Abstract
Persons that develop Alcohol Use Disorder (AUD) experience behavioral changes that include compulsion to seek and take alcohol despite its negative consequences on the person's psychosocial, health and economic spheres, inability to limit alcohol intake and a negative emotional/ motivational state that emerges during withdrawal. During all the stages of AUD executive functions, i.e. the person's ability to direct their behavior towards a goal, working memory and cognitive flexibility are eroded. Animal models of AUD recapitulate aspects of action selection impairment and offer the opportunity to benchmark the underlying circuit mechanisms. Here we propose a circuit-based approach to AUD research focusing on recent advances in behavioral analysis, neuroanatomy, genetics, and physiology to guide future research in the field.
1. Introduction
Acute alcohol intoxication and AUD are characterized by neurocognitive disorders affecting the perceptual-motor and executive function domains. Neurocognitive impairments emerge during the development of AUD, contributing to key clinical manifestations including loss of control over alcohol intake, compulsions centered on alcohol and negative emotional states [40]. Furthermore, neurocognitive impairments might persist following sustained abstinence. Understanding the neural substrates underlying the emergence of inflexible behavior that contribute to compulsive alcohol seeking, uncontrolled drinking, relapse and altered executive function during sustained abstinence is warranted to inform the refinement of targeted therapeutic approaches for AUD.
Adaptive behavior requires moment-to-moment action selection to master one's environment. Actions performed to obtain a specific outcome are defined as ‘goal-directed’, driven by Action-Outcome (AO) associations. Actions performed in response to a triggering environment based on past reinforcement history are defined as ‘habitual’, driven by stimulus-response (SR) associations. Cooperation between these two modalities enables the optimization of action performance and the learning of novel actions. An increased reliance on habitual action control has been proposed to contribute to behavioral inflexibility in AUD.
Fundamental brain circuits for action control have been identified in the Basal Ganglia (BG). In this review, we discuss translational research into the mechanisms underlying BG circuit dysfunctions induced by acute or chronic alcohol in adults. All the BG circuits have important roles in effects of alcohol and AUD. However, we will focus on the the associative and sensorimotor CB loops, as these circuits have been implicated in AO and SR instrumental conditioning as well as effects of alcohol on these behaviors. In parallel, we discuss fundamental research studies on the organization and function of basal ganglia circuits to identify novel avenues for future research.
2. The role of associative and sensorimotor basal ganglia loops in action control
2.1. The organization of associative and sensorimotor basal ganglia loops
Distinct BG loops share basic organizational motifs and are structured in parallel channels. Cortical and thalamic glutamatergic inputs and midbrain neuromodulatory inputs are integrated in the striatal microcircuitry by downstream projecting spiny projection neurons (SPNs) and locally projecting GABAergic and cholinergic interneurons [7], [63]. The dopaminergic inputs from the substantia nigra compacta and ventral tegmental area densely innervate the dorsal and ventral striatum, respectively.
In the dorsal striatum, SPNs expressing the dopamine D1 receptor form the direct pathway (dSPNs) and project to the substantia nigra reticulata (SNr), entopeduncular nucleus (EPN, the rodent homolog of the primate globus pallidus internal segment, GPi) and through bridge collaterals to the globus pallidus external segment (GPe). SPNs expressing the dopamine D2 receptor originate the indirect pathway (iSPNs) by targeting the GPe, which in turn innervates the SNr and subthalamic nucleus (STN), the latter ultimately projecting to the SNr. Direct and indirect pathway projections converge in the basal ganglia output nuclei, the GPi and SNr, that mainly project to brainstem, midbrain, and thalamic regions.
Extra-striatal cortical and amygdalar inputs are integrated in the GPe. The STN integrates extra-pallidal inputs, including the cortical ‘hyperdirect pathway’ and thalamic inputs. Hence, the GPe and STN are not only integral nuclei in the BG circuitry, but also input structures. Further, the GPe and STN possess specific extra-BG targets and feedback projections to the striatum and GPe, respectively. Two main GPe neuronal subtypes have been identified based on their projection targets and cellular identity: downstream (SNr) projecting ‘prototypic’ neurons and striatum projecting ‘arkypallidal’ neurons. Genetic markers of prototypic GPe neurons include the transcription factor NK2 homeobox 1(NKX2.1) and Parvalbumin (PV), whereas genetic markers of arkypallidal GPe neurons include the neuronal PAS domain protein 1 (Npas1) and Forkhead-box-Protein 2 (FoxP2). Prototypic neurons have been shown to be preferentially targeted by striatal iSPNs, whereas arkypallidal neurons have been shown to be the main recipient of the bridge collateral dSPN projections.
Circuit topography is preserved throughout parallel BG loops. The associative loop originates from associative cortices (notably medial prefrontal, mPFC and orbitofrontal, OFC) and targets the dorsomedial striatum (DMS) alongside amygdalar projections. A medial bias is maintained in the striatal downstream projections to the GPe and SNr. The sensorimotor loop originates from sensorimotor cortices (motor, sensory cortices) targeting the dorsolateral striatum (DLS), and a lateral bias is maintained in the downstream projections to the GPe and SNr. Other loops which will not be extensively discussed in this review include the ventrolateral loop involving the ventrolateral striatum (VLS) and the limbic loop involving the nucleus accumbens (NAc). The organization and function of the cortico-striatal branch of the associative and sensorimotor loops has received relatively more attention than its downstream branches until recently. In the SNr, DMS-connected and DLS-connected neurons are spatially segregated and target common outputs (thalamus, pedunculopontine nucleus, midbrain reticular formation, superior colliculus) while maintaining spatial segregation.. In addition to their common targets, functionally and spatially distinct subpopulations of SNr neurons have been shown to collateralize throughout the midbrain and brainstem to innervate specialized targets.
A funnel-like organization that allows for dimensionality reduction of output information has been proposed for basal ganglia circuits. In the context of this model, context-dependent, state-dependent and high-order inputs are integrated through the striatal microcircuitry and transferred to downstream structures that innervate specialized targets to influence motor and cognitive processes. Evidence from in-vivo imaging studies led to the hypothesis that different ‘ensembles’, functionally and spatially related subgroups of striatal SPNs, are organized to control specific aspects of action performance and are selectively recruited during behavior. The recruitment of specific ensembles for action performance requires the interplay of excitatory cortical and thalamic inputs, neuromodulation by dopamine and acetylcholine as well as lateral inhibition between SPNs. Here we propose that the role of striatal ensembles for action is a key part of models of BG circuit dysfunction in AUD and should be examined in depth in future research (see section 2).
2.2. Functional roles of associative and sensorimotor basal ganglia loops
Associative and sensorimotor circuits are key mediators of goal-directed and habitual behavioral control, respectively, as reviewed elsewhere and have been investigated in human and animal studies examining alcohol effects on action control.
While BG loops have been implicated in Pavlovian conditioning, the associative and sensorimotor circuits have especially prominent roles in instrumental learning and control thereof by outcomes, reinforcement history and the environment. Furthermore, the literature on the effects of misused substances on associative and sensorimotor circuit function in the context of instrumental learning is more detailed than that examining Pavlovian conditioning, although the role of Pavlovian to instrumental transfer (PIT) is an emerging theme. Thus, we have chosen to focus on instrumental learning in this review. Instrumental conditioning paradigms have allowed investigators to operationally define goal-directed and habitual action control. In instrumental conditioning paradigms, AO/goal-directed and SR/habitual associations are established by pairing an action (e.g., lever press) with a salient outcome (e.g., sucrose pellet). Briefly, instrumental training is performed to consolidate the associations and afterwards action performance is tested in brief sessions under extinction conditions (no outcome delivered) using outcome devaluation procedures. The outcome devaluation procedures are designed to assess if the outcome drives the behavior. The training schedule can be varied to bias the animal toward goal-directed strategies or habitual strategies using random ratio (sensitive to outcome devaluation) or random interval schedules (insensitive to outcome devaluation), respectively. Animals that respond to the outcome devaluation by decreasing action performance are thought to maintain an AO strategy of action performance, as opposed to animals that continue performing the same action using an SR strategy. Other methods to assess AO versus SR action strategies include contingency degradation and omission procedures. In a contingency degradation paradigm, rewards are delivered randomly with no relation to action performance (e.g., lever pressing). Animals that decrease pressing during these sessions are thought to be using an AO strategy, while those that maintain pressing are likely using an SR strategy. In the omission test, animals are rewarded for not producing an action. In this paradigm, animals using an AO strategy will decrease their pressing faster than those using an SR strategy. Instrumental conditioning paradigms have been used to assess goal-directed and habitual alcohol seeking and to assess action control following chronic alcohol exposure. Further, they have been used to test for reversal learning using dual lever-press paradigms. In dual lever pressing paradigms, the contingency can be reversed after training and the performance assessed as a measure of behavioral flexibility.
3. Neuroadaptations in the associative striatum following acute and chronic alcohol exposure
We will first review the Associative Circuit. In model 1, we consider changes in the integrative properties of dSPNs and iSPNs in the DMS that contribute to increased alcohol seeking and taking (Fig. 1). In model 2, we consider the role of altered top-down cortical control of the DMS in behavioral flexibility deficits following chronic alcohol exposure (Fig. 2).
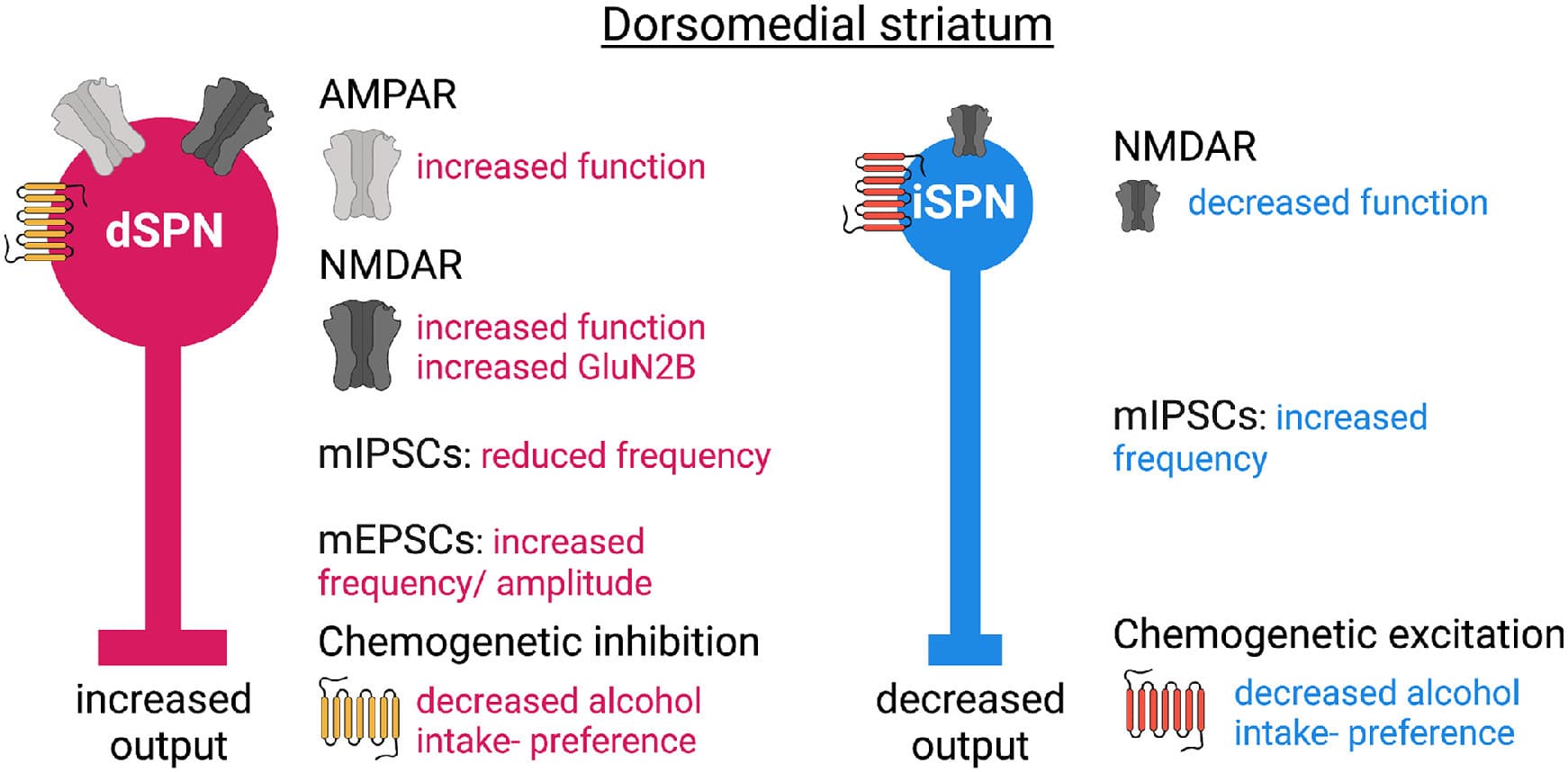
Fig. 1. Model 1, neurotransmitter imbalance in the DMS. dSPN: direct pathway spiny projection neuron. iSPN: indirect pathway spiny projection neuron.
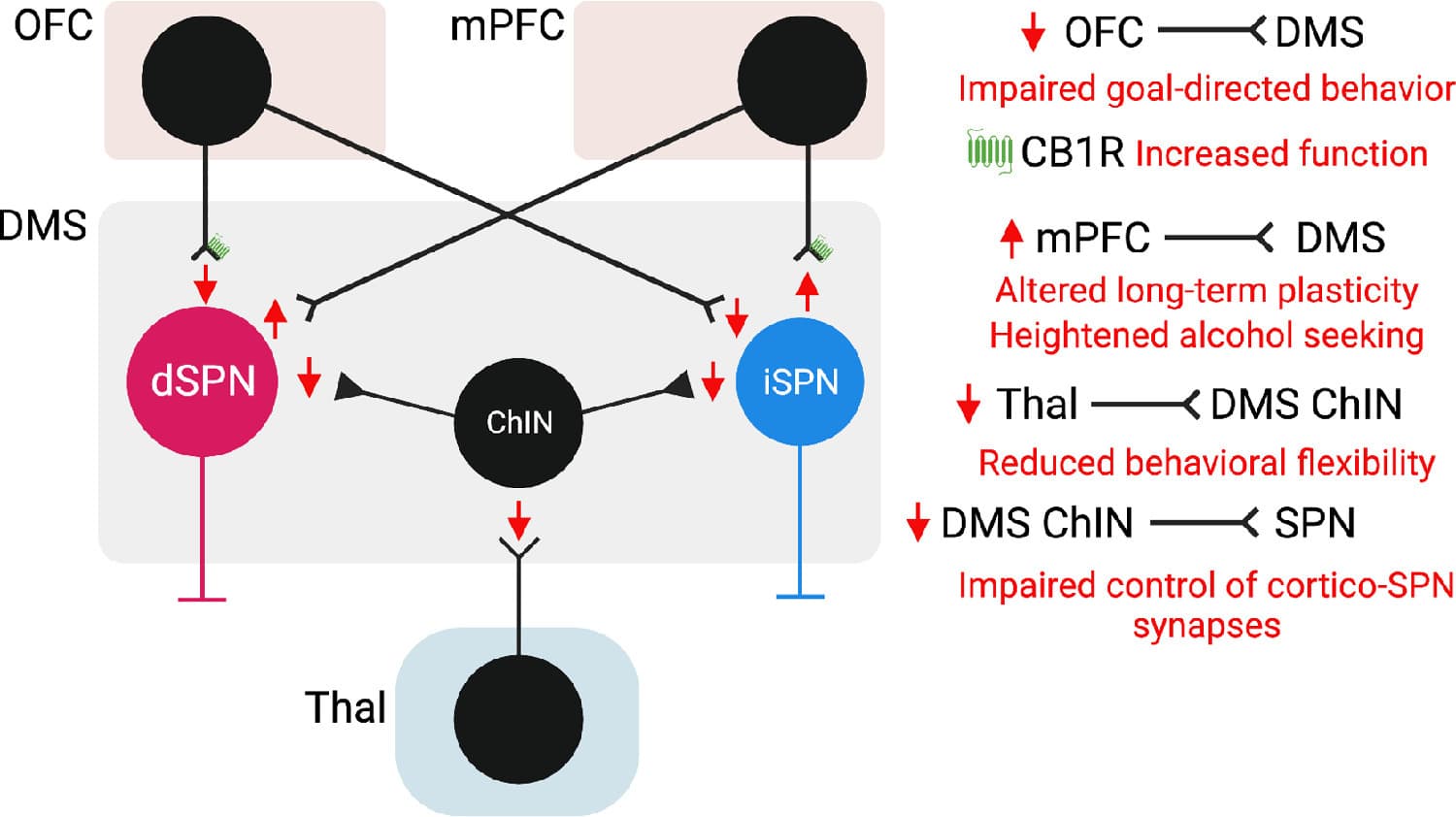
Fig. 2. Model 2, cortico-striatal and thalamo-striatal deficits contributing to altered behavioral flexibility and alcohol seeking following chronic alcohol exposure. OFC: orbitofrontal cortex. mPFC: medial prefrontal cortex. DMS: dorsomedial striatum. Thal: thalamus. ChIN: Cholinergic Interneuron. dSPN: direct pathway spiny projection neuron. iSPN: indirect pathway spiny projection neuron.
3.1. The role of GABAergic and Glutamatergic transmission in the DMS in alcohol seeking and taking
Acute alcohol has been shown to enhance GABAergic miniature inhibitory postsynaptic current (mIPSC) frequency in DMSSPNs in mice. Conversely, chronic binge-like alcohol drinking was shown to decrease the frequency of mIPSCs in DMSSPNs whereas no changes were observed in miniature excitatory postsynaptic currents in DMSSPNs in mice. The postsynaptic characteristics of mIPSCs were found to be altered independently from the age of drinking onset in the caudate of macaques, whereas mixed effects were observed in the frequency of mIPSCs which increased only in monkeys that begun drinking as young adults. The frequency of miniature excitatory postsynaptic currents (mEPSCs) was found to be increased by alcohol drinking in the caudate of rhesus monkeys.
Changes reported in DMSdSPNs following chronic alcohol drinking in mice include altered morphology and altered glutamatergic input integration. The spine density and dendritic branching was found to be increased and he function of AMPA receptors and the frequency and amplitude of mEPSCs was shown to be increased. The function of NMDA receptors and in particular GluN2B-containing NMDARs was reported to be increased. Further, alcohol self-administration was found to produce increased NMDAR function in dSPNs. In DMSiSPNs, NMDAR function was shown to be decreased, whereas GABAergic transmission was increased.
Hence, bidirectional changes occur in DMSdSPNs and DMSiSPNs following chronic alcohol drinking or self-administration. An increased excitatory drive DMSdSPNs might contribute to increased alcohol drinking and seeking behaviors, and accordingly the chemogenetic inhibitory modulation of DMSdSPNs was found to decrease alcohol intake and preference in mice previously exposed to chronic drinking. Conversely, an increased inhibitory drive was reported DMSiSPNs and accordingly chemogenetic excitatory modulation of DMSiSPNs was found to decrease alcohol intake and preference in mice previously exposed to chronic drinking.
Overall, a first model of DMS circuit dysfunctions following chronic alcohol drinking indicates a predisposition of DMS circuits towards increased direct pathway output and decreased indirect pathway output driving alcohol seeking and taking behaviors (Fig. 1). However, the recruitment of striatal SPNs for action control depends on cortical inputs and on the neuromodulation particularly from dopaminergic inputs and cholinergic interneurons. The role of altered neuromodulation and top-down cortical control following chronic alcohol exposure will be summarized in model 2.
3.2. DMS circuit dysfunctions underlying altered behavioral flexibility following chronic alcohol exposure
Mice previously exposed to chronic intermittent alcohol (CIE) vapor were shown to have habit-like responding to sucrose reward in an outcome devaluation test following random ratio training, while preserving habit formation following random interval training. Further, mice exposed to CIE were reported to use different action strategies than untreated mice to perform lever press sequences during random ratio training. In another study, rats chronically exposed to alcohol drinking and trained to lever press for two different outcomes on two different levers were found to show a reversal learning deficit.
The strength of OFC cortico-striatal inputs was found to be reduced following chronic alcohol exposure, and later it was shown that an increased CB1 receptor function mediated increased inhibition of OFC inputs. The activity of OFC terminals in the striatum measured in vivo as Ca2+ measurements was reported to be increased during action performance (lever press) in an operant task but decreased at reward delivery in CIE mice compared to control mice. Decreased activity of OFC terminals at reward delivery might impair the encoding of reward value, contributing to the loss of value-based goal directed behavior following chronic alcohol exposure in mice. Normalizing CB1 signaling restored OFC terminal activity at reward delivery and goal-directed behavior in chronic alcohol exposed mice. Overall, loss of top-down control over DMS function by the orbitofrontal cortex impairs goal-directed behavior. A weakened OFC-DMS projection has also been proposed to contribute to alcohol seeking behaviors, as in vivo optogenetic long-term potentiation (LTP) of OFC to DMS projections was shown to decrease alcohol self-administration in rats.
The mPFC-DMS projection is critical for the cognitive control of action performance. Bidirectional in vivo control of mPFC-DMS synaptic plasticity has been shown to exert opposite effects on alcohol seeking behavior. The induction of endocannabinoid-dependent long-term depression (LTD) of mPFC-dSPN synapses was shown to decrease alcohol seeking. Dopamine D1 and D2- receptor dependent LTD at mPFC-SPN synapses transiently increased alcohol seeking and LTD induction in the presence of D1 and D2 receptor antagonists was reported to induce a lasting suppression of alcohol seeking.. Conversely, LTP of mPFC-dSPN projections was found to increase alcohol seeking. Taken together these findings indicate that altered plasticity at mPFC-DMS projections contributes to perseveration in alcohol seeking behaviors.
Thalamic inputs target dSPNs and iSPNs and preferentially innervate striatal cholinergic interneurons (ChINs) over other striatal interneurons. Thalamic inputs to DMSChINs play a central role in behavioral flexibility. Decreased thalamic input strength to DMSChINs was observed following chronic alcohol drinking and was associated with impaired cholinergic control over cortical inputs to the DMS and ChIN firing. LTP of thalamic inputs to the DMS was found to ameliorate reversal learning deficits following chronic alcohol drinking.
Overall, in model 2 we summarize altered cortico-striatal and thalamo-striatal circuits that contribute to reduced behavioral flexibility and altered value-based decision making following chronic alcohol exposure in animal models (Fig. 2).
Altered DAergic control over associative striatal circuitry might further contribute to circuit dysfunctions induced by chronic alcohol. A study in rhesus macaques reported that chronic alcohol consumption alters dopamine release in a sex-specific manner. Dopamine release was found to be increased in the caudate of female macaques, whereas in males dopamine release was not changed in the caudate nucleus. Following abstinence, dopamine release in males was significantly decreased in the caudate. Dopamine uptake was increased in the caudate and putamen of female but not male macaques. Control of dopamine release by D2/D3 receptors was decreased in the caudate of male but not female macaques, but no difference was observed in cholinergic regulation of DA release. In another study where DA release was examined in chronic drinking macaques, decreased release and uptake was observed in the dorsal lateral caudate nucleus (a portion of the macaque DMS) compared to controls. Taken together, dopamine dynamics in the associative striatum are altered in a sex-specific manner and might contribute to drive divergent neuroadaptations during alcohol consumption and following abstinence.
The availability of dopamine D2 receptors was shown to be decreased in individuals with AUD. Interestingly, mice lacking D2 receptors in iSPNs were shown to have enhanced sensitivity to ethanol stimulation, higher preference and escalation of drinking. Low D2 receptors in iSPNs was reported to produce hypersensitivity of striatal dopamine D1 receptors selectively in the DMS, and accordingly knock-down of D1Rs in the DMS but not ventral striatum reduced ethanol stimulation and preference. Hence, altered striatal DA receptor signaling further contributes to AUD-related phenotypes and striatal circuit dysfunctions.
Future studies shall identify how in vivo striatal ensemble function is altered by acute and chronic alcohol: how does perseveration emerge, and which circuit- based strategies are best suited to reduce it? It is also critical to understand how an altered striatal output will impact on downstream circuitry.
4. Neuroadaptations in the sensorimotor striatum following acute and chronic alcohol exposure
We will now turn our attention to the sensorimotor circuit. In model 3, we describe that a disinhibition of the cortical projections to the DLS has been implicated in habitual alcohol seeking and taking and in the maintenance of binge alcohol drinking (Fig. 3).
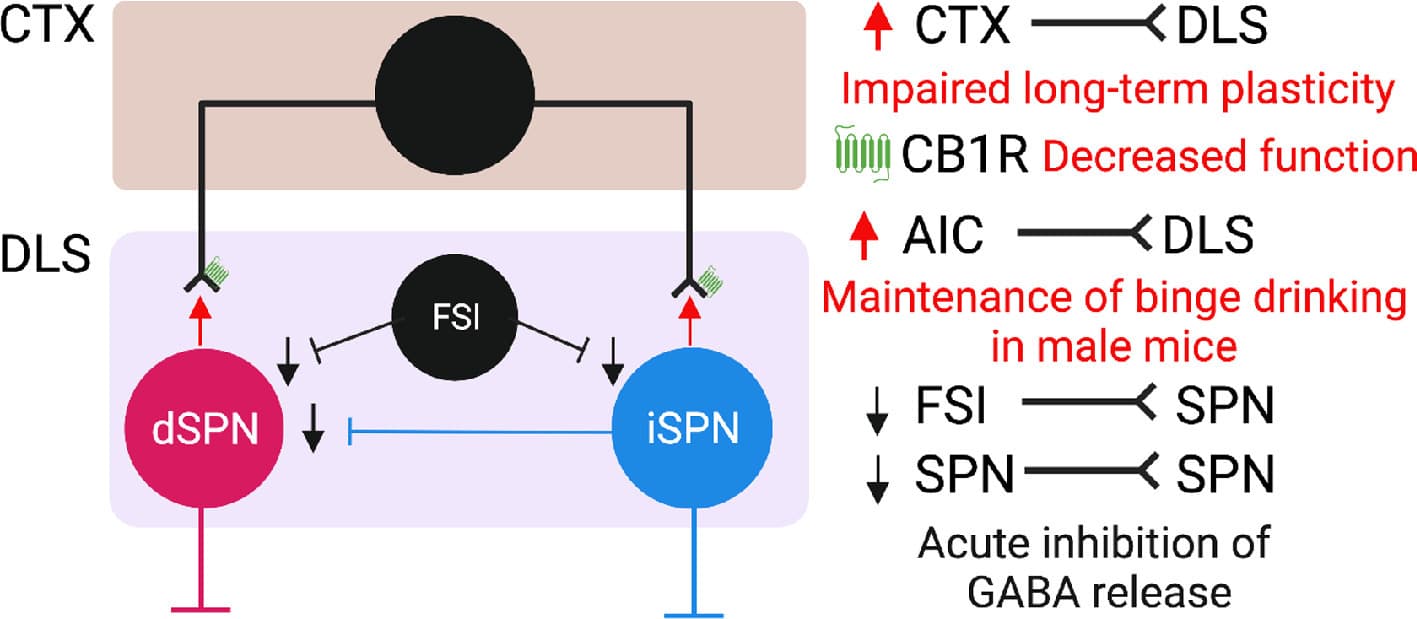
Fig. 3. Model 3, acute and chronic alcohol effects on the sensorimotor striatal circuitry. CTX: motor and somatosensory cortex. AIC: anterior insular cortex. DLS: dorsolateral striatum FSI: fast spiking interneuron. dSPN: direct pathway spiny projection neuron. iSPN: indirect pathway spiny projection neuron.
Operant responding for alcohol but not for sucrose in rats was demonstrated to become habitual after 4 weeks of training. Inactivation studies where a GABAA agonist was infused in the DMS or DLS shown that whilst the inactivation of the DMS accelerated the emergence of habitual alcohol seeking, inactivating the DLS prevented it. Similar results were obtained through the local infusion of AMPA and D2 receptor antagonists in the DLS. In a more recent study using a seeking-taking chained design in alcohol-preferring rats, seeking responses in a subpopulation of rats which developed compulsive alcohol seeking were reduced through the inhibition of DA receptors in the DLS. Overall, these studies demonstrated that abnormal DLS function participates to habitual and compulsive alcohol-seeking behaviors and put the accent on the role of neurotransmitter and neuromodulator systems in this region.
Acute alcohol exposure was shown to reduce GABAergic mIPSC frequency in the mouse DLS. Acute bath application of alcohol was reported to inhibit optogenetically- evoked GABAergic transmission at synapses made by fast-spiking interneurons onto DLSSPNs. Furthermore, acute alcohol was shown to inhibit SPN-SPN synapses. Interestingly, ablation of FSIs results in reduced ethanol consumption and disrupts drinking microstructure. Neuroadaptations in the putamen of chronic drinking macaques measured during abstinence include increased glutamatergic mEPSCs and increased excitability of striatal SPNs, together with decreased GABAergic mIPSCs. These changes were accompanied by increased dendritic arborization and spine density of SPNs in the putamen. The dendritic arborization of DLSSPNs was also found increased in a mouse model of passive (vapor) alcohol exposure. Following prolonged binge-like drinking in mice, mIPSCs frequency in DLSSPNs was found to be decreased whereas no change was observed in mEPSCs. These changes were accompanied by a reduction in the sensitivity of synapses on SPNs to acute alcohol application. Hence, an overall disinhibition of DLSSPNs following chronic alcohol drinking was observed across species.
Equal to the associative striatum, the activity of DLSSPNs is driven by cortical and neuromodulatory inputs. A reduction in striatal endocannabinoid (eCB)-dependent plasticity on cortical inputs to the DLS was observed following chronic alcohol exposure. Mice chronically exposed to alcohol displayed heightened performance in a visual discrimination and reversal learning task and the activity of DLS neurons during a reversal learning task was increased in the late phases of reversal training. Hence, circuit adaptations following chronic alcohol exposure predispose towards an increased engagement of DLSSPNs in action control. A recently characterized projection from the anterior insular cortex (AIC) to the DLS has been investigated in the context of a mouse model of binge alcohol drinking. A first study reported that μ-opioid receptor dependent long-term depression is lost at insular cortex projections to the DLS following binge alcohol drinking The AIC projection to the DLS was found to be potentiated following binge alcohol drinking and implicated in the maintenance of alcohol binge drinking in male but not female mice. Photostimulation of AIC to DLS projections reduced binge drinking.
Dopamine release was reported to be unchanged in the putamen of chronic drinking female macaques, whereas in male dopamine release was decreased in the putamen. Following abstinence, dopamine release in males was significantly decreased in the putamen, and dopamine uptake was increased in the putamen of females but not male macaques. Control of dopamine release by D2/D3 receptors was decreased in putamen of male but not female macaques, but no difference was observed in cholinergic regulation of DA release in the putamen.
In summary, changes reported in DLSSPNs predispose the sensorimotor striatum towards an increased output. Loss of plasticity at cortico-striatal synapses can impact the learning of novel actions and behavioral flexibility. It remains to be established how these changes affect DLSdSPNs and DLSiSPN specifically. Further, it will be important to investigate how DLS ensemble function is altered following chronic alcohol exposure and how these changes impact downstream circuitry, as discussed in the final part of this review.
5. Novel vistas: consequences of striatal circuit dysfunctions on downstream circuitry following chronic alcohol exposure
5.1. Associative circuits: behavioral flexibility with a sweet spot for sleep and arousal
In this paragraph we review recent anatomical and functional studies on the direct, bridge collateral and indirect pathway portions of the associative circuit. We highlight novel research avenues needed to understand how altered cortico-striatal and striatal function (Fig. 1, 2) will impact action control via abnormal recruitment of downstream circuitry.
DMSdSPN-SNr projections target medial SNr neurons, whose specific molecular identity remains undefined. Most SNr neurons are projection neurons that express GABAergic markers including the vesicular GABA transporter (VGAT) and glutamate decarboxylase 2 (GAD2), except for a subpopulation of dopaminergic neurons whose connectivity remains undefined (∼5 % of neurons). A large number (∼85%) of SNr neurons are parvalbumin (PV) expressing neurons that are enriched in the lateral tier of this nucleus. It is currently unclear whether PV+ or PV− neurons in the SNr are differentially innervated by different striatal subregions. SNr projection neurons collateralize so that each SNr neuron targets ‘canonical’ SNr targets (motor and intralaminar thalamus, midbrain reticular formation, pedunculopontine nucleus and zona incerta) while simultaneously targeting specialized midbrain and brainstem targets. In the case of medial SNr neurons, specialized targets include neuromodulatory regions such as the dorsal raphe, locus coeruleus, and ventral tegmental area, and the pain-related periaqueductal gray region. Functional manipulation and Ca2+ imaging of SNr PV neurons or medial GAD2-expressing neurons suggests that, while both are involved motor control, medial GAD2-expressing neurons are involved in promoting sleep. Hence, altered DMSdSPN-SNr output following chronic alcohol exposure might not only impair action control but also play a role in changing control of brain states including arousal and sleep, and pain modulation.
DMSiSPN-GPe projections were shown to preferentially target prototypic neurons in the GPe. Further, GPePV neurons innervated by the DMS preferentially target the Pf thalamus and their activation impairs reversal learning. In model 1, we reviewed the idea that GABAergic inhibition of DMSiSPNs is increased following chronic alcohol exposure. As a result, a disinhibition of GPePV-Pf thalamus projections following chronic alcohol exposure might occur, contributing to reversal learning deficits reported in rodent models of chronic alcohol exposure.
DMSdSPN-GPe projections were shown to preferentially target Npas1- expressing ‘arkypallidal’ neurons in the GPe, whose main output is the striatum. Functional manipulations indicate that DMSdSPN-GPe projections might be implicated in promoting movement via the inhibition of GPeNpas1 neurons. Arkypallidal projections to the striatum provide a “stop signal” for action suppression. A study from our laboratory has reported that moderate, acute alcohol doses can decrease the firing of arkypallidal GPe neurons via a mechanism involving large conductance voltage- and Ca2+-gated (BK) potassium channels. The stop signal provided by Npas1-neurons to striatal SPNs might therefore be relieved during alcohol intoxication. In model 1, we summarized studies indicating a disinhibition of DMSdSPNs following chronic alcohol exposure and in model 2 studies indicating an impaired top-down cortical control of the DMS microcircuitry. Hence, enhanced DMSdSPN-Npas1 connectivity might alter stop signals conveyed to the striatum, contributing to heightened alcohol seeking and taking behaviors.
5.2. Sensorimotor circuit: habits and movement
Here, we review recent anatomical and functional studies on the direct, bridge collateral and indirect pathway portions of the sensorimotor circuit. DLSdSPN-SNr projections target the lateral SNr, and lateral SNr neurons target primarily motor-related regions. A disinhibition of DLSdSPN-SNr projections following chronic alcohol exposure (Fig. 3) might direct context-dependent action control towards habitual alcohol seeking and taking.
DLSdSPN-GPe projections preferentially target Npas1-expressing ‘arkypallidal’ neurons in the GPe. One consequence of the DLS plasticity summarized in Fig. 3 might be that an increased striatal output induces abnormally strong inhibition of GPeNpas1 neurons, further contributing to disinhibition of DLS output.
DLSiSPN-GPe projections preferentially target parvalbumin expressing neurons in the GPe. GPePV neurons are enriched in the dorsolateral portion of the nucleus and GPePV neurons targeted by the DLS preferentially target the SNr promoting locomotion. One possibility is that an increased iSPN-GPe output (Fig. 3) might induce abnormally excessive inhibition of GPePV neurons.
Overall, key questions remain as to how DLS plasticity reverberates in downstream targets to disinhibit habitual action control. It will be important to establish whether distinct plasticity occurs at synapses made by DLSdSPNs and DLSiSPNs. Further, in vivo studies should clarify how neurons in the GPe and SNr encode action control following chronic alcohol exposure.
6. Conclusions
A corpus of animal studies has identified several cellular and circuit mechanisms underlying alcohol effects on associative and sensorimotor basal ganglia circuits. Fundamental research is helping to identify and diversify the broad neuronal networks embedded in associative and sensorimotor basal ganglia circuits, unraveling novel targets and neural processes influenced by these circuits. Future studies should determine how altered striatal function influences downstream targets whose circuit organization and functional roles are now beginning to be mapped to a greater extent. The impact of acute and chronic alcohol on striatal ensembles and on the modulation of striatal circuits by dopamine and other neuromodulators can now be studied in greater detail thanks to the advances and scalability of optical imaging techniques.
Here, we reviewed translational and fundamental studies and identified pressing questions to be addressed in future research into alcohol effects on the associative and sensorimotor circuits. We provide several examples of studies that detailed the acute and chronic effects of alcohol on cells, molecules and circuits of the basal ganglia identifying novel targets for translational studies on AUD related phenotypes including alcohol seeking and taking.