Abstract
Objective: Methamphetamine and cannabis are two widely used, and frequently co-used, substances with possibly opposing effects on the central nervous system. Evidence of neurocognitive deficits related to use is robust for methamphetamine and mixed for cannabis. Findings regarding their combined use are inconclusive. We aimed to compare neurocognitive performance in people with lifetime cannabis or methamphetamine use disorder diagnoses, or both, relative to people without substance use disorders. Method: 423 (71.9% male, aged 44.6 ± 14.2 years) participants, stratified by presence or absence of lifetime methamphetamine (M-/M+) and/or cannabis (C-/C+) DSM-IV abuse/dependence, completed a comprehensive neuropsychological, substance use, and psychiatric assessment. Neurocognitive domain T-scores and impairment rates were examined using multiple linear and binomial regression, respectively, controlling for covariates that may impact cognition. Results: Globally, M+C+ performed worse than M-C- but better than M+C-. M+C+ outperformed M+C- on measures of verbal fluency, information processing speed, learning, memory, and working memory. M-C+ did not display lower performance than M-C- globally or on any domain measures, and M-C+ even performed better than M-C- on measures of learning, memory, and working memory. Conclusions: Our findings are consistent with prior work showing that methamphetamine use confers risk for worse neurocognitive outcomes, and that cannabis use does not appear to exacerbate and may even reduce this risk. People with a history of cannabis use disorders performed similarly to our nonsubstance using comparison group and outperformed them in some domains. These findings warrant further investigation as to whether cannabis use may ameliorate methamphetamine neurotoxicity.
Introduction
Methamphetamine and cannabis are two widely used, and frequently co-used, substances with possibly opposing effects on aspects of central nervous system functioning. United States population prevalence estimates show that of people who have used methamphetamine in the past year, an overwhelming majority (78.5%) also used cannabis in the past year, and though not a majority, a substantial percentage (39.1%) of people meeting past-year DSM-5 methamphetamine use disorder criteria also met criteria for past-year cannabis use disorder (U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2022).
The connection between methamphetamine use and neurocognitive deficits has been well documented, with prominent deficits observed in episodic memory, executive functioning, information processing speed, and visuospatial abilities (Daldegan-Bueno et al., 2021; Naveed et al., 2022; Reback et al., 2018; Saloner et al., 2020; Scheffler et al., 2022; Watson et al., 2020). Research on cannabis use and neurocognitive outcomes is less conclusive than for methamphetamine use. Whether cannabis use results in performance deficits and/or impairments may be dependent on the neurocognitive domain in question. A meta-analysis of acute cannabis effects (i.e., those conferred by acute cannabis intoxication) studies using neurocognitive assessments found evidence for lower performance on measures of psychomotor functioning, attention, verbal learning and memory, but results are still mixed for working memory, verbal fluency, and executive functioning domains (Broyd et al., 2016). The latter authors and Ramaekers et al. (2021) argue that inconsistent findings for acute effects can be at least partially attributed to sample heterogeneity in terms of prior cannabis exposure patterns, acute dose delivered, mode of delivery, and age of cannabis use initiation.
As for long-term effects, meta-analyses generally demonstrate that long-term cannabis use is associated with small but detectable detrimental effects in both adults and adolescents, relative to demographically matched controls (Grant et al., 2003; Schreiner & Dunn, 2012; Scott et al., 2018). However, it is unclear whether these effects can be differentiated from those of acute intoxication or short-term residual effects (i.e., following acute intoxication but <72 hours after use). When examining neurocognitive performance after periods of cannabis abstinence that range from 72 hours (Scott et al., 2018) to at least one month (Schreiner and Dunn, 2012), these relative neurocognitive deficits appear absent, suggesting that residual effects of cannabis on neurocognition are likely reversible after discontinuing use.
Given mixed evidence surrounding neurocognitive deficits and cannabis use, it comes as no surprise that findings regarding combined methamphetamine and cannabis use are inconclusive. Preclinical evidence suggests that cannabis may protect against aspects of methamphetamine neurotoxicity by inhibiting neural nitric oxide synthase via CB1-dependent mechanisms and by activating astrocytes via CB1-independent mechanisms (Castelli et al., 2014). Acutely, stimulation of the endocannabinoid system via CB2 receptors was found to decrease methamphetamine neurotoxicity resulting from an overdose administration in mice (Nader et al., 2014). Preclinical studies of chronic co-administration of cannabis and methamphetamine have found that exposure to methamphetamine induces cognitive impairment that may be improved by chronic cannabinoid administration (Razavi et al., 2020). Findings from human studies are mixed due in part to heterogeneity of design, participant characteristics (e.g., age, gender, diagnoses), cannabinoids used (e.g., THC, CBD) and differences in the operationalization of cannabis and methamphetamine study groups. Human participants research has primarily focused on subpopulations in which the use of methamphetamine and cannabis is relatively more prevalent than in the general populations (e.g., people with schizophrenia, people living with HIV). Evidence from these clinical populations follows much the same pattern: methamphetamine use confers risk for lower neurocognitive performance, but the effects of cannabis use are variable, and may be beneficial, detrimental, or unrelated to neurocognitive performance (Daldegan-Bueno et al., 2021; Naveed et al., 2022; Reback et al., 2018; Saloner et al., 2020; Scheffler et al., 2022; Watson et al., 2020).
In one of the few studies to compare neurocognitive performance and impairment rates among people who use methamphetamine and/or cannabis, Gonzalez et al., (2004) found that people with both methamphetamine and cannabis lifetime substance use disorder diagnoses displayed statistically equivalent neurocognitive performance and likelihood of neurocognitive impairment (NCI) to demographically matched comparison participants. On the other hand, participants with only methamphetamine diagnoses displayed significantly worse neurocognitive performance and impairment likelihood relative to the control group. This early investigation displayed trends resembling evidence from the preclinical literature, suggesting that cannabis may protect against methamphetamine-related neurological damage. With access to a larger sample and an additional cannabis use disorder comparison group, we aimed to compare global and domain-specific (i.e., verbal fluency, executive functions, information processing speed, learning, memory, working memory, and motor function) neurocognitive performance and deficits in people with lifetime cannabis and/or methamphetamine use disorder diagnoses, relative to people without these lifetime substance use disorder diagnoses.
Method
Sample
Participants included 423 adults enrolled in various NIH-funded research studies (see acknowledgments section for details).
Participants provided written, informed consent to undergo study procedures, which were approved by the UCSD Institutional Review Board and completed in accordance with Helsinki Declaration. Participants were included in the present analyses if they completed a comprehensive neuromedical, neurocognitive, psychiatric, and substance use assessment. Participants were stratified into four groups based on the presence or absence of lifetime methamphetamine (M+/M−) and cannabis (C+/C−) use abuse or dependence diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders : DSM-IV, 1994): M−C- (n = 170), M−C+ (n = 59), M+C− (n = 78), and M+C+ (n = 116).
Participants were excluded from inferential analyses according to the following criteria: (1) participant was HIV+; (2) participant presented for their assessment with a positive Breathalyzer test or urine drug screen other than THC for C+ groups, or METH for the M+ groups, (3) participant met criteria for DSM-IV alcohol or other (non-cannabis, non-METH) substance abuse/dependence within a year of assessment, (4) Presence of any known active major neurological (e.g., seizure, stroke) or psychiatric conditions (i.e., lifetime psychosis, unstable psychiatric diagnoses in the past 6 months, and changes to psychiatric medication in the past six months), learning disabilities, or dementia diagnosis that may confound their performance on neurocognitive measures; (5) Wide Range Achievement Test-4 (WRAT-4; Wilkinson and Robertson, 2006) reading subtest standard scores <80.
Measures
All study participants were administered standardized neuromedical, neurocognitive, and psychiatric evaluations. The neuromedical evaluation included a standardized medical history interview to assess for current and past medical conditions (e.g., Hepatitis C Virus [HCV], diabetes, hypertension, hyperlipidemia) using a combination of self-report and laboratory measurements, as well as a Breathalyzer to assess for recent alcohol use and specimen collection (blood, urine) for routine clinical labs, diagnostic (e.g., HIV, HCV) tests, and urine toxicology screening. No participants had a positive Breathalyzer test on the morning of the evaluation.
Psychiatric and substance use history
The Composite International Diagnostic Interview (Robins et al., 1988) was administered to assess for presence of current and lifetime substance use and mood disorders (e.g., major depressive disorder [MDD]) based on DSM-IV criteria as well as age at first and most recent diagnosis. Detailed lifetime cannabis (Robinson et al., 2014) and methamphetamine (Rippeth et al., 2004) use characteristics, including age at first use, years since last use, age at first cannabis/methamphetamine use disorder, years since last use disorder, and total lifetime duration of use (days) and total lifetime quantity (grams) were gathered using a semi-structured timeline follow-back substance use interview. Timeline follow-back data were missing for a subset of participants in the C+ (n = 4, 2.3%) and M+ (n = 12, 6.2%) groups. Cumulative density of use was calculated using estimates of total grams of a substance used per estimated days used over the participants’ lifetime. Participants’ current prescription medication usage was also assessed, and these variables were used to better inform medical comorbidity variables (e.g., hypertension, diabetes, lipidemia), based on whether participants were actively using medications to alter/ manage these conditions. Current self-reported depressive symptoms were assessed using the Beck Depression Inventory – 2nd version (Beck et al., 1996).
Neurocognitive performance
Participants completed a standardized battery of tests to evaluate neurocognitive functioning, which included an estimate of premorbid verbal IQ (i.e., Wide Range Achievement Test-4 [WRAT-4] Reading subtest). The battery included 14 tests assessing seven domains relevant to methamphetamine and/or cannabis (Rippeth et al., 2004; Watson et al., 2020) including verbal fluency, information processing speed, executive functions, learning, memory, attention/working memory, and motor skills (for a list of tests, see Heaton et al., 2010). Raw scores from individual tests were converted to T-scores (Mean = 50; SD = 10) which are demographically adjusted for age, education, sex, and race/ethnicity as appropriate based on published normative samples (Heaton, 2004; Heaton et al., 2003; Norman et al., 2011), Individual test T-scores were averaged within domain to obtain domain neurocognitive T-scores and together to obtain a global neurocognitive T-Score. Global and domain neurocognitive T-scores were used to assess neurocognitive performance across the study groups, where higher values indicate better performance.
Neurocognitive impairment (NCI)
Demographically corrected T-scores from individual tests were also converted into deficit scores, ranging from 0 (T-score >39, no impairment) to 5 (T-score <20, severe impairment) and averaged to create domain deficit scores (DDS) and a global deficit score (GDS), which were used as outcome variables in analyses. To classify global impairment, we used a cutoff of greater than or equal to 0.5, a score that represents performance that is at least mildly impaired on at least half of the tests in the battery (Carey et al., 2004; Heaton et al., 1995); domain impairment DDS cutoff is >0.5.
Data analysis
All analyses were conducted using R (version 4.1.1). Descriptive statistics are listed in Table 1. For neuromedical and psychiatric variables previously shown to confound neurocognitive performance (i.e., all medical, psychiatric, and lifetime substance variables listed in Tables 1 and 2) that were also observed to be different among the groups, we generated Pearson correlation matrices with global and neurocognitive domain T-scores to determine which variables justified inclusion as covariates in subsequent modeling procedures. Current depressive symptoms (BDI-II Total Score), hepatitis C infection, and lifetime alcohol use disorder, cocaine use disorder, and opioid use disorder were observed to significantly correlate with neurocognitive measures and were included as covariates in all inferential models. Covariates were subsequently trimmed from individual models if they did not significantly improve model fit. Demographic and education-related variables were omitted as model covariates, as response variables (T-scores and rates of impairment) were demographically adjusted for age, education, sex, and race/ethnicity prior to modeling. Sensitivity checks were conducted to ensure that the inclusion of WRAT-4 test scores did not alter model inferences.
Separate multiple linear regression models of Global and Domain neurocognitive T-scores were used to examine differences in performance across groups. Model contrast codes were determined from two primary comparisons of interest and from examining descriptive neurocognitive performance results among the groups. We were initially interested in determining how people with both lifetime methamphetamine and cannabis use disorder differed from those with lifetime methamphetamine use disorder and from those with neither use disorder (i.e., M+C+ vs. M+C− and M+C+ vs. M−C−). Additionally, to examine whether lifetime cannabis use disorder was associated with poorer neurocognitive performance for those with no methamphetamine use history, we compared M−C+ and M−C−. Contrasts were operationalized using backwards difference coding. Using the same group contrasts, covariates, and model comparison strategy, we used multiple logistic models to examine if differences in impairment rate could be attributed to substance use disorder groups. Variance inflation factors were computed for all models to ensure that multiple collinearities did not inflate model error.
Lastly, to determine whether neurocognitive performance and/or impairment group differences were being driven by underlying differences in cannabis and/or methamphetamine use characteristics (i.e., age at first use, recency of use, cumulative density of use, age at first diagnosis, and recency of diagnosis), we modeled the unique effects of these substance use characteristics on neurocognitive performance (T-scores) and impairment within the C+ (i.e., M−C+ and M+C+ groups combined; n = 175) and M+ (i.e., M+C− and M+C+ groups combined; n = 194) subsamples. In the event of these variables being significantly associated with neurocognitive performance and impairment measures, we conducted sensitivity analyses to examine whether inclusion of these variables into the previously employed multiple regression changed group contrast effects. Because these substance use variables were intercorrelated, each was entered as a single predictor into each model, which included substance use group membership. Interaction terms were also examined to test for differential associations between groups.
Results
Participant characteristics
Participant demographic, medical comorbidity, and psychiatric characteristics for each substance use group and for the overall sample are provided in Table 1. The sample consisted of 423 participants who were on average 44.6 ± 14.2 years of age, majority male (n = 304, 71.9%), majority non-Hispanic White (n = 255, 60.3%), and educated 13.6 ± 2.6 years on average. The groups were balanced on age, sex, and race/ethnicity, but there was a significant discrepancy in education years and Wide Range Achievement Test (WRAT-4) scores, such that M−C− and M−C+ had more years of education (p < .001) and higher estimated premorbid IQ (WRAT-4) scores (p < .001) than both M+ groups. Hypertension (n = 85, 20.1%) and hepatitis C infection (n = 83, 19.7%) were the most prevalent medical comorbidities, and these did differ significantly between groups, with hypertension being more prevalent in M-C+ and M-C- groups (p = .016) and hepatitis C infection being more prevalent in the M+C+ group (p < .001). Rates of diabetes and chronic pulmonary disease were comparable between groups. While rates of lifetime and current major depressive disorder diagnoses were comparable overall, BDI-II scores did significantly differ between groups. M−C+ and M−C− displayed significantly lower current depressive symptom scores than the M+ groups (p < .001).
Substance use characteristics
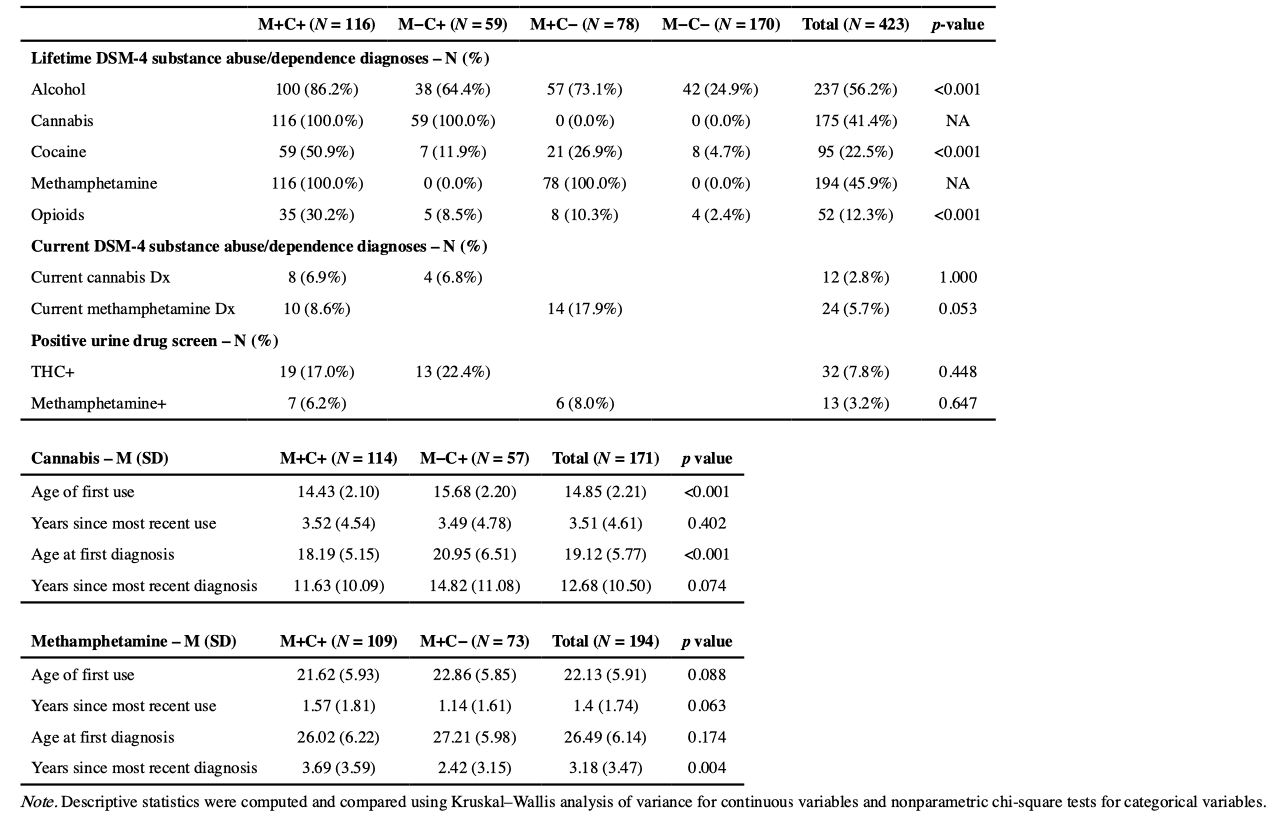
Group and overall sample substance use characteristics are provided in Table 2. Lifetime DSM-4 abuse/dependence diagnoses at least one year removed from data collection significantly differed between groups for alcohol (p < .001), cocaine (p < .001), and opioids (p < .001). A history of cocaine and opioid abuse/ dependence was most prevalent in M+C+, with M+C− also having an elevated rate of cocaine abuse/dependence compared to M −C+ and M−C−. C+/ M+ groups all displayed elevated lifetime alcohol abuse/dependence relative to M−C−. Rates of current methamphetamine and cannabis abuse/dependence diagnoses and positive urine drug screens were statistically equivalent between M+ (p = .65) and C+ groups (p = .45). Positive urine drug screen variables were not significantly related to neurocognitive T-scores or rates of impairment.
Cannabis and methamphetamine use: descriptive characteristics
Cannabis use.
Descriptive results for group cannabis use characteristics are provided in the latter half of Table 2. M+C+ and M−C+ differed in their age of first cannabis use (14.4 v. 15.7 years old, p < 0.001) and age of first cannabis use disorder diagnosis (18.2 v. 21.0 years old, p < 0.001), such that M+C+ was younger at cannabis initiation and younger at first cannabis use diagnosis. Years since last cannabis use and years since last cannabis use disorder diagnosis were comparable between M+C+ and M−C+ (p = .40). On average, participants were >1 year removed from their most recent period of cannabis use (3.51 ± 4.61 years), but a majority of C+ participants were <1 year removed from their most recent cannabis use (n = 98/171, 57.3%).
Methamphetamine Use.
Descriptive results for group methamphetamine use characteristics are provided in the latter half of Table 2. M+C+ and M+C− were comparable in terms of their age at first use, years since most recent use, and age at first methamphetamine use disorder diagnosis, only displaying differences in their years since last methamphetamine use disorder diagnosis (6.4 v. 3.6, p = 0.004). On average, participants were >1 year removed from their most recent period of methamphetamine use (1.4 ± 1.74 years), but many participants were <1 year removed from their most recent methamphetamine use (n = 90/188, 47.9%).
Effects of cannabis and/or methamphetamine use characteristics on neurocognition
Next, we performed analyses in the C+ and M+ subgroups to assess possible contributions of cannabis and methamphetamine use characteristics that differed significantly across the respective study groups.
Cannabis use.
For the C+ subgroup. age at first cannabis use disorder diagnosis was associated with global neurocognitive performance (β= 0.27, p < .001), such that for every year cannabis use disorder was delayed, T-scores increased. Individual neurocognitive domains associated with age at first cannabis use diagnosis included learning (β = 0.37, p < 0.001), memory (β= 0.32, p < 0.01), motor (β=0.41, p < 0.001), executive functions (β = 0.24, p < 0.05), and verbal fluency (β = 0.23, p < 0.05). No model interaction terms were significant, indicating that these relationships were the same for both M−C+ and M+C+ groups. No other significant associations between cannabis use characteristics and NC outcomes were observed (ps > 0.10).
Methamphetamine use.
Years since most recent methamphetamine use, age at first cannabis use, years since most recent CUD, and density of cannabis use (g/day) use were not significantly associated with neurocognitive performance or impairment. Age at first methamphetamine use was associated with learning T-scores (β = 0.19, p = .04), such that an increase in age at first use corresponds to an increase in learning T-scores (i.e., better performance). Years since most recent methamphetamine use, age at first methamphetamine use disorder diagnosis, years since most recent methamphetamine use disorder diagnosis, and lifetime density of methamphetamine use (g/day) were not significantly associated with neurocognitive performance or impairment. Sensitivity analyses were conducted to ensure that results detailed below were robust to the inclusion of age at first methamphetamine use as a covariate in multiple regression models. In these analyses, inferences for M+C+ v. M+C− contrasts (detailed below) remained unchanged and inclusion of age at first methamphetamine use did not significantly increase the proportion of variance explained (p = .07–.41). Results of the subgroup analyses of global NC outcomes are available in the supplementary materials.
In all performance and impairment models, we controlled for current depressive symptoms, hepatitis C virus infection, and lifetime alcohol, opioid, and cocaine abuse/dependence. Of these, only current depressive symptoms (i.e., BDI-II total scores) were significantly related to neurocognitive performance, such that higher depressive symptom scores were associated with lower global, executive function, and information processing speed performance. In impairment models, hepatitis C virus infection was associated with significantly increased odds of executive function impairment (OR = 1.98, p = .013).
Neurocognitive performance
Neurocognitive performance model results are provided in the first half of Table 3.
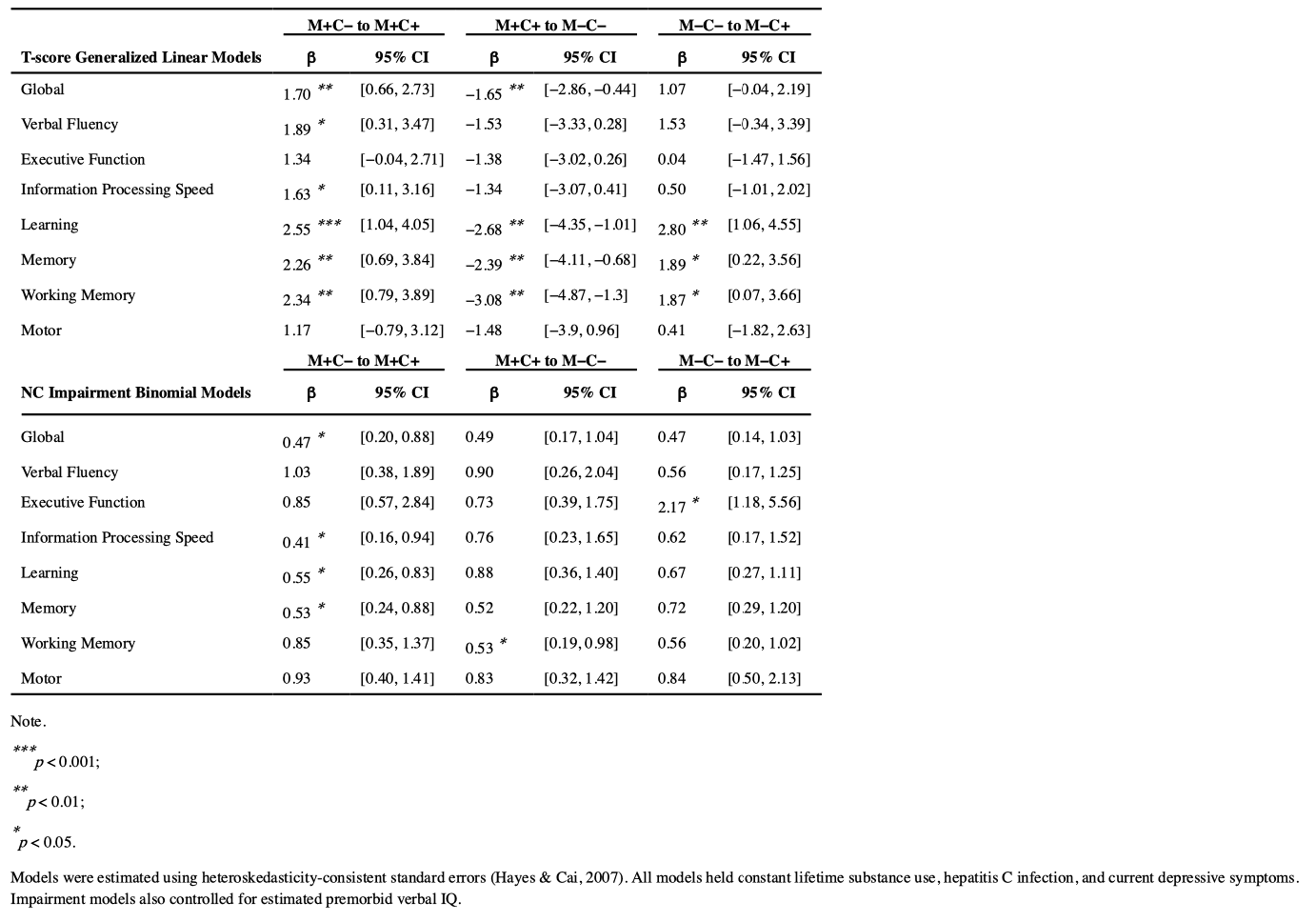
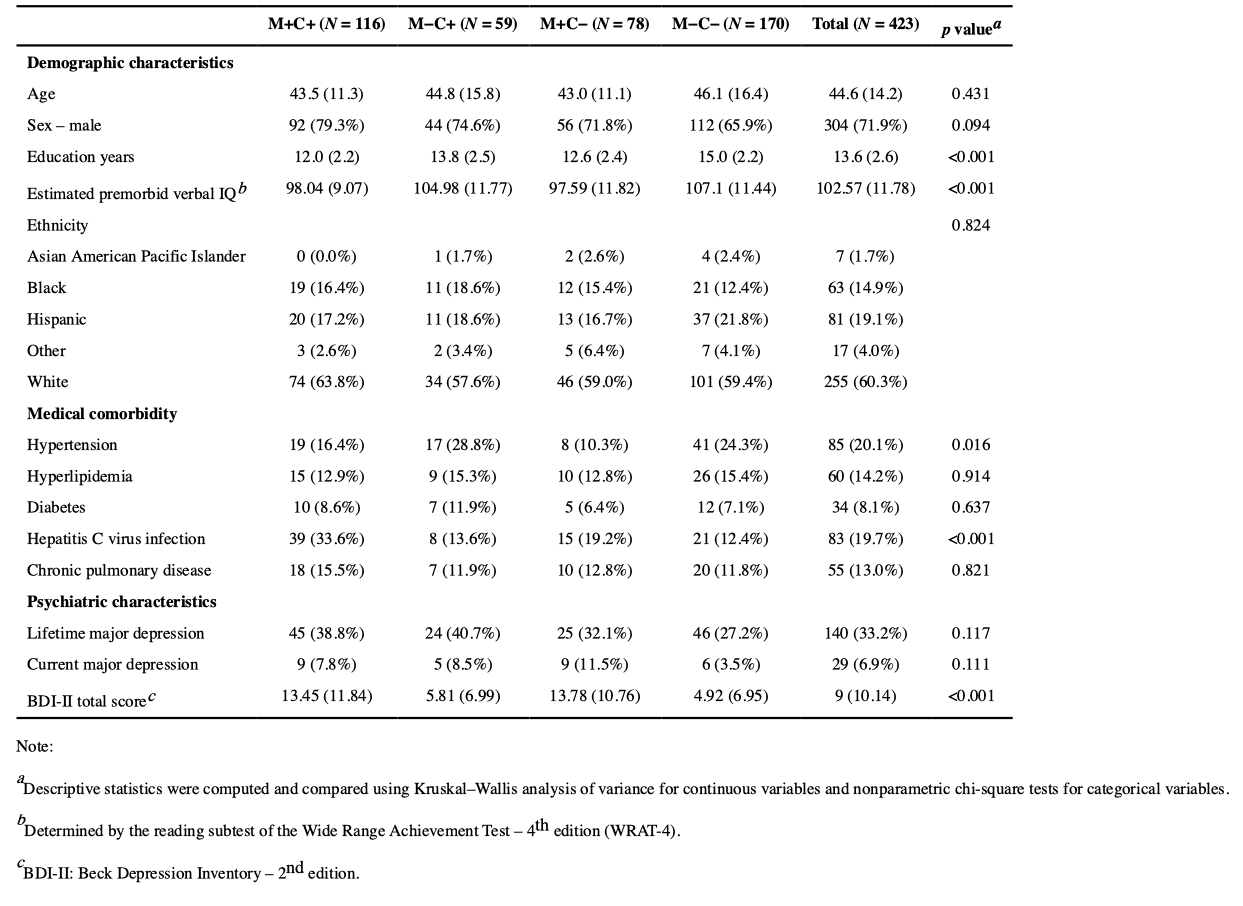
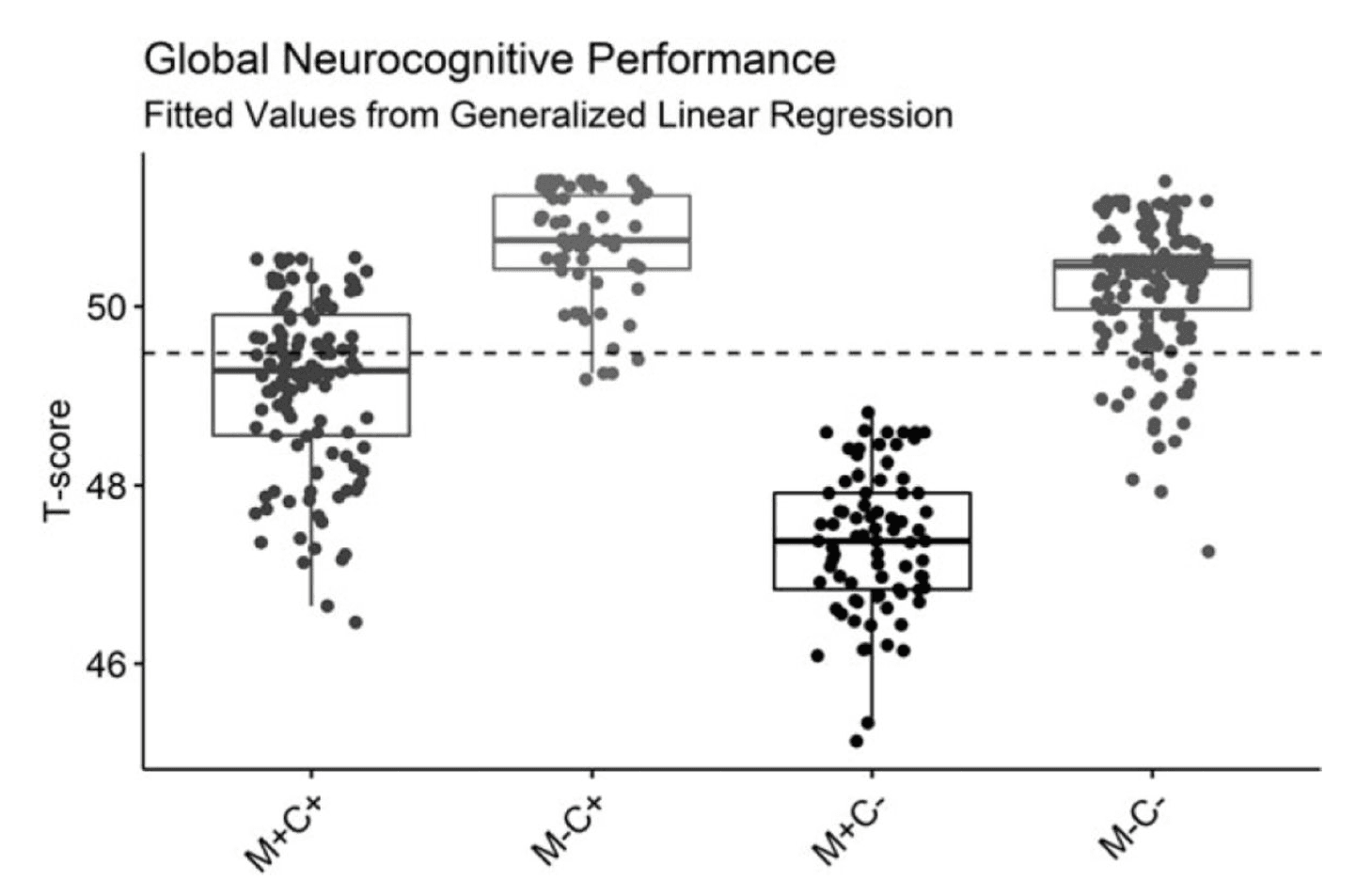
Global performance T-scores were significantly different between groups. M+C− showed the lowest overall performance, performing significantly worse than M+C+ (Mdiff = 1.70, p = .003), who performed significantly worse than the M−C− group (Mdiff =−1.65, p = .011). For a visual representation of these contrast effects, a box and whisker plot of model fitted global T-scores is provided in Figure 1.
A similar pattern of results was observed for performance across neurocognitive domains, and a profile plot of domain T-scores is displayed in Figure 2. M+C− displayed lower performance relative to M+C+ on measures of verbal fluency (Mdiff = 1.89, p = .025), information processing speed (Mdiff = 1.63, p = .038), learning (Mdiff = 2.55, p < .001), memory (Mdiff = 2.26, p = .005), and working memory (Mdiff = 2.34, p = .006). M−C− performed better than M+C+ on measures of learning (Mdiff =−2.68, p < .001), memory (Mdiff =−2.39, p = .007), and working memory (Mdiff =−3.08, p = .002); however, M−C− performed worse than M−C+ on measures of learning (Mdiff = 2.80, p < .001), memory (Mdiff = 1.89, p= .034), and working memory (Mdiff = 1.87, p = .046). No group differences were observed for the motor domain.
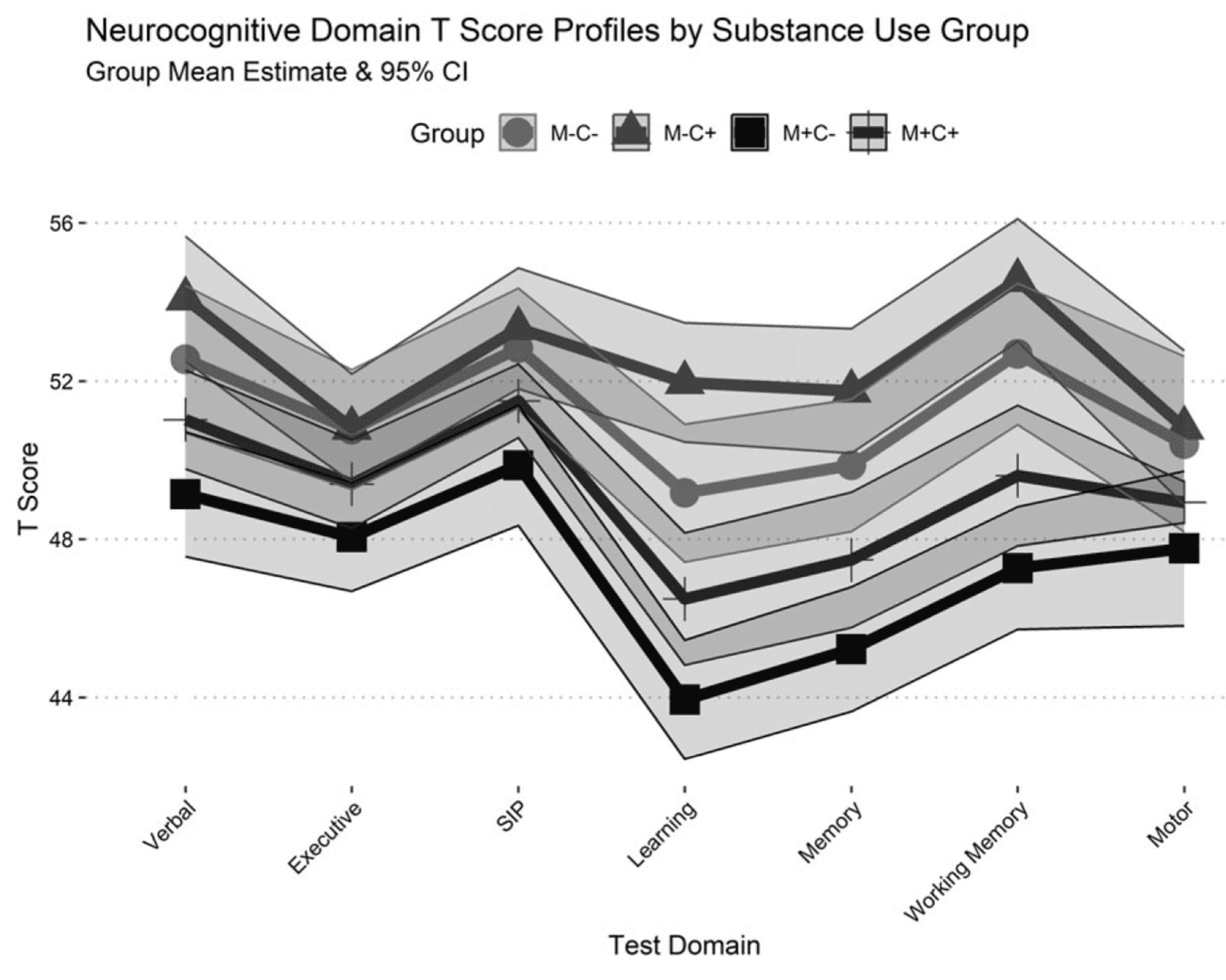
Neurocognitive impairment
Detailed results for all models of NCI are provided in the second half of Table 3. As visually displayed in Figure 3, model fitted probability of global NCI was highest for M+C−, and the M+C+ group was 53% less likely to display global impairment than M+C − (OR = 0.47, p = .028). There was no significant difference in odds of global impairment between M+C+ and M−C− (p = .099) or between M−C+ and M−C− (p = .101).
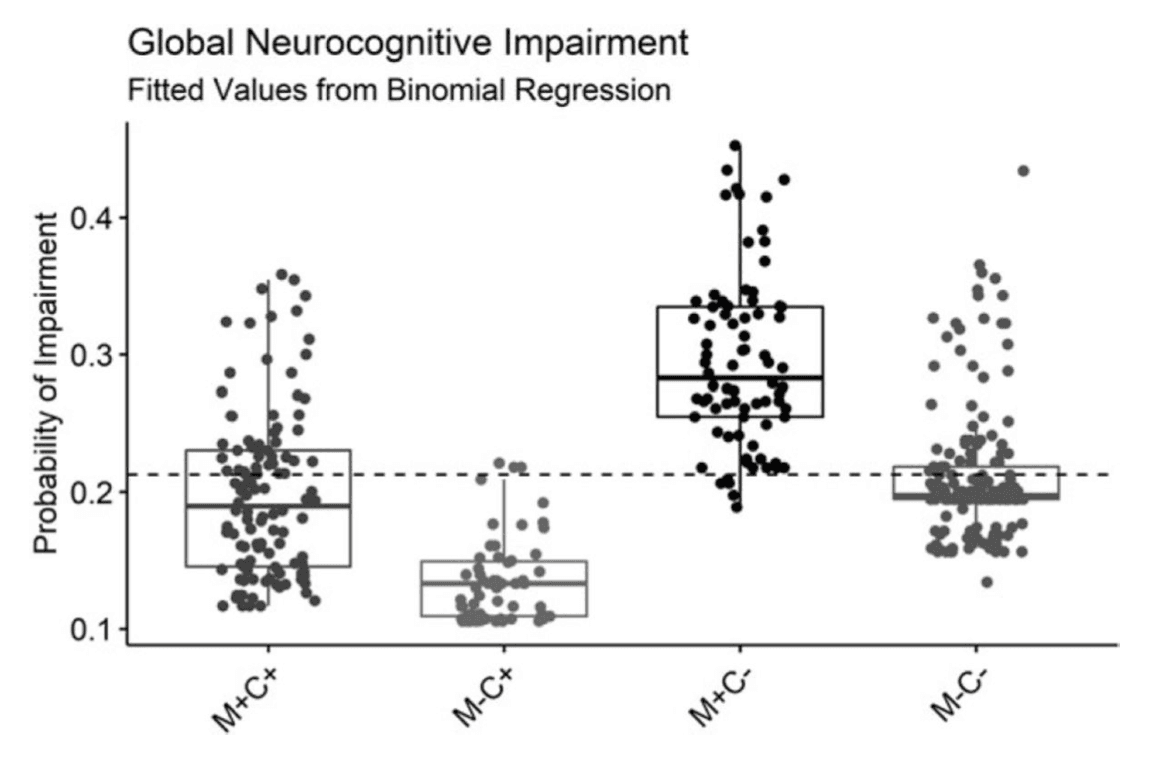
A profile plot of domain impairment probability fitted values from binomial regression models is displayed in Figure 4. The higher likelihood of global impairment among M+C− participants was particularly evident in their M+C+ was less likely to display information processing speed (OR = 0.41, p = .019), learning (OR = 0.55, p = .036), and memory impairment (OR = .053, p = .027) compared to the M+C− group. M−C− was significantly less likely than M+C+ to display working memory impairment (OR = 0.53, p = .047), and they were also less likely than M−C+ to display executive functioning impairment (OR = 2.17, p = .018). Similar to performance metrics, no group differences were found for impairment rates within the verbal fluency or motor domains.
Discussion
Existing evidence suggests that worse neurocognitive performance and higher rates of impairment can result from methamphetamine use (Daldegan-Bueno et al., 2021; Naveed et al., 2022; Reback et al., 2018; Saloner et al., 2020; Scheffler et al., 2022; Watson et al., 2020), and our present results are consistent with these findings. Lifetime methamphetamine use disorder was associated with worse neurocognitive performance and higher rates of NCI, relative to lifetime cannabis use disorder or neither substance use disorder. However, methamphetamine-associated deficits in neurocognitive performance appeared to be significantly less severe in people with contemporaneous cannabis use disorder. We found that people with histories of both methamphetamine and cannabis use disorder (M+C+) performed better on neurocognitive measures and displayed fewer impairments than those with a history of methamphetamine use disorder without associated cannabis disorder (M+C−). Compared to M+C−, M+C+ displayed better neurocognitive performance on a global index and measures of verbal fluency, information processing speed, learning, memory, and working memory. M+C+’s better neurocognitive functioning was also evidenced by their significantly lower likelihood of global impairment or impairments in information processing speed, learning, memory domains. Methamphetamine-associated domain impairments observed in this sample are largely consistent with results from meta-analyses (Potvin et al., 2018; Scott et al., 2007).
To the extent possible, we ensured that these performance and impairment differences were not attributable to differential histories of methamphetamine exposure between M+C− and M+C+. These M+ groups’ methamphetamine use characteristics were largely equivalent, only differing in their years since most recent methamphetamine use diagnosis, with those of M+C− being more recent on average (2.4 vs 3.7 years ago). However, in our focused, subgroup (M+/C+ sample) models, time since last methamphetamine use disorder diagnosis was not significantly associated with any of the neurocognitive outcome measures. After controlling for other relevant confounds in the data (i.e., current depressive symptoms, hepatitis c virus infection, other lifetime abuse/dependence diagnoses) and with the groups being balanced on participant demographics, a significant history of cannabis use emerged as the most salient characteristic distinguishing M+C+ from M+C−.
While observational studies cannot demonstrate causality, it is of interest to consider whether the findings may be consistent with plausible mechanisms. In this case it is generally accepted that methamphetamine can be neurotoxic via multiple mechanisms, including induction of hypertension with consequent injury to cerebral arteries and microvasculature, excessive dopamine release, microglial activation, reactive oxygen and nitrogen species, and mitochondrial injury (Cadet & Krasnova, 2009; Jayanthi et al., 2021). In terms of possible cannabis interactions with these processes, THC, the principal psychoactive ingredient, is an agonist of both CB1 and CB2 receptors. CB1 activation has been suggested to be neuroprotective via reduction of dopamine release and through downregulation of glutamate mediated excitotoxic cascades (Sánchez-Blázquez et al., 2014; Vahidinia et al., 2021). In addition, CB2 stimulation has anti-inflammatory effects that include a shift of macrophage phenotype from the proinflammatory M1 to anti-inflammatory M2 (Beltramo et al., 2001; Razavi et al., 2020; Saloner et al., 2020). While these are plausible mechanisms whereby cannabis might exert protective effects against methamphetamine induced neural injury, further preclinical studies are needed to clarify these interactions.
Our results also indicate that, in non-methamphetamine users, a lifetime cannabis use disorder history was not detrimental to neurocognitive performance. Rather, M−C+ generally displayed better performance than M−C−, particularly on measures of learning, memory, and working memory. While we posit that some factor related to cannabis use may have been implicated in better performance among those with methamphetamine use disorder, there may be other mechanisms at play for our groups outside of the methamphetamine abuse/dependence context.
A small portion of our sample (n = 12, 6.9%) met criteria for current cannabis abuse/dependence at the time of their neurocognitive assessment, and cannabis may yield different acute and residual neurocognitive performance in people who use cannabis daily and/or people who have developed tolerance. Namely, cannabis tolerance results in less prominent neurocognitive performance deficits from acute and/or residual cannabis effects (Ramaekers et al., 2009, 2011), perhaps especially for attention, memory, and impulse control domains (Colizzi & Bhattacharyya, 2018). Apart from blunted acute and residual effects, current evidence also suggests that performance deficits from cannabis use can be expected to dissipate after a brief periods of abstinence (Schreiner & Dunn, 2012; Scott et al., 2018). For the cannabis use disorder subset of our sample, most had used cannabis in the past year (57.3%) but few displayed positive urine screens for cannabis. Given that M−C+ displayed better performance in some domains than M−C−, our results are consistent with current meta-analytic findings that cannabis-related neurocognitive performance deficits may dissipate after brief (>72 hours) periods of abstinence.
Though group comparisons showed no evidence for long-term (i.e., those persisting after 72 hours of abstinence) cannabis use effects, focused C+ subgroup analysis of cannabis use characteristics suggests that younger age of first cannabis use disorder diagnosis was associated with worse global neurocognitive performance, which is consistent with studies showing cannabis exposure earlier in adolescence can be linked to poorer neurocognitive performance later in life (Colizzi & Bhattacharyya, 2018; Fontes et al., 2011; Jacobus et al., 2015). While the presence/ absence of a cannabis use disorder was not associated with worse neurocognitive performance or impairment compared to people without a cannabis use history, people who met use disorder criteria earlier in life displayed lower average performance than those who met criteria later in life.
Limitations
Substance use, especially use of an illicit substance like methamphetamine does not occur in isolation and the cross-sectional nature of this investigation did not allow us to effectively control for lifestyle variables which must be observed longitudinally. While we controlled for differences in methamphetamine and cannabis use exposure between groups in all statistical models, these variables were cross-sectionally estimated using timeline follow-back techniques, which have inherent self-report limitations, and may decrease in reliability as the reporting time period becomes further removed from the present.
Our findings may also be limited in terms of generalizability to the present cannabis market, in terms of changes in the modalities and THC concentrations among new and emerging cannabis products. These data were collected between 2007 and 2015, and with much of the data being collected prior to legalization in California, cannabis concentrates and other high-potency THC products weren’t as widely available. Our sample was primarily composed of people reporting smoked cannabis flower in rolled cigarettes as their primary cannabis use modality. Furthermore, many of our participants met criteria for cannabis use disorder when THC concentrations in cannabis were generally lower than what is presently available.
Importantly, the majority male composition of our sample may limit generalizability to women who use cannabis and/or methamphetamine.
Future directions
To more precisely estimate how cannabis use interacts with methamphetamine use, longitudinal investigation should seek to quantify cannabis and methamphetamine exposure amount, duration, and the degree to which these drugs are used simultaneously. Both preclinical and clinical evidence suggest that a cannabis and methamphetamine drug interaction may depend on whether the two are co-administered. Evidence for a potential protective effect of cannabis in people who use methamphetamine could be further supported by examining the effects of combined methamphetamine and cannabis use on the endocannabinoid system and whether subsequent effects can be observed in inflammatory markers and cognition. Thus far, preclinical studies have shown differential effects of methamphetamine and cannabis on CBRs, metabolizing enzymes, and endocannabinoids (Alizamini et al., 2022; Saloner et al., 2020).
Conclusions
In summary, we found that people who met criteria for lifetime methamphetamine abuse/dependence exhibited multiple neurocognitive deficits, as has been previously reported. However, those also meeting criteria for lifetime cannabis abuse/dependence performed significantly better than those without co-occurring cannabis diagnoses. Given that the dually diagnosed group did not differ in characteristics that might independently be associated with divergent neurocognitive profiles, the results raise the possibility that aspects of cannabis use may be involved in ameliorating methamphetamine induced brain injury.
Our results also support previous meta-analytic findings that cannabis use, even to the degree of meeting criteria for cannabis use disorder, does not necessarily result in neurocognitive performance deficits or impairment following periods of abstinence. People with lifetime cannabis use disorder showed better performance than a comparison group with no history of significant cannabis use on measures of learning, memory, and working memory. Otherwise, those groups’ neurocognitive performance was comparable.