Abstract
Importance: Neuroimaging studies have documented racial and ethnic disparities in brain health in old age. It remains unclear whether these disparities are apparent in midlife.
Objective: To assess racial and ethnic disparities in magnetic resonance imaging (MRI) markers of cerebrovascular disease and neurodegeneration in midlife and late life.
Design, Setting, and Participants: Data from 2 community-based cohort studies, Washington Heights–Inwood Columbia Aging Project (WHICAP) and the Offspring Study of Racial and Ethnic Disparities in Alzheimer Disease (Offspring), were used. Enrollment took place from March 2011 and June 2017, in WHICAP and Offspring, respectively, to January 2021. Of the 822 Offspring and 1254 WHICAP participants approached for MRI scanning, 285 and 176 refused participation in MRI scanning, 36 and 76 were excluded for contraindications/ineligibility, and 4 and 32 were excluded for missing key variables, respectively.
Main Outcomes and Measures Cortical thickness in Alzheimer disease–related regions, white matter hyperintensity (WMH) volume.
Results: The final sample included 1467 participants. Offspring participants (497 [33.9%]) had a mean (SD) age of 55 (10.7) years, had a mean (SD) of 13 (3.5) years of education, and included 117 Black individuals (23.5%), 348 Latinx individuals (70%), 32 White individuals (6.4%), and 324 women (65.2%). WHICAP participants (970 [66.1%]) had a mean (SD) age of 75 (6.5) years, had a mean (SD) of 12 (4.7) years of education, and included 338 Black individuals (34.8%), 389 Latinx individuals (40.1%), 243 White individuals (25.1%), and 589 women (65.2%). Racial and ethnic disparities in cerebrovascular disease were observed in both midlife (Black-White: B = 0.357; 95% CI, 0.708-0.007; P = .046) and late life (Black-Latinx: B = 0.149, 95% CI, 0.068-0.231; P < .001; Black-White: B = 0.166; 95% CI, 0.254-0.077; P < .001), while disparities in cortical thickness were evident in late life only (Black-Latinx: B = −0.037; 95% CI, −0.055 to −0.019; P < .001; Black-White: B = −0.064; 95% CI −0.044 to −0.084; P < .001). Overall, Black-White disparities were larger than Latinx-White disparities for cortical thickness and WMH volume. Brain aging, or the association of age with MRI measures, was greater in late life compared with midlife for Latinx (cortical thickness: B = 0.006; 95% CI, 0.004-0.008; P < .001; WMH volume: B = −0.010; 95% CI, −0.018 to −0.001; P = .03) and White (cortical thickness: B = 0.005; 95% CI, 0.002-0.008; P = .001; WMH volume: B = −0.021; 95% CI −0.043 to 0.002; P = .07) participants but not Black participants (cortical thickness: B = 0.001; 95% CI, −0.002 to 0.004; P =.64; WMH volume: B = 0.003; 95% CI, −0.010 to 0.017; P = .61), who evidenced a similarly strong association between age and MRI measures in midlife and late life.
Conclusions and Relevance: In this study, racial and ethnic disparities in small vessel cerebrovascular disease were apparent in midlife. In Latinx and White adults, brain aging was more pronounced in late life than midlife, whereas Black adults showed accelerated pattern of brain aging beginning in midlife.
Introduction
Exposure to conditions that increase stress and risk for poor health, such as material hardship, interpersonal discrimination, institutional racism, residential segregation and pollution, and personal danger, are more common among Black and Latinx people than among White people. The weathering hypothesis suggests that repeated exposure to stress, suboptimal environments, and social disadvantage contributes to accelerated wear and tear on the body, leading to a faster rate of biological aging in Black and Latinx people. The weathering hypothesis provides a framework to understand differential health outcomes across race and ethnicity groups across the life span, including differential brain aging and differential neurodegenerative disease.
Cortical thickness and cerebrovascular disease, measured in vivo with magnetic resonance imaging (MRI), are common in aging and neurodegenerative disease. White matter hyperintensity (WMH) burden is a marker of small vessel cerebrovascular disease and is associated with risk of stroke, cognitive decline, incident and prevalent clinical Alzheimer disease (AD), and rate of AD progression. Similarly, cortical thickness is considered a criterion standard biomarker of neurodegeneration in amyloid-tau neurodegeneration cascade models for AD. Because they are established biomarkers that are anchored in clinical outcomes, WMH and cortical thickness are well suited as outcome measures in observational studies in racially and ethnically diverse cohorts.
While previous studies suggested worse brain health in minoritized racial and ethnic groups in late life, they did not examine the differences in markers of brain health in midlife compared with later life relative to non-Latinx White adults. The purpose of this study was to examine racial and ethnic differences in cortical thickness and WMH volume in midlife and late life. Second, we examined the association of age with markers of neurodegeneration and cerebrovascular disease in midlife and late life to assess different patterns of brain aging across race and ethnicity groups.
Methods
Participants
We included participants from 2 community-based studies of cognitive aging and dementia that enrolled older and middle-aged adults, the Washington Heights–Inwood Columbia Aging Project (WHICAP) and the Offspring Study of Racial and Ethnic Disparities in Alzheimer Disease (Offspring). Full descriptions of study procedures have been reported previously. Briefly, WHICAP participants were residents of northern Manhattan, New York, 65 years and older, and fluent in English and/or Spanish and were recruited in 3 waves, beginning in 1992, 1999, and 2009. The current analysis included WHICAP participants recruited from the 2009 cohort who received scans with 3-T MRI beginning in 2011. Offspring study participants were adult children of WHICAP participants, 25 years and older, and fluent in English and/or Spanish. This analysis includes Offspring participants enrolled through January 2021 who received MRI scanning. The design did not require that a WHICAP participant have an MRI scan to recruit their offspring or to include offspring in the MRI substudy. These analyses include participants without a diagnosis of dementia at the time of scanning. Additional inclusion criteria were that participants (1) underwent neuropsychological evaluation near the time of MRI; (2) self-reported their race and ethnicity as Black, African American, or African and non-Latinx (hereafter referred to as Black); Hispanic or Latino/a/x of any race (hereafter referred to as Latinx); or White and non-Latinx (hereafter referred to as White)17; and (3) had complete MRI data and covariates of interest. Race and ethnicity were used as a marker of racism and discriminatory beliefs and policies that increase weathering and AD risk, not as a proxy for genetic variation. All participants gave written informed consent. The Institutional Review Board at Columbia University approved these studies. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Measures
At time of MRI scan, history of diabetes, hypertension, heart disease (arrhythmias, coronary artery disease, and congestive heart failure), and clinical stroke was ascertained by self-report. These 4 dichotomous variables were summed to create a cardiovascular disease (CVD) count variable (range, 0-4). Cognition was evaluated with neuropsychological tests, including the Selective Reminding Test (SRT). In this study, we focused on the delayed recall score of the SRT because of its strong association with cognitive aging and dementia progression.
MRI Acquisition and Processing
Participants in the WHICAP cohort were scanned on a 3-T MRI scanner (Intera; Philips) at Columbia University between 2011 and 2019. Parameters for the T1-weighted anatomical scans included repetition time (TR) = 6.6 milliseconds; echo time (TE) = 3.0 milliseconds; flip angle = 8°; field of view (FOV) = 256 × 200 × 165 mm3; resolution = 1 mm3. T2-weighted fluid-attenuated inversion recovery (FLAIR) images were acquired in the axial orientation with the following parameters: TR = 8000 milliseconds; TE = 337 milliseconds; inversion time (TI) = 2400 milliseconds; FOV = 240 × 240 × 180 mm3 with 1-mm slice thickness. Participants in the Offspring study were scanned on a 3-T MRI scanner (Prisma; Siemens) at Columbia University between 2018 and 2020. Parameters for the T1-weighted anatomical scans included TR = 2300 milliseconds; TE = 2.26 milliseconds; flip angle = 8°; FOV = 256 × 256 × 192 mm3; resolution = 1 mm3. T2-weighted FLAIR images were acquired in the axial orientation with the following parameters: TR = 5000 milliseconds; TE = 387 milliseconds; TI = 1800; FOV = 230 × 230 × 192 mm3 with 0.90-mm slice thickness.
Regional cortical thickness was quantified with FreeSurfer version 6.0 using T1-weighted images. A single AD signature measure was derived for each participant by calculating mean cortical thickness values across hemisphere in 9 regions previously shown to best reflect AD neurodegeneration. These regions included rostral medial temporal lobe, inferior parietal lobe, inferior frontal lobe, inferior temporal lobe, temporal pole, precuneus, supramarginal gyrus, superior parietal lobe, and superior frontal lobe (Figure 1A).
Figure 1. Neuroimaging Measures
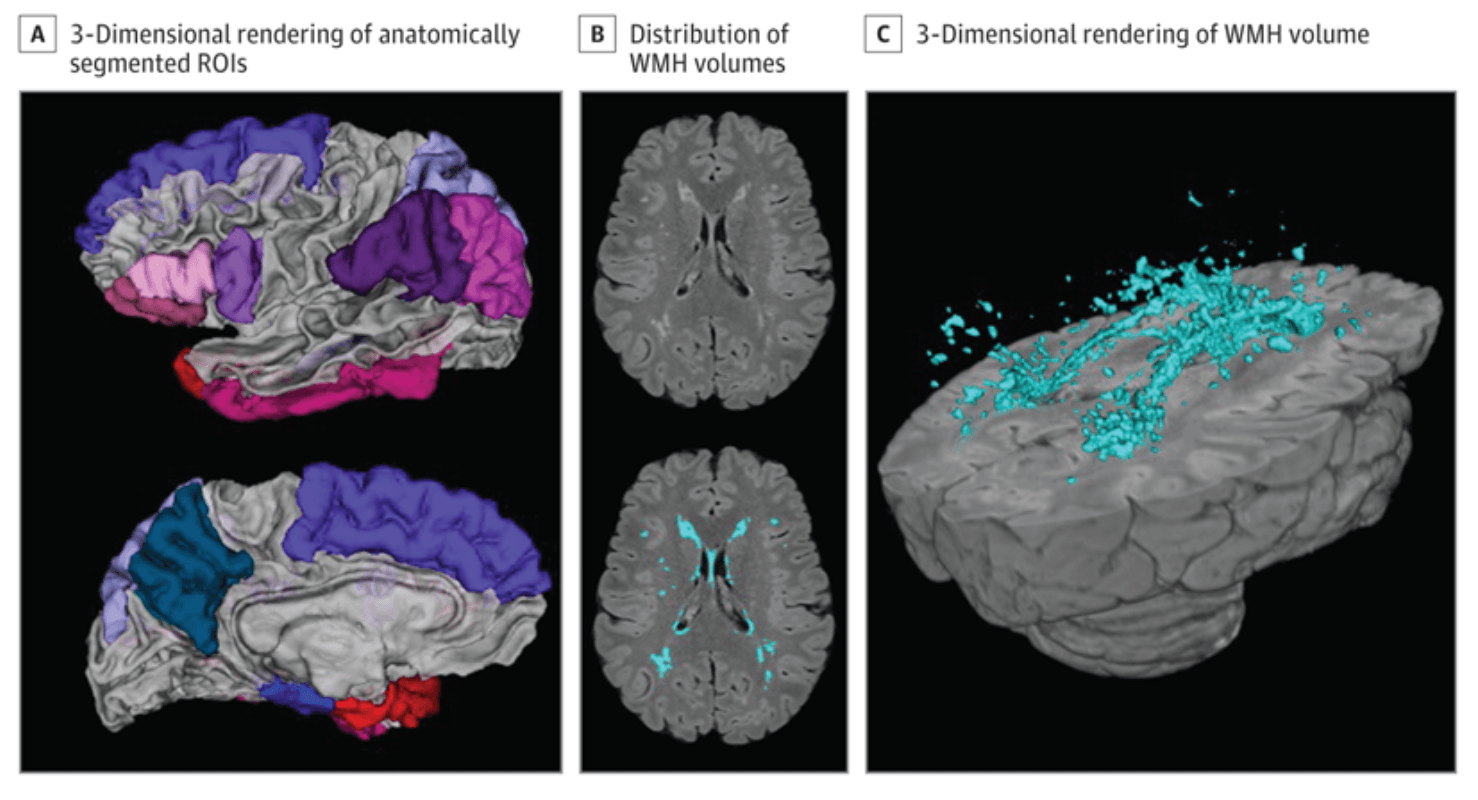
A 3-dimensional rendering of the anatomically segmented regions of interest included in the Alzheimer disease signature composite (A). An axial slice displaying distribution of white matter hyperintensity (WMH) volumes unlabeled (B, top) with in-house developed software and WMH labeled (B, bottom). A 3-dimensional rendering of the labeled WMH volume (C). ROI indicates region of interest.
Whole-brain WMH volumes were quantified from T2-weighted FLAIR images with in-house developed software. Briefly, in the WHICAP study, images were brain extracted and the intensity histogram was fit with a Gaussian curve. Voxels with intensities greater than 2.1 SDs above the image mean were labeled as WMH. In the Offspring study, FLAIR images were brain extracted, then corrected for intensity inhomogeneities. The intensity histogram was high pass filtered at the mode, log-transformed, and fit with a half Gaussian mixture model where the upper full Gaussian represented WMH. WMH were visually inspected and manually corrected if needed (Figure 1B and C); labeled voxels were summed and multiplied by voxel dimensions to yield volumes in cm3.
Statistical Analysis
We used t tests for continuous variables and χ2 tests for categorical variables to evaluate differences in baseline demographic characteristics between participants in each study cohort. We used multivariable linear regression models in Mplus version 8 to compare MRI markers between Black, Latinx, and White participants and multiple-group models to determine if these estimates differed as a function of study cohort, adjusting for self-reported gender, age (study cohort-specific mean-centered age), and intracranial volume (for WMH models). Multiple group models used mixture estimation procedures with a single latent class comprising known classes (Black midlife, Latinx midlife, White midlife, Black late life, Latinx late life, and White late life). This grouping variable was incorporated into the model as a moderator, allowing model parameters to vary independently within each group. The multiple-group approach is preferable to treating race and ethnicity and study cohort as covariates in the model, which would impose equalities between groups that may not be valid. It also minimizes the association of MRI protocol differences by treating each group separately. For each model, intercept and residual variance parameters were allowed to vary across groups. Differences in intercept and age slopes were examined with the model constraint option in Mplus through 2 models: (1) race and ethnicity differences in mean MRI markers within each study cohort (eg, Black individuals in Offspring vs White individuals in Offspring) and (2) study cohort differences in the association between age and MRI markers within race and ethnicity (eg, White individuals in Offspring vs White individuals in WHICAP).
The eAppendix in the Supplement (1) compared demographic data between those included in the analyses with those who were not, (2) confirmed the clinical relevance of the neuroimaging markers by assessing their association with memory, (3) evaluated the CVD risk factors independently, and (4) conducted sensitivity analyses by both restricting the sample to ages that overlap between the Offspring and WHICAP study cohorts and by removing individuals with overlapping ages between the study cohorts. Two-sided P values were statistically significant at .05.
Results
Demographic Characteristics
Of 1467 individuals, 970 (66.1%) were in the WHICAP cohort (mean [SD] age, 75 [6.5] years, 338 Black individuals [34.8%], 389 Latinx individuals [40.1%], 243 White individuals [25.1%], and 589 women [65.2%]) and 497 (33.9%) were in the Offspring cohort (mean [SD] age, 55 [10.7] years, 117 Black individuals [23.5%], 348 Latinx individuals [70%], 32 White individuals [6.4%], and 324 women [65.2%]). Characteristics of participants across race and ethnicity groups and within the study cohort are shown in Table 1. In the Offspring cohort, participants were similar in age and comprised a similar proportion of women across race and ethnicity groups. White participants had more years of formal education, followed by Black participants, then Latinx participants. White participants had the highest SRT delayed recall scores, followed by Latinx participants, then Black participants. Summary measure of CVD factors (ie, CVD count) was similar across groups, but individual CVD factors varied. White participants were less likely to report a history of diabetes and hypertension than Black participants, followed by Latinx participants. History of stroke and heart disease was similar across groups.
Table 1. Demographics Across Race and Ethnicity Within the Study Cohort
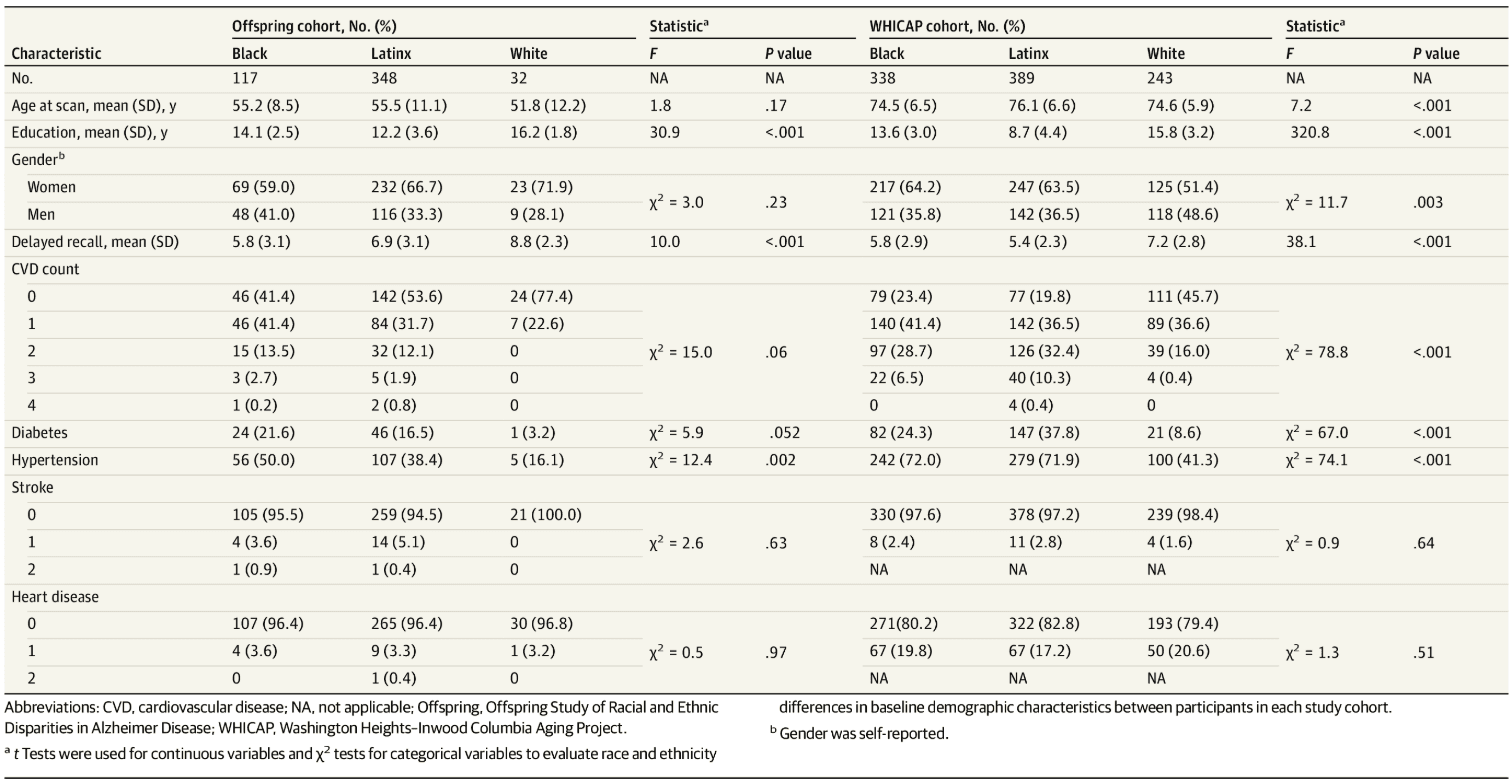
In the WHICAP cohort, Latinx participants were older than Black and White participants, and the proportion of women was similar across groups. White participants had the highest SRT delayed recall scores and more years of formal education, followed by Black participants, then Latinx participants. White participants had lower CVD count, followed by Black participants, then Latinx participants. Latinx participants were more likely to report a history of diabetes, followed by Black participants, then White participants. Similarly, Black and Latinx participants were more likely to report hypertension history than White participants. Self-reported stroke and heart disease were similar across groups. Differences in demographics and CVD factors were noted between those included in MRI analyses compared with those who were not (eResults and eTable 1 in the Supplement).
Imaging Results
Cortical thickness and WMH volume differences between race and ethnicity groups within the study cohort are presented in Table 2 and Figure 2. In midlife (ie, Offspring study), there were no reliable race and ethnicity differences in cortical thickness. In late life (ie, WHICAP study), White participants had greater cortical thickness than Latinx participants, who in turn had greater cortical thickness than Black participants. WMH volume differed by race and ethnicity in midlife and late life. In midlife, White participants had lower WMH volume than Black participants, and there was no significant difference between Latinx and White or Black and Latinx participants. In late life, Black participants had greater WMH volume than Latinx and White participants.
Table 2. Differences in Estimated Marginal Means of Cortical Thickness and WMH Volume Within the Study Cohort Across Race and Ethnicitya
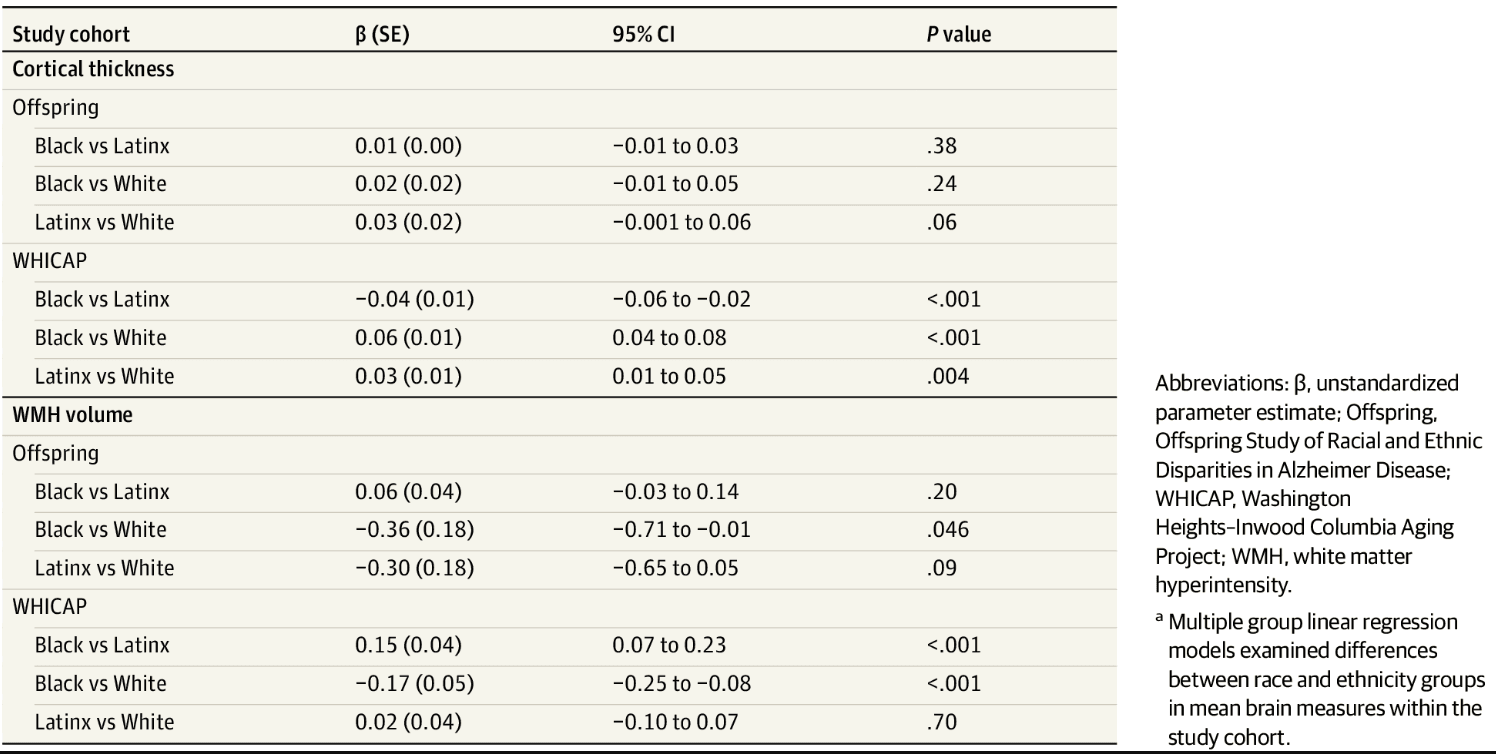
Figure 2. Racial and Ethnic Disparities in Cortical Thickness and White Matter Hyperintensity (WMH) Volume
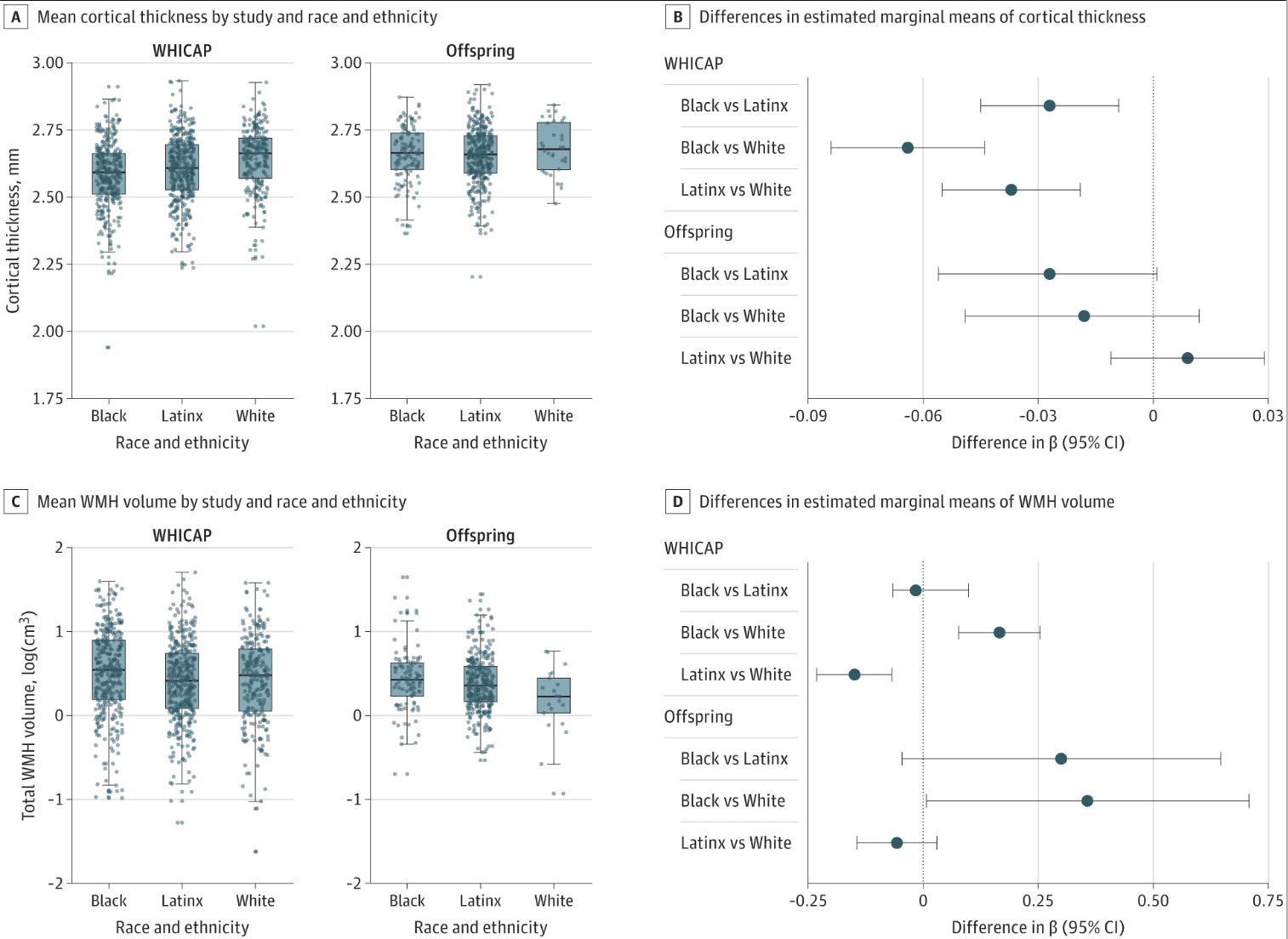
Multiple group linear regression models examined differences between race and ethnicity groups in mean magnetic resonance imaging markers within the study cohort. Racial and ethnic disparities in cortical thickness are more strongly apparent in late life (A). Offspring indicates Offspring Study of Racial and Ethnic Disparities in Alzheimer Disease; WHICAP, Washington Heights–Inwood Columbia Aging Project.
The association of age with MRI markers differed by study cohort and race and ethnicity. Older age was associated with lower cortical thickness similarly across race and ethnicity within midlife and late life participants (ie, similar slopes). When comparing across study cohort, the association of age with cortical thickness was greater in late life compared with midlife but only in White participants (0.005; 95% CI, 0.002-0.008; P = .001) and Latinx participants (0.006; 95% CI, 0.004-0.008; P < .001). In Black participants, the association of age with cortical thickness was similar across midlife and late life (0.001; 95% CI, −0.002 to 0.004; P = .64). Notably, unlike Latinx and White participants, the age association among Black midlife participants was comparable with that observed in late life among all participants (Figure 3A). Largely, older age was associated with increased WMH volume in midlife and late life, similarly across race and ethnicity. When comparing across study cohort, the association between age and WMH volume was stronger in late life than midlife but only in Latinx participants (−0.010; 95% CI, −0.018 to −0.001; P = .03) and marginally in White participants (−0.021; 95% CI, −0.043 to 0.002; P = .07) (Figure 3B). In Black participants, the association of age with WMH volume was similarly strong in midlife compared with late life (0.003; 95% CI, −0.010 to 0.017; P = .61) (Figure 3B).
Figure 3. Age-Related Associations With Cortical Thickness (CT) and White Matter Hyperintensity (WMH) Volume
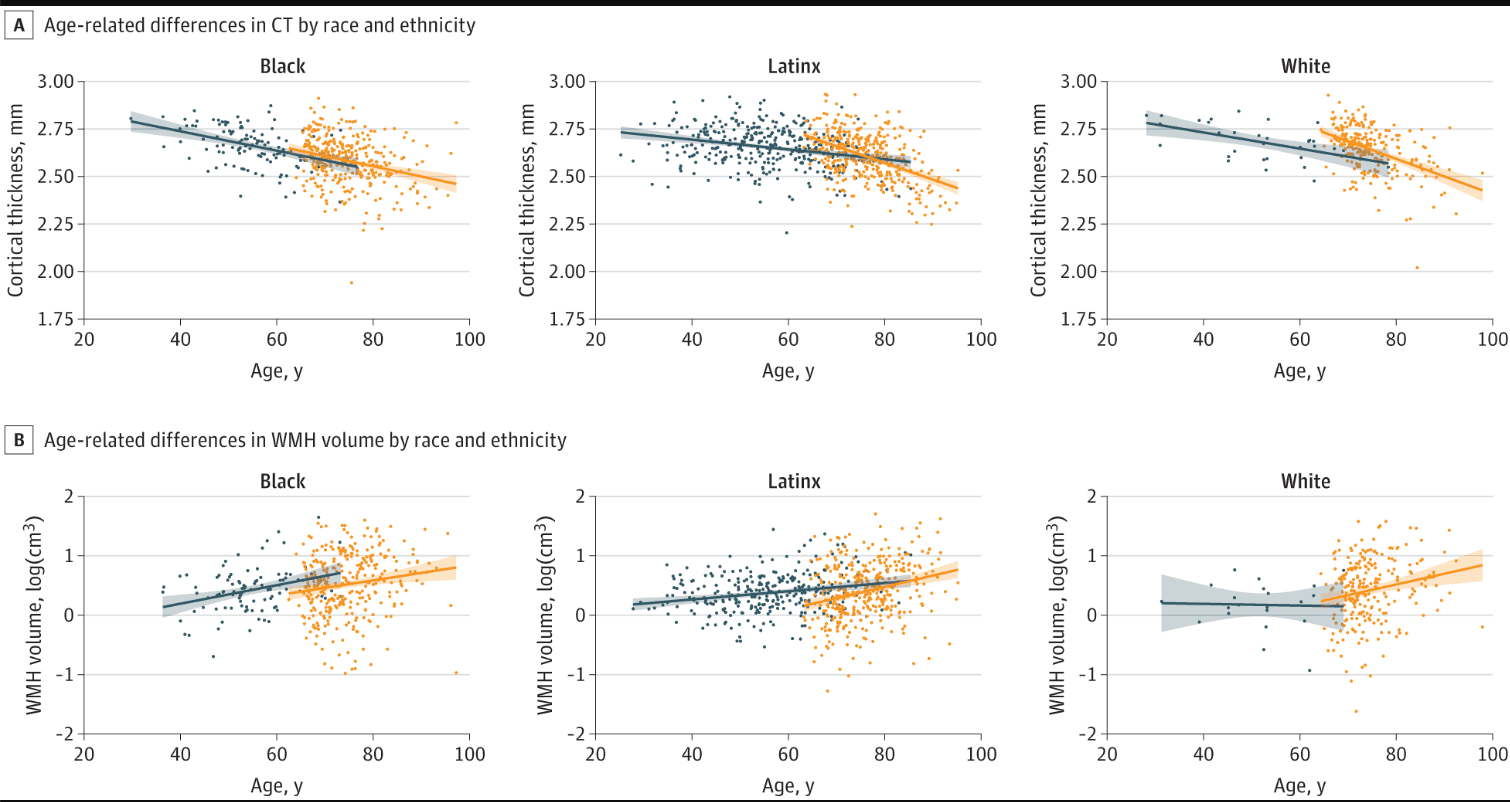
Multiple group linear regression models examined age associations with magnetic resonance imaging markers within race and ethnicity, across study cohort.
The eAppendix in the Supplement confirmed that the imaging markers studied are associated with cognition and thus clinically anchored, showed some attenuation of results after adjustment for hypertension but not other vascular factors (eResults and eTables 2-3 in the Supplement) and showed that the pattern of results was similar in sensitivity analyses (eResults and eTable 4 in the Supplement).
Discussion
In 2 large community-based studies of middle-aged and older adults, we found that racial and ethnic disparities in cerebrovascular disease occurred in both midlife (ie, Offspring cohort) and late life (ie, WHICAP cohort), while disparities in cortical thickness only occurred in late life. Overall, comparable with other reports Black-White disparities were larger than Latinx-White disparities for both measures, while Black-Latinx disparities were minimal. Lastly, brain aging (ie, the association of age with cortical thickness and WMH volume) was greater in late life than midlife for Latinx and White participants but not Black participants, who exhibited a similar magnitude of brain aging in midlife and late life. In other words, among Latinx and White participants, there was an inflection in age slopes for both measures from midlife to late life, while there was no difference or inflection in age slopes between midlife and late life Black participants, suggesting accelerated brain aging in middle-age Black individuals. White matter hyperintensities and cortical thickness are well known determinants or correlates of cognitive health, including in the current study, and the results have obvious implications for cognitive aging.
We observed greater magnitude of race and ethnicity disparities in WMH volume in midlife than late life. This observation may be due to differential survival across race and ethnicity. Previous findings show that Black adults have higher mortality and morbidity rates and lower life expectancy, resulting in fewer Black adults surviving to older ages, compared with White adults. Thus, midlife Black adults with disproportionately high WMH volume may be less likely to survive to older ages or participate in research studies, resulting in hardier survivors (ie, less brain pathology), and observed narrowing of racial and ethnic disparities at older ages.
We used race and ethnicity as a marker of exposure to all forms of racism, which increases weathering and AD risk. The cumulative impact of social, physical, and economic adversities, often faced by individuals from historically excluded populations lead to earlier health deterioration and advanced biological aging, which may be caused by chronic or reoccurring stressors. Previous studies showed that lower childhood socioeconomic status and environmental adversity, such as household material hardship, are associated with greater cognitive decline in late life, memory impairment, and reduced hippocampal volumes. Markers of neighborhood level socioeconomic disadvantage are associated with poorer cognition. Experiences of interpersonal racism and discrimination and coping styles such as John Henryism, also have a negative influence on late life health.
While the weathering hypothesis focused on allostatic load, the cumulative effects of oppression, environmental adversity, and psychological stress can also affect the brain and may lead to greater cerebrovascular disease and neurodegeneration. Previous studies reported increased prevalence of dementia risk factors, cerebrovascular disease, and AD and related dementias among Black and Latinx people compared with White people. We postulate that race and ethnicity disparities in brain aging are due to lifetime cumulative exposure to structural and social forces that elevate subsequent exposure to risk factors for brain pathology. Future studies should incorporate measurement of these forces across the life course to determine whether they mediate disparities observed in brain health, their functional consequences, and secular trends over time. Future studies should also include biomarkers reflecting pathology (eg, amyloid and tau) to clarify risk factors and potential pathways that contribute to race and ethnicity disparities in brain aging and AD.
Limitations
There are a few limitations that must be addressed. First, while the racial and ethnic diversity in both cohorts is a strength, the proportion of White participants in the Offspring cohort was lower than in WHICAP, which may limit statistical power for statistical contrasts. We are confident that the results are generalizable because demographic and health characteristics among White participants in the 2 cohorts were as expected. That is, the midlife White participants were younger and healthier with respect to vascular risk factors yet had similar years of education compared with the late-life White group, reducing the concern of selection bias. Future studies would be strengthened by nationally representative, larger sample sizes of all racial and ethnic groups across the life span. Another limitation is the cross-sectional design of our study. A longitudinal design would help infer causality and eliminate sources of bias. Further, deconstructing the social determinants that drive disparities in brain health is an aim of our future work to identify the multiple pathways that mediate the observed disparities in brain aging. Our current findings, showing evidence of differential brain aging across race and ethnicity, provide an essential foundation to further investigate the factors that drive these disparities.
Participants in WHICAP and Offspring were scanned on different MRI scanners with harmonized but slightly different image acquisition parameters. Scanner differences between study cohorts may have influenced the findings somewhat, but this limitation cannot explain observed race and ethnicity disparities in brain aging. Systematic differences between study cohorts, like the MRI scanner used, likely did not influence the results because the primary analysis addressed differences among race and ethnicity groups within the cohort, not between study cohorts. Although age-association analyses compared race and ethnicity differences across study cohorts, these analyses were conducted for all race and ethnicity groups, and the results do not appear to be systematically biased. Coupled with the sensitivity analyses, which replicated the overall findings among an overlapping age group of Offspring and WHICAP participants, we are confident that scanner differences between cohorts did not confound our results.
Conclusions
The WHICAP and Offspring studies include participants who have been historically excluded from studies of brain aging and AD. Data from both community-based studies provide a unique opportunity to examine the role of race and ethnicity across a wide age range of participants (age 25-98 years), who also have health status, economic, and educational backgrounds that are representative of their communities. This study shows that race and ethnicity disparities observed at older ages are apparent in midlife, suggesting accelerated brain aging in Black participants. Future efforts will further disentangle the mechanisms underlying potential racial and ethnic disparities in brain aging, which in turn, may improve early detection, diagnosis, and treatment of AD.