Abstract
Late adolescence and emerging adulthood (specifically ages 15–24) represent a period of heightened sexual risk taking resulting in the greatest annual rates of sexually transmitted infections and unplanned pregnancies in the US population. Ongoing efforts to prevent such negative consequences are likely to benefit from a deepening of our understanding of biological mechanisms through which sexual risk taking emerges and biases decision making during this critical window. Here we present a neuroscience framework from which a mechanistic examination of sexual risk taking can be advanced. Specifically, we adapt the neurodevelopmental triadic model, which outlines how motivated behavior is governed by three systems: approach, avoidance, and regulation, to sexual decision making and subsequent risk behavior. We further propose a testable hypothesis of the triadic model, wherein relatively decreased threat-related amygdala reactivity and increased reward-related ventral striatum reactivity leads to sexual risk taking, which is particularly exaggerated during adolescence and young adulthood when there is an overexpression of dopaminergic neurons coupled with immature top-down prefrontal cortex regulation. We conclude by discussing how future research based on our adapted triadic model can inform ongoing efforts to improve intervention and prevention efforts.
Introduction
Even though 15- to 24-year-olds make up only 25% of the sexually active population, they account for 50% of all new cases of sexually transmitted infections (STIs) and have the highest rates of unintended pregnancies (CDC, 2012; Eaton et al., 2008; Guttmacher Institute, 2014). These negative health behaviors are likely a result of the low rate of condom use and the high number of new sexual partners among this age group (Gavin et al., 2009; Johnston, O’Malley, Bachman, & Schulenberg, 2010). Young people between the ages of 15 and 24 are experiencing significant brain development resulting in incentive-motivated behavior to assure exposure to unfamiliar contexts to promote learning for future behavior. With repeated experiences in certain contexts, adolescents and young adults are prepared to better value risk and reward to make safer decisions (Luciana & Collins, 2012). In many ways, then, these neurobiological changes are crucial for healthy development, and more often than not, do not result in negative health outcomes (Sercombe, 2014). In particular, recent research considering a “sex-positive framework” for adolescent sexuality underscores how consensual sexual activities in adolescence is not only developmentally normative but also associated with many positive psychosocial outcomes, including pleasure, intimacy, competence, and general well-being (Harden, 2014). However, specific to sexual risk behavior, even while most adolescents and emerging adults are capable of mature decision making, including being able to precontemplate and prepare for sexual encounters (Reece et al., 2010), many are unable to translate these rationale forethoughts into action “in the moment” that would lead to abstinence or proper condom use (Reyna & Farley, 2006). These specific, emotionally salient “heat of the moment” situations occur when cognitive processes interact with emotional and physiologic drives that can bias decisions (Blakemore & Robbins, 2012; Casey, Getz, & Galvan, 2008; Casey, Jones, & Hare, 2008), especially during sexual decision making (Ariely & Loewenstein, 2006; Bancroft et al., 2004). In this way, risk taking may occur when decisions are not necessarily impulsive or unplanned (Willoughby, Tavernier, Hamza, Adachi, & Good, 2014). For instance, young adults may consciously engage in sexual behavior with the awareness that there are potential negative consequences such as romantic partner rejection, STIs, unplanned pregnancies, and potential social reputation concerns. We define risk taking in this review then as engaging in behavior with potential rewarding outcomes, but also with significant potential negative consequences (Padmanabhan & Luna, 2014).
While multiple studies suggest that emotionally charged or reinforcing contexts (e.g., social and sexual interactions) can modulate cognitive control abilities, only very recently have researchers started to investigate the multiple dynamics involved in “heat of the moment” sexual decision making and exclusively with behavioral only tasks with young adult samples (Abbey, Saenz, & Buck, 2005; Ariely & Lowenstein, 2006; George et al., 2009; MacDonald, MacDonald, Zanna, & Fong, 2000; Prause, Staley, & Finn, 2011). The preponderance of research on sexual risk behavior has utilized psychosocial models, targeting key personality (e.g., sensation seeking), social (e.g., peer and family influence, partner norms, and relationship status), and motivation/intention factors (e.g., self-regulatory goals and self-control) to understand risk behavior (e.g., Aalsma et al., 2013; Deckman & DeWall, 2011; Noar, Zimmerman, Palmgreen, Lustria, & Horosewski, 2006). While there are important strengths in utilizing such models, sexual decision making involves not only social and cognitive factors but also biological components, including brain function and physiologic arousal.
Here, we wish to extend an empirically validated neurodevelopmental model, the triadic model, to better understand the propensity for heightened sexual risk behavior, often resulting from decisions made under “emotionally charged” situations, during the uniquely high window of vulnerability represented by adolescence and emerging adulthood. To do this, we will review and integrate evidence from the rich literature on three related constructs: threat sensitivity, reward sensitivity, and behavioral control. Threat sensitivity reflects individual differences in the neural circuits supporting the experience of heightened motivation and negative arousal leading to avoidance of potentially threatening or dangerous stimuli. In contrast, reward sensitivity captures variability in neural circuits supporting the experience of heightened motivation and positive arousal in the service of seeking rewards (Casey, Jones, & Somerville, 2011; Galvan, 2013). Finally, variability in behavioral or cognitive control is associated with neural circuits supporting the ability to suppress inappropriate, often reflexive, actions in favor of those that are goal directed (Casey, Galvan, & Hare, 2005; Casey, Thomas, Davidson, Kunz, & Franzen, 2002).
To support the integration of these three constructs in the service of better understanding and predicting sexual risk behavior in adolescence and emerging adulthood, we will first introduce the triadic model, a neural systems model wherein heightened sensation-seeking behavior in adolescence is postulated to result from an imbalance between reward sensitivity through the ventral striatum (VS) and threat sensitivity through the amygdala emerging through inadequate “top-down” behavioral control and goal-directed planning through an immature prefrontal cortex (PFC; Ernst & Fudge, 2009; Ernst, Pine, & Hardin, 2006). Next, we will outline how neuroscience research with adolescents and emerging adults reveals patterns in risk behavior that are consistent with the triadic model (i.e., imbalance between the VS, amygdala, and PFC). Given the recent critiques and suggestions regarding the triadic model (see Crone & Dahl, 2012; Luciana & Segalowitz, 2014), we will provide evidence for an extension of the triadic model to include a more nuanced understanding of neural development to include cognitive, affective, and social processing. Specifically, we outline considerations for physiological (sexual) arousal by reviewing research on the relationship between neural circuit function and sexual risk behavior as it fits within the framework of the triadic model (Stoleru, Fonteille, Cornelius, Joyal, & Moulier, 2012). We will also extend Ernst’s original model to include not only the imbalance of frontal and subcortical neural development leading to heightened risk behavior but also considerations for the role of dopaminergic contributions to subcortical regions (e.g., Luciana & Collins, 2012; Padmanabhan & Luna, 2014), as well as hormonal and social–contextual changes (Blakemore, Burnett, & Dahl, 2010; Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; Crone & Dahl, 2012; Peper & Dahl, 2013; Steinberg, 2008). Finally, in Ernst’s original framing of the triadic model, risk behavior was posited to result from approach-related drives from the VS; however, we will explore data from our lab, along with others (e.g., Spielberg, Olino, Forbes, & Dahl, 2014), to suggest not only that heightened approach-related VS drives coupled with decreased avoidance-related amygdala drives lead to risk behavior but also that decreased approach-related drives and increased sensitivity to negative consequences can result in increased risk-taking behaviors.
Only one neuroimaging study to date (Goldenberg, Telzer, Lieberman, Fuligni, & Galvan, 2013) has included an adolescent sample, so the majority of the studies discussed will include emerging adult samples. In addition, given the very few studies that explicitly address the relationship between neural circuit function and sexual risk behavior, we augment this approach by reviewing how changes in brain development supporting threat sensitivity, reward sensitivity, and behavioral control broadly may impact sexual decision making and risk behavior specifically. We further consider evidence that supports how sexual decision making involves uniquely powerful emotional and physiologic drives that may further accelerate subcortical (i.e., VS and amygdala) drives, which overwhelm the limited capacity for behavioral control through an immature PFC, ultimately resulting in significant risk for negative sexual health decisions. This unique arousal component to sexual risk behavior, we postulate, creates an even greater imbalance in the neural nodes of the triadic model, compared to other types of risk behavior occurring during adolescence and emerging adulthood (e.g., drug and alcohol use, monetary risks).
Triadic Model of Adolescent Risk Behavior
In the last decade, remarkable research has been conducted in the field of developmental neuroscience to provide a richer understanding of brain function and development during adolescence and emerging adulthood (cf. Romer, 2010). Most notable is the protracted maturation of the PFC in which, around age 11, the PFC begins a period of prolonged pruning of neuronal connections (Giedd, 2004; Paus et al., 1999; Sowell et al., 2004). This pruning helps to sculpt information processing within neural circuits in response to changing environmental contexts, resulting in increased speed of communication (Giedd et al., 1999; Sowell, Thompson, Tessner, & Toga, 2001). In contrast to the PFC, multiple cross-sectional and longitudinal studies support an earlier (Casey, Thomas, et al., 2002; Luna & Sweeney, 2001) and curvilinear development of subcortical brain areas, including the VS and amygdala, with a peak in activity during adolescence (Casey, Getz, et al., 2008; Ernst & Fudge, 2009; Ernst et al., 2006; Somerville & Casey, 2010; Somerville, Jones, & Casey, 2010; Steinberg, 2008).
While there are other neurobiological approaches to why adolescence and emerging adulthood serves as a critical developmental period of heightened risk behavior (cf. dual systems model; see Casey, Getz, et al., 2008; Somerville & Casey, 2010; Somerville et al., 2010; for a critique of the approach, see Pfeifer & Allen, 2012), the triadic model delineates specific roles for the amygdala and VS that are particularly useful for understanding the emergence of risk (Ernst & Fudge, 2009; Ernst et al., 2006). More specifically, the triadic model outlines a cortical “cognitive regulatory system” that modulates, through top-down influences, the activity of a subcortical “emotional/motivational system,” which is further separated into two modules: a positive (approach) and negative (avoidance) module (Ernst et al., 2006), with different qualitative and quantitative patterns of functioning (Richards, Plate, & Ernst, 2013). The approach module includes the VS, which largely functions to facilitate reward learning and express approach-related behaviors (for reviews, see Kringelbach, 2005; Wise, 2004). The avoidance module includes brain regions that have been shown to consistently respond to emotionally charged stimuli, especially the amygdala, and facilitate threat learning and stress responsiveness (for reviews, see LeDoux, 2000; Phelps, 2006). Finally, the control module includes PFC subregions implicated in “top-down” behavioral control, including higher cognitive abilities associated with decision making and goal-directed planning (Casey, Tottenham, & Fossella, 2002), as well as inhibition of inappropriate thoughts or behaviors (Chikazoe, Konishi, Asari, Jimura, & Miyashita, 2007) and conflict detection and monitoring (Carter & van Veen, 2007).
Because the motivational and emotional subcortical connections develop earlier than do connections supporting prefrontal control and self-regulation, the triadic model underscores the importance of imbalance between threat sensitivity and reward sensitivity subsequent to poor top-down regulation in the emergence of heightened risk behavior (Ernst & Fudge, 2009). This imbalance reflects not only greater VS-related appetitive drives related to positive outcome expectancies but also decreased amygdala-related response to danger or threat through reduced harm avoidance behavior (Ernst et al., 2005). Through the lens of the triadic model, risky decision making occurs through neural coding of potential options based on somatosensory and autonomic signals integrated through the amygdala and VS. Therefore, depending on the valence and context in which decisions are made, adolescent and emerging adult responses may be biased more toward the amygdala or VS (for a review, see Ernst & Paulus, 2005). In other words, the triadic model proposes that the neural imbalance between the VS and amygdala associated with weak PFC control manifests as generally increased risk behaviors and immature “self-regulatory competence” (Steinberg, 2004).
Research conducted with 18- to 22-year-old university students provides further support for the importance of separating the VS and amygdala when mapping the neural basis of risk-related behaviors. For instance, Nikolova and Hariri (2012) found that higher reward-related VS reactivity resulted in higher levels of problem drinking in emerging adults, but only if subjects also had lower threat-related amygdala reactivity. They have recently extended this work in a larger sample to demonstrate that the opposite pattern of low VS reactivity and high amygdala reactivity also predicts problem drinking (Nikolova, Mihic, & Hariri, 2013). It is hypothesized that the balance between these core neural phenotypes is critical for normal behavioral responses and that an imbalance in either direction contributes to risky decision making, possibly including sexual risk behavior. Consistent with this pattern, we have found that among individuals reporting low impulsivity, the intrinsic (i.e., in the absence of specific tasks or stimulation) activity of cortical structures, including the PFC, are highly correlated with the intrinsic activity of subcortical regions, including the amygdala and VS (Davis et al., 2013). In contrast, intrinsic activity of cortical control regions is less correlated with subcortical drive regions in individuals exhibiting high impulsivity (Davis et al., 2013). The relative decrease in the correlated intrinsic activity between cortical and subcortical regions suggests that cognitive control over affective drives may be more effortful in highly impulsive emerging adults. That is, it may be more difficult for highly impulsive individuals to engage synchronized activity across these brain regions in response to stimulation.
In summary, the triadic model supports a relationship during adolescence and emerging adulthood wherein immature PFC regulation of avoidance and approach drives could result in an imbalance, such that reward-related drives are preferred and increased risk behavior can occur. We hypothesize that this relationship is further modulated by particularly strong subcortical drives, such as heightened physiologic arousal to sexual cues, that could lead to sexual risk behavior, especially in adolescents and emerging adults with immature cognitive and self-regulatory skills (see Figure 1, in the online-only color version, the purple line represents social and motivational contexts, such as sexually appetitive cues).
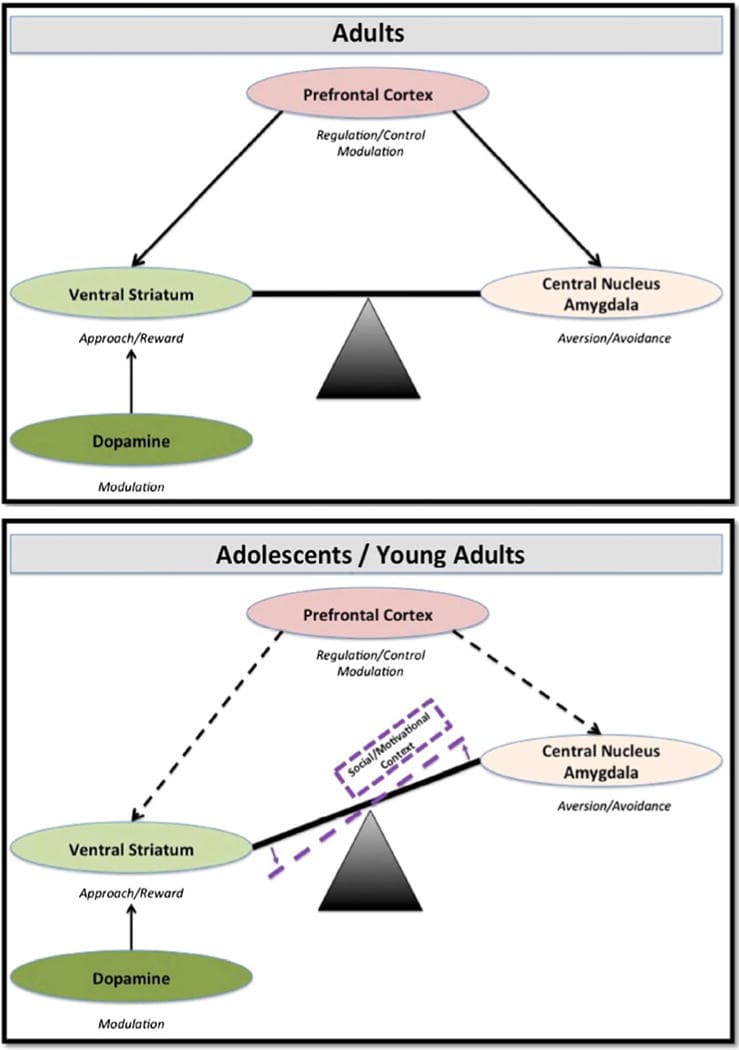
Extending the Triadic Model: The Role of the Amygdala
One limitation of the triadic model as originally proposed is that it does not reflect that the amygdala is both structurally and functionally heterogenous with multiple subregions participating in the generation of both approach and avoidance behaviors (Whalen & Phelps, 2009). Although well beyond the scope of this paper, the basolateral complex of the amygdala (BLA) serves as a sensory gateway to not only the central nucleus of the amygdala (CeA), which mediates reflexive and autonomic responses to threat including avoidance, but also the VS, which as reviewed above, supports reward learning and approach behaviors. Thus, increased reactivity of the BLA, particularly to positive stimuli such as sexual images is not at all inconsistent with the broader model of approach–avoidance balance. Neither then is increased reactivity of the CeA, particularly to negative stimuli such as threat-related facial expressions. To further underscore the diverse role of the amygdala, Morrison and Salzman (2010; see also Belova, Paton, & Salzman, 2008), posit that neurons in the amygdala encode “state value,” including valence inputs from an array of internal and external sources (e.g., context specific, as well as individual specific, such as hunger cues). We therefore propose within our adapted triadic model, a further specification that increased threat-related reactivity of the amygdala, particularly the CeA, should contribute to decreased sexual risk behaviors (see Figure 1), while increased reward-related reactivity of the amygdala, particularly the BLA, should contribute to increased risk. In addition to such stimulus- and context-specific contributions of increased BLA and CeA reactivity to sexual risk behavior, increased reactivity of a third subregion, the medial nucleus, contributes directly to reproductive behaviors and coincides with pubertal maturation (Perlman, Webster, Kleinman, & Weickert, 2004; Roselli, Klosterman, & Resko, 2001). Unfortunately, measurement of such subregional specificity of amygdala development and function, while critical for understanding the emergence of risk behavior, has not been generally adopted in the research on sexual risk behavior. It is our hope that in better delineating the subregional specificity of the amygdala within the triadic model, that future researchers will attempt to measure the relative activation of the BLA and CeA (and possibly medial nucleus) in paradigms assessing risk behavior in the context of highly arousing stimuli such as sexual images.
We now review evidence specific to each of the three nodes of the triadic model as well as their interactions, with an eye toward studies of particular relevance for understanding sexual risk behavior.
Evidence supporting the role of the PFC in risky decision making and behavior
Neuroimaging studies utilizing a variety of self-control paradigms (including go-no/go, Stroop, flanker, and antisaccade tasks) suggest that the slower development of the PFC compared to subcortical regions often results in a greater inability to inhibit prepotent responses (e.g., Adleman, 2002; Casey et al., 1997; Durston et al., 2006; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Somerville, Hare, & Casey, 2011). However, some studies have found evidence of age-related decreases in frontal cortical activity in adolescents compared to children and adults (Geier, Garver, Terwilliger, & Luna, 2009; Libertus, Brannon, & Pelphrey, 2009), while others have reported that the PFC was engaged to the same extent in participants of different age groups depending on experimental conditions (Cohen et al., 2010; Crone, Zanolie, Van Leijenhorst, Westenberg, & Rombouts, 2008; van den Bos, Guroglu, van den Bulk, Rombouts, & Crone, 2009; van Duijvenvoorde, Zanolie, Rombouts, Raijmakers, & Crone, 2008; Velanova, Wheeler, & Luna, 2008), suggesting that one hypothesis for the unstable nature of the PFC is that cognitive control processes in adolescence are strongly influenced by motivational salience of context (e.g., factors such as task instructions, presence of peers, and appraisal of the value of task performance) or individual factors.
For instance, Cservenka, Herting, Mackiewicz, Hudson, and Nagel (2013) found that adolescents scoring high on trait sensation seeking showed significant differences in PFC activity when comparing reward receipt versus reward absence, such that high sensation seekers showed a hyporesponsive pattern to reward absence. The authors suggest that this decreased PFC activity in high sensation-seeking adolescents could reflect deficits in attention to negative feedback during goal-directed behavior, which could have critical implications for sexual risk behavior.
Neuroimaging studies further suggest increased functional connectivity between the PFC and VS mediates the ability to exert control and inhibit responses (e.g., Christakou, Brammer, & Rubia, 2011; Durston et al., 2006; Fair et al., 2009; Hwang, Velanova, & Luna, 2010; Liston, Matalan, Hare, Davidson, & Casey, 2006; Somerville et al., 2011). Given that older adolescents report often engaging in health risk behavior in the presence of peers (Steinberg et al., 2009), socially relevant environmental stimuli may serve to further increase an adolescent or young adult’s approach behaviors, especially when friendship and romantic relationship salience are heightened (see reviews by Albert, Chein, & Steinberg, 2013; Blakemore, 2008; Pfeifer & Allen, 2012; Romer, 2010). Multiple neuroimaging studies have examined the relationship between adolescent risk taking under peer influence providing some initial evidence that peer presence may bias adolescents and young adults toward negative risk behavior (Cascio et al., 2014; Chein et al., 2011; Falk et al., 2014; Galvan, Hare, Voss, Glover, & Casey, 2007; Peake, Dishion, Stormshak, Moore, & Pfeifer, 2013; Pfeifer et al., 2011; Rodrigo, Padron, de Vega, & Ferstl, 2014; see review by Albert et al., 2013; for exception see Segalowitz et al., 2012).
For instance, Chein et al. (2011) found that adolescents took more risks in an incentive-based simulated driving task in the social (presence of peers) than in the nonsocial context compared to adults. The degree of risks (e.g., percentage of risky decisions and number of crashes) was positively correlated with VS and orbitofrontal cortex activity in adolescents, but only when the adolescents were aware that their friends were watching them (Chein et al., 2011; note that Rodrigo et al., 2014, found no relationship between the VS and risk behavior in the presence of a peer). In contrast, adults showed no differences in activation of these brain regions as a function of social context; instead, adults showed stronger recruitment of the lateral PFC during the task regardless of social context (Chein et al., 2011). Cascio et al. (2014) found more recently that individual differences among late adolescents in response inhibition brain regions (right inferior frontal gyrus and basal ganglia) during a go/no-go task were associated with moderating the effects of risky simulated driving in the presence of a cautious peer 1 week later. Increased activity in these cognitive control regions was not associated with risk taking in the presence of a risky peer. These findings suggest an important role for social context in the relationship between risky behavior and individual differences in neural function; for instance, when making decisions about risk behaviors, young adults may often be faced with how to weigh the impact of their decision on their peers’ perception of them (e.g., their reputation, such as being admired, rejected, etc.).
Evidence supporting the VS in risky decision making and behavior
Neuroimaging studies across development have observed an inverted U-shaped curve in VS activity associated with reward, such that adolescents show hyperresponsivity compared to both children and adults (cf. Christakou et al., 2011; Ernst et al., 2005; Eshel, Nelson, Blair, Pine, & Ernst, 2007; Galvan et al., 2006; Geier et al., 2009, 2010; Padmanabhan, Geier, Ordaz, Teslovich, & Luna, 2011; Somerville et al., 2011; Van Leijenhorst, Moor, et al., 2010; Van Leijenhorst, Zanolie, et al., 2010). However, discrepant findings have also emerged, where adults showed greater activation than adolescents in the striatum during reward expectation or anticipation (Bjork et al., 2004; Bjork, Smith, Chen, & Hommer, 2010).
The relative increase in VS activity during adolescence is also positively correlated with increases in reported trait sensation seeking (Nelson et al., 2002; Zuckerman, 1994). For example, adolescents show a temporally extended reward response in the VS relative to adults (for review of findings see Fareri, Martin, & Delgado, 2008) and an exaggerated VS response (positively correlated with subjective happiness) when winning large rewards (Ernst et al., 2005).
Taking into account the role of emotionally salient cues, Somerville et al. (2011) found that adolescents showed a nonlinear pattern of VS activity in a version of a go/no-go task involving emotional faces. Specifically, adolescents showed linear improvement in impulse control with age to neutral faces, but showed a nonlinear reduction in impulse control with age to happy faces. PFC recruitment showed a linear increase with age for all trials and correlated with overall task accuracy. The PFC was engaged to a greater degree in individuals who had the most difficulty accomplishing response suppression (i.e., children). Functional connectivity findings supported a ventral frontostriatal circuit in task performance, including the VS, such that adolescents, relative to children and adults, exhibited greater between-subjects VS coactivation for appetitive (happy) versus neutral cues. Somerville et al. (2011) point out that connectivity, especially between the PFC and VS, may be one mechanism through which teens can engage these regions to effectively suppress approach (e.g., potentially risky) behaviors.
In another emotionally charged task, Figner, Mackinlay, Wilkening, and Weber (2009) found that adolescents performed worse on a card-sorting task (Columbia card task) compared to adults under conditions in which they were receiving immediate feedback (“hot” conditions) on their selections, versus no feedback (“cold” condition). The tendency to play increasingly from the disadvantageous decks of cards followed an inverted U shape, peaking in middle to late adolescence. The author’s postulated that this behavior reflected an adolescent bias toward potentially rewarding approach behavior, even when the behavior may have negative consequences. In contrast to performance under emotionally salient or hot conditions, performance in cold condition tasks evidenced no age-related differences. This research underscores the potential ways in which contextual factors may moderate behavioral and brain connections.
Finally, Galvan et al. (2007) found, across youth ages 12–24, that individual differences in the likelihood of engaging in future risky behaviors (e.g., heavy drinking, aggressive and illegal behaviors, irresponsible academic/work behaviors) was positively correlated with VS activity in anticipation of reward during a delayed-response two-choice task. In addition, individual differences in risk assessment was related to both VS activity and the likelihood of engaging in risky behavior, such that individuals who expected a negative consequence to result from a risky behavior showed diminished VS activity in anticipation of reward and were less likely to engage in risky behavior in the future (outside the scanner). These individual differences in risk assessment highlight the importance of considering malleable attitudes and psychosocial traits when examining brain–behavior relationships related to reward sensitivity and real-world risk taking.
Evidence supporting the amygdala in risky decision making and behavior
Adolescents show significantly greater amygdala reactivity to facial expressions of negative emotions (e.g., fear, sadness, or disgust), as well as general negative cues (such as omission of a large monetary reward), relative to adults and children (e.g., Ernst et al., 2005; Guyer et al., 2008; Hare et al., 2008; Killgore, Oki, & Yurgelun-Todd, 2011; Monk et al., 2003; Pfeifer et al., 2011; Williams et al., 2006). Moreover, Hare et al. (2008) found that amygdala–PFC functional connectivity mediated adolescent’s ability to exert control in the face of emotional cues during a go/no-go task, such that poorer performing adolescents exhibited greater amygdala reactivity and decreased PFC recruitment during the tasks.
Specific to risk taking, Ernst et al. (2005) found that during reward omission in a “wheel of fortune” task, the amygdala was significantly more reactive in adults compared to adolescents, whereas the VS tended to be more active in adolescents compared to adults. One interpretation of this finding is that the adult’s heightened amygdala reactivity to negative feedback in a risk-taking paradigm may be protective, whereas the adolescent’s heightened VS response may result in further approach behavior toward risky and potentially dangerous outcomes. This is consistent with the pattern observed by Nikolova et al. (2013) predicting problem drinking in university students.
Spielberg et al. (2014) recently found that in a sample of 11- to 12-year-old girls and 12- to 13-year-old boys, pubertal increases in testosterone over 2 years of early adolescence predicted increased activation in the amygdala and the VS to threatening faces. Moreover, the researchers found that increased threat reactivity over time in the amygdala was associated with decreased trait anxiety and increased trait sensation seeking only in adolescents who also showed increased VS reactivity to threat. The authors postulated that these seemingly paradoxical findings support the notion that adolescence involves a maturational shift toward more complex processing of threatening cues, which may contribute to increased risk-taking behaviors (e.g., experiencing potentially threatening situations as rewarding). Such research may be particularly pertinent for understanding sexual risk behavior, because threatening cues (e.g., not knowing one’s partner’s risk status or not having a condom available) may be experienced as novel and thrilling during adolescence and emerging adulthood.
Evidence linking the triadic model and real-world risk behavior
One major criticism of neuroimaging findings is the lack of external validity (cf. Berkman & Falk, 2013; Bjork, Lynne-Landsman, Sirocco, & Boyce, 2012). However, in the few studies that have collected measures of individual real-world behavior, brain activation patterns do map onto real-world individual differences in adolescent and emerging adult health behaviors, including stealing and binge drinking (Berkman & Falk, 2013), smoking (Berkman, Falk, & Lieberman, 2011; Chua et al., 2011), sexual risk behavior (Demos, Heatherton, & Kelley, 2012; Goldenberg et al., 2013), gambling (Chambers & Potenza, 2003), and self-reported likelihood of engaging in other current and future risky health behaviors (Galvan et al., 2007). For instance, as mentioned previously, Nikolova et al. (2013) found that low VS activity and high amygdala reactivity are associated with future problem drinking behaviors. In addition, Galvan et al. (2006) found that the magnitude of adolescent VS activity was positively associated with degree of self-reported risk taking; VS activity has also been associated with estimates of future risk-taking behavior (Chein et al., 2011). In contrast to the VS, Eshel et al. (2007) found that the degree of PFC activity during risky decision-making functional magnetic resonance imaging (fMRI) tasks was positively correlated with less risk taking in both adults and adolescents. Finally, studies of high-risk populations (i.e., individuals with a positive family history of alcohol dependence) suggest that impairments in PFC-related functioning are present before drug use (Monti et al., 2005; Pulido, Brown, Cummins, Paulus, & Tapert, 2010) and predict later substance abuse (Deckel & Hesselbrock, 1996; Tarter et al., 2003).
Extending the Triadic Model: The Role of Dopamine (DA)
While the triadic model largely focuses on the imbalance between prefrontal and subcortical brain areas in facilitating adolescent propensity for risk-taking behavior, other approaches (e.g., Chambers, Taylor, & Potenza, 2003; Luciana & Collins, 2012; Padmanabhan & Luna, 2014) suggest that dopaminergic contributions to incentive motivation should be considered, if not equally emphasized, in driving adolescent behavior. In this manuscript, we have extended the triadic model to include the role of DA (see Figure 1). Although it is unknown whether and to what extent age-related behavioral changes could be accounted for by changes in neurochemistry (due largely to difficulties in measuring chemical substrates using noninvasive techniques), evidence from behavioral neuroimaging and especially neurogenetic studies underscores the potential role the neurotransmitter DA plays in adolescent risk behavior (for reviews, see Ernst, Romeo, & Anderson, 2009; Luciana, Wahlstrom, Porter, & Collins, 2012; Wahlstrom, Collins, White, & Luciana, 2010; Wise, 2004).
DA functions within and across limbic, striatal, and frontal circuitry and is largely involved in the promotion of incentive-guided behavior and regulation through the mesocorticolimbic system (Depue & Collins, 1999). Of particular relevance for the triadic model are DA projections originating in the midbrain ventral tegmental area and terminating in the nucleus accumbens and PFC (Bjorklund & Dunnett, 2007). The role of DA in appetitive behavior has largely been understood as resulting from increases in mesolimbic/striatal DA activity that increase an individuals approach toward incentive-motivated behaviors, while also impacting the individual’s ability to learn from positive and negative feedback experiences in the context of reinforcement-based learning (see reviews by Holroyd & Coles, 2002; Schultz, Dayan, & Montague, 1997). While DA also contributes to the modulation of amygdala function (Rosenkranz & Grace, 2001), the mapping out of this modulatory effect onto risk-related behaviors is poorly understood, and in contrast to dopaminergic modulation of appetitive behaviors through the striatum and PFC, the developmental variation in this effect is not well studied. Thus, we focus further consideration of dopaminergic modulation on appetitive behaviors through the striatum and PFC.
Overlapping, but functionally segregated, frontal–striatal circuits function through excitatory projections from the PFC to the striatum and back via the thalamus, resulting in direct and indirect DA transmission pathways (Di Martino et al., 2008; Postuma & Dagher, 2006). Dopaminergic neuromodulation occurs through both pathways by either disinhibiting the thalamus (direct pathway involving excitatory D1 receptors toward favored behaviors) or inhibiting the thalamus (indirect pathway involving inhibiting D2 receptors to decrease undesirable behaviors). During adolescence, there are peaks in DA tissue concentrations (Andersen, Dumont, & Teicher, 1997), alterations in DA transporter density (Coulter, Happe, & Murrin, 1996; Moll et al., 2000), and changes in D1 and D2 receptors in the striatum and PFC (Andersen, Thompson, Krenzel, & Teicher, 2002; Seeman et al., 1987; Tarazi, Tomasini, & Baldessarini, 1998), leading to an overall excitatory effect on the brain and an increase in DA dependent behaviors. While studies of DA activity and DA concentrations in the human cortex are unavailable, animal and postmortem human literature underscore how adolescence and young adulthood may be a particularly vulnerable time because there is an overexpression of receptors for DA (Lidow & Rakic, 1992), an increase in the density of interneurons (Lewis, 1997), and an increase in levels of GABA (Hedner et al., 1984), all of which serve to alter the excitatory–inhibitory balance of neuronal signaling that lead to more refined cognitive control (Padmanabhan & Luna, 2014). In addition, while increases in prefrontal DA concentrations and dopaminergic innervation in the PFC increase during adolescence (Benes, Taylor, & Cuningham, 2000; Rosenberg & Lewis, 1995), DA concentrations in the striatum either decrease with age or undergo no developmental changes (cf. Haycock et al., 2003). While these developmental changes may seem counterintuitive to risk-seeking behavior, evidence suggests that DA transmission has a small window of optimal functioning, wherein both excessive and deficient levels of DA impair performance (Cools, Sheridan, Jacobs, & D’Esposito, 2007; Kimberg, D’Esposito, & Farah, 1997).
Luciana and Collins (2012) hypothesized that increases in tonic DA during adolescence, which is largely independent of environmental triggers and driven by genetic regulatory factors, leads to weak or inconsistent learning signal detection (Robinson, Zitzman, Smith, & Spear, 2001). Simply put, during adolescence and young adulthood, immature function of frontostriatal circuits coupled with increases in tonic DA could result in competition between the two DA pathways and therefore suboptimal decision making, especially among adolescents with higher DA receptor availability at baseline (Padmanabhan & Luna, 2014).
Striatal DA neurons are mainly involved in reinforcement learning by responding to primary rewards, coding reward prediction in response to cues that signal reward delivery and providing cues to reward prediction fails (see in depth reviews of DA function and development and implications for adolescent behavior: Luciana et al., 2012; O’Donnell, 2010; Spear, 2000; Wahlstrom et al., 2010). Wahlstrom et al. (2010) proposes that while theoretical accounts of DA functioning assume that the D1 “go” state activity will be linked to appropriate behavior, little research has considered how DA activity involves potentiation of a neural input or output selection when an individual perceives stimuli as salient, despite the context being inappropriate. This scenario underscores what might be occurring during adolescence, when strong reward signals from striatal/limbic DA interact with undersynchronized (due to immature pruning) “no-go” PFC (Wahlstrom et al., 2010); this may be occurring even more frequently for adolescents and young adults when contexts contain both positive and negative cues, such as within sexual risk contexts.
One aspect of DA neurotransmission that is important for understanding individual differences in adolescent behavior is that biologically based differences impact functional DA activity within a given brain region at any time. For instance, while maturational changes in DA function have not been directly mapped onto adolescent decision making or behaviors, genetic predispositions for higher levels of DA in neural synapses have been associated with increased levels of brain activation in response to rewards in neuroimaging tasks (Dreher, Kohn, Kolachana, Weinberger, & Berman, 2009; see also review by Hariri, 2009). In addition, levels of DA have been linked to variability in related behavioral phenotypes (aggression in Eley, Lichtenstein, & Moffitt, 2003; disruptive behavior disorders in Lee et al., 2007; novelty seeking in Zald et al., 2008; for a review, see Nemoda, Szekely, & Sasvari-Szekely, 2011).
We propose that future empirical research consider not only the role of immature PFC regulation over subcortical regions in driving adolescent risk behavior but also how and to what extent DA signaling influences or is influenced by the differences in maturation of cortical and subcortical systems. Understanding the nature of individual differences in DA (e.g., tonic levels, receptor densities, clearance, and degradation rates) may prove especially important in gaining a deeper appreciation for adolescent health risk behavior (Luciana et al., 2012; Padmanabhan & Luna, 2014).
Studies Specific to Brain Function and Sexual Behavior
In the last 15 years, more extensive research has been conducted on the relationship between brain function and adult sexual arousal. This research has shed important light not only on the potential etiology of sexual disorders and mechanisms of orgasm but also on the brain’s response to erotic material, or how the anticipation of a sexual encounter (e.g., rewarding stimuli), may impact decision making, mood, and behavior (for an extensive meta-analysis and review, see Stoleru et al., 2012). Sexual arousal is defined as the physical (i.e., genital response) and psychological (i.e., sexual desire) readiness to perform a sexual behavior (Rosen & Beck, 1988). Sexual arousal includes the pleasure one feels during the state of arousal (i.e., liking), as well as the anticipated desire for more stimulation and other potential interpersonal rewards (i.e., wanting; Berridge, 1996). Adolescence has been cited as the most critical phase in sexual development, as individuals begin to learn to associate stimuli such as bodily features, personality, and contextual cues with genitally induced sexual pleasure (Georgiadis, Kringelbach, & Pfaus, 2012; Pfaus et al., 2012).
Because of ethical considerations, little is known about the role of physiologic sexual arousal on brain function and subsequent sexual risk behavior or decision making among adolescents under the age of 18. However, some cross-sectional and experimental research with adolescents and emerging adults underscores the important role of sexual arousal in impeding self-regulation, potentially resulting in increased risk behavior (e.g., Abbey, Saenz, & Buck, 2005; Ariely & Lowenstein, 2006; George et al., 2009; Janssen, Goodrich, Petrocelli, & Bancroft, 2009; Lindgren, Shoda, & George, 2007; MacDonald et al., 2000; Prause et al., 2011). For instance, experimental studies found that heterosexual men reported lower STI risk perception after exposure to sexually appealing women (Blanton & Garrard, 1997) and lower reported likelihood of using a condom after self-reported increased sexual arousal (Ariely & Loewenstein, 2006). To date, only three studies (see Table 1) have directly investigated the role of brain circuitry in sexual decision making or risk behavior in adolescents or emerging adults. We next describe how these studies support involvement of the PFC, VS, and amygdala in sexual risk behavior, and further consider evidence from other fMRI studies involving exposure to sexually explicit video clips in emerging adults.
Evidence supporting the PFC in risky sexual behavior
While some adult cross-sectional self-report data suggests that difficulties in impulse control are associated with risky sexual behavior (e.g., Clift, Wilkins, & Davidson, 1993; Pinkerton & Abramson, 1995), very few behavioral or fMRI studies have been conducted to examine the relationship among impulse control, sexual arousal, and decision making. Macapagal, Janssen, Fridberg, Finn, and Heiman (2011) found that more impulsive emerging adults committed significantly more errors (e.g., failed to inhibit a response) compared to less impulsive subjects in a go/no-go task involving the presence of sexual stimuli. More specifically, more impulsive subjects had greater difficulty inhibiting a button press for sexual stimuli especially after viewing sexually arousing videos.
In the first of the studies summarized in Table 1, Rupp et al. (2009) conducted the only fMRI study to date in which subjects were making hypothetical sexual decisions in the scanner (i.e., indicating the extent to which they were willing to have sex with the person presented in a photo). This study did not explicitly ask subjects their motivations for their willingness to engage in sex with a potential partner (e.g., sexual attraction, potential for relationship), making it difficult to tease out the various reasons driving the riskiness of the female subjects’ decisions. Rupp et al. (2009) found that emerging adult heterosexual women had stronger activation in the anterior cingulate cortex (ACC), a PFC region involved in conflict monitoring and top-down regulatory control (Carter & van Veen, 2007), when making sexual decisions about low-risk men versus high-risk men. These findings suggest that greater effortful control may be necessary to offset risky sexual decision making in women. Furthermore, activation in the ACC was positively related to women’s subjective ratings of their likelihood of having sex with high-risk men.
Prevost, Pessiglione, Metereau, Clery-Melin, and Dreher (2010) extended these findings using a delay and effort-discounting paradigm, involving passive delay periods and real physical effort using a hand grip as sources of delay and effort for viewing erotic pictures. The authors found that distinct valuation subsystems for different types of reward costs were reflected in brain function, such that greater PFC activity was associated with greater effort and delay required for longer viewing of an erotic image. In the second study summarized in Table 1, Goldenberg et al. (2013) found that sexually riskier adolescents, based on self-reported contraception use at last sexual encounter, showed less activation in the PFC during response inhibition in a standard go/no-go task. These studies provide initial support for the importance of the PFC node of the triadic model in sexual decision making and risk behavior, such that adolescents and emerging adults appear to engage the PFC to a greater extent in decisions presenting potentially greater sexual risk. In addition, youth reporting greater sexual risk behavior in their personal lives show PFC engagement when trying to suppress impulses in cognitive control tasks.
Evidence supporting the VS in risky sexual behavior
Imaging studies provide evidence that just showing emerging adults physically attractive photos (Aharon et al., 2001; Cloutier, Heatherton, Whalen, & Kelly, 2008) or sexually explicit images or video clips (Hamann, Herman, Nolan, & Wallen, 2004; Karama et al., 2002) activates the VS and amygdala. Costumero et al. (2013) recently found that trait reward sensitivity (similar to trait sensation seeking) correlated positively with VS reactivity to sexually explicit pictures in a sample of emerging adult heterosexual males. The authors postulated that these results reflect the hypothesis that individuals who are more sensitive to rewarding cues (like erotic stimuli) may attribute greater reward value to the stimuli and have increased motivation to pursue sexual behaviors. In the final study summarized in Table 1, Demos et al. (2012) found VS reactivity to sexual images specifically correlated positively with increases in sexual activity 6 months later and individual scores of sexual desire. More specifically, greater VS reactivity at baseline correlated with an increase in number of sexual partners 6 months later.
Evidence supporting the amygdala in risky sexual behavior
Stoleru et al. (2012) conducted a meta-analysis of 21 studies including over 200 emerging adult males to find that the amygdala is particularly reactive during exposure to sexually explicit material and subsequent self-reported sexual arousal. Subsequent to this meta-analysis, Sescousse, Caldu, Segura, and Dreher (2013) conducted another meta-analysis and review of human functional neuroimaging studies examining how erotic rewards reflect similar, yet unique, functional brain activations to other primary and secondary rewards, including food and monetary rewards. Across 87 studies (26 of which included erotic material) including 1,452 subjects, brain responses to monetary, erotic, and food reward outcomes significantly engaged a common brain network, including the PFC, VS, and amygdala. Compared to food and monetary rewards, the amygdala responded exclusively to erotic pictures and videos. Sescousse et al. (2013) postulated that the erotic reward differences likely reflect the extent to which these stimuli are affectively laden reinforcers (i.e., greatly impacting amygdala response). In one of the first studies using sexual images in the scanner, Beauregard, Levesque, and Bourgouin (2001) found that emerging adult heterosexual males showed increased amygdala reactivity during passive viewing of sexual images. They also found heightened recruitment of the PFC when participants were asked to specifically inhibit arousal after exposure to these sexual images, a pattern consistent with top-down executive control of the PFC over amygdala reactivity (Ochsner & Gross, 2005). Unfortunately, the neuroimaging data to date has reported specific nuclei of the amygdala in their results to determine to what extent the amygdala-related responses are driven from the BLA or CeA.
The above results in general and the specific results in Table 1 collectively lend support for the utility of the triadic model in further understanding sexual risk behavior in adolescents and emerging adults. Given the relative dearth of imaging studies relating brain function to real-world sexual decision making and behavior, there is clearly a need for further research examining the brain mechanisms through which sexual decisions are made and how brain activation to sexual cues influences subsequent real-world sexual behavior. A reasonable next step in this research would be to explore the extent to which individual differences in neural cue reactivity, specifically associated with reward motivation to sexual cues, relates to actual sexual behavioral outcomes (i.e., proclivity to sexual promiscuity).
Future Directions: A Brain-Based Phenotype for Sexual Risk Behavior
Farris, Akers, Downs, and Forbes (2013) call for translational research that integrates neuroscience, ecological systems theory, and decision science with adolescent sexual behavior. The authors argue that interventions in sexual health need to account for the salience of social rewards, reward-driven decision making, and sensitivity to peer or social contexts (points that have been well established in the neurodevelopmental specialty area of adolescent risk taking). Georgiadis et al. (2012) point out that “sexual incentive motivation built on genital reward will lead to new avenues of human sexual brain research, including the investigation of novel paradigms that investigate how the brain mediates sexual learning” (p. 496). Finally, Berkman and Falk (2013) argue that the “brain-as-predictor” approach, wherein brain measures of activation, structure, and connectivity are used as independent variables in models that predict longitudinal behavioral outcomes as dependent variables, “broaden our ability to test theory and facilitate the translation of basic neuroscience results” (p. 46).
Against this background, we encourage research explicitly examining how a combined neural phenotype of relatively high VS reactivity to reward and low amygdala (specifically CeA) reactivity to threat maps onto sexual risk behavior, especially in combination with high trait-level sensation seeking and low trait-level self-control. If these patterns are observed, the findings would suggest important, yet complex, interactions among arousal, personality, and brain response to both threat and reward. Brain and behavioral data collected from such studies could then be analyzed along with actual sexual behavior changes over time to determine their relationship. A focus on the relative contribution of these processes in adolescents and emerging adults, who have immature top-down PFC cognitive and behavioral control (Somerville & Casey, 2010; Somerville et al., 2010), may be particularly important for understanding risk behavior as bottom-up drives from the amygdala and VS that exert greater bias on information processing in the absence of “effective” PFC functioning (Heatherton & Wagner, 2011). Of particular importance in our application of sexual decision making and risk behavior to the triadic model is that sexual risk is a unique health behavior that involves even greater emotional arousal to environmental stimuli and interoceptive physiologic cues, biasing the VS to reward-seeking behaviors in the absence of mature PFC control (see purple line online in Figure 1).
Subsequently, we propose that future research should address variability in the relative engagement of these three brain regions (i.e., VS, amygdala, and PFC) to map individual differences in sexual decisions and risk behavior. Brain-based investigations of “real-time” sexual decision making in emerging adult men and women could then inform differential strategies for reducing risky decision making that is unique to each individual (e.g., decreasing relatively high limbic drive versus increasing relatively low PFC regulation). Given the many cognitive, hormonal, emotional, and physical changes that adolescents and young adults experience, which likely bias rational decision making, an important next step in advancing prevention and intervention efforts for sexual risk behavior may be to leverage key findings in developmental neuroscience (Suleiman & Brindis, 2014). Imaging research supports the potential protective role of increased striatal response during reward-related preparation for inhibition in adolescents compared to adults and children (Geier et al., 2010; Hardin et al., 2009). Therefore, prevention programs could capitalize on inhibitory control reinforcement efforts that focus on upregulation of the immature PFC inhibitory regions to facilitate safer health choices (Eldreth, Hardin, Pavletic, & Ernst, 2013). For example, the Good Behavior Game, a universal school-based intervention, which teaches children to inhibit impulses and regulate emotions to obtain rewards, serves as an example of how a self-regulatory skills-based program could help to reduce aggressive and off-task behaviors, as well as high-risk behaviors, like substance abuse (e.g., Kellam et al., 2008; Poduska et al., 2008).
Ultimately, the extent to which a relationship exists between brain function and sexual risk behavior remains unknown; however, it is likely that current sexual health intervention and prevention efforts will have a limited chance of success without better understanding the complex interaction of neural development and sexual decisions made within the context of highly affective and spontaneous states (Suleiman & Brindis, 2014). Suleiman and Brindis have begun to outline how previous developmental affective neuroscience research could inform sex education, based largely on adolescent risk-related neuroscience concepts that have not specifically been investigated in the context of sexual risk behavior (see Suleiman & Brindis for examples of potential sex education innovations integrating neuroscience concepts). However, if a clearer relationship between brain function and risky sexual decision making can be established, it may prove fruitful in testing and creating more innovative and individually tailored sexual health efforts. Crone and Dahl (2012) wrote, “Progress in identifying the neurodevelopmental underpinnings of [differences in motivational priorities] are relevant to understanding the development of healthy versions of inspired passions as well as vulnerabilities for developing unhealthy versions” (p. 647). This statement highlights the gap in our current understanding of adolescent and young adult motivations to engage in or refrain from health risk behaviors and underscores the potential for the use of neurobiological markers to better understand these crucial individual differences in development.
Limitations
Foremost, due to the limited number of studies examining the links between brain function and sexual risk behavior, it is not possible to draw generalizations from this literature. However, we believe that these studies support overarching neurobiological models of adolescent and emerging adult brain development, namely, the triadic model, that lead to increased health risks in some individuals during this developmental period of life. Our overall assessment of empirical research is that there are sufficient grounds to continue research in this area, and it is our hope that this paper will stimulate further research by providing readers with suggestions for further study.
While this paper focuses on neural factors, we recognize and acknowledge the importance of environmental (situational), individual psychosocial trait level, physiologic, pubertal, and genetic factors on sexual decision making and risk behavior in adolescence and emerging adulthood. In particular, prior sexual experiences, socioemotional influences, and environmental/social context (for reviews, see Fischhoff, 2008; Kotchick, Shaffer, Forehand, & Miller, 2001) have been shown to greatly impact adolescent and emerging adult sexual risk behavior. Self-report data supports how peer norms regarding sexual behavior impacts individual sexual behavior over time (e.g., Coley, Lombardi, Lynch, Mahalik, & Sims, 2013; Huebner, Neilands, Rebchook, & Kregels, 2011; Romer et al., 1994; Sieving, Eisenberg, Pettingell, & Skay, 2006). In addition, future research may also consider the relationship context (e.g., nature and quality of adolescent/young adult and his/her partner) in which sexual decisions are made because this variable is likely an important moderator of brain to behavior outcomes. Previous cross-sectional research has shown that “hooking up” (sexual relationships outside of committed romantic relationships) is associated with increased depressive symptoms (Mendle, Ferrero, Moore, & Harden, 2013) and longitudinally associated with delinquent behavior (Harden & Mendle, 2011), but sex within a committed relationship is not associated with delinquency, substance use, or poorer academic achievement (McCarthy & Casey, 2008; McCarthy & Grodsky, 2011). Future research is needed to ascertain how these various social environments and relationship contexts may moderate the associations between adolescent and emerging adult brain development and risk behavior (Willoughby et al., 2014; Willoughby, Good, Adachi, Hamza, & Tavernier, 2013). Such research might be able to better address the gaps in our current understanding of risk behavior within certain groups of young people. For instance, social environments and contexts may explain why despite brain and psychosocial trait-level rationale for adolescents being at heightened risk for health-compromising behaviors, college students, whose risk for these behaviors should be low, report higher levels of health risk behaviors on average than teens or emerging adults not enrolled in college (Willoughby et al., 2013). In the same vein, underlying mechanisms driving variability in brain circuit function (e.g., increased serotonin signaling predicting increased amygdala reactivity) should be further examined as potential moderators in regional brain activation and trait-like behavior relationships (Hariri, 2009).
While it is beyond the scope of this paper, the role of pubertal hormones on brain developmental and function is likely intimately tied to individual differences in sexual risk behavior and should also be further investigated in future research on the role of neural function in sexual risk (for a more detailed review, see Sisk & Zehr, 2005; see also the reviews by Blakemore et al., 2010; Crone & Dahl, 2012; Eisenegger, Haushofer, & Fehr, 2010; Peper & Dahl, 2013). During puberty there is a significant increase in gonadal hormones, leading to sexual maturation (Spear, 2000), which may sensitize neural circuits to hormone activation allowing for the development of sexual behaviors (Romeo, Wagner, Jansen, Diedrich, & Sisk, 2002; Sisk & Zehr, 2005; Steinberg, 2008). More specifically, Scherf, Behrmann, and Dahl (2012) reported that secondary sex characteristics and sexual dimorphisms affect modulation of limbic circuitry, particularly the amygdala, such that adolescents are able to master new developmental tasks, including forming deeper friendships and romantic relationships.
Pubertal maturation, commonly associated with increases in sensation seeking (Galvan et al., 2007), may play a critical role in PFC recruitment during decision making. For example, Forbes et al. (2010) found decreased VS and increased PFC activity in response to reward outcome in adolescents with more advanced pubertal maturation compared to their same-aged peers with less advanced pubertal maturation. Vermeersch, T’Sjoen, Kaufman, and Vincke (2008a, 2008b, 2009) found that acute increases in gonadal hormones in adolescent boys and girls was positively correlated with greater affiliation with risk-taking peers and higher social dominance. Wood (2004) posited that androgens have reinforcing effects that increase the salience of rewarding stimuli, which has been demonstrated in naturally elevated androgen levels in adolescents and young adults (Forbes et al., 2010; Op de Macks et al. 2011; Stanton, Liening, & Schultheiss, 2011), as well as artificial testosterone administration (van Honk et al., 2004). One interesting, but understudied, area of hormonal investigation with human subjects focuses on the role of the oxytocin–vasopressin system to social-bonding motivation and behavior (Peper & Dahl, 2013; see reviews by Carter, 2003; Gordon, Martin, Felman, & Lechman, 2011). Given the social and emotional changes occurring during adolescence and young adulthood, particularly in the realm of early sexual and romantic relationships, the role of oxytocin may prove particularly promising as a hormonal biomarker for sexual risk behavior. Further research should help clarify whether and to what extent onset and changes across pubertal development impact cognitive and affective neural pathways, which are likely intimately tied to sexual behavior and decision making.
In more broadly thinking about pubertal development and changes on sexual risk behavior, research should also consider how gender differences from these biological and other psychosocial factors impact differences in sexual behavior between young men and women. For instance, experimental studies show that men are willing to discount higher future monetary rewards in favor of smaller immediate monetary rewards (Wilson & Daly, 2004), wait longer, exchange more money, and expend more effort than women to look at attractive faces of the opposite sex (Hayden, Parikh, Deaner, & Platt, 2007), compared to women. These findings support evolutionary perspectives that when selecting sexual partners, men value attractiveness more so than women (facial attractiveness is believed to indicate genetic and reproductive fitness; cf. Fink & Penton-Voak, 2002; Li, Bailey, Kenrick, & Linsenmeier, 2002; Rhodes, 2006; Sprecher, Sullivan, & Hatfield, 1994). Across both genders, Gunther Moor, van Leijenhorst, Rombouts, Crone, and van der Molen (2010) found that social rejection in an fMRI task was associated with activation of the insula and dorsal ACC across children, adolescents, and adults; however, only adults showed additional recruitment of the dorsolateral PFC, likely supporting a stronger capacity to regulate social rejection. Unfortunately, only one neuroimaging study to date has investigated gender differences in social decision-making tasks (Rodrigo et al., 2014); they found no gender differences behavioral decision making in the task (similar to other laboratory studies on individual decision making in nonsocial contexts; see Galvan et al., 2007; Gardner & Steinberg, 2005; Van Leijenhorst, Moor, et al., 2010), but did find that young adult women elicited more activation in the right insula and superior temporal gyrus than young men in the risky decision conditions, suggesting greater emotional engagement in anticipation of potential aversive outcomes (Clark et al., 2008). Future neuroimaging studies should consider how gender differences in sexual arousal and social drives (e.g., social acceptance and avoiding social rejection) may interact with or moderate the role of neural function on sexual risk behavior.
Finally, we need to extend studies to include more ethnically and racially diverse populations, especially in the realm of sexual risk behavior where African Americans between ages 18 and 26 are at a significantly higher risk for contracting HIV compared to White Americans (CDC, 2012). We need to observe the extent to which neurobiological factors vary as a function of not only race but also gender. For instance, males show higher trait-level sensation seeking compared to females (Zuckerman & Kuhlman, 2000), yet small sample sizes limit our ability to properly tease out how personality factors may mediate gender differences in brain function and behavior. Across the few fMRI studies exploring gender differences to sexually explicit material, Stoleru et al.’s (2012) meta-analysis found that visual sexual stimuli activated the amygdala and the thalami to a greater extent in men than in women. Longitudinal studies should also be extended to better determine how developmental shifts in brain pathways mediate individual differences in behavior over time, using within-subjects designs that provide more statistical power than cross-sectional designs.
Conclusion
Although sexual risk behavior is common among adolescents and emerging adults, such risk may be more highly expressed in individuals with relative imbalance between reward-related VS reactivity and threat-related amygdala reactivity coupled with immature PFC capacity for behavioral control. With the recent increase in studies demonstrating that measures of neural circuit function can predict health behavior outcomes (e.g., drug and alcohol use) over time, it is our hope that the approach presented in this review can be used to further reveal important connections between brain function in laboratory contexts and longer-term, ecologically valid sexual health behaviors and outcomes (Berkman & Falk, 2013). The demonstration of such predictive links can then better inform ongoing efforts to prevent the negative consequences of sexual risk behavior during this developmental window of heightened vulnerability.