Abstract
Alcohol use disorder (AUD) is a worldwide problem and the most common substance use disorder. Chronic alcohol consumption may have negative effects on the body, the mind, the family, and even society. With the progress of current neuroimaging methods, an increasing number of imaging techniques are being used to objectively detect brain impairment induced by alcoholism and serve a vital role in the diagnosis, prognosis, and treatment assessment of AUD. This article organizes and analyzes the research on alcohol dependence concerning the main noninvasive neuroimaging methods, structural magnetic resonance imaging, functional magnetic resonance imaging, and electroencephalography, as well as the most common noninvasive brain stimulation - transcranial magnetic stimulation, and intersperses the article with joint intra- and intergroup studies, providing an outlook on future research directions.
Introduction
Alcohol is the most commonly used addictive substance in the world and, because it is estimated that 107 million people worldwide suffer from alcohol use disorders (AUD) and that it also causes 2.8 million premature deaths every year, AUD has emerged as a major public health issue on a global scale (Wigger et al., 2022). AUD is a type of substance use disorder, mainly manifested by excessive and uncontrollable cravings for alcohol, and a common disorder: it can accelerate the course of other clinical or psychiatric disorders, thereby shortening the patient's life expectancy by >10 years (Schuckit, 2009).
Alcohol use affects the gray and white matter of the brain and also alters the electrophysiology of the brain, which then leads to addiction through the process of neuroplasticity (a trait of the nervous system that permits lifelong adaptation to change) (Khan et al., 2021). The neuronal alterations of alcohol exposure have been found extensively. Acute alcohol exposure selectively over-activates primary motor cortex neurons resulting in reduced motor performance (Zhang et al., 2022a), and repeated alcohol exposure over-activates dentate gyrus neurons of the hippocampus along with spatial memory damage (Zhang et al., 2022b). Many existing studies have revealed molecular targets for AUD at the genetic level, but because different alcoholics have different genetic substrates, are at different social levels, and experience different life circumstances, these factors pose significant limitations to research in areas such as risk genes in alcoholics (Ferraguti et al., 2015). Therefore, we want to investigate structural and functional alterations in the brain of AUD patients by a new approach—neuroimaging—that could overcome these limitations while also expanding our systematic knowledge of the physiology and pathology of the human nervous system.
Neuroimaging research has evolved from a focus on relatively isolated functional brain regions, where different brain regions corresponded to different brain functions, to the integration of functional regions, where there are many functional and efficacy connections across functional regions, even at a more subtle level (Oberlin et al., 2020). In this paper, we focus on the main noninvasive neuroimaging methods, structural magnetic resonance imaging (MRI), functional MRI (fMRI) and electroencephalography (EEG), to analyze and summarize the research on alcohol addiction. As more researchers concentrate on simultaneous transcranial magnetic stimulation (TMS) and neuroimaging devices, we introduced transcranial magnetic techniques, which are known as one of the four major technological tools for studying brain science in the 21st century, to investigate the impact of transcranial magnetic techniques on the examination and intervention of patients with AUD (Table 1).
Table 1: Summary of the four brain techniques.
Technique | Category | Location | Mechanism | Dominance | Brain change |
MRI | Noninvasive neuroimaging Techniques | Brain sectional anatomy | Reconstruction imaging of signals generated by resonance of atomic nuclei in a magnetic field | High spatial resolution | Cerebellar atrophy and decreased gray and white matter volume |
fMRI | Noninvasive neuroimaging Techniques | Specific cortical regions of brain activity | BOLD response neuronal activity | Better temporal and spatial resolution | Abnormal FC, impaired function of specific neural pathways |
EEG | Noninvasive neuroimaging Techniques | Scalp | Spontaneous electrical activity of the brain | High temporal resolution | Visual and auditory P3 amplitude reduction, δ, theta, and resting beta power abnormalities |
TMS | Noninvasive brain stimulation | Cortical regions of interest | Dopamine release makes neurons active | Joint neuroimaging techniques | Neural target: dorsolateral prefrontal cortex |
Brain structural alterations in AUD
There is a consensus that the volume density of the prefrontal lobe, especially the medial frontal lobe, is reduced in patients with AUD. Structural MRI studies provide ample evidence of reduced gray matter (GM) volume associated with alcohol dependence, with the most pronounced damage in the frontal lobes (Yang et al., 2016). An analysis by anatomical likelihood estimation found that GM changes in patients with AUD were distributed in different parts of the cingulate and medial frontal gyri, the paracentral lobe, the left postcentral and precentral gyri, the left anterior and right posterior insulae, and the left superior frontal gyrus (Spindler et al., 2021). With these findings, we hypothesize that the reduction of GM in AUD may disrupt network communication and lead to neurocognitive impairment associated with chronic alcohol consumption. However, studies have also shown that the associated brain imaging deficits are also reversed and partially recovered, usually after a few weeks to months of abstinence from alcohol, which emphasizes the importance of abstinence from alcohol (Nutt et al., 2021). An analysis of the effects of gender and age on the GM of alcoholics' brains using voxel-based morphometry and surface-based morphometry found that women had more adverse effects from alcohol use on the left orbitofrontal cortex thickness than men did, and had a more pronounced negative correlation between age and right insula volume (Galandra et al., 2018, Thayer et al., 2016). The influence of gender, age, and other factors on the structure and function of the brain in alcohol-addicted patients could also be a major breakthrough in our understanding of this disease.
There is mounting evidence that AUD patients experience significant changes in their white matter in addition to the commonly described defects in the GM. In individuals with AUD, an investigation using anatomical likelihood estimation found four separate sets of aggregated macro- and microstructural white matter changes in the fornix, the anterior and posterior cingulum, the right posterior limb of the internal capsule, and the genu and body of the corpus callosum (Spindler et al., 2022). For different levels of alcohol consumption, studies have found that any level of alcohol consumption affects brain volume and white matter microstructure, especially the corpus callosum, resulting in altered cognitive function in patients (Nutt et al., 2021). We hope to learn more about how white matter changes as the disease progresses in future studies.
Of interest, another recently discovered brain region associated with alcoholism is the cerebellum. Alcoholics are often associated with shrinkage of the cerebellum. The cerebellar Purkinje fibers, granule cells, and white matter fibers are the main targets of neurodegeneration in alcoholics, with the most pronounced atrophy in the anterior middle part of the vermis (de la Monte & Kril, 2014). This atrophy is progressive. In a recent study, cerebellar volume loss was found to increase with age in patients with additional neurological complications such as Wernicke's Korsakoff syndrome (Nutt et al., 2021). In functional connectivity (FC) analysis, Abdallah et al. abandoned previous static FC measurements and used the sliding window method and multilayer community assay to investigate dynamic brain-cerebellar FC in AUD patients and found that the AUD group showed significant FC variability between the cerebellum and both the frontoparietal executive control network and ventral attention network, with significantly less cerebellar flexibility and greater integration (Abdallah et al., 2021). However, the small sample size limits the reliability of the findings, and in the future, we hope to explore the characteristics of dynamic FC at the level of cerebellar sub-modules to gain a more comprehensive understanding of cerebellar alterations associated with AUD.
Brain functional alterations in AUD
Since its introduction in the early 1990s, fMRI has grown in popularity. fMRI has a much higher spatial resolution when looking at the activation of brain regions, even to the order of seconds in temporal resolution. Blood-oxygen-level-dependent- (BOLD-)fMRI is one of these methods, which uses the ratio of oxyhemoglobin to deoxyhemoglobin in the local blood to represent neuronal activity in the brain (Zakiniaeiz et al., 2017). The temporal sensitivity of the physiological blood flow response largely determines the extent to which active neurons can be detected in BOLD-fMRI (Weingarten & Strauman, 2015).
Task-state fMRI is an fMRI of the brain while performing a specific task, which necessitates the use of a complex task paradigm and can reflect different activation patterns of brain regions under different tasks. The tapping finger task state fMRI results revealed reduced FC between the prefrontal cortex and parts of the cerebellum in alcoholics, implying that alcoholism is associated with dysfunction of thalamocortical cerebellar neural pathways (Dupuy & Chanraud, 2016). Another finger-tapping experiment found that AUD patients did not commandeer the anterior cerebellar network as normal during maximal self-paced tapping, but instead recruited parietal function to perform the tapping task (Parks et al., 2010). According to the studies mentioned before, alcohol-dependent patients have impaired neural pathway function and require more compensatory increases in brain areas to complete the assigned task. Applying a monetary incentive delay task to participants, some scholars found that the ventral striatum and posterior cingulate cortex in AUD patients were associated with reduced reward responsiveness, while the anterior cingulate cortex and dorsal striatum were associated with reduced punishment responsiveness (Aloi et al., 2019). This suggests that the severity of AUD patients is negatively correlated with the activity of reward processing neural circuits. A recent substance-related visual cueing trial using alcohol versus nonalcoholic beverages showed that AUD patients showed more BOLD responses in the left posterior cingulate cortex when confronted with drinking behavior (Fukushima et al., 2020); this also provides evidence that patients with AUD have different patterns of brain activity in response to different visual stimuli, which may help clinicians develop treatments for patients with AUD.
Resting-state fMRI is the focus of current fMRI research, and of the total energy consumed by the brain, resting-state energy expenditure is much higher than task-related neuronal metabolic activity (O'Connor & Zeffiro, 2019). In terms of FC, evidence suggests that alcohol-dependent individuals in withdrawal show significantly enhanced BOLD signals in reward-related anterior striatal brain regions, particularly the prefrontal cortex, ventral striatum, orbitofrontal cortex, and anterior cingulate cortex, and, conversely, BOLD responses to anticipated nondrug rewards become blunted in the ventral striatum and dorsal striatum (Nutt et al., 2021). The default mode network is a major hotspot in the study of resting-state functional brain networks, with abnormal default mode network connectivity in nonwithdrawn AUD patients, impaired posterior cingulate cortex-cerebellar connectivity, and increased connectivity with the midbrain (Fritz et al., 2022), and abnormal FC between the prefrontal, parietal, and cerebellar lobes (Liu et al., 2018). Another finding was that at the limbic level, the FC strength of the cerebellar–thalamic–striatal–cortical circuit altered in patients with AUD, suggesting a disruption in the topology of the patient's motor executive network, which may underlie AUD-related movement disorders (Zhu et al., 2018). Liu et al. used the receiver operating characteristic curve and the Pearson correlation to show that amplitude of low frequency fluctuations (ALFF) differences in specific brain regions of AUD patients have high sensitivity and specificity, and that ALFF analysis can be used as a biological indicator to detect spontaneous brain activity in alcohol-dependent patients (Liu et al., 2018). In another study related to the ALFF, Hong et al. found that the frame frontal cortex of 56 sober alcoholics and 56 healthy controls varied in frequency-dependent oscillatory power, and that low scores on psychomotor and situational memory tests were significantly correlated with abnormal frame frontal high-frequency power in alcoholics, suggesting that overactivation of the frame frontal cortex contributes to increased relapse (Hong et al., 2018).
For the heterogeneity of AUD, some scholars have conducted phenotypic analysis by the underlying motivation of individuals to drink alcohol, and it was found that remission/habitual drinkers (i.e. negative reinforcement/normalization) showed greater dorsal striatum activation to visual alcohol cues than reward drinkers (i.e. positive reinforcement), while cue-induced ventral striatum activation did not differ significantly between groups (Burnette et al., 2021). Our understanding of regional brain activation has improved thanks to the widespread use of fMRI, which will also play a part in predicting the clinical outcome of AUD medication therapy (Table 2).
Table 2: Studies about MRI for AUD.
Studies | n | State | Experimental design | Results |
Zakiniaeiz et al., 2017 | 45 AUD/30 C | Task-state | Prospective research (90 days post-discharge); imagery paradigm; alcohol, stressful, or neutral/relaxing states (1.5-min quiet baseline, 2.5-min imagery period, 1-min quiet recovery)×6. | Blunted posterior cingulate cortex during alcohol cues. |
Zakiniaeiz et al. 2017 | 30 AUD/30 C | Task-state | Imagery paradigm; alcohol, stressful, or neutral/relaxing states; (1.5-min quiet baseline, 2.5-min imagery period, 1-min quiet recovery)×6. | Reduced cingulate connectivity during alcohol and stress cues. |
Weingarten & Strauman, 2015 | 10 AUD/10 C | Task-state | Self-paced tapping stimulus and externally paced tapping tasks; 300 s. | AUD patients did not commandeer the anterior cerebellar network as normal but instead recruited parietal function to perform the tapping task. |
Aloi et al., 2019 | 109 AUD/41 C | Task-state | Monetary incentive delay task (48 reward trials, 48 punish trials, and 12 neutral trials, yielding 108 total trials). | AUD score is negatively related to activity in reward processing neuro-circuitry in adolescents. |
Fukushima et al., 2020 | 24 AUD/15 C | Task-state | Substance-related visual cueing trial (juice, drinking juice, sake, drinking sake, blurred images); 2 sessions, 120-s per session. | AUD patients showed more BOLD responses in the left posterior cingulate cortex when confronted with drinking behavior. |
Liu et al., 2018 | 29 AUD/29C | resting-state | ALFF. | Significantly elevated ALFF values in the right inferior parietal lobule and right supplementary motor area. |
Zhu et al., 2018 | 19 AUD/20C | resting-state | Graph theoretical approaches; the topological properties of the two groups are compared. | The topological architecture of the motor execution network is disrupted in AUD patients. |
Hong et al., 2018 | 56 AUD/56 C | resting-state | Frequency power quantification approach; ALFF. | Alcoholics exhibited greater frequency oscillation power in the orbitofrontal cortex and less power in the posterior insula within the HF bandwidth than controls. |
Burnette et al., 2021 | 122 RD/62 r/HD | Task-state | 720-s visual alcohol cue-reactivity task (alcoholic beverage images, nonalcoholic beverage images, blurred images). | r/HD showed greater dorsal striatum activation to visual alcohol cues than RD. |
Abdallah et al., 2021 | 18 AUD/18 C | resting-state | Sliding window approach; multilayer community detection; flexibility and integration of the cerebellum. | Significant FC variability between the cerebellum and both the frontoparietal executive control network and ventral attention network, with significantly less cerebellar flexibility and greater integration. |
EEG applications in AUD
EEG is a noninvasive test of brain activity that captures electrical impulses from the brain (Table 3). A study about EEG, event-related potentials, and event-related oscillations discovered that early fast activity associated with sensory reception occurred in the visual cortex (i.e. occipital lobe), while slower activity associated with higher cognitive function involved the parietal and frontal lobes (Porjesz & Begleiter, 2003). Decreased visual and auditory P3 amplitude commonly occurs in alcohol-dependent patients, and to a lesser extent in women than in men (Cofresí et al., 2022). It has long been shown that in addition to P3, delta oscillations, theta oscillations, and resting beta power are also abnormal in alcoholics and even in the offspring of alcoholics (Rangaswamy et al., 2004). Alcoholics exhibited higher energy in the theta to high beta bands than controls, and the magnitude and anterior–posterior range of these effects varied between bands (Fein & Allen, 2005). Meanwhile, dimensional complexity can be used as a measure of EEG complexity, with significant increases in EEG dimensional complexity values in frontal (F3, F4), right posterior temporal (T6), and occipital (O1, O2) regions after viewing alcohol cues in alcoholics (Kim et al., 2003). These regions could be targeted brain areas for future studies of alcohol craving and addiction.
Table 3: Studies about EEG for AUD.
Studies | n | State | Number of channels | Sampling rate (Hz) | Record time | Results |
Rangaswamy et al., 2004 | 171 HR/204 LR | resting-state | 19 | 256 | 4.25 min | Resting beta power is abnormal in alcoholics and even in the offspring of alcoholics |
Kim et al., 2003 | 15 AUD/10 C | Task-state | 16 | 500 | 32.678 s | Changes in EEG complexity are induced in frontal, right posterior temporal, and occipital regions when participants are exposed to alcohol cues |
Fein & Allen, 2005 | 51 TxNA/51 C | resting-state | 40 (n = 87) 64 (n = 15) | 250 | 5 min | Alcoholics exhibited higher energy in the theta to high beta bands than controls |
Mumtaz et al., 2018 | 30 AUD/30 C | resting-state | 19 | 256 | 10 min | With 513 features obtained by synchronization likelihood calculation of 19-channel EEG, synchronization likelihood features can be used as objective indicators for AUD patients and healthy controls. |
Minnerly et al., 2021 | 23 AUD/20 C | resting-state | 19 | 256 | 10 min | Data conversion and reorganization in the topographic way have an impact on EEG spectral powers |
Recently, several researchers have proposed a machine learning approach based on resting-state EEG data that uses synchronization likelihood features as objective markers for screening AUD patients and healthy controls, and this machine learning approach suggests that EEG-based computer aided design tools could be developed that could help make AUD screening an automated and standard procedure (Mumtaz et al., 2018). However, due to the lack of a deep learning architecture for extracting spatiotemporal characteristics from EEG data, Neeraj et al. presented a combination of fast Fourier transform, a convolutional neural network, long short-term memory, and an attention mechanism. This design has a 98.83% accuracy in determining whether an individual is an alcoholic or not (Neeraj et al., 2021). Meanwhile, the wavelet scattering transform together with the intentional classifier can replace the convolutional neural network with extremely high accuracy and sensitivity, where the features based on the occipital and parietal regions of wavelet scattering transform are most beneficial to distinguish alcoholic patients from normal people (Buriro et al., 2021).
The alteration of EEG differences is multifaceted, and it is recommended to improve the accuracy of observing EEG differences from a combination of multiple perspectives and techniques. It has been suggested to use machine learning and artificial intelligence to analyze EEG signals from at least five perspectives, including individual electrodes, cortical subregions, left and right hemispheres, anterior and posterior axes, and the entire cortex, to diagnose and explore the prognosis of patients with substance use disorder (Minnerly et al., 2021). The combination of artificial intelligence and EEG promises to be a powerful tool for the rapid and low-cost diagnosis of mental health in AUD patients. Although the magnetoencephalographic is used as a superior form of EEG, some researchers recommend synchronized magnetoencephalographic-EEG experiments to better meet the traceability requirements of experimental data by taking into account the detection of surface and deep sources on the one hand, and improving the spatial resolution of EEG data on the other (Hauk et al., 2022). However, due to the inherent limitations of magnetoencephalographic or EEG, its traceability results cannot be very accurate.
TMS applications and future directions
TMS is accomplished by passing a rapidly alternating current via a coil close to the scalp, which forms a magnetic field in a targeted area of the brain below and generates a current in the brain via neuronal depolarization, thus influencing metabolism and neural electrical activity in the brain. The duration of the ipsilateral silent phase and contralateral silent phase are potential indicators of central nervous system hyperexcitability. In one study, it was found that participants at high-risk of AUD had a significantly shorter contralateral silent phase (indicating diminished intracortical inhibition) and ipsilateral silent phase (indicating diminished interhemispheric, transcallosal inhibition) compared to participants at low risk for AUD (Muralidharan et al., 2008).
TMS stimulation of the dorsolateral prefrontal cortex (DLPFC) has been shown to be effective as a target of action (Table 4). A single-blind, sham-controlled randomized controlled trial of patients with AUD showed a significant reduction in alcohol craving (Mishra et al., 2010), a significant increase in days of abstinence, and a significant reduction in alcohol consumption after high-frequency rTMS (HF-rTMS) in the right DLPFC compared to sham surgery (Antonelli et al., 2021), whereas no significant differences were found in the reduction of craving and alcohol intake for the HF-rTMS intervention on left-sided DLPFC (Del Felice et al., 2016, Hoppner et al., 2011). However, at the same time, some scholars also randomly divided 20 AUD patients into two groups, one group had the left side of DLPFC stimulated and the other group had the right side stimulated, and found a significant reduction in craving after the TMS in both (Ceccanti et al., 2015, Mishra et al., 2015). This difference in results may be due to bias in single-blind trials, limitations of small sample sizes, or participants receiving treatment that interfered with the assessment of craving. For the mechanistic study of rTMS for alcoholism, a randomized, double-blind, placebo-controlled study randomized 18 alcoholics into two groups: nine in the true stimulation group, and nine in the sham stimulation group. The true stimulation group indicated that rTMS significantly decreased cortisol levels and prolactin levels, thus suggesting an increase in dopamine, while the sham stimulation group showed no significant effect (Ceccanti et al., 2015). Observations on the modulation of the dopamine system will be useful for the study of AUD. A recent small randomized trial showed the most significant efficacy of HF-rTMS in right-sided DLPFC at a 3-month follow-up (Belgers et al., 2022). It gives us a direction for future research to explore how to change the intervention protocol, such as stimulation frequency, duration of each stimulation, and stimulation interval, to induce a longer treatment effect.
Table 4: Studies about rTMS of the DLPFC for AUD.
Studies | n | Design | Number of sessions | Stimulation site | Frequency (Hz) | Percentage MT (%) | Total pulses per session | Effect |
Mishra et al., 2010 | 45 | Single-blind, sham-controlled | 2 (1 Ac and 1S) | Right DLPFC | 20 | 110 | 1000 | Significant reduction in alcohol craving. |
Hoppner et al., 2011 | 19 | Randomized, sham-controlled | 2 (1 Ac and 1S) | Left DLPFC | 20 | 90 | 1000 | No significant differences. |
Ceccanti et al., 2015 | 18 | Randomized, double-blind, placebo-controlled | 2 (1 Ac and 1S) | Medial prefrontal cortex | 20 | 120 | 1500 | Significantly reduced blood cortisol levels and decreased prolactinemia. Cravings and drinking have decreased. |
Belgers et al., 2022 | 30 | Randomized controlled, single-blind, sham-controlled | 2 (1 Ac and 1S) | Right DLPFC | 10 | 110 | 3000 | Significant reduction in alcohol craving, and differences in craving between groups were most prominent three months after treatment. |
Del Felice et al., 2016 | 17 | Randomized, sham-controlled | 2 (1 Ac and 1S) | Left DLPFC | 10 | 100 | 1000 | Improve inhibitory control task and selective attention and reduce depressive symptoms but not reduce craving and alcohol intake. |
Mishra et al., 2015 | 20 | Randomized, single-blind, parallel-group | Ten daily sessions | Left and right DLPFC | 10 | 110 | 1000 | Significant reduction in craving after the TMS in both groups. |
Simultaneous TMS-EEG and TMS-fMRI experiments are technically feasible and provide insights into brain function beyond what is possible when each method is used alone (Table 5). TMS-EEG can assess various properties of the cortex such as excitability and connectivity (Tremblay et al., 2019). Through the combination of TMS-EEG, it was more clearly discovered that postabstinence AUD patients have changed cortical function related to GABAergic neurotransmission (Kaarre et al., 2018), including decreased frontal cortex excitability and increased motor cortex excitability (Naim-Feil et al., 2016). In addition, in another TMS-EEG trial of ethanol consumption in 10 healthy participants, ethanol was found to possibly alter the FC between the prefrontal and motor cortices (Kahkonen et al., 2001). TMS-fMRI is a viable tool that can explore the potential mechanisms of TMS-mediated neuronal modulation (Mizutani-Tiebel et al., 2022). Hanlon et al. found by comparing the effects of TMS on BOLD signals before and after continuous theta-burst stimulation of the frontal pole in alcoholics that continuous theta-burst stimulation of the frontal pole significantly reduced activity in the orbitofrontal region and indirectly reduced activity in several functionally relevant nodes in the salience network, such as the anterior insula and anterior cingulate gyrus, which becomes a powerful new adjunct to addiction treatment (Hanlon et al., 2017). The combination of EEG and fMRI covers both temporal and spatial resolution, and recent technical improvements have demonstrated the feasibility of simultaneous TMS-EEG-fMRI (Janssens & Sack, 2021, Peters et al., 2020, Peters et al., 2013). A study covering four healthy right-handed volunteers also showed us that TMS-EEG-fMRI can directly monitor the relationship between oscillatory states and signal propagation in the entire cortical–subcortical network (Peters et al., 2020), opening a new avenue for studying dynamic cognitive loops and their dysfunction (Fig. 1).
Table 5: Studies on the integration of TMS, EEG, and fMRI in patients with AUD.
Studies | n | Stimulation site | TMS parameters | EEG recordings | fMRI | Main result(s) |
Kaarre et al., 2018 | 27 AUD/25 C | The motor cortex (M1) | Single pulse (90% rMT) | 64-channel sampling rate 5 kHz | None | Significant increase in GABAergic N45 amplitude in patients with AUD. |
Naim-Feil et al., 2016 | 12 AUD/14 C | Left and right DLPFC | Single/paired-pulse; biphasic pulses | 24-channel sampling rate 20 kHz | None | Inhibition of the frontal cortex and increased excitability of the motor cortex in patients with AUD after alcohol withdrawal. |
Kahkonen et al., 2001 | 10 healthy volunteers | The left motor cortex | Single pulse | 60 scalp electrodes, sampling rate 1450 Hz | None | Ethanol alters FC between the prefrontal and motor cortices. |
Hanlon et al., 2017 | 24 AUD | The left frontal pole | Single pulse (110% rMT); biphasic pulses | None | Measurement of baseline-evoked BOLD signal immediately before and after real and sham CTBS. | Real cTBS significantly reduced BOLD-evoked signals in the left orbitofrontal, insula, and lateral sensorimotor cortex. |
Peters et al., 2020 | 4 healthy volunteers | The right dorsal premotor cortex | Triple-pulse (95% rTM) | 64-channel, sampling rate 5000 Hz | Participants tapped their left index finger at a 500 Hz sine tone during the “motion execution” interval; the same auditory pacing tone was given, while the participants were instructed not to perform the corresponding finger taps. | Accurate and direct monitoring of the causal relationship between oscillatory states and signal propagation throughout the cortico-subcortical networks. |
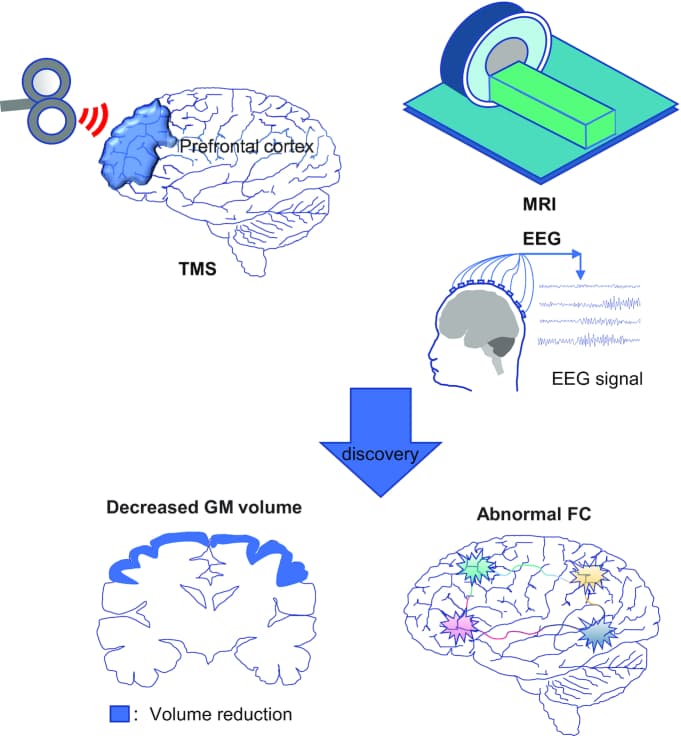
Conclusion and Future Perspectives
With the development of neuroimaging techniques, an increasing number of studies have revealed alterations in brain structure and function in alcohol dependence, providing neuroimaging evidence for early diagnosis, treatment, assessment of efficacy, and alcohol withdrawal in patients with AUD. Noninvasive brain stimulation techniques have also been used to explore the effects of DLPFC activity on cognitive processes and can focus on targeting the cortical cortex to improve cognitive function and treat related disorders (Liu et al., 2021). Among them, structural MRI allows clear visualization of morphological changes in the brain, meanwhile, fMRI and EEG are the most commonly used techniques, and both can be combined with TMS (the most commonly used noninvasive physical therapy) for efficacy evaluation and are widely used.
Current research has found that patients with alcohol dependence often have reduced GM, and the damage is most pronounced in the frontal lobes (Yang et al., 2016). Many studies have targeted the prefrontal cortex for addiction treatment, and various studies have confirmed that stimulation of this area is beneficial in reducing cravings and improving cognitive function changes due to substance dependence (Antonelli et al., 2021, Belgers et al., 2022, Mishra et al., 2010). After several weeks to months of abstinence, the corresponding brain imaging deficits were reversed (Nutt et al., 2021), affirming the importance of abstinence and the feasibility of treatment.
In future research, we would like to consider the following questions: (i) how to conduct studies with larger sample sizes to increase the likelihood of detecting small effects and improve the generalizability of findings; (ii) how machine learning techniques can improve the prediction of AUD across modalities; (iii) what additional evidence comparison of left- and right-sided DLPFC stimulation using a randomized double-blind controlled study would obtain for brain regions; (iv) how existing sham stimuli and real stimuli differ in some ways, and why the nature of sham stimuli remains a limitation of our study; and (v) how to conduct more joint TMS and neuroimaging studies to further investigate the mechanism of AUD.