Abstract
The capacity to anticipate and detect rewarding outcomes is fundamental for the development of adaptive decision‐making and goal‐oriented behavior. Delineating the neural correlates of different stages of reward processing is imperative for understanding the neurobiological mechanism underlying alcohol use disorder (AUD). To examine the neural correlates of monetary anticipation and outcome in AUD patients, we performed two separate voxel‐wise meta‐analyses of functional neuroimaging studies, including 12 studies investigating reward anticipation and 7 studies investigating reward outcome using the monetary incentive delay task. During the anticipation stage, AUD patients displayed decreased activation in response to monetary cues in mesocortical‐limbic circuits and sensory areas, including the ventral striatum (VS), insula, hippocampus, inferior occipital gyrus, supramarginal gyrus, lingual gyrus and fusiform gyrus. During the outcome stage, AUD patients exhibited reduced activation in the dorsal striatum, VS and insula, and increased activation in the orbital frontal cortex and medial temporal area. Our findings suggest that different activation patterns are associated with nondrug rewards during different reward processing stages, potentially reflecting a changed sensitivity to monetary reward in AUD.
1. INTRODUCTION
Alcohol use disorder (AUD) is a common and potentially lethal disorder (Becker et al., 2017). According to a recent systematic analysis of a global burden of disease study from 1990 to 2016, AUD has become the most common drug use disorder worldwide, with an estimated 100.4 million cases and 2.8 million deaths attributed to alcohol use (Degenhardt et al., 2018). The Diagnostic and Statistical Manual of Mental Disorders‐5 (DSM‐5) has created significant changes in the definition of AUD, including merging the abuse and dependence criteria into a single diagnosis of AUD, removing the legal criterion, and adding an item to assess craving (Beseler et al., 2012; Kerridge et al., 2013). As a core feature of craving, the strong desire to consume is a conditioned response to cues that have been learned to be associated with alcohol use. Alcohol‐related cues can reliably predict rewards and hijack dopamine reward pathways through classical conditioning learning to ultimately evoke alcohol craving in individuals with AUD (Cofresi et al., 2019; Wrase et al., 2007; Xie et al., 2022). A recent meta‐analysis of the neuroimaging literature concerning alcohol cue activity task performance by our group indicated that AUD patients showed an increased brain response in the reward system during alcohol‐related cue exposure but reduced activation after alcohol treatment compared to controls (Zeng et al., 2021), suggesting that alcohol dependence may induce excessive sensitivity to alcohol‐associated incentive salience. However, a large body of literature has also reported that addiction risk is characterized by dysfunctional incentive motivational neurocircuitry, even by nondrug‐related incentive cues or deliveries (Balodis & Potenza, 2015; Hommer et al., 2011; Luijten et al., 2017). Thus, it is interesting to study the neural bases of motivated approach behaviors driven by nondrug‐related reinforcers.
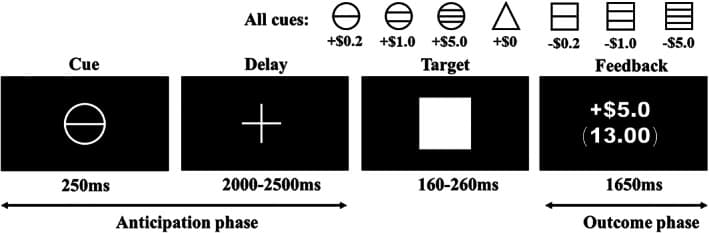
Monetary reinforcement is nearly universally valued and can be learned by association, which provides experimental flexibility (Knutson & Wimmer, 2007). The monetary incentive delay (MID) task is a widely used and validated reward processing task for investigating motivational salience processes in response to monetary stimuli in healthy individuals and those with mental disorders (Beck et al., 2009; Becker et al., 2017; Bjork, Smith, & Hommer, 2008; Knutson et al., 2000; Wrase et al., 2007). Importantly, the MID task allows reward processing to be parsed into at least two distinct components, namely, “anticipation” and “outcome” (Knutson et al., 2001; Knutson et al., 2007; Wilson et al., 2018). During the MID task, the anticipation stage is defined as the period when the subject prepares to make a motor response to stimuli to obtain potential monetary rewards or avoid losses (Yau et al., 2012), whereas the outcome stage occurs when rewards are delivered (Bjork, Smith, & Hommer, 2008). In the MID task published by Knutson and colleagues (Knutson et al., 2000; Knutson et al., 2001), the subjects were presented with incentive cues (cue, 250 ms) of seven possible values (gain of $0.2, $1.0, or $5.0; loss of $0.2, $1.0, or $5.0; or no change $0). Then, the individuals fixated on a cross‐hair as they waited a variable interval (delay, 2000–2500 ms). Next, a target appeared for a variable length of time (target, 160–260 ms) during which the subjects made a button‐press response in an attempt to gain or avoid losing money. During the subsequent outcome phase, the participants received performance feedback (outcome, 1650 ms). A schematic of the MID paradigm is presented in Figure 1. This task requires a relatively low cognitive demand and simple learning content, which minimizes cognitive confounds (Balodis et al., 2015). Moreover, this task has the advantage of assessing and explaining the interaction between valence and the temporal phase by modeling in the MID task (Oldham et al., 2018). Importantly, this design disentangles the motivational from hedonic aspects of reward in addicted populations; on a neurobiological level, this task demonstrates different striatal and limbic region recruitments during reward anticipation and consumption (Balodis et al., 2015; Beck et al., 2009). Given that alcohol dependence is often associated with impulsivity and an altered sensitivity to rewarding outcomes and situations, mapping the neural correlates of different stages of reward processing in alcohol dependence is important.
However, whether patients with AUD have different or overlapping monetary‐related brain activity dysfunction during the anticipation and consumption domains remains unclear. Considerable evidence suggests that the ventral striatum (VS), as a central node in the reward system, is less activated by nondrug reward cues (Beck et al., 2009; Hagele et al., 2015; Wrase et al., 2007). Furthermore, these studies show that a reduced VS response to monetary cues is significantly correlated with the severity of alcohol craving (Wrase et al., 2007) and impulsivity (Beck et al., 2009). These findings support the notion that there is diminished motivation to pursue nondrug rewards and a weakened strength of inhibitory cognitive control in alcohol‐dependent patients. However, some studies have also reported that alcohol‐dependent participants displayed hyperactivation in the VS (Becker et al., 2017; Grodin et al., 2016) or dorsal striatum (DS) (Romanczuk‐Seiferth et al., 2015) compared with controls during reward anticipation. In contrast, regarding reward outcome, the findings were more inconclusive. Brain activation in response to monetary reward receipt varied across brain regions, including the anterior cingulate cortex (ACC), insula, hippocampus and amygdala, in people with AUD compared to healthy controls (HC) (Bjork, Smith, & Hommer, 2008; Nestor et al., 2017; Romanczuk‐Seiferth et al., 2015). The heterogeneity of subjects and variability in experimental paradigms and imaging protocols have led to discrepancies in describing the etiology of the disorder.
Given these relatively inconsistent findings, two recent meta‐analyses have attempted to address the question of the neural bases of reward processing in addiction, including gambling disorder and substance use disorder (SUD), from the perspective of the distinct stages of reward processing. Nevertheless, despite the findings of the previous two meta‐analyses, whether disorder‐specific abnormalities exist in AUD remains unknown. First, compared to SUD, gambling disorder showed different motivation and behavior toward gambling cues and monetary rewards, which could serve as stronger conditioned reinforcers (Flack & Morris, 2015; Lostutter et al., 2019). Individuals with gambling disorders have been shown to display greater attentional bias to monetary cues and gambling environments (such as flashing lights and sounds) (Noseworthy & Finlay, 2009). Gambling disorder sensitizes the incentive value attributed to gambling and particularly cues that have been associated with gambling. The same applies to alcohol such that alcohol‐related cues also trigger cue approach and alcohol‐seeking behavior (Zeng et al., 2021). Therefore, monetary‐related cue reactivity in gambling disorder appears to be more similar to the alcohol cue reactivity response than the monetary neural response in alcohol addiction. As proposed in a recent review, the widespread use of money as a reinforcer creates some neglected conceptual problems in gambling and substance addiction research (Clark et al., 2019). Considering the effect of the reinforcer type on behavior, it is necessary to clarify the unique neural correlates of monetary reinforcement processing in alcoholism. Second, abundant neuroimaging evidence suggests that the neural processes underpinning reward processing differ between pathological gambling and SUD (Romanczuk‐Seiferth et al., 2015; Tanabe et al., 2007; Worhunsky et al., 2014). As shown in one study involving gambling disorder patients, significantly higher activation during loss anticipation in the posterior proportion of the striatum was reported compared with the AUD patients (Romanczuk‐Seiferth et al., 2015). Additionally, Genauck and colleagues observed altered loss‐related activity in lateral prefrontal regions in AUD subjects and altered amygdala‐prefrontal functional connectivity in gambling patients (Genauck et al., 2017). Furthermore, within the SUD group, several studies have demonstrated that there are differences in the activation of reward circuits induced by monetary rewards among patients with alcohol dependence, cocaine dependence, and nicotine dependence (Addicott et al., 2019; Balodis & Potenza, 2020; Bustamante et al., 2014; Grodin et al., 2016). Third, several methodological aspects of the included studies, such as the group specification (e.g., different patient samples) and processes (e.g., different task paradigms), should be considered in the interpretation and generalizability of the meta‐analysis results (Muller et al., 2018). Regarding the included samples, the authors applied broad inclusion criteria and included a series of addiction studies (including alcohol, cannabis, cocaine, nicotine, and gambling addictions) (Luijten et al., 2017; Qiu & Wang, 2021). The inclusion of mixed samples may have introduced confounding factors and biased the results of the two studies. Regarding the included task paradigms, two recent meta‐analysis studies were performed across relatively large, different types of experimental paradigms (monetary incentive delay task, Iowa gambling task, reward prediction task, and card guessing game) (Luijten et al., 2017; Qiu et al., 2021). The presence of notable heterogeneity in the task paradigms combined with the bias introduced by including region of interest (ROI) analyses may explain the inconsistencies among studies. To map the potentially distinctive neural profiles associated with AUD, it is necessary to investigate the disorder‐specific biological mechanisms underpinning AUD during different stages of reward responding.
To address this issue, we conducted a voxel‐based meta‐analysis of the available whole‐brain functional magnetic resonance imaging (fMRI) studies to determine the most prominent and replicable areas to elucidate disorder‐specific brain responses to monetary reward during the anticipation phase and outcome phase in AUD patients and controls. The analyses were restricted to studies that recruited AUD patients and used the MID paradigm to reduce sample and task heterogeneity. We also explored the impact of demographic or clinical factors on MID‐related brain responses. Based on the literature reviewed above, we hypothesized that different stages of reward processing could be characterized by different brain activity patterns in response to nondrug rewards in AUD patients.
2. METHODS
2.1. Search strategy and data sources
The purpose of this meta‐analysis was to compare MID‐related brain activation differences between AUD patients and HC. A systematic and comprehensive two‐step literature search was completed by two investigators to identify relevant articles. First, we searched PubMed (https://www.pubmed.org), ScienceDirect (https://www.sciencedirect.com), Google Scholar (https://www.scholar.google.com), and Web of Science (https://www.webofknowledge.com) for all articles published in English from January 2000 to June 2022. The following search terms were used as keywords: (1) “alcohol dependence” OR “alcoholism” OR “alcohol abuse” OR “alcohol use disorder” OR “AUD” OR “AD”; (2) “monetary incentive delay” OR “MID” OR “modified monetary incentive delay”; and (3) “functional magnetic resonance imaging” OR “neuroimaging” OR “fMRI”. Second, we manually checked the reference lists of the retrieved articles to identify additional relevant articles. The corresponding authors were invited by e‐mail to provide additional details not included in the original manuscript. The above procedures followed the recommended guidelines defined in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (Moher et al., 2009).
2.2. Selection criteria and data extraction
Studies were eligible for inclusion if they met the following criteria: (1) AUD patients were diagnosed based on the diagnostic criteria in the DSM or International Statistical Classification of Diseases and Related Health Problems (ICD) diagnostic criteria; (2) whole‐brain functional activity was compared between AUD patients and HC; (3) the MID or modified MID task was used; and (4) the Montreal Neurological Institute (MNI) or Talairach coordinates were used.
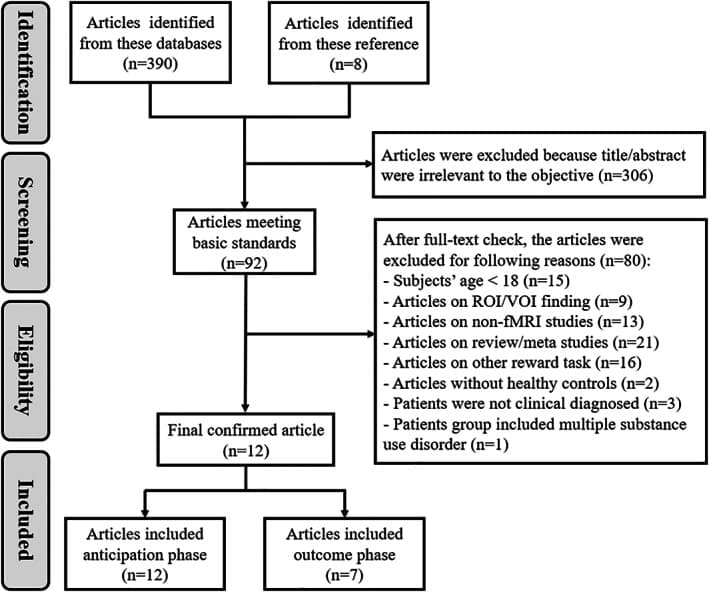
Studies were excluded from the final meta‐analysis for the following reasons: (1) subjects were younger than 18 years; (2) only ROI or volume of interest findings were reported; (3) the study was a literature review or meta‐analysis; (4) the article was based on non‐fMRI techniques; and (5) the study used other reward tasks. One study was excluded because the addiction populations consisted of alcohol‐dependent individuals and patients dependent on multiple substances (e.g., cocaine and opioids) (Nestor et al., 2020). Three other studies were excluded because the problem drinkers in the patient group did not meet any diagnostic criteria (Grodin et al., 2018; Tong et al., 2020; Yau et al., 2012). Two studies without a control group were not included in our analysis (Perini et al., 2020; Quelch et al., 2017).
2.3. Voxel‐wise meta‐analysis
We conducted meta‐analysis comparisons between AUD patients and HC during different reward phases using seed‐based d mapping (SDM) software (version 5.15, https://www.sdmproject.com, formerly “signed differential mapping”). Briefly, SDM uses standard effect size and variance‐based meta‐analysis calculations and allows the combination of statistical parametric maps and peak coordinates from the original studies (Radua et al., 2014; Radua & Mataix‐Cols, 2009). SDM adopts and combines various positive features of activation likelihood estimate (ALE) and multilevel kernel density analysis, two main tools for meta‐analysis of neuroimaging data. Compared to ALE, SDM has the following advantages or differences. First, SDM allows the combination of studies with available statistical parametric maps and studies that report only peak coordinates, constituting an improvement over ALE. Second, regarding effect sizes, SDM aims to estimate the effect size, whereas ALE aims to estimate the peak likelihood (Radua et al., 2014). Third, SDM can be used to recreate maps of the signed (i.e., positive and negative) functional activation or differences between patients and HC by using the reported peak coordinates, which makes SDM an optimal method for comparing AUD patients and HC without biasing the results (Radua et al., 2009). This feature of SDM prevents spurious overlap between the two categories of localization information in ALE, that is, a particular voxel erroneously reported as simultaneously exhibiting both increased and decreased activation (Eickhoff et al., 2012). Fourth, unlike ALE, studies reporting no significant group differences can also be included in SDM. Fifth, regarding study weighting, SDM implements random‐effects models in which each study is weighted by the inverse of the sum of its variance plus the calculated between‐study variance (Radua et al., 2012a).
The meta‐analysis strictly followed the following steps (Radua et al., 2012b). First, we extracted the peak coordinates and corresponding effect sizes of the task‐evoked brain activation differences between AUD patients and HC from the original research articles and prepared text files for SDM software. Notably, we converted the Z‐value into a t‐value by an online converter (https://www.sdmproject.com/utilities/?show=Statistics). Second, we recreated a standard MNI map of activity aberrances by using an anisotropic unnormalized Gaussian kernel for each study. Third, the maps were combined with a random‐effects generalized linear model and weighted by the sample size, intra‐study variability and inter‐study heterogeneity such that studies with larger sample sizes or lower variance contributed more (Radua et al., 2012b). Finally, the statistical significance of each voxel was determined using standard randomization tests (Radua et al., 2010). The default kernel size and thresholds in SDM (p < .005 with a peak height Z > 1 and a cluster extent >10 voxels) were used in this meta‐analysis, which was considered to optimally balance the sensitivity and specificity (Radua et al., 2012b). To further limit the risk of false‐positive errors in meta‐analyses arising from multiple comparisons, we also corrected our default threshold of p < .005 with the Bonferroni method, giving p < .0025 (.005/2 = .0025).
2.4. Conjunction analysis and contrast analysis
Moreover, conjunction analysis and contrast analysis were performed to reveal the shared or distinct reward responses between the anticipation phase and outcome phase, as described in corresponding meta‐analyses (Brandl et al., 2022; Fouragnan et al., 2018; Yaple et al., 2021). Specifically, we ran a conjunction analysis between the two components to formally quantify the degree of overlap between anticipatory and outcome brain activity and ran a contrast analyses between anticipation and outcome groups to identify the areas unique and specific to the anticipation and outcome stages.
2.5. Analyses of subgroups
To control for any possible differences observed across the studies, the analyses were repeated several times to include only those studies that were methodologically homogenous. Therefore, the analyses were repeated for studies including AUD individuals without other Axis I psychiatric comorbidities, studies including AUD patients without SUD comorbidities, except for nicotine, studies including off‐medication patients, and studies using SPM software.
2.6. Sensitivity analysis
Additionally, we performed whole‐brain voxel‐based jackknife sensitivity analysis to assess the robustness of the results. The reliability analysis of the pooled analysis results consisted of repeating the main statistical analysis several times during the reward anticipation stage and reward outcome stage while removing individual studies and calculating the stability of the findings using the remaining studies. The jackknife sensitivity analysis revealed the percentage of study combinations that produced a significant result out of all study combinations in one specific cluster. It is concluded that the results are highly replicable if previously significant brain regions remain significant in all or most study combinations (Radua et al., 2009).
3. RESULTS
3.1. Brain response differences between AUD patients and HC during the reward anticipation phase
3.1.1. Included studies and sample characteristics
Figure 2 shows the flow of the identification and attrition of the studies. Eleven studies enrolled in this meta‐analysis comprised a total of seventeen datasets comparing patients with AUD and HC during the reward anticipation phase (Beck et al., 2009; Becker et al., 2017; Bjork, Knutson, & Hommer, 2008; Bjork et al., 2012; Grodin et al., 2016; Groefsema et al., 2020; Hagele et al., 2015; Murphy et al., 2017; Musial et al., 2023; Nestor et al., 2017; Romanczuk‐Seiferth et al., 2015; Wrase et al., 2007). These studies included 263 patients with AUD (mean age = 38.88; percentage of men = 79.82%) and 317 HC (mean age = 37.73; percentage of men = 76.93%). There were no significant differences in age (t = −.291, p = .774) or sex (χ 2 = .447, p = .504) between the AUD patients and HC. Table 1summarizes the clinical and demographic data from all included studies.
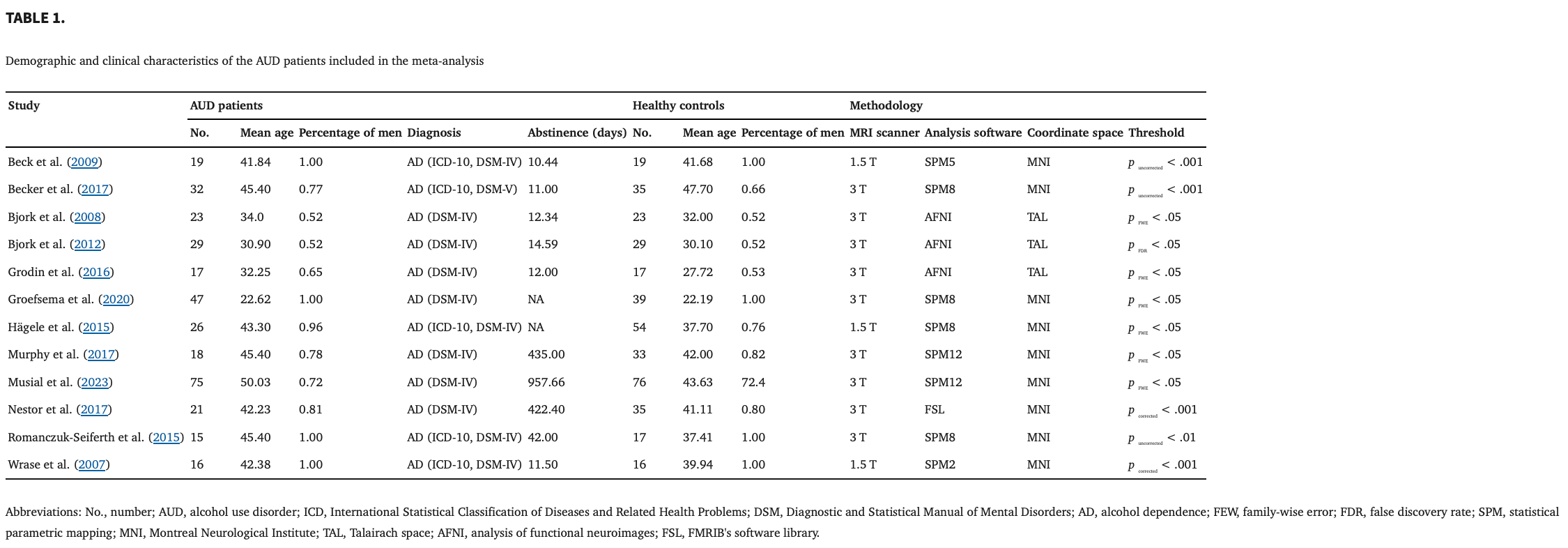
3.1.2. Main meta‐analysis
Relative to the HC, the AUD patients exhibited reduced activity in the right VS, right orbital frontal cortex (OFC), ACC, right insula, right hippocampus, left inferior occipital gyrus (extending into the cerebellum), right supramarginal gyrus, left lingual gyrus and left fusiform gyrus during the reward anticipation phase (Figure 3 and Table 2). Bonferroni correction did not change the significance of any brain regions in the anticipation meta‐analysis.
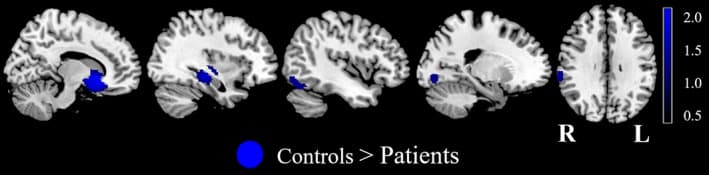 Table 2
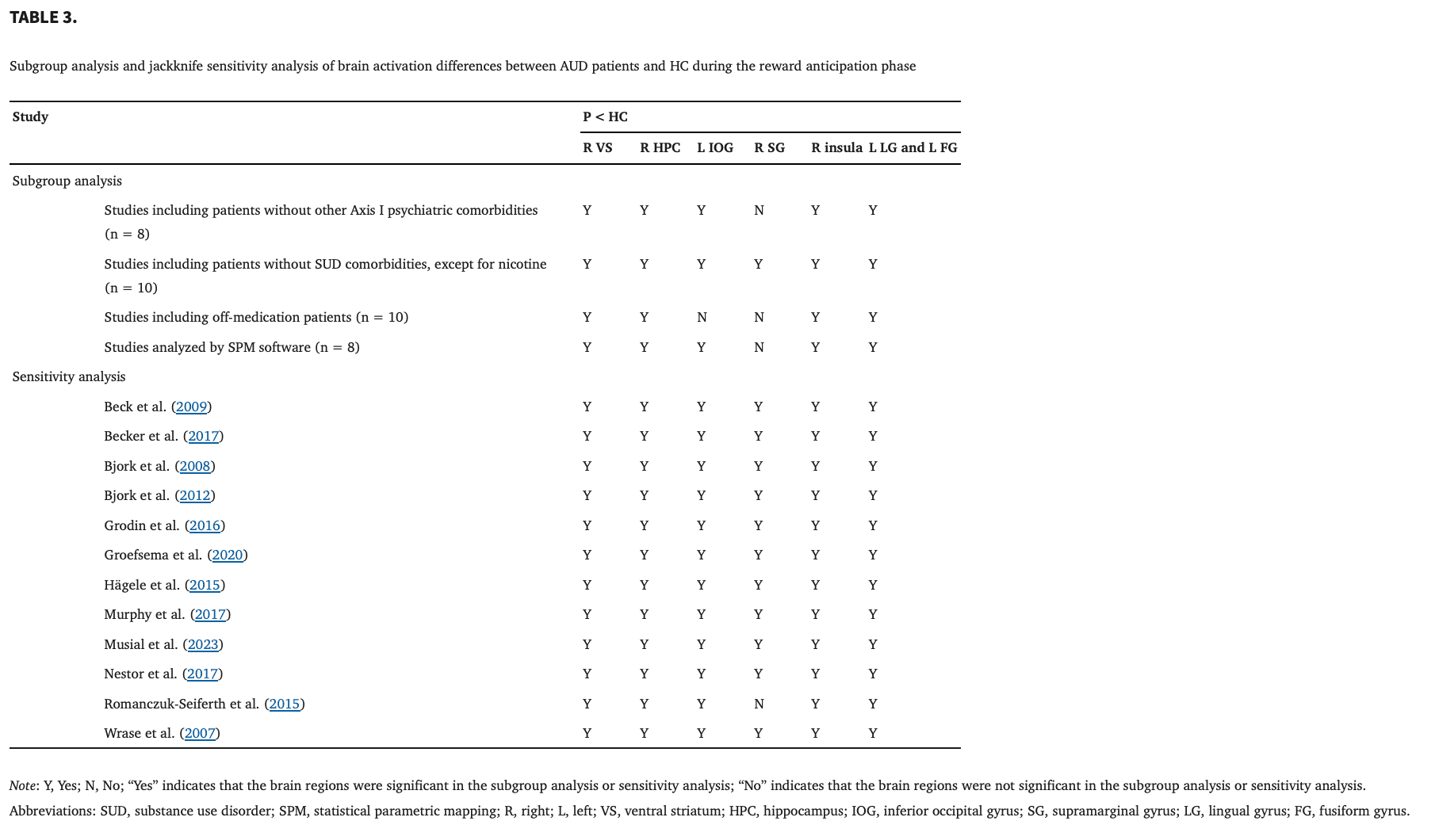
Table 23.1.3. Analyses of subgroups
The above results remained largely unchanged when the analyses were repeated and limited to methodologically homogenous groups of studies. The results of the subgroup analysis of the studies including patients without other Axis I psychiatric comorbidities shared all clusters with the results of the pooled meta‐analysis, except for the right supramarginal gyrus. The results of the subgroup analysis of the studies including patients without SUD comorbidities, except for nicotine, shared all clusters with the results of the pooled meta‐analysis. The results of the subgroup analysis of the studies including off‐medication patients shared all clusters with the results of the pooled meta‐analysis, except for the left inferior occipital gyrus and right supramarginal gyrus. The results of the subgroup analysis of the studies analyzed by SPM software shared all clusters with the results of the pooled meta‐analysis, except for the right supramarginal gyrus (Table 3).
3.1.4. Sensitivity analysis
As shown in Table 3, the whole‐brain jack‐knife sensitivity analysis showed that the decreased blood oxygen level‐dependent (BOLD) activations in the right VS, right insula, right hippocampus, left inferior occipital gyrus, left lingual gyrus and left fusiform gyrus were highly replicable and preserved in all 12 combinations of studies. The findings in the left supramarginal gyrus were significantly replicable in all combinations of studies, except for one.
3.2. Brain response differences between AUD patients and HC during the reward outcome phase
3.2.1. Included studies and sample characteristics
During the reward outcome phase, in total, 12 datasets were extracted from seven studies (Beck et al., 2009; Bjork, Knutson, & Hommer, 2008; Bjork et al., 2012; Grodin et al., 2016; Groefsema et al., 2020; Nestor et al., 2017; Romanczuk‐Seiferth et al., 2015) comprising 171 AUD patients (mean age = 33.05; percentage of men = 79.53%) and 179 HC (mean age = 32.55; percentage of men = 78.61%). There were no significant differences in age (t = .022, p = .983) or sex (χ 2 = .044, p = .834) between the AUD patients and HC.
3.2.2. Main meta‐analysis
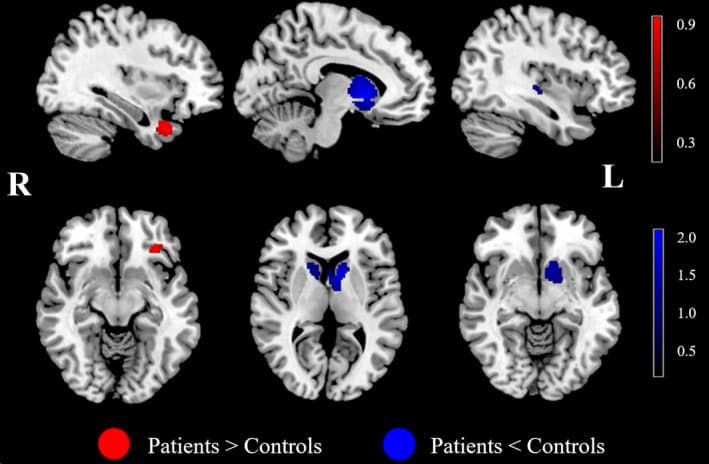
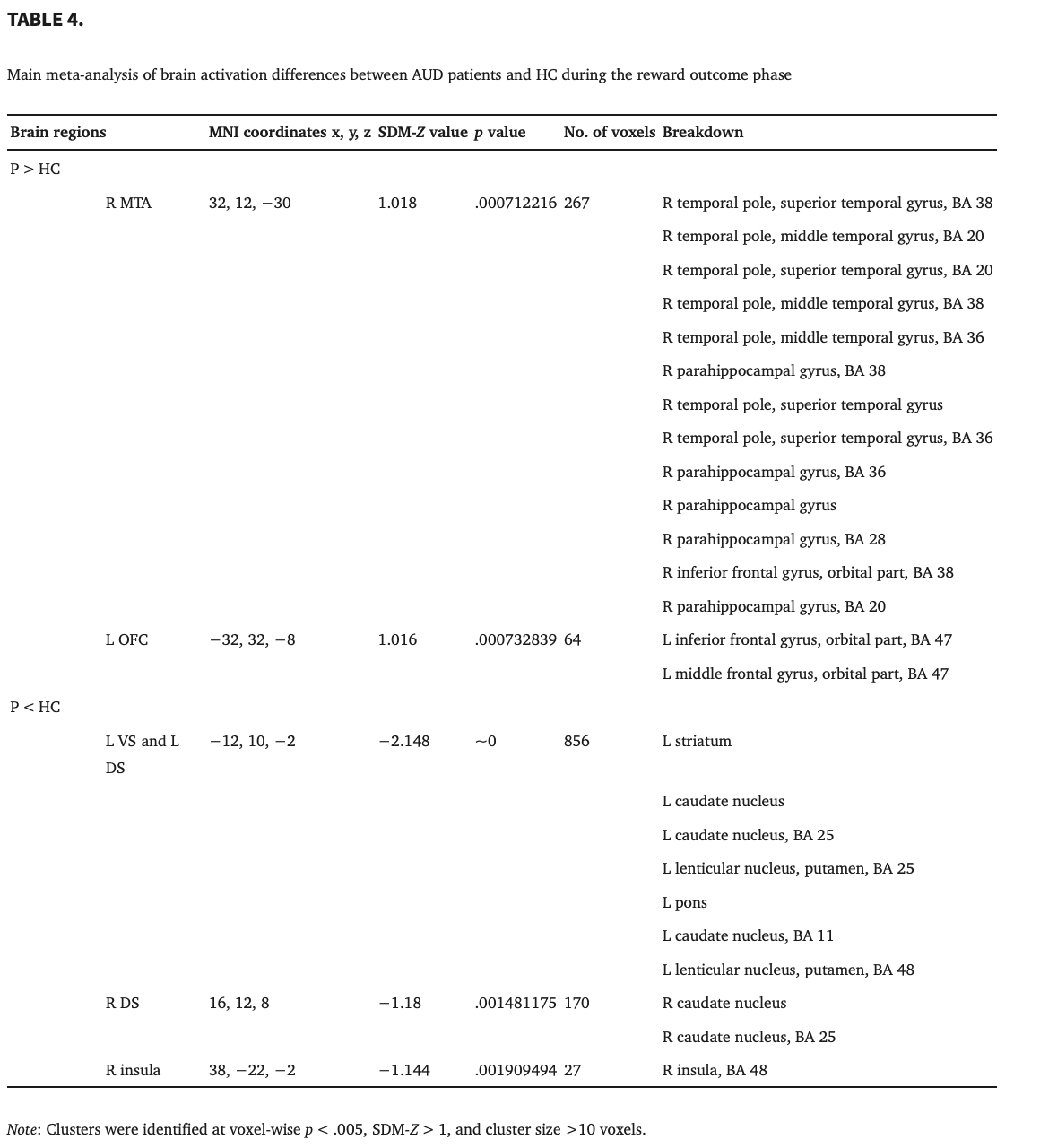
The AUD patients showed higher activations in the left OFC and right medial temporal areas (MTA, including the superior temporal gyrus, parahippocampal gyrus and temporal lobe) than the HC. In contrast, significant deactivation was observed in the bilateral DS, left VS and right insula during the reward outcome phase (Figure 4 and Table 4). These significant results survived Bonferroni correction, except for the insula in the outcome phase meta‐analysis.
3.2.3. Analyses of subgroups
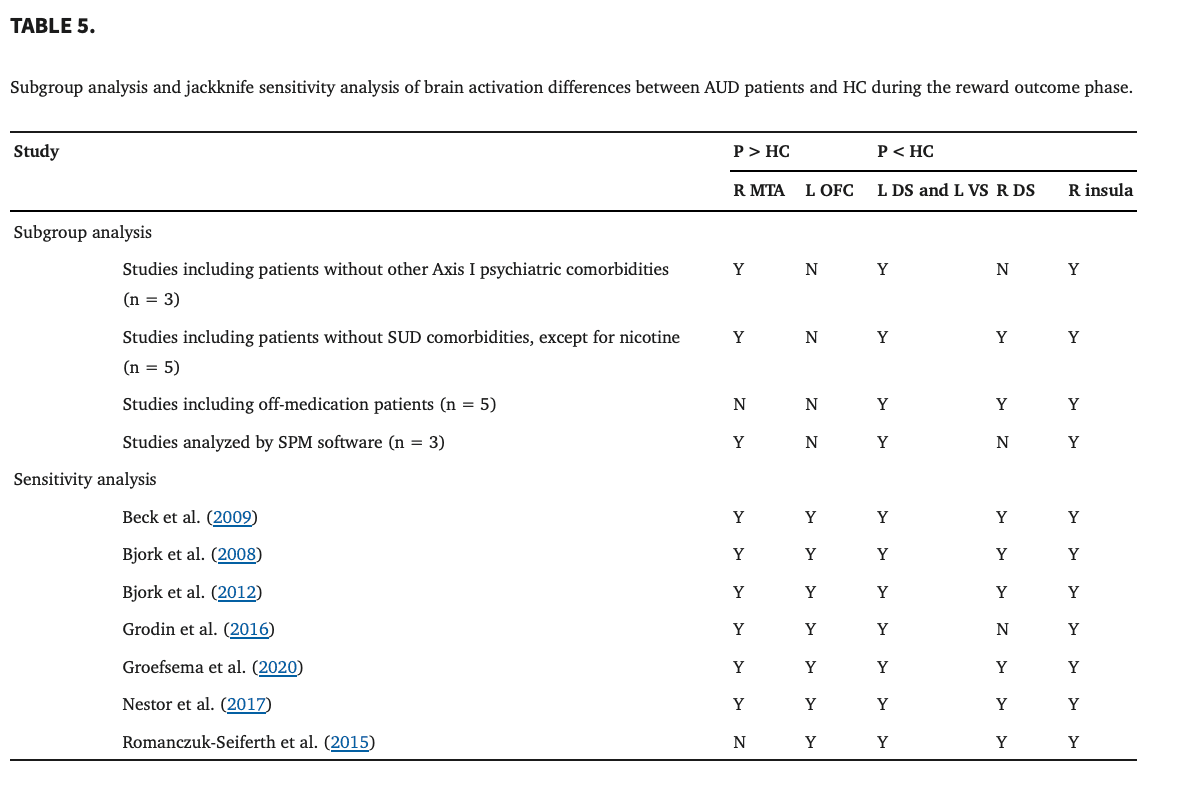
These results were broadly consistent with the main findings. The results of the subgroup analysis of the studies including patients without other Axis I psychiatric comorbidities and studies analyzed by SPM software shared all clusters with the results of the pooled meta‐analysis, except for the left OFC and right DS. The results of the subgroup analysis of the studies including patients without SUD comorbidities, except for nicotine, shared all clusters with the results of the pooled meta‐analysis, except for the left OFC. The results of the subgroup analysis of the studies including off‐medication patients shared all clusters with the results of the pooled meta‐analysis, except for the right MTA and left OFC (Table 5).
3.2.4. Sensitivity analysis
The results in the right MTA with increased BOLD activity showed high replicability in the combinations of all studies, except for one, by using a whole‐brain jack‐knife sensitivity analysis. Moreover, the whole‐brain jack‐knife sensitivity analysis showed that hypoactivation in the left DS and left VS remained significant in all combinations of studies. The right DS was significant in all combinations of studies, except for one. The right insula was significant in all combinations of studies (Table 5).
3.3. Shared or distinct regions between reward anticipation and reward outcome
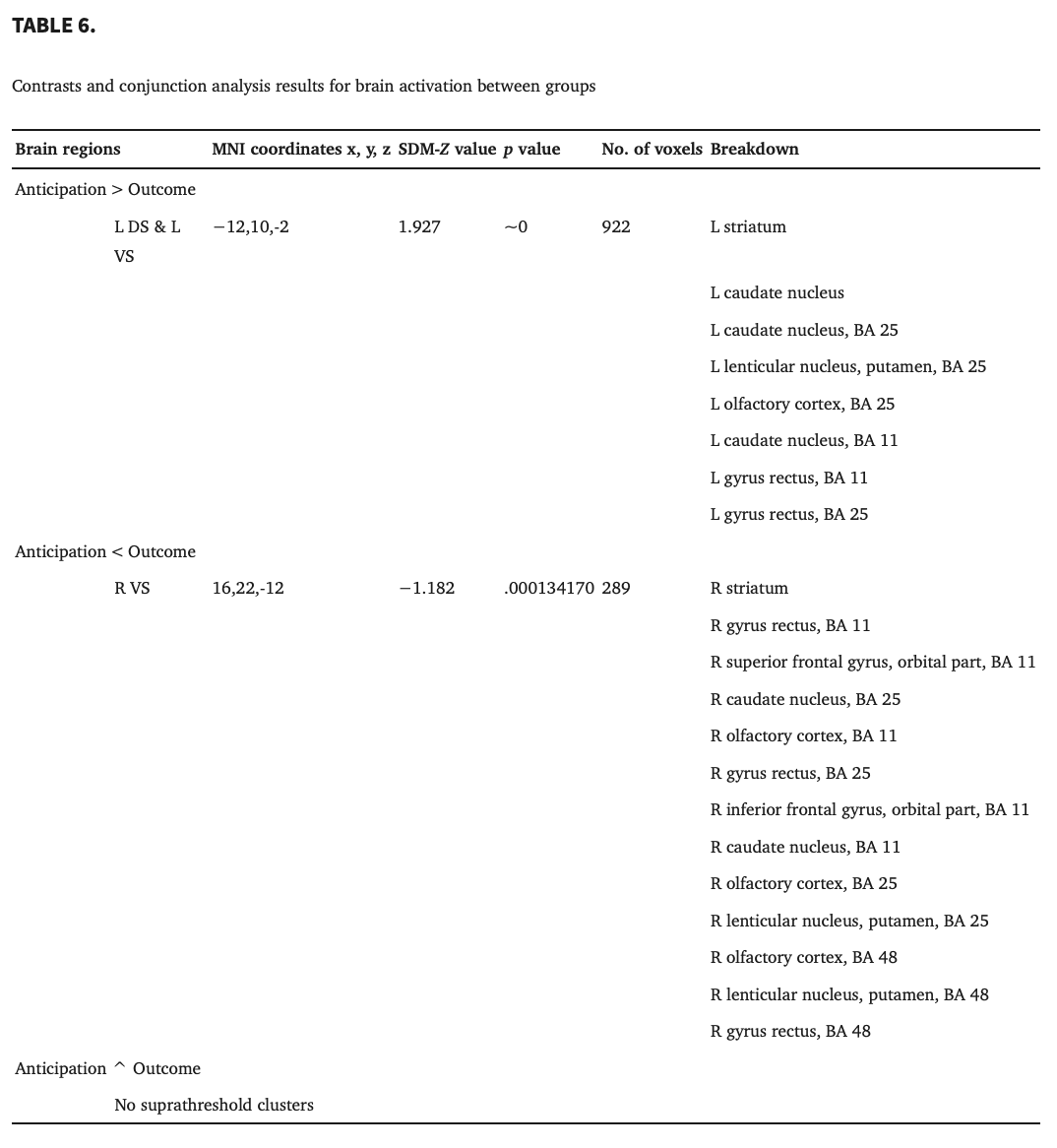
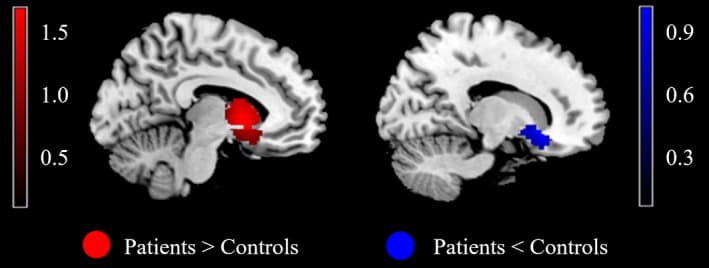
Conjunction analysis of brain activity between reward anticipation and outcome revealed no significant finding (Table 6). The statistical map resulting from the overlay of two separate statistical maps for anticipation and outcome (as shown in Figures 3 and 4) is shown in Figure 5 and Table 6. A comparison of the anticipation > outcome contrast revealed greater concordant activity within the left VS and left DS. The right VS was found to exhibit the reverse pattern (anticipation < outcome).
4. DISCUSSION
In the present voxel‐based meta‐analysis study, our results revealed different brain activation patterns in AUD patients during the anticipation and outcome phases, perhaps suggesting a disrupted dopaminergic motivational response toward nondrug rewards. During the anticipation phase, compared with the HC, individuals with AUD showed attenuated activation in the mesocortical‐limbic circuit and sensory system, including the VS, insula, hippocampus, inferior occipital gyrus, supramarginal gyrus, lingual gyrus and fusiform gyrus. During the outcome phase, the AUD patients showed increased activation in response to monetary reward in the OFC and MTA and reduced activation in the DS, VS and insula. In brief, these results suggest altered recruitment of mesolimbic incentive neurocircuitry by cues and deliveries of nondrug rewards, and these differences help explain the impairment in incentive motivational processing toward conventional rewards in AUD patients.
4.1. Monetary cue‐elicited anticipatory response
Our meta‐analysis revealed that during reward anticipation, the patients with AUD showed more robust hypoactivation in the mesocortical‐limbic circuit, including the VS, OFC, ACC, hippocampus and insula, than the controls. This circuit, which has been extensively implicated due to its involvement in the rewarding properties of both neutral stimuli and drug‐related stimuli in addiction (Feltenstein & See, 2008), consists of dopamine projections from cell bodies in the ventral tegmental area (VTA) to limbic structures (that is, the mesolimbic pathway, which includes the VS, amygdala, insula, and hippocampus) and cortical areas (that is, the mesocortical pathway, which includes the prefrontal cortex [PFC], OFC and ACC). The mesolimbic dopaminergic pathway between the VTA and the nucleus accumbens plays a central role in motivational behaviors and reinforcement learning (Yang et al., 2018), whereas in the mesocortical pathway, the OFC and ACC primarily project to the ventral and medial parts of rostral striatum cortical regions, which are associated with long‐term strategic planning and habit formation (Haber, 2014). The VS encodes stimulant value, regulates reward‐related behavior, and plays a key role in regulating incentive salience and reward learning (Knutson et al., 2001; Liu et al., 2011; Minogianis et al., 2019; Zink et al., 2004). Consistent with previous data, decreased volume in the VS (Makris et al., 2008; Yang et al., 2016), reduced VS activation (Beck et al., 2009; Wrase et al., 2007), and weaker functional connectivity between the striatum and prefrontal regions (Becker et al., 2017; Courtney et al., 2013) have been observed in alcohol‐dependent individuals. According to reward deficiency syndrome theory (Blum et al., 2000; Blum et al., 2012), addicted individuals have deficits in the recruitment of the dopaminergic motivational circuitry by nondrug rewards such that drugs are uniquely able to normalize the dopamine levels in the VS to readily motivate drug‐taking behavior. In fact, in AUD individuals, these systems are often “hijacked” by drug cues and show increased sensitivity in motivational neurocircuitry but decreased sensitivity toward nondrug rewards. As previously suggested by incentive sensitization theory (Robinson & Berridge, 2008), addiction is maladaptive stimulus–response learning that involves enhanced drug‐related incentive salience at the expense of natural reward‐related incentive salience. The blunted activation in the mesocortical‐limbic circuit in AUD may indicate that alcohol‐related cues hijack and reorganize the priorities of the reward circuitry such that these cues induce more appetitive behavior than cues for conventional rewards. Taken together, our meta‐analysis confirms that alcohol‐dependent patients show blunted brain responses to monetary anticipation in the mesolimbic and mesocortical pathways, which may underlie the impairment in incentive motivation to conventional rewards.
The recruitment of limbic regions, including the insula and hippocampus, during anticipation reward processing was also observed in our meta‐analysis. The insula has been implicated in incentive motivational processes and risk prediction possibly because of its role in awareness of the interoceptive state (Naqvi & Bechara, 2009). Additionally, compatible with incentive sensitization theory, the AUD patients showed higher insular activity and oversensitized conditioned responses to drug‐related cues but blunted responses to nondrug cues (Nestor et al., 2017; Zeng et al., 2021). The observed diminished insular activation may have resulted from decreased attention arousal toward nondrug cues, suggesting reduced reward sensitivity and altered stimulus salience.
The hippocampus is especially critical for many domains of learning and memory and, thus, has been implicated in the acquisition, consolidation, and expression of context‐dependent drug memories (Volkow & Fowler, 2000). Numerous studies have shown that excessive alcohol intake impacts the structure and function of hippocampal circuitry (Mechtcheriakov et al., 2007; O'Daly et al., 2012; Stavro et al., 2013; Stephens & Duka, 2008; Vetreno et al., 2011). The neural substrates of memory and conditioned learning are among the major circuits underlying the aberrant neuroadaptations in response to chronic drug exposure. Even low‐dose ethanol intake may result in hippocampal dysfunction, including disrupted short‐term and long‐term memories of drug‐context associations, which may contribute to future alcohol intake (Kutlu & Gould, 2016). Therefore, disruption of memory reconsolidation in hippocampal function may help erode the strong associations between context and drugs. Regarding nondrug rewards, the AUD patients showed a reduced response to natural reinforcers, which may contribute to alcohol addiction by interfering with the acquisition of adaptive behavior. Such observations have provided insight into the disruption of memory reconsolidation during stimulus salience processing.
The present study also found that dysfunction associated with the monetary anticipated response recruited several sensory areas, including the inferior occipital gyrus, supramarginal gyrus, lingual gyrus and fusiform gyrus. Prior studies indicated that monetary reward processing can be reflected in early visual processing as shown by the reduced responsiveness in the occipital lobe, parietal regions and related visual cortex during the pictorial presentation of monetary cues (Qiu et al., 2021). This result emphasizes the importance of the attentional processing of visual information and sensory neurocircuits for nondrug cues with reward properties in alcohol addiction.
4.2. Monetary cue‐elicited outcome responses
Regarding reward outcomes, our results showed attenuated activation in the mesolimbic dopaminergic pathway, including the DS, VS and insula, and enhanced activation in the temporal lobe and OFC in the AUD patients compared with the controls. The AUD patients exhibited decreased BOLD signals in the striatal‐limbic circuitry during monetary reward receipt, which is similar to the pattern observed in the anticipation stage but stronger in the dorsal part of the striatum rather than the ventral part. Similar to animal studies, learning about actions and their reward consequences tends to involve the DS more than appetitive learning, which has been found to depend on the VS (Balleine et al., 2007; Lex & Hauber, 2010; Yin et al., 2005). The caudate is involved in action‐outcome learning that subserves goal‐directed action, whereas the putamen appears to link cognitive functions that are more limited to stimulus–response coding or habit learning (Grahn et al., 2008; O'Doherty et al., 2004). During the outcome evaluation, the comparison of the actual outcome with the expectation generates a reward prediction error signal, which is thought to regulate flexible decisions by updating the reward values associated with available actions (Beylergil et al., 2017; Schultz, 2015). If the goal value changes or becomes less appropriate for satisfying the needs of the individual, then the goal changes and behavior is adapted accordingly. The responsiveness of the DS to monetary incentives may be decreased because monetary stimuli no longer represent an appetitive goal. This notion is supported by the findings of altered sensitivity in the dorsal caudate nucleus to the magnitude of reinforcement during the feedback phase in individuals who engage in problem drinking (Joseph et al., 2015). The insula has been previously shown to be recruited by task conditions involving uncertain outcomes, such as choosing a risky response option in a decision‐making task. Previous evidence has revealed insular hypoactivation during the feedback phase (Nestor et al., 2017; Romanczuk‐Seiferth et al., 2015). These outcome findings are also consistent with the reward deficiency syndrome hypothesis, which might suggest that individuals with AUD have deficits in recruiting the dopaminergic motivational circuitry in response to nondrug rewards, which hinders striatal‐limbic circuitry learning and influences subsequent decisions.
Alcohol‐dependent patients also exhibited significantly increased brain activation in the OFC compared with the controls during the outcome phase. The OFC has important functional connections with the striatum and is known to control flexible, goal‐directed behavior and be associated with reward identification and acquisition (Kringelbach, 2005). The encoding of stimuli is also strongly modulated by and associated with value in the OFC, which, in turn, contributes to the overall value of outcomes and facilitates subsequent decisions and actions (Moorman, 2018). A previous study showed that the OFC was more strongly activated in a series of tasks with some uncertainty in outcomes (Schnider et al., 2005). The hyperactivity in the OFC might suggest its role in encoding appetitive value and monitoring reward outcomes.
Our meta‐analysis also revealed notably increased activity within the MTA, including the superior temporal gyrus, and adjacent structures, such as the parahippocampal gyrus and temporal lobe. Since medial temporal cortical regions are strongly interconnected with most multimodal areas, this cortical area can be viewed as the supramodal cortex, where all sensory cortical channels converge (Strange & Dolan, 2006). Additionally, medial temporal lobe subsystems become engaged when decisions involve constructing a mental scene based on memory (Andrews‐Hanna et al., 2010). Our results are consistent with the fMRI findings of greater activation in the anterior portion of the superior temporal gyrus and the temporal pole observed in marijuana users (Smith et al., 2010). Altogether, these results suggest that alcohol‐dependent patients recruit temporal areas when receiving a reward involving sensory and memory‐related processing and for successfully performing the task.
4.3. Difference between anticipation responses and outcome responses induced by monetary cue
Supporting the idea that separate neural systems participate in reward anticipation and outcome, our contrast analysis revealed enhanced responses in the left VS and DS for the anticipation > outcome contrast and reduced activity in the right VS for the anticipation < outcome contrast. Accumulating evidence suggests that the striatum is a subcortical structure rich in dopaminergic neurons and important in reward learning (Chen et al., 2015). It has been proposed that the VS is associated with assigning salience to reinforcers, incentive motivation, and tracking reward value (Liu et al., 2011; Tricomi & Lempert, 2015; Zink et al., 2004), whereas the DS is more involved in habit formation and encoding stimulus–reward associations (Yin et al., 2005), especially the (rewarding) consequences of actions (O'Doherty et al., 2004). We found that patients with AUD exhibited reduced activity predominantly in the dorsal corticostriatal circuit during the outcome stage, which may indicate disrupted encoding of the response outcome during reward learning. The dysfunction of “dorsal stream” during response‐outcome association learning contributes to the transition from initially volitional use of alcohol to ultimately compulsive and chronic alcohol consumption in AUD patients (Chen et al., 2011; Klugah‐Brown et al., 2020). This result aligns with the reward deficiency syndrome hypothesis, which proposes that habit formation exerts a greater influence during reward outcome. Additionally, the meta‐analysis revealed that AUD patients showed attenuated activation in the DS and VS, supporting this hypothesis. The activation differences in striatal subregions observed in the contrast analysis as well as the nonsignificant results of the conjunction analysis, may indicate that the VS and DS play different roles in anticipation and outcome. Therefore, future research should focus on the distinct contribution of striatal subregions in different stages of reward processing.
4.4. Effect of medication
Accumulating evidence has shown that detoxification treatment, even on an acute time scale, contributes to the normalization of brain function and structure in patients with AUD (Burnette et al., 2021; George et al., 2008; Lukas et al., 2013; Son et al., 2015; Vollstadt‐Klein et al., 2019; Wang et al., 2018). To explore the potential effects of medication on the MID‐related brain response, it is better to directly compare treated patients with treatment‐naïve individuals or perform pretreatment and posttreatment comparisons or treatment‐placebo comparisons. In fact, most treatment studies investigating neuroimaging markers of AUD did not examine reward paradigms but rather focused on alcohol‐related stimulus processing using cue‐reactivity paradigms (Beck et al., 2018; Logge et al., 2021). To address these issues, we conducted subgroup analyses of AUD patients who underwent an off‐medication period before MRI scanning given that there were insufficient data to perform comparisons between drug‐treated and drug‐naïve groups, between pretreatment and posttreatment groups, and between treatment and placebo groups. Most of the results of the off‐medication subgroup analyses were consistent with the pooled analysis, except for the blunted response to reward in the left inferior occipital gyrus and right supramarginal gyrus during the anticipation stage. Pharmacological manipulation may slightly change sensory processing and normalize attention distribution in AUD patients, followed by the transient off‐medication period, probably due to the delayed onset of drug action and maintenance (Chen & Skolnick, 2007). Our findings are in line with evidence from imaging studies indicating that detoxification treatment can influence neural activation and functional connectivity in patients with AUD (Burnette et al., 2021; Lukas et al., 2013; Vollstadt‐Klein et al., 2019). Previous functional MRI studies have indicated improved supramarginal gyrus activity in AUD patients in the emotional faces task after receiving nalmefene (Vollstadt‐Klein et al., 2019). In addition, evidence suggests that compared to the placebo group, patients with cocaine dependence exhibited enhanced cue‐elicited activation in occipital areas after receiving d‐cycloserine (Prisciandaro et al., 2013). During the reward outcome stage, the results of the off‐medication subgroup analyses were consistent with the pooled analysis, except for brain activity in the right MTA and left OFC. A recent study observed that OFC activation was significantly decreased after 6 weeks of topiramate treatment in heavy drinkers (Wetherill et al., 2021). Our results may suggest that pharmacological intervention could help normalize the hyperactivation of the OFC in patients with AUD. The MTA is strongly implicated in novelty and unexpected processing (Murty et al., 2016). This area has been shown to be involved in emotional memory, impacting craving symptoms (Kakko et al., 2019; Koob & Volkow, 2010). Many studies using a multimodal imaging approach consistent with our results have shown that detoxification treatment contributes to the normalization of temporal lobe function (George et al., 2008; Morris et al., 2018; Son et al., 2015; Wang et al., 2018). Thus, we cannot fully rule out the effect of medication given the known different medication use profiles and complicated side effects profiles.
4.5. Shared or distinct neural mechanisms underlying reward processing among patients with AUD, schizophrenia, and depression
Altered brain activation during reward processing in similar brain regions is frequently observed in patients with AUD and other mental disorders, such as schizophrenia and depression. Human studies have attempted to elucidate the shared or distinct reward processing features to understand the biological mechanisms of neuropsychiatric disorders. Interestingly, BOLD fMRI studies in AUD showed some reward‐related mesocorticolimbic abnormalities similar to those observed in depression and schizophrenia. Accordingly, several recent studies and meta‐analyses reported reduced VS activation during reward anticipation in schizophrenia (Chase et al., 2018; Radua et al., 2015; Schwarz et al., 2020); decreased activation in subcortical and limbic regions during both monetary anticipation and outcome in depressed adults (Ng et al., 2019; Pizzagalli et al., 2009; Zhang et al., 2013); and decreased striatal activation during reward anticipation in addiction (Luijten et al., 2017). Similarly, a blunted striatal response during reward anticipation is also associated with greater negative symptom severity in schizophrenia (Simon et al., 2010; Waltz et al., 2010) and anhedonic symptoms in schizophrenia and depression (Dowd & Barch, 2010; Keedwell et al., 2005). The dopaminergic mesocorticolimbic pathway is important for mediating reinforcement learning and decision‐making processes (Pizzagalli et al., 2008). It is assumed that blunted reward responsiveness leads to decreased engagement in pleasurable activities and a decreased motivational drive to obtain future rewards (Clery‐Melin et al., 2019; Pizzagalli et al., 2008). Thus, VS blunting may suggest a reduced sensitivity of the reward system and the possibility of a common psychobiological substrate among AUD, schizophrenia and depression. Furthermore, clinical and epidemiologic studies have found a high frequency of the co‐occurrence of AUD and psychiatric disorders (Hasin et al., 2005; Hunt et al., 2018). As previously suggested, the shared reward‐related brain circuit could be due to the association of many chronic psychiatric illnesses with increased substance abuse, particularly alcohol abuse and increased smoking, which can lead to compromised brain abnormalities.
In contrast, during feedback of monetary processing, AUD individuals showed greater activity in temporal regions and the orbital frontal lobe. These regions are involved in the computation of expected values and multisensory perception and have been widely reported in the monetary reward processing literature. In contrast, it has been suggested that depressed patients display increased activation in the middle frontal gyrus and ACC during reward anticipation (Zhang et al., 2013). However, other studies report hyperactivity or hypoactivity in the striatum during monetary receipt in schizophrenia (Abler et al., 2008; Kirschner et al., 2018; Li et al., 2018). These findings reveal distinct neural substrates for reward processes and their relevance for mental illness.
4.6. Translational implications
The National Institute of Mental Health (NIMH) has launched the Research Domain Criteria (RDoC) initiative to identify pathophysiological mechanisms that are common across multiple psychiatric disorders and mechanisms that are unique to specific psychiatric symptoms (Insel et al., 2010; Insel & Cuthbert, 2015). There is growing interest in utilizing a translational approach to understand reward processing abnormalities in psychiatric illness. Within the Positive Valence Systems domain in the RDoC, reward processing abnormalities in the dopaminergic coding of uncertainty could account for the neurobiological dysfunctions in several psychiatric disorders (Nusslock & Alloy, 2017). Importantly, considering reward a multifaceted process, progress has been achieved in not only parsing the psychological components of reward but also identifying the neural substrates associated with each component (Baskin‐Sommers & Foti, 2015). Thus, rather than conceptualizing abnormal reward processing as a relatively global dysfunction (i.e., decreased vs. increased reactivity to rewards overall), our current findings indicate that AUD is characterized by the following specific neural patterns of monetary processing: hypoactivation in the mesocortical‐limbic network and sensory areas during the anticipation stage and reduced activation in the striatum and insula and enhanced activation in the OFC and MTA during the outcome stage. Reward anticipation is delineated within RDoC in terms of incentive salience, which motivates approach toward rewards (Musser & Raiker, 2019). The midbrain dopamine system appears to be primarily responsible for mediating the motivation to obtain monetary rewards (Schott et al., 2008). As previously mentioned, the blunted striatum response during anticipation may suggest a reduced sensitivity to the conditioned reward cue and the possibility of a shared psychobiological substrate among AUD, schizophrenia and depression (Hagele et al., 2015; Wrase et al., 2007). Among individuals with schizophrenia or schizoaffective disorder, alcohol dependence comorbidity is common (Hasin et al., 2005; Hunt et al., 2018). Significant psychiatric comorbidity has reciprocal impacts on the development of each disorder and may partly explain the shared and marked VS dysfunction in salience detection. In contrast, the outcome stage represents the hedonic impact of information and taps the “liking” components of rewards. Within the RDoC framework, the “liking” profile may be associated with hedonic responses and the culmination of reward seeking (Nusslock et al., 2017). The AUD patients exhibited distinctly reduced activation in the DS, VS and insula and increased activation in the MTA and OFC. Hypoactivity in the striatal‐limbic circuitry may indicate impaired reward value updating and adaptive learning in expectation of reward outcomes. Meanwhile, the OFC is critically involved in demanding learning tasks and is important for encoding appetitive value and monitoring reward outcomes. As central to sensory/memory‐related processing, MTA regions are overactivated by monetary cues and may underlie hedonic vulnerability in alcohol addiction. Thus, the present meta‐analysis results extend beyond the limitations of a single‐study approach (e.g., low power and generalizability) and provide converging evidence of partially separable neural circuits for these distinct reward processes.
As a DSM‐5 diagnostic criterion, craving is one of the most selective and specific symptoms across SUD (APA, 2013). In fact, clinical studies have shown that craving is associated with the severity of AUD and relapse to drinking following treatment (Chakravorty et al., 2010; Murphy et al., 2014). At the neural level, neuroimaging studies increasingly investigate the drug craving state and its relevance in drug use and relapse risk (Koob & Volkow, 2016; Sinha, 2013). Cue‐induced craving appears to involve the activation of similar circuits (Seo et al., 2013). The research presented in our recent meta‐analysis suggested that neural cue reactivity may have some clinical relevance as craving was associated with striatolimbic hyperfunctioning during the cue reactivity task (Zeng et al., 2021). Based on these data, these results may imply that craving symptoms are associated with specific neurofunctional alterations in motivational systems and elucidate the clinical significance of craving for improving illness diagnosis in the domain of reward processing.
4.7. Limitations
Several limitations of this study should be highlighted, some of which are inherent to all meta‐analysis methods. First, we could not completely rule out the possibility of publication bias, although we adopted a comprehensive literature search strategy and attempted to include as many unpublished AUD studies as possible, even if their results were negative (Cheung, 2019). Second, the results may not be as accurate as those of image‐based meta‐analyses because voxel‐based meta‐analyses are based on summarized data from published studies (i.e., peak coordinates and effect sizes) rather than raw statistical brain maps from original studies (Salimi‐Khorshidi et al., 2009). However, it is difficult to obtain and analyze original images from these studies. Third, stress is a well‐known risk factor in the development of alcohol dependence and alcohol dependence relapse vulnerability (Heinz et al., 2017). However, we cannot rule out the potential influence of stress on our results. Fourth, few included studies reported the doses of medication or the percentage of participants who had received alcohol treatment, which prevented further meta‐regression analyses. These results should be interpreted with caution and require very careful clinical observation to examine the effects of treatment on reward response. Fifth, as most included studies have different durations of abstinence from alcohol (varied from 0 days to approximately 1.2 years) in our meta‐analysis, special attention should be given to the time course of the incubation of craving along with abstinence. Further longitudinal neuroimaging studies are warranted to clarify the mutual neural substrates during monetary processing involved in the incubation of alcohol craving. Sixth, we included only studies involving adults with AUD in our analysis. Our findings need to be considered with caution when applied to children/adolescents. Finally, whether these brain responses to monetary stimuli in AUD can be distinguished from those in other psychiatric disorders should be simultaneously investigated in patients with AUD and other related illnesses using univariate methods or multivariate pattern analyses in future studies. Similarly, the differences and similarities of dopaminergic monetary‐related reward processes in gambling disorder and SUD need to be further investigated in disorder‐specific studies and transdiagnostic comparison approaches.
5. CONCLUSION
Taken together, this meta‐analysis revealed that individuals with AUD show reduced activation in mesocortical‐limbic circuits and sensory areas during monetary anticipation, and this decreased activation in the striatum was associated with alcohol craving. Furthermore, individuals with AUD displayed stronger reduced activations in the DS, VS and insula and increased activation in the temporal and frontal cortices during outcome receipt. The observed activation patterns during the different reward stages could help elucidate the mechanisms underlying nondrug incentive processing in individuals with AUD that contribute to the pathophysiology of alcohol dependence.