Abstract
Opioid use disorder (OUD) is a chronic brain disease which originates from long-term neuroadaptations that develop after repeated opioid consumption and withdrawal episodes. These neuroadaptations lead among other things to the development of a negative affect, which includes loss of motivation for natural rewards, higher anxiety, social deficits, heightened stress reactivity, an inability to identify and describe emotions, physical and/or emotional pain, malaise, dysphoria, sleep disorders and chronic irritability. The urge for relief from this negative affect is one of major causes of relapse, and thus represents a critical challenge for treatment and relapse prevention. Animal models of negative affect induced by opioid withdrawal have recapitulated the development of a negative emotional state with signs such as anhedonia, increased anxiety responses, increased despair-like behaviour and deficits in social interaction. This research has been critical to determine neurocircuitry adaptations during chronic opioid administration or upon withdrawal. In this review, we summarize the recent literature of rodent models of (i) acute withdrawal, (ii) protracted abstinence from passive administration of opioids, (iii) withdrawal or protracted abstinence from opioid self-administration. Finally, we describe neurocircuitry involved in acute withdrawal and protracted abstinence.
Introduction
Opiates are powerful drugs consumed by humans for centuries (Darcq and Kieffer, 2018; Stein, 2018). The use of opium, the latex extracted from the opium poppy (Papaver somniferum), is known for at least a millennium BC and was used at the medicinal level to relieve pain and induce sleep. Morphine is the major active ingredient in opium, and this prototypic opioid drug, together with its many synthetic analogues, remains the most prescribed and effective pain treatment in current clinical practice. This pain-relieving outcome of opioids is often associated with euphoric and rewarding effects which contribute to misuse and may lead to opioid use disorders (OUDs). Two decades ago, pharmaceutical companies over-prescribed synthetic opioids, such as oxycodone, as pain-relievers with reduced addiction liability. Consequently, the prescription rate of opioids has increased dramatically, which was the first step to the so-called “opioid epidemic”, (Compton et al., 2016; Kolodny et al., 2015). Medicinal opioids, therefore, are at the center of the rising opioid crisis in North America (Compton et al., 2016; Kolodny et al., 2015) and now recently in Europe including France (Chenaf et al., 2019; Jannetto et al., 2019), which face an alarming increase in transition to heroin and fentanyl addiction and escalation of deaths by overdose. Thus, a better understanding of neural mechanisms contributing to OUDs and animal models are crucial to face this harmful and devastating “opioid epidemic”.
OUDs are chronic brain disorders, which are hypothesized to be produced by long-term neuroadaptations that developed after repeated opioid exposure and withdrawal episodes (Koob and Volkow, 2010, 2016). OUDs are often theorized as a 3-stage cycle. OUDs start when recreational or medicinal use of opioid switches to intoxication/binge episodes (Koob and Volkow, 2010, 2016; Welsch et al., 2020). Then, intoxication/binge episodes are often followed by withdrawal when the opioid drug clears out. The third stage is preoccupation/anticipation, in which urge for relief from withdrawal and/or craving, causes the planification of the next intoxication episode. Relapse to drug seeking during abstinence is another major step in the development of addiction. Distinct brain networks and brain centers responsible of each of the three stages have been identified (Koob and Volkow, 2010, 2016).
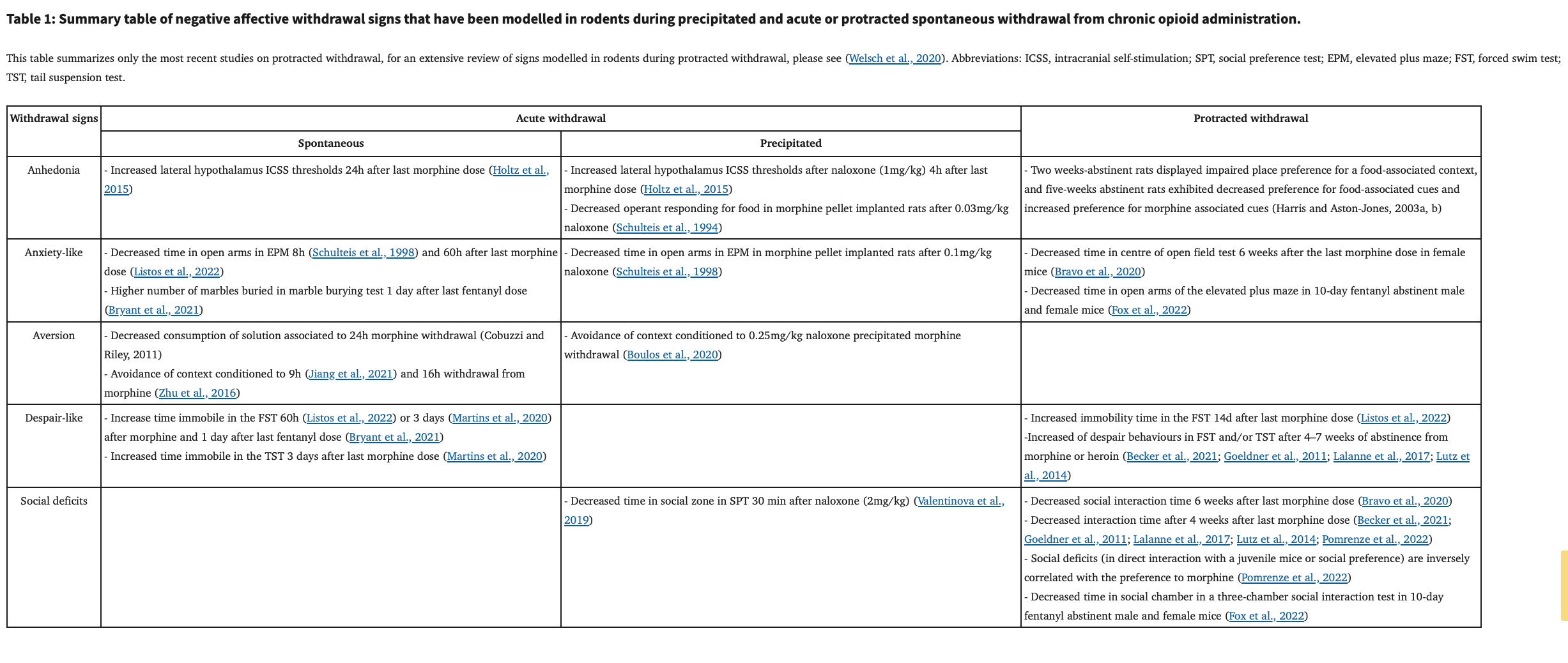
In humans (see Figure 1), acute withdrawal symptoms are experienced during the detoxification process (Kleber, 2007), when there is an abrupt cessation or decrease in the dose of opioid use (Kosten and Baxter, 2019). Acute withdrawal is characterized by intense somatic and affective symptoms that manifest within 12h after the last opioid dose (Kosten and Baxter, 2019). Signs disappear within 5–7 days, but may last up to 14 days in the case of withdrawal from long-acting opioids such as buprenorphine or methadone (Kosten and Baxter, 2019). Somatic signs of withdrawal include flu-like symptoms, body tremors, hyperalgesia, insomnia, muscle aches, and gastrointestinal symptoms, while signs of negative affect include anxiety, malaise, irritability and overall dysphoria (Pergolizzi et al., 2020). Protracted withdrawal, also referred as to protracted abstinence, occurs when withdrawal is maintained for a long time period, during which there is no physical dependence anymore. Protracted abstinence is characterized mainly by psychological symptoms associated with negative affect including a loss of motivation for natural rewards (George and Koob, 2017; Volkow et al., 2010), heightened stress reactivity (Ghitza, 2016), irritability, fatigue and/or insomnia (Chakravorty et al., 2018) and dysphoria (Martin and Jasinski, 1969). To relieve negative affect, abstinent individuals may relapse and consume opioids again, making negative affect and emotional symptoms one of the major causes of relapse with impulsive decision making and craving (Blum et al., 2013). Furthermore, it was suggested in humans that non-physical signs of opioid withdrawal such as negative affect are a higher predictor of drug relapse than somatic symptoms of withdrawal (Jasinski et al., 1985; Kandasamy et al., 2017). In consequence, negative affect caused by acute and protracted withdrawal phases requires better treatment, as inadequate management can increase the risk of relapse (Volkow and Blanco, 2020).
It is therefore important to understand the mechanism underlying both acute withdrawal and protracted abstinence, in order to better manage OUDs. Here we review the recent literature on rodent models of acute withdrawal and protracted abstinence from administration of opioids. For most of these models, withdrawal is studied after passive opioid administration, but other models involve voluntary opioid self-administration, and these will also be reviewed in a separate section. Finally, neurocircuitry involved in the acute withdrawal and the protracted abstinence phases will be also summarized.
I. Progress in rodent models of acute withdrawal after passive administration of opioids
Most rodent models of acute withdrawal have used passive administration of opioids (acute or chronic), and withdrawal may be spontaneous or precipitated with an opioid antagonist. This passive administration of opioids may mimic some aspects of opioid-based pain treatment in humans. Rodent models of acute opioid withdrawal effectively recapitulate many signs of the human physical withdrawal syndrome, including somatic signs such as tremors, muscle spasms/twitching, diarrhea, lacrimation, rhinorrhea and piloerection (Boulos et al., 2020; Gellert and Holtzman, 1978; Kosten and Baxter, 2019). Rodent models also recapitulate affective syndrome and negative affective signs such as anxiety and dysphoria observed in humans (Koob, 2020). The most rapid and strongest manifestations of negative affect during withdrawal in rodent models are aversion to withdrawal associated-contexts or cues, anxiety-like behaviour, and reward/hedonic deficits. This section will overview rodent models of spontaneous and antagonist-precipitated acute withdrawal following passive opioid administration (acute or chronic).
A. Rodent models of spontaneous withdrawal
Rodent models of spontaneous withdrawal from opioids (morphine, heroin and fentanyl) in mice and rats replicate the aversive, anxiety-like, dysphoric-like and anhedonia-type effects of spontaneous opioid withdrawal in humans (Koob, 2020; Schulteis et al., 1994).
A single dose of an opioid is enough to induce a withdrawal syndrome (Harris and Gewirtz, 2005). Withdrawal from an acute dose of morphine has been shown to be less severe in intensity compared to somatic and affective signs from withdrawal after chronic opioid administration in rats (Harris and Gewirtz, 2004; Kalinichev and Holtzman, 2003; Liu and Schulteis, 2004; Schaefer and Michael, 1983, 1986). Studies report that spontaneous withdrawal from acute morphine is aversive as it produces place aversion in rats (Vargas-Perez et al., 2009; Vargas-Perez et al., 2007), and increases anxiety-like behaviour demonstrated as enhanced acoustic startle reflex in rats (Harris and Gewirtz, 2004; Radke et al., 2011; Rothwell et al., 2009). Heroin administration led to similar signs of negative affect, as rats had potentiated acoustic startle 4 hours after a single heroin dose or within 10–20 hours of spontaneous withdrawal from a 12-hour heroin self-administration session (Park et al., 2013).
While spontaneous opioid withdrawal from a single opioid dose leads to signs of negative affect, the observation of physical signs of withdrawal is less obvious, and requires chronic opioid exposure. For this reason, rodent models were developed using repeated opioid administration. In recent works, rats (Cobuzzi and Riley, 2011) and mice (Jiang et al., 2021; Zhu et al., 2016) expressed conditioned place and taste aversion to the spontaneous morphine withdrawal-paired context (Cobuzzi and Riley, 2011; Jiang et al., 2021; Zhu et al., 2016). Increased anxiety after opiate withdrawal was found in several anxiety-type behaviours including elevated plus maze in rats exposed to morphine (Kotlinska et al., 2019) and mice exposed to morphine (Listos et al., 2022; Masukawa et al., 2020), and marble burying test in mice treated with fentanyl (Bryant et al., 2021). Fear responses, reflective of anxiety, were also increased by spontaneous withdrawal from heroin in rats, as short-term heroin withdrawal was shown to enhance fear learning (Parekh et al., 2021) and fear-potentiated startle in rats after withdrawal from a long-acting opiate (methadone derivative l-alpha-acetylmethydol) (Hamilton et al., 2013). In addition to increased anxiety-like behaviours after acute withdrawal, some recent studies detected increased despair-like behaviour from one to three days of spontaneous morphine or fentanyl withdrawal in mice (Bryant et al., 2021; Martins et al., 2020) in the forced swim and tail suspension tests. However, these despair-like behaviours more typically appeared after one week of protracted abstinence from morphine in mice (See part II and (Goeldner et al., 2011; Hodgson et al., 2010)).
B. Animal models of antagonist-precipitated withdrawal
Administration of an opioid receptor antagonist in an opioid-dependent individual precipitates a withdrawal syndrome (Kanof et al., 1992). The two main opioid antagonists used frequently in the clinical setting are naloxone and naltrexone. Naloxone is used to prevent death from opioid overdose, and naltrexone is one of the three FDA approved medications for OUDs treatment (Volkow and Blanco, 2020). Antagonist exposure precipitates and exacerbates the withdrawal that would occur spontaneously when the opioid drug clears out. Antagonist precipitated opioid withdrawal models allow us to enhance and to temporally control the appearance of withdrawal signs (somatic and negative affect). Antagonist-precipitated withdrawal in dogs and rodents has been commonly used to induce somatic withdrawal symptoms, verifying the presence of physical dependence (Blasig and Herz, 1977; Frenois et al., 2002; Martin et al., 1974; Matthes et al., 1996; Wikler and Carter, 1953).
Antagonist-precipitated withdrawal symptoms can be measured after a single opioid exposure (Harris and Gewirtz, 2005). This phenomenon was first revealed after observing antagonist-precipitated somatic withdrawal-like signs in dogs 1 hour after a single injection of morphine (Wikler and Carter, 1953), and it has since been demonstrated in humans and rodents (Bickel et al., 1988; Harris and Gewirtz, 2005; Meyer and Sparber, 1977). The extent of withdrawal signs after an acute dose is highly dependent on the doses of agonist and antagonist, and the interval between the two administrations (Harris and Gewirtz, 2005; Zhang and Schulteis, 2008). Antagonist administration after acute administration of morphine increased anxiety-like responses from 2 hours (acute withdrawal phase) and up to 80 days (protracted withdrawal phase) (Rothwell et al., 2012; Zhang and Schulteis, 2008). Many signs of antagonist-precipitated withdrawal identified in chronic opioid procedures were also found after only a unique acute administration, including aversion to withdrawal-conditioned taste and place avoidance (Azar et al., 2003; Parker et al., 2002; Radke and Gewirtz, 2012; Rothwell et al., 2012), increased anxiety-like responses demonstrated by enhanced acoustic startle reflex (Cabral et al., 2009; Hamilton et al., 2013; Harris et al., 2006; Harris and Gewirtz, 2004; Radke and Gewirtz, 2012; Rothwell et al., 2012) and decreased open arm exploration in the elevated plus maze (McElligott et al., 2013; Zhang and Schulteis, 2008)), and anhedonia and reward deficits (Amitai et al., 2006; Schulteis et al., 1997; Schulteis et al., 2003). Altogether, studies suggest that negative affect during withdrawal from an acute opioid dose may be qualitatively similar to signs of withdrawal from prolonged opioid treatment.
After chronic opioid administration (morphine, heroin), different signs of precipitated withdrawal are detectable in function of the dose of antagonist. High doses of antagonist (>0.03mg/kg of naloxone) can lead to somatic symptoms such as escape attempts by jumping, ptosis, piloerection, and diarrhea, that could obscure negative affective signs (Frenois et al., 2002; Maldonado et al., 1992). Lower doses of antagonist (<0.03mg/kg of naloxone) avoid strong somatic symptoms and allow the study of negative affect without the confounding factor of somatic symptoms (Boulos et al., 2020; Frenois et al., 2002). Higher doses of antagonist (>10mg/kg) induced somatic withdrawal signs and also induced conditioned place aversion in opioid-naive rodents (Bechara et al., 1995; Boulos et al., 2020), due to the antagonism of the endogenous opioid tone.
Antagonist-precipitated opioid withdrawal from chronic opioids induces negative affective signs. The aversive withdrawal experience was often associated to a cue or context (Boulos et al., 2020; Frenois et al., 2002; Noe et al., 2019). During the test phase, avoidance of the withdrawal-associated context is a sensitive cue of the intensity of the aversiveness of opioid withdrawal (Schulteis et al., 1994; Wang et al., 2017). Additionally, cue or context-conditioning models are used to probe withdrawal and cue-associated memory consolidation, retrieval, and extinction (Garcia-Carmona et al., 2015; Wang et al., 2021; Yu et al., 2021; Zhu et al., 2016). These mechanisms are of particular importance to understand as exposure to withdrawal-paired environmental cues or contexts (conditioned withdrawal) can drive opioid use and contribute to relapse in individuals with OUDs (Koob, 2020; Pantazis et al., 2021; Yu et al., 2021). Anxiety-like deficits were also detected, as antagonist-precipitated withdrawal induced anxiogenic behaviours in the elevated plus maze procedure (McElligott et al., 2013; Schulteis et al., 1998). While social deficits, along with other signs of despair-like behaviour, are usually expressed in models of protracted abstinence (See part II), a recent study showed naloxone-precipitated morphine withdrawal decreased time spent in the social zone in the social preference test 30 min after naloxone administration in mice (Valentinova et al., 2019).
The timing of the appearance of signs of opioid withdrawal may be variable in rodent models. Rothwell and colleagues showed that naloxone-precipitated withdrawal increased anxiety-like responses until 80 days after the exposure to one morphine injection of 10mg/kg, but naloxone conditioned place aversion is present only until 24h and not 20 days after morphine (Rothwell et al., 2012). This study points at the complexity of negative affect during opioid withdrawal, emphasizing the importance of using a panel of animal models to cover all the different aspects of withdrawal.
C. Comparison of spontaneous vs antagonist-precipitated withdrawal
Here we summarize studies that directly compared the signs of negative affect after spontaneous or antagonist-precipitated withdrawal to determine whether both withdrawal syndromes show signs of negative affect, and to which extent. In studies testing withdrawal-induced reward deficits, both naloxone-precipitated and spontaneous withdrawal elevated medial forebrain bundle intracranial self-stimulation (ICSS) thresholds in morphine pretreated rats, with no evident differences between the two withdrawal induction methods (Holtz et al., 2015). As for the effect of both forms of withdrawal on anxiety-like responses, spontaneous and naloxone-precipitated morphine withdrawal decreased open arm time on the elevated plus maze in rats (Schulteis et al., 1998). Contrarily, other studies reported differences between naloxone-precipitated and spontaneous withdrawal. Rothwell and colleagues compared two models of withdrawal-induced negative affect, conditioned place aversion and acoustic startle reflex. Both place aversion and enhanced acoustic startle reflex were induced by naloxone-precipitated morphine withdrawal at similar time points in rats (Rothwell et al., 2009). However, during spontaneous withdrawal, enhanced acoustic startle was observed at 4 hours, but no place aversion was present due to the lasting rewarding effects of morphine (Rothwell et al., 2009). In this study, naloxone produced an aversive but not an anxiogenic aspect of the withdrawal process (Rothwell et al., 2009). In another study, naloxone-precipitated methadone withdrawal had no effect on acoustic startle reflex, while spontaneous withdrawal blunted the circadian variation in startle reflex, reflecting another different outcome with the two methods of withdrawal induction in rats (Hamilton et al., 2013).
Additional studies revealed that naloxone-precipitated withdrawal did not only accelerate, but also intensified negative affect when compared to spontaneous withdrawal. Morphine withdrawal induced enhanced acoustic startle and decreased ultrasonic vocalizations were higher in magnitude during naloxone-precipitated compared to spontaneous withdrawal (Kalinichev and Holtzman, 2003). In addition, Schaefer and colleagues compared ICSS in the medial forebrain bundle during naloxone-precipitated and spontaneous morphine withdrawal in rats. While both groups decreased lever pressing, precipitated withdrawal decreased lever pressing to a greater extent and increased stimulus thresholds, indicating exacerbated anhedonia deficits (Schaefer and Michael, 1983, 1986). To conclude, intensity of negative affect signs of opioid withdrawal depends on the method of withdrawal induction, timing and dosing regimens. Models of naloxone-precipitated withdrawal provide preclinical tools to uncover new therapeutical targets that would allow diminishing the negative affect and physical signs, and facilitating recovery from acute withdrawal.
II. Progress in rodent models of protracted abstinence after passive administration of opioids
Chronic opioid administration has strong consequences on hedonic balance and mood states as it was reviewed in details recently in (Welsch et al., 2020). Evidence from rodent models indicate that the intensity of negative affect increases with time during protracted abstinence (Welsch et al., 2020). We will here briefly summarize and update this review with recent studies. Deficits of social behaviours, hedonic homeostasis and mood responses were studied after 6 days to 5 weeks of protracted abstinence. After 6 days of abstinence of chronic morphine rats showed increased despair-like behaviour in the forced swim test (Anraku et al., 2001). After 10 days of chronic morphine abstinence, rats displayed an anhedonia and a lower motivation for a natural reinforcer as the sucrose self-administration was reduced (Zhang et al., 2007). Stress-induced anxiety was also identified after one and 7 days of abstinence in rats (Blatchford et al., 2005). Effects of 7 days abstinence from chronic morphine in mice are similar to rats, and morphine abstinent mice presented deficits in negative affect in forced swim and sociability and elevated plus maze tests (Zanos et al., 2014). In rats, two and five weeks of opioid abstinence decreased place preference for food-associated cues, whereas five weeks abstinence increased preference for morphine associated cues (Harris and Aston-Jones, 2003a, b). In mice, 4 weeks of abstinence from chronic morphine increased despair-like behaviour, decreased social interactions and increased self-grooming in the presence of an unknown interactor (Goeldner et al., 2011). Importantly, these alterations were not present after one week of abstinence, suggesting that negative affect deficits incubate and develop with the duration of abstinence (Goeldner et al., 2011). Social interaction deficits and increased despair behaviour were also found after 4–7 weeks of abstinence from heroin (Lalanne et al., 2017; Lutz et al., 2014). Recently, social interaction and preference deficits induced by three weeks abstinence of chronic morphine were shown to be inversely correlated with morphine-induced place preference directly after the chronic morphine and after the three weeks of abstinence (Pomrenze et al., 2022). These social deficits are associated with increased preference to the morphine-associated chamber three weeks after last morphine exposure (Pomrenze et al., 2022). Social deficits are also induced by a 10-day abstinence from 5-day oral fentanyl administration in both male and female mice (Fox et al., 2022).
Increased anxiety-like responses were described after 4 weeks abstinence from morphine in mice, using the marble burying and novelty suppressed feeding tests (Becker et al., 2017; Becker et al., 2021), the open field or the elevated plus maze (Listos et al., 2022; Ma et al., 2018). Increased anxiety-like responses were also recently reported in the elevated plus maze 10 days after chronic fentanyl exposure (Fox et al., 2022). Furthermore, it was shown that re-exposure to the context of opioid administration induces strong anxiety after abstinence from chronic morphine (4 weeks) using the elevated plus maze (Masukawa et al., 2020). Recently, heroin abstinence (8–14 days) was shown to be sufficient to produce enhanced fear learning after severe electric footshocks, at a similar level that could be induced by post-traumatic stress disorder (Parekh et al., 2021; Parekh et al., 2020). Acute exposure to morphine decreased anxiety responses observed in the elevated plus maze in male but not in female, 6 weeks after administration (Bravo et al., 2020). Together, rodent models of protracted abstinence recapitulate major features characterizing the negative affect of abstinence such as increased anxiety-like responses, despair-like behaviour and social interaction deficits. Protracted abstinence procedures are beneficial to test novel treatments to improve the quality of life for OUDs long-term recovery.
III. Progress in rodent models of withdrawal and protracted abstinence after self-administration of opioids
Drug self-administration models of addiction have been increasingly used since the 1960s (Mello and Negus, 1996; Weeks, 1962), as they offer refined animal models of voluntary intake and may better mimic the pattern of opioid consumption in humans. Here, we will thus summarize studies characterizing opioid withdrawal after self-administration. Classical somatic withdrawal signs can be observed after spontaneous withdrawal from heroin self-administration in rats. It was recently reported that, after disruption of heroin self-administration (for 14 3-h sessions), somatic signs of withdrawal (including: body shakes, chewing, diarrhea, digging, escape attempts, eye blinks, foot licks, genital licks, grooming, head shakes, jumping, piloerection, ptosis, rearing, teeth chatters and writhing) appeared in male and female rats at the 16 and 48 hour timepoints following the last session (Gipson et al., 2021). The expression of withdrawal signs at these timepoints was higher in female compared to male rats (Gipson et al., 2021).
The aversive state induced by opioid antagonists (naltrexone or naloxone) in dependent or non-dependent rats had no effect on heroin-seeking behavior (Shaham et al., 1996; Shaham and Stewart, 1995; Stewart and Wise, 1992), whereas spontaneous 24-h withdrawal increased heroin-seeking in dependent rats (Shaham et al., 1996). Furthermore, self-administration models allow to compare animals that can escalate their drug use throughout extended sessions (Long Access, LgA) versus others that can maintain a low and stable drug use over shorter sessions (Short Access, ShA). Recently, a study compared heroin withdrawal in rats with moderate vs excessive intake (Vendruscolo et al., 2011). Both spontaneous and naloxone-precipitated somatic signs of withdrawal (body weight loss in 60 min, escape attempts, wet dog shakes, and abdominal constrictions, defecation/diarrhea, teeth chattering, swallowing movements, salivation, ptosis, penile erection/ejaculation/grooming, hyperirritability upon touch and abnormal posture) were observed in rats that self-administered heroin for 12-h sessions but these signs were minimal in ShA-rats (Vendruscolo et al., 2011). Naloxone also increases heroin intake in both ShA and LgA rats (Carmack et al., 2019; Kenny et al., 2006) but with a greater extent in LgA rats (Carmack et al., 2019). LgA to heroin induced a progressive increase of intracranial self-stimulation thresholds which reflect the appearance of deficits in brain reward function compared to ShA (Kenny et al., 2006). Naloxone-induced precipitated withdrawal (associated with cues) increased ICSS thresholds specifically in heroin-dependent animals and further cues’ presentation without naloxone administration could mirror reward function deficits (Kenny et al., 2006). This suggests that cues previously paired with naloxone acquired a negative emotional valence in heroin dependent rats.
Park et al. 2013 showed that acute 10–20h withdrawal from heroin self-administration increased acoustic startle response in LgA-rats versus ShA-rats, suggesting that spontaneous acute withdrawal from heroin self-administration leads to increased anxiety levels that can be associated with a ‘drug-dependent’ state (Park et al., 2013). Also, the startle response during withdrawal after a unique passive injection of heroin is of similar amplitude to the one observed after a history of LgA heroin self-administration (Park et al., 2013). This led the authors to speculate that withdrawal from an acute self-administered dose of heroin is a reliable model for further investigation of mechanisms underlying opioid withdrawal-induced potentiated startle.
The strong desire to use an addictive substance such opioid is referred to a subjective state called craving (Fredriksson et al., 2021). Incubation of craving has been modelled in rodents by the intensification of drug-seeking behaviour in response to previously opioid-paired cues over the course of abstinence. After a 10-day heroin self-administration (9h/day) in rats, heroin seeking behaviour was observed after 1, 6, 12, 25 and 66 days of abstinence during extinction training. During extinction, heroin seeking on abstinence days 6, 12 and 25 was higher than on day 1 of withdrawal, demonstrating a difference in drug seeking behaviour in acute and protracted periods of heroin withdrawal (Shalev et al., 2001). Then, Shalev and colleagues measured stress-induced reinstatement of heroin seeking, reporting foot-shock stress induced reinstatement of heroin seeking after 6, 12, 25 and 66 days of heroin withdrawal but not after 1 day, with the maximal responses on days 6 and 12 (Shalev et al., 2001). Recently, Mayberry and colleagues reported that a 30-day abstinence period after 10 days of heroin self-administration increased active lever pressing and locomotion in response to previously opioid-paired cues in the absence of opioid in both female and male rats (Mayberry et al., 2022). Zhou and colleagues also found that when withdrawal period was increased from 1 to 14 days after a 14-day LgA heroin self-administration session, heroin-conditioned cue-induced reinstatement of drug seeking was increased, yet again demonstrating withdrawal period effects on drug seeking behaviour (Zhou et al., 2009).
Recent studies in self-administration models have studied an interesting phenomenon in which rodents have the choice between the self-administered opiate and a nondrug reward (palatable food or social interaction), also called voluntary abstinence (Fredriksson et al., 2021; Venniro et al., 2020). This model is relevant as humans with OUDs may remain abstinent voluntarily by obtaining non-drug rewards (Katz and Higgins, 2003). In one of the first studies on voluntary abstinence in the context of opioid self-administration, rats were trained to self-administer LgA heroin for 12 days, followed by an extinction of this behaviour (Venniro et al., 2017). Rats were then either forced into abstinence with the possibility to seek heroin or given the choice between palatable food or heroin self-administration. Rats readily chose palatable food over heroin administration the majority of the time, however while the authors observed drug seeking in forced abstinent rats when comparing withdrawal days 1 and 21, as described in previous reports, voluntarily abstinent male and female rats did not seek drugs (Venniro et al., 2017). In another study from the same authors, not only food reward was sufficient to induce voluntary abstinence, but so was social reward in a social self-administration procedure (Venniro et al., 2019). A recent study showed that the pattern of heroin self-administration could be crucial for the expression of cue-induced craving behaviour (D’Ottavio et al., 2022). Indeed, rats that self-administered heroin intermittently (6 h/day; 5-min access every 30-min) for 10 days further showed higher cue-induced heroin seeking behaviour compared to rats that could self-administer heroin continuously (6h/day; 10 days). However, this effect did not incubate after 21 days of forced or voluntary abstinence. Intermittent but not continuous heroin self-administration revealed sex differences with female rats seeking for heroin more than male rats (D’Ottavio et al., 2022).
Altogether, despite advances in opioid self-administration research, models of negative affect during withdrawal after opioid self-administration are limited. And, it is not clear whether withdrawal after passive or voluntary opioid administration are qualitatively and quantitively similar. Self-administration models have proven valuable in the preclinical opioid addiction field and highly relevant to OUDs in humans, therefore withdrawal from this form of opioid exposure adds a necessary dimension to the field of negative affect induced by opioid withdrawal and increases the translatability and scientific value of animal models of opioid withdrawal. Voluntary abstinence models are useful to the development of novel medications for relapse prevention.
IV. Neurocircuitry involved in opioid withdrawal and protracted abstinence
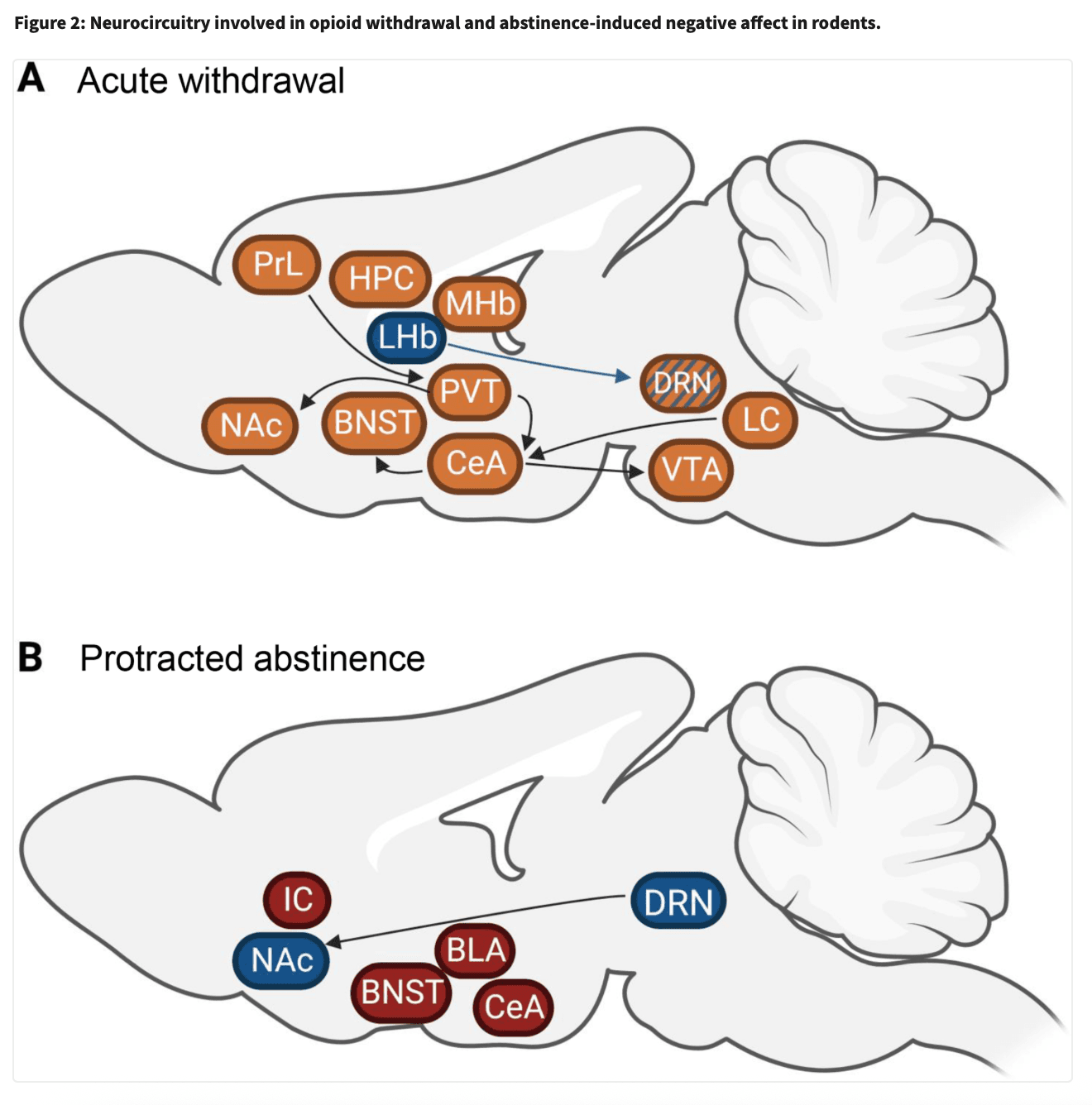
These rodent models were used to identify brain centers, neurocircuitry and neurotransmitters that underlie negative affect following opioid withdrawal (Figure 2).
A. Amygdalar circuitries
The amygdala is a key brain center involved in mood regulation and negative affect (Janak and Tye, 2015; Phelps and LeDoux, 2005). Studies have identified the importance of the amygdala in negative affect during opioid withdrawal, with implications of all its subregions, including the basolateral amygdala and the nuclei of the extended amygdala (i.e. central amygdala (CeA), bed nucleus of the stria terminalis (BNST) and nucleus accumbens (NAc) shell) (Nakagawa et al., 2005; Russell et al., 2016; Valero et al., 2018). C-fos mRNA responses occurred specifically in the extended amygdala and BLA only when opiate withdrawal was precipitated by naloxone doses that induced an aversive state without somatic signs (Frenois et al., 2002). Re-exposure to a withdrawal-paired environment induced c-fos responses specifically in the extended amygdala suggesting that this circuitry mediates the affective value of withdrawal (Frenois et al., 2005). The BNST has received the most attention as an area of particular importance, with several neurotransmitter systems (such as endocannabinoid, noradrenaline, gabaergic) contributing to the negative affect of opioid withdrawal (Fox et al., 2017; Luster et al., 2020; Wills et al., 2017). In particular, noradrenergic signaling in the BNST has been widely shown to mediate the aversive component of opioid withdrawal (Delfs et al., 2000). Both lesion of medullary noradrenergic neurons that project to the BNST and intra-BNST alpha2 agonist injection attenuated naloxone-conditioned place aversion in morphine-dependent rats (Delfs et al., 2000). Another study showed bilateral excitotoxic lesions of the CeA and BNST in rats attenuated naloxone-conditioned place aversion, and c-Fos analyses revealed the likelihood that the CeA to BNST projection mediates this aversive effect of withdrawal (Nakagawa et al., 2005). In fact, the CeA, containing majoritarily inhibitory outputs, seems to be a key mediator of negative affect in opioid withdrawal. A recent study found optogenetic inhibition of inhibitory CRHCeA->VTA neurons during spontaneous withdrawal-induced place aversion abolished avoidance of the withdrawal-paired compartment, and increased the time spent in open arms in the elevated plus maze (Jiang et al., 2021). This population of neurons, increasingly activated with chronic morphine administration, was shown to project onto dopaminergic morphine-responsive ensembles of the VTA, demonstrating morphine-induced plasticity mechanisms leading to the development of negative affect once the drug has left the system. Optogenetic activation of morphine-responsive VTA ensembles was rewarding in an ICSS procedure, and alleviated negative affect during morphine withdrawal, indicating that activation of drug-reward encoding ensembles could also be an avenue for relief from withdrawal-induced negative affect. The central amygdala is not only a key area regulating negative affect, but is also involved in aversive opioid withdrawal conditioned memory retrieval. A recent study found blockade of the lateral division of the CeA, along with chemogenetic inhibition of inputs from the locus coeruleus and the paraventricular thalamus (PVT), prevented the retrieval of conditioned context-induced spontaneous morphine withdrawal memory in a CPA procedure (Wang et al., 2021). A recent study from Carmack and colleagues studied BOLD responses to olfactory cues previously associated with opioid withdrawal after self-administration (Carmack et al., 2019). Rats trained to self-administer LgA (12h/day) or ShA (1h/day) heroin were presented olfactory cues after naloxone injections (120mg/kg), associating odor with precipitated withdrawal. These odors were then presented to lightly anesthetized mice during fMRI acquisitions, and the authors uncovered increased BOLD signals in the paraventricular nucleus and ventromedial nucleus of the hypothalamus and amygdala nuclei in LgA rats but a decrease in signal in ShA rats. These results are in line with previously reported increased HPA axis activity during opioid withdrawal in rats, which may in turn activate extrahypothalamic brain stress systems in the amygdala. Altogether, amygdalar circuitry is a commonly reported substrate in the negative affective component of opioid withdrawal (Valero et al., 2018; Welsch et al., 2020; Zhou et al., 2013).
B. Thalamic circuitries
The PVT has indeed received increased interest in opioid withdrawal since an elegant 2016 study by Zhu and colleagues determined a PVT to NAc pathway mediates the aversive aspect of both spontaneous and naloxone-precipitated morphine withdrawal in the conditioned place aversion procedure (Zhu et al., 2016). A study from the same lab also showed activation of inputs into the PVT from the prelimbic cortex was necessary for the conditioned context-induced retrieval of naloxone-precipitated morphine withdrawal memory, placing the PVT as not only a regulator of opioid withdrawal aversion, but also aversive memory (Yu et al., 2021). This study was one of the few forays on cortical involvement in negative affect of opioid withdrawal.
C. Monoaminergic circuitries
A large portion of the literature in brain areas involved in negative affect of opioid withdrawal places focus on opioid-responsive subcortical areas, particularly the monoaminergic dopamine and serotonin systems. There is now evidence of the involvement of the VTA, the source of dopaminergic innervation in the mesocorticolimbic pathway, and the dorsal raphe nucleus (DRN), the brain’s source of serotonergic neurons, in not only reward, but also negative affect associated with opioid exposure (García-Pérez et al., 2019; Jiang et al., 2021; Lutz et al., 2014; Radke et al., 2011; Welsch et al., 2020). In an interesting convergence of the two systems, a recent study by Lin and colleagues demonstrated that a population of extra-VTA dopaminergic neurons in the DRN is involved in the formation and expression of morphine withdrawal memory by optogenetic inhibition during a spontaneous conditioned place aversion procedure (Lin et al., 2020). As for projections to the DRN, neurons from the lateral habenula (LHb) projecting to the DRN encode acute withdrawal induced social deficits in morphine-dependent mice (Valentinova et al., 2019). Specifically, chemogenetic inhibition of LHb to DRN neurons enhanced social avoidance elicited by naloxone-precipitated withdrawal (Valentinova et al., 2019). Altogether, LHb, DRN, NAc and PVT neuronal adaptations contribute to the negative affect observed after acute opioid withdrawal (see Figure 2A).
Finally, VTA neuronal adaptations have also been identified after spontaneous withdrawal and abstinence from chronic morphine in rats (Georges et al., 2006; Kaufling and Aston-Jones, 2015). After 24 hours of withdrawal or 2 weeks of abstinence, dopaminergic neurons of the VTA did not respond to morphine as in naïve rats (absence of firing), suggesting a long lasting tolerance to the acute effect of morphine (Georges et al., 2006). In contrast, GABAergic neurons of the tail of VTA did not show long lasting tolerance to the acute effect of morphine after 2 weeks of abstinence, but the capacity for disinhibitory control of these GABAergic neurons was impaired after 2 weeks of abstinence, indicating that alterations in GABAergic neurons of the tail VTA contribute to long-term negative affective states during abstinence (Kaufling and Aston-Jones, 2015). Altogether, mechanisms and neuronal populations underlying social deficits in opioid abstinent animals start to be elucidated (see Figure 2B), and gain interest as it is considered a key factor for relapse in humans.
D. Direct and indirect circuitries of the nucleus accumbens
Recently, a mGlu4 positive allosteric modulator was shown to reverse social and anxiety deficits in morphine-abstinent mice (Becker et al., 2021). This beneficial effect may be due to the repression of excessive activity of D2-MSNs in the NAc (Becker et al., 2021). Furthermore, NAc D1-MSNs but not D2-MSNs exhibited dendritic atrophy, an increase in excitatory drive and a downregulation of a cluster of dendritic morphology genes that are transcriptionally coregulated by E2F1 after fentanyl abstinence (Fox et al., 2022). E2F1 viral-expression in NAc D1-MSNS was sufficient to prevent abstinence-induced dendritic atrophy, altered physiology, social and anxiety-like deficits. Finally, chemogenetic inhibition of striatal MOR positive neurons rescued fentanyl withdrawal-induced physical symptoms and anxiety-like behaviors (Wang et al., 2023). Altogether, these studies are providing mechanistic evidence of NAc involvement in abstinence-induced negative affect.
E. Opioid circuitries
Besides monoaminergic systems, the opioid system and brain regions rich in opioid receptors, particularly the mu opioid receptor (MOR), are promising areas in the field of opioid withdrawal. An area of particular interest is the habenula, as it is one of the sites with the densest expression of MOR in the brain (Gardon et al., 2014). While the lateral habenula has received much of the focus with regards to negative affect and drug withdrawal, due to its key connections in regulating monoaminergic midbrain areas (Clerke et al., 2021), recent literature is establishing the medial habenula as a center highly implicated in aversion and negative affect (Bailly et al., 2022; Boulos et al., 2020; Cho et al., 2020; Otsu et al., 2019; Xu et al., 2018). Specifically, a conditional knockout of MORs in nicotinic receptor B4 sub-unit expressing neurons of the medial habenula was sufficient to drastically reduce conditioned place aversion in morphine-dependent and drug-naïve mice (Boulos et al., 2020). An additional promising avenue in the field of opioid research is the study of opioid-responsive neurons and their adaptations to chronic opioid exposure, now possible thanks to the development of three mouse MOR-Cre lines (Bailly et al., 2020; Martin et al., 2019; Okunomiya et al., 2020) and one rat MOR-Cre line (Bossert et al., 2023). One of these mouse lines (Bailly et al., 2020) permitted the study of the behavioural role of opioid responsive neurons in a known aversion center, the habenula (Bailly et al., 2022). The study revealed opto-stimulation of habenular MOR-expressing neurons that project to the interpeduncular nucleus induces avoidance in the real-time place preference test and increased despair-like behaviour in the tail suspension procedure (Bailly et al., 2022). Opto-stimulation of habenular MOR neurons projecting to the DRN however increased anxiety-like responses in the elevated plus maze and marble burying tests, revealing two distinct pathways of habenular MOR neurons controlling negative affect (Bailly et al., 2022). This study demonstrates the involvement of MOR neurons in negative affective processes in the drug naïve state, what left to be determined is uncovering the role of opioid responsive neurons in negative affect in context of opioid dependence. Additionally, a knock-out of MOR in glutamatergic neurons expressing Vglut2 abolished naloxone-precipitated somatic withdrawal symptoms, further emphasizing the role of MOR in an aversive aspect of opioid withdrawal (Zhang et al., 2020). Studies into dynorphin/kappa opioid receptor (KOR) signaling have established it as important in negative affect regulation in the drug-naïve state and in the development of social impairment during protracted heroin abstinence (Baird et al., 2021; Lalanne et al., 2017; Margolis and Karkhanis, 2019). More recently, a study found blockade of KOR by injection of norBNI in the dorsal hippocampus decreased naloxone conditioned place aversion in morphine-dependent mice, suggesting that an upregulation of dynorphin/KOR is involved with aversion in the acute opioid withdrawal phase as well (Chen et al., 2022). Altogether, several neuronal circuits regulating reward and aversion processing and expressing opioid receptors (MORs and KORs) are important for adaptations leading to negative affect after acute withdrawal.
Longer abstinence from chronic morphine (4 weeks) is associated with a decrease of serotonin levels in the dorsal raphe (Goeldner et al., 2011). Importantly, treatment with an inhibitor of the serotonin reuptake (Fluoxetine) during the abstinence period inhibits the development of despair-like behaviour and social deficits (Goeldner et al., 2011). Using a similar model of abstinence from chronic heroin, both fluoxetine and a KOR antagonist, norBNI, inhibit negative affect induced by heroin abstinence (Lalanne et al., 2017). Specifically, when fluoxetine and the KOR antagonist were administered during abstinence, they prevented the development of negative affect, and when administered after 4 weeks of abstinence, fluoxetine and norBNI administration treated and reversed the established negative affect (Lalanne et al., 2017). These two neurotransmitter systems have been associated with negative affect, as serotonin deficits are strongly associated with mood disorders (Dayan and Huys, 2009; Yohn et al., 2017) and KOR activation induces dysphoria and despair-like responses (Bruchas et al., 2010; Chavkin and Koob, 2016). Using a corelative multi-modal behavioural mouse model of protracted morphine abstinence, Pomrenze et al. dissect the circuits mechanisms underlying social deficits induced by morphine abstinence (Pomrenze et al., 2022). First, they demonstrated that kappa opioid receptor blockade, with the KOR antagonist norBNI, infused in the nucleus accumbens medial shell and the specific deletion of prodynorphin neurons from the dorsal raphe prevent social avoidance (Pomrenze et al., 2022). Next, using optogenetic and photometry experiments they identified a dynorphin-producing neuronal population (DRPdyn neurons) of the dorsal raphe projecting to the nucleus accumbens medial shell, which promotes social deficits due to abstinence. Finally, they showed that these social deficits are caused in part by presynaptic KOR activation, on the serotoninergic neurons terminals from the dorsal raphe, and induced by dynorphin release from DRPdyn neurons, which is limiting the release of serotonin in the nucleus accumbens (Pomrenze et al., 2022).
Perspectives and conclusions
To conclude, rodent models of opioid withdrawal, using both acute and chronic passive administration, or self-administration of several opioids (morphine, heroin, fentanyl), recapitulate a number of behavioural signs of negative affect, such as anhedonia, social interaction deficits, increased anxiety levels and despair like behaviours (see Figure 1), opening the way to better understanding and treating the negative affect in OUD. Important also, are rodent models developed to examine the choice between continuing to opioid self-administer, or shifting to self-administration of palatable food or a social reward. Rodent research has successfully identified neurocircuitry and neurotransmitter systems (dynorphin, 5HT) implicated in emotional negative states of opioid withdrawal and in the longer-term neuroadaptations of protracted opioid abstinence. Brain regions that contribute to this negative state were also determined (NAc, Amygdala, VTA, BNST and the DRN). Specific circuits that encode for the different signs start to be elucidated but remain poorly identified, and deserve to be better studied using neuronal manipulation or in-vivo recording. Functional magnetic resonance imaging studies in rodents are few in the context of opioid withdrawal and abstinence, and may help to improve the translation of animal research to human. Indeed, fMRI studies in humans have suggested that the opioid-abstinent human brain shows alterations of functional connectivity strength between brain networks responsible for reward and stress (Liu et al., 2009; Xie et al., 2011; Yuan et al., 2010; Zhai et al., 2015) and some alterations are present even after multiple years of abstinence (Zou et al., 2015). Also, more efforts should be put to investigate sex differences in the neuroadaptations underlying negative affect of opioid withdrawal, as sex factors seem to influence some signs of despair or anxiety (Bravo et al., 2020). Finally, it may be crucial to determine whether new compounds activating MOR signaling developed with the goal to reduce euphoric effects of opioids and avoid addiction liability (Darcq and Kieffer, 2018; Ehrlich and Darcq, 2021; Ehrlich et al., 2019) lead to the same alterations of negative emotional deficits. Overall, animal research on negative affect of withdrawal in OUDs has and will hopefully continue to elucidate mechanisms involved with this highly aversive aspect of OUDs, translating to potential new clinical targets to stop the OUDs cycle.