Abstract
Few studies have examined the neural correlates of emotion regulation across adolescence and young adulthood. Existing studies of cognitive reappraisal indicate that improvements in regulatory efficiency may develop linearly across this period, in accordance with maturation of prefrontal cortical systems. However, there is also evidence for adolescent differences in reappraisal specific to the activation of “social‐information processing network” regions, including the amygdala and temporal‐occipital cortices. Here, we use fMRI to examine the neural correlates of emotional reactivity and reappraisal in response to aversive social imagery in a group of 78 adolescents and young adults aged 15–25 years. Within the group, younger participants exhibited greater activation of temporal‐occipital brain regions during reappraisal in combination with weaker suppression of amygdala reactivity—the latter being a general correlate of successful reappraisal. Further analyses demonstrated that these age‐related influences on amygdala reactivity were specifically mediated by activation of the fusiform face area. Overall, these findings suggest that enhanced processing of salient social cues (i.e., faces) increases reactivity of the amygdala during reappraisal and that this relationship is stronger in younger adolescents. How these relationships contribute to well‐known vulnerabilities of emotion regulation during this developmental period will be an important topic for ongoing research.
Introduction
Adolescents’ emotional lives differ from those of children or adults: they experience frequent and intense negative emotions in daily life [Larson et al., 1980, 2002; Larson and Lampman‐Petraitis, 1989], encounter unstable peer and romantic relationships [Brown, 2004; Collins et al., 2009; Hardy et al., 2002], and are at greater risk of mental health issues marked by emotional dysfunction [Arnett, 1999; Dahl and Gunnar, 2009]. These issues are thought to stem largely from an immaturity in adolescents’ emotion regulation abilities underpinned by a “still developing” neural architecture [Casey et al., 2010; Silk et al., 2003]. Specifically, neuroscientific research suggests that such immaturity may result from the combination of (a) an intensification of “bottom‐up” social‐affective processing, reflecting the heightened engagement of subcortical brain regions, such as the amygdala, to socially relevant stimuli, and (b) immaturity of prefrontal cortical systems responsible for generating “top‐down” regulatory control [Casey et al., 2008; Somerville et al., 2010; Steinberg, 2008]. While both are clearly relevant, some have argued that the former neural changes (i.e., subcortical changes that facilitate intensified social‐engagement) and also changes to a broader network of social‐brain regions, may be relatively more important for understanding emotion‐related vulnerabilities during the middle adolescent period [Blakemore, 2008; Crone and Dahl, 2012].
In adult populations, the neural basis of emotion regulation has been extensively examined [Carter, 2009; Kanske et al., 2011; Phillips et al., 2008]. Most studies have focused on the regulation of emotion via cognitive reappraisal, a highly adaptive technique that involves cognitively challenging one's initial negative interpretation of an aversive stimulus in order to reduce negative emotion [Ochsner et al., 2002]. During reappraisal, the generation of new appraisals consistently activates a distributed network of prefrontal and parietal cortical brain regions [Kalisch, 2009; Ochsner and Gross, 2008], which in turn modulates (or “down‐regulates”) neural indicators of affective responding, such as functional activation in the amygdala [Goldin et al., 2008; McRae et al., 2008; Schaefer et al., 2002].
Neurodevelopmentally, there is evidence to suggest that reappraisal ability improves linearly across the adolescent period [McRae et al., 2012; Pitskel et al., 2011]. This notion was endorsed by Pitskel et al.'s [2011] study of children and adolescents (aged 10–17), which reported that younger participants had reduced suppression of amygdala and visual cortical responses to disgust‐inducing images, indicative of poorer reappraisal. In a subsequent study of 10‐ to 22‐year‐old participants, McRae et al. [2012] reported a significant linear relationship between age and reappraisal success that was accompanied by linearly increased activation of the left ventrolateral prefrontal cortex. McRae et al. also observed significant non‐linear age effects in the activation of midline and temporal cortical regions (posterior cingulate, dorsomedial prefrontal cortex, temporal pole and superior temporal sulcus), broadly linked to social‐affective processes, including mental state attribution and self‐other perspective taking [Carrington and Bailey, 2009; Frith and Frith, 2006; Singer, 2006]. Compared to children and young adults, adolescents (aged 14–17 years) demonstrated lower emotional reactivity‐related activation of these regions when looking at aversive (vs. neutral) imagery, but higher reappraisal‐related activation, raising the possibility that they engage qualitatively distinct social‐affective processes during reappraisal tasks. Thus, in addition to observed improvements in reappraisal efficiency across adolescence and young adulthood, neurobiological research to date suggests that functional changes to the “social‐information processing network” [Nelson et al., 2005]—including amygdala, medial, and temporal‐occipital regions—may be critical to understanding the developmental trajectory of reappraisal ability.
The aim of the current investigation was to build on these two previous imaging studies by examining the neurodevelopment of emotional reactivity and reappraisal in a large sample of adolescents and young adults (aged 15–25 years). In particular, our study focused on the neurodevelopmental aspects of reappraisal in response to social‐affective stimuli. That is, while previous reappraisal studies have intermixed social (e.g., funeral scenes) and non‐social imagery (e.g., snakes), we used only social‐affective imagery in recognition of the heightened salience of interpersonal events during the adolescent period [Davey et al., 2008]. Specifically, we sought to investigate reappraisal of human emotions and intentions as conveyed by sad/distressed individuals in complex social scenes. Social stimuli are by far the most salient form of information to young people and the ability to regulate negative social information is particularly important to mental health outcomes during this period [Brown, 2004; Larson and Richards,1994; Prinstein and Aikins, 2004].
Further, unlike previous developmental studies of reappraisal, which have included children, we intentionally focused our enquiry on post‐pubescent adolescence. Our motive was to characterize a period synonymous with social change and heightened incidence of a number of affective disorders, including depression [Hecht et al., 1998; Monroe and Harkness, 2005; Rao et al., 1995]. Finally, neuroimaging studies of human brain development have consistently shown that maturation of prefrontal and lateral temporal regions continues well into early adulthood and thus developmental studies of emotion regulation abilities should ideally extend into this period [Gogtay et al., 2004; Pujol et al., 1993; Sowell et al., 2003].
Our hypotheses were twofold. Firstly, we anticipated developmentally mediated linear improvements in the efficiency of reappraisal, either in the form of age‐related increases in prefrontal cortical engagement [McRae et al., 2012] and/or age‐related increases in the down‐regulation of amygdala activity [Pitskel et al., 2011]. Secondly, as adolescence is a period of dramatic social‐emotional change that is associated with ongoing development of large‐scale neural networks, we hypothesized developmental effects in a broader network of neural regions, particularly regions within the “social‐information processing network,” that are associated with social processing and attention to biologically salient visual imagery [Burnett et al., 2011; Scherf et al., 2012].
Methods and Materials
Participants
Ninety‐six adolescents and young adults (15–25 years) were recruited for the current study. Participants were recruited via multiple channels including advertisements on websites, social media, university and hospital notice boards, and via community newspapers. Participants were considered eligible if they were (i) without current or past diagnosis of an Axis I psychiatric, substance use, or neurological disorder (SCID‐non patient version; [First et al., 1997]), (ii) native English speakers, (iii) not taking psychoactive medication, (iv) not pregnant, and (v) had no further contraindications to magnetic resonance imaging (MRI). Participants (or their parents if under 18 years of age) provided their informed consent to participate in the study, which was approved by the Melbourne Health Human Research Ethics Committee, Victoria, Australia. All participants were compensated for their time and travel expenses. Of the 96 participants who completed the full study protocol, 5 were subsequently excluded due to excessive head movement during scanning (see Image Acquisition and Pre‐Processing section); 10 due to an inability to cognitively reappraise (see Behavioral Analysis section); and 3 due to incidental neurological findings on MRI. The final composition of the sample was 78 participants (44 females), with a mean age of 19.91 years (SD = 2.78; range 15–25, see Supporting Information Fig. 1). Additional sample characteristics have been summarized in Supporting Information Table 1.
Experimental Task
We developed a blocked‐design cognitive reappraisal task that emulated the general features of many previously published tasks [Phan et al., 2005]. Consistent with such prior studies, the task involved three conditions—“look‐neutral,” “look‐negative,” and “reappraise”—presented in an ABC design with eight blocks per condition (i.e., a total of 8 × 3 = 24 blocks). At the beginning of each block, a word appeared for 2 s in the middle of the screen (see below for details) instructing participants to either “reappraise” or “look.” If the instruction was to “look,” the images that followed were either negative or neutral in content (depending on the condition), and participants were required to simply attend to these images without trying to alter their affective response. If the instruction was to “reappraise,” the images were always negative and participants were instructed to reduce the intensity of their negative affect using the reappraisal strategies described below. All blocks consisted of four consecutive images (each image was presented on screen for 6 s, no inter‐stimulus interval), immediately followed by a prompt to rate negative affect (cue: “How bad do you feel?”), to which participants responded by pressing 1 to 4 (1 = not at all bad; 4 = very bad) with their dominant hand. The task blocks were interspersed with rest periods in which participants viewed a fixation cross (10 s).
Stimuli
The task was presented with Paradigm software (http://www.paradigmexperiments.com), running on a Dell computer. The LCD screen that presented stimuli was visible via a reverse mirror mounted to the participants’ head coil and behavioral responses were captured using a button‐box. Picture stimuli contained complex imagery of people and were taken from the International Affective Picture System (IAPS) [Lang et al., 2008], the Empathy Picture System database [Geday et al., 2003], and online sources. To ensure comparability of pictures selected from different sources, individual images were independently rated (n = 10) for valence and arousal using a standardized 9‐point Self‐Assessment Manikin Scale (1 = most unpleasant/least arousing and 9 = most pleasant/most arousing). A total of 64 negative and 32 neutral pictures were selected based on these ratings (see Supporting Information Table 2). An analysis of variance (ANOVA) confirmed that negative pictures were significantly more arousing than neutral pictures (M = 5.74 (SD = 0.72) vs. M = 3.73 (SD = 0.46); F 1,94 = 204.33, P < 0.001) and valence ratings revealed they were significantly more aversive (M = 2.10 (SD = 0.66) vs. M = 4.98 (SD = 0.30); F 1,94 = 556.56, P < 0.001). Negative picture stimuli were divided into two picture‐sets that were assigned to the aversive stimulus conditions (i.e., “look‐negative” and “reappraise”). These picture‐sets were matched for valence and arousal (t tests, P > 0.23), and their assignment to the “look‐negative” and “reappraise” conditions was counterbalanced across participants. Picture stimuli (both negative picture‐sets and neutral pictures) were also selected to match for general content (including number of faces and figures) and differences in luminance and complexity were kept minimal.
Reappraisal Training
In the hour prior to scanning, participants familiarized themselves with reappraisal strategies. Specifically, in a training protocol adapted from the work by McRae et al. [2012], they were prompted to reappraise several practice images (not appearing in the experiment) by narrating aloud their re‐interpretation of each image. Three types of re‐interpretations were suggested: (i) it is not real (e.g., it is just a scene from a movie); (ii) things will improve with time (e.g. whatever is going wrong will resolve over time); and (iii) things are not as bad as they appear to be (e.g., the situation looks worse than it is, it could be a lot worse, or at least it is not me in that situation). If a participant's responses indicated that they were using a non‐cognitive strategy (such as looking away or attending to non‐emotional aspects of the picture), the participant was re‐directed to the three example strategies mentioned above. Once the experimenter determined from a participant's narration that they could utilize appropriate reappraisal strategies within the desired time (i.e., 6 s per image), the participant independently completed several practice blocks of the experimental task in preparation for the scan.
Compliance with reappraisal strategies during the fMRI‐task was subsequently confirmed using a brief post‐scan questionnaire. Our post‐scan questionnaire was designed to assess both participants’ perceived frequency of reappraisal strategy use, as well as their use of avoidance strategies (i.e., only looking at the non‐emotional aspects of the picture, looking away or closing their eyes), which participants rated on a scale from 1 to 5 (1 = Never, to 5 = Always). While the occasional use of other strategies was not considered an exclusion criterion over and above reappraisal success, avoidance strategies are known to confound the results of fMRI visual provocation studies and thus were of interest. In particular, we sought to determine whether individual variation in use of avoidance strategies during reappraisal was predicated by age or associated with any activation effects observed during fMRI.
Image Acquisition and Pre‐Processing
A 3T General Electric Signa Excite system equipped with an 8‐channel phased‐array head coil was used in combination with ASSET parallel imaging (Sunshine Hospital, Western Health, Melbourne). The functional sequence consisted of a single shot gradient recalled EPI sequence in the steady state (repetition time, 2,000 ms; echo time, 35 ms; and pulse angle, 90°) in a 23‐cm field of view, with a 64 × 64‐pixel matrix and a slice thickness of 3.5 mm (no gap). Thirty‐six interleaved slices were acquired parallel to the anterior–posterior commissure line with a 20° anterior tilt to achieve more optimal coverage of ventral prefrontal cortical brain regions. The total sequence time was 16 min, corresponding to 485 whole brain echo‐planar imaging volumes. The first four volumes from each run were discarded to allow for T1 equilibration effects. Additionally, a T1‐weighted high‐resolution anatomical image was also acquired for each participant to assist with functional time‐series co‐registration using the following 3D BRAVO sequence: 140 contiguous slices; repetition time, 7,900 ms; echo time, 3,000 ms; flip angle, 13°; in a 25.6‐cm field of view, with a 256 × 256 pixel matrix and a slice thickness of 1 mm (no gap). To assist with noise reduction, all participants used foam insert earplugs. To assist with head immobility, foam‐padding inserts were placed around the participants’ head inside the coil.
Imaging data were transferred and processed on a Linux platform running MATLAB version 8.2 (The MathWorks Inc., Natick, MA). Pre‐processing was performed with Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, UK). Motion correction was performed by aligning each participant's time series to the first image using least squares minimization and a six‐parameter (rigid body) spatial transformation. Participants’ data were excluded if movement in the translational or rotational planes exceeded 3 mm or 3°, respectively. These realigned functional images were then co‐registered to each participant's respective T1 anatomical scan, which were segmented and spatially normalized to the International Consortium for Brain Mapping template using the unified segmentation approach (re‐sliced to 2 mm isotropic resolution). Functional images were smoothed with a 6‐mm (full‐width, half maximum) Gaussian filter.
Behavioral Analysis
In‐scanner mean negative affect ratings were derived for each participant corresponding to the “look‐negative,” “look‐neutral,” and “reappraise” conditions. Emotional reactivity and reappraisal success scores were estimated for each participant by computing simple differences between mean condition ratings (“look‐negative minus look‐neutral,” and “look‐negative minus reappraise,” respectively). If a participant's reappraisal difference score indicated that they were unable to reduce negative affect with reappraisal (i.e., difference score < 0), they were excluded from the current analyses (as stated above). The 10 participants excluded due to reappraisal ability were no different from the remaining sample in terms of age (t 86 = 0.18, P = 0.22) or gender (χ 2 (1, N = 88) = 0.05, P = 0.83). To assess the relationship between age and behavioral indices of emotional reactivity and reappraisal success, difference scores were regressed against age effects using multiple regression models in Statistical Package for the Social Sciences (SPSS; Chicago, IL; version 20). We also examined for any potential relationships between age and avoidance strategies (e.g., looking away).
Imaging Analysis
For each participant, the primary task conditions (“look‐negative,” “look‐neutral,” “reappraise”) were specified as individual predictors in an SPM8 “first‐level” time‐series analysis, in addition to specifying conditions of no interest (fixation, cue, and affect rating periods). The resulting model was convolved with a canonical hemodynamic response function with 1/128 s high‐pass filter applied in order to remove low‐frequency noise. Maximum likelihood parameter estimates were calculated at each voxel using the general linear model and an AR(1) model of serial autocorrelations.
First‐level contrast‐images were estimated for the following primary effects of interest: (i) “look‐negative > look‐neutral” to identify brain regions associated with emotional reactivity to aversive social‐affective images; (ii) “reappraise > look‐negative” to identify brain regions activated during reappraisal; and (iii) “look‐negative > reappraise” to identify brain regions that were significantly down‐regulated during reappraisal. Contrast images for each participant were then carried forward to the “second level” using the summary statistics approach to random‐effects analyses (one‐sample t tests). The resulting group statistical parametric maps were thresholded using a false‐discovery rate error‐correction of P FDR < 0.05 across the whole‐brain volume with a minimum cluster extent of 10 contiguous voxels (K E, ≥ 10 voxels). To identify brain regions that were specifically down‐regulated by reappraisal, the group level results corresponding to the “look‐negative > reappraise” contrast were inclusively masked to include only those voxels identified as significantly activated by the aversive imagery (i.e., as determined by the “look‐negative > look‐neutral” contrast), at P FDR < 0.05 (K E, ≥ 10 voxels, whole‐brain corrected). We also investigated within subjects the extent to which reappraisal success significantly predicted down‐regulation of amygdala activation reported for the primary study contrast “reappraise > look‐negative”. To do so, participants' reappraisal success scores were entered as a regressor in the corresponding second‐level analysis thresholded at P FDR < 0.05 (K E, ≥ 10 voxels, whole‐brain corrected).
The relationship between age and brain activation was assessed by repeating the above group level analyses with linear and quadratic age effects as covariates of interest. We examined age associations within regions identified in the former analyses as significantly activated across the entire group. For example, to identify the influence of age on brain activation associated with the “reappraise > look‐negative” contrast, we inclusively masked age analyses by the estimated “reappraise > look‐negative” main effect. Significant age‐modulated effects are reported if they survived P FDR < 0.05 (K E, ≥ 10 voxels, whole‐brain corrected).
Results
Behavioral Ratings
As expected, negative affect was significantly higher in the “look‐negative,” as compared to the “look‐neutral” condition, indicative of significant emotional reactivity within subjects (M = 2.81 (SD = 0.69) vs. M = 1.03 (SD = 0.10); t 77 = 22.77, P < 0.001). Importantly, negative affect was significantly lower in the “reappraise,” as compared to the “look‐negative” condition, indicative of successful reappraisal within subjects (M = 1.97 (SD = 0.53); t 77 = 14.01, P < 0.001). There was no significant influence of gender on emotional reactivity to aversive stimuli (t 76 = 0.55, P = 0.88), or reappraisal success (t 76 = −1.13, P = 0.79) as indicated by the negative affect difference scores (“look‐negative minus look‐neutral” and “look‐negative minus reappraise,” respectively). With regard to the modulatory effects of age, there was no significant relationship between age and reappraisal success (β = −0.08, P = 0.46), however, there was a tendency toward reducing emotional reactivity with age (β = −0.1, P = 0.09). Finally, there was no relationship between age and reported use of reappraisal and avoidance strategies as assessed by our post‐scan measure.
fMRI Analyses
For the “look‐negative > look‐neutral” contrast, significant activation was observed bilaterally across a large expanse of visual association cortex, including the fusiform gyrus and lateral occipital cortex, extended hippocampus‐amygdala complex, dorsal midbrain (∼periaqueductal gray), hypothalamus, medial thalamus, caudate head, ventral anterior insular cortex, pre‐supplementary and lateral premotor cortices, dorsomedial and dorsolateral prefrontal cortex, and the intraparietal sulcus extending to primary somatosensory cortex (Fig. (Fig.1A,1A, Supporting Information Table 3).
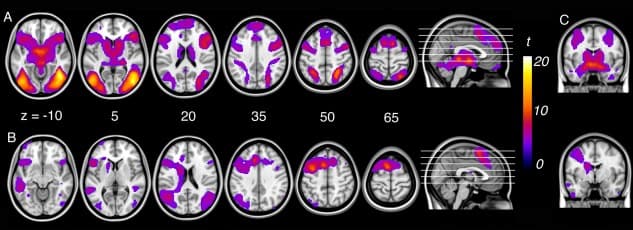
Figure 1
A: Activation corresponding to emotional reactivity (“look‐negative > look‐neutral”) and (B) reappraisal (“reappraise > look‐negative”) within subjects. C: Amygdala activation for emotional reactivity (top‐right) and reappraisal (bottom‐right).
For the “reappraise > look‐negative” contrast, significant activation was observed in predominantly left‐lateralized regions including the left pre‐supplementary area extending to the cingulofrontal cortex (at the junction of dorsal anterior cingulate cortex and dorsomedial frontal cortex), dorsal premotor cortex extending to dorsolateral prefrontal cortex, frontal operculum and ventral anterior insular cortex, left caudate body, bilateral angular gyrus, posterior‐superior temporal gyrus, and fusiform gyrus (Fig. (Fig.1B,1B, Supporting Information Table 3).
Examination of the “look‐negative > reappraise” contrast revealed that reappraisal led to significant reductions in activation (i.e., down‐regulation) of brain regions previously identified as responsive to the aversive social‐affective imagery. This included the left and right primary somatosensory cortex (area 2 and 3b); right dorsal amygdala extending to ventral pallidum and putamen; and secondary visual cortex (Fig. (Fig.2,2, Supporting Information Table 3). Examination of brain–behavioral associations within subjects revealed no significant relationship between down‐regulation of the amygdala during reappraisal and behavioral indices of reappraisal success.
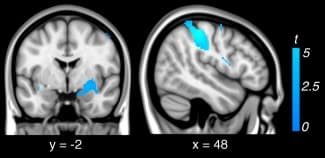
Figure 2
Regions down‐regulated during reappraisal (i.e., look‐negative > reappraise) within subjects.
We did not observe any significant effects of age on the activation of brain regions previously identified in the “look‐negative > look‐neutral” contrast. However, we observed a significant effect of age on brain regions activated by reappraisal. Activation of temporal and parietal cortical regions, lateral occipital cortex, and bilateral fusiform gyrus were significantly linearly modulated by age in the “reappraise > look‐negative” contrast (Table (Table1,1, Fig. Fig.3A),3A), with younger age predicting greater activation of these regions. No significant quadratic associations between age and brain activation were observed for the “look‐negative > look‐neutral” or “reappraise > look‐negative” contrast.
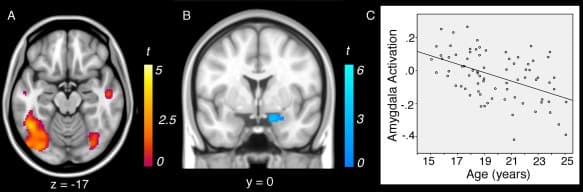
Figure 3
Negative linear associations between age and brain activation during reappraisal (“reappraise > look‐negative”): (A) younger participants showed enhanced activation of reappraisal‐related regions and (B) reduced down‐regulation of the amygdala during reappraisal. C: Scatterplot of linear association between age and amygdala activation during reappraisal.
Table 1
Linear effects of age on reappraisal activation. Younger age predicted greater neural activation in both regions whose activation increases (left) and decreases (right) during reappraisal (vs. look negative) within subjects
Reap > Look Neg | Anatomy | Stats | Look Neg > Reap | Anatomy | Stats | ||||||||
x | y | z | K E | Z | BA | x | y | z | K E | Z | BA | ||
Inferior temporal gyrus | −48 | −52 | −12 | 2146 | 4.69 | 37 | Amygdala/ | 14 | −2 | −18 | 102 | 4.67 | 28 |
Lateral occipital cortex | −24 | −88 | 2 | 4.33 | 18 | Parahippocampal gyrus | |||||||
Fusiform gyrus | −34 | −78 | −18 | 4.04 | 19 | ||||||||
Fusiform gyrus | 34 | −72 | −20 | 222 | 3.81 | 19 | |||||||
Lateral occipital cortex | 32 | −86 | −10 | 3.61 | 19 | ||||||||
Fusiform gyrus | 30 | −74 | −6 | 3.53 | 19 | ||||||||
Superior parietal lobe | −24 | −70 | 60 | 39 | 3.77 | 7 | |||||||
Middle temporal gyrus | 52 | −12 | −14 | 34 | 3.63 | 20 | |||||||
Middle temporal gyrus | −54 | −10 | −12 | 22 | 3.60 | 22 | |||||||
Temporal pole | 44 | 20 | −32 | 14 | 3.43 | 38 | |||||||
Middle temporal gyrus | 54 | −70 | 12 | 36 | 3.57 | 37 | |||||||
Angular Gyrus | −42 | −68 | 26 | 11 | 3.54 | 39 |
Anatomical co‐ordinates (x, y, z) are given in MNI space (mm). Magnitude and extent statistics correspond to a minimum threshold of P FDR < 0.05, KE, ≥ 10 voxels. Upper‐case Z values correspond to SPM Z‐score statistics. BA= approximate Brodmann Area.
The magnitude of right amygdala down‐regulation during reappraisal (as identified with the “look‐negative > reappraise” contrast) was also significantly linearly modulated by age (Fig. (Fig.3B,C,3B,C, Table Table1).1). Specifically, younger age was associated with significantly weaker down‐regulation of amygdala activity during reappraisal (as compared to look‐negative). No other regions identified for the “look‐negative > reappraise” contrast were significantly modulated by age, nor were there any significant quadratic associations with age.
To assess whether age‐related effects in the right amygdala were associated with age‐related differences in reappraisal success or strategy use (including avoidance), we next correlated peak activation of this region with behavioral variables assessed online and with our post‐scan questionnaire. No significant associations were observed in relation to these measures.
fMRI Mediation Analysis
As reported above, we observed developmental variation in the extent to which amygdala activity was effectively down‐regulated during reappraisal, but no developmental changes in reappraisal‐related prefrontal cortical activation. Therefore, in further analyses, we sought to characterize whether developmental changes in other brain regions might partly explain the observed amygdala findings. To do so, we utilized Mediation Effect Parametric Mapping [Wager et al., 2008]. MEPM allowed us to identify, on a voxel‐wise basis, any brain region(s) activated during reappraisal that also satisfied formal criteria for mediators in a standard three‐variable path‐modeling framework. In our model, Path a signifies the relationship between age (X) and brain regions activated during reappraisal (M). Path b signifies the relationship between brain regions activated during reappraisal (M) and corresponding level of amygdala activity (Y), controlling for age (X). Finally, Path a×b signifies the test of mediation, that is, whether the direct X→Y relationship is significantly reduced by including M in the path model. For brain regions to be considered significant mediators, we required that they reach statistical significance in each of the three tests comprising the path model (Paths a, b, a×b). The false positive rate was controlled using a voxel‐wise FDR at q < 0.05 across all estimated effects in the path model (a, b, a×b), which corresponded to minimum P = 0.001 and at least three contiguous voxels [Wager et al., 2008]. Regional path effects from this analysis are reported as significant if surviving P uncorrected < 0.001. For both analysis approaches, statistical significance of the peak voxel path effects were computed via a bootstrap test (10,000 permutations), as described by Wager et al. [2008].
Our MEPM analysis identified left fusiform gyrus activation as a significant neural mediator of age‐related changes in amygdala reactivity during reappraisal. As depicted in Figure Figure4,4, fusiform gyrus activation demonstrated a significant negative path coefficient for Path a (r = −0.46) and a significant positive coefficient for Path b (r = 0.57). That is, reduced modulation of amygdala activation during reappraisal in younger participants was explained by a greater relative engagement of the left fusiform gyrus (a = −0.04 (0.01), Z = −3.58; b = 0.46 (0.07), Z = 3.72; a×b = −0.02 (0.01), Z = −3.30, q < 0.05 FDR). Alternatively stated, linear reductions in activation of the left fusiform gyrus across mid‐adolescence to young adulthood predicted observed improvements in amygdala down‐regulation during reappraisal.
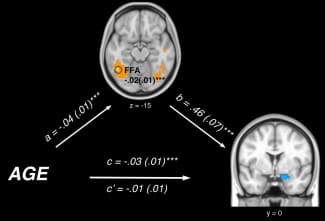
Figure 4
Path diagram showing the relationships between variables in the path model. Age (bottom‐left) predicts increased activation in the fusiform face area (FFA, top; a path), which acts as a mediator of the relationship between age and amygdala activation. The lines are labeled with path coefficients and standard errors are shown in parentheses. The mediator's connection to amygdala activation (right) is denoted by the b path, calculated controlling for age. ***P < 0.001 two tailed. The direct path is the c′ path, and this is calculated controlling for the mediator.
PPI Analysis
To further explore the age‐related changes in the amygdala, we sought to confirm whether our activation effects, including the mediating influence of the fusiform gyrus, might be related to changes in functional coupling of these regions across development (i.e., “functional connectivity”). Given the lack of influence of age on activation of the prefrontal cortex, we were also interested to confirm this finding by assessing whether any age‐related changes in functional connectivity between the amygdala and prefrontal cortex were observed. To do so we performed a psychophysiological interactions (PPI) analysis in SPM8 [Friston et al. 1997] to assess task‐related changes (“reappraise > look‐negative”) in the functional connectivity of the amygdala as our primary “seed” region of interest (ROI) and the fusiform gyrus and dorsolateral prefrontal cortex as our “target” ROIs. For an extended description of the PPI analysis and uncorrected whole‐brain results, see Supporting Information Materials (Supporting Information Tables 4 and 5). Briefly, the placement of the seed amygdala ROI was informed by the age effects reported for the “reappraise > look‐negative” contrast (x, y, z = 14, −2, −18). The placement of the target ROIs corresponded to the left fusiform gyrus cluster also identified from the age‐analysis (“reappraise > look negative”; x, y, z = −48, −52, −12, K E, = 2,217 voxels) and the left dorsolateral prefrontal cortex (dlPFC) cluster identified from main‐effects analysis (“reappraise > look negative”; x, y, z = −8, 10, 62, K E, = 10,555 voxels).
Task‐dependent increases in functional connectivity strength were observed between the right amygdala and the left fusiform gyrus (P FDR < 0.05, Z = 3.32, (x, y, z = −36, −68, −14); K E, = 45 voxels, small‐volume corrected) during “look‐negative > reappraise”. In other words, reappraisal was associated with significant functional decoupling between amygdala and fusiform activity compared to when viewing negative images. We observed that this relationship was significantly influenced by age (P FDR < 0.05, Z = 3.07, (x, y, z = −46, −76, −8); K E, = 231 voxels, small‐volume corrected) with younger age associated with less amygdala‐fusiform decoupling during reappraisal (see Supporting Information Figure 2). No significant changes in functional connectivity were observed between the amygdala and left dlPFC during reappraisal, nor was this relationship significantly influenced by age.
Discussion
The use of social‐affective imagery to examine the neuromaturational underpinnings of emotional reactivity and reappraisal in adolescents and young adults generated several key findings. First, we observed no significant influence of age on brain responses associated with emotional reactivity to aversive social‐affective stimuli. Second, in relation to the reappraisal of aversive social‐affective stimuli, we observed significant linear associations with age, including greater activation of the amygdala among younger participants. Third, we observed that left fusiform gyrus activation significantly accounted for age‐related changes in amygdala activation across adolescence and young adulthood—with greater activation of this region predicting greater amygdala activation during reappraisal. Finally, our findings were corroborated by the results of a functional connectivity analysis, which also revealed significantly greater fusiform‐amygdala coupling among younger participants for the “reappraise > look‐negative” contrast.
In reappraisal studies, the amygdala has been identified as the brain region most frequently modulated by reappraisal [see recent meta‐analyses, Buhle et al., 2014; Frank et al., 2014]. This observation is generally consistent with the hypothesized role of the amygdala in bottom‐up encoding and generation of responses to salient stimuli [Anderson and Phelps, 2001; Cunningham and Brosch, 2012; Fitzgerald et al., 2006; Sergerie et al., 2008]. It has been theorized that reappraisal alters the signaling properties of aversive stimuli, thereby modulating emotional responding in the amygdala accordingly [Diekhof et al., 2011; Ochsner and Gross, 2005; Ray et al., 2005]. Conversely, poorer down‐regulation of the amygdala during reappraisal (or greater amygdala activation) typically reflects poorer ability to modify salience‐related processing and thus reduce negative affect [Johnstone et al., 2007; Ochsner et al., 2004; Urry et al., 2006]. The observed relationship between age and amygdala responding in the current study is therefore compelling, as it indicates that efficiency in modulation of the amygdala (via reappraisal), and thus, related flexibility in modulating internal representations of social‐affective stimuli, may improve linearly across adolescence and young adulthood.
In the context of this study, enhanced amygdala activation among younger adolescents during reappraisal may reflect greater sensitivity to emotive social cues. This fits with a body of work suggesting that mid‐adolescence is associated with a general intensification of affective processing, particularly for social stimuli [Davey et al., 2008; Somerville et al., 2010]. Consistent with this notion, activation within visual sensory, temporal, and parietal regions implicated in social‐affective processing was also greater among younger adolescents during reappraisal. This included both regions implicated in the encoding of perceptual features of social stimuli (fusiform gyrus, inferior occipital) [Gauthier et al., 2000; Haxby et al., 2002] and also those involved in social perception, including thinking about another's mental state (superior temporal sulcus, lateral temporal regions, angular gyrus, and the temporal poles) [Allison et al., 2000; Olson et al., 2007; Van Overwalle and Baetens, 2009; Vigneau et al., 2006; Young et al., 2010]. Importantly, recent work has indicated that temporal cortical regions, as identified in our age‐related analysis, might be particularly important to transformations of stimulus meaning that enable emotional change [Buhle et al., 2014; Ochsner et al., 2012]. Thus, it may be that perceptual and semantic encoding of social‐cues, which is essential to constructing alternative internal representations of stimuli [Buhle et al., 2014], is enhanced among younger adolescents. Reappraisal‐related developmental change within perceptual and semantic systems is also consistent with ongoing development of the “social‐information processing network,” and in particular, the protracted structural development of lateral temporal regions [Giedd et al., 1999; Nelson et al., 2005]. Whether our age‐related effects in activation of temporal and occipital cortical regions reflect differences in neural efficiency of reappraisal processes, or differences in the ability to appropriately modulate attention to social‐affective stimuli is a question that warrants further enquiry.
Given the established role of the amygdala in reappraisal processes, determining the precise region(s) that mediated age‐related changes in amygdala activation was of particular interest. Thus, a notable finding in our study is that left fusiform gyrus activity significantly mediated the relationship between age and right amygdala activation during reappraisal. That is, age‐dependent amygdala reactivity during reappraisal was significantly accounted for by age‐dependent changes in left fusiform gyrus activation, such that in younger participants, heightened fusiform activation accounted for reduced amygdala down‐regulation. The association between fusiform and amygdala activation is consistent with results from emotional face tasks, which suggest that the fusiform face area is an important feed‐forward modulator of amygdala responding [Fairhall and Ishai, 2007; Pujol et al., 2009]. Indeed, the strength of functional coupling of these regions during reappraisal (versus look‐negative) was found to be negatively associated with age, highlighting the possibility that such feed‐forward modulation may be greater among younger participants. The role of the fusiform gyrus in reappraisal is also consistent with the use of complex social images in our task, which overwhelmingly contained human faces [Kawasaki et al., 2012; Monroe et al., 2013; Vrtička et al., 2011].
While age effects in the fusiform gyrus were bilateral, only the left fusiform was found to significantly mediate activation in the amygdala. This result is interesting as past research suggests that activation of the left, but not the right fusiform gyrus may require attention to be proximal to placement of facial stimuli, implicating a role for attention in activation of this region [Vuilleumier et al., 2001]. If this is the case, age‐related differences in fusiform gyrus activity could reflect differential attention to emotion‐relevant features that are essential for triggering one's emotions. This hypothesis is consistent with other work showing that, compared to adults, adolescents show less ability to modulate affective brain regions like the amygdala during face processing in accordance with attentional instructions [Monk et al., 2003].
One possible interpretation of the observed effect in the left fusiform gyrus is that younger adolescents, relative to older adolescents and young adults, may experience social material (faces in particular) as more perceptually salient during reappraisal—potentially interfering with the cognitive modulation of the amygdala. Such observations may reflect developmental differences in strategies used to reappraise. For example, while younger adolescents may demonstrate greater reliance on elaborating social perceptual cues when thinking about alternative interpretations of stimulus depictions, older adolescents may be more practiced at referencing stored semantic information (regarding the causes and outcomes of certain situations), and therefore may be less reliant on stimulus‐driven reinterpretations. Importantly, while this study acquired additional self‐reported data on avoidance‐type strategy use (i.e., looking away, looking at non‐emotional features of the stimuli, or closing one's eyes), we identified no evidence of developmental differences in use of such strategies, suggesting that any age‐related attentional differences during reappraisal are likely to be subtle.
It is of interest that age‐related reductions in amygdala activation were observed for reappraisal, but were not associated with general emotional reactivity to aversive social‐affective stimuli. This is inconsistent with numerous studies that have reported heightened amygdala activation among adolescents, versus adults, to facial expressions [Gee et al., 2013; Guyer et al., 2008; Hare et al., 2008; Killgore et al., 2001; Monk et al., 2003] and IAPS images [Vasa et al., 2011; Vink et al., 2014]. However, unlike the majority of these studies, we used a paradigm designed to distinguish developmental changes in emotional reactivity from developmental changes in reappraisal. We achieved this by explicitly instructing participants to either attend to stimuli (and not alter their emotions in any way), or to deliberately cognitively reappraise their emotional response. The only other study of reappraisal to observe an age‐effect in the amygdala, similarly found this to be evident when participants were attempting reappraise, but not in association with emotional reactivity to aversive stimuli [Pitskel et al., 2011]. While further work is needed to corroborate our findings, it appears that age‐related differences in amygdala reactivity and “bottom‐up” neural processing may become particularly evident when participants attempt to cognitively regulate emotions. This suggestion is broadly consistent with evidence indicating that adolescents’ vulnerability to negative emotional states is often perpetuated by failures of emotional regulation rather than emotional reactivity [Silk et al., 2003; Silvers et al., 2012; Yap et al., 2010].
Our experimental design also differs from the protocol of a past developmental study of reappraisal that found age‐related increases in prefrontal cortical activity [McRae et al., 2012]. Differences between our results and those of McRae et al. may be due to methodological factors. For example, while McRae et al. focused on the age period between childhood and young adulthood, we focused on post‐pubertal adolescence to young adulthood, during which time changes in prefrontal regulatory control associated with task performance may be less pronounced [Adleman et al., 2002; Crone et al., 2006; O'Hare et al., 2008]. In addition, our task contained shorter trial durations and a greater number of trials, which may have influenced the nature of cognitive processes engaged, or cognitive load accordingly. Differences may also be attributed to our sole use of social‐affective stimuli, as compared with work that has intermixed social and non‐social‐affective stimuli. In general, the impact of inclusion of social stimuli on age‐related differences in prefrontal engagement remain remarkably understudied [Geier et al., 2009; Silvers et al., 2012].
The present study is not without limitations. First, the study was cross‐sectional, meaning that longitudinal validation of our age‐related finding is desirable. Second, our study did not employ eye‐tracking during fMRI. As such, we cannot confirm how gaze and attentional deployment may have contributed to our age effects in the fusiform gyrus and amygdala [Manera et al., 2014; van Reekum et al., 2007]. Finally, due to social‐desirability bias associated with self‐report, our study may have been limited in its ability to detect important age‐related differences in reappraisal success. As such, future studies may benefit from the addition of more objective measures of reappraisal success, such as indicators of arousal obtained from physiological recordings (i.e., skin conductance, heart rate).
In summary, we observed heightened amygdala activation in younger participants during attempts to reappraise. Interestingly, enhanced amygdala activation among younger participants during reappraisal occurred in the absence of functional deficits in assumed prefrontal cortical control. Rather, these developmental differences were mediated by age‐related activation of the fusiform gyrus. Thus, our study provides evidence for the notion that regions important to encoding of social‐affective stimuli may have an influence on reappraisal processes during adolescence in a manner that is independent from prefrontal cortical immaturity. Our finding that amygdala and fusiform gyrus modulation improves across adolescence and young adulthood may have important implications for understanding adolescent affective vulnerabilities. Specifically, while activation in the amygdala and fusiform gyrus may reflect adaptive engagement with salient social stimuli, it also creates a perceptual bias toward aversive social stimuli that may overwhelmingly increase propensity for negative affective experiences [Barrett et al., 2007]. Importantly, reduced ability to modulate these regions during reappraisal may be problematic, as it indicates a reduced ability to disengage from processing aversive stimuli when required. Difficulty disengaging processing of negative social information is a feature of many psychiatric disorders [Cardoner et al., 2011; Mathews et al., 1996; Monk et al., 2008], including social anxiety disorder, whereby individuals demonstrate heightened amygdala and fusiform responses to aversive emotional faces [Campbell et al., 2007]. Thus, for individuals with a pre‐existing perceptual bias toward negative information, the transitional period from adolescence to young adulthood may represent a significant period of neural vulnerability to emotion dysregulation. This hypothesis is consistent with the rapid increase in mood and affective disorders seen in adolescence [Insel and Fenton, 2005; Paus et al., 2008] and aligns with growing evidence that points to the importance of changes in social‐affective processing, as crucial to understanding emergent adolescent vulnerabilities [Crone and Dahl, 2012; Nelson et al., 2005].