Abstract
Cannabis use often co-occurs with attention-deficit/hyperactivity disorder and other internalizing and externalizing disorders. Treatment planning, including pharmacologic and psychosocial interventions, for these comorbid disorders require thorough diagnostic evaluation to determine the extent of social, emotional, and behavioral impairments, severity of substance use, and motivation for change. Improved understanding of these comorbid disorders will inform treatment planning that address current symptoms and behaviors and may also prevent the development of mental health and substance use disorders in early adulthood.
INTRODUCTION
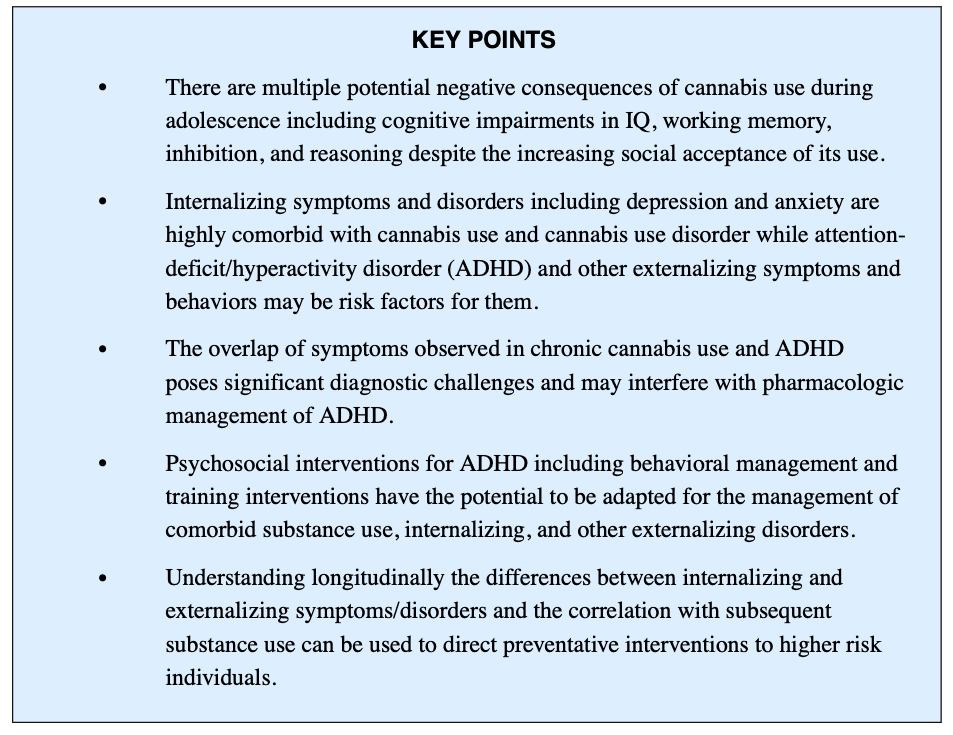
Cannabis use is a growing area of concern for pediatric mental health given the ongoing neurodevelopment throughout children and adolescents. Negative consequences of cannabis use during this vulnerable period include cognitive impairments in IQ, working memory, inhibition, and reasoning. Despite the adverse effects and long-term outcomes from cannabis use, individuals continue to seek its primary intoxicating or euphoric effects of feeling “high.” Adolescents have reported reasons for ongoing use to include euphoric effects, means of coping, and social cohesion. Additionally, some individuals who use cannabis cite temporary relief from symptoms of anxiety, depression, and pain as a motivation for continued use.
Adolescents aged 12 to 18 years who use cannabis at least twice a month for 6 months or longer are also at increased risk for poor academic performance, delinquent behavior, psychiatric problems, and arrests. Further, cannabis use is associated with poorer mental health outcomes, and comorbid mental health disorders are associated with problematic cannabis use. Individuals who use cannabis may experience more severe and persistent depression and anxiety, as well as worse cannabis withdrawal and cravings. Among individuals with major depressive disorder (MDD), cannabis use has been associated with decreased quality of life, reduced efficacy of pharmacologic treatments, and poorer daily functioning. Although the biologic cause and directionality of comorbid disorders remains unclear, it is vital to accurately diagnose and manage these co-occurring disorders.
One notable clinical challenge is the diagnosis and management of comorbid attention-deficit/hyperactivity disorder (ADHD) and cannabis use disorder (CUD). Soler Artigas and colleagues reviewed summary statistics in meta-analyses studying the relationship between ADHD and substance use and found cannabis was the most used among these youth. Problematic cannabis use and ADHD exhibit overlapping signs and symptoms and may result in similar academic and behavioral challenges. Other mental health disorders are often present in adolescents with problematic cannabis use and ADHD. Further, adolescents commonly endorsed 2 or more psychiatric syndromes when entering treatment of cannabis use. The most common internalizing disorders are depression and anxiety, with conduct disorder (CD) and oppositional defiant disorder being the most common externalizing disorders.
Due to the interconnectedness of these various disorders, a well-developed breadth of clinical interventions including both pharmacologic and psychosocial becomes essential. Although the cause of comorbid disorders, including ADHD and CUD, is not well understood, clinicians face the challenge of diagnosing and managing these co-occurring disorders. The purpose of this article is to provide a greater understanding of the insights into the complex nature of cannabis use, ADHD, and comorbid internalizing and externalizing disorders to serve as guidance for the management of an often undertreated population.
ATTENTION-DEFICIT/HYPERACTIVITY DISORDER
ADHD is common, with a prevalence of approximately 4 million among school-aged youth in the United States. Youth meeting criteria for ADHD demonstrate behavioral deficits in attention, self-regulation, and social competence and higher rates of executive functioning deficits including processing speed and working memory. ADHD is classified as a neurodevelopmental disorder, with symptoms typically present in early childhood. However, for numerous reasons (eg, access to care, adaptive functioning), many children go undiagnosed until adolescence. Further, CUD is common among youth with ADHD, with an estimated prevalence of 33% to 38%. Research has suggested that individuals with ADHD are 7.9 times more likely to use cannabis than non-ADHD peers. Both CUD and ADHD are independently associated with academic challenges, including disruptive classroom behavior, learning, and time management difficulties, often resulting in poor grades. These overlapping cognitive deficits and social-behavioral challenges highlight the clinical importance of better understanding the intersectionality of these 2 populations to inform treatment planning and interventions. Early diagnosis of ADHD before the initiation of cannabis use may also reduce potential long-term problems associated with these co-occurring disorders.
The significant symptom overlap of chronic cannabis use and ADHD poses significant diagnostic challenges. Initiation of cannabis use before the diagnosis of ADHD may result in more pronounced maladaptive effects of cannabis than non-ADHD peers. There is also interest in understanding the shared biology of ADHD and problematic cannabis use. The individual heritability factors of ADHD and cannabis use initiation have been observed to be between 70% and 80% and 40% and 48%, respectively. There are also significant overlapping features of the neurocircuitry implicated in both problematic cannabis use and ADHD, including dysfunction in frontal cortical regions responsible for executive functioning and response inhibition, and the dopaminergic reward systems. Chronic cannabis use is associated with hyper-activation of frontal cortical regions, which impairs working memory and contributes to deficits in executive functioning similar to those found in ADHD. Thus, differentiating the neurocognitive deficits of problematic cannabis use and ADHD may not be feasible or practical.
In addition, societal perception of cannabis use and its harmful effects have evolved during the past few decades. There is growing perceptions of its therapeutic use and decreased perceived harm or deviance. These changes in perception become increasingly important among youth with ADHD, who have an earlier age of onset of substance use and increased likelihood for developing a substance use disorder (SUD). Many adolescents think that cannabis improves their ADHD symptoms or remedies side effects from stimulant medications, whereas the potential risks and interactions from combining cannabis and stimulants are often not considered. Yet, those individuals with hyperactive/impulsive symptoms may be at a higher risk for cannabis misuse or dependence given a self-medication function while those with inattentive subtype of ADHD may actually experience worsening symptoms. Further, ADHD symptoms are correlated with greater severity of cannabis cravings and associated with greater risk of relapse. For clinicians, it becomes even more challenging to circumvent the societal normalization of cannabis despite a growing literature of associated poor health outcomes.
INTERNALIZING SYMPTOMS AND DISORDERS
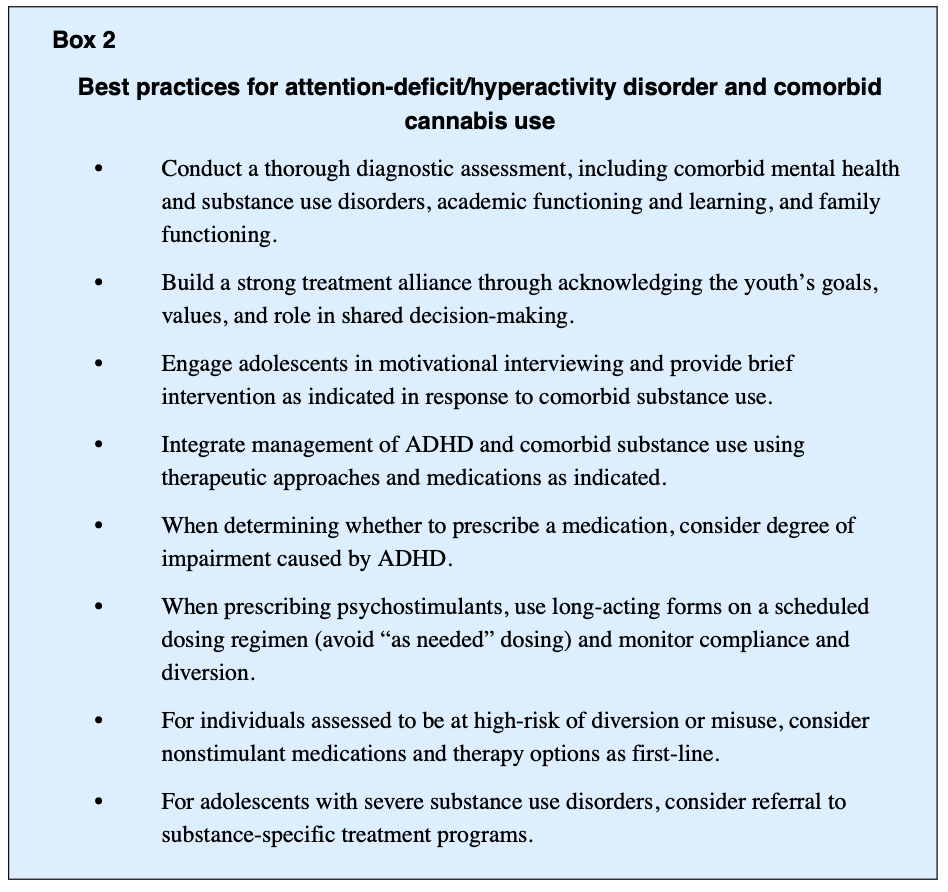
Youth with ADHD experience increased social and emotional deficits, which limits availability of coping resources and increases the risk of experiencing anxiety and depression (Box 1). Family history of depression and anxiety is further associated with increased risk of internalizing symptoms and disorders. Approximately 13% to 51% of youth with ADHD have comorbid internalizing disorders and 25% of youth with ADHD exhibit an anxiety disorder. Longitudinal studies estimate up to 50% of individuals with predominantly ADHD inattentive type have an anxiety disorder, which often goes undiagnosed. Given the high prevalence of comorbidity, understanding the developmental pathway of ADHD and anxiety is important. Overlapping symptoms in ADHD and anxiety disorders include restlessness, irritability, difficulty concentrating, and sleep disturbance. The term “anxiety” relates to worry, separation fears, and obsessive thoughts, which are not core features of ADHD. In youth with ADHD, subsequent anxiety disorders may also arise from a collection of failure experiences related to ADHD deficits that contribute to chronic fear and worry. Further, “anxious impulsivity” may present as restless behavior that is misinterpreted as hyperactivity. Considering the interrelatedness of symptomatology and high rates of comorbidity, it becomes important to screen for anxiety disorders and develop a thorough timeline of symptoms to inform treatment planning and monitor clinical progression.
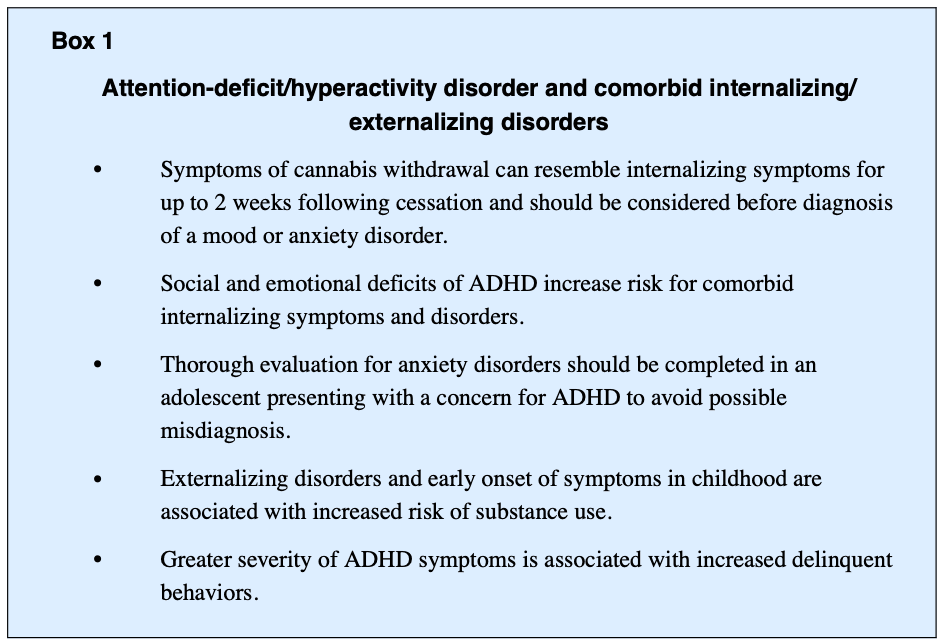
Adolescents and young adults with ADHD also frequently report significant sleep disturbances, which are not well understood. Individuals with ADHD are 3 times more likely to have circadian rhythm sleep disturbances as well as longer sleep-onset latencies. This typically manifests in a variety of ways, including shifts in sleep patterns with inability to fall asleep and difficulty staying asleep or waking up early. Poor sleep can then yield increased mood and cognitive problems, as well as fatigue and exhaustion, which may exacerbate symptoms of depression, anxiety, and ADHD.
The relationship between cannabis use and internalizing symptoms and disorders has also been a focus of research and clinical interest (see Box 1). The symptomatology of these disorders includes depression, anxiety, somatic features, traumatic distress, and suicide. Although problematic cannabis use is often associated with worsening symptoms of internalizing disorders, direction of the relationship or causality is unknown. Further, studies have shown internalizing symptoms associated with ADHD are also risk factors for problematic substance use. Internalizing disorders may also be a moderator of ADHD-related risk for developing problematic substance use and subsequent SUD and are associated with prolonged course of illness. However, the effect of internalizing symptoms on an individual’s well-being tends to be the primary reason treatment is sought among those with problematic cannabis use, rather than the substance use itself.
The directionality of relationships between cannabis use with mood disorders and anxiety disorders continues to be obscure. Studies have found rates of comorbid MDD and generalized anxiety disorder to be 3 times higher among those with CUD. Cannabis use is also associated with higher rates of lifetime MDD and suicide attempt. It is valuable to understand the discontinuation or sudden reduction of cannabis use can lead to withdrawal that may mimic internalizing symptoms and occur one to 2 days after the last use and can last up to 2 weeks if heavy use. It is not uncommon for youth to have limited resources to maintain a constant supply of cannabis and may have recurrent periods of withdrawal that can present with anxiety or nervousness, restlessness, irritability, anger, fatigue, dysphoria, depressed mood, decreased appetite, amotivation, and sleep difficulty. In fewer cases, suicidal ideation, nightmares, and strange dreams may also occur. If there are comorbid anxiety and depressive disorders, withdrawal periods may exacerbate those symptoms and complicate the diagnostic assessment.
EXTERNALIZING SYMPTOMS AND DISORDERS
There is a rather complex relationship between ADHD, CD, and SUD risk. Both ADHD and CD are independent risk factors for the development of an SUD. A common predisposing factor may be the general disinhibitory behavioral processes (ie, hyperactivity/impulsivity), which are features of both ADHD and CD. One study found youth with ADHD were also 4 to 5 times more likely to progress to heavy cigarette and cannabis use compared with youth without ADHD. Further, children with persistent and worsening delinquent behaviors were shown to be at higher risk for problematic substance use. When disruptive behaviors and high conflict occur primarily within the family or with parents, these youth were more likely to engage in heavier substance use in late adolescence when compared with youth without high levels of conflict. Understanding patterns of symptom progression and quality of parent–child relationships may provide insight into the likelihood of an SUD developing and play a direct role on the escalation of overall risk behavior.
Externalizing disorders, particularly CD and related behaviors, in individuals with ADHD have also been investigated as risk factors for substance use. A recent meta-analysis found the odds of perpetrating violence was 2.01 to 2.62 times higher among adolescents who use cannabis compared with adolescents who do not, with the highest odds among persistent heavy users (OR = 2.81, 95% CI 1.26–3.23). The cognitive effects of cannabis that may contribute to the risk of behavioral dysregulation include impaired ability to reduce aggressive impulses, anxiety or panic, feelings of paranoia, and increased arousal. Symptoms related to disruptive disorders of childhood, personality traits related to behavioral disinhibition, and traits of CD were also negatively correlated with constraint adherence and traditional moral values while positively correlated to impulsive thrill-seeking behaviors. A history of abuse and maltreatment, poor parental monitoring/involvement, affiliation with deviant peers, and weak attachment to their schools have also been associated with disruptive behaviors. Cumulative exposure to multiple risks is hypothesized to contribute to externalizing disorders in youth and subsequent problematic substance use.
TREATMENT
Pharmacologic Management
Pharmacologic management of ADHD includes stimulant and nonstimulant medications, with stimulant medications typically considered first line. A recent meta-analysis establishing evidence to support the use of extended-release methylphenidate and amphetamine formulations, atomoxetine, and extended-release guanfacine for the treatment of noncomorbid ADHD in adolescents. Although treatment of ADHD improves academic, economic, and social outcomes, less is known about the efficacy of pharmacologic management of ADHD among youth with comorbid CUD. Treatment providers might insist the youth be abstinent before treating comorbid mental health conditions. They may also be hesitant to use particularly psychostimulant medications in these youth. Because cannabis is also associated with executive dysfunction, including impairments of attention and working memory, it is unclear if ADHD medications will be effective if the youth is still using cannabis. There may also be concerns about misuse and diversion of psychostimulants by adolescents with CUD, although few studies may begin to address these clinical concerns.
Stimulant medications
To date, 2 randomized controlled trials (RCTs) have investigated long-acting formulations of methylphenidate (MPH-SODAS and OROS-MPH) in adolescents with ADHD and SUDs. In a randomized, placebo-controlled crossover study design, Szobot and colleagues evaluated MPH-SODAS in adolescents with ADHD and comorbid cannabis use (N = 16, 100% male), 43.8% of whom also used cocaine.Participants had greater reductions in ADHD symptoms when taking MPH compared with placebo, although there was no significant change in substance use. Riggs and colleagues subsequently completed a multisite RCT of osmotic-release methylphenidate (OROS-MPH) combined with cognitive behavioral therapy (CBT) in adolescents with ADHD and comorbid SUD (N = 303, 79% male, 66.7% cannabis dependence, and 29.7% alcohol dependence). Adolescents in both the OROS-MPH and placebo groups showed clinically significant improvements in ADHD and substance use, although no significant between-group differences were found. Although the effectiveness of OROS-MPH versus placebo was unclear, parents reported significantly lower ADHD symptoms in adolescents in the OROS-MPH group, and these adolescents showed significant improvements in problem-solving ability and acquisition of focused coping skills compared with the placebo group. Overall, OROS-MPH was relatively well tolerated. Adolescents who use cannabis more frequently (>40% of days) were more likely to report side effects from OROS-MPH, although no specific side effect and no serious adverse drug events were associated with cannabis use. Of note, a recent study by Skoglund and colleagues found individuals with ADHD and comorbid SUD typically require higher doses of methylphenidate compared with individuals without SUD and postulated that lack of efficacy may be due to inadequate dosing.
To investigate the risk of misuse and diversion of OROS-MPH among adolescents with ADHD and comorbid SUD, Winhusen and colleagues analyzed these outcomes by baseline substance use severity. Cannabis and tobacco were the most frequently used substances (11/28 days and 24/28 days, respectively). There were higher subjective ratings of effectiveness of the medication (P<.001) and feeling high (P<.01) but not in craving the medication (P = .18) or craving substances (P = .06) among youth who received OROS-MPH. However, neither cannabis use nor substance use severity was significantly associated with misuse or diversion. Further, studies have shown that pharmacologic treatment of ADHD does not seem to increase the risk for developing a SUD. Treatment of ADHD and comorbid substance use may also reduce the risk of alcohol and substance use ADHD and may prevent the development or progression of SUD in adolescence and young adulthood. In adults with ADHD, an RCT of extended-release mixed amphetamine salts found a significant decrease in the proportion of participants using cannabis over time, compared with placebo. Thus, research suggests that treatment with a stimulant medication serves as a protective factor for substance use. Nonetheless, stimulant medications do have potential to be misused or abused, with a risk of diversion, particularly in unstructured or unsupervised settings, which may be mitigated with parental involvement and close clinical follow-up.
Nonstimulant medications
Three nonstimulant medications have been investigated in small studies of adolescents with ADHD and comorbid substance use: atomoxetine, bupropion, and guanfacine. Thurstone and colleagues completed a single-site RCT of atomoxetine versus placebo, combined with motivational interviewing and CBT in both groups for adolescents with ADHD and comorbid SUD. Both treatment groups demonstrated improvements in ADHD scores and the number of days nonnicotine substances were used, with no significant between-group differences.
In 2 small studies of bupropion, Riggs and colleagues (N = 13, 100% male) and Solhkhah and colleagues (N = 14, 64% male) found adolescents with comorbid substance use tolerated bupropion well, with high medication compliance (85% and 90%, respectively). In both studies, there was significant reduction in clinician-rated ADHD symptoms from baseline. The study by Riggs and colleagues was conducted in a residential substance use program, where all adolescents received substance use programming; no substance use outcomes were reported. In the study by Solhkhah and colleagues, 57% of participants received some form of counseling or psychotherapy, with significant reductions in substance abuse at the 6-month endpoint compared with baseline.
Although guanfacine has shown promising results in a 6-day preclinical trial, its efficacy as a treatment option for adolescents with ADHD and comorbid cannabis use remains unknown.
Psychosocial Interventions
To date, no psychosocial interventions have been studied specifically for adolescents with ADHD and comorbid CUD. However, several psychosocial interventions for ADHD show potential to be adapted for the management of comorbid SUD, including CUD. Psychosocial interventions for ADHD are broadly categorized as behavioral management or training interventions. Behavioral management often uses selective reinforcement to enhance desired behaviors and selective ignoring to decrease problematic behaviors. Conversely, training interventions are skills-based programs that can be implemented in a variety of settings, including clinic and school. Training interventions may also incorporate family components to optimize the home environment and provide behavioral management.
Family-focused psychoeducation models have been proven to be helpful for youths with ADHD by increasing medication and behavioral treatment effects and adherence. Peer programs further provide an opportunity for youth with ADHD to improve social skills, promote social competence, and reduce the risk of peer rejection. These models have shown a direct positive effect on ADHD symptoms and overall prosocial functioning.
Considering the clinical trials by Riggs and colleagues and Thurstone and colleagues, integration of therapy targeting substance use may also be effective for the management of substance use and comorbid mental health disorders, including ADHD. In these studies, the authors postulated the lack of between-group differences between atomoxetine or OROS-MPH and placebo may be due to the impact of the background CBT treatment received by both groups. Although not specific to ADHD, such therapeutic interventions, including motivational enhancement therapy (MET) combined with CBT (MET/CBT) and the Adolescent-Community Reinforcement Approach, may be more broadly applicable in adolescent mental health treatment programs.
SUMMARY
Concern about cannabis use among youth has become more prevalent during the past decades as access and social acceptance of it have increased. Problematic substance use rarely presents on its own in adolescence and frequently co-occurs with ADHD, as well as other internalizing and externalizing disorders. Individually these disorders can be challenging to treat, and comorbid ADHD and CUD are associated with a greater symptom burden and treatment complexity. Approaching treatment planning with a biopsychosocial perspective can allow for a better understanding of the interconnectedness of these disorders. Although limited, evidence to date posits the standard of care should be comanagement of these disorders to improve outcomes and maximize the likelihood of adequate management or remission of both disorders. Consideration of studies of pharmacologic and psychosocial interventions in adolescents with ADHD and CUD support an integrated approach for managing these comorbid disorders (Box 2).