Abstract
Prior research suggests that increased adolescent risk-taking in the presence of peers may be linked to the influence of peers on the valuation and processing of rewards during decision-making. The current study explores this idea by examining how peer observation impacts the processing of rewards when such processing is isolated from other facets of risky decision-making (e.g. risk-perception and preference, inhibitory processing, etc.). In an fMRI paradigm, a sample of adolescents (ages 14–19) and adults (ages 25–35) completed a modified High/Low Card Guessing Task that included rewarded and un-rewarded trials. Social context was manipulated by having participants complete the task both alone and while being observed by two, same-age, same-sex peers. Results indicated an interaction of age and social context on the activation of reward circuitry during the receipt of reward; when observed by peers adolescents exhibited greater ventral striatal activation than adults, but no age-related differences were evinced when the task was completed alone. These findings suggest that, during adolescence, peers influence recruitment of reward-related regions even when they are engaged outside of the context of risk-taking. Implications for engagement in prosocial, as well as risky, behaviors during adolescence are discussed.
1. Introduction
One hallmark of adolescent risk taking is that, more often than not, it occurs in the presence of peers (for recent review, see Albert et al., 2013). Although the customary explanation of this phenomenon assumes that it arises from explicit peer pressure to engage in risky behaviors, experimental studies of the “peer effect” on adolescent risk taking have demonstrated that the mere presence of peers can increase adolescents’ risk taking even when the adolescents are prohibited from directly communicating with each other (Chein et al., 2011, Smith et al., 2014), an effect that is not seen among adults. This finding suggests that a process other than explicit encouragement to behave recklessly explains why adolescents, but not adults, are more likely to take risks when with their friends.
One explanation suggested by prior work is that, during adolescence, the presence of peers affects the way in which rewards are valuated and processed. In behavioral studies, for example, adolescents who are being watched by peers are more oriented toward immediate than delayed rewards (O’Brien et al., 2011, Weigard et al., 2013), and more inclined to pursue rewards even in the face of likely negative outcomes (Smith et al., 2014). Prior neuroimaging work further shows that during a risk-taking task, being observed by peers produces heightened activation selectively in brain areas associated with reward processing (e.g., the ventral striatum, VS), and not in other brain regions engaged by the task (e.g., lateral prefrontal cortex, lPFC) (Chein et al., 2011). Consistent with the behavioral evidence, this increased activation during peer observation is found among adolescents, but not among adults.
These findings suggest that adolescents’ relatively stronger inclination to behave recklessly in the presence of peers is due specifically to the impact of peers on reward sensitivity, which is likely mediated by engagement of reward processing regions, specifically the VS. However, the paradigms previously used to investigate this effect conflate reward processing with other facets of risky decision making, such as risk preference and self-regulation, making it difficult to determine whether reward processing per se is specifically impacted by the presence of peers, or whether some more complex interaction between self-regulatory and affective processes operative during risky decision making might underlie this effect. In the present study, we therefore examine the peer effect on reward processing using a task in which no explicit risk is involved.
There are no prior studies investigating how peers impact age differences during reward processing, but there have been several studies of age differences in reward sensitivity when individuals are alone (e.g., Bjork et al., 2004, Galvan et al., 2006, Padmanabhan et al., 2011, Van Leijenhorst et al., 2009). Several such studies report age differences in striatal engagement during reward processing. The majority of studies show that relative to both children and adults, adolescents are more sensitive to rewards and show greater striatal activation in brain regions typically associated with reward processing (Barkley-Levenson and Galvan, 2014, Christakou et al., 2011, Galvan et al., 2006, Galvan and McGlennen, 2013, Geier et al., 2010, Hoogendam et al., 2013, Jarcho et al., 2012, Padmanabhan et al., 2011, Van Leijenhorst et al., 2009). There are also several studies, however, reporting a dampened striatal response to reward during adolescence (Bjork et al., 2004, Bjork et al., 2010, Hoogendam et al., 2013, Lamm et al., 2014) and others that do not find any effect of age on striatal response (Benningfield et al., 2014, Krain et al., 2006, Teslovich et al., 2013, Van Leijenhorst et al., 2006). Despite these inconsistencies in the literature, which are likely due to differences in the specific tasks employed and the specific stages of reward processing under investigation (e.g., anticipation or receipt) (for recent review, see Richards et al., 2013), the weight of the available evidence seems to indicate increased striatal responding to rewards during adolescence. Whether this age difference in the activation of reward circuitry is moderated by the presence of peers, and whether any such moderating influences arise during reward anticipation, reward receipt, or both, is unknown.
The current study uses functional magnetic resonance imaging (fMRI) to examine age differences in neural engagement during peer observation when participants perform a reward-processing task that involves no risk taking (i.e., there is no response that can be thought of as inherently more “safe” or more “dangerous”). We tested three hypotheses. First, we hypothesized that adolescents would show greater activation than adults in the VS during the anticipation and receipt of reward. Second, we hypothesized that adolescents’ activation of this region (either during the anticipation or receipt of reward) would be greater in the presence of peers than when alone. And third, we hypothesized that the impact of peers on activation of the VS would be seen among adolescents, but not adults.
2. Method
2.1. Participants
Twenty adolescent participants (ages 14–19 years, M = 16.7, SD = 1.5, 10 females), and 20 adult participants (ages 24–32 years, M = 26.7, SD = 2.3, 10 females) provided data for the study. The demographics were as follows: 43% Caucasian, 25% African American, 20% Asian, and 12% Unknown. The two age groups did not differ with respect to race, X2(3,40) = 5.48, p = 0.14. Informed consent was obtained from each participant aged 18 and older, and parental consent and youth assent were obtained from each participant aged 17 and younger. All procedures were reviewed and approved by the university's Institutional Review Board. Participants received monetary compensation ($35) for their participation. To keep participants motivated throughout the experiment, they were informed that an additional bonus payment (up to $15) would be provided based on their overall task performance. In actuality, all participants received the bonus.
2.2. Procedure
The current study was part of a larger fMRI experiment in which we systematically varied the social context (i.e., alone versus peer observation, as described below) under which individuals were tested. While in the scanner, participants completed 6, 8-min rounds of the High/Low Card Guessing Task (described below) and 2, 5-min rounds of a Delay Discounting Task. The order of tasks was the same for all participants: 3 runs of the Card Guessing Task, followed by 2 runs of Delay Discounting, then the social condition was switched and 3 additional runs of the Card Guessing Task (but no additional runs of Delay Discounting) were completed. Only the results of the Card Guessing Task are presented in this manuscript, since the Delay Discounting task was not administered with a within-subjects social context manipulation.
2.3. Task design
The current study employed a modified version of the High/Low Card Guessing Task (adapted from Delgado et al., 2003) administered on a computer inside the scanner (Fig. 1a). This reward processing task required participants to make a series of uninformed guesses about whether a number hidden on the reverse side of each card in a virtual stack of cards would be higher or lower than 5. It is important to note that although the task does involve a simple decision (a guess) that may encourage some degree of internal deliberation, it does not involve risky decision making, because the guesses made by the participants are not reliably related to any specific outcome contingencies, or a choice between a risky versus safe option.
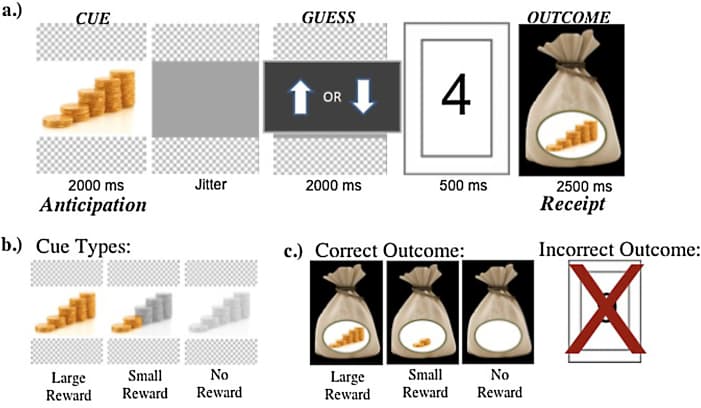
Fig. 1. High/Low Card Guessing Task. (a) We used a modified version of the High/Low Card Guessing Task (original task, Delgado et al., 2003). (b) Anticipatory cues used in the task. (c). Receipt of reward (correct outcome) and failure to receive potential reward (incorrect outcome).
At the beginning of each trial, the back face of the card was shown along with an image that specified the potential reward value of the trial, serving as a reward “cue” (2000 ms). Each reward cue showed a stack of coins colored according to the magnitude of the reward that would be earned with a correct guess about that card, with three cue types: large reward (all of the coins in the stack colored gold), small reward (a small portion of the coins colored gold, the remainder in gray), or no reward (all coins shown in gray) (Fig. 1b). Participants were told that bonus payments would be based upon correct guessing in rewarded trials, but were not told the exact monetary value of the reward that could be obtained on each trial.
The cue was followed by a blank screen which lasted for a jittered interval ranging between 2 and 14 s (exponentially distributed), after which time a prompt appeared on the screen (2000 ms) to signal that the participant should press the button corresponding to his or her guess (higher or lower than 5). The reverse side of the card was then revealed, showing the number on the card (500 ms), and then feedback was provided – either a stack of coins indicating a correct guess (colored according to the reward magnitude presented in the cue), or a card with an ‘X’ drawn through it to indicate an incorrect guess (2500 ms; see Fig. 1c). Each trial was followed by a jittered inter-trial interval, again ranging between 2 and 14 s.
On trials where participants did not respond within the 2000 ms window the remainder of the trial occurred as though the participant had responded incorrectly, and these trials were excluded from all analyses. Non-response trials were infrequent (M = 2.55, SE = 0.46) and the number of missed trials did not differ by age (F(1,38) = 0.28, p = 0.60, hp2 = 0.007) or social context (F(1,38) = 0.61, p = 0.44, hp2 = 0.02).
An important aspect of the task is that, despite appearing random to the participant, the outcomes of each trial were experimentally predetermined such that each participant experienced the same outcome on each trial, regardless of his or her guess. This “fixed” event sequencing assured a common reward history for all participants and avoided performance differences that could otherwise contaminate effects of interest (e.g., age and social context differences).
Participants completed 6, 8-minute long rounds of the task (42 trials each); 3 in each social context. Behavioral data from the scanner was acquired and temporally aligned to fMRI acquisitions using E-Prime, interfaced with an LCD display and a button-press unit (Psychology Software Tools, Pittsburgh, PA).
2.4. Manipulation of social context
The social context manipulation was very similar to that used in Chein et al. (2011), with the addition of a laptop camera used to enhance the peer interaction between tasks (described below). Participants brought two, same-age, same-sex friends with them to the scanning session, but were not told in advance how the peers would be used in the experiment. In the Peer condition, participants were informed that their friends were going to observe them playing the task on a monitor from the scanner control room and would be making predictions about how they expected the participant to perform on the task. Prior to the beginning of the Peer condition, and between each 8-minute round of the task, the peers were asked to communicate with the target participant via the scanner's intercom system and the laptop camera. The peers were encouraged to speak naturally while indicating their presence in the control area, their ability to observe the participant's task performance on the monitor, and the fact that they had made predictions about the participant's pending performance (without providing any details about these predictions). The peers were instructed to make these specific points during the interaction (e.g., “How are you doing in there?” “We’re watching you play.” “Good luck.”), and to avoid comments that might explicitly or intentionally bias the participant's behavior. In the Alone condition, participants completed the task with no observers. All participants completed the task both in the Alone condition and the Peer condition, with the order of social context counterbalanced across participants.
2.5. fMRI data acquisition
Subjects were scanned using a 3-Tesla Siemens magnet located at Temple University Hospital, equipped with a 12-channel phased array transmit/receive head coil. A T-1 weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) image, collected in the sagittal plane, provided high-resolution structural images for co-registration of functional images and inter-subject normalization. Each functional scan of the task included 240 acquisitions collected with a whole brain T2*-weighted echoplanar imaging (EPI) sequence (TR = 2000 ms, TE = 30 ms, flip = 90°, 4-mm slice thickness with no gap, 220 field of view, 3.4 mm × 3.4 mm in-plane resolution).
2.6. fMRI data analysis
All fMRI data analyses were performed using AFNI (Cox, 1996). The functional data were preprocessed in several steps. First, data were interpolated to adjust for slice time acquisition effects. Next, a six-parameter rigid-body motion correction was applied and the motion-corrected functional and structural images were co-registered. All participants included in the analysis exhibited less than 3-mm of motion and less than 3 degrees of rotation in any direction/axis over the course of the scan. Motion-corrected images were smoothed with a 6-mm full-width half maximum Gaussian kernel before applying a mask to exclude all voxels outside of the brain. Data were then converted based on voxel-wise percent signal change (relative to the mean value for each voxel across the run), and finally, all functional scans were normalized into MNI space using an automated 12-parameter nonlinear transformation.
The preprocessed data from each participant were analyzed in an event-related fashion using a general linear model (GLM) approach, with fMRI time-series collected during Alone and Peer social contexts modeled separately. For each social context, both cue-dependent and outcome-dependent activities were modeled. Initial analyses indicated no magnitude-dependent effects (i.e., no differences in activity following large vs. small reward cues or large vs. small reward outcomes) in task-dependent regions of interest; therefore, large-reward and small-reward trials were collapsed into a single “reward” condition, and contrasted with “no reward” trials. In addition, there were no main or interactive effects in no reward trials for either cue or feedback phases in regions of interest (the only significant findings were from a main effect of age in the postcentral gyrus, precentral gyrus, and posterior insula); therefore we used the no reward condition as a common baseline.
BOLD activity evoked in response to each reward cue type (reward, no reward) was time-locked to the onset of each cue, and convolved with a canonical model of the hemodynamic response function (HRF, Boynton et al., 1996). Based on the combined accuracy of the guess and the type of reward offered on a given trial, four outcome types were also modeled: correct reward trials, correct no reward trials, incorrect reward trials, and incorrect no reward trials. BOLD activity associated with each trial outcome was time-locked to the onset of outcome presentation, convolved with a canonical HRF function. Although all four outcome types were included in the GLM model, analyses focused on the contrast between correct-reward and correct-no-reward trials, which provides the most direct test of sensitivity to the receipt of reward. In addition to task-dependent regressors, the full model also included nuisance covariates representing each of the 6 estimated motion time series provided by motion correction.
2.7. Region of interest analyses
Several lines of evidence point to the nucleus accumbens (NAcc) and surrounding areas of the VS as the likely locus for the interaction between social context, development, and reward processing. In particular, the VS/NAcc has been implicated in many prior studies of reward processing (including those using the High/Low Card Guessing Task, Delgado et al., 2003, May et al., 2004), in studies exploring developmental differences during reward processing using a whole-brain analysis (e.g., Barkley-Levenson and Galvan, 2014, Galvan et al., 2006) as well as those employing an ROI-based method (e.g., Geier et al., 2010, Hoogendam et al., 2013), and in our own prior work examining the impact of social context manipulations during a risk-taking task (Chein et al., 2011). Accordingly, we focused our primary analyses on this region by creating anatomical masks of the bilateral NAcc, and used these masks as regions-of-interest (ROI) to examine possible main and interactive effects of age and social context. Bilateral NAcc masks were localized at MNI coordinates (±) 14, 10, −10, and consisted of 6 contiguous voxels in each hemisphere (see Fig. 2a). As a comparison, we also explored activity in a prefrontal region that in prior work had exhibited age-dependent effects, but no sensitivity to a social context manipulation (Chein et al., 2011). This control ROI was based on an anatomical mask of Brodman's Area 46 bilaterally, centered at (±) 45, 37, 19, and included 10 voxels in each hemisphere.
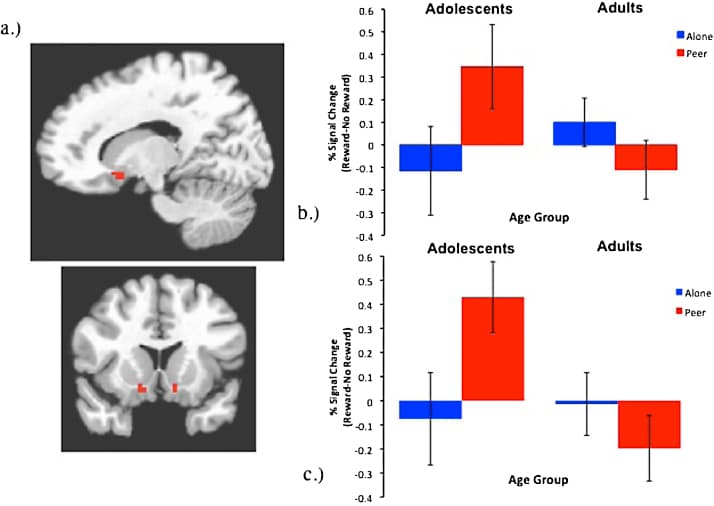
Fig. 2. Region of interest analysis. (a). NAcc masks were anatomically defined at 14, 10, −10 and −14, 10, 10. A repeated measures GLM revealed an interaction between age and social context during receipt of reward in the (b) right NAcc F(1, 36) = 4.04, p = 0.052, hp2 = 0.10, and a marginal interaction in the (c) left NAcc, F(1, 36) = 3.94, p = 0.055, hp2 = 0.10.
Separate ROI analyses were conducted for the cue-dependent and outcome-dependent phases of the task. To explore effects in each ROI, for each social context (Peer, Alone) and phase (cue, outcome), we extracted the estimated percent signal change difference associated with reward vs. no reward events (i.e., the difference in the beta coefficient estimates for each regressor type; Cue: reward minus no-reward; Outcome: correct-reward minus correct-no-reward), and averaged these values across all voxels included in the ROI. The individual subject average ROI data were then submitted to repeated-measures ANOVA conducted in SPSS, with age group as a between-subjects factor and social context as a within-subject factor.
2.8. Whole-brain analyses
Whole-brain group analyses were performed using individual subjects’ voxel-wise parameter estimates (beta coefficients) from a contrast of reward vs. no reward events in each phase (cue and outcome). Group analyses were performed to identify regions exhibiting main and interactive effects of age and social context. For these analyses we used a two-way, mixed-effects ANOVA with age group as the between-subjects factor and social context as the within-subjects factor. Separate ANOVAs were run for anticipation (cue) and receipt (outcome) of reward. All group maps were corrected for multiple comparisons using a voxel-wise probability threshold (p < 0.005) and a contiguity requirement (21 adjacent voxels) that, based on Monte Carlo simulations, resulted in a family-wise error rate of p = 0.05. All results are reported in MNI coordinates.
3. Results
3.1. Region of interest analyses
Two participants were excluded from the analyses because their extracted average percent signal change within the ROI represented significant outliers (>2.5 standard deviations from the mean) within their age group.1
3.1.1. Anticipation of reward
A repeated-measures GLM examining the effects of age and social context on anticipation of a possible reward (vs. no reward) revealed no significant main effects of age on activation in the right NAcc, F(1,36) = 0.09, p = 0.76, hp2 = 0.003 or left NAcc, F(1,36) = 0.12, p = 0.73, hp2 = 0.003. There was also no main effect of social context in the right NAcc, F(1,36) = 1.61, p = 0.21, hp2 = 0.04, but there was a significant main effect of social context in the left NAcc, F(1,36) = 7.14, p = 0.01, hp2 = 0.17, resulting from greater relative NAcc activation in the Alone condition during anticipation of reward. However, further inspection of this effect showed that activity in this ROI was not different from zero in either the Alone or Peer condition (M = 0.197, SD = 0.57; M = −0.079, SD = 0.66, respectively), suggesting that this apparent main effect may be spurious. Finally, no interaction effects between age and social context were found in the right or left NAcc, F(1,36) = 2.15, p = 0.15, hp2 = 0.06, and F(1,36) = 1.28, p = 0.27, hp2 = 0.03, respectively.
Analysis of the prefrontal ROI yielded no main effect of social context in the right hemisphere (F(1,36) = 1.24, p = 0.73, hp2 = 0.003); however, there was a marginal effect in the left lPFC (F(1,36) = 3.97, p = 0.06, hp2 = 0.09). In addition, we found no significant effects of age (right: F(1,36) = 0.26, p = 0.61, hp2 = 0.007, left: F(1,36) = 0.31, p = 0.58, hp2 = 0.008), and no interaction between age and social context (right: F(1,36) = 0.02, p = 0.89, hp2 = 0.001, left: F(1,36) = 2.45, p = 0.13, hp2 = 0.06), during the anticipation of a possible reward, compared to no reward.
3.1.2. Receipt of reward
A repeated measures GLM examining the effects of age and social context following a correct guess and the consequent receipt of reward (correct rewarded vs. no reward outcome) revealed no main effects of age in the right NAcc, F(1,36) = 0.63, p = 0.43, hp2 = 0.02, but there was a significant main effect of age in the left NAcc, F(1,36) = 4.79, p = 0.04, hp2 = 0.12, (Adolescents: M = 0.18, SE = 0.09; Adults: M = (−)0.12, SE = 0.09), with adolescents demonstrating greater NAcc recruitment than adults. There was no main effect of social context in the right or left NAcc, F(1,36) = 0.56, p = 0.46, hp2 = 0.02 and F(1,36) = 0.86, p = 0.36, hp2 = 0.02, respectively. However, there was an interaction between age and social context in both the right and left NAcc, F(1,36) = 4.04, p = 0.052, hp2 = 0.10, and F(1,36) = 3.94, p = 0.055, hp2 = 0.10, respectively (see Fig. 2b). Follow-up t-tests indicated that adolescents exhibited a greater bilateral striatal response in the presence of peers compared to adults (right NAcc: t(36) = 2.00, p = 0.052, d = 0.67; left NAcc: t(36) = 3.133, p = 0.003, d = 1.04), while no age effect was observed in the alone condition (right NAcc: t(36) = −0.97, p = 0.34, d = 0.33; left NAcc: t(36) = −0.27, p = 0.79, d = 0.09), Paired samples t-tests of social context did not reach significance within either group (Adolescents: right NAcc: t(18) = 1.54, p = 0.14, d = 0.73; left NAcc: t(18) = 1.77, p = 0.093, d = 0.83; Adults, right NAcc: t(18) = −1.42, p = 0.18, d = 0.67; left NAcc: t(18) = −0.93, p = 0.37, d = 0.44). The pattern of this interaction was consistent with the hypothesized peer effect, with adolescents exhibiting greater activation of the NAcc during peer observation compared to adults; however the difference in adolescents’ VS response did not reach significance for the Alone versus Peer conditions, and therefore did not fully support our hypotheses.
When probed for main and interactive effects on correct outcome activation (i.e., receipt of reward), the prefrontal ROI exhibited no patterns of interest. Tests for the main effect of social context (right: F(1,36) = 0.06, p = 0.81, hp2 = 0.002, left: F(1,36) = 0.02, p = 0.89, hp2 = 0.001), the main effect of age (right: F(1,36) = 2.35, p = 0.13, hp2 = 0.06, left: F(1,36) = 0.94, p = 0.34, hp2 < 0.001), and the interaction between age and social context (right: F(1,36) = 1.30, p = 0.26, hp2 = 0.04, left: F(1,36) = 0.009, p = 0.93, hp2 = 0.03) were all non-significant.
3.2. Whole-brain analyses
3.2.1. Anticipation of reward
A two-way ANOVA during anticipation of reward produced no regions exhibiting a main effect of age. There was a significant main effect of social context in only one cluster, located in the posterior cingulate cortex (−1, −35, 7). Follow-up tests showed that this region was activated to a greater extent when participants completed the task alone than with peer observers. No regions exhibited a significant interaction between age and social context.
3.2.2. Receipt of reward
A two-way ANOVA (age and social context) on receipt of reward (vs. correct no reward outcome) yielded several regions whose activity indicated a significant main effect of age (see Table 1), including the left VS, with adolescents exhibiting increased activation compared to adults. No regions demonstrated a significant main effect of social context in whole-brain analyses, and no interactive effects of age and social context were found after correcting whole-brain data for overall family-wise error rates.Table 1. Whole-brain tests for main and interactive effects of receipt of reward and social context.
Region | Coordinates | ||||
Empty Cell | k | x | y | z | F |
Main effect of age | |||||
Adolescents>adults | |||||
Middle frontal gyrus | 134 | 32 | 16 | 42 | 9.05 |
Inferior parietal lobule | 38 | 34 | −34 | 36 | 13.67 |
Precentral gyrus | 25 | 54 | −8 | 25 | 10.62 |
Ventral striatum | 21 | −7 | 3 | −12 | 9.64 |
Main effect of social context | No above threshold activation | ||||
Age × social context | No above threshold activation |
Threshold used was p < 0.005 with a 21 voxel extent, which provided an FWE corrected p < 0.05. k = cluster size; x, y, and z = MNI coordinates, left–right, anterior–posterior, interior–superior, respectively; F = F-value at the peak coordinate.
4. Discussion
In this study we explored the hypothesis that adolescents’ increased engagement of the reward system in the presence of peers is not specific to situations that involve risky decision-making, but rather, is a fundamental characteristic of reward processing during this stage of development. While we did not find age differences in striatal engagement during reward anticipation, ROI analyses revealed that, compared to adults, adolescents exhibited greater engagement of the VS during receipt of reward when their peers were watching.
The fact that we did not see age and/or social-context dependent differences in NAcc engagement in response to the reward cue might seem surprising in light of some prior evidence indicating age differences in reward anticipation (e.g., Bjork et al., 2004, Galvan et al., 2006, Galvan and McGlennen, 2013, Van Leijenhorst et al., 2009), as well as our own prior discovery that peers impact activity in this region in the moments leading up to a risky decision, before the outcome of the decision was determined (Chein et al., 2011). However, the reward literature provides considerable evidence that the timing of striatal activation is dependent on the reliability of reward cues (e.g., Schultz et al., 2007, Tricomi et al., 2004). Specifically, when a cue reliably predicts the eventual receipt of a reward, the striatum will activate in response to the cue, and before the reward is delivered (Tricomi et al., 2004). However, when the cue is not reliable (i.e., sometimes the cue predicts reward and sometimes it does not), striatal activity remains yoked to the actual delivery of the reward. In the present card-guessing paradigm, we fixed the outcomes such that half of the time a reward cue was rewarded (correct guess), but half the time it was not (incorrect guess). Accordingly, the cue in this paradigm did not provide a reliable signal regarding the payout of rewards, which may explain why no age differences were observed in the VS during this anticipatory phase, and why in this paradigm we find that adolescents engaged the VS to a greater extent than adults during the receipt of rewards.
In line with our predictions about the age-dependence of peer effects, adolescents demonstrated greater VS activation during receipt of reward during peer observation compared to their adult counterparts. While we had also expected that adolescents’ response to receipt of reward would be greater during the Peer condition than during the Alone condition, this expectation was only partially borne out – the within-group test for adolescents did not reach statistical significance despite a fairly substantial increase in VS engagement in the Peer condition and despite fairly large and consistent effect sizes in the bilateral NAcc (right, d = 0.73; left, d = 0.83), suggesting that the lack of statistical significance may have been due to limited power.
This is the first study to find that peer observation increases striatal sensitivity to receipt of rewards, and importantly, that this effect is present outside of the context of risk-taking. While our prior behavioral work suggests that peers influence adolescents’ reward sensitivity even when no risk is involved (O’Brien et al., 2011, Weigard et al., 2013), the present study extends these findings by providing evidence that behavioral reward sensitivity under peer observation may be associated with increased engagement of the VS. Importantly, the current study begins to disentangle reward sensitivity from other aspects of risky decision-making that may engage overlapping brain circuitry. For instance, striatal engagement during risk-taking may be a result of calculations associated with risk predictions and prediction errors, rather than simply increasing the perceived benefits of engaging in a risky behavior, which we have posited in our account of peer influence. The Card Guessing Task used in this study requires subjects to make a decision between two choices that are equivalent with regard to the likelihood of possible negative and positive outcomes (high vs. low) rather than choose between a risky or safe option, providing further evidence that heightened striatal activation during peer observation may be related to increased reward sensitivity rather than changes in other aspects of the risk-taking process. While we cannot dismiss the possibility that participants employed strategies that engaged deliberative processes, especially in light of a marginal peer effect in the lPFC during the decision-making phase of the task, the presence of this effect during receipt of reward, when no decisions are rendered, further supports the idea that during adolescence peers alter the experience of receiving rewards.
Our finding that peers enhance adolescents’ striatal response to rewards, which may indicate increased reward sensitivity, even in contexts that involve no explicit risk-taking, has a number of implications for our understanding of peer influence during adolescence. Most importantly, this finding suggests that adolescents’ social environment may affect their behavior beyond the decision to engage (or not) in reckless activities. For instance, adolescents’ reward sensitivity, and the manner in which it is influenced by the social context, could also affect adolescents’ involvement in prosocial behaviors. For example, feeling rewarded by the mere presence of peers could motivate adolescents to engage in philanthropic activities when there is an opportunity to do so with friends. Furthermore, education strategies aimed at providing explicit rewards to students during classroom and extracurricular activities where peers are present may prove beneficial for student engagement.
Both our current and past findings provide evidence that reward sensitivity and, importantly, activation of the VS, are a mechanism through which social context influences decision making behavior during adolescence. An important future direction will be to determine whether the degree of striatal engagement during decision-making and receipt of rewards in the presence of peers serves as a predictor of future behavior. By developing a better understanding of the neural mechanisms that mediate peer influences on adolescent behavior we may be more successful in the implementation of informed interventions aimed at reducing risky behaviors, and promoting positive, prosocial behaviors during this developmental period.