Abstract
Background Anxiety disorders often emerge in adolescence and are associated with risk aversion. Risk aversion conflicts with the typical adolescent approach-motivated phenotype and can interfere with learning and contribute to symptom maintenance. Methods We investigated the neural and behavioral correlates of risk avoidance in a diverse sample of adolescents (N = 137; MAge = 11.3; 34.3 % white, 22.1 % Latino, 20 % Asian, 14.3 % Black, 9.3% Mixed Race) as they completed a task involving risky decision-making and response inhibition during fMRI. Voluntary cautious choice was compared to successful response inhibition to isolate the neural systems underlying the decision to avoid a risk and identify their relation to risk-taking and anxiety in adolescents. Results Anxious adolescents self-reported more avoidance but demonstrated normative risk-taking on the laboratory task. Interestingly, they responded quickly during response inhibition but took longer to decide in the face of risk. All youth showed widespread recruitment of decision-making and salience network regions when deciding to avoid risk. The neural mechanisms driving avoidance differed based on anxiety such that left inferior frontal gyrus (IFG) activation was linked to risk avoidance in adolescents with low anxiety and risk-taking in anxious adolescents, while striatal connectivity was linked to risk avoidance in anxious adolescents and risk-taking in those with low anxiety. Limitations This work is cross-sectional and therefore cannot speak to causality or directionality of effects. Conclusions These results suggest that the neural mechanisms contributing to adolescent risk-taking may function to promote avoidance in anxious youth, increasing vulnerability to maladaptive avoidance and further anxiety development.
1. Introduction
Anxiety disorders commonly emerge during adolescence (Kessler et al., 2007; Merikangas et al., 2010) and demonstrate considerable heterogeneity in presentation and symptom course, making them complex and often difficult conditions to treat (Strawn et al., 2021). Counter to the approach-motivated behavior observed in typically-developing adolescents, anxiety is often marked by avoidance which contributes to much of the interference caused by these disorders. Overly avoidant behavior can become reinforcing and habitual (LeDoux et al., 2017) and contribute to symptom maintenance (Arnaudova et al., 2017), especially in a critical time of life when approach behaviors such as risk-taking are important for promoting independence, learning, and goal-directed behavior (Casey et al., 2008). Nonetheless, studies of adolescent risky decision-making and anxiety have primarily been conducted separately, leaving their complex reciprocal associations relatively unexplored. This knowledge is crucial for understanding how adolescent development promotes both vulnerability to and resilience against anxiety and to identify factors contributing to the emergence of pathological anxiety. Here, we examined the neural correlates of avoidance in a large community sample of adolescents across the anxiety continuum to elucidate the mechanisms driving avoidance and their implications for adolescent anxiety.
While adolescence is characterized by behavioral activation, or motivation to approach novel stimuli, anxiety is characterized by fear and avoidance of unfamiliar stimuli (Fox et al., 2005). Fearing threats is common early in development, and anxiety disorders such as separation anxiety and specific phobias often onset in childhood (Beesdo et al., 2009). Conversely, anxiety disorders such as panic disorder, generalized anxiety, and social anxiety often emerge during adolescence (Beesdo et al., 2009) and increase risk for negative outcomes such as depression, substance dependence, and suicide (Woodward and Fergusson, 2001). Although high levels of anxiety during childhood often predict anxiety disorders in adolescence (Essex et al., 2010), not all youth who exhibit them go on to develop clinical anxiety in puberty (Henderson et al., 2015), suggesting that the developmental window of adolescence is crucial for determining youth anxiety trajectories.
When considering adolescent avoidance, it is important to distinguish adaptive from maladaptive behavior. For example, a tendency to avoid in response to threat can help promote safety, such as braking at a red light to avoid a crash. Indeed, research suggests that anxiety can sharpen inhibitory ability in situations requiring harm avoidance (Grillon et al., 2017). The adaptive function of certain adolescent avoidance behaviors is important to consider given the high mortality rates in adolescents, with the leading danger being motor vehicle crashes (CDC, 2017). As adolescents tend to impulsively react rather than retreat when faced with threat compared to children and adults (Dreyfuss et al., 2014), it is possible that a certain degree of anxiety might drive adolescents to retreat rather than react to threat, increasing harm avoidance and therefore benefitting healthy development.
Conversely, decisions involving uncertain outcomes—such as risk-taking—can spur approach-avoidance conflict in which engaging in goal-directed behavior becomes difficult for anxious youth (Barker et al., 2019; Peris and Galván, 2021). As previous work has found increased risk aversion, but not loss aversion, in anxious adults (Charpentier et al., 2017), it may be the uncertainty inherent in risk rather than the potential for negative outcomes that is uniquely aversive for individuals with anxiety. Interestingly, it is precisely the uncertainty of risk that appeals to typically-developing adolescents (Tymula et al., 2012), highlighting yet another paradox in these overlapping yet opposing phenotypes.
Learning to avoid an aversive stimulus prior to its onset can provide momentary reductions in anxiety (Hofmann and Hay, 2018). However, over time, avoidance can become habitual and resistant to extinction, especially in adolescents (Klein et al., 2020). Compared to adults, adolescents demonstrate elevated fear generalization and fear responses to safety during avoidance learning (Klein et al., 2020, Klein et al., 2021), increasing vulnerability to persistent or habitual avoidance and precluding the opportunity for potential rewarding experiences (Arnaudova et al., 2017). Fear overgeneralization is moderated by anxiety in adolescents but not adults, suggesting anxiety and avoidance are especially linked at this time (Klein et al., 2020). Therefore, adolescents are uniquely sensitive to aversive in addition to appetitive cues, both of which show downstream effects on learning and behavior.
Adolescent motivated behavior is explained neurobiologically by the Triadic model in which approach (ventral striatum; VS), avoidance (amygdala), and regulatory (prefrontal cortex; PFC) systems interact and compete to influence response to positive and aversive cues (Ernst et al., 2009). When adolescents face approach-avoidance conflict, this model posits that striatal sensitivity and developing regulatory systems will bias behavior towards approach (Ernst et al., 2009). However, the striatum and its connections are also crucial for avoidance (Delgado et al., 2008) and implicated in adolescent anxiety (Bar-Haim et al., 2009; Benson et al., 2014; Guyer et al., 2012). While avoidance is often attributed to the amygdala, animal models suggest habitual or persistent avoidance forgoes the amygdala and instead correlates with activity in the striatum and PFC (Bravo-Rivera et al., 2015). Anxious adolescents demonstrate reduced striatal response during risk-taking in conditions of potential reward (Galván and Peris, 2014) and upon receiving rewards during passive avoidance learning (Bashford-Largo et al., 2021), suggesting altered associations between the striatum and risk and reward in anxiety. Therefore, the combination of striatal sensitivity and still-developing fronto-striatal circuits in adolescence may bias behavior towards either approach or avoidance depending on adolescent anxiety.
In a study examining the neural bases of approach-avoidance conflict in adults (Zorowitz et al., 2019), response time (RT) during decision-making and inferior frontal gyrus (IFG) activation were positively correlated with approach-avoidance conflict. Previous work implicates the IFG in inhibition: stimulation of the right IFG diminishes impulsive behavior (Jacobson et al., 2011) and IFG connectivity relates to adolescent self-control (Pyeon et al., 2021). Perhaps the neural regions involved in approach-avoidance conflict overlap with response inhibition regions such that the IFG inhibits prepotent motor responses to facilitate longer deliberation in the face of conflict. Conversely, it might be specifically engaged in conflict resolution and involved in biasing behavior towards approach or avoidance. IFG functioning has implications for anxiety; left IFG activation correlates with anxious apprehension during decision-making (Engels et al., 2007) and anxious adults demonstrate IFG dysregulation via weakened IFG-ventromedial PFC (vmPFC) communication (Cha et al., 2016). In this circuit, the IFG evaluates stimulus meaning and informs the vmPFC in inhibiting the amygdala (Cha et al., 2016), suggesting it exerts indirect influence on amygdala response. The extensive development and sensitivity of IFG-subcortical circuits occurring in puberty plays a role in vulnerability to anxiety, as early pubertal timing has been linked to later anxiety symptoms via increases in positive IFG-amygdala coupling (Barendse et al., 2020). Measuring IFG-subcortical functioning during adolescent decision-making is a crucial next step for delineating the shift from normative to pathological anxiety from a neurobiological perspective.
Here, we measured anxiety, risk-taking, and response inhibition in 137 adolescents (9-13y/o) as they played the Driving Game, a decision-making task, during functional magnetic resonance imaging (fMRI). Participants were instructed to “go” at green lights, “stop” at red lights, and choose whether to stop or go at yellow lights, thereby measuring response inhibition (stopping a prepotent response when the light turns red) and risk-taking in the same design. Participants ranged across a spectrum of normative anxiety levels with over-sampling from those at the cusp of clinical diagnosis who are vulnerable to developing anxiety in adolescence. As anxiety has been linked to risk aversion (Charpentier et al., 2017), we hypothesized that anxiety would correlate negatively with risk-taking. As anxious youth show normative response inhibition (Lipszyc and Schachar, 2010), we predicted anxiety would not affect false-alarm rate. As anxiety can sharpen inhibitory ability in response to clear threats (Grillon et al., 2017), we hypothesized that anxious youth would respond faster during response inhibition. As approach-avoidance conflict can interfere with decision-making in anxiety (Barker et al., 2019), we predicted that faster RT during inhibition would be paralleled by longer RT during cautious choice. Given the role of fronto-striatal circuitry in approach, avoidance and anxiety (Bar-Haim et al., 2009; Bashford-Largo et al., 2021; Benson et al., 2014; Bravo-Rivera et al., 2015; Delgado et al., 2008; Ernst et al., 2009; Galván and Peris, 2014; Guyer et al., 2012), we predicted that neural responding in areas involved in approach-avoidance conflict (e.g., IFG) and their connections with the striatum during decision-making would explain the behavioral differences observed in high versus low anxiety.
2. Methods and materials
2.1. Participants
171 youth were recruited from a large metropolitan area to complete a clinical interview and an fMRI scan. Recruitment aimed to capture the full spectrum of anxiety symptom severity assessed via the Screen for Child Anxiety and Related Emotional Disorders (SCARED) (Birmaher et al., 1997). Eligible participants were 9-13y/o, right-handed, free of metal, had no medical or psychiatric conditions contraindicating study participation (e.g., suicidality, head trauma), did not use psychotropic medication, and were not claustrophobic. Youth were compensated $100 and could win $10 during the fMRI tasks. Participants completed the Anxiety and Related Disorders Interview Schedule-IV (ADIS-IV) (Silverman and Albano, 1996) with a clinician trained to criterion.
Twenty-five youth did not complete the scan: 13 visits were canceled due to the COVID-19 pandemic and 12 youth were uncomfortable with the MR environment. Data from 7 participants were unusable due to too few trials, and data from 2 participants were unusable due to technical errors during data collection. Data were excluded if the participant exceeded 1 mm mean relative motion (1 run for 1 participant; no youth excluded). Data are presented for 137 participants (MAge = 11.3, SDAge = 1.41; 61 girls; 35 % white, 22.2 % Latino, 20.4 % Asian, 13.9 % Black, 9.5 % Mixed Race; Table 1).
2.2. Compliance with ethical standards
Informed consent and assent were obtained from all legal guardians and study participants in accordance with the Institutional Review Board.
2.3. Anxiety severity
Participants completed the 41-item self-report SCARED (Birmaher et al., 1997) as a dimensional measure of anxiety severity. They rated statements describing anxiety symptoms (e.g., “I feel nervous around people I don't know very well”) on a 3-point Likert scale ranging from 0 (Not True or Hardly Ever True) to 2 (Very True or Often True) based on how often the symptoms were true for them. Raw total SCARED scores were used to index anxiety.
2.4. Adolescent decision-making
Participants also completed the 30-item self-report Flinders Adolescent Decision Making Questionnaire (ADMQ) (63) to assess their perceptions of their avoidant decision-making patterns. Participants read a series of statements regarding their perceptions of their decision-making (e.g., “I put off making decisions until it's too late”) and rated each statement on a 4-point Likert scale ranging from 0 (not at all true of me) to 3 (almost always true). The ADMQ is composed of 5 sub-scales that broadly measure adaptive decision-making patterns—i.e., decision self-esteem (confidence in one's decisions) and vigilance (care one takes while making decisions)—and maladaptive decision-making patterns including panic (panic when making decisions), cop out (avoidance of decisions), and complacency (preferring others to make decisions for you). The cop out and complacency scales were summed to create a measure of self-reported decision-making avoidance for each participant.
2.5. fMRI task
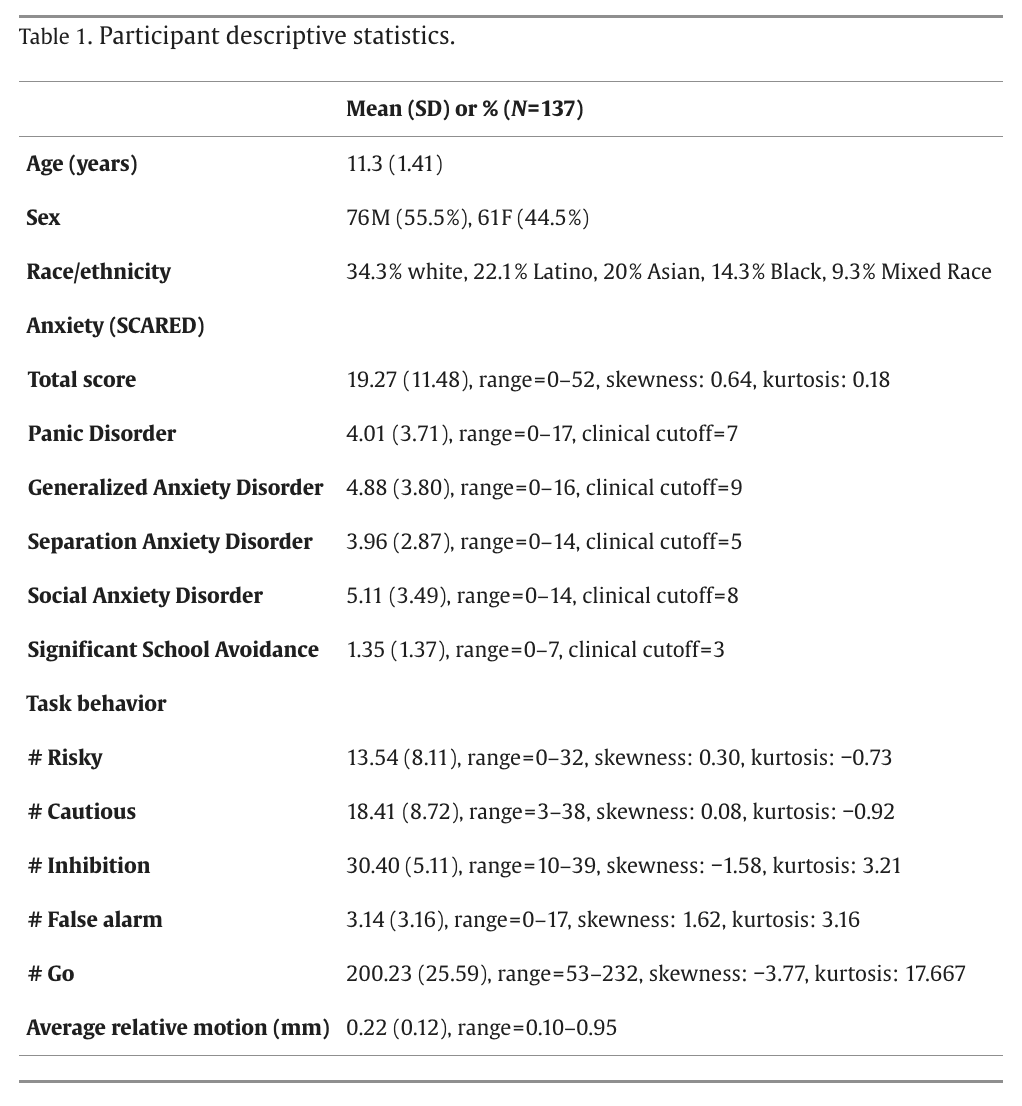
Participants played two 8-minute runs of the Driving Game, an adapted version of the Stoplight Task (Chein et al., 2011) involving decision-making at randomly-presented traffic lights and trying to finish quickly to maximize monetary reward ($5; Fig. 1). Each trial begins with 2–4 green lights and ends with a yellow or red light. Lights are presented for 1 s or until participant response and followed by a jittered inter-trial interval (ITI; 0.5–0.5 s). Participants were instructed to press “1” to go at green lights and “2” to stop when the light turns red. Failure to stop resulted in a crash (+6 s). At yellow lights, participants could choose to press “1” to go (risky choice) or “2” to stop (cautious choice). Stopping made the light turn red (+3 s). Going led to a 50/50 chance of a safe crossing and a reward or a crash (+6 s). RT was duration in milliseconds from stimulus onset to participant response.
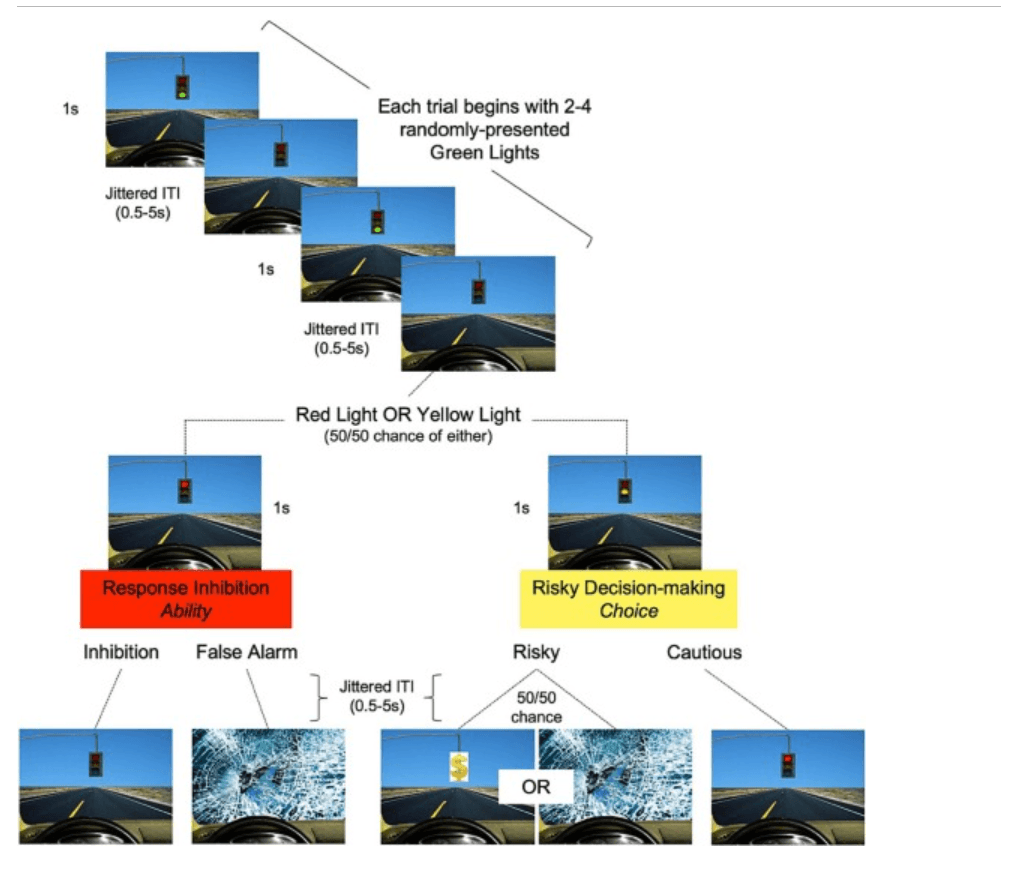
Fig. 1. The Driving Game. Participants encountered green, red, and yellow stoplights in the laboratory task and were instructed to press “1” to go for green lights, “2” to stop for red lights, and either “1” to go (risky choice) or “2” to stop (cautious choice) for yellow lights. A jittered inter-trial (ITI) stimulus followed each event. All trials began with 2–4 green lights and ended with either a red or yellow light (50/50 chance). A false alarm at a red light was followed by a crash. A risky choice at a yellow light was followed by either a reward (50 % chance), getting to the finish line faster and earning more money, or a crash (50 % chance), adding a 6 s delay. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.6. fMRI acquisition
A 20-channel head coil was used for scanning on a 3-Tesla Siemens Trio MRI machine. Participants completed a mock scan and were screened with a metal detector before entering. The task was in E-Prime, which collects responses and RTs. A Magnetization-Prepared Rapid Gradient-Echo (MPRAGE) scan (TR = 1900 ms, TE = 2.26 ms, FOV = 250 mm, slices = 176, slice thickness = 1 mm, voxel size = 1.0 × 1.0 mm, interleaved) was used for registration. For B0 distortion correction, participants received 2 T2*-weighted gradient-echo field-map scans with opposite phase encoding directions (AP, PA; TR = 8000 ms, TE = 66 ms, FOV = 208 mm, slices = 72, slice thickness = 2 mm, voxel size = 2x2mm, interleaved). Two runs of the T2*-weighted task fMRI sequence (TR = 800 ms, TE = 37 ms, FOV = 208 mm, slices = 72, slice thickness = 2 mm, voxel size = 2x2mm, interleaved) were acquired during the task. A single-band reference (SBRef) image was acquired before each run.
2.7. fMRI preprocessing
FEAT V6 within FSL (FMRIB Software Library; https://fsl.fmrib.ox.ac.uk/fsl/ ) (Smith et al., 2004) was used for preprocessing including non-brain removal (FSL BET), high-pass filtering (100 s), and spatial smoothing using a Gaussian kernel of FWHM 5 mm. Rigid body motion correction with 6° of freedom was performed using MCFLIRT. AP and PA field-map images were combined using FSL's topup (Andersson et al., 2003) and multiplied by 2π to convert to rad/s. A magnitude image was created using the unwarped field map and brain-extracted. Rad/s and magnitude images were used for B0 unwarping in FEAT. Each participant's functional data was registered to their SBRef, to the MPRAGE, and to Montreal Neurological Institute (MNI) stereotaxic space with 12° of freedom using FSL's nonlinear registration method FNIRT. FSLMotionOutliers (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers ) detected timepoints corrupted by motion (box-plot cutoff = P75 + 1.5 ∗ IQR). The resulting confound matrices were entered as regressors of no interest in the general linear model (GLM), removing the effects of these timepoints.
2.8. Behavioral analysis
Behavioral analyses were conducted using the lme4 package in R. To investigate how anxiety relates to self-reported avoidance in adolescence, we conducted a linear regression analysis with ADMQ avoidance scores as the dependent variable, anxiety as a covariate of interest, and age and sex as covariates of no interest. To investigate whether anxiety impacted the likelihood of avoiding traffic lights across risk-taking (yellow lights) and response inhibition (red lights) task conditions, we conducted a mixed-effects logistic regression analysis with decision (1 = avoid, 0 = approach) as the dependent variable, random intercepts to account for repeated measures within participants, task condition (yellow vs. red lights) as a within-subjects factor, and anxiety, age, and sex as covariates. To test whether anxiety influenced the time it took for participants to make their decisions, we conducted a mixed-effects linear regression analysis with RT as the dependent variable, random intercepts to account for repeated measures within participants, task condition (yellow vs. red lights) and action (approach vs. avoid) as within-subjects factors, and anxiety, age, and sex as covariates. To test our hypothesis that anxiety would specifically impact the time it takes to avoid across risk-taking vs. response inhibition conditions, we conducted a separate analysis restricting RT to only avoidance trials (i.e., the trials represented in the Cautious vs. Inhibition task contrast).
2.9. fMRI analysis
2.9.1. Whole-brain activation
A GLM was defined in FEAT with 11 regressors: Go (“1” at green), Inhibition (“2” at red), False Alarm (“1” at red), Red Crash (crash following false alarm), Risky (“1” at yellow), Cautious (“2” at yellow), Anticipation (period between risk and feedback), Yellow Crash (crash following risk), Reward (reward following risk), Finish (3 s finish line), and Junk (trials of no interest or without responses). Events were modeled with a canonical double-gamma hemodynamic response function (HRF) for a variable duration dependent on participant behavior. Rest periods and ITIs were not explicitly modeled and served as the implicit baseline of interest. Temporal derivatives for regressors, standard and extended motion parameters (6 standard motion parameters, their temporal derivatives, and squares of the above), and motion outliers were included as covariates of no interest. Individual-level models were defined with 3 contrasts: Inhibition vs. Baseline, Cautious vs. Baseline, and Cautious vs. Inhibition to identify the neural correlates of avoidance across conditions. First-level analyses were conducted using fixed-effects modeling with FLAME-1. Runs were combined using a fixed effect voxel-wise second-level model in FEAT.
Group-level activation analyses were performed using FMRIB Local Analysis of Mixed Effects (Beckmann et al., 2003). Anxiety, risk-taking, false-alarm rate, and the anxiety ∗ risk-taking interaction were included in the design matrix as covariates of interest. Age and sex were covariates of no interest. Thresholded Z-statistic images were generated to visualize clusters determined by a corrected, cluster-forming threshold of Z > 3.1 and an extent threshold of p < .05 family-wise error (FWE) corrected using the Theory of Gaussian Random Fields (Poline et al., 1997). Statistical maps were projected onto a standard MNI brain and visualized using MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/ ).
2.9.2. Striatal connectivity
Beta series correlation analyses (Rissman et al., 2004) were conducted in FSL to examine differences in VS functional connectivity across task conditions. For each run, one GLM was defined for Cautious trials and another for Inhibition trials. Trials of interest were separated into their own regressor (resulting in n regressors for n trials). Trials of no interest were represented by one regressor per trial type to preserve the original task design. Parameter estimates were combined within conditions, registered to standard space, and extracted from a bilateral VS seed (Harvard-Oxford subcortical probabilistic atlas, 50 % probability). The resulting timeseries was correlated with every other voxel in the brain and Fisher transformed using 3dTcorrelate in AFNI (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dTcorrelate.html ). Z-transformed Inhibition maps were subtracted from Z-transformed Cautious maps and concatenated for group analysis.
Group-level analysis was performed using FSL's randomise (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise/UserGuide ) with Threshold-Free Cluster Enhancement and 5000 permutations. Anxiety, risk-taking, false-alarm rate, and the anxiety ∗ risk-taking interaction were covariates of interest; age and sex were covariates of no interest. Thresholded Z-statistic images were generated to visualize clusters (Z > 3.1, p < .05). Statistical maps were projected onto a standard MNI brain and visualized using MRIcron.
3. Results
3.1. Behavioral results
3.1.1. Anxiety
SCARED scores ranged from 0 to 52 (MAnx = 19.27, SDAnx = 11.48; Fig. S1), with higher scores indicating more anxiety. In community samples, scores ≥25 have been found to indicate an anxiety disorder, suggesting that 26.3 % of participants showed signs of anxiety when using self-report to describe their symptoms (Birmaher et al., 1999; Canals et al., 2012). On the SCARED, 20.4 % of participants scored in the clinical range for Panic Disorder (PD), 19.7 % scored in the clinical range for Generalized Anxiety Disorder (GAD), 38.7 % scored in the clinical range for Separation Anxiety Disorder (SAD), 24.1 % scored in the clinical range for Social Anxiety Disorder (SOCAD), and 17.5 % scored in the clinical range for Significant School Avoidance (SSA; Table 1).
An independent samples t-test revealed a significant sex difference in total SCARED score (t(135) = 2.07, p = .04) with girls scoring 4 points higher than boys on average. Girls scored significantly higher than boys on PD (t(135) = −3.01, p = .002), but not on other subscales. Total SCARED score was not associated with age (r(137) = 0.13, p = .15), although older participants scored higher on GAD (r(137) = 0.19, p = .03) and lower on SAD (r(137) = −0.18, p = .04).
3.1.2. Self-reported avoidance
Regression results revealed that adolescents with higher anxiety self-reported higher avoidant decision-making practices (e.g., avoiding/putting off decisions or making others decide; b = 0.29, p < .0001) as compared to their low-anxious peers (Fig. S2).
3.1.3. Task behavior
On average, participants made 18.41 cautious choices and took 13.54 risks at yellow lights, whereas they made 30.4 successful inhibitions and 3.14 false alarms (accidental approach) at red lights. Mixed-effects logistic regression results revealed a significant main effect of task condition on likelihood of avoidance such that participants were more likely to avoid at response inhibition (red light) trials than at risk-taking (yellow light) trials (b = −2.25, p < .001; Fig. S3a). Girls were more likely to avoid than boys across conditions (b = 0.89, p < .001; Fig. S3b). Contrary to hypotheses, anxiety was unrelated to overall levels of task avoidance (b = 0.01, p = .92), and the interaction between anxiety and task condition was not significant (b = 0.02, p = .84; Fig. S3c).
3.1.4. Task response time
On average, participants spent 0.52 s making risky choices at yellow lights (note: n = 3 participants took 0 risks), 0.63 s making cautious choices at yellow lights, 0.59 s successfully inhibiting at red lights, and 0.45 s accidentally going at red lights (note: n = 22 participants made 0 false alarms). Mixed-effects linear regression results revealed a significant interaction between task condition and action on RT (b = −0.04, p < .001; Fig. S4a) such that participants spent approximately 0.05 s longer at yellow lights than at red lights and were 0.12 s faster to approach than avoid, with participants responding especially quickly when making false alarms (accidental approach at red lights). Older participants responded more quickly across conditions (b = −0.02, p < .001; Fig. S4b). Anxiety interacted with task condition such that anxious participants took relatively longer at yellow lights than at red lights (b = 0.01, p = .02; Fig. S4c).
When restricting our analyses to only RT during avoidance (i.e., the trials represented in the Cautious vs. Inhibition contrast), the anxiety ∗ condition effect was strengthened (b = 0.02, p = .005; Fig. 2) such that anxious participants took relatively longer to avoid risk at yellow lights than to inhibit their response at red lights.
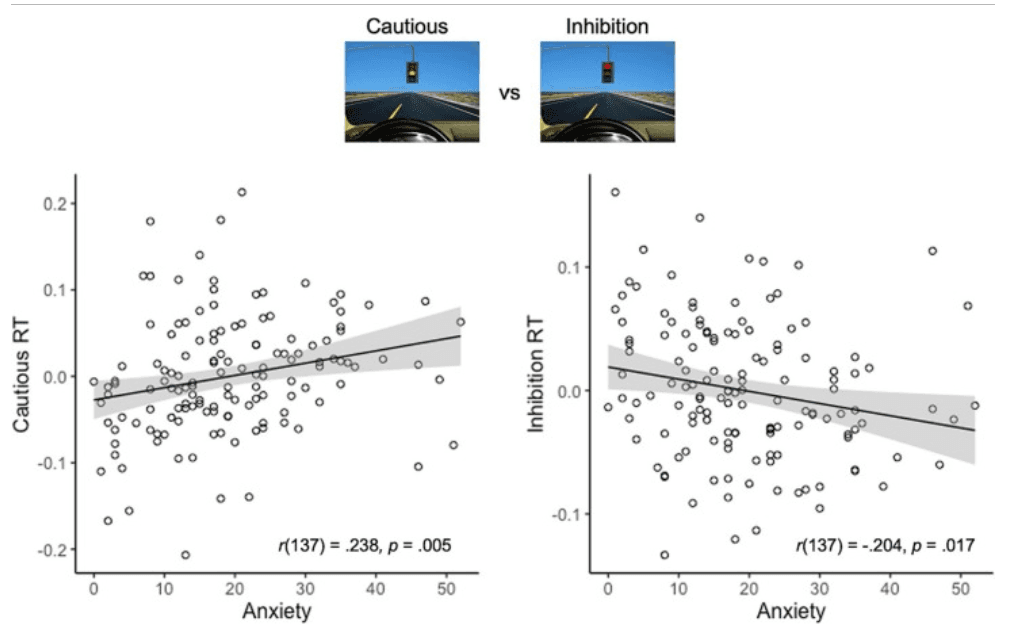
Fig. 2. Anxiety and response time during Cautious vs. Inhibition. Youth with higher anxiety took relatively longer to avoid risk at yellow lights (Cautious) than to inhibit a prepotent response at red lights (Inhibition), suggesting that anxiety may aid in response inhibition, but interfere with decision-making once outcomes are uncertain. RT = response time. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.1.5. Self-reported vs. task avoidance
The interaction between self-reported avoidance on the ADMQ and task condition on task behavior was marginally significant (b = 0.12, p = .06; Fig. S3d) such that participants who reported avoiding decision-making frequently in daily life showed slightly higher avoidance in the risk-taking condition. They were also faster to respond across conditions (b = −0.02, p = .01; Fig. S4d), perhaps indicating a higher tendency for habitual or automatic responding spurred by their dislike of decision-making. Interestingly, the interaction between anxiety and task condition on RT remained significant when self-reported avoidance was included in the model (b = 0.02, p = .004), suggesting that anxiety severity (rather than its association with avoidance) was driving the slower RT observed at yellow light trials.
3.2. fMRI results
3.2.1. Whole-brain activation
The Cautious > Inhibition contrast isolated avoidance stemming from risky decision-making processes (cautious choice at yellow lights) from avoidance in a response inhibition, harm reduction context (inhibiting at red lights). Whole-brain analysis revealed activation of the dorsal anterior cingulate cortex (dACC), posterior cingulate cortex (PCC), precuneus, anterior insula, IFG pars opercularis, angular/supramarginal gyri, paracingulate gyrus, middle frontal gyrus (MFG), precentral gyrus, and frontal pole, while the Inhibition > Cautious contrast revealed activation of the lingual gyrus, orbitofrontal cortex (OFC), IFG pars triangularis, lateral occipital cortex (LOC), and temporal fusiform gyrus (Fig. 3, Table 2).
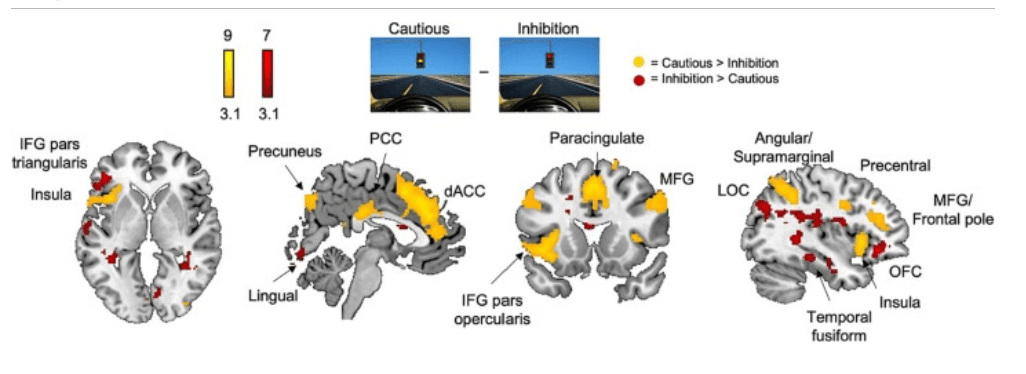
Fig. 3. Whole-brain neural activation during Cautious vs. Inhibition. Whole-brain activation for the Cautious > Inhibition contrast revealed activation of the anterior insula, posterior cingulate cortex (PCC), precuneus, dorsal anterior cingulate cortex (dACC), inferior frontal gyrus (IFG) pars opercularis, paracingulate gyrus, middle frontal gyrus (MFG), angular and supramarginal gyri, precentral gyrus, and frontal pole. Conversely, whole-brain activation for the Inhibition > Cautious contrasts revealed activation of the left IFG pars triangularis, lingual gyrus, lateral occipital cortex (LOC), temporal fusiform cortex, and orbitofrontal cortex (OFC). Cluster-corrected at Z > 3.1, p < .05.
Neural activation during Cautious > Inhibition did not differ significantly as a function of task risk-taking frequency or anxiety severity. However, participants who made more false alarms (accidental approach) at red lights showed reduced activation in the lingual gyrus, occipital pole, and intracalcarine cortex during Cautious > Inhibition (Fig. S3), suggesting that lower activation in the visual system during decision-making was associated with more impulsive behavior.
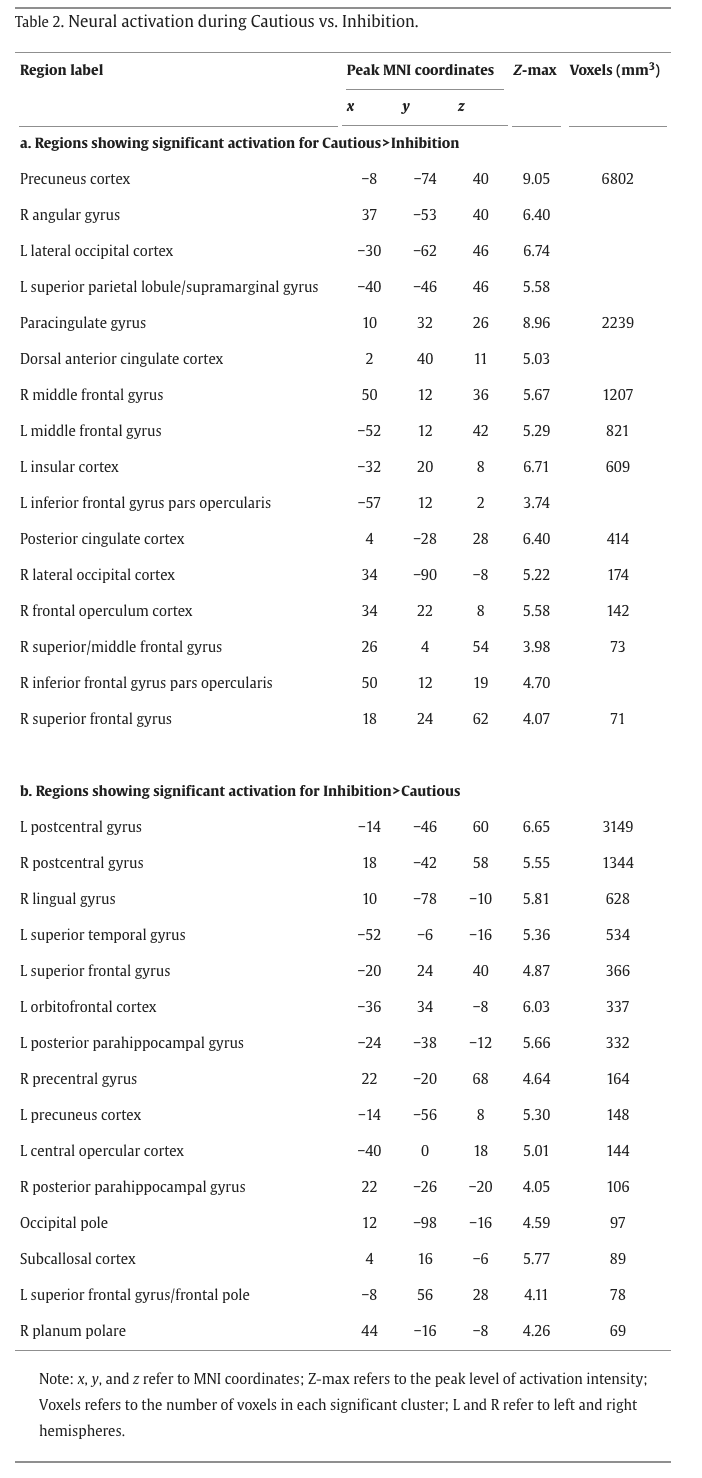
We found a significant interaction between risk-taking and anxiety on left IFG pars opercularis activation: greater IFG recruitment during Cautious > Inhibition was associated with increased risk-taking in higher anxious youth and decreased risk-taking in lower anxious youth (Fig. 4), as revealed with a whole-brain analysis for the Cautious > Inhibition contrast. Exploratory whole-brain analyses probing neural activation during approach behaviors (risky choices and false alarms) can be found in Figs. S11 and S12, although caution should be taken when interpreting the results given the low base rate of approach behaviors in the task.
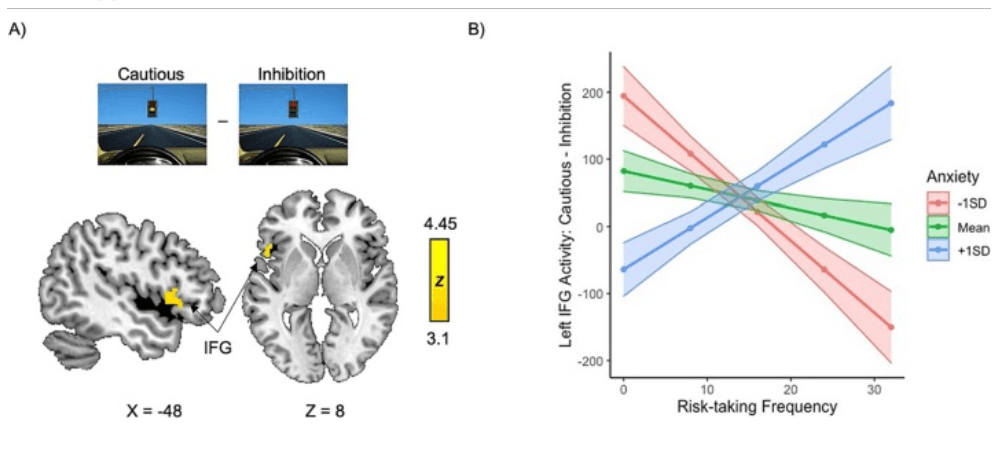
Fig. 4. Left IFG recruitment during Cautious > Inhibition differentially facilitates risk-taking depending on youth anxiety. A) The interaction between anxiety and risk-taking frequency on whole-brain activation for the Cautious > Inhibition contrast revealed a cluster encompassing the left inferior frontal gyrus (IFG) pars opercularis and extending into the anterior insula that showed opposing associations with risk-taking frequency in youth with high vs. low anxiety. B) Visual depiction of the interaction between anxiety and risk taking on left IFG activity averaged over the entire significant cluster. More IFG recruitment is associated with greater cautious behavior in youth with low anxiety and more risky choices in youth with high anxiety. Cluster-corrected at Z > 3.1, p < .05.
3.2.2. Striatal connectivity
The VS showed greater positive coupling with regions including the dACC, thalamus, hippocampus, brainstem, insula, and IFG pars triangularis during Cautious > Inhibition (Fig. S4). As VS-right IFG connectivity increased, task risk-taking frequency decreased, suggesting that regulation of the VS by the IFG promoted risk avoidance in this sample (Fig. S5). As false-alarm rate increased, VS-medial prefrontal cortex (mPFC) connectivity decreased, suggesting that VS-mPFC communication was important for successful response inhibition (Fig. S6).
Anxiety was not significantly associated with striatal connectivity during Cautious > Inhibition. However, there was a significant interaction between anxiety and risk-taking on connectivity: VS connectivity with limbic regions such as the amygdala, putamen, subcallosal, OFC, hypothalamus, mPFC, and insula was associated with risk-taking in youth with low anxiety and risk avoidance in youth with high anxiety, suggesting that VS-limbic connectivity contributes differently to adolescent approach-avoidance decisions depending on anxiety (Fig. 5).
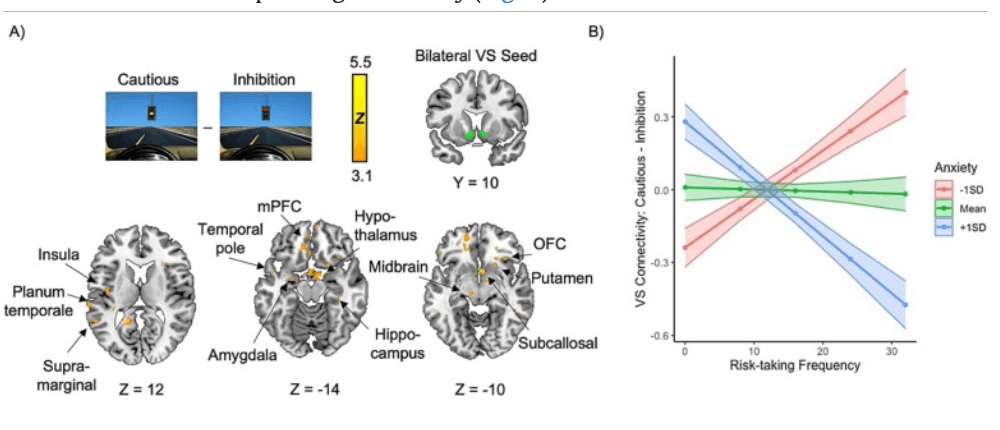
Fig. 5. Interaction between anxiety and risk-taking frequency on VS-limbic functional connectivity. A) More VS connectivity with a range of limbic regions during decision-making was associated with heightened risk-taking in youth with low anxiety, whereas it was associated with risk avoidance in youth with higher anxiety. B) Visual depiction of the interaction between risk-taking and anxiety on VS connectivity averaged over the entire significant cluster. SD = standard deviation, VS = ventral striatum, STG = superior temporal gyrus, OFC = orbitofrontal cortex, mPFC = medial prefrontal cortex. Cluster-corrected at Z > 3.1, p < .05. 3.2.3. Self-reported avoidance and neural metrics
Exploratory whole-brain analyses probing associations between self-reported avoidance on the ADMQ and neural responding during Cautious > Inhibition revealed that participants who reported avoiding decision-making in daily life demonstrated heightened activity in regions including the left precentral gyrus, left anterior insula/frontal operculum cortex, and right supramarginal gyrus, as well as reduced activity in the right cerebellum, during Cautious > Inhibition (Fig. S9). Even further, they showed heightened striatal connectivity with the right precuneus/LOC and reduced striatal connectivity with a range of regions including the left brainstem, thalamus, temporal pole, and frontal pole, the right precentral and postcentral gyri, as well as the anterior cingulate cortex and the paracingulate gyrus, during Cautious > Inhibition (Fig. S10).
4. Discussion
In this study, we examined the neural mechanisms underlying avoidance across risk-taking and response inhibition conditions in a sample of 137 adolescents who varied in anxiety severity. Although anxious adolescents self-reported more decision avoidance, anxiety was unrelated to risk-taking frequency or false-alarm rate in the laboratory task. Anxiety was associated with the time it took adolescents to avoid across conditions: anxious youth were able to inhibit a prepotent response quickly but took longer to decide in the face of risk. The neural mechanisms driving risk-taking differed by anxiety such that greater left IFG recruitment was associated with greater risk-taking in those with high anxiety, while heightened striatal-limbic connectivity was associated with greater risk-taking in those with low anxiety. We also identified a circuit between the VS and the right IFG that promoted risk avoidance regardless of anxiety levels. Together, these results suggest that fronto-striatal influences on adolescent risk-taking may differ as a function of youth anxiety severity.
Anxiety was neither related to risk-taking frequency nor false-alarm rate in the laboratory task. However, anxious adolescents reported disliking and avoiding making decisions in their daily lives, suggesting that act of decision-making itself can be aversive for anxious youth. In the task, anxious adolescents took relatively longer at risky decision-making compared to response inhibition trials, suggesting that the uncertainty inherent to risk-taking may have spurred approach-avoidance conflict and slowed their responding in the risk-taking (but not the response inhibition) condition. As anxiety can co-occur with and show reciprocal associations with attention-deficit/hyperactivity disorder (ADHD) (Murray et al., 2022), tasks involving both risk-taking and response inhibition such as this one might prove useful for a study examining comorbid trajectories.
Participants showed widespread activation in salience network and decision-making regions such as the dACC, PCC, precuneus, angular gyrus, and insula during Cautious > Inhibition, while left IFG pars triangularis and LOC activity was greater for Inhibition > Cautious. IFG pars triangularis is thought to mediate response inhibition through bottom-up and top-down reprogramming of action plans (Lenartowicz et al., 2011), while occipital activity may demonstrate visual attention to switching task demands. Activation was modulated by false-alarm rate such that increasing amounts of false alarms were associated with decreasing activation in regions of the visual system including the lingual gyrus, occipital pole, and intracalcarine cortex. This lower visual response during decision-making may have indicated task inattention, resulting in poorer response inhibition.
rucially, the regions modulated by risk-taking were dependent on anxiety. Regulatory systems in the left IFG played opposing roles in risk-taking depending on anxiety: IFG recruitment was associated with risk avoidance in low anxiety, but risk-taking in high anxiety. Research has identified left IFG dysregulation in anxiety associated with weaker connectivity between the IFG and the vmPFC where the IFG evaluates stimulus meaning and informs the vmPFC in inhibiting the amygdala (Cha et al., 2016). Perhaps increased IFG recruitment in anxious youth was compensatory and served to inhibit the amygdala, promoting risk-taking. Alternatively, perhaps bottom-up mechanisms drive risk-taking in neurotypical adolescents, while top-down processes guide risk-taking in anxious youth. As left IFG activity has been linked to anxious apprehension during decision-making (Engels et al., 2007), greater IFG recruitment in anxious risk-takers may have indicated deliberation and apprehension regarding potential outcomes, whereas anxious avoiders may have engaged in more automatic/habitual avoidance not requiring IFG involvement. In adolescents with low anxiety, IFG activity may have indicated harm avoidance tendencies, explaining its association with cautious behavior in these youth.
Risk-taking and avoidance have both been linked to functioning of the VS, which is also implicated in anxiety. Here, striatal connectivity with the right IFG was associated with risk avoidance regardless of anxiety severity. This is consistent with previous research implicating the right IFG in inhibition (Lenartowicz et al., 2011) and approach-avoidance conflict (Zorowitz et al., 2019), further highlighting its relevance for studies of decision-making and anxiety. As false-alarm rate increased, VS-mPFC connectivity decreased, suggesting that VS-mPFC communication is important for successful response inhibition in adolescence.
VS connectivity with limbic regions including the amygdala, putamen, OFC, hypothalamus, mPFC, and insula was associated with risk-taking in low anxiety and risk avoidance in high anxiety. In lieu of anxiety-related differences in frequency of behavior, this suggests that anxious adolescents engage in risk-taking as frequently as their peers, but the neural mechanisms driving risk-taking work in opposing ways depending on anxiety symptoms. Future longitudinal work will probe how these mechanisms contribute to anxiety development over adolescence, and whether more pronounced behavioral differences emerge when anxiety reaches a clinical level.
The VS translates evaluative signals from the amygdala into value-based action (Fareri and Tottenham, 2016). Here, greater VS-amygdala connectivity was associated with risk-taking in those with low anxiety and risk avoidance in those with high anxiety, suggesting that amygdala signaling to the VS may be interpreted differently in adolescents depending on their anxiety; perhaps the “thrilling” aspect of uncertainty inherent in risk drives risk-taking in typically-developing adolescents but is perceived as threatening in higher anxiety. While VS response has been associated with risk-taking (Chein et al., 2011), it is also important for avoidance (Loewke et al., 2021) and shows associations with anxiety (Levita et al., 2012). The considerable development of frontal-subcortical connections in adolescence contributes to both the emergence of anxiety and the refinement of motivated behavior.
These findings should be interpreted in the context of several limitations. First, these analyses are cross-sectional and anxiety was observed rather than experimentally manipulated, limiting the ability to make causal inferences about the effects of anxiety on brain functioning. Although we identified altered brain-behavior associations amongst anxious participants, longitudinal tracking will be important for assessing how fronto-striatal functioning during avoidance contributes to anxiety development later in adolescence. It is also important to note the limitations of the fMRI task. Although it provided a compelling comparison between voluntary risk avoidance and instructed harm avoidance, including risk-taking and response inhibition in the same design along with the decision-making nature of the task meant that certain conditions had lower than the recommended number of trials. Comparing voluntary approach at yellow lights (risky choice) to accidental approach at red lights (false alarm) could shed insight into factors contributing to adaptive and maladaptive approach behaviors and their associations with anxiety, but these analyses were not feasible as the false-alarm rate was low. Future studies may want to utilize tasks with difficult inhibitory conditions and more risk-taking trials. Finally, while we did find task-modulated functional connectivity, we did not measure effective connectivity and therefore cannot speak to the direction of these effects. We can hypothesize top-down or bottom-up effects, but future work using approaches such as dynamic causal modeling will be important for inferring the causal architecture of these systems.
Nonetheless, this study provides insight into the mechanisms driving avoidance in adolescents at risk for developing anxiety. The findings presented here suggest that fronto-striatal mechanisms in adolescence contribute to both approach and avoidance phenotypes depending on anxiety symptoms, adding to the literature implicating the striatum in anxiety and underscoring the importance of considering the multifaceted nature of adolescent brain and behavioral development when studying adolescent-onset anxiety.