Abstract
Background Substance use disorder (SUD) shares common clinical features, including impulsive and compulsive behaviors, which are associated with dysfunctions in the brain’s reward circuit. Resting-state functional magnetic resonance imaging (rs-fMRI) studies have shown inconsistent results due to variability in the substances and stages of addiction. Identifying common neurobiological patterns in SUD could improve both our understanding of the disorder and the development of treatment strategies.
Methods We conducted a comprehensive meta-analysis of 53 whole-brain rs-fMRI studies involving SUD patients. The Seed-based d Mapping toolkit was used to analyze connectivity patterns of key brain regions in the reward circuit: anterior cingulate cortex (ACC), prefrontal cortex (PFC), striatum, thalamus, and amygdala. Additionally, we explored correlations between resting-state functional connectivity (rsFC) patterns and impulsivity scores.
Results The meta-analysis included 1700 SUD patients and 1792 healthy controls (HCs). Compared with HCs, SUD patients exhibited significant dysfunctions in the cortical-striatal-thalamic-cortical circuit. The ACC exhibited increased connectivity with the inferior frontal gyrus (IFG), lentiform nucleus, and putamen. The PFC demonstrated hyperconnectivity with the superior frontal gyrus (SFG) and striatum, as well as hypoconnectivity with the IFG. The striatum showed hyperconnectivity with the SFG and hypoconnectivity with the median cingulate gyrus (MCG). Thalamic connectivity with the SFG, dorsal ACC, and caudate nucleus was reduced. The amygdala exhibited hypoconnectivity with the SFG and ACC. Alterations in connectivity were also observed between several seed regions and the parahippocampal gyrus. Notably, the total score of the BIS-11 in SUD patients was significantly negatively correlated with reduced rsFC between the striatum and MCG. After family-wise error (FWE) correction, dysfunctions in the cortical-striatal-cortical circuit persisted.
Conclusions Our findings revealed specific network abnormalities in SUD patients, highlighting disrupted connectivity within the brain’s reward circuit. These abnormalities were associated with impulsivity and may provide a theoretical basis for effective interventions to restore normal connectivity patterns.
Introduction
Substance use disorder (SUD) is a complex, multifactorial condition that poses a significant challenge to public health. It is characterized by compulsive substance use, loss of control, and significant impairments in cognitive and emotional regulation. SUD leads to severe physical, psychological, and social consequences, including overdose, infectious diseases, mental health disorders, and an increased risk of socioeconomic instability. Despite advances in understanding SUD, its neurobiological mechanisms remain poorly understood, primarily due to the variability in substances and addiction stages, which leads to inconsistent findings across studies. This highlights the need for a more systematic and integrated approach to identifying common neurobiological patterns in SUD, which could facilitate the development of new therapeutic strategies.
Disruptions in the brain’s reward circuitry are thought to contribute to the compulsive and impulsive behaviors observed in SUD. The brain’s reward circuitry, comprising the anterior cingulate cortex (ACC), prefrontal cortex (PFC), striatum, thalamus, and amygdala, plays a pivotal role in mediating reward processing, goal-directed behavior, and cognitive control. The ACC is essential for emotional regulation and impulse control, often displaying altered connectivity in SUD patients, which reflects their difficulties with these functions. The PFC is integral to executive functions and decision-making processes. Previous research has shown that dysregulation of the PFC is linked to impaired executive control in SUD patients. The striatum, particularly the nucleus accumbens, is central to reward processing and has been consistently implicated in the compulsive aspects of SUD. The thalamus functions as a relay station for sensory and motor signals, playing a role in the regulation of consciousness and alertness. Altered thalamic connectivity has been observed in SUD, contributing to cognitive deficits and sensory processing alterations. The amygdala is involved in emotional processing and memory formation, with disruptions in this region linked to the emotional dysregulation seen in SUD. Therefore, studying specific functional connectivity patterns between these key nodes and whole-brain regions can provide insights into the fundamental mechanisms driving compulsive and impulsive behaviors in addiction.
Resting-state functional magnetic resonance imaging (rs-fMRI) has become a powerful tool for exploring the brain’s functional connectivity, providing insights into the intrinsic activity of neural networks. However, rs-fMRI captures only the brain’s spontaneous activity and does not provide a direct measure of task-induced or real-time behavioral responses, limiting our ability to understand the dynamic processes underlying addiction. Furthermore, inferences regarding the association between connectivity changes and SUD behaviors are limited. Previous rs-fMRI studies have identified disruptions in the reward circuitry of SUD patients. A study of abnormalities in the nucleus accumbens (NACC) rsFC in patients with long-term heroin withdrawal observed significantly enhanced connectivity with the right ventromedial PFC. In contrast, connectivity was reduced between the NACC and the left putamen, left precuneus, and the accessory motor area. Ge, et al. found that the dorsolateral PFC exhibited decreased rsFC with the right insula and left inferior frontal gyrus in nicotine-dependent smokers, which was associated with cravings and impulse suppression. An important question arises as to whether patients with different types of SUD exhibit specific and consistent rsFC abnormalities in the reward circuit. However, results regarding rsFC changes in the same seed region have been inconsistent. For example, some striatal rsFC studies have reported increased frontostriatal connectivity, while others have observed decreased connectivity. This inconsistency may stem from heterogeneity across studies in terms of addictive substances, stages of addiction, sample sizes, and populations.
To address this, we conducted a systematic meta-analysis of whole-brain rs-fMRI studies published before April 2023 in PubMed, Web of Science, Scopus, EMBASE, and ScienceDirect databases. Using the Seed-based D-mapping toolkit, we analyzed the connectivity patterns of key brain regions in the reward circuit, including the ACC, PFC, striatum, thalamus, and amygdala. This approach helped mitigate issues related to small sample sizes and group heterogeneity. Furthermore, we included studies on various substances (e.g., alcohol, cocaine, amphetamine, heroin, ketamine, nicotine, betel nut, and cannabis) and addiction stages (e.g., dependence, abuse, initial abstinence, and prolonged abstinence) to identify common neural patterns in SUD patients. Our goal is to provide a theoretical foundation for targeted interventions designed to restore normal brain connectivity. Additionally, given that impulsivity is a hallmark symptom of SUD, leading to compulsive substance use and relapse. To elucidate the neurobiological mechanisms underlying impulsivity in SUD, we also explored the correlation between rsFC patterns and impulsivity scores. We hypothesize that SUD patients exhibit specific and consistent rsFC abnormalities in the reward circuit, and that higher impulsivity scores are associated with greater disruption of these neural circuits.
Materials and methods
Literature search
We conducted a comprehensive literature search up to April 2023 across PubMed, Web of Science, Scopus, EMBASE, and Science Direct databases using the following keywords: “addiction OR abuse OR dependence OR substance use disorder” OR “alcohol OR ethanol” OR “caffeine” OR “inhalants” OR “opioids OR opiate OR opium OR heroin OR methadone OR codeine” OR “stimulants” OR “cocaine” OR “sedatives OR hypnotics” OR “cannabis OR marijuana OR THC” OR “amphetamine OR methamphetamine OR ecstasy OR hallucinogens OR phencyclidine OR arylcyclohexylamine” OR “tobacco OR cigarette OR smoker OR nicotine” AND “fMRI OR functional magnetic resonance imaging OR neuroimaging” AND “rest OR resting-state OR functional connectivity”. Additionally, we conducted manual searches of the reference lists from both original studies and review articles to identify potentially eligible studies.
Study selection
Eligible studies included original fMRI studies using seed-based methodologies (such as ACC, PFC, striatum, amygdala, and thalamus seeds) and whole-brain rsFC analysis, comparing SUD patients with healthy controls (HCs). Inclusion criteria were: (1) participants aged over 18 years; (2) a formal diagnosis of SUD according to DSM or ICD criteria, or other objective laboratory or clinical assessments (e.g., urine drug tests); (3) studies published in English. Exclusion criteria were: (1) absence of a HC group; (2) SUD comorbidities with serious mental illness or neurological disorders; (3) recreational drug use or gambling; (4) high-risk family addiction groups; (5) studies using duplicate data, with preference given to those with higher quality and larger sample sizes; (6) non-seed-based methodologies, such as independent component analysis (ICA), graph theory, voxel-mirrored homotopic connectivity (VMHC), amplitude of low-frequency fluctuations (ALFF), regional homogeneity (ReHo), spectral dynamic causal modeling (spDCM), and psychophysiological interaction (PPI); (7) acute intervention studies; (8) longitudinal studies lacking baseline data; (9) non-original research or studies without full text availability, including guidelines, conference proceedings, reviews, books, or evaluations; (10) studies where peak coordinates and effect values for differential brain areas could not be obtained after author contact.
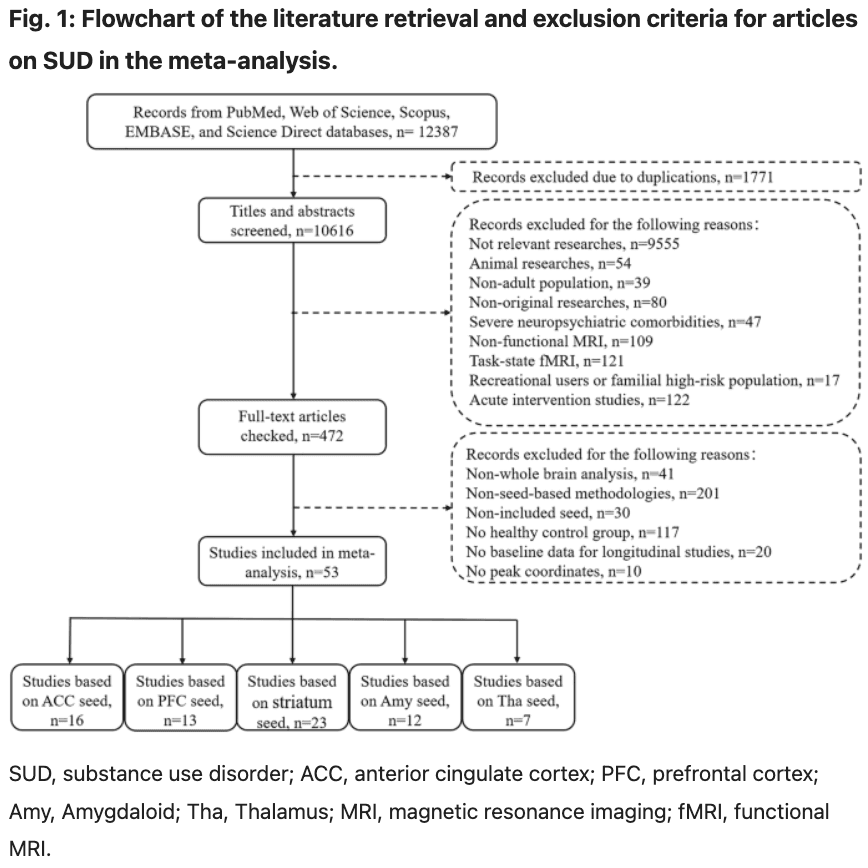
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA, http://www.prisma-statement.org/). The Flowchart of literature retrieval is presented in Fig. 1.
Meta-analysis
To investigate brain rsFC patterns in individuals with SUD, we performed a meta-analysis using the Seed-based d Mapping with Permutation of Subject Images toolbox (SDM-PSI, version 6.22; http://www.sdmproject.com/). This is a robust statistical tool for analyzing differences in brain activity or structure, based on peak coordinates and effect sizes. First, we selected regions of interest (ROIs) in the brain, including the striatum, ACC, PFC, thalamus, and amygdala. These ROIs were defined based on coordinates derived from the included studies. Second, regions that showed statistical significance at the whole-brain level were included in the meta-analysis, and their peak coordinates and effect t-values were extracted. P- or z-values from some studies were converted to t-values using the online SDM tool. Third, the SDM tool used anisotropic non-normalized Gaussian kernels to convert the peak coordinates to Hedge’s effect sizes and recreate voxel-level rsFC difference maps for each study. Given the large differences in age and sex between studies, we employed a random-effect model to generate the mean effect-size map across studies, covarying for age and sex, and weighted to account for sample size. To minimize the false positive rate, we set a conservative threshold. Only abnormal clusters with peak height threshold |z | >1, voxel p < 0.005, and cluster width threshold >10 voxels were reported. Additionally, we applied family-wise error (FWE) rate correction to obtain more robust results, with a peak height threshold |z | >1 and voxel p < 0.05. Furthermore, we subdivided the striatum into the ventral striatum (VS) and NACC subgroups, exploring the potential rsFC patterns within these subgroups through meta-analysis. Data extraction was performed independently by two investigators and subsequently double-checked.
Sensitivity analysis
To evaluate the reliability and reproducibility of the results, we performed a jackknife sensitivity analysis. In each iteration, one study was excluded, and the meta-analysis was repeated n times. The consistency of the affected brain regions was assessed using a reproducibility threshold, which is typically set at 75 ~ 80% in meta-analyses. Accordingly, we used a threshold of 80%.
Publication bias
For each significant cluster in the SUD-HCs comparisons, the Egger test was performed to assess funnel plot asymmetry as a means of identifying potential publication bias.
Meta-regression analysis
To explore the relationship between the meta-analysis results and impulsive behavior, we performed a meta-regression analysis on SUD patients. The total score on the Barratt Impulsiveness Scale (BIS-11) served as the regressor, while striatal rsFC was used as the covariate, with a significance threshold of p < 0.05. FWE correction was applied (p < 0.05).
Results
Included studies and sample characteristics
Our search strategy identified 63 eligible studies. However, the peak coordinates and effect sizes for differentially expressed brain regions were not available in 10 studies after contacting the authors. As a result, these studies were excluded from our meta-analysis. Ultimately, 53 studies were included (Fig. 1), encompassing nine substance types: alcohol, nicotine, cocaine, cannabis, heroin, ketamine, amphetamine, areca nut, and methamphetamine. As each study included multiple seed regions, 23, 16, 13, 12, and 7 studies were incorporated into the meta-analysis of the striatum, ACC, PFC, amygdala, and thalamus, respectively. In total, these studies included 1,792 healthy controls (HCs) (mean age: 37.98 years; 530 women [30%]) and 1,700 patients with SUD (mean age: 39.09 years; 321 women [19%]). Significant differences in age (t = −3.283, p = 0.001) and gender (X² = 54.126, p < 0.001) were observed between the two groups. Further details on the study and sample characteristics are provided in Table S1 in the supplementary materials.
Abnormal rsFC in multiple seed regions in SUD patients compared with HCs
Anterior cingulate cortex (ACC)
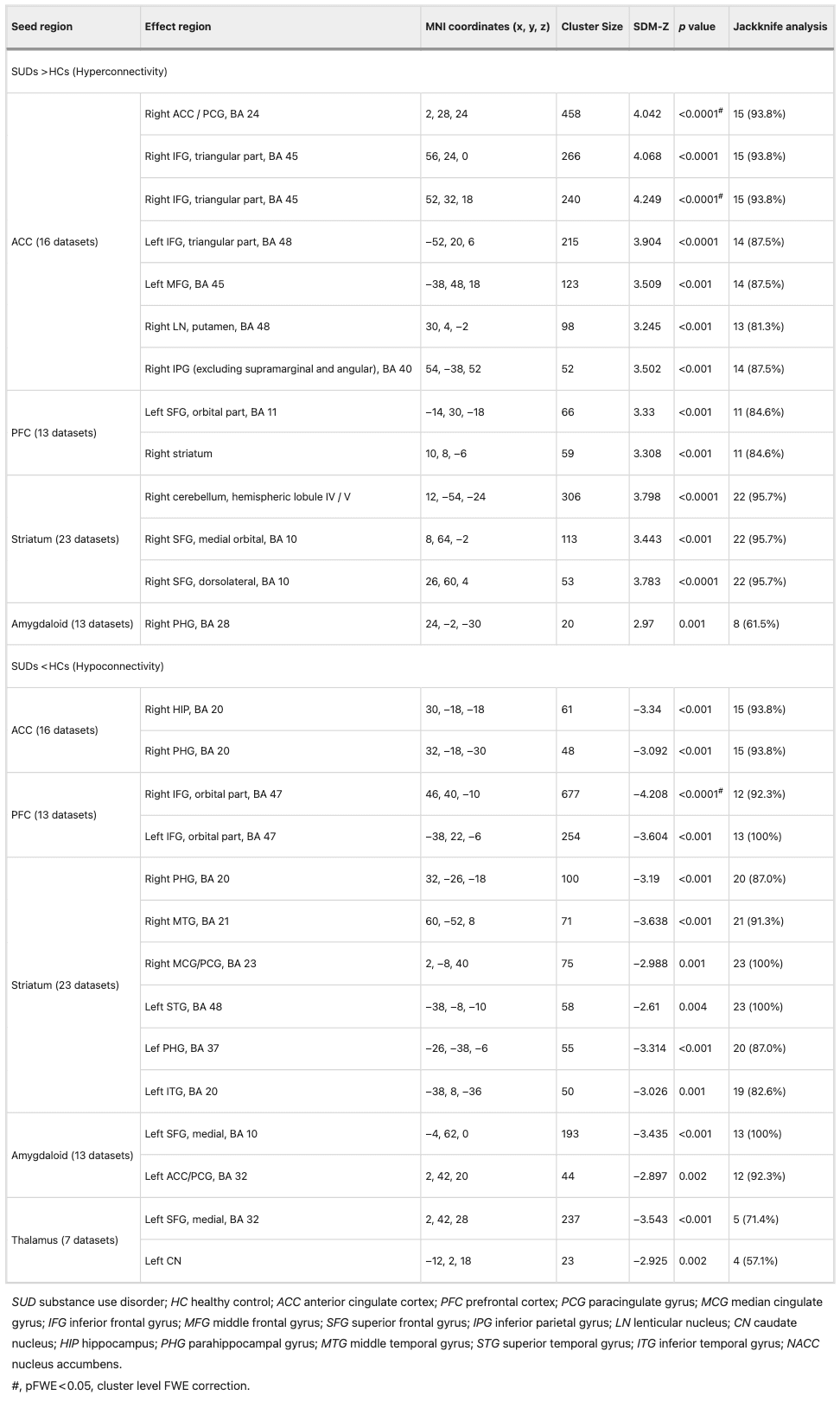
The study selected bilateral ACC and its subregions (dorsal [dACC], ventral [vACC], rostral [rACC], subcallosal [sACC], and perigenual [pACC]) as seed regions. Compared with HCs, SUD patients exhibited significantly increased connectivity of the ACC with the right paracingulate gyrus (PCG), bilateral inferior frontal gyrus (IFG), left middle frontal gyrus (MFG), right lentiform nucleus (LN), and right inferior parietal gyrus (IPG), along with decreased connectivity with the right hippocampus (HIP)/parahippocampal gyrus (PHG). After FWE correction (p < 0.05), the hyperconnectivity between the ACC and right PCG (z = 4.042, p = 0.021) and right IFG (z = 4.249, p = 0.034) remained significant (Table 1).
Prefrontal cortex (PFC)
The study selected bilateral PFC, dorsolateral PFC (dlPFC), medial PFC (mPFC), and orbitofrontal cortex (OFC) for seed analysis. Compared with HCs, SUD patients exhibited hyperconnectivity between the PFC and the left superior frontal gyrus (SFG) as well as the right striatum, and hypoconnectivity between the PFC and the bilateral IFG. After FWE correction (p < 0.05), the hypoconnectivity between the PFC and right IFG (z = −4.208, p = 0.008) remained significant (Table 1).
Striatum
The seed regions in this study included the ventral and dorsal striatum and their subregions (caudate, ventral and dorsal putamen, globus pallidus, and nucleus accumbens [NACC]). Compared with HCs, the striatum of SUD patients exhibited significantly increased connectivity with the right cerebellum and right SFG, along with decreased connectivity with the bilateral PHG, right middle cingulate gyrus (MCG)/PCG, left superior temporal gyrus (STG), right middle temporal gyrus (MTG), and left inferior temporal gyrus (ITG) (Table 1).
Amygdaloid
The bilateral amygdala and its subregions (basolateral and medial cortex) were selected for seed analysis. Compared with HCs, SUD patients exhibited hyperconnectivity between the amygdala and the right PHG, and hypoconnectivity between the amygdala and the left SFG as well as the left ACC/PCG (Table 1).
Thalamus
The bilateral thalamus and its mediodorsal subregion were selected for seed analysis. Compared with HCs, SUD patients exhibited hypoconnectivity between the thalamus and the left SFG, left dACC, and left caudate nucleus (CN) (Table 1).
Subgroup analysis: abnormal rsFC in ventral striatum and NACC
Ventral striatum (VS)
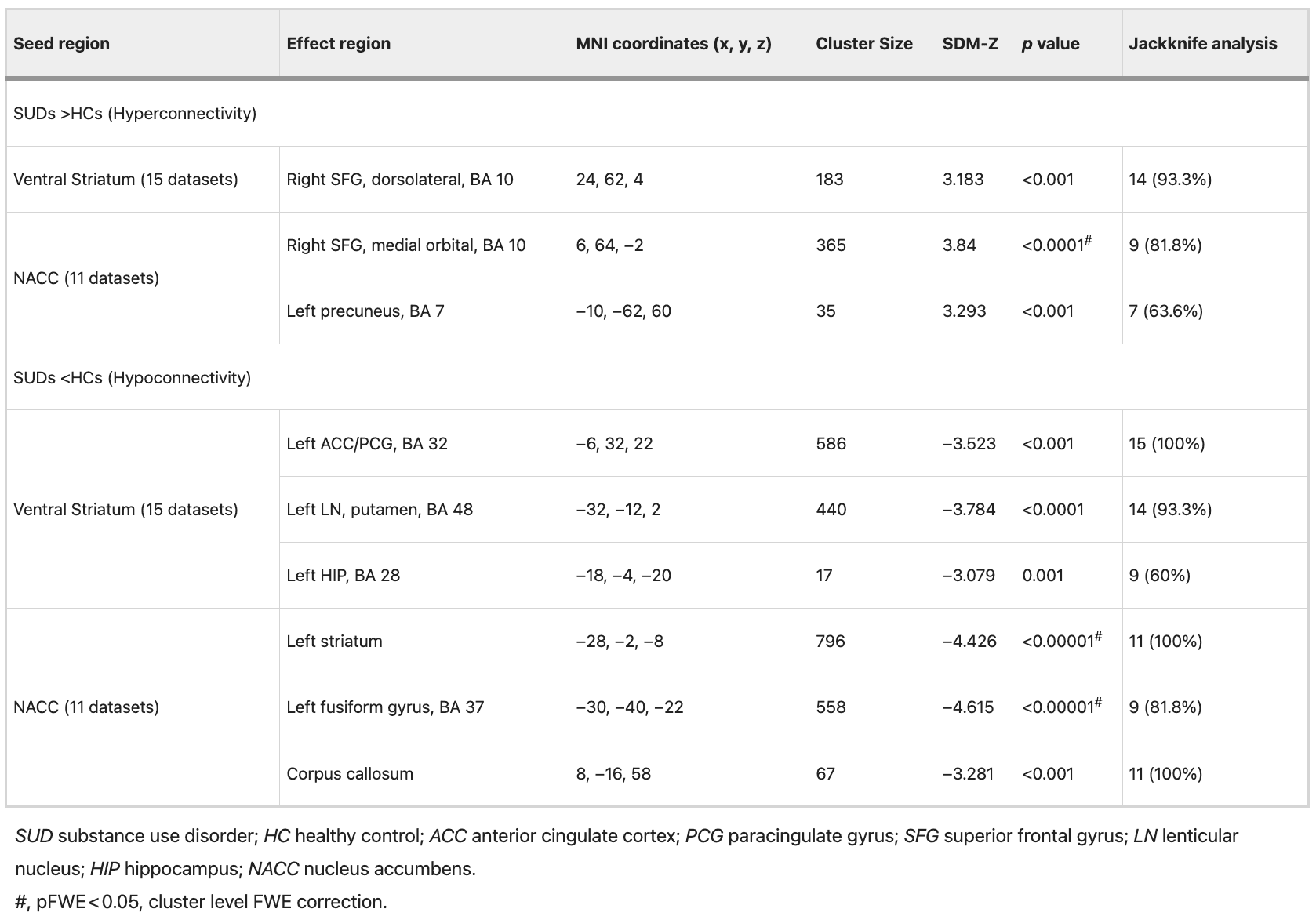
The ventral striatum and its subregions (ventral caudate nucleus, ventral putamen, NACC) were selected as seed regions (See Table S2 in Supplementary Material). In patients with SUD, abnormal rsFC in the ventral striatum mirrored that observed in the global striatum. Compared with HCs, it exhibited hyperconnectivity with the right SFG and hypoconnectivity with the left ACC/PCG, left medial temporal lobe (BA48), and left HIP (Table 2).
Nucleus accumbens (NACC)
The bilateral NACC and its subregions (shell and core) were selected as seed regions (See Table S2 in Supplementary Material). Compared with HCs, the NACC of SUD patients exhibited significantly increased connectivity with the right SFG and left precuneus, and decreased connectivity with the left striatum, left fusiform gyrus, and corpus callosum. After FWE correction (p < 0.05), hyperconnectivity between NACC and the right SFG (z = 3.84, p = 0.039) remained significant. Additionally, hypoconnectivity between the NACC and left striatum (z = −4.426, p = 0.002), as well as the left fusiform gyrus (z = −4.615, p = 0.005), also remained significant (Table 2).
Further subgroup analysis revealed similar abnormal rsFC patterns in the striatum, ventral striatum, and NACC in SUD patients. Hyperconnectivity with the SFG and hypoconnectivity with the medial temporal lobe or HIP were observed in all the aforementioned regions.
Meta-regression analysis
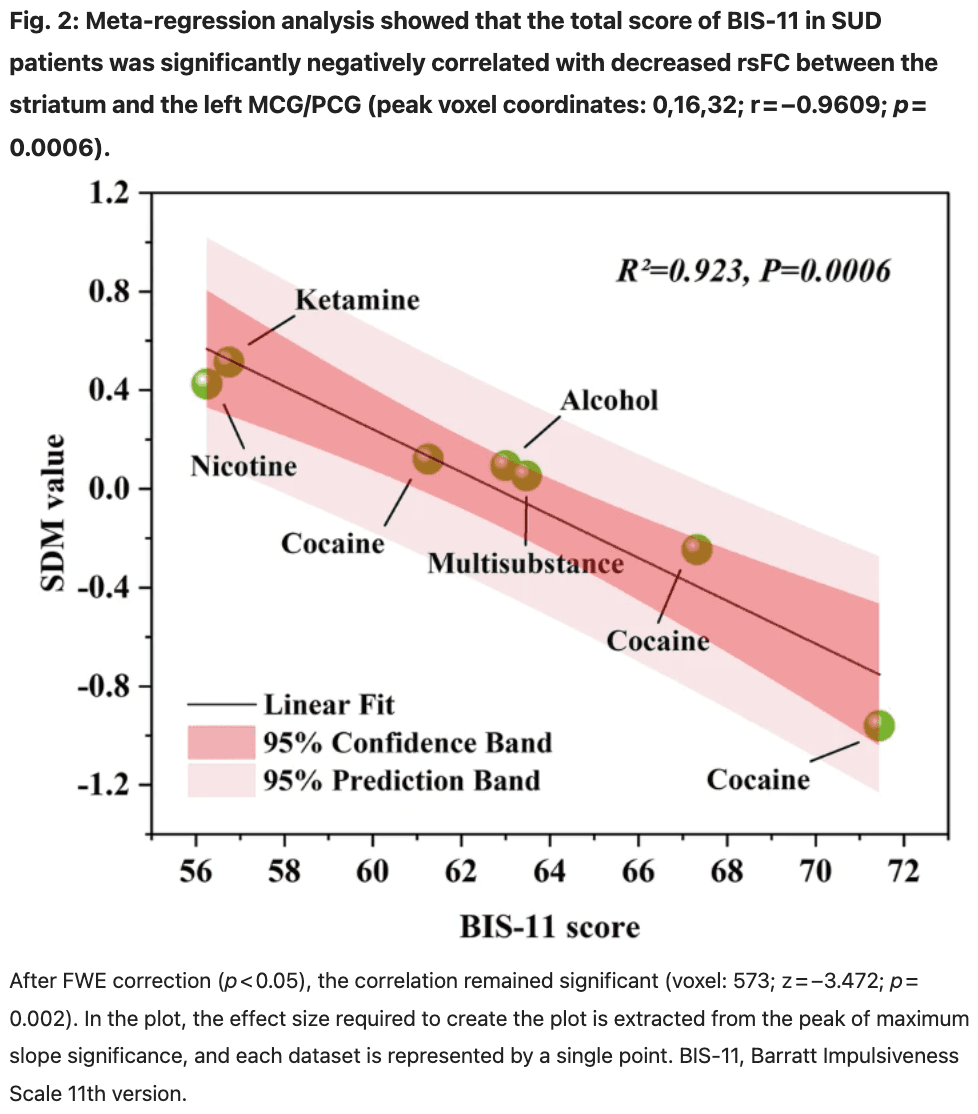
Seven studies from the striatum seed region, which included BIS scores, were selected for further meta-regression analysis (see Table S3 in Supplementary Material). The results showed a significant negative correlation between the total BIS-11 score in SUD patients and the decrease of rsFC between the striatum and left MCG/PCG (peak voxel coordinates: 0, 16, 32; r = −0.9609; p = 0.0006) (Fig. 2). After FWE correction (p < 0.05), the correlation remained significant (voxel: 573; z = −3.472; p = 0.002). In other words, in multiple SUD studies involving cocaine, nicotine, alcohol, and ketamine, higher impulsivity scores were associated with lower rsFC between the striatum and left MCG/PCG. Decreased frontostriatal connectivity was associated with impulsive behavior.
Disruption of the reward circuit
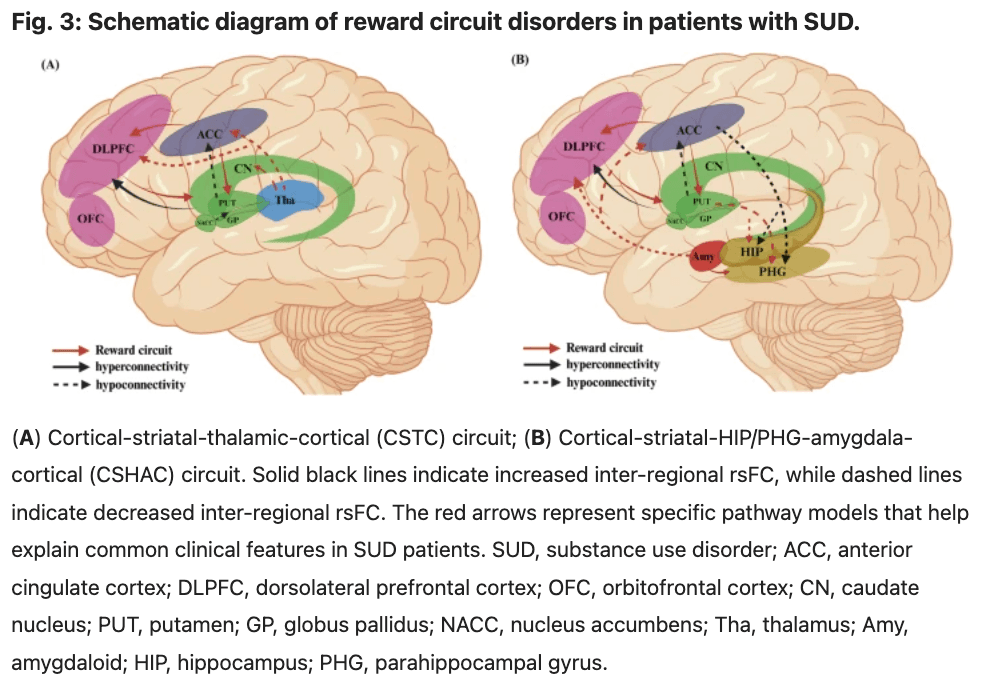
Our study identifies disruptions in two critical reward circuits: the cortical-striatal-thalamic-cortical (CSTC) circuit and the cortical-striatal- HIP/PHG-amygdala-cortical (CSHAC) circuit. The structural connectivity of the CSTC circuit originates in the frontal cortex, including the ACC, dlPFC, and OFC. The striatum receives information from the frontal cortex and transmits it to the CN. This information is then projected back through the thalamus to the frontal cortex, forming a closed loop (Fig. 3A). The CSHAC circuit also originates in the frontal cortex. However, unlike the CSCT circuit, the striatum sends information to the HIP/PHG, which then projects it back to the frontal cortex via the amygdala (Fig. 3B).
Jackknife analysis
In the rsFC models based on the ACC, PFC, and striatum, the jackknife sensitivity analysis revealed that all affected brain regions showed high reproducibility (all >80%). In the striatal subgroup analysis, the abnormal rsFC between the NACC and the left precuneus exhibited lower reproducibility (63.6%), as did the abnormal rsFC between the ventral striatum and the left hippocampus (60%). In the amygdala-based rsFC model, the reproducibility of the right PHG was relatively low (61.5%). In the thalamus-based rsFC model, the robustness of the left SFG (71.4%) and left CN (57.1%) was slightly lower. Overall, the results for most affected brain areas were robust under the jackknife sensitivity analysis (Tables 1, 2).
Publication bias
The results of Egger’s test were not significant (p > 0.05), suggesting no publication bias (see Tables S4 in the supplementary material for details).
Discussion
Through a comprehensive meta-analysis, we present, for the first time, a specific pattern of network abnormalities in SUD patients based on key nodes of the reward circuit, offering new insights into functional deficits within and between these networks. We found significant dysfunction in the cortical-striatal-thalamic-cortical circuit in SUD patients compared with HCs. Additionally, in SUD patients, we found that the total score of BIS-11 was significantly negatively correlated with decreased rsFC between the striatum and the left MCG/PCG.
Abnormal rsFC patterns of ACC seed
Compared with HCs, the ACC in SUD patients showed significantly increased connectivity with the right PCG, bilateral IFG, left MFG, right LN, and right IPG, while showing decreased connectivity with the right HIP/PHG. The ACC is a key brain region of the salience network and is associated with reward-related decision making, impulse monitoring, and false awareness. The PCG is a critical region involved in emotional and pain processing. The enhanced connectivity between the ACC and PCG may reflect an exaggerated response in emotional regulation or an abnormal focus on drug-related emotional stimuli in SUD patients. One study examined the relationship between regional network connectivity and neuropsychological performance in alcoholics, finding that increased rsFC between the ACC and IFG/caudate nucleus was associated with worse visuospatial working memory. Increased rsFC between the ACC and MFG/caudate in alcoholics is associated with slower perceptual-motor processing.
Abnormal rsFC patterns of PFC seed
Relative to HCs, SUD patients showed hyperconnectivity between the PFC and the left SFG, as well as the right striatum, and hypoconnectivity between the PFC and the bilateral IFG. These findings are consistent with previous research suggesting that the PFC plays a crucial role in cognitive control and reward processing, both of which are often dysregulated in addiction. Hyperconnectivity between the PFC and SFG may reflect an overactivation of executive functions, as the SFG is a key region involved in decision-making, impulse inhibition, and cognitive control. Such overactivation could contribute to the heightened sensitivity to drug-related cues observed in SUD patients. Furthermore, the increased connectivity between the PFC and the right striatum supports the idea that addiction involves disrupted reward processing, as the striatum is integral to reinforcement learning and habit formation. In contrast, reduced connectivity between the PFC and bilateral IFG may indicate a dysfunction in cognitive control, particularly in areas related to inhibition and self-regulation. The IFG is involved in controlling impulsive behavior and regulating emotional responses, and its weakened connectivity with the PFC may contribute to the impulsivity and poor decision-making that characterize SUD. Taken together, these findings suggest that SUD is associated with an imbalance in both cognitive control and reward processing, further emphasizing the need for targeted interventions aimed at addressing these mechanisms.
Abnormal rsFC patterns of striatum and its subregions seed
Compared with HCs, the Striatum of SUD patients showed significantly increased connectivity with the right cerebellum and right SFG. It showed decreased connectivity with the bilateral PHG, right MCG/PCG, left STG, right MTG, and left ITG. Further subgroup analysis revealed similar abnormal rsFC results. Additionally, we found reduced rsFC between the VS and the left medial temporal lobe, left hippocampus, and left ACC. Connectivity between the NACC and the left precuneus increased, while connectivity between the NACC and the left striatum, left fusiform gyrus, and corpus callosum decreased. The VS/NACC is a key region in the subcortical reward network (RW). By connecting with other brain regions, they collectively support learning reward contingencies, hedonic responses, generating motivation to pursue rewards and goals, forming and implementing plans to obtain rewards, and adjusting behaviors and plans according to changing contingencies. The striatum plays a central role in motivation and reward, and its hyperconnectivity with the SFG may indicate an overactive response to drug-related cues or an imbalance in cognitive control mechanisms, a hypothesis supported by previous studies. The MCG/PCG is involved in processing of pain and emotional responses, and decreased connectivity with the striatum may indicate impaired emotional regulation, which is commonly observed in addiction. Additionally, in SUD patients, we observed that the total score on the BIS-11 was significantly negatively correlated with the decline in striator-right MCG/PCG rsFC, which further supports the above conclusions. However, this meta-regression analysis included only seven studies, and the small number of studies may lead to unstable results and an increased risk of false positives. Future studies should incorporate more research to enhance the statistical power of meta-regression and further explore the neural mechanisms underlying impulsive behavior in SUD. Moreno‐López, et al. found coupling between the striatum and cerebellar regions in alcohol addicts, suggesting an unusually strong correlation between feedback representations and exercise habits. Muller-Oehring, et al. found that alcoholics showed increased frontostriatal connectivity and decreased connectivity with the limbic system (HIP/PHG, temporal lobe, fusiform gyrus) in the RW network. This may be related to heightened anxiety symptoms and increased alcohol cravings.
Abnormal rsFC patterns of amygdaloid seed
Compared with HCs, SUD patients showed significantly increased amygdala connectivity with the right PHG and decreased connectivity with the left SFG and left ACC/PCG. As a key hub in emotional processing, the amygdala plays a crucial role in the mechanisms of addiction. It mediates withdrawal symptoms of addiction, including the generation of negative emotions such as stress, anxiety, and depression. The decreased rsFC between the amygdala and the left SFG suggests a top-down inhibitory effect of the SFG. The SFG primarily guides decision-making and goal-directed behavior in response to emotional or rewarding stimuli, while the amygdala is involved in detecting the valence of emotional stimuli. Both play a synergistic role in regulating objective behavior.
Abnormal rsFC patterns of thalamus seed
SUD patients showed hypoconnectivity between the thalamus and the left SFG, left dACC, and left CN relative to HCs. These results are consistent with previous studies, emphasizing the critical role of the thalamus in SUD. The reduction in thalamus-SFG rsFC may be linked to impaired cognitive control. The SFG is a critical area for executive functions and top-down cognitive control, and its dysfunction may impair the ability of SUD patients to suppress impulsive drug-seeking behavior. Previous studies have also shown that the prefrontal-thalamic network is dysfunctional in SUD patients, closely associated with deficits in decision-making and impulse control. The reduction in thalamus-dACC rsFC may reflect deficits in emotion regulation and conflict monitoring, both associated with addiction. The dACC plays a crucial role in regulating motivationally driven behaviors and error monitoring, and its dysfunction may lead to increased attention to drug-related cues. Moreover, structural connectivity between the thalamus and ACC is enhanced in smokers, while functional connectivity is reduced, suggesting that chronic substance use may lead to maladaptive changes in the thalamus-ACC network. The reduction in thalamus-CN rsFC supports the hypothesis of dysfunctional reward processing in SUD. The thalamus influences reward learning by modulating dopamine signaling in the striatum, and the CN plays a central role in reinforcement learning and habit formation (Everitt & Robbins, 2005). Our findings are consistent with previous studies showing impaired thalamus-striatum connectivity in SUD, which may be related to increased craving and severity of drug dependence.
In summary, our study identifies disruptions in two critical reward circuits: the cortical-striatal-thalamic-cortical (CSTC) circuit and the cortical-striatal- HIP/PHG-amygdala-cortical (CSHAC) circuit. The CSTC circuit is crucial for mediating reward-related behaviors, emotional regulation, and addiction mechanisms. Furthermore, the CSTC circuit is associated with impaired decision-making, a hallmark feature of addiction. The CSHAC circuit integrates sensory information from the amygdala and episodic memory from the hippocampus and projects back to the frontal cortex via the striatum. This loop’s involvement in emotional regulation and memory formation suggests its crucial role in reward-based learning and addiction. While the CSTC loop has been extensively studied, the role of the CSHAC loop in addiction and reward processing remains relatively underexplored. The identification of these disrupted reward circuits aligns with existing theories on the neural mechanisms underlying SUD. Volkow, et al. propose that addiction involves alterations in brain circuits related to reward, motivation, memory, and inhibitory control. The disruptions in the CSTC and CSHAC loops observed in our study support this model, highlighting the critical neural pathways involved in SUD. Previous studies have also suggested that insights derived from mapping intrinsic brain connectivity networks can provide a framework for understanding various aspects of human behavior.
Limitations
Our study has several limitations. First, the included studies varied in sample characteristics, imaging protocols, and analysis methods, which may introduce variability and affect the robustness of our results. Second, most of the results in this study were uncorrected, which could affect the generalizability of our findings. Third, studies involving severe psychiatric disorders in the context of SUD were excluded, and this exclusion strategy may limit the external validity of the findings, particularly regarding their applicability to patients with SUD and co-occurring psychiatric disorders in clinical practice. Fourth, due to the scarcity of rsFC studies using the dorsal striatum (DS) as a seed region, we did not include striatal subgroup analysis. Future research should further investigate the interaction and functional differentiation between the VS and DS in the reward system of SUD. Finally, the cross-sectional design of the included studies limits our ability to infer causal relationships between rsFC alterations and SUD. Longitudinal studies are required to determine whether the observed connectivity changes are a cause or consequence of SUD. Future studies should consider other connectivity measures and multimodal imaging approaches to provide a more comprehensive understanding of the neurobiological mechanisms underlying SUD.
Conclusion
We identified common cortical-striatal-thalamic-cortical functional connectivity patterns in SUD patients through a seed-based whole-brain rsFC meta-analysis. This circuit mediates reward processing, goal-directed behavior, cognitive control, and impulsive behaviors, which are central to current research on the neurobiological mechanisms of addiction. Our study provides a neurobiological foundation for personalized treatment strategies for SUD and contributes to the innovation and optimization of therapeutic methods, such as deep brain stimulation, repetitive transcranial magnetic stimulation, electrical stimulation, and deep brain-machine interfaces in key brain regions.