reports about 51 patients with BD and comorbid SUD, stating that taking part in the AST program has also improved quality of life (QoL) and diverse functionality measures (98).
For contingency management and motivational therapy in comorbid BD and SUD, only low-level evidence exists, e.g., non-randomized, prospective studies, case series or retrospective studies. In the CANMAT guidelines they are only recommended as second-choice in situations where first choice treatments are not indicated or cannot be used, or when first-choice treatments have not worked (89).
In adolescents with comorbid BD and SUD, inclusion of the family appears crucial. Family-focused treatment (FFT) with psychoeducation is recommended and effective (99).
In summary, only few psychotherapeutic interventions have been studied in a randomized study design and mostly only by one research group. The most recent Cochrane review on psychotherapies of mental illness and comorbid SUDs examined 41 RCTs and concluded that it was impossible to rule in favor of any specific psychosocial treatment, because of a large array of methodological differences and difficulties impeding data pooling as well as interpretation (100).
E-Mental Health Approaches
Not only in times of pandemics, but for the sake of high visibility, easy access and cost-effectiveness digital media are increasingly on the rise in health care, and have been used for screening and supplementing psychotherapy in affective disorders comorbid with AUD during recent years (101). For unipolar depression, efficacy for depressive symptoms as well as drinking behavior (cumulative duration of abstinence) was reported in a study comparing SMS twice daily (n = 26) vs. control condition (14-day “thank you” —SMS, n = 28). After 3 months of study completion, both Beck Depression Inventory scores and cumulative abstinence were significantly improved in the experimental group (102); however, the effect did not last (103). A subsequent, slightly larger study (n = 95) included comorbid patients both with unipolar and bipolar depression. Unfortunately, numbers and outcomes are not reported separately for unipolar and BD subjects. The study found a significant reduction in depression scores (p = 0.02) and perceived stress scores (p < 0.01) 3 months after completing a 30-day rehabilitation program in the intervention group. The intervention group- again receiving twice daily supportive text messages- also showed a significantly greater reduction in units per drinking day from baseline to 6 months after completion of the rehabilitation program. treatment point compared to the control group with a medium effect size (P = 0.03). At follow up 6 months after stopping text messaging (12 months after completion of the rehabilitation program) group differences in drinking or mood measures had vanished, again suggesting that the effect of text messaging was transient but not lasting.
The use or digital media and “blended care” is likely to increase in the future across treatment settings and will facilitate diagnosis and treatment of mental disorders including comorbid conditions. It's usefulness in BD patients comorbid with AUD, however, still needs to be further investigated.
Pharmacological Options
Besides psychotherapy an individually tailored pharmacotherapy is essential in almost all BD patients with comorbid AUD. For BD, pharmacotherapy is an essential component to stabilize mood and prevent recurrences, whereas its role for treating AUD beyond controlling acute withdrawal symptoms is less clear. Randomized controlled studies in BD traditionally exclude patient with concurrent SUD. Thus, the evidence for choosing a mood stabilizer in BD with comorbid AUD is rather weak; strictly speaking, high levels evidence consists of altogether three placebo-controlled studies in this patient group (104–106). To make any suggestion (not even recommendations) about best available treatments we therefore rely on additional low-level evidence from open or retrospective studies and expert opinion.
In general, treatment-refractory patients are over-represented in the group of BD patients with comorbid SUD (107). As with most treatments, concurrent SUD including AUD is thus a predictor for inferior response to lithium. However, as shown in adolescents, achieving more mood stability with lithium can result in lower levels of alcohol or drug consumption (108). Positive effects of lithium on SUD apart from indirect effects via mood stabilization could not be substantiated so far (109).
Anticonvulsants, namely valproate, carbamazepine and lamotrigine, had been first line alternatives in BD to lithium for a long time. An open pilot study with valproate by Brady and colleagues in 1995 suggested a reduction of drinking days in parallel to a reduction of manic and depressive symptoms (110). Subsequently, Brady and colleagues conducted a 12-week, double-blind, placebo-controlled trial (105) in a small group of BD patients with comorbid AUD (n = 29). In this study, the valproate group had a significantly smaller percentage of subjects who relapsed to heavy drinking, but otherwise there were no significant differences in other alcohol-related outcomes. As far as BD symptomatology was concerned, there was only a significantly greater decrease in irritability. In the following, Salloum and colleagues conducted a 24-week, double-blind, placebo- controlled study with a slightly greater number of patients (n = 59). Valproate failed to demonstrate improvement in mood stabilization. The number of heavy drinking days, number of drinks per heavy drinking day, and serum biomarker levels of alcohol use in the valproate-treated group, however, were significantly reduced compared to the placebo group (106). Finally, the study by Kemp and colleagues in rapid-cycling (RC) BD patients with comorbid SUD (alcohol, cannabis or cocaine) consisted of an open label stabilization (up to 24 weeks) with all patients receiving lithium plus valproate, follow by a 6-month double- blind phase with randomization of stabilized patients to either lithium alone or lithium plus valproate. The study failed to demonstrate any effect of valproate on mood- related parameters (104). As far as AUD is concerned, the authors reported that of the 19 subjects with AUD, 58% no longer met criteria for active abuse or had entered into early full remission while in the open-label phase. Due to the very small number of subjects entering the double-blind phase (n = 31, corresponding to 21% of the original sample), there are no reliable data whether adding valproate to lithium is superior to lithium monotherapy, neither for mood-nor alcohol related outcomes.
For lamotrigine, only open-label evidence exists. In this small study (n = 28), lamotrigine improved mood symptoms, as well as decreased craving for alcohol and decreased carbohydrate deficient transferrin over 12 weeks (111). Unfortunately, so far, no confirmative controlled studies in BD + AUD with lamotrigine have been conducted (112). The only placebo-controlled study with lamotrigine in BD comorbid with SUD (cocaine) was negative across mood and substance related outcomes (113).
Carbamazepine has been traditionally used in acute alcohol withdrawal to reduce the risk of seizures and ameliorate physical symptoms. However, there are no reliable data whether it is of any usefulness in the long-term treatment of BD + AUD. Carbamazepine is metabolized by the liver and can, by itself, induce an increase in liver transaminases (ALAT, ASAT, γGT) and, in rare cases, cause liver failure. Thus, its use might put active alcohol users on risk.
A controlled study with topiramate in BD + AUD failed due to slow recruitment (114).
Atypical antipsychotics (aAP) have increasingly become a treatment of choice in BD. There was some expectation that their dopamine-stabilizing effects might also lead to a reduction of craving; however, a meta-analysis of the use of aAP as a group in primary AUD without comorbidities could not find any effects on drinking behavior or craving (115). The only exception was aripiprazole which reduced significantly number of drinks and heavy drinking days in one study (116).
Retrospective data suggested that, similar to aripiprazole (117), quetiapine might relieve alcohol graving in patients with BD and concomitant cocaine use (118). Subsequently, the same group conducted a double-blind, placebo-controlled study (119) in patients with BD + AUD. At the time of the study, 82% of subjects were in a depressive state. Quetiapine add-on to treatment as usual (TAU) had no effect on any alcohol-related outcomes, but produced a faster and significantly greater decrease of depressive symptoms. This finding is of note as many antidepressant treatment modalities are less effective in BD patients with comorbid AUD. The lack of efficacy of quetiapine against AUD was also confirmed in another placebo- controlled study (120). No controlled data for other aAP or antidepressants have, so far, been generated (see Table 1).
Table 1. Randomized controlled studies on pharmacological treatments of comorbid BD and AUD.
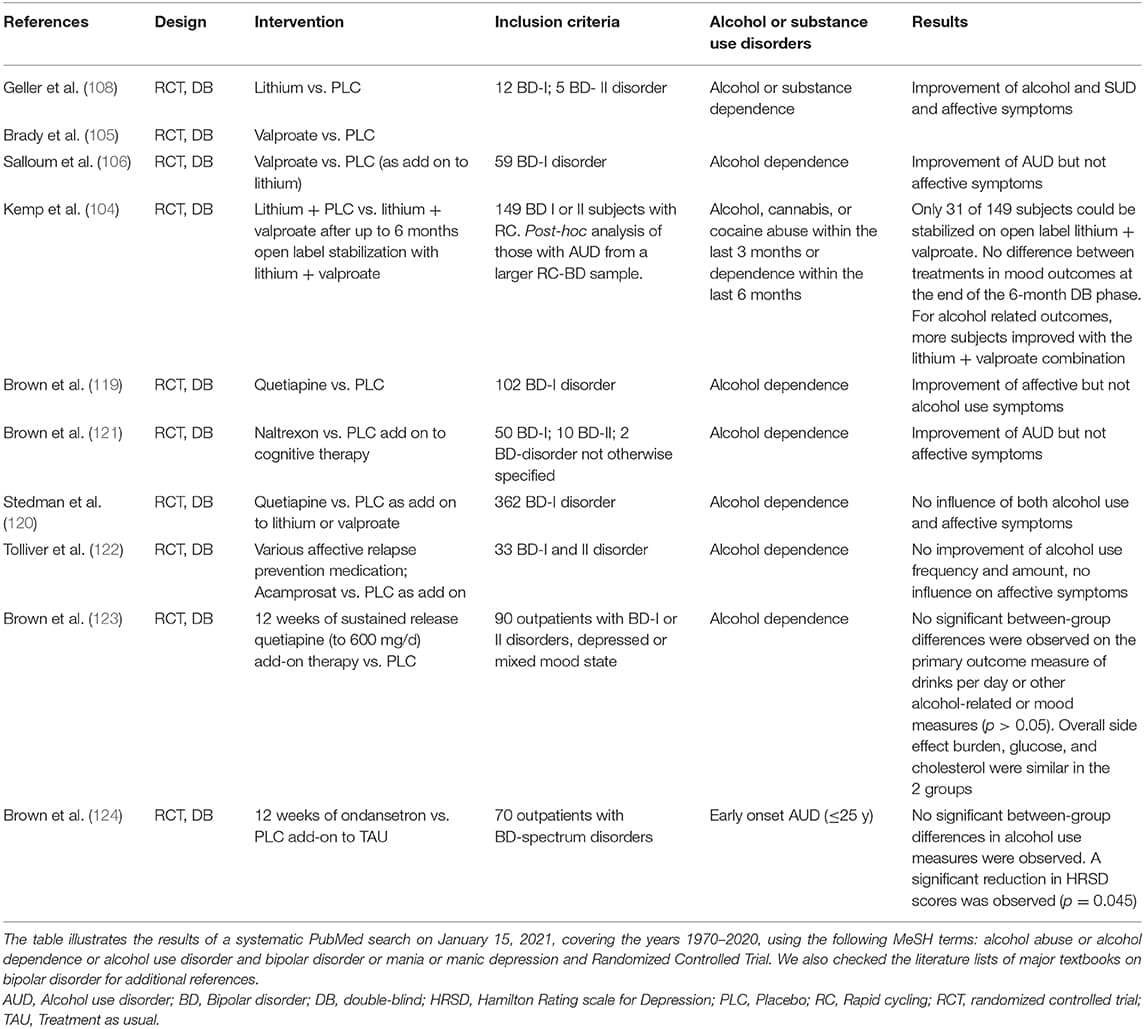
Limited data exist on the effect of anti-craving medication in AUD with comorbid BD. Results of an open study suggested a reduction of both craving and stabilization of mood with naltrexone in patients with BD + AUD (125). However, improvement of mood was not confirmed in a double-blind study with naltrexone add-on to cognitive behavioral therapy, and there was only a trend toward less alcohol consumption (121). Similar disappointing results have been reported from a controlled study with acamprosate in BD + AUD (122).
A recent experimental approach used ondansetron. Ondansetron is a 5-HT3 receptor antagonist used to prevent nausea and vomiting caused by chemo- or radiation therapy. A controlled study suggested a reduction of alcohol consumption with ondansetron (126). However, in a randomized, double-blind, placebo-controlled trial in outpatients with BD and early onset AUD (124) no reduction of alcohol use measures were observed; but interestingly, ondansetron led to an improvement of depressive symptomatology measured with the Hamilton Depression rating scale [HDRS (127)].
Table 1 supplies an overview of double-blind, randomized pharmacological studies for comorbid bipolar affective and AUDs, based on a systematic PubMed search.
Conclusion
In BD, comorbid SUD and especially AUD are rather the rule than the exception. Pharmacological and integrated psychotherapeutic approaches that give equal weight to both disorders, while still scarce, are recommended. CBT and IGT have the best, but still insufficient evidence- base as psychosocial treatments. Figure 1 depicts a proposed therapy algorithm based on the evidence presented in this article. Supportive pharmacotherapy should be mainly centered around BD, with mood stabilizer, e.g., lithium and valproate, still the treatment of choice. However, there is clearly more research needed to develop reliable treatment algorithms for comorbid BD and AUD.
Figure 1. Proposed treatment and support algorithm for patients with comorbid AUD and BD. aAP, atypical antipsychotics; AC, Anticonvulsants; AD, Antidepressants; AST, Assertive community treatment; AUD, Alcohol use disorder; BD, Bipolar Disorder; BZD, benzodiazepines; CBT, Cognitive behavioral therapy; ECT, Electroconvulsive therapy; EMH, E-Mental Health; FFT, Family-focused therapy; IGT, Integrated group therapy; MS, Mood stabilizer.