Abstract
Cannabis use disorder (CUD) is an underappreciated risk of using cannabis that affects ~10% of the 193 million cannabis users worldwide. The individual and public health burdens are less than those of other forms of drug use, but CUD accounts for a substantial proportion of persons seeking treatment for drug use disorders owing to the high global prevalence of cannabis use. Cognitive behavioural therapy, motivational enhancement therapy and contingency management can substantially reduce cannabis use and cannabis-related problems, but enduring abstinence is not a common outcome. No pharmacotherapies have been approved for cannabis use or CUD, although a number of drug classes (such as cannabinoid agonists) have shown promise and require more rigorous evaluation. Treatment of cannabis use and CUD is often complicated by comorbid mental health and other substance use disorders. The legalization of non-medical cannabis use in some high-income countries may increase the prevalence of CUD by making more potent cannabis products more readily available at a lower price. States that legalize medical and non-medical cannabis use should inform users about the risks of CUD and provide information on how to obtain assistance if they develop cannabis-related mental and/or physical health problems.
Cannabis is the third most commonly used controlled substance worldwide after alcohol and tobacco (first and second, respectively). In 2018, the United Nations estimated that 192 million persons or 3.9% of the global adult population had used cannabis in the previous year. High-income countries have the highest prevalence of cannabis use, with a lower but increasing use in low-income and middle-income countries. Approximately 9.9% of individuals who reported cannabis use in the past year were daily or near-daily users. Cannabis use disorder (CUD) is broadly defined as the inability to stop consuming cannabis even when it is causing physical or psychological harm. Global data on CUD are incomplete, but according to the most recent global estimate 22.1 million persons met diagnostic criteria for CUD in 2016 (289.7 cases per 100,000 people).
In this Primer we use ‘cannabis’ to refer to the cannabis plant material or its extracts that contain substantial amounts of Δ9-tetrahydrocannabinol (THC), the compound that interacts with the cannabinoid CB1 receptor in the brain to produce the euphoric effects (the ‘high’) sought by people who use cannabis. The ‘high’ can produce a desire for repeated use, which in some users develops into CUD. People who use cannabis may mistakenly believe that cannabis does not produce a dependence syndrome or withdrawal symptoms; however, the effects of regular cannabis use on the endocannabinoid system and a considerable body of behavioural and clinical research indicate otherwise. In addition, CUD occurs in approximately 1 in 10 regular users and as many as one-third of those who use daily. Persons with CUD also have higher risks of poor mental health, psychoses and bronchitis.
The optimal treatments for most substance use disorders (SUDs) combine psychosocial and pharmacological interventions. No effective pharmacological approaches for CUD are available. Psychosocial-based interventions, including cognitive behavioural therapy (CBT), motivational enhancement therapy (MET) and abstinence-based contingency management combined with CBT and MET, are, therefore, the first-line treatment for adolescents and adults. There is mixed support for prevention approaches such as media campaigns, and school-based, family-based and community-based programmes.
Non-medical cannabis use is illegal in most parts of the world but, to date, 12 US states, Uruguay and Canada have legalized recreational cannabis use by adults. Medical cannabis use has been legalized in many more jurisdictions globally. It is too early to assess the full effects of legalization of commercial supply, but experience with alcohol strongly suggests that increasing access to cheaper, more potent cannabis products will increase the prevalence of regular cannabis use, poorer health and consequently CUD.
This Primer reviews the epidemiology of cannabis use and CUD and evidence on effective prevention and treatment approaches. It also discusses the biological and social mechanisms underlying the development of CUD and considers the potential impacts of global trends to allow legal access to cannabis use. The Primer concludes with the major outstanding research questions in the field, and considers how researchers may advance these areas. Cannabidiol (CBD) products that contain no or very small amounts of THC are not reviewed. CBD has generated a great deal of interest in its potential therapeutic use because it does not produce euphoria and it has low abuse or dependence potential.
Epidemiology
Prevalence
In 2018, the United Nations estimated that 192 million persons had used cannabis in that year. The prevalence of past-year cannabis use varies substantially across countries and regions, with higher estimated use in North America (12.4%), West and Central Africa (12.4%) and Oceania (10.3%) than across Asia (1.8%), North Africa (4.3%) and Eastern and Southern Europe (2.4%). Within Europe, western and central regions have higher rates of use than eastern and south-eastern regions, and cannabis use has been stable in western and central Europe over the past decade. The prevalence of cannabis use is low in Asia compared with other regions, but use has increased in low-income and middle-income regions, such as Uruguay, since 2011. In the USA, the number of individuals who used cannabis declined between the late 1970s and the early 1990s. However, use has increased among adults over the past decade, as has the proportion of people who use cannabis who use daily or near daily.
In 1992, the lifetime risk of dependence among those who had ever tried cannabis was estimated at 9%, which was lower than the risks for tobacco (32%), heroin (23%), cocaine (17%) and alcohol (15%). This risk increases to 30–40% among those with a history of daily cannabis use. More recently, nationally representative data from the USA suggest that ~30% of those who use cannabis may develop CUD. This finding may reflect increases in cannabis potency, changes to legal status and societal acceptance of cannabis use over time.
The short period of time since the legalization of cannabis in the Americas (BOX 1) makes it difficult to evaluate its full effects on the prevalence of cannabis dependence. In US household surveys from 1992 to 2015, adults who used cannabis reported using cannabis more often than in the preceding decade but prevalence of adult CUD has been relatively stable between 2002 and 2014. Cannabis use among adolescents and young adults did not increase from 2010 to 2016. Among school-aged youths, US national survey data spanning 2017–2019 suggest an increase in daily use and vaping of cannabis, but not an overall increase in the prevalence of cannabis use. Legalization of cannabis has sharply reduced cannabis prices and increased the sale of high-potency cannabis such as cannabis edibles, oils, extracts and waxes containing >70% THC.
Age of onset
The onset of cannabis use most often occurs in late adolescence, with the median onset age in the Americas, Europe, Asia, New Zealand, the Middle East and Africa at 18–19 years (mean 15–16 years). Initiation of cannabis use before 16 years of age increases the risk of developing CUD, the rate of progression to CUD, other SUDs and anxiety disorders. Use of cannabis before 18 years of age is associated with increased risk of car accidents, antisocial behaviour, polysubstance use and early school dropout compared with non-cannabis users or those who begin to use cannabis at a later age. Twin studies have observed that earlier age of onset is influenced by genetic factors, even after accounting for other substance use, conduct disorder, depression and anxiety. Consistent with other types of illicit drug use, common genetic or shared environmental factors, including social and developmental vulnerability, contribute to an early age of cannabis use onset..
Consumption patterns
The risk of progression from cannabis use to CUD increases with frequency of use. In the USA, adults with CUD, on average, use cannabis 6.2 out of 10 days over a year. Approximately 17.0% of weekly and 19.0% of daily cannabis smokers met the criteria for cannabis dependence. In addition, in a longitudinal study almost 1 in 19 (9.7%) non-dependent weekly cannabis users progressed to dependence within a year. The co-use of tobacco and cannabis is associated with a higher risk of CUD, greater number of withdrawal symptoms and lower rates of cessation than those who use cannabis without tobacco.
Co-occurring mental health disorders
In a national stratified Australian sample of persons aged 18 years and older, 7 in 10 persons with CUD had another psychiatric disorder, compared with 4 in 10 among cannabis users without CUD and 1.5 in 10 individuals who did not use cannabis. Similarly, in nationally representative US surveys, the presence of CUD in the past 12 months was significantly associated with a high risk of any mood disorder (odds ratio (OR) 3.8), anxiety disorder (OR 2.8), post-traumatic stress disorder (PTSD) (OR 4.3) and personality disorder (OR 4.8). Of persons diagnosed with CUD in the past 12 months, 40.5% met criteria for an anxiety disorder in a nationally representative Australian household survey. Anxiety disorders were reported in 20.8% of cannabis users without CUD and 11.2% of individuals who did not use cannabis. US population surveys found a prevalence of 8.9% for generalized anxiety disorder, 8.4% for social anxiety, 7.7% for panic disorder and 16.4% for specific phobia in individuals with CUD.
A meta-analysis of epidemiological and clinical studies predominantly in the USA and Europe found that 12% of persons who had been treated for, or diagnosed with, major depressive disorder had CUD. In clinical and population studies of persons with bipolar disorder, 24% use cannabis and 20% have CUD. Approximately one in four (26.6%) patients with schizophrenia have current CUD or met criteria for a life-time CUD. The prevalence varies substantially by region with the highest prevalence of comorbid CUD and schizophrenia in the UK (36.7%), followed by Australia (35.2%), Europe (27.8%), North America (23.5%) and all other regions (4.5%). Data on the comorbidity of CUD with other psychiatric disorders are less consistent. For example, among individuals with PTSD, a national US survey found a prevalence of 9.4% for 12-month CUD and a prevalence of 17.6% for lifetime CUD; however, a lower prevalence (3%) has been reported in Danish population-based psychiatric register studies.
Cannabis and other substance use
In a nationally representative US sample, persons with CUD in the past year were more likely to have an alcohol use disorder (OR 6.0) and any other drug use disorder (OR 9.0), after adjusting for sex, race, and sociodemographic and other variables. Of persons with CUD in the past year, 83.5% of men and 82.9% of women had another SUD, and 59.4% of men and 59.5% of women had alcohol use disorder. Rates of other SUDs were lower in those with mild and moderate CUD according to Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 criteria compared with severe CUD.
In cannabis users who seek treatment, the prevalence of other drug problems varies with drug availability, cultural practices, drug policy, cost, purity, and health and psychiatric risk profiles. Typically, three in four of those undertaking CUD treatment also have another SUD. Some individuals use other substances to enhance cannabis effects, reduce withdrawal symptoms (such as anxiety and agitation), or use cannabis to reverse or reduce psychostimulant-induced hyperactive states, relieve pain (usually in conjunction with prescribed medication) and reduce the adverse effects of prescribed medications. Cannabis users who use more drug types have a greater number and severity of psychotic symptoms and more severe depression, anxiety and mania than cannabis users who use fewer drug types.
The reasons for the high rates of comorbidity between cannabis use and other drug use are debated. One possibility is the gateway hypothesis (BOX 2) according to which cannabis use increases the risk of using other illicit drugs and developing other SUDs. It is unclear from epidemiological and animal studies whether cannabis has a causal effect on the risk of using other drugs or whether the association is explained by a shared liability to engage in different types of drug use, or increased access to other illicit drugs via drug markets or affiliating with other illicit drug users.

Little is known about interactions between the effects of cannabis and other drugs. Adverse pharmacodynamic (between drugs with similar effects) and pharmacokinetic (between drugs that alter metabolic enzymes) interactions can complicate clinical presentation. Clinical assessments should give priority to reducing concurrent use of substances that elevate the risks of severe withdrawal symptoms (such as central nervous system (CNS) depressants) or overdose (such as opioids, particularly when used concurrently with CNS depressants).
Mechanisms/pathophysiology
Our current understanding of the neurobiological mechanisms involved in CUD derives from preclinical and clinical studies. Although the utility of animal models of addiction has been questioned by some investigators, preclinical studies have revealed how THC exposure can affect the brain and behaviour. Although no single preclinical model captures all the aspects of SUD, various models have been invaluable in understanding addiction processes. These models suggest that, as the severity of SUDs increases, the more involved and dysfunctional neurobiological systems become. Various human studies (genetic, imaging, pharmacological studies and randomized clinical trials) have also provided evidence on the role of altered brain activity and functional networks in the onset and maintenance of CUD. This section describes the key neurobiological systems that animal studies have implicated in cannabis use and CUD.
Endocannabinoid systems
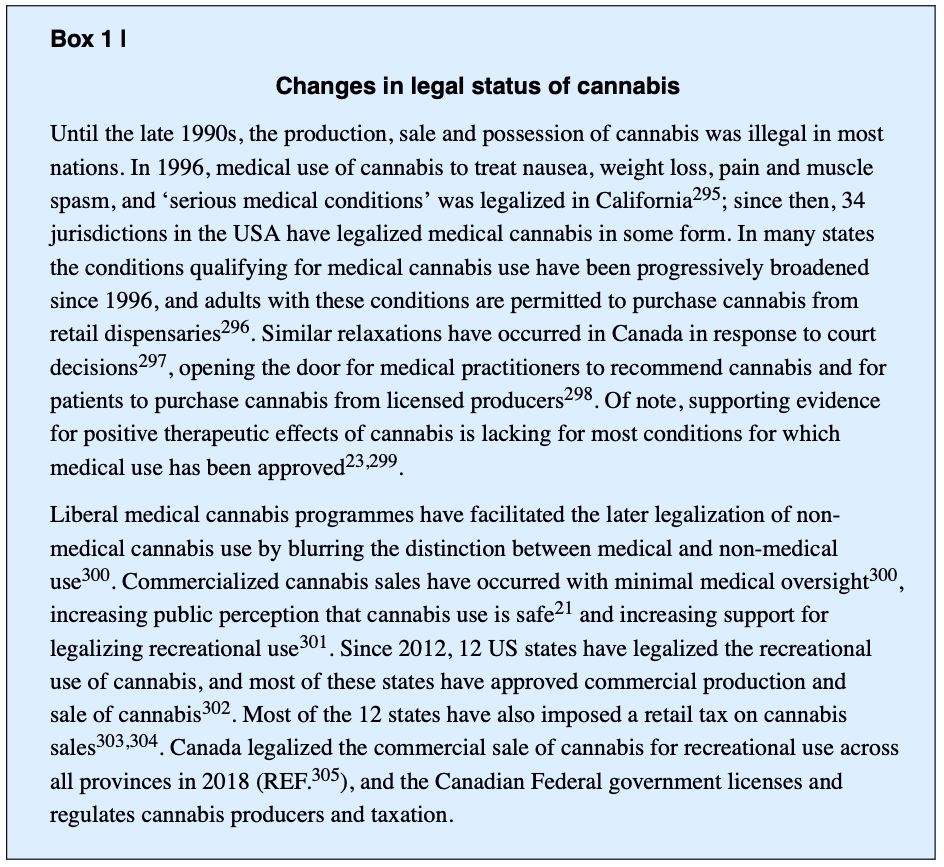
The abuse-related properties of cannabis are mediated by THC. The primary target of THC is the cannabinoid CB1 receptor, the most abundant G-protein coupled receptor in the brain. THC also acts on the cannabinoid CB2 receptor, which is primarily found in immune cells and is much less densely expressed in the brain than the CB1 receptor (FIG. 1). THC is a partial agonist of CB1 and CB2 receptors. Endogenous cannabinoids (endocannabinoids), the most abundant of which are anandamide and 2-arachidonoylglycerol (2-AG) are neurotransmitters that are synthesized by N-acylphosphatidylethanolamine phospholipase D and diacylglycerol lipase, respectively. Enzymes that degrade the endocannabinoids include fatty acid amide hydrolase (FAAH) for anandamide and monoacylglycerol lipase for 2-AG. THC is metabolized in the liver by various cytochrome P450 enzymes.
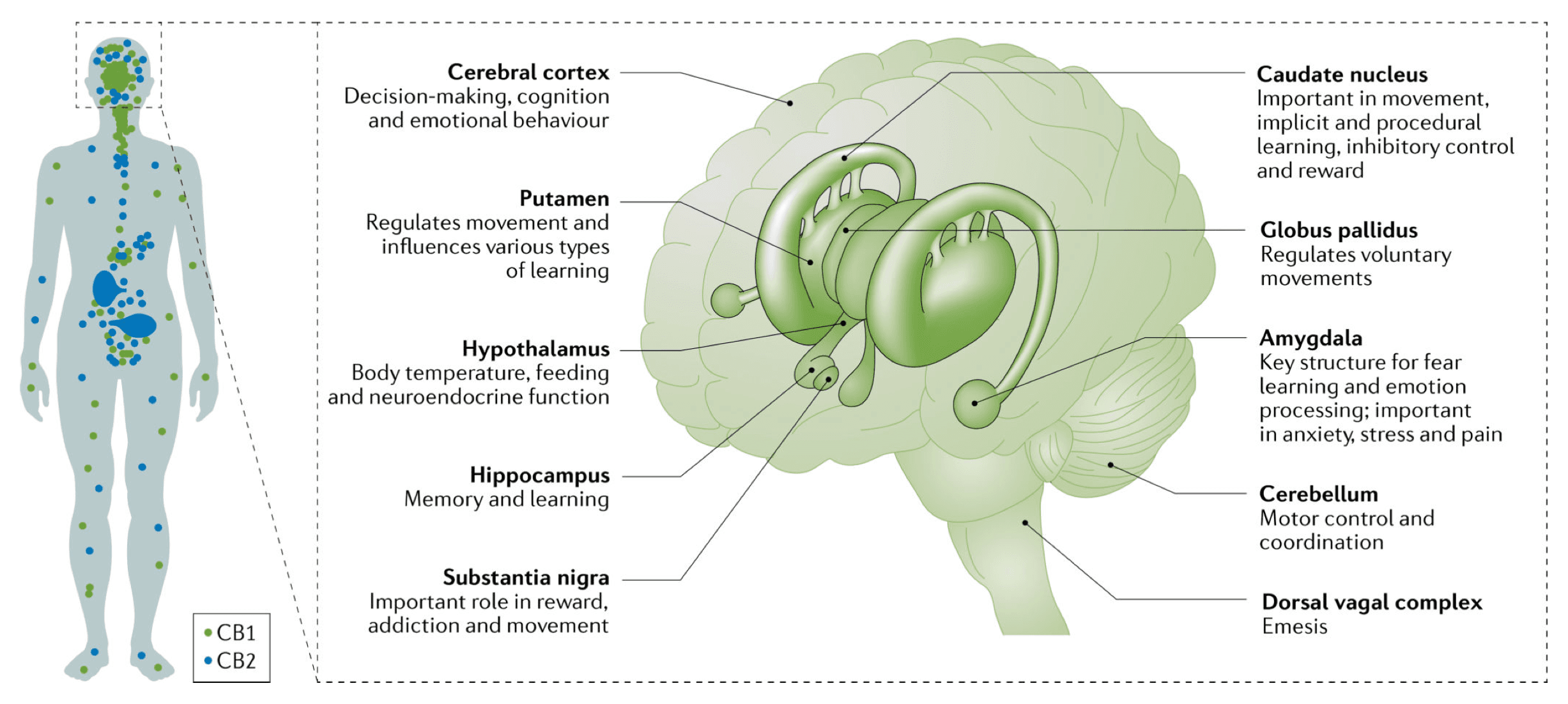
THC and endogenous cannabinoids modulate brain function primarily through the CB1 receptor. The first intracellular process is the inhibition of adenylyl cyclase activity through activation of Gi/o protein. CB1 can also activate other cellular targets (such as β-arrestins, MAPK, various ions channels, and extracellular regulated kinases) leading to a complex response that seems to depend on the neuronal type.
The cellular localization of CB1 controls its function. Most functions of CB1 receptors in the brain are mediated by receptors located on presynaptic terminals. However, CB1 can also be expressed in astrocytes, mitochondria and somatodentritic compartments of neurons. Those CB1 receptors could also have a role in mediating some central effects of CB1 (such as memory function). The most important CB1 receptors in the brain are the presynaptic CB1 receptors that mediate the role of endogenous cannabinoids as retrograde signalling messengers (FIG. 2). Following activation of post-synaptic neurons, endogenous cannabinoids are released from post-synaptic neurons into the synaptic cleft and act on presynaptic CB1 receptors. Those CB1 receptors then reduce neurotransmitter release through either Ca2+ channels or vesicular release mechanisms. The effect of such retrograde signalling depends on the type of neurons involved. The highest expression of CB1 is found in glutamate and GABAergic neurons, but other neuron types also express CB1 (such as serotoninergic and noradrenergic neurons).
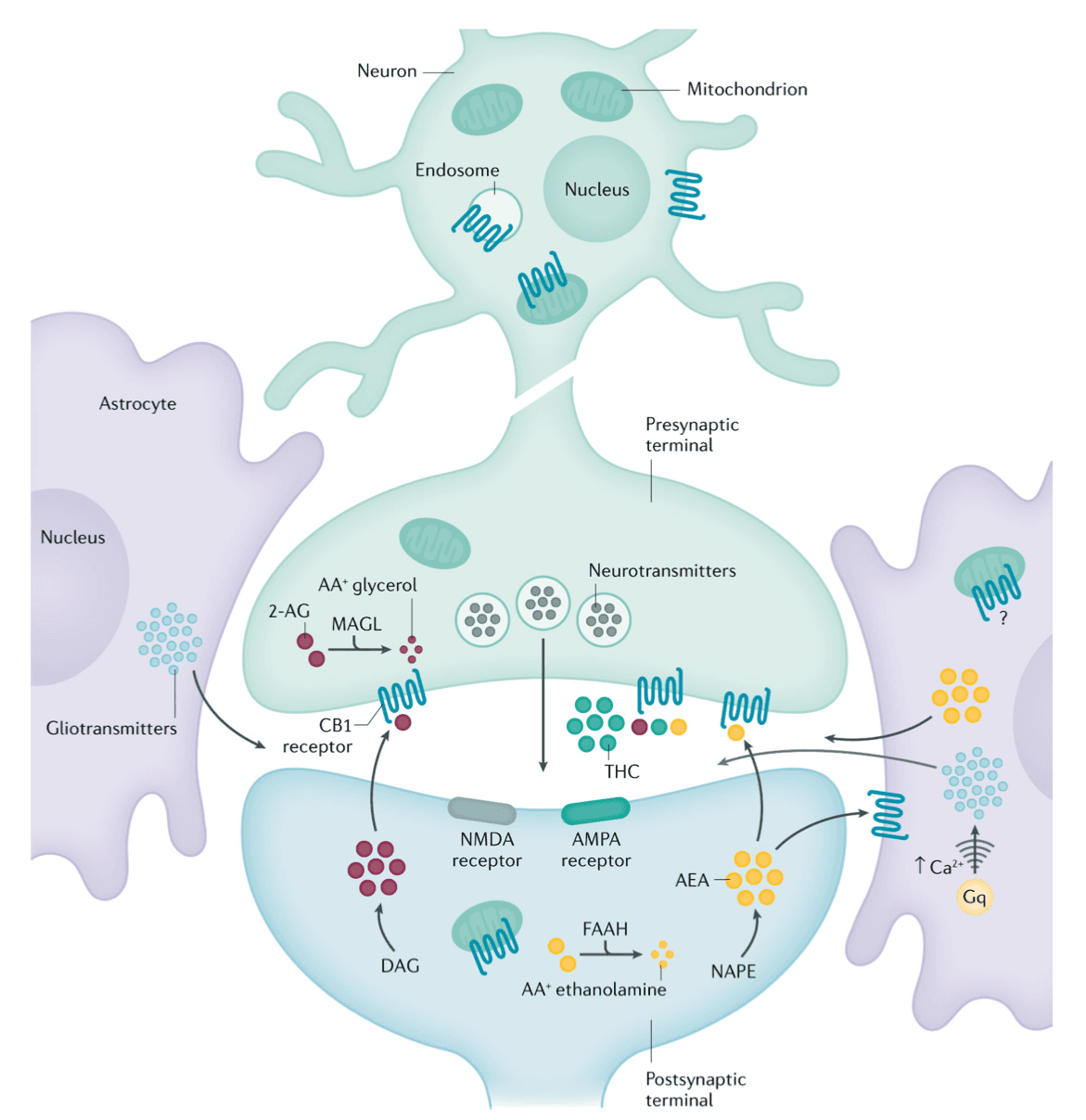
In this retrograde signalling process, excessive stimulation of a post-synaptic neuron releases endogenous cannabinoids that act on presynaptic receptors located on glutamatergic excitatory neurons to reduce hyper-excitability and possibly prevent seizures. However in vivo, the circuitry is often more complex, with presynaptic CB1 receptors located on both GABAergic and glutamatergic terminals. The endogenous cannabinoids modulate neuronal excitability of brain circuits by regulating both GABA and glutamate release. The overall net effect depends on multiple factors such as the degree of expression of CB1 in GABAergic versus glutamatergic neurons, the anatomy of the local circuit and the signalling efficacy in each neuron, which may differ based on brain areas.
Endocannabinoids and the reward system
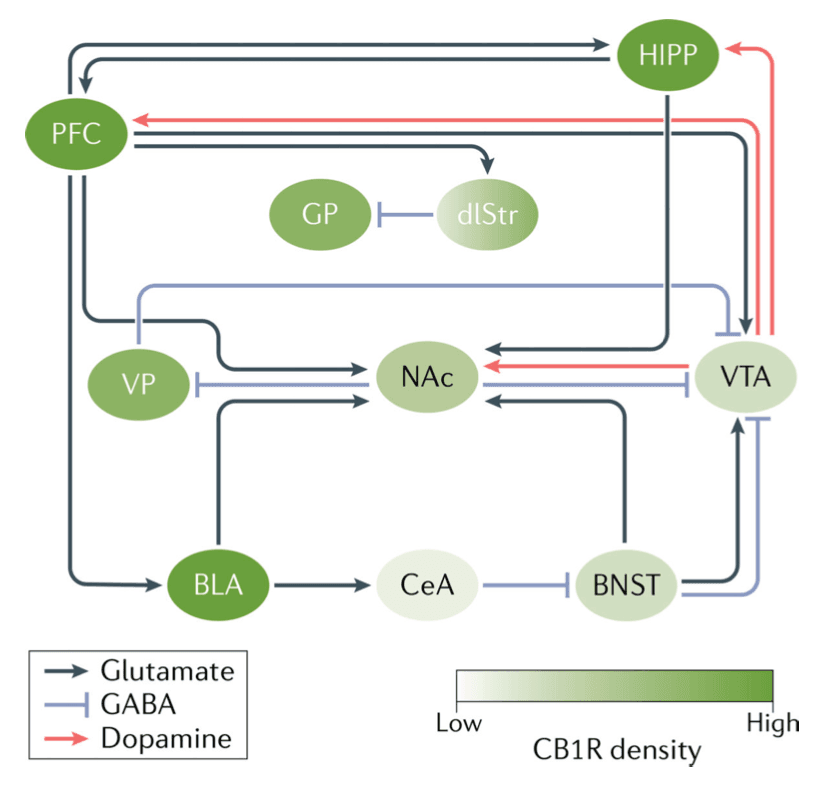
CB1 receptor stimulation can indirectly activate the dopaminergic system that mediates the rewarding effects of many drugs (FIG. 3). Although CB1 receptors are widely expressed throughout the brain, dopaminergic neurons in the midbrain do not express CB1 receptors. It is most likely that THC indirectly increases dopaminergic activity by influencing the firing of dopaminergic neurons in the midbrain. In the ventral tegmental area (VTA), CB1 receptors are primarily located on GABAergic neurons (as opposed to glutamatergic neurons). One proposal is that THC activation of presynaptic CB1 receptors on VTA GABAergic neurons inhibits presynaptic GABA release, allowing dopaminergic neurons in the VTA to fire. In two small human PET imaging studies, THC significantly increased dopamine release in the limbic striatum. The increase in dopamine levels was much smaller than that elicited by psychostimulant drugs and more like the changes produced by alcohol.
Cannabinoid CB1 antagonists block the effects of THC in drug discrimination procedures in rats and monkeys. In humans, a CB1 antagonist (rimonabant) reduces the subjective effects of cannabis, validating the role of CB1 in mediating the ‘high’ produced by cannabis.
Animal studies have explored the neurobiological circuitry that mediates the rewarding effects of THC. Place conditioning is a paradigm that tests the rewarding effects of substances by measuring whether or not an animal spends time in an environment previously associated with the effects of the drug. Most drugs of abuse produce a conditioned place preference in animals, but this has been difficult to demonstrate with THC. Indeed, most rodent studies have not found a significant place preference, although some found a place preference with lower doses of THC (ranging from 0.075 to 4 mg/kg) and no effect or a conditioned aversion with high doses of THC. The lower doses were those at which THC increased dopamine release or had anxiolytic effects; THC doses that produced aversive effects also increased anxiety, corticosterone levels, conditioned taste aversions and impaired motor function (catalepsy).
A self-administration paradigm (during which animals typically press a lever to obtain intravenous drug infusion) has been the ‘gold-standard’ in animal studies demonstrating the reinforcing effects of drugs. In these paradigms, rodents self-administer most drugs that humans self-administer, but rats do not reliably self-administer THC. This may be because THC has partial agonist effects, as rodents will self-administer a full CB1 agonist. The squirrel monkey is so far the only tested animal species that reliably self-administers THC. Self-administration of THC is decreased by CB1 receptor and prolonged μ-opioid receptor blockade in squirrel monkeys and in humans. Other receptor targets have been studied in animal models but these results require validation in human subjects.
In humans, the most challenging aspect of addiction treatment is maintaining abstinence (that is, preventing relapse). Relapse has been modelled in animal studies using the self-administration paradigm and drug-seeking after abstinence. In humans, relapse is often observed after exposure to drugs, drug-related stimuli or stressful events. The same stimuli can reinstate drug-seeking in laboratory animals so these studies can help to understand why people who use cannabis relapse. CB1 receptors are implicated in relapse in squirrel monkeys and rats. Relapse is a complex phenomenon that likely involves multiple brain areas such as the nucleus accumbens, amygdala, prefrontal cortex and insula (FIG. 3). Those various stimuli (drug exposure, cue exposure and stress) modulate the neuronal activity in some cortical areas that control the ability to resist drug-taking and ultimately trigger the decision to use the substance. Human laboratory studies have also tested medications that may reduce cannabis intake. As an example, the CB1 receptor agonist nabilone reduces cannabis intake, suggesting a possible therapeutic role for agonists in treating cannabis dependence. Quantity and frequency of dosing require further investigation, and this drug has not been approved for the treatment of CUD.
Administering a CB1 antagonist to animals that have been repeatedly exposed to THC, will produce behavioural withdrawal symptoms (such as scratching, face rubbing, licking and wet-dog shakes). Humans who cease regular cannabis use can also experience a withdrawal syndrome (see Diagnosis, screening and prevention, below). In some PET imaging studies, the availability of CB1 receptors was negatively associated with severity of withdrawal symptoms, suggesting a direct role for CB1 receptors in cannabis withdrawal. The intensity of cannabis withdrawal was reduced by CB1 agonists such as dronabinol or nabilone or nabiximols (which is a ~1:1 combination of THC and CBD). The blockade of FAAH to enhance anandamide levels is another potential way to reduce withdrawal symptoms.
Brain alterations
Chronic administration of THC or CB1 receptor agonists decreases CB1 receptor availability in the limbic system and neocortex in animal and human post-mortem studies (FIG. 2). CB1 receptors are downregulated in individuals with CUD and, in some studies, an inverse association has been found between CB1 receptor density in cortical areas and the duration of cannabis smoking. Other studies have reported that CB1 density normalizes a few days to 4 weeks after cannabis withdrawal, suggesting that the effects of chronic cannabis use on CB1 receptors may be reversible. In addition to downregulation on CB1 receptor density, the endogenous cannabinoid anandamide is downregulated in striatal areas after repeated administration of THC in rodents. Lower levels of anandamide have also been found in the cerebrospinal fluid of people who use cannabis, although the activity of FAAH is lower in the brain of people who use cannabis (FIG. 4). The full effects of chronic cannabis exposure in the cannabinoid system have not yet been elucidated.
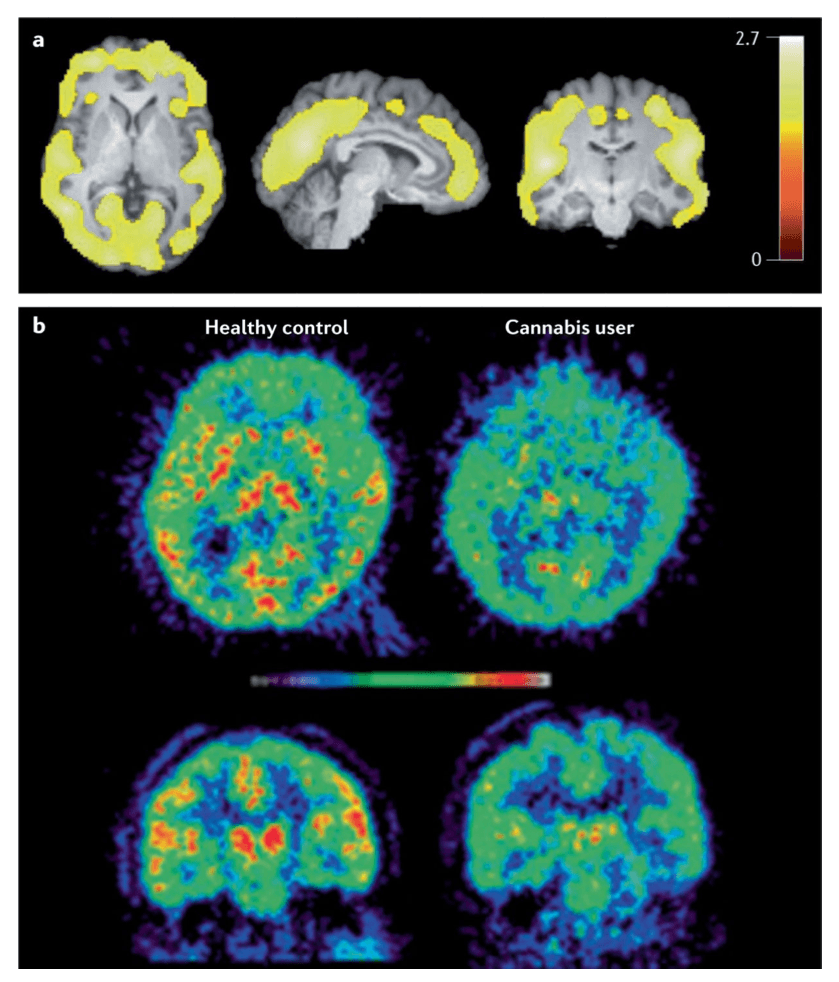
Most PET imaging studies of the dopaminergic system in the brains of cannabis users have not identified one of the most consistent changes in other types of drug dependence, namely, a lower availability of striatal D2 and D3 receptors. In addition, chronic THC administration does not affect D2 and D3 receptor availability in nonhuman primates. Chronic cannabis users may have lower capacity to synthesize dopamine as some studies have found lower dopamine release, notably in striatal areas and the globus pallidus, in response to an amphetamine challenge in chronic cannabis users. This may not be the case in individuals with mild to moderate cannabis dependence. In addition, cannabis users have lower dopamine transporter availability than controls in the dorsal striatum, ventral striatum, midbrain, middle cingulate and thalamus. Whether these changes reflect vulnerability factors or neuro-adaptations to cannabis exposure is unclear. Very few other neurobiological systems have been investigated so this area requires more exploration.
Multiple studies have investigated the effects of chronic and acute cannabis use on functional brain activation and connectivity. Synthesis of those findings is difficult because most functional studies have used different cognitive paradigms and had small sample sizes. However, one meta-analysis of functional activations revealed that cannabis users had increased brain activation in the striatum, along with frontal and other limbic areas. By contrast, decreased activation was observed in the anterior cingulate cortex and the dorsolateral prefrontal cortex, areas associated with cognitive control and attention-related processes. Interestingly, it appears that the ventral striatal response may be associated with heavy cannabis use, while reactivity in the dorsal striatum may mediate the shift towards habit formation and CUD.
Other studies have explored the effects of cannabis on brain anatomy. The anatomical effects of regular cannabis use are more subtle and difficult to detect than neurochemical or functional effects. One meta-analysis indicated that chronic users have significantly smaller volumes in the hippocampus, orbitofrontal cortex and lateral cortex than non-users but there was a large overlap between cannabis users and controls. A review of studies performed in adolescents found some anatomical changes in fronto-parietal areas, but it was unclear whether these anatomical effects are directly related to cannabis use or to other factors such as depression. Altogether, it appears that anatomical effects of cannabis are more modest and much less than those created by regular alcohol exposure, which produces more substantial anatomical brain changes.
Acute and chronic cannabis use has been associated with reduced cognitive performance in a number of domains in adults, young adults and adolescents. Psychomotor function is the cognitive domain most affected by acute cannabis intoxication. Other key domains affected include short-term memory, attention and inhibition. One meta-analysis found a low, but significant correlation between chronic cannabis use and impairment in cognitive (but not motor) impulsivity, cognitive flexibility, attention, short-term memory and long-term memory. There is some evidence that cognitive impairment in chronic cannabis use can improve after sustained abstinence, particularly in the domains of learning and memory impairment, but these studies have rarely extended follow-up beyond 4 weeks so well-controlled, longer follow-up studies are required.
Aetiology
Genetics.
Heritability and family-based linkage studies have indicated that cannabis use runs in families, but differences in populations and diagnostic classifications do not permit consistent estimates of genetic contributions across studies. Twin studies that have estimated the effects of shared and unshared environmental factors on cannabis use provide more consistent evidence of unique genetic liability. A meta-analysis of these studies found that genetic factors contribute 40% in females and 48% in males to vulnerability to the onset of cannabis use and 59% in females and 51% in males to cannabis use with abuse and dependence symptoms. In addition, data from an Australian cross-sectional study of 3,303 twins suggests that genetic heritability of cannabis abuse and dependence is substantial, but largely overlaps with influences that affect opportunity and frequency of use.
Genes that seem to be involved in cannabis use and CUD have been implicated in dopamine regulation (such as DRD2, also implicated in susceptibility to other SUDs), those encoding the cannabinoid receptor (CNR1), FAAH or transporter genes and clock genes. Genome-w ide association studies (GWAS) of cannabis dependence have not reliably detected risk alleles. In one meta-analysis of eight GWAS, several common genetic variants associated with lifetime cannabis use accounted for 11% of the observed variance. The variants with the strongest associations were those associated with ‘risk-taking’ and ‘substance abuse’. Genetic variants identified in these GWAS may have limited or no functional effect on behaviour. Recent research suggests that gene expression may be influenced by cannabis exposure during key periods of brain development such as pre-gestational and prenatal periods. These effects could underlie intergenerational transmission of risk of cannabis use, CUD and other psychiatric disorders.
Psychological learning processes.
Social and cognitive learning processes can explain the onset, course and maintenance of addictive behaviour. Balanced placebo studies can isolate the pharmacological effects of a substance from expected (learnt) cognitive changes. These designs typically lead participants to expect that they are consuming alcohol or drugs when some participants are given a placebo (non-active substance). Social learning theory, which emphasizes the role of social modelling, states that outcome expectancies (for example, cannabis use has benefits) can be learned by observing the behaviour of others. Central to this theory is self-efficacy, that is, a person’s evaluation of his or her ability to perform a task (for example, belief in capacity to resist using cannabis). Individual differences, such as biological make-up, social skills and management of emotions, interact with environmental influences, such as peers, cultural norms and positive portrayals of cannabis in media, contribute to the risk of cannabis use and CUD.
Positive experiences of reward or reinforcement can maintain cannabis use after experimentation because, according to instrumental learning (also known as operant conditioning), behaviour is controlled by its consequences. On the basis of this model, if a person finds cannabis use rewarding they are more likely to continue or increase their use than if cannabis use had no positive consequences. Reinforcement can be positive (such as physical satisfaction) or negative (such as relief of discomfort). Punishment decreases the likelihood of the behaviour (for example, through aversive consequences such as pain or loss of positive consequences). The frequency and regularity of the consequences affects learning; for example, a cannabis user who smokes 5 joints and has 10 puffs per cigarette receives 50 reinforcements per day.
Humans and animals rapidly learn cues that predict drug availability. In classical conditioning, repeated association of a neutral stimulus (such as a bell) with a stimulus that evokes a physiological reflex (such as food and salivation) leads the neutral stimulus to evoke a similar response to the stimulus that evokes a reflex (for example, ringing a bell produces salivation). Reward-associated learning has a crucial role in the development of addiction. Indeed, the development of frequent drug-seeking involves multiple parallel learning and memory systems. Repeated pairing of environmental cues (such as smell, cannabis paraphernalia, use locations and cannabis-using friends) and positive reward (perceived benefits of cannabis use) enhances subjective and physiological responses. Once learned, cues and contexts associated with cannabis use predict reward, initiate drug-seeking, craving and relapse in animal models and human clinical studies. The risk of relapse may remain high even after long periods of abstinence.
The VTA, nucleus accumbens, prefrontal cortex, hippocampus and basolateral amygdala are all critical brain areas for learning, attention, memory, decision-making, executive functions, motivation and motion. In all these brain regions, preclinical and imaging studies have found neuroplastic and functional changes that underlie the development and maintenance of addictive behaviours. One hypothesis is that the neuronal basis for conditioning of drug–cue associations sensitizes the mesolimbic dopaminergic system, leading to decreased value of natural rewards and shifting attention towards drug-associated cues. There are many variations on the dopamine theory of positive reinforcement but all highlight the importance of dopamine receptors in the nucleus accumbens. An enhanced glutamatergic drive in response to drug-associated stimuli also contributes substantially to the maintenance of addictive disorders. The learning processes mediated by the brain reward system can be modified by behavioural therapies (see Psychosocial treatments).
Risk and protective factors.
Cannabis use and CUD have similar risk factors to other substance use and SUDs. For example, an individual’s family can be either a protective (for example, with clear rules, roles, open communication and individual support) or a risk factor (for example, separation of parents, death of one parent, growing up without parents, traumatic events and conflict-ridden family life circumstances). Other factors that increase risk of SUDs are parental use of drugs, permissive attitudes towards drug use, mental disorders, poor relationships and unfavourable child-rearing. Peer substance use, attitudes and behaviours have an important role in adolescents. Psychosocial risk factors include social disadvantage, early onset behavioural difficulties and adverse peer affiliations, moving away from home, dropping out of education, behavioural deviance and acts of violence. The number and type of negative life events are also independent predictors of CUD incidence.
The more risk factors an adolescent has, the greater their risk of a CUD diagnosis in young adulthood. An individual’s risk of CUD can in addition be influenced by cultural norms, values, rules and the price, availability and supply of drugs, and drug policy, legislation, prosecution, prevention and access to treatment. Personality traits and temperament may also have a role in vulnerability to cannabis use and CUD, for example, antisocial behaviour, novelty seeking and impulsivity.
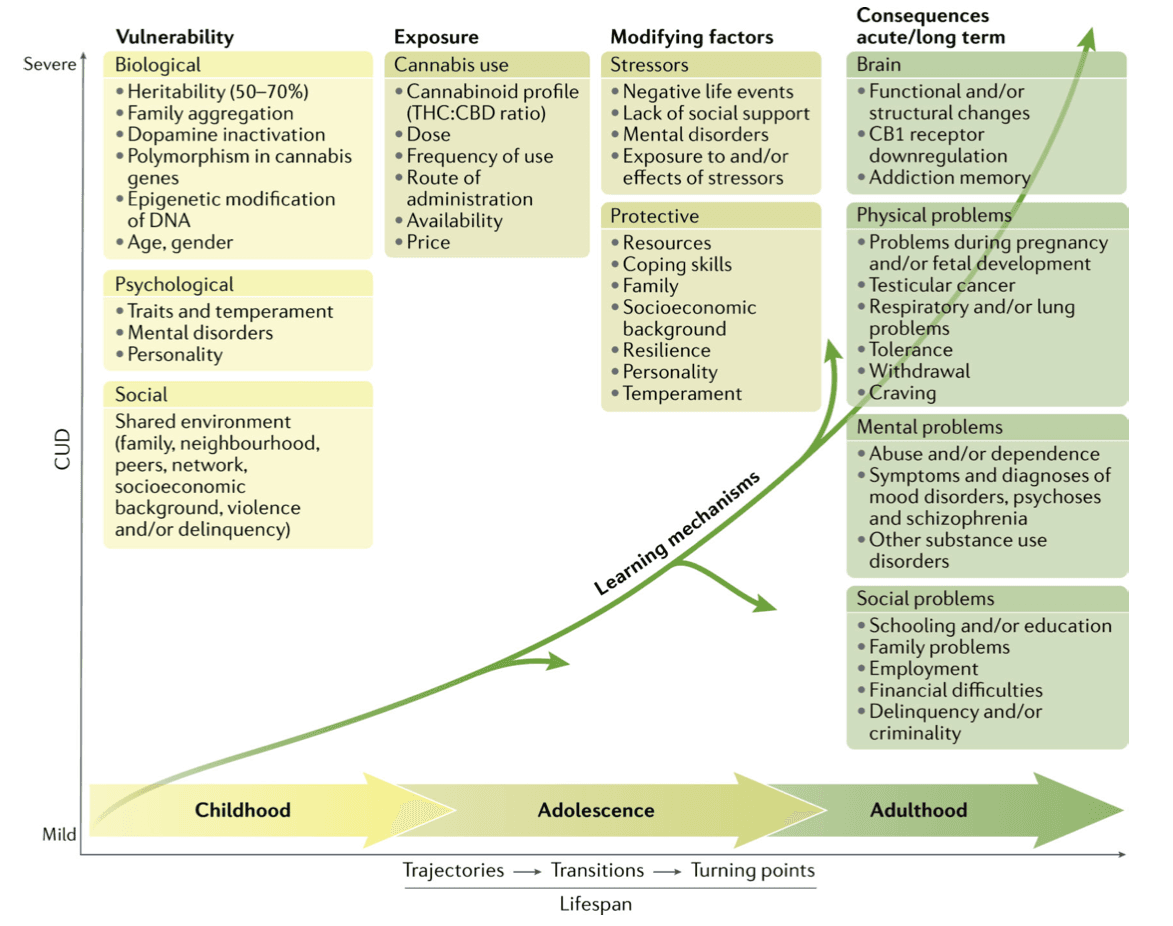
Multifactorial model of CUD
Overarching theories of SUDs have been proposed, but there is no specific framework for CUD. A multifactorial disease model (FIG. 5) is proposed that integrates evidence from epidemiological studies, neurobiological, psychological and social factors, and individual vulnerability and environmental influences and depicts common trajectories and transitions from cannabis use to CUD over the life span.
Diagnosis, screening and prevention
Diagnostic systems
Problematic cannabis use is marked by persistent use despite negative effects on the social functioning and physical or mental health of the user or the health of other individuals. Two diagnostic systems classify and define the severity of CUD: the DSM and the International Classification of Diseases (ICD). An understanding of the most recent and previous diagnostic classifications for CUD is important because most clinical trials and epidemiological studies have used these classifications (FIG. 6).
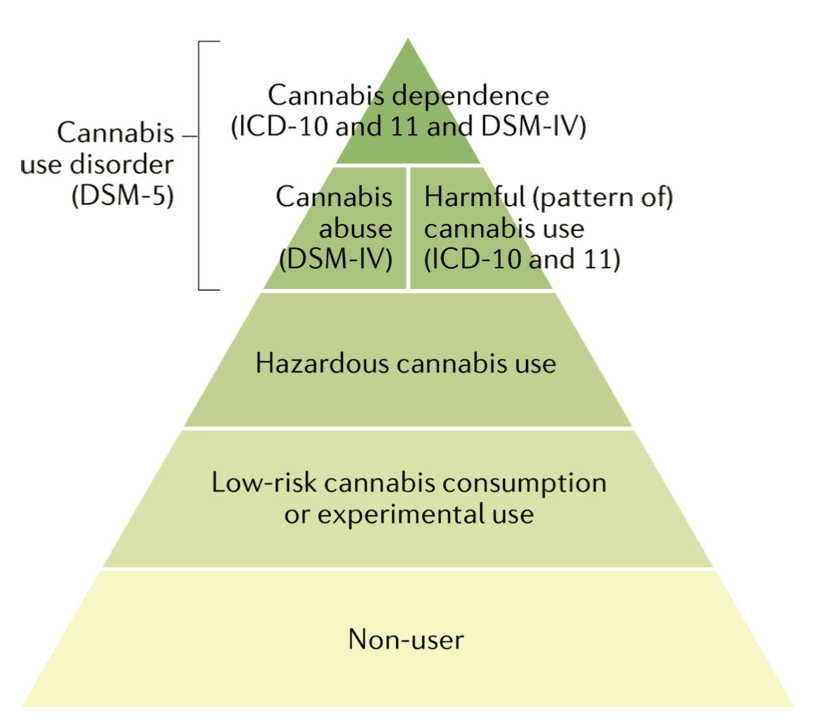
Until DSM-5, the DSM and ICD classification systems both included ‘Cannabis dependence’. However, in the most recent edition of the DSM (DSM-5) there is only one CUD category of ‘Cannabis use disorder’, based on statistical evidence that the symptoms of cannabis abuse and dependence fall on a single severity dimension (TABLE 1). A diagnosis of DSM-5 CUD requires the presence of 2 of the 11 symptoms that have produced marked clinical impairment or distress over the past 12 months, and the severity of CUD is assessed by symptom count (TABLE 1). Of note, remission specifiers can be used for patients who previously met CUD criteria. By contrast, ICD-11 classifies cannabis use into Hazardous cannabis use (potential to cause harm), Harmful pattern of cannabis use (causing harm, similar to ‘Cannabis abuse’ in DSM-IV-TR) and Cannabis dependence (similar to ‘Cannabis dependence’ in DSM-IV-TR). ICD-11 uses diagnostic guidelines that can allow more scope for clinical judgement and cultural variations.
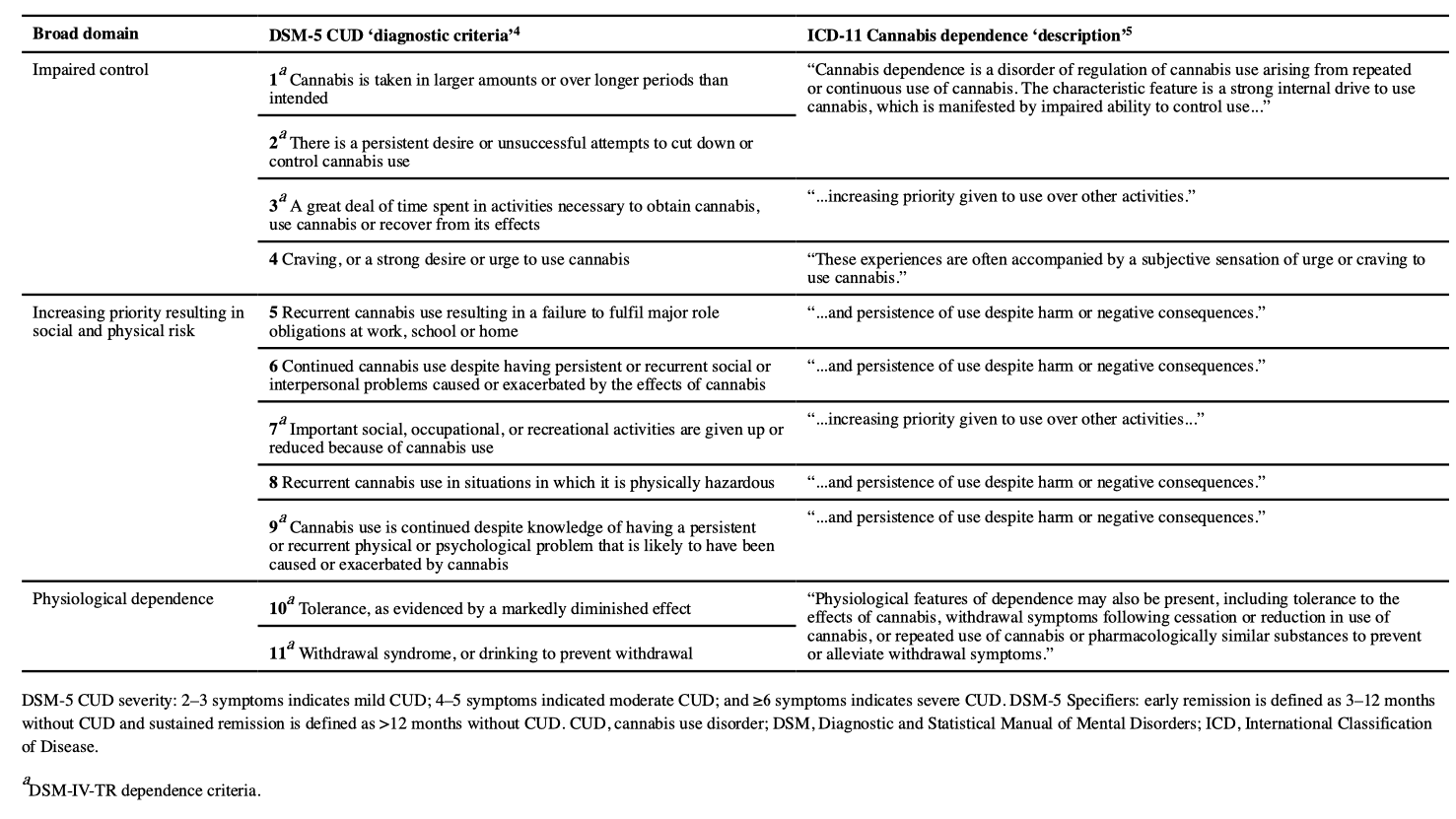
DSM-5 has included diagnostic criteria for cannabis withdrawal as evidence for the syndrome has accumulated. Cannabis withdrawal symptoms typically begin 24–48 hours after cessation, peak within the first week and last for 1–2 weeks. Three or more of the following signs must occur within 1 week of cannabis cessation for a diagnosis of cannabis withdrawal based on DSM-5 criteria: irritability, anger or aggression; nervousness or anxiety; sleep difficulties (such as insomnia or disturbing dreams); decreased appetite or weight loss; restlessness; depressed mood; and at least one physical symptom causing severe discomfort from abdominal pain, shakiness or tremors, sweating, fever, chills or headache. In addition, these signs should cause clinically severe distress or impairment in a social or occupational setting, or other important areas of functioning. In a nationally representative US sample, 12% of individuals who frequently used cannabis had clinical symptoms of DSM-5 cannabis withdrawal in the past 12 months. Many of these symptoms can occur in other types of substance withdrawal and/or as symptoms of other mental disorders.
Screening and assessment
There is no consensus on whether cannabis use should be routinely screened for in general populations. The US Preventive ServicesTask Force recommended screening for illicit drug use in adults ≥18 years, in pregnant and postpartum women and in adolescents aged 12–17 years in primary care settings, if follow-up care can be offered. Good clinical practice would include, as a minimum, assessment of the quantity, frequency and mode of cannabis administration, and if possible, an estimate of the active compounds (THC and CBD) in the products being used. Use of products with a higher proportion of THC is of greater concern than products with a high proportion of CBD and little or no THC. To assess the active compounds, a patient can be asked about their preference of cannabis products: a preference for ‘strong’ products (such as Sativa strains, or parts of the plant, including the crystal resin that coats the plant, or flowering parts of the plant) provides an indirect indicator of high THC and low CBD content.
Screening high-risk populations (such as patients with psychiatric or forensic histories) is considered good clinical practice. A systematic review of screening measures in emergency departments found that a single screening question (“In the past year, how often have you used cannabis?”) was as effective as multi-item measures. In treatment-seeking populations, cannabis use should be addressed early in the consultation. In the general population, cannabis use can be included in routine ‘lifestyle’ history-taking (including, for example, other substance use, diet and exercise).
If a person reports recent cannabis use, a more comprehensive clinical interview should assess whether use fits on the spectrum of hazardous use, harmful use and CUD (FIG. 6). This clinical interview may also include psychometric cannabis and mental health scales, a physical examination and urine toxicology screening for recent substance use. The clinical assessment should also determine the presence of comorbid mental and physical health problems and other SUDs.
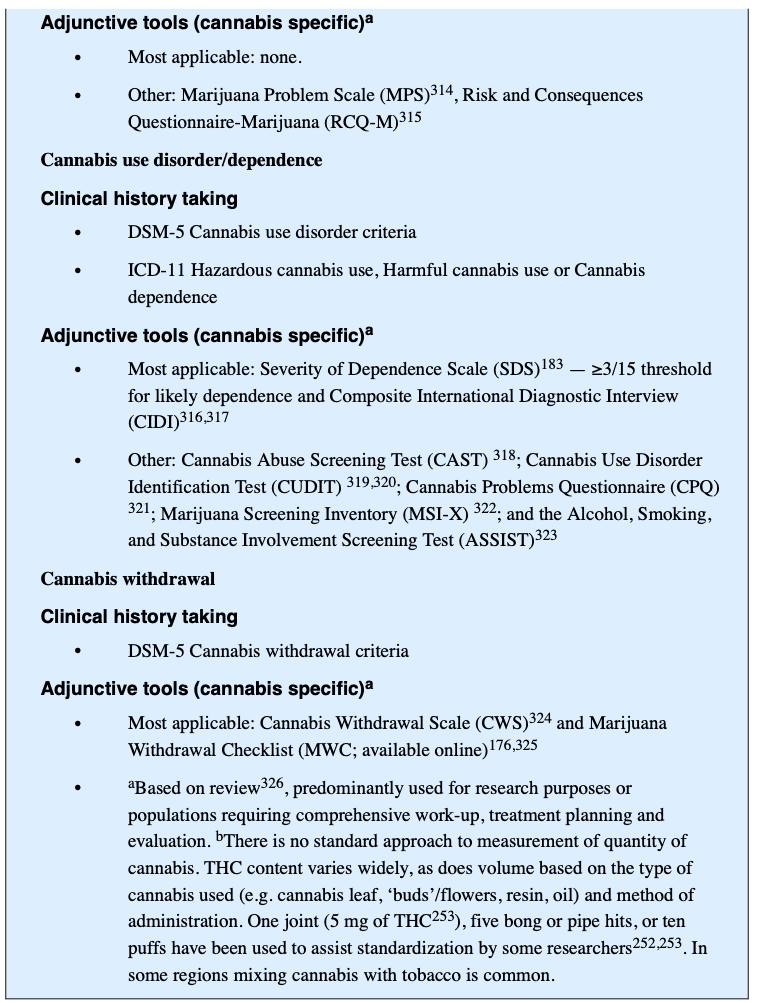
A diagnosis of CUD requires clinically significant impairment for a minimum period, usually 12 months (DSM-5, ICD-11) or 1 month if use has been daily or almost daily (ICD-11). Psychometric scales can supplement a structured interview and CUD diagnostic criteria. The routine use of these scales is limited, in part, by time, absence of standardized dose metrics, restricted timeframes, inconsistent validity and reliability, poor scale development, and a lack of gender, age and cultural calibration.
The Timeline Follow-Back (TLFB) and the five-item Severity of Dependence Scale (SDS) can be used to supplement CUD diagnostic criteria (BOX 3). The TLFB is a clinician-guided interview that uses a calendar to assist patients in accurately identifying when they used cannabis. For cannabis, the concordance rate between the TLFB and biological markers of cannabis use such as urine screens is ~90%. The SDS is a 5-item self-report scale that discriminates between regular cannabis users who do and do not meet dependence criteria (applying DSM-III-R criteria) with a sensitivity of 64% and specificity of 82%. Of note, the SDS and TLFB may not be reliable if the patient has reasons to understate their use, such as in assessing their fitness for work, forensic matters, disability support or welfare. In these cases, more weight may be given to corroborating data from family, work, medical records and to biological markers of cannabis use.

Differential diagnosis
Psychiatric and physical disorders that co-occur with CUD can present similarly to cannabis intoxication, dependence or withdrawal. Cannabis intoxication can impair coordination, memory and reaction time, and produce confusion, nausea, vomiting, distorted perception, hallucinations, agitation and anxiety. These symptoms can also occur, for example, in alcohol withdrawal-related delirium tremens, which is a medical emergency. In individuals with these symptoms, a priority is to determine which substance or substances have been used, when, by what route of administration and in what quantity. For patients who are heavily intoxicated or unconscious, or suspected of using other illicit drugs, corroboration from friends and family or biological markers of substance use are required.
Common symptoms of CUD include episodic or chronic mood changes (also found in depressive disorders), anxiety (also seen in anxiety disorders) and thought disturbances (also seen in schizophrenia spectrum and other psychotic disorders or other substance-induced intoxication). A differential diagnosis requires information on the temporal sequence of regular cannabis use, and the exclusion of cannabis-induced mental disorders (psychosis, bipolar disorder, depression, anxiety, obsessive–compulsive disorder, sleep disorders, sexual dysfunction, delirium or neurocognitive disorders). A dose–response relationship exists between cannabis use and psychosis risk, and there is evidence that cannabis use exacerbates schizophrenia symptoms. The case for the causal effect of cannabis on psychosis is contested because it may be a consequence of shared environmental and genetic risk for developing both CUD and schizophrenia. Cannabis-induced psychosis is one of the more challenging differential diagnoses as patients with primary psychotic disorders often use cannabis. The temporal sequence of cannabis use followed by novel psychotic symptoms is the central feature. Of note, patients with substance-induced psychotic disorder typically have greater insight into their illnesses, less often have a family history of psychotic disorder, fewer positive and negative symptoms, and more severe depression and anxiety, than cannabis users with a primary psychotic disorder.
Recurrent vomiting is a symptom of cannabis hyperemesis syndrome (CHS), which has been reported in emergency department patients presenting with cyclical vomiting and a current or recent history of cannabis use. The differential diagnosis of CHS and other cyclical vomiting disorders is underdeveloped owing to poorly specified and overlapping symptoms, but almost all patients with CHS are heavy weekly cannabis users and 90% report that hot baths and abstinence from cannabis relieve their symptoms. Cannabis use can affect the cardiovascular, gastrointestinal, immune, neuro muscular, ocular, reproductive and cognitive systems, but the major adverse physical health effect of cannabis smoking is on the respiratory system.
Prevention
The most effective prevention approaches for alcohol and tobacco are to reduce supply (for example, through pricing, taxation and introducing legal restrictions on minimum purchasing age) and to restrict advertising. The same strategies are likely to be effective in jurisdictions that have legalized the retail sale of medicinal and recreational cannabis (BOX 1). In regions where cannabis is illegal, prevention approaches have included media campaigns, and primary (universally applied) and secondary (selectively applied to higher risk populations, including cannabis users) individual-based, school-based, family-based and community-based programmes. Mass media approaches to prevention are typically delivered as short ‘advertisement’ campaigns that present positive role models who reject substance use. There is conflicting evidence on whether these campaigns reliably reduce drug use.
Few drug prevention programmes solely address cannabis. Most aim to reduce all substance use and are implemented in schools, which provide easy access to young people. A meta-analysis of primary prevention programmes that included cannabis-specific content found that half of the programmes reported significant but modest effects on cannabis use (median Cohen’s d = 0.12). The comparative median effect size for cannabis use in general drug prevention programmes was 0.30. Substance use prevention programmes seem to have reduced cannabis use in most but not all studies but these studies have generally weak methodology, low fidelity of programme implementation, poor validity of outcome measures and statistical procedures. Community-based interventions that aim to mobilize community ‘champions’, leaders and organizations have limited to no effect on 12-month cannabis use, although very few controlled studies have been carried out.
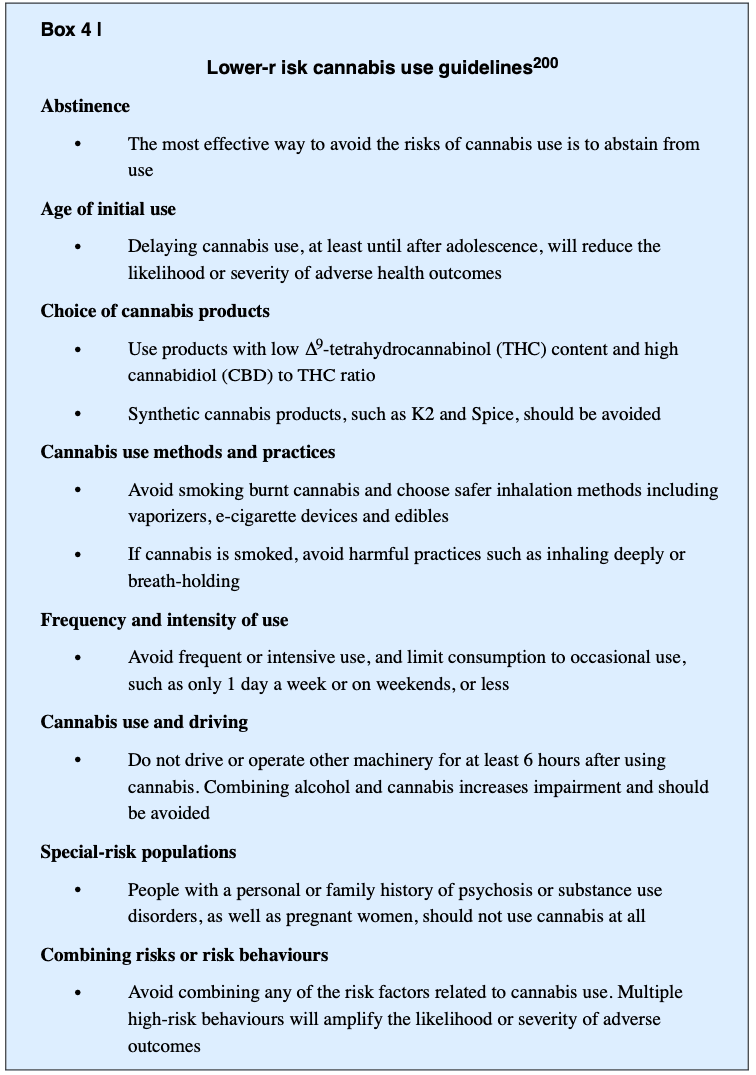
Lower-risk cannabis use.
Lower-risk cannabis use guidelines were endorsed by Canadian health organizations in 2017 as a source of information to the public and to health-care providers after the legalization of cannabis. These guidelines comprise ten recommendations (BOX 4) that are similar to the low-risk guidelines developed for alcohol, nutrition or sexual behaviour. The guidelines assume that individuals who continue to use cannabis, despite advice to abstain, may be prepared to modify their use to minimize harms, including CUD.
Management
Cannabis withdrawal
A cannabis withdrawal syndrome increases the difficulty of quitting and may precipitate relapse. Data predominantly from North America estimate the prevalence of cannabis withdrawal syndrome in the general population of cannabis users at 12–17%. By contrast, in patients with CUD seeking treatment, 54% of outpatients and 87% of inpatients report clinically severe withdrawal. The majority of patients seeking treatment for CUD, including adolescents, report a history of cannabis withdrawal symptoms.
In the absence of medical or psychiatric comorbidities, cannabis withdrawal does not pose serious risks to individuals, and most persons with CUD require only supportive care. Behavioural approaches to withdrawal management include psychoeducation and coping skills training, which normalizes the experience by informing the patient about expected signs, symptoms and time course, and suggests ways to manage specific symptoms (such as exercise or hot baths to manage irritability, avoiding excessive caffeine to address restlessness, consuming nutritious food to counter decreased appetite, and reminding patients that symptoms are temporary).
Pharmacotherapy trials for CUD have investigated agonist-like medications that target the CB1 receptor (substitution therapies) such as dronabinol or nabiximols. They appear to reduce the severity of cannabis withdrawal symptoms. Although no guidelines have been developed specifying which patients are good candidates for these CB1 agonist medications, those who may benefit are patients who previously reported severe withdrawal symptoms or failed quit attempts because of withdrawal symptoms. In addition, zolpidem and other benzodiazepines (nitrazepam) have been used to treat withdrawal-related sleep disturbances. Of note, some medications for mood, sleep or craving that reduce withdrawal symptoms have not produced commensurate reductions in the amount of cannabis use or increased the duration of cannabis abstinence, but only a few studies have been conducted.
Psychosocial treatments
Psychosocial approaches for adults with CUD include CBT, MET including brief MET (bMET), contingency management, social support counselling, drug education counselling, relapse prevention, mindfulness meditation and mutual help groups, based on the 12-step approaches (such as Marijuana Anonymous) (TABLE 2).
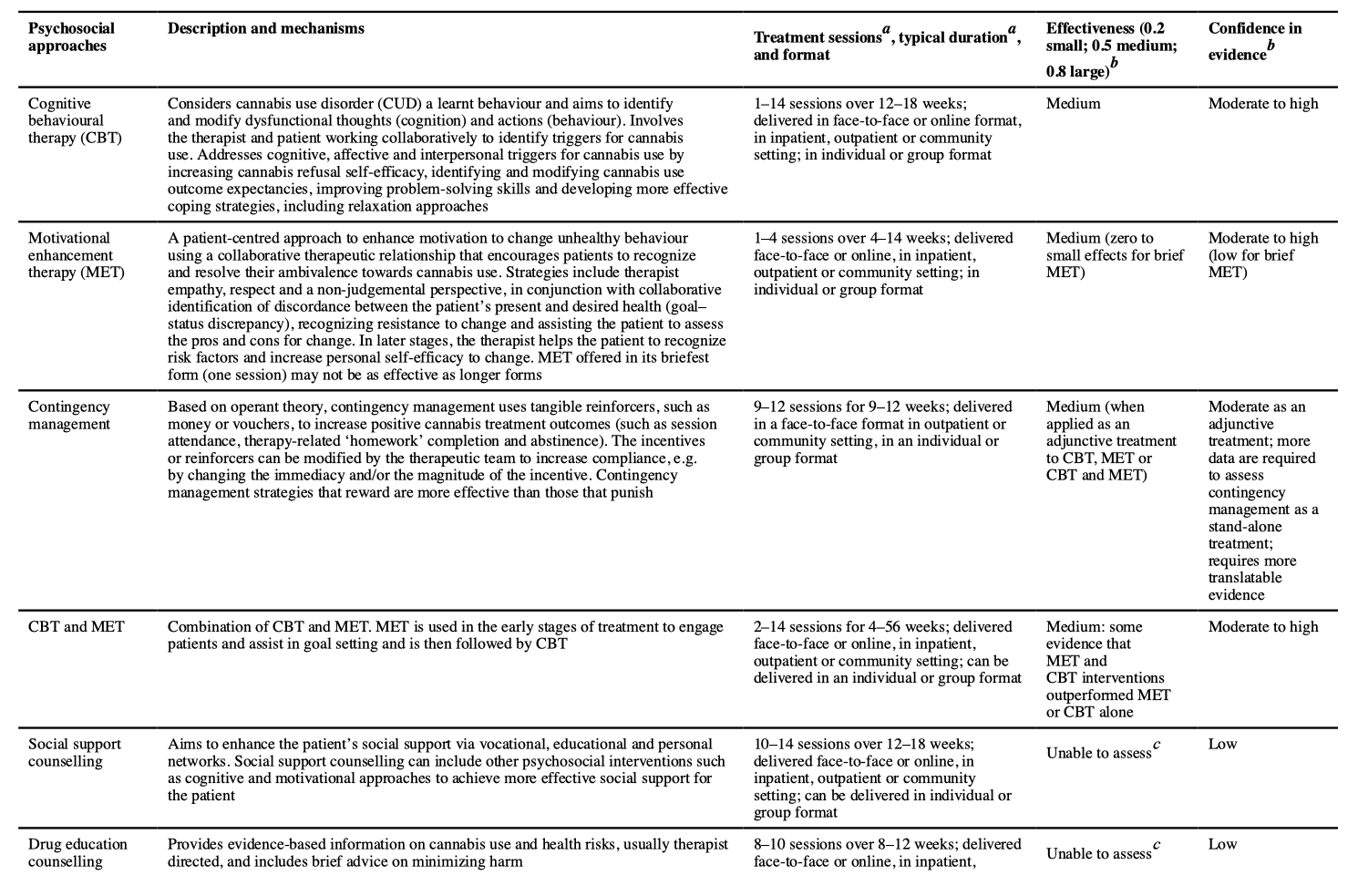
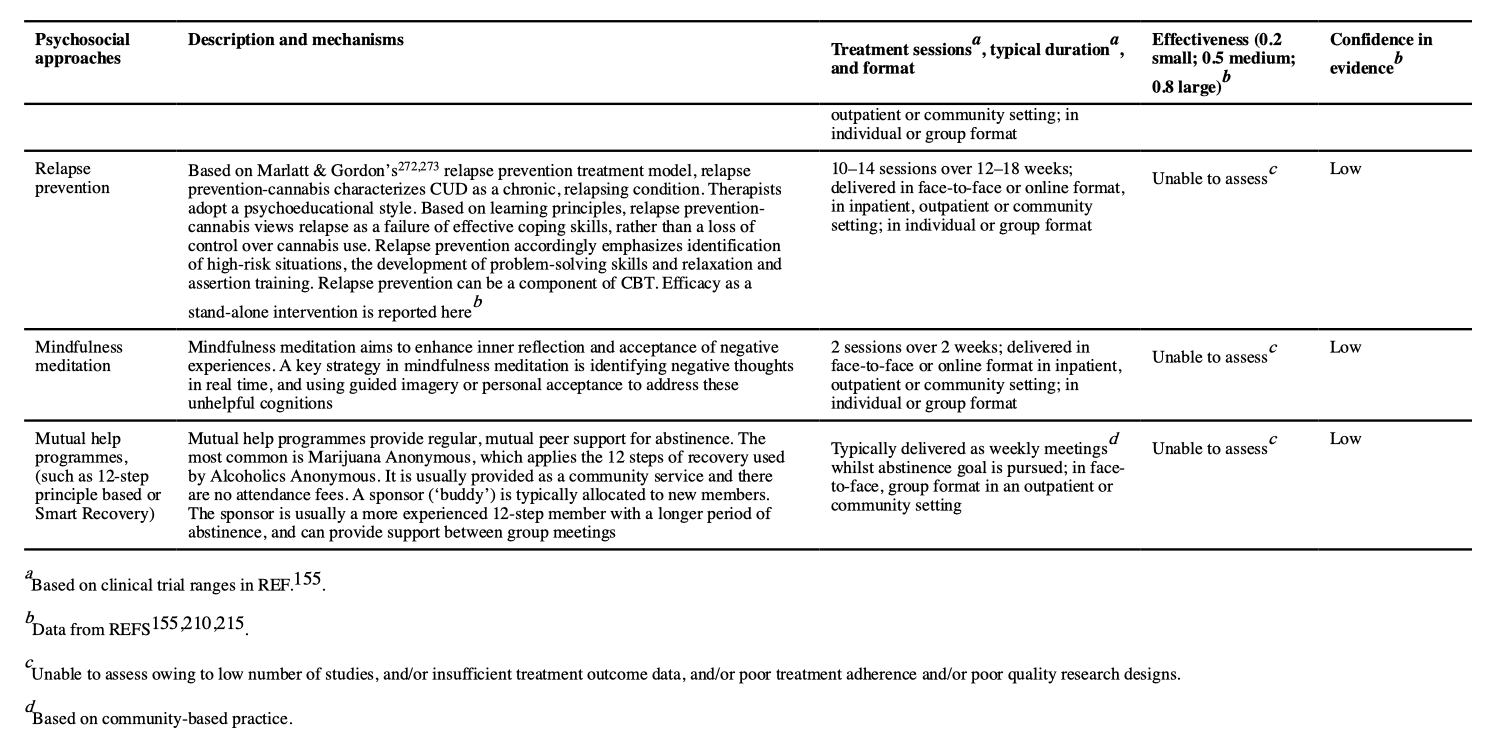
A pooled meta-analysis of CBT, MET, relapse prevention and contingency management found an overall moderate effect size (Hedges’ g = 0.44) at 2–14 weeks follow-u p compared with controls (which included waiting list, psychological placebo and treatment as usual). Of note, these effect sizes did not differ between those with cannabis abuse and those with cannabis dependence. An earlier meta-analysis of psychosocial cannabis interventions found a larger effect (Cohen’s d = 0.81). The efficacy of CUD psychosocial interventions is similar to that for psychosocial interventions in alcohol use disorders (Hedges’ g = 0.15 to Cohen’s d = 0.77) and major depression (Hedges’ g = 0.38–1.10).
CBT and MET have similar efficacy in reducing cannabis use and CUD. Some studies have found that combining CBT and MET is more effective than either treatment alone. Augmenting CBT or MET, or combining CBT and MET with abstinence-oriented contingency management further reduces frequency of use and cannabis problem severity than either intervention alone. Most studies that applied adjunctive contingency management also reported improved abstinence rates, but more studies are required. Despite its potential benefits for CUD treatment, contingency management has largely been used in research studies because of perceived concerns with cost, provider burden and the lack of familiarity with the approach. Too few studies on treating CUD with social support counselling, drug education counselling, relapse prevention, mindfulness meditation and mutual help groups have been carried out to reliably assess their efficacy (TABLE 2).
In the short term (median 4 months), combined MET and CBT produces a 25% reduction in frequency of cannabis use and doubles abstinence rates compared with non-active treatment. Neither MET nor CBT were superior to each other at 6 months follow-up. Few psychosocial interventions maintain treatment gains after 9-month follow-up . Some evidence supports that more than four sessions of CBT, MET or CBT plus MET over longer than 1 month are more effective than fewer sessions, over a shorter period. One study found that very brief interventions (two or fewer sessions or ≤60 minutes of intervention time) applying principles of MET did not significantly reduce the frequency of cannabis use or dependence severity. As CBT typically involves more sessions over a longer period, this may explain why CBT shows improvement over MET in some studies, but it is unclear whether this reflects more treatment or differential efficacy. In adults, there are not enough data to recommend group-based over individual psychosocial treatment, or interventions delivered by telephone or the internet. The most effective treatment for adults with CUD seems to be a combination of face-to-face CBT and MET (with more than four sessions over longer than 1 month), preferably with contingency management.
Psychosocial approaches for adolescents (10–18 years of age) with CUD include individual, group-based and family-based interventions. Multidimensional family therapy, functional family therapy, MET and CBT, and contingency management integrated with MET and CBT have good supporting evidence. Combining evidence-based approaches seems to enhance outcomes. Brief interventions and innovative digital health interventions are being tested to extend the reach and enhance the efficacy of interventions for adolescents with CUD.
Pharmacotherapy
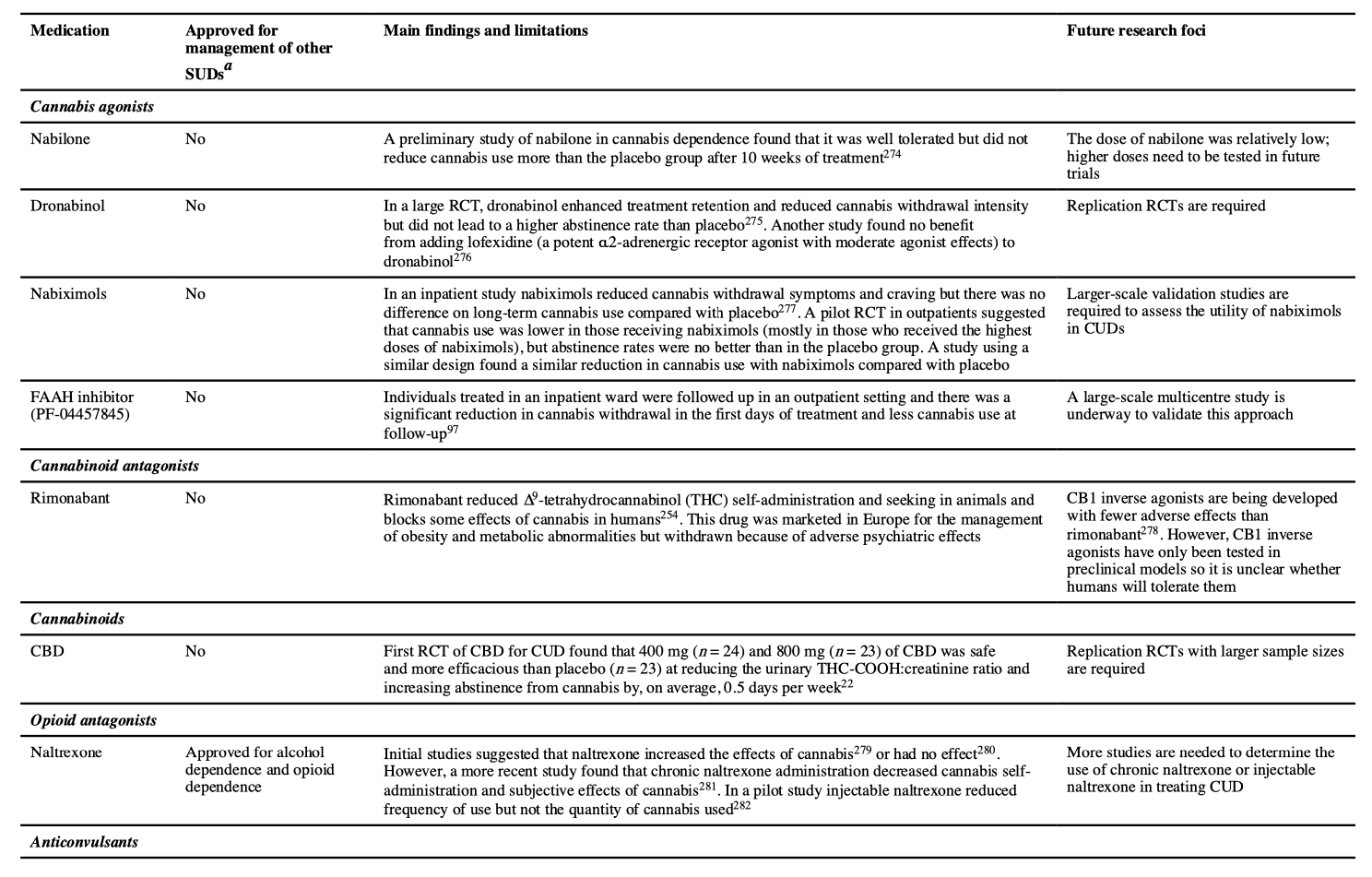
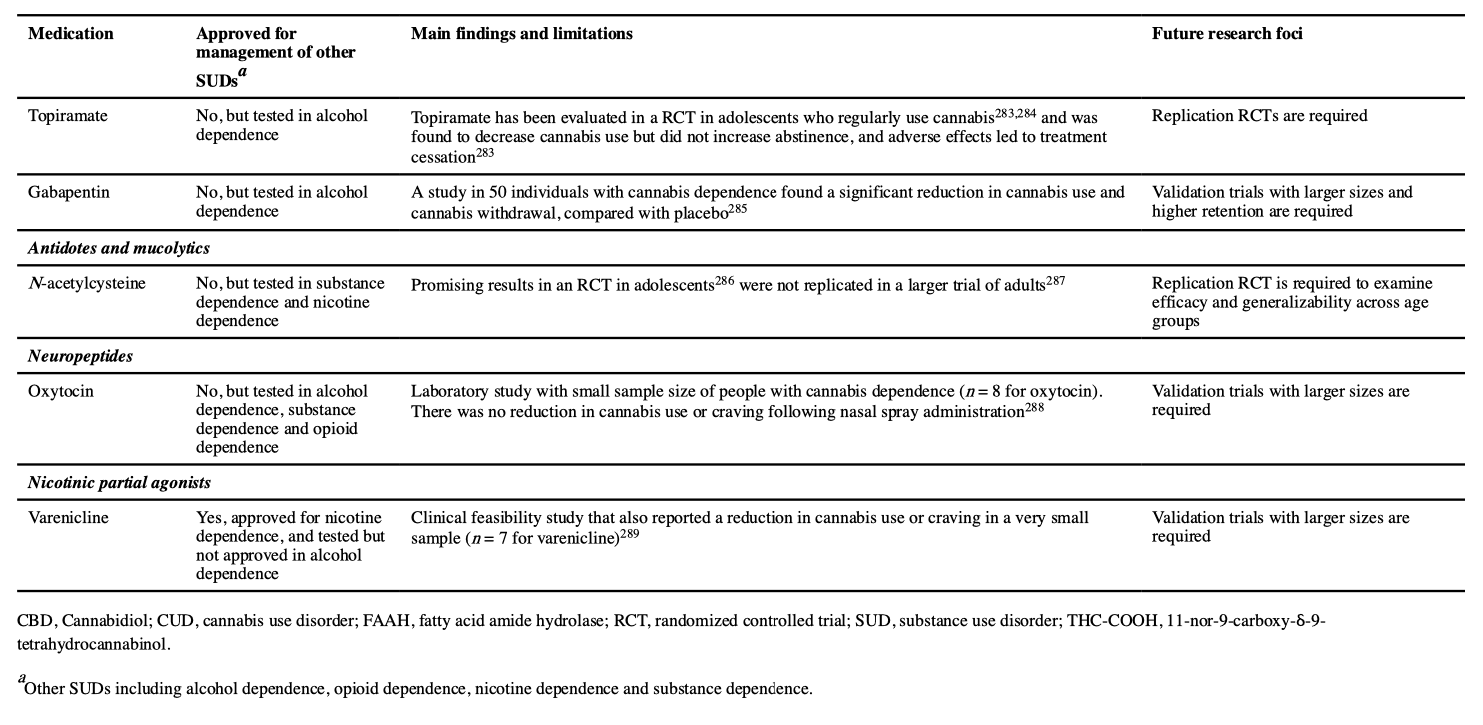
No pharmacological treatments have been approved for CUD. Various classes of drugs have been tested in treating cannabis withdrawal and/or cannabis use and promoting abstinence. Of these evaluated drugs, Cochrane and other reviews have found limited support for selective serotonin reuptake inhibitors, the antidepressant bupropion, the anxiolytic buspirone and the selective noradrenaline reuptake inhibitor atomoxetine. THC substitution (agonist) and antagonist treatments have produced some positive short-term results in reducing cannabis use, as have a number of agents used for addiction and non-addiction management but the number of studies is small and their quality is poor.
Quality of life
Quality of life (QOL) assessments can monitor subjective and objective functioning and well-being in CUD treatment. QOL and health-related QOL (HRQOL) provide broad multi-dimensional assessments of well-being that can supplement more specific assessments of functioning (BOX 3). QOL in individuals with CUD has not been as extensively examined as QOL in those with other mental health disorders. To evaluate QOL, studies usually apply self-report QOL or HRQOL scales or, by proxy, assess behaviours that are associated with QOL or HRQOL to measure well-being in people who use cannabis. The most studied areas include the relationship between QOL and recreational cannabis use, residual effects of cannabis, cannabis-related psychiatric disorders and distress.
There is a dose–response relationship between heavier cannabis use and poorer QOL. Individuals who meet criteria for CUD or psychometrically assessed cannabis-related problems report poorer QOL than individuals without CUD. Whether QOL improves after CUD treatment or cessation is uncertain. Some studies have found no improvement in QOL associated with a remission of, or reduction in, cannabis use in individuals with CUD, whereas other studies have reported significant improvements in QOL after both abstinence and reduction in cannabis use. Abstinent users have reported improved sleep, anxiety and self-reported cognitive function compared with heavy cannabis users.
A comparison of monozygotic twins of whom one had no history of cannabis use andthe other was a former heavy cannabis user found no differences between the two in educational attainment, employment, physical or mental health or HRQOL, suggesting that the adverse cognitive effects of cannabis may be reversed by sustained abstinence.
Other acute adverse effects of cannabis use include anxiety, depression, and psychotic, cardiovascular and gastrointestinal symptoms. Cannabis-impaired driving is of public health concern. There is a dose–response relationship between cannabis intoxication and cognitive and psychomotor impairment, and epidemiological data indicate that cannabis use makes a small contribution to motor vehicle crashes. Chronic bronchitis is reliably associated with regular cannabis smoking after controlling for tobacco smoking. Case series suggest that heavy cannabis smoking can result in hyperemesis syndrome and increase the risk of myocardial infarction and stroke in predisposed persons. Some epidemiological evidence supports that fetal growth and development may be adversely affected if cannabis is used during pregnancy.
Outlook
Future effects of legal cannabis
It may be more than a decade before legal commercial recreational cannabis markets in the USA and Canada mature, and their effects on CUD may not be fully assessed until the 2030s. If alcohol regulation serves as a guide, the commercialization of cannabis sales is likely to increase the frequency of use among current users owing to greater accessibility and acceptability of cannabis and lower prices for higher potency products. There are indications that this may be happening. A serious concern is that more potent novel cannabis products will be marketed to young people in ways that increase the risk of CUD and cannabis-related social and health problems. For example, the legal sale of THC-infused alcoholic drinks and cannabis edibles (for example ‘gummies’, sodas, candy bars and cookies) may appeal to young non-smokers who want to try cannabis.
The legal cannabis industry is becoming a multibillion-dollar enterprise in North America that the alcohol and tobacco industries have begun to invest in. Like the alcohol industry, the cannabis industry will seek to maximize its profits by increasing the number of regular, heavy users who comprise its best customers. The legal cannabis industry is now lobbying governments to reduce cannabis taxes, opposing caps on cannabis potency, campaigning for cannabis-vaping lounges and home delivery services, and making unsubstantiated claims about the medical benefits of cannabis use. Countries planning cannabis legalization should approach it with primarily a public health lens using approaches shown to be effective in reducing tobacco and alcohol-related harm. A referendum in New Zealand and a vote in the federal parliament in Germany have recently rejected bills to legalize recreational cannabis use.
Potential risks of high-potency products
The health effects of using more potent cannabis products in states and provinces with medical and recreational cannabis dispensaries require careful monitoring over the next decade. In surveys, individuals who report using higher-potency cannabis extracts report more symptoms of dependence and mental distress than users of herbal cannabis. In the Netherlands, the number of persons seeking help to quit cannabis use increased as cannabis potency increased and later fell when it declined. A major concern is that the use of high-potency cannabis may increase the risk of psychotic disorders. Some approaches such as restrictions on high-potency products, taxes based on the THC content of the product, clear labelling on dosage and risks, and robust monitoring of sales and impacts could reduce the negative effects of the marketing of these products.
Cannabis consumption measures
A major limitation of epidemiological research on cannabis use is the absence of adequate measures of the amount of THC consumed. Unlike alcohol, where we can discriminate between high-risk and low-risk drinking, only the frequency of cannabis is typically assessed. This makes it difficult to investigate the risks and benefits of cannabis use and to assess the effects of policy changes and treatment outcomes in clinical trials of CUD. There are recommendations to quantify a standard THC unit as 5 mg. A major challenge in developing better measures of THC consumption are the increased variety of cannabis products, changes in methods of use and the lack of technologies that reliably and cost-effectively quantify use in clinical and non-laboratory-based research.
Epidemiologists and economists would benefit from better measures to study the effect of cannabis laws on attitudes, markets, use and harms. Clinical researchers require better measures of cannabis use to guide treatment, assess outcomes and advise patients on safer patterns of use. In addition, health educators and policy-makers need better information to plan intervention and prevention programmes, design cannabis regulation and provide accurate information about cannabis risks to the public.
Pharmacotherapy
Research on pharmacotherapies for CUD is less developed than for other drugs of abuse. The main classes of medications that have been investigated are summarized in TABLE 3. The most promising are cannabinoid agonists that can be used in the same way as the nicotine patch for tobacco smoking (cessation), or as long-acting opioid agonists such as methadone and buprenorphine in heroin dependence (maintenance). The pharmacological agonists offset cannabis withdrawal symptoms and reduce the motivation to use cannabis by occupying CB1 receptors. Administering CB1 antagonists may also block the effects of cannabis and support abstinence. Preclinical studies show strong interactions between opioid and cannabinoid systems, suggesting that opiate antagonists such as naltrexone may reduce cannabis reward and intake. Other promising agents reviewed in TABLE 3 include topiramate, N-acetylcysteine, gabapentin, oxytocin and varenicline. Further investigation of replacement or substitution/agonist pharmacotherapy should personalize treatment to patient characteristics (such as gender and age) and symptomatology (for example, severity of cannabis use). Studies of new or repurposed agents will require similar advances in accompanying psychosocial interventions.
Psychosocial interventions
Screening and assessment tools are needed to personalize prevention and treatment. The development requires the identification of mechanisms that respond to psychosocial interventions. Research should explore the incremental benefits of combining different treatment components and identify how best to treat common comorbid psychiatric disorders.
The stigma arising from cannabis use and CUD needs to be reduced to increase treatment seeking. This may include refining online and self-help approaches that can be delivered in the privacy of one’s own home and better communicating about higher-quality prevention and treatments to increase public confidence in their effectiveness. Successful online programmes need to be more accessible as less than one in six programmes that have shown benefits in online RCTs are available for general use.
Supply reduction, advertising and education
In jurisdictions where adult cannabis use is legal, governments can minimize heavy use and adolescent uptake using taxation, legal restrictions on minimum purchase age and controls on advertising. A priority for cannabis research should be providing accurate information to assist the community and policy-makers to avoid ideologically influenced decision-making. Improved education needs to address the misinformation about and exaggerations of the medical benefits of cannabis. More effective communication of accurate information through social media and other forms of media is needed to reach youth and young adults to counter the promotional activities of the legal cannabis industry.
Mechanisms of CUD
Research on the endocannabinoid system should provide insight into the aetiopathogenesis of CUD, addiction vulnerability and comorbidity with other mental disorders. Understanding the roles of endogenous and exogenous cannabinoids may increase our knowledge of the developmental trajectories of different addictive substances if THC modifies the dopaminergic reward system to make other substances more rewarding.
Genetic research may help to understand the contribution of the endocannabinoid system to CUD vulnerability. Some GWAS of cannabis dependence have identified significant individual risk alleles but these have not been replicated across studies. The largest GWAS have not been able to identify specific genetic loci significantly associated with lifetime cannabis use or dependence, and individual SNPs typically explain <1% of the variance in risks of CUD. These findings limit pharmacogenomic approaches to treatment. A greater insight into the functional role of genes is needed, for example, clock genes, which may regulate dopamine transmission via change in circadian rhythms. Polygenic risk scores that combine SNPs associated with cannabis use and treatment response may be useful in targeting behavioural and pharmacological approaches. There may be incremental gains from combining polygenic risk scores and social and individual risk factors for developing CUD. Cannabis exposure during critical developmental periods may influence gene expression, affecting the nature and severity of CUD. In addition, cannabis exposure may produce epigenetic alterations in functional genes in pre-gestational and adolescent periods that increase the risks of developmental disorders in children, and psychiatric and SUDs in adolescents and adults.