Abstract
Importance: Possible associations between stimulant treatment of attention-deficit/hyperactivity disorder (ADHD) and subsequent substance use remain debated and clinically relevant.
Objective: To assess the association of stimulant treatment of ADHD with subsequent substance use using the Multimodal Treatment Study of ADHD (MTA), which provides a unique opportunity to test this association while addressing methodologic complexities (principally, multiple dynamic confounding variables).
Design, setting, and participants: MTA was a multisite study initiated at 6 sites in the US and 1 in Canada as a 14-month randomized clinical trial of medication and behavior therapy for ADHD but transitioned to a longitudinal observational study. Participants were recruited between 1994 and 1996. Multi-informant assessments included comprehensively assessed demographic, clinical (including substance use), and treatment (including stimulant treatment) variables. Children aged 7 to 9 years with rigorously diagnosed DSM-IV combined-type ADHD were repeatedly assessed until a mean age of 25 years. Analysis took place between April 2018 and February 2023.
Exposure: Stimulant treatment of ADHD was measured prospectively from baseline for 16 years (10 assessments) initially using parent report followed by young adult report.
Main outcomes and measures: Frequency of heavy drinking, marijuana use, daily cigarette smoking, and other substance use were confidentially self-reported with a standardized substance use questionnaire.
Results: A total of 579 children (mean [SD] age at baseline, 8.5 [0.8] years; 465 [80%] male) were analyzed. Generalized multilevel linear models showed no evidence that current (B [SE] range, -0.62 [0.55] to 0.34 [0.47]) or prior stimulant treatment (B [SE] range, -0.06 [0.26] to 0.70 [0.37]) or their interaction (B [SE] range, -0.49 [0.70] to 0.86 [0.68]) were associated with substance use after adjusting for developmental trends in substance use and age. Marginal structural models adjusting for dynamic confounding by demographic, clinical, and familial factors revealed no evidence that more years of stimulant treatment (B [SE] range, -0.003 [0.01] to 0.04 [0.02]) or continuous, uninterrupted stimulant treatment (B [SE] range, -0.25 [0.33] to -0.03 [0.10]) were associated with adulthood substance use. Findings were the same for substance use disorder as outcome.
Conclusions and relevance: This study found no evidence that stimulant treatment was associated with increased or decreased risk for later frequent use of alcohol, marijuana, cigarette smoking, or other substances used for adolescents and young adults with childhood ADHD. These findings do not appear to result from other factors that might drive treatment over time and findings held even after considering opposing age-related trends in stimulant treatment and substance use.
Introduction
Childhood attention-deficit/hyperactivity disorder (ADHD) carries risk for elevated substance use and substance use disorder (SUD) by adulthood. Stimulant medications, a first-line treatment for ADHD, should decrease substance use given the prominence of impulsivity in models of addiction and the acute efficacy of stimulants for ADHD symptoms, including impulsivity. However, early exposure to stimulants may cause neurobiological and behavioral sensitization to other drugs and thus increase the risk for harmful substance use. A 2013 meta-analysis found no associations consistent with either protective or adverse effects of stimulants on substance use or SUD. After further individual studies with mixed results, a 2014 meta-analysis supported protective associations for cigarette smoking, and large epidemiologic studies demonstrated protective associations for SUD. In all observational studies, a vexing methodologic barrier to inferring causal effects of stimulants on substance use is the confounding influence of factors presumed to drive ADHD treatment and substance use. Recent studies have increased scientific rigor, yet none have comprehensively and prospectively assessed both substance use and the wide range of demographic, clinical, and psychosocial factors contributing to both substance use and treatment. The current study was designed to remedy this.
Age confounds the stimulant–substance use association. As substance use escalates through adolescence, well before most SUD diagnoses, adolescents with ADHD are increasingly unlikely to continue taking prescribed stimulants. Thus, without adjustment for age, associations between stimulant medication and substance use may be spurious. Groenman and colleagues found no differences in adolescent daily smoking across age-matched stimulant treatment profiles. A further complication is that some adolescents resume stimulant medication after months or years. A within-participant analysis of filled stimulant and atomoxetine prescriptions found that substance use–related emergency department visits were less frequent during medicated, vs unmedicated, periods for adolescents and adults with ADHD. However, data excluded frequent substance use, class of substance was not measured, data were limited to the commercially insured, and most substance use does not lead to hospitalization. Thus, additional research must address the effects of age through adolescence to the peak period of substance use (early adulthood), specificity of substance use class, and medication continuation, cessation, resumption, or stable termination.
Other factors driving both stimulant treatment and substance use are numerous, including sociodemographics, symptom severity, psychiatric comorbidities, functional impairment, psychosocial treatment and factors affecting it (eg, insurance), and parent characteristics (psychopathology, parenting practices/attitudes). Some confounders have been covaried in prior studies but only at single time points. Adjustment for changing influences over time is needed to test another popular treatment hypothesis: that younger age of treatment initiation and longer duration improve substance use outcome. For the first time, to our knowledge, we apply a causal analytic method, marginal structural models, to address this dynamic confounder problem.
In this study, we use prospective longitudinal data from a randomized multisite clinical trial of ADHD treatments in childhood to study the association between stimulant treatment and substance use through adolescence into early adulthood. We focus on substance use due to its critical role in SUD ontogeny. We implement a 2-part analytic series investigating the impact of age adjustment when examining contemporaneous associations between stimulants and substance use (part 1) and whether cumulative stimulant treatment from childhood through early adulthood predicts early adult substance use by comprehensively adjusting for time-varying covariates that may underlie stimulant–substance use associations (part 2).
Methods
Participants were children aged 7 to 9 years with DSM-IV combined-type ADHD recruited between 1994 and 1996 from 6 sites in the US and 1 in Canada. Children (95-99 per site) were randomly assigned to 1 of 4 treatment groups: medication management, multicomponent behavior therapy, their combination, or referral to usual community care. The Multimodal Treatment Study of ADHD (MTA) timeline, recruitment, diagnostic procedures, treatment, and sample demographics appear in prior publications. Demographics, including race and ethnicity, were reported by parents at baseline when participants were children. Informed consent was monitored by the 7 respective university institutional review boards. Treatment group assignment was not associated with later substance use.
Participants were assessed at baseline prior to randomization, at 3 and 9 months, at conclusion of the 14-month treatment, and at 2, 3, 6, 8, 10, 12, 14, and 16 years after baseline. Substance use data were provided at least once in adulthood (12, 14, and/or 16 years after baseline) by 81.3% of participants (mean [SD] age, 25.1 [1.07] years at 16-year assessment) and 95% were reassessed at least once between the 2- and 16-year assessments. Number of waves participated and substance use were nominally associated; these results and wave-specific retention are available elsewhere.
Measures
Substance Use
The self-report Substance Use Questionnaire, adapted for the MTA, included frequency of use for alcohol, marijuana, cigarettes, and several illicit and prescription drugs. Substance Use Questionnaire data were from the 2-year (mean [range] age, 10.5 [8.8-12.5] years) through 16-year (mean [range] age, 25.1 [22.5-28.6] years) assessments. Our harmonization procedure across development, and additional measure description, is detailed by Molina et al. A National Institute of Health Certificate of Confidentiality facilitated honest reporting.
Heavy drinking was the higher score of 2 items assessing frequency of binge drinking and drunkenness (How many times…[1] did you drink 5 or more drinks when you were drinking? …[2] have you gotten drunk or very, very high on alcohol?). Marijuana use was assessed with 1 frequency of use question. Past-year frequency ratings for each of these variables were coded to 1 of 4 levels: 0 (none), 1 (<once per month), 2 (at least monthly; less than weekly), and 3 (once per week or more). Daily smoking was a binary variable (0 = no, 1 = yes) reflecting 1 or more cigarettes per day. For other substance use, participants reported misuse of prescription medications including stimulants, sedatives, and opioids and use of heroin, inhalants, hallucinogens, cocaine, and “other substances to get high.” Maximum frequency of any illicit substance use (or prescription drug misused) was calculated; past-year frequency was coded to 1 of 3 levels: 0 (none), 1 (<once per month), and 2 (once per month or more often).
Stimulant Treatment
From the 14-month assessment to age 18 years, stimulant treatment information was collected via the Services for Children and Adolescents Parent Interview. After age 18 years, participants self-reported treatment. Stimulant treatment was analyzed as a binary variable reflecting a minimum of 10 mg/d d,l-methylphenidate–equivalent of any stimulant for 50% or more of the days in the past year across the longitudinal follow-up (see eMethods 1 in Supplement 1 for details and rationale). Medication use prior to entering the MTA, retrospectively reported by parents, was binary (presence = 1, absence = 0). Prior publications extensively describe medication assessments.
Analytic Plan
Parts 1 and 2 of this study used the substance use and stimulant medication measures described above. Part 1 models added age and socioeconomic disadvantage in a hierarchical fashion. These analyses tested the hypothesis that contemporaneous (past-year), interacting with recent (1 year prior) medication status would be associated with substance use before and after controlling for age. Part 2 tested the association of cumulative stimulant medication use with adult substance use using causal inference methods that adjusted for potential time-varying confounds. We repeated these analyses for alcohol, marijuana, and other SUD to check extension of our results to diagnosable SUD.
Part 1
We estimated generalized multilevel linear models for ordered categorical outcomes in 4 steps. First, effects of stimulant medication use at the current assessment (past-year), year prior, and their interaction were estimated to capture unadjusted effects of concurrent stimulant use and patterns of medication continuation and discontinuation. Second, we added index variables marking the passage of time over assessments and capturing differential rates of change through adolescence and early adulthood (as in Molina et al2 ). Third, we added participants’ ages relative to others at the same assessment. Finally, we added baseline parents’ combined household income (1, <$10 000 to 9, ≥$75 000) and household (dis)advantage covariates reflecting family structure (single- vs 2-parent households) and parents’ education (see eMethods 2 in Supplement 1 for variable coding details). Models accommodated missing data with full information maximum likelihood estimation.
Part 2
We fit a series of marginal structural models that adjusted for 75 covariates (eTable 1 in Supplement 1) that may confound the estimated effect of stimulant use on substance use, vis-à-vis their associations with both stimulant treatment and substance use. Marginal structural models yield unbiased estimates of causal effects assuming ignorability (ie, no unmeasured confounding), positivity (ie, individuals have a nonzero probability of treatment), and consistency (ie, an individual’s treatment status does not affect another individual’s outcome). Covariates included baseline variables and time-varying covariates measured repeatedly. The substance use outcomes for these analyses were the 16-year observations. We used multiple imputation for missing data from baseline to the 16-year assessment. We used a bayesian multiple imputation by chained equations approach for nested data in Mplus (version 8.3), saving 100 imputed data sets. We then estimated time-varying propensity scores reflecting individuals’ propensities to take stimulant medications at each assessment based on the covariates (propensity scores near 1 or 0 indicate that taking stimulant medication is extremely likely or unlikely, respectively). We used the super learner package in RStudio version 2022.07.0 (R Foundation) (a suite of machine learning tools) to derive a flexible propensity score model comprising the covariates in eTable 1 in Supplement 1 (eg, determine necessary interactions among covariates, specify covariates’ functional forms) without overfitting to the data. Finally, we estimated linear probability regression models for substance use outcomes at the 16-year assessment. Estimated effects were adjusted for confounders by weighting cumulative stimulant treatment, stimulant treatment prior to study entry, and their interaction by the propensity scores. Specifically, they were weighted by the product of the inverse of the time-varying propensity scores, which were stabilized to protect against potential bias due to observations with extreme probabilities. Thus, stimulant treatment became statistically disaggregated from the covariates, mirroring random treatment assignment, to ensure that any remaining differences in substance use among individuals with varying levels of stimulant treatment over time must be due to stimulant treatment itself; thus, a causal effect of cumulative stimulant treatment on substance use could be estimated under the assumptions described above). First, we estimated the effect of total cumulative years of stimulant medication exposure across the follow-up period. Second, we estimated the effect of the number of consecutive years of stimulant medication exposure. Analyses were conducted in RStudio version 2022.07.0 (RStudio PBC).
Two-sided P values were statistically significant at .05. All study questions and analysis plans are documented elsewhere.32 The part 2 strategy was preregistered before data analysis.44 Analysis took place between April 2018 and February 2023.
Results
Rates of Substance Use and Stimulant Medication Use
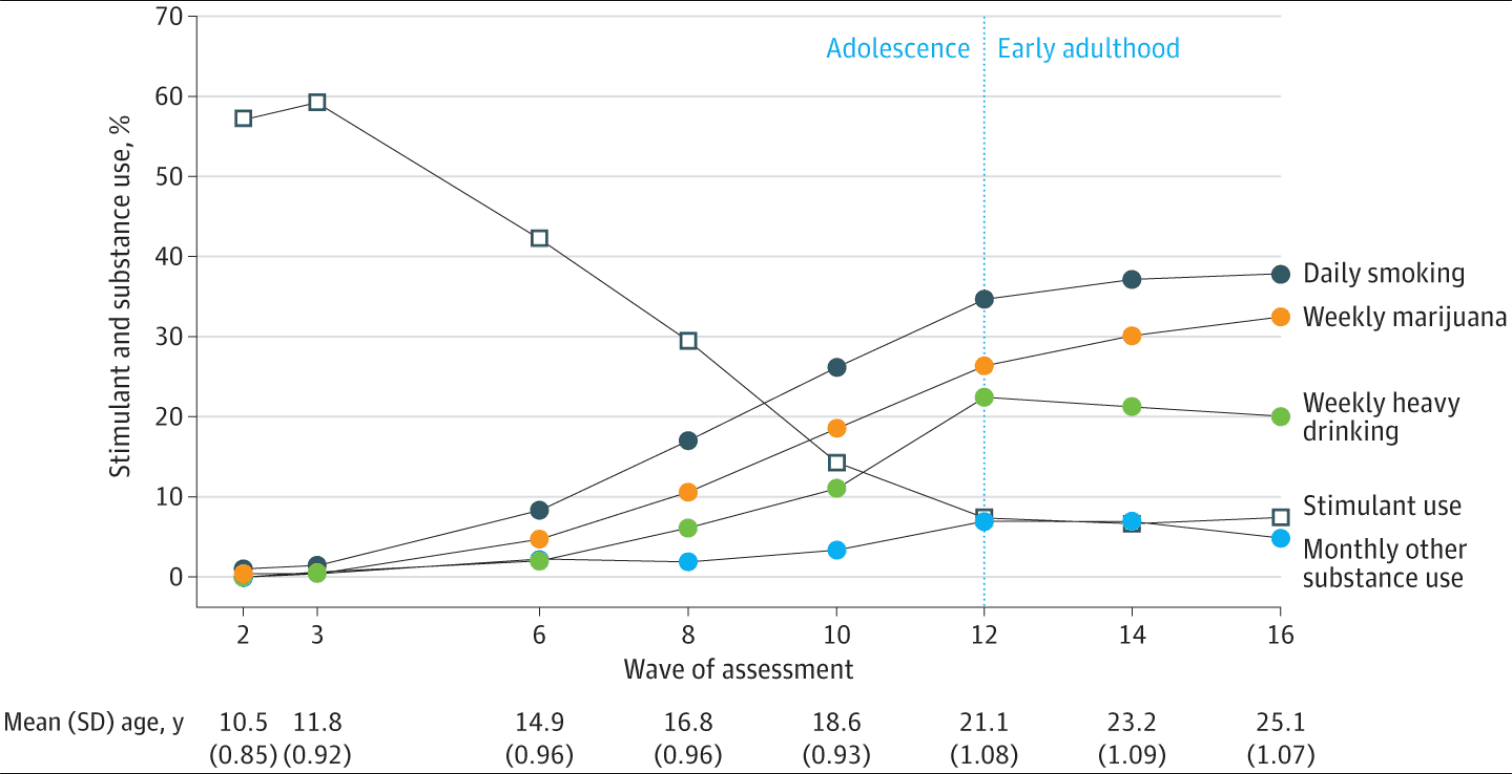
A total of 579 children with DSM-IV combined-type ADHD were recruited with a mean (SD) age of 8.5 (0.8) years at baseline. A total of 115 participants (20%) were African American; 48 (8%), Hispanic; and 351 (61%), White. The Figure shows the rates of substance use and stimulant use across all assessments (and mean age by assessment). As reported elsewhere, substance use increased steadily through adolescence and remained stable through early adulthood. Mean percentages across the 12-, 14-, and 16-year follow-up assessments were 36.5% for daily smoking, 29.6% for marijuana use at least weekly, 21.1% for heavy drinking at least weekly, and 6.2% for other substance use at least monthly (eTable 2 in Supplement 1). The Figure shows that the share of adolescents using stimulant medication declined precipitously through adolescence from nearly 60% at the 2- and 3-year assessments to 7.2% on average in early adulthood (eTable 2 in Supplement 1).
Figure. Rates of Stimulant and Substance Use at the 2-Year Through 16-Year Follow-up Assessments of the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder
Part 1
Initial models predicting substance use from contemporaneous and prior year stimulant medication use and their interaction showed that stimulant use was associated with reduced odds of heavy drinking, using marijuana, daily smoking, and other substance use (Table 1, model 1). Consistent with stimulant use patterns described above, however, these associations were no longer present after accounting for developmental change in substance use through adolescence into early adulthood (model 2; eTable 3A-D in Supplement 1). Model 3 added relative age, and the same pattern emerged: neither contemporaneous nor prior year stimulant medication use was associated with any substance outcome, and the absence of interaction effects indicated no evidence of association between medication continuation or discontinuation with elevated or reduced substance use. In general, standard errors for stimulant use effects were large, indicating low precision in the estimates of stimulant-substance use associations.
Table 1. Selected Results of Generalized Multilevel Linear Models Testing Effect Sizes of Stimulant Use on the Log Odds of Heavy Drinking, Marijuana Use, Daily Smoking, and Other Substances Used, When Time and Age Trends are Absent (Model 1) vs Present (Model 3)
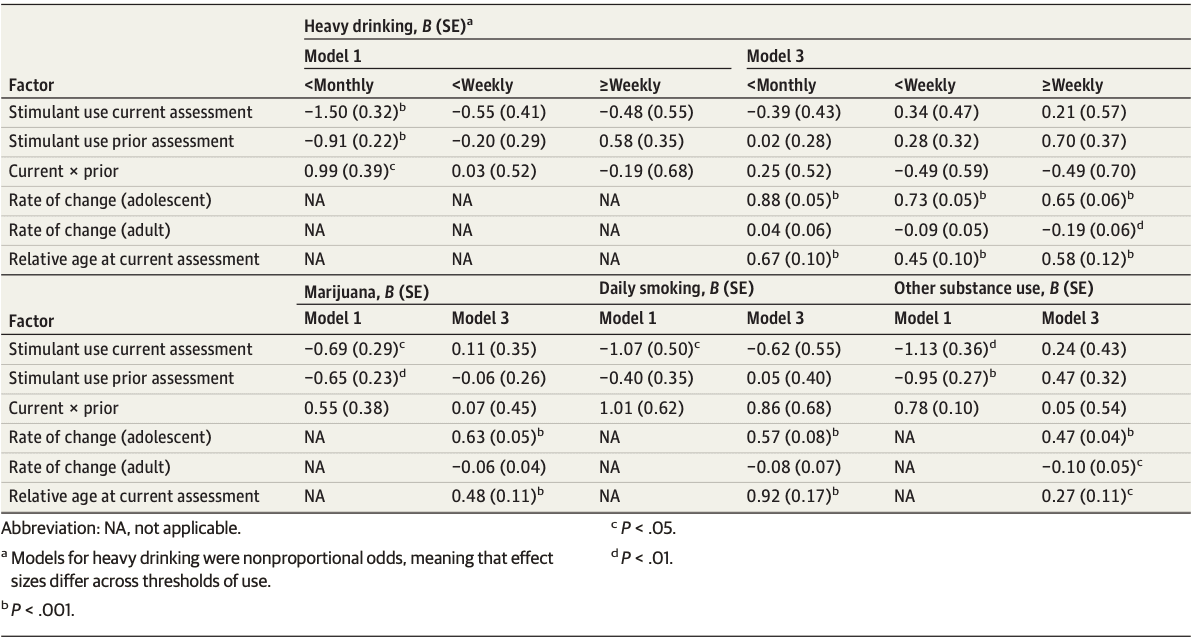
In addition to the substance use trends, the estimated effects of relative age showed that youth who were older than average within a given assessment were most likely to use substances. For example, the odds of daily smoking in a given year were 2.5 times higher for people a year older than average during that assessment (Table 1, model 3: B = 0.92; odds ratio, 2.51). The same pattern persisted after adding income and household (dis)advantage in model 4 (eTable 3A-D in Supplement 1). Given the absence of any statistically significant estimated effects of stimulant medication at this stage. we did not pursue additional sociodemographic, clinical, or familial covariates. These were comprehensively assessed in part 2.
Part 2
As displayed in Table 2, results of the marginal structural models provided no evidence of an effect of cumulative years of stimulants with daily smoking, marijuana use, or other substance use at a mean of age 25 years. Each additional year of cumulative stimulant medication was estimated to increase individuals’ likelihood of any binge drinking/drunkenness vs none in the past year by 4% and of monthly vs less frequent binge drinking/drunkenness by 3.5% (95% CI, 0.01-0.08; P = .03 and 95% CI, 0.0003-0.07; P = .048, respectively). However, using a familywise Bonferroni-corrected α level for multiple tests (P = .006), these 2 effects (of 18) became nonsignificant. Results showed no evidence that stimulant medication use prior to study entry or its interaction with cumulative years of stimulants increased the likelihood of any substance use at a mean age of 25. Similarly, in Table 3, results did not provide evidence that more years of continuous, uninterrupted stimulant medication use or its interaction with stimulant use prior to study entry had effects on any substance use at a mean age of 25 years. Results accounted for inconsistently medicated treatment patterns (see full models in eTable 4 in Supplement 1).
Table 2. Marginal Structural Model Results: Cumulative Stimulant Medication Usea

Table 3. Marginal Structural Model Results: Continuous, Uninterrupted Stimulant Medication Usea

Results from parts 1 and 2 were robust to substitution of substance use with alcohol, marijuana, and other SUD (eTables 5A-C, 6A-B in Supplement 1).
Discussion
Recent reviews concluded that stimulant medication protects against well-established substance use risk for children with ADHD, but critical methodologic limitations were also identified. Using prospective data, the current study addressed 2 key weaknesses of previous reports: (1) adjustment for age across the developmental phases of adolescence and early adulthood when substance use escalates and (2) adjustment for time-varying confounders of the association between stimulant treatment and substance use using causal inference methods.
Part 1 analyses, correcting for age, did not provide evidence that individuals currently or recently taking stimulants report lower rates of substance use than individuals not taking stimulants, although large standard errors prevent us from completely ruling out possible protective (or harmful) effects. Findings highlighted opposing developmental trends in substance use and stimulant medication through adolescence into early adulthood: as substance use escalates, stimulant medication use declines. Extensive research has documented these trends separately but never adjusted for them when studying association of stimulant use with substance use. Without adjusting for developmental trends, we might have mistakenly concluded a protective association in our longitudinal analyses. We also found no contemporaneous by prior year stimulant treatment interaction, indicating no evidence that patterns of stimulant continuation or discontinuation were related to substance use.
The results suggest that more and different treatment may be needed for individuals with ADHD to reduce escalating substance use known to precede SUD. Substance use prevention must begin early when reward seeking and social context drive initial substance use and precede habit formation. Preventive interventions may also need to directly address perceptions that some substances (eg, marijuana) are therapeutic. Our findings may differ from recent US commercial health care claims data because we examined more prevalent substance use behavior vs rare presentations to the emergency department. A Swedish registry study showing protective associations also relied on hospital visits and death and criminal records. However, our results extended to diagnosable SUD, which is more proximal clinically to emergency department visits. Similar to our findings, Groenman and colleagues also did not find protection by any stimulant treatment patterns for daily smoking. The large database studies provide reassurance that the most serious substance use–related end points (eg, hospitalization, death, adjudication) are not elevated as a function of recent stimulant treatment.
Another long-standing hypothesis is that early, continuous stimulant treatment should protect children with ADHD from harmful substance use. Part 2 analyses did not show that longer duration of stimulant treatment predicts less substance use in adulthood. In fact, cumulative stimulant treatment was associated with increased heavy drinking, although the effect size was small and did not survive experimentwise error correction. The marginal structural models adjusted for a comprehensive suite of time-varying confounders measured longitudinally from childhood to adulthood, and results persisted whether or not children were taking stimulant medication before study entry. Even if stimulants were initiated before the mean age of 8 years, our results were not consistent with the hypotheses of protection or harm in relation to substance use or SUDs. Mannuzza and colleagues found that early treatment (before age 9 years) predicted lower SUD in adulthood, and Groenman and colleagues found protection from SUD in 103 adolescents treated early and consistently at a high dose (53.4-mg methylphenidate). Both studies were limited by not comprehensively adjusting for the wide range of confounding variables that drive decreasing stimulant treatment and increasing substance use over time.
A key MTA (and other longitudinal studies of children with ADHD) finding is that most patients no longer take stimulants in adulthood. Yet, if stimulant treatment creates long-term protection, that benefit should extend beyond treatment cessation. (In previous reports, we did find long-term effects in another domain, growth impairment, even though only 53 participants were consistently taking medication to the 10-year assessment, and fewer were taking medication after this point.) Stimulants may not permanently change some important substance use risk processes, including impulsivity and poor delay of gratification. Tamminga et al recently reported that methylphenidate-driven improvements in cognitive performance, including response inhibition and delay aversion while receiving medication, disappeared once treatment ceased. There still remain possible benefits to combined pharmacotherapy (including stimulants), psychoeducation, and psychotherapy for individuals with current ADHD and SUD.
Limitations
Limitations include that we lacked medical records to verify medication history, although confidence in our data is supported by studies showing concordance between parent report and medical records. Nonmarijuana illicit substance use and prescription misuse rates were very low, precluding testing associations within classes of other substance use. Finally, although our multisite sample included female individuals (20%) and children identified as African American (20%) and Hispanic (8%), it was insufficiently powered for a strong test of moderation by sex, race, or ethnicity.
Conclusions
Taken together, our 16-year prospective, comprehensive, and developmentally sensitive analyses of stimulant medication associations with substance use failed to support any hypotheses of substance use protection or harm from stimulant treatment for ADHD. Although these results contrast with recent conclusions of protection found in other data sets, across all studies the findings lend a measure of comfort in the consistent lack of evidence that stimulant treatment predisposes children with ADHD to later substance use.